Abstract
Rheumatoid arthritis (RA) is an autoimmune inflammatory disease characterized by joint infiltration and bone damage. The aim of the present study was to evaluate the beneficial effects of losartan in adjuvant-induced arthritis (AIA). Arthritis was induced in rats by subcutaneous injection of 0.2 mL of Complete Freund’s adjuvant (CFA) in the planter surface of the hind paw. Arthritic rats were allocated into three groups (n = 10), the first group (arthritis control), received 1% of tween 80, the second and the third groups received prednisolone (10 mg/kg/day; p.o) and losartan (20 mg/kg/day; p.o) respectively for two weeks. A fourth group (vehicle control) received 1% tween 80. At the end of the experiment, blood samples were collected for biochemical, oxidative stress, and hematological analysis. Histopathological and macroscopical examinations on joints were also performed. Our results revealed that losartan significantly reduced serum levels of tumor necrosis factor alpha (TNFα), interleukin-6 (IL-6) , rheumatoid factor (RF), and erythrocytes sedimentation rate (ESR).It significantly decreased serum malondialdehyde and increased blood glutathione .Losartan exhibited significant decrease in serum level of total cholesterol (TC), triglycerides (TG) and low density lipoprotein (LDL) coupled with marked increase in high density lipoprotein (HDL).Furthermore, losartan decreased white blood cell cells (WBC’s) count and increased red blood cells (RBC’s) , hemoglobin (Hb) , platelets, and hematocrit (Hct) counts. These findings were further supported by histopathological and macroscopical examinations. It could be concluded that losartan was able to repress biochemical, oxidative and hematological changes associated with AIA. These effects could be attributed to anti-arthritic, hypolipidemic, antioxidant and anti-anemic properties.
Key Words: Complete Freund’s adjuvant, Losartan, Prednisolone, Rheumatoid Arthritis
Introduction
Rheumatoid arthritis (RA) is a common multisystem autoimmune disease characterized by chronic inflammation in particular synovial tissue and associated with over production of proinflammatory cytokines. These cytokines are directly implicated in many of immune processes correlated with the pathogenesis of rheumatoid arthritis (1).
Proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) produced by the rheumatoid synovium can diffuse within the bloodstream and may alter the function of numerous tissues including fat, skeletal muscle, liver, and vascular endothelium (2). These functional alterations can induce changes, such as insulin resistance, increased oxidative stress, endothelial dysfunction, dyslipidemia, and anemia (3).
Critical issues concerning the effect of therapy were to control symptoms and signs of the disease as well as the capacity to retard the damaging effect of inflammation on articular cartilage, hematology, and lipids deterioration induced by RA (4).
Prednisolone is a very fast-acting glucocorticoid , potent anti-inflammatory drug where short-term use of it can reduce synovitis and its long-term use decreases joint damage (1). Substantial adverse risks, such as infections and osteoporosis, and also their overall risk/benefit ratio was deemed unfavorable (5). In addition, glucocorticoids could increase the risk of cardiovascular disease in patients with RA due to their potential deleterious effects on lipid, glucose tolerance as well as development of hypertension and/or obesity (1).
Angiotensin II (Ang II) AT1 receptor was implicated in up-regulation of pro-inflammatory cytokines and production of vascular endothelial growth factor (VEGF) which promote angiogenesis, increase vascular permeability, and act as chemotactic for monocytes (6). Accordingly, Ang II may contribute to pathogenesis of RA, therefore Ang II AT1 receptors blockade, by a specific inhibitor such as losartan, may represent a more effective therapeutic target for RA treatment.
The present study was designed to investigate the anti-arthritic, antioxidant, hypolipidemic and anti-anemic effects of losartan against AIA in rats in order to find an alternative safe new medical treatment with more efficacies and less side effects.
