CD56 is a fundamental marker in the determination of human natural killer (NK) cell subsets. The degree of CD56 expression is ubiquitously used to define human NK cell maturation, functional, and tissue-specific subsets, yet a unifying implication for the degree of CD56 expression in NK cells remains elusive. Peripheral blood (PB)-NK cells are dichotomized into CD56bright, which highly express CD56, and CD56dim, which have lower CD56 expression. Classically, CD56bright NK cells are considered to be a less mature, immunoregulatory subset with a greater propensity for cytokine production, whereas CD56dim are more mature with greater cytotoxic abilities. While PB-NK cells are primarily CD56dim with only a minor CD56bright population, NK cells in secondary lymphoid and other tissues are predominantly CD56bright.1 It has been shown that the interaction of CD56 with human fibroblast cells can promote the differentiation of CD56bright to CD56dim NK cells.2 However, considering the importance and the extent of use of this marker in NK cell biology, relatively little is known about how downregulation of CD56 contributes to NK cell maturation, what role the degree of CD56 expression plays in contexts separate from NK cell maturation, and whether CD56 has an active functional role in mature NK cells. Moreover, while CD56bright NK cells were classically considered immunoregulatory, evidence is emerging to suggest that CD56bright NK cells also comprise the most highly cytotoxic of NK cell subsets, producing contradictory implications for CD56 brightness. Given this, we ask: is there a unifying implication for the degree of CD56 brightness across NK cells? Herein, we explore the wide-ranging functions of CD56bright NK cells in order to determine a unified meaning for the degree of CD56 brightness and shed new light on the implications of CD56 brightness in NK cell biology.
In different settings, CD56bright NK cells can have vastly divergent functions. In their most classic definition, they are immunoregulatory and play important regulatory roles in numerous contexts. A seminal study by Hanna et al. revealed that decidual NK cells (dNK), which are superbright for CD56 expression, play a critical role in tissue remodeling during pregnancy.3 They demonstrated that noncytotoxic CD56superbright dNK cells promote trophoblast invasion through secretion of IL-8 and IP-10, and support angiogenesis through secretion of VEGF and PLGF. In contrast to the classic anti-tumor and anti-viral functions induced by NK cell activation, receptor-mediated activation of dNK cells enhanced the secretion of these tissue-modeling factors, revealing a distinct activation-induced functional profile. Due to these tissue-remodeling capabilities, dNK cells in fact improved tumor growth and vascularization of trophoblast choriocarcinomas.3 Indeed, regulatory mechanisms have important implications that also extend to NK cells in the context of cancer. In contrast to anti-tumor splenic NK cells, our group has demonstrated that tumor-associated murine NK cells have a less mature phenotype and exhibit reduced expression of activation receptors, perforin, and granzyme B.4 Similarly, in humans, tumor-associated NK cells are enriched in the less mature CD56bright subset and in many ways resemble dNK cells. The presence of CD56bright cells within a tumor correlates with poor prognosis and reduced time to disease recurrence following therapy.5,6 In addition, Crome et al. demonstrated that CD56+ TILs have impaired anti-tumor cytotoxicity and negatively regulate T cell expansion, TNFα, and IFN-γ production.5 The regulation of T cells by CD56bright NK cells has also been reported to play an important role in the attenuation of autoimmune disease. For example, Bielekova et al. demonstrated that daclizumab therapy, a mAb against the IL-2Rα chain, exerted its therapeutic effects in patients with multiple sclerosis by inducing the expansion of CD56bright NK cells.7 The expansion of CD56bright cells is correlated with reduced brain inflammation and reduced T cell populations. While regulatory CD56bright NK cells are considered to have poor cytotoxicity against tumor targets, these CD56bright NK cells inhibited the survival of activated autologous T cells through direct cytotoxicity.7 Thus, in these studies, CD56bright cells have a strong propensity for regulatory function in response to activation signals.