Experimental
Experimental animals
Adult female Wistar rats, weighing 170–200g were purchased from the Modern Veterinary Office for Laboratory Animals (Cairo, Egypt). Rats were housed in well ventilated opaque propylene cages with free access to commercial diet and tap water. Rats were maintained at 22 ± 2 °C under light and dark cycle and they were allowed to acclimatize for one week prior to the study. Handling of animals and animal care were done according to the guidelines of Beni-Suef Animal House approved by the Pharmacology and Toxicology Department, Faculty of Pharmacy, Beni-Suef University, which were based on the guidelines suggested by the recommendations of the National Institutes of Health (NIH) Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985). All experimental protocols were approved by Research Ethics Committee at the Faculty of Pharmacy, Beni-Suef University (Egypt).
Drugs, chemicals and diagnostic kits
Losartan, prednisolone, and Complete Freund’s Adjuvant (CFA) were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO, USA). Serum rheumatoid factor (RF) ELISA kit and erythrocyte sedimentation rate (ESR) kits were purchased from CliniLab Company (Cairo, Egypt). Serum TNF-α and IL-6 ELISA kits were obtained from Glory Science Company (St. Del Rio, USA). Serum high density lipoprotein (HDL), total cholesterol (TC), and triglycerides (TG) enzymatic reagent kits were obtained from Assaypro LLC (St. Charles, MO, USA).
Serum glutathione (GSH), malondialdehyde (MDA) colorimetric reagent kits and complete blood count kit were purchased from Bio Diagnostic Company (Giza, Egypt). All other chemicals included were of the highest analytical grade available. Drugs used were suspended in 1% Tween 80 shortly before administration to animals.
Experimental design
After one week of acclimatization to the laboratory conditions, fourty rats were randomly allocated into four groups (n = 10) placed in individual cages and classified as follows: Group 1 served as vehicle control and received an injection of 0.2 mL of paraffin oil into the right hind paw every four days for twelve days and 1% of tween 80 in 0.9% physiological saline solution (0.1mL/ 100g body weight;p.o) on day thirteen for two weeks. The remaining three groups received subcutaneous injection of 0.2 mL of CFA (heat killed powdered mycobacterium cells suspended with liquid paraffin) in the planter surface of the right hind paw (7) every four days for twelve days. On day thirteen, the treatment with various drug regimens started and continued up to two weeks. The second group was assigned as AIA control and received 1% tween 80 dissolved in 0.9% physiological saline solution (0.1mL/ 100g body weight;p.o). Groups 3 and 4 received prednisolone (10 mg/kg/day;p.o.) (1) and losartan (20 mg/kg/day;p.o.) (4) Respectively.
Induction of adjuvant arthritis
In order to induce a chronic rat model of arthritis and exclude the presence of just temporary inflammatory reaction, an aggressive modified model of adjuvant arthritis was used. Complete Freund’s adjuvant (5 mg of heat killed, powdered mycobacterium tuberculosis cell was suspended in liquid paraffin to get 5mg/mL suspension) was used to induce arthritis in rats. Pilot studies with the aid of a histopathological study on joint sections were performed to assess the success of the model.
Blood collection and processing of samples
At the end of the experimental period (day 27), blood samples were collected from orbital venous plexus from animals under light sodium phenobarbital (40 mg/kg) anesthesia sodium. Each sample was divided into two portions; the first portion was used for erythrocyte sedimentation rate (ESR) evaluation and hematological parameters including assessment of white blood cells (WBCs), red blood cells (RBCs), hemoglobin (Hb), hematocrit (Hct) and platelets values. The second part was collected into non heparinized tubes and centrifuged at 3000 rpm for 10 min using a cooling centrifuge (Sigma 3-30k, USA). Then, serum samples were separated, collected in clean tubes, and stored at -80 °C until use. The Serum was used for the determination of the levels of tumor necrosis factor (TNF-α), interleukin-6 (IL-6), rheumatoid factor (RF), glutathione (GSH), malondialdehyde (MDA), total cholesterol (TC), triglyceride (TG), high density lipoprotein (HDL), and low density lipoproteins (LDL).
Determination of serum TNF-α and IL-6 activities
Serum TNF-α and serum IL-6 were determined using Enzyme Linked Immunosorbent Assay (ELISA) kits according to the method described by Paine et al. (2012) and Rysz et al. (2006) respectively (8,9).
Determination of ESR and RF levels
ESR and RF were determined using diagnostic kits according to the methods described previously (10;11) and following the manufacturer’s instructions.
Determination of oxidative stress parameters
Serum GSH and MDA levels were determined according to the method described by Pedrini et al. (2012) (12) and Padurariu et al. (2010) (13) respectively using diagnostic reagent kits.
Determination of serum levels of TC, TG, HDL and LDL
Serum TC, TG, and HDL levels were estimated using diagnostic kits according to the methods described by Akbas et al. (2010) and Zheng et al. (2011) (14;15) respectively. Low density lipoprotein (LDL) level was calculated according to the formula described previously (16) :
LDL = total cholesterol−HDL−TG/5.
Determination of hematological parameters
Blood smears containing EDTA were prepared as soon as possible after blood collection on a glass slide, quickly dried, and stained with Giemsa and May-Grunwald stain for the determination of total number of WBCs, RBCs, Hb concentration, platelets count, and Hct value. All were estimated by adopting standard procedures according to the method of Flouris et al. (2012) (17).
Hind paw processing and histopathological examination
Immediately, after blood withdrawal, the rats were anesthetized with thiopental sodium (50 mg/kg) and killed by decapitation. Whole knee joints, including synovium, adjacent tissues and bones, were carefully exposed, separated, and preserved in 10% formalin solution in saline in labeled well-sealed containers. After tissue hardening, the joints were transversely sectioned (4-5μm thick sections) and stained with standard Hematoxylin and Eosin (H&E) dye and evaluated microscopically using a light microscope (18) .
Statistical analysis
The data were expressed as means ± standard deviation (SD). The statistical analysis was performed using Graphpad prism software program, version 5.01 (GraphPad Softwae, Inc, San Diego, USA).
One-way analysis of variance (ANOVA) followed by Tukey-Kramer multiple comparisons test was used for comparison of means of different groups. The level of statistical significance was set at
p < 0.05.
Results
Effects on serum levels of TNF-α and IL-6
The planter injection of CFA in rat model led to a significant elevation in inflammatory mediators, TNF-α and IL-6 levels to about 356.92% and 376.72% respectively when compared to vehicle control group. Interestingly, treatment of AIA rats with losartan for two weeks inhibited the production of TNF-α and IL-6 by 60.74% and 55.08% respectively as compared to CFA control group. It is worth noting that losartan reduced IL-6 serum level without difference with respect to prednisolone group (Table1).
Table 1.
Effect of two weeks administration of prednisolone (10 mg/kg;p.o) and losartan (20 mg/kg; p.o) on serum levels of TNF-α , IL-6, RF and ESR in a rat model of adjuvant -induced arthritis
| Experimental groups | TNF-α ( pg/mL ) | IL-6 ( pg/mL ) | RF ( IU/mL ) | ESR ( mm/hr) |
|---|---|---|---|---|
| Vehicle control (10% tween 80) | 32.78 ± 3.95 | 34.76 ± 3.86 | 11.66 ± 0.98 | 6.75 ± 1.01 |
| AIA | 117.55 ± 5.53* | 130.95 ± 15.30* | 50.78 ± 3.32* | 44.21 ± 3.51 * |
| Prednisolone | 57.74 ± 9.33 *@ | 58.88 ± 13.66*@ | 17.52 ± 2.02 *@ | 13.46 ± 2.37 *@ |
| Losartan | 71.40 ± 9.83 *@$ | 72.13 ± 10.59*@ | 21.45 ± 3.64 *@ $ | 19.48 ± 4.08*@$ |
Each group consisted of 10 rats.