In contrast to their classic regulatory roles, recent evidence has demonstrated that in certain contexts, CD56bright NK cells have the capacity for anti-viral and anti-tumor cytotoxicity, which plays an important role in driving responses to therapies. For instance, in patients with chronic hepatitis B infection, Pegylated Interferon-Alpha (PegIFNα) therapy induced preferential expansion of CD56bright NK cells compared to CD56dim cells. PegIFNα treatment enhanced the expression of activation receptors, degranulation, and IFN-γ production in CD56bright NK cells. Importantly, this increase in degranulation and IFN-γ was only observed in patients who responded to therapy and was not observed in non-responders.8 While CD56bright NK cells are typically considered to have poor cytotoxicity against tumor cells, a recent study demonstrated that IL-15-priming enhanced degranulation and cytotoxicity of CD56bright PB-NK cells against tumor cells to levels that surpassed even those of IL-15-primed CD56dim NK cells. In fact, these CD56bright NK cells had a more comprehensive anti-tumor functional profile as they combined a superior ability to secrete high levels of IFN-γ with enhanced cytotoxic function.9 Similarly, in the clinical setting, IL-15 infusion in cancer patients was shown to predominantly impact CD56bright NK cells. IL-15 infusion induced greater expansion of CD56bright NK cells and these expanded CD56bright cells upregulated anti-tumor cytokine secretion and displayed strong anti-tumor cytotoxicity.10 Ex vivo expansion of NK cells has also been shown to induce the growth of a highly cytotoxic CD56bright NK cell subset. Indeed, following expansion with K562 feeder cells that express 4-1BBL and membrane-bound (mb)IL-21, the majority of the expanded NK cell population consisted of CD56superbright cells, again with a combined capacity for high levels of anti-tumor cytokine production and cytotoxicity.11 In fact, expanded CD56bright NK cells exhibited greater degranulation in response to tumor targets than expanded CD56dim NK cells.11 These recent studies expose a new facet of CD56bright NK cells as critical cytotoxic effectors.
Collectively, a functional dichotomy emerges for CD56bright NK cells: they play important immune and homeostatic regulatory roles but can also be endowed with potent cytotoxic functions that drive responses to anti-viral and anti-cancer therapies. Furthermore, CD56bright NK cells can interconvert between these distinct functional subsets: our group has demonstrated that regulatory, poorly cytotoxic CD56bright tumor-associated NK cells can be converted through ex vivo expansion to the highly cytotoxic CD56superbright subset.11,12 While the transition from CD56bright to CD56dim is indicative of maturation, and activation can convert CD56dim cells to CD56bright, it remains to be determined what fundamental transformations differentiate regulatory CD56bright and proinflammatory CD56bright NK cells and whether transition through an intermediate CD56dim subset occurs in the process.
An important question arises in light of these disparate functions: is there a unifying implication for CD56 brightness in NK cells? If we place NK cell functional potential on a spectrum from regulatory and homeostatic to proinflammatory and cytotoxic, CD56superbright NK cells seem to occupy the very far ends of this spectrum (Fig. 1). In contrast, CD56dim NK cells typically fit within the proinflammatory and cytotoxic section, but given the superior cytotoxic potential of CD56bright NK cells that has recently been demonstrated, we suggest that the cytotoxic potential of CD56dim NK cells does not reach that of CD56bright. Thus, we propose that the brightness of CD56 in NK cells indicates a heightened potential for activation and function in both regulatory and proinflammatory contexts. To further explore this theory, we stratified mbIL-21 expanded NK cells based on the degree of CD56 expression into CD56dim, CD56bright, and CD56superbright subsets. In support of our hypothesis, expanded NK cells had a specific increase in expression of activation receptors, IFN-γ, and degranulation in response to tumor targets with increasing CD56 expression, whereas expression of inhibitory receptors did not change with CD56 brightness.11 This demonstrates that in the context of cytotoxic expanded NK cells, the degree of CD56 expression corresponds to heightened activation and anti-tumor functions. Moreover, this indicates that considering CD56 expression as a spectrum, rather than dichotomized subsets, can reveal further relationships between CD56 expression and NK cell function. Future exploration into whether the degree of CD56 expression within CD56bright regulatory NK cells corresponds to enhanced regulatory function would address whether this concept holds true in the context of regulatory CD56bright NK cells.
Fig. 1.