Arthritis was induced by subcutaneous injection of 0.2 mL of Complete Freund’s adjuvant (5 mg of heat killed powdered mycobacterium cells suspended with liquid paraffin) in the planter surface of the right hind paw every four days for twelve days.
TNF-α: Tumor necrosis factor alpha.
IL-6: Interleukin -6.
RF: rheumatoid factor.
ESR: Erythrocyte sedimentation rate.
AIA: Adjuvant-induced rat model of arthritis.
Data are presented as mean ± SD.
Statistical analysis was carried out using one-way analysis of variance (ANOVA) followed by Tukey Kramer multiple comparison test.
Significantly different from vehicle group at p < 0.05.
Significantly different from AIA group at p < 0.05.
Significantly different from prednisolone group at p < 0.05.
Effects on blood levels of RF and ESR
As shown in Table 1, there was approximately four and six fold increase in blood levels of RF and ESR respectively in AIA control group in comparison to the vehicle control group. A substantial decrease in blood levels of RF and ESR to about 42.24% and 44.06% respectively was observed following the administration of losartan for two weeks as compared to AIA group (p < 0.05).
Effects on oxidative stress biomarkers
The rats subjected to CFA showed approximately three-fold decrease in blood GSH level coupled with eleven-fold increase in serum MDA level indicating severe oxidative stress. The two therapeutic regimens successfully restored the increase in MDA level and the decrease in GSH level compared to
AIA control group (Table 2).
Table 2.
Effect of two weeks administration of prednisolone (10 mg/kg; p.o) and losartan (20 mg/kg; p.o) on oxidative stress biomarkers in a rat model of adjuvant -induced arthritis.
| Experimental groups | GSH ( μmol/L) | MDA ( nmol/mL ) |
|---|---|---|
| Vehicle control (10%tween 80) | 55.37 ± 2.91 | 1.11 ± 0.22 |
| AIA | 17.05 ± 1.80 * | 12.24 ± 1.52 * |
| Prednisolone | 41.93 ± 6.64 *@ | 3.88 ± 1.29 *@ |
| Losartan | 31.35 ± 6.67*@$ | 4.55 ± 1.52 *@ |
Each group consisted of 10 rats.
Arthritis was induced by subcutaneous injection of 0.2 mL of Complete Freund’s adjuvant (5 mg of heat killed powdered mycobacterium cells suspended with liquid paraffin) in the planter surface of the right hind paw every four days for twelve days.
GSH: Glutathione.
MDA: Malondialdehyde.
AIA: Adjuvant-induced rat model of arthritis.
Data are presented as mean ± SD.
Statistical analysis was carried out using one-way analysis of variance (ANOVA) followed by Tukey Kramer multiple comparison test.
Significantly different from vehicle group at p < 0.05.
Significantly different from AIA group at p < 0.05.
Significantly different from prednisolone group at p < 0.05.
Effects on serum levels of TC, TG, HDL and LDL
As represented in Table 3, subplanter injection of CFA induced hyperlipidemia as manifested by marked rise in serum levels of TC from 128.71 ± 4.52 to 172.59 ± 26.75,TG from 65.60 ± 9.49 to 108.56 ± 9.83 and LDL from 62.03 ± 1.77 to 121.05 ± 20.93 in arthritic rats with concurrent decrease in serum HDL level from 53.56 ± 3.51 to 29.76 ± 4.55.Treatment with prednisolone or losartan alleviated the hyperlipidemic effect as evidenced by significant reduction in serum TC, TG, and LDL levels with significant increase in serum HDL in comparison with AIA group. It is to be noted that losartan showed no significant difference in lowering serum level of TC and TG compared to prednisolone treated group.
Table 3.