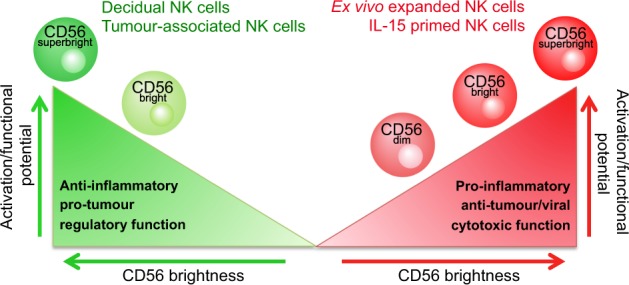
CD56superbright NK cells occupy the far ends of the NK cell functional spectrum. Schematic illustrating the concept that increasing CD56 brightness in NK cells corresponds to an increased potential for activation and function in both proinflammatory and regulatory contexts
While assessing NK cell function with respect to the degree of CD56 brightness has demonstrated that these two aspects are associated, it does not address another important question: does the degree of CD56 expression have an active role in regulating NK cell activation potential, or is it strictly a surrogate marker with expression that changes as a result of changes to activation potential? Knockdown of CD56 expression in CD56bright NK cells would be instrumental in addressing this question.
NK cells play important roles in homeostasis, disease, and many emerging therapies for a variety of diseases that function by modulating NK cells. Furthermore, it is becoming increasingly apparent that specific NK cell subsets are responsible for the respective therapeutic responses. Given that the degree of CD56 brightness is integral to defining human NK cell subsets, determining what the CD56 brightness inherently represents across all NK cells would further our understanding of the functional potential of different NK cell subsets. This knowledge would lead to better identification of which subsets should be therapeutically targeted. Furthermore, it would facilitate efficient harnessing of specific subsets, potentially even by directly modulating CD56 expression, to improve therapeutic responses. If it holds true that increased CD56 expression corresponds to heightened activation and functional potential in both regulatory and cytotoxic contexts, this indicates that subsets with the highest CD56 expression hold profound potential for therapeutic modulation.
Acknowledgements
A.A.A. holds a grant from the Juravinski Hospital and Cancer Center Foundation and a Tier 1 Canada Research Chair. S.M.P. holds an Ontario Women’s Health Scholars Award funded by the Ontario Ministry of Health and Long-Term Care.
Author contributions
A.A.A. and S.M.P. designed and wrote the article and produced the figure.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan A, et al. CD56bright human NK cells differentiate into CD56dim cells: role of contact with peripheral fibroblasts. J. Immunol. 2007;179:89–94. doi: 10.4049/jimmunol.179.1.89. [DOI] [PubMed] [Google Scholar]
- 3.Hanna J, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat. Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 4.Krneta T, Gillgrass A, Chew M, Ashkar AA. The breast tumor microenvironment alters the phenotype and function of natural killer cells. Cell. Mol. Immunol. 2016;13:628–639. doi: 10.1038/cmi.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crome SQ, et al. A distinct innate lymphoid cell population regulates tumor-associated T cells. Nat. Med. 2017;23:368–375. doi: 10.1038/nm.4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mamessier E, et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J. Clin. Invest. 2011;121:3609–3622. doi: 10.1172/JCI45816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bielekova B, et al. Regulatory CD56(bright) natural killer cells mediate immunomodulatory effects of IL-2Ralpha-targeted therapy (daclizumab) in multiple sclerosis. Proc. Natl Acad. Sci. USA. 2006;103:5941–5946. doi: 10.1073/pnas.0601335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gill US, et al. Interferon alpha induces sustained changes in NK cell responsiveness to hepatitis B viral load suppression in vivo. PLoS Pathog. 2016;12:e1005788. doi: 10.1371/journal.ppat.1005788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner JA, et al. CD56bright NK cells exhibit potent antitumor responses following IL-15 priming. J. Clin. Invest. 2017;127:4042–4058. doi: 10.1172/JCI90387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubois S, et al. IL15 infusion of cancer patients expands the subpopulation of cytotoxic CD56(bright) NK cells and increases NK-cell cytokine release capabilities. Cancer Immunol. Res. 2017;5:929–938. doi: 10.1158/2326-6066.CIR-17-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poznanski S. M., et al. Expanded CD56superbrightCD16+ NK cells from ovarian cancer patients are cytotoxic against autologous tumor in a patient-derived xenograft murine model. Cancer Immunol. Res. 10.1158/2326-6066.CIR-18-0144 (2018). [DOI] [PubMed]
- 12.Nham T, et al. Ex vivo-expanded NK cells from blood and ascites of ovarian cancer patients are cytotoxic against autologous primary ovarian cancer cells. Cancer Immunol. Immunother. 2018;67:575–587. doi: 10.1007/s00262-017-2112-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