Effect of two weeks administration of prednisolone (10 mg/kg;p.o) and losartan ( 20 mg/kg; p.o) on serum levels of TC, TG, HDL and LDL in a rat model of adjuvant -induced arthritis
| Experimental groups | TC ( mg/dL) | TG ( mg/dL) | HDL ( mg/dL ) | LDL ( mg/dL) |
|---|---|---|---|---|
| Vehicle control (10% tween 80) | 128.71 ± 4.52 | 65.60 ± 9.49 | 53.56± 3.51 | 62.03 ± 1.77 |
| AIA | 172.59 ± 26.75* | 108.56 ± 9.83 * | 29.76 ± 4.55 * | 121.05 ±20.93* |
| Prednisolone | 140.85 ± 9.01 @ | 81.75± 5.34*@ | 47.69 ± 4.46 *@ | 76.81 ± 3.16*@ |
| Losartan | 146.66 ± 7.62 *@ | 87.89 ± 5.09*@ | 37.15 ± 3.95 *@$ | 91.93 ± 3.54*@ $ |
Each group consisted of 10 rats.
Arthritis was induced by subcutaneous injection of 0.2 mL of Complete Freund’s adjuvant (5 mg of heat killed powdered mycobacterium cells suspended with liquid paraffin) in the planter surface of the right hind paw every four days for twelve days.
TC: Total cholesterol.
TG: Triglycerides.
HDL: High density lipoprotein.
LDL: Low density lipoprotein.
AIA: Adjuvant-induced rat model of arthritis.
Data are presented as mean ± SD.
Statistical analysis was carried out using one-way analysis of variance (ANOVA) followed by Tukey Kramer multiple comparison test.
Significantly different from vehicle group at p < 0.05.
Significantly different from AIA group at p < 0.05.
Significantly different from prednisolone group at p < 0.05.
Effects on hematological parameters
The current results demonstrated that thearthritic rats showed a rise in WBC’s count along with reduced RBC’s count, Hb concentration, platelet count, and Hct value in comparison with vehicle control rats. All these measured parameters indicate the anemic condition commonly noted in arthritis. Both implemented treatments ameliorated the anemic condition as marked by reversing back WBCs count and increasing RBCS count, HB concentration, and Hct value compared to AIA group.
On the other hand, the rats treated with prednisolone resulted in slight increase in platelet count while treatment with losartan showed no effect on platelet count compared to AIA group (Table 4).
Table 4.
Effect of two weeks administration of prednisolone (10 mg/kg; p.o) and losartan (20 mg/kg; p.o) on hematological parameters in a rat model of adjuvant -induced arthritis
| Experimental groups | WBCs ( 103/μL) | RBCs ( 106/mcl ) | Hb ( g/dL ) | Platelets(billion/L) | Hct (%) |
|---|---|---|---|---|---|
| Vehicle control | 8.55 ±1.93 | 3.18± 0.57 | 13.93 ± 0.51 | 351.25 ± 31.46 | 53.28 ± 3.86 |
| AIA | 21.74 ±3.60 * | 1.63 ± 0.44 * | 8.21 ± 0.85 * | 277.63 ± 31.40 * | 38.90 ± 7.05* |
| Prednisolone | 14.01± 1.20 * @ | 2.95± 0.28 @ | 10.99± 0.95 * @ | 309.63 ± 9.01* @ | 46.81 ± 4.21* @ |
| Losartan | 16.89± 2.75 * @$ | 2.36 ± 0.44 * @ $ | 9.40 ± 1.20* @ $ | 304.00 ± 20.08 * | 45.8 ± 3.51 * @ |
Each group consisted of 10 rats.
Arthritis was induced by subcutaneous injection of 0.2 mL of Complete Freund’s adjuvant (5 mg of heat killed powdered mycobacterium cells suspended with liquid paraffin) in the planter surface of the right hind paw every four days for twelve days.
WBCs: White blood cells.
RBCs: Red blood cells.
Hb: Hemoglobin.
Hct: Hematocrit.
AIA: Adjuvant-induced rat model of arthritis.
Data are presented as mean ± SD.
Statistical analysis was carried out using one-way analysis of variance (ANOVA) followed by Tukey Kramer multiple comparison test.
Significantly different from vehicle group at p < 0.05.
Significantly different from AIA group at p < 0.05.
Significantly different from prednisolone group at p < 0.05.
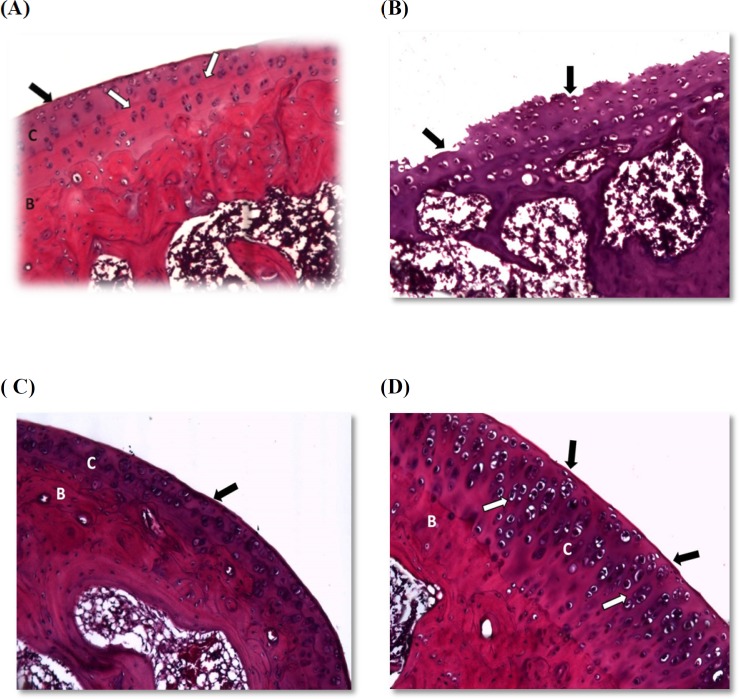
Histopathological examination
Histopathological examination for H&E stained sections affirmed that joints obtained from vehicle control rats showed a smooth articular surface with a regular tide mark separating the articular from the underlying subchondral bone. All chondrocytes were embedded in a homogenous matrix (Figure 1A). Joints obtained from AIA group showed disrupted articular surface, erosion of articular cartilage, and degeneration of chondrocytes with pyknotic nuclei (Figure 1B). Sections prepared from prednisolone treated rats exhibited smooth articular surface with an increase in articular cartilage layers. Also, normal chondrocytes were embedded in homogenous matrix and subchondral bone can be observed (Figure 1C). The joints from losartan treated rats showed smooth articular surface with thickened articular cartilage and subchondral bone. The chondrocytes showed hypercellularity and aggregation. All of the chondrocytes are embedded in a homogenous matrix (Figure 1D).
Figure 1.
Photomicrographs for joint sections stained with H& E
(A) Normal architecture of the joints showing smooth articular surface (black arrow) with a regular tide mark (white arrow) separating the articular cartilage (c) from the underlying subchondral bone (B). (B) Section from the AIA group demonstrating disrupted articular surface, erosion of articular cartilage and degeneration of chondrocytes with pyknotic nuclei. (C) Joint section in the prednisolone group showing smooth articular surface (black arrow) with an increase in articular cartilage layers (C) and subchondral bone (B) can be observed. (D) Losartan treated rats showed smooth articular surface (black arrow) with tide marks (white arrow) and thickened articular cartilage (C) and subchondral bone (B). The chondrocytes showed hypercellularity and aggregation. All chondrocytes are embedded in a homogenous matrix. (at x 400 magnification).
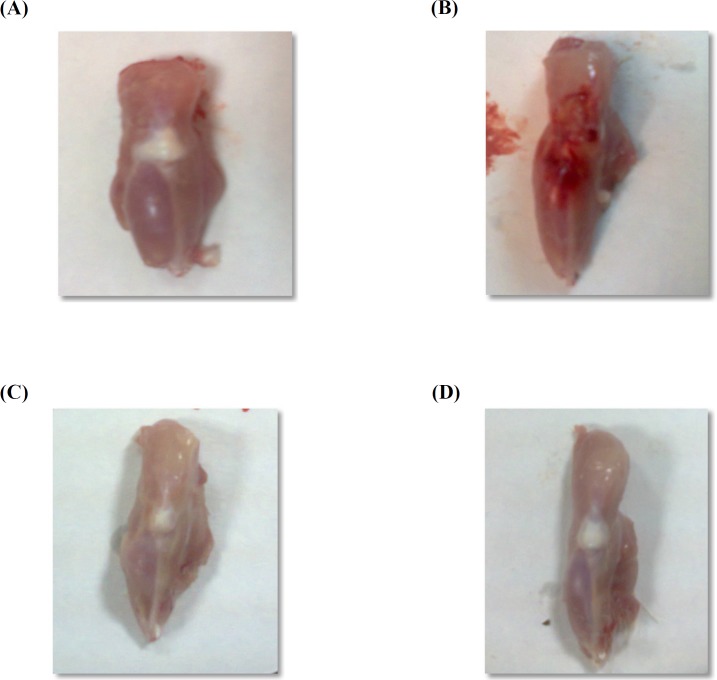
Macroscopical examination
Macroscopical changes of joints obtained from AIA group exhibited severe inflammatory area on joint surface associated with edema as compared to vehicle control group (Figure 2B). Treatment of rats with prednisolone or losartan resulted in disappearance of this inflammation associated with edema inhibition (Figures 2C& D).
Figure 2.
Photographs of joints demonstrating macroscopical changes
(A) Vehicle control group with no inflammation. (B) Joint from AIA group showing severe inflammation. (C) Prednisolone treated rats showing disappearance of inflammation. (D) Joint in losartan group showing relief of inflammation.
Discussion
Although RA is properly considered a disease of the joints, abnormal immune responses can cause a variety of extra-articular manifestations. In some cases, occurrence of severe anemia and alteration of atherogenic profile that cause hyperlipidemia contribute to extra-articular findings. The use of traditional medications, unfortunately have many undesired gastrointestinal, renal, and other adverse effects (19). Therefore, new drug classes for treatment of RA are highly required. In the present research, anti-arthritic, antioxidant, anti-anemic, and hypolipidemic effects of losartan against p-induced arthritis in rats were studied.
The presence and up-regulation of Ang II AT1 receptors has been described in synovium samples obtained from RA patients (20). Therefore, Ang II AT 1 receptors blockade, by a specific inhibitor such as losartan, may present a novel and more effective therapeutic target for rheumatoid arthritis treatment (21).
The current study showed that losartan alleviated the symptoms of AIA as well as the progress of macroscopic and microscopic symptoms, most probably through targeting and suppressing AT1 receptor expression and also increasing AT2R expression in the splenocytes and synovium (22).
Losartan relieved inflammation as it induced significant decrease in serum levels of TNFα and IL-6 when administered to arthritic rats, these findings are confirmed by the work of Wang et al. (2013) who reported similar decrease in TNFα and IL-6 after losartan treatment (22). Refaat et al. (2013) revealed that down regulation of Ang II AT1 receptors by losartan was responsible for down regulation of proinflammatory cytokines such as TNF α and IL-6 (4).
Side by side, inhibition of AT1 receptors lead to increase in local Ang II levels, resulting in increased activation of AT2 receptors, which in turn may confer additional benefit since AT2 receptor activation was considered to oppose the actions of the AT1 receptor, such that increased AT2 activation could exert antiarithritic actions (23) along with significant decrease in ESR (24) and RF (25). These findings come in agreement with our results which revealed that losartan administration in AIA rat model attenuated the increase in ESR and RF.
According to the results of the present investigation, losartan elicited a significant protective antioxidant effect by reducing MDA level and increasing GSH level. These results are in agreement with Heeba et al. (2011) revealing similar results (26). These findings suggest a profound antioxidant role of losartan to restore cellular defense mechanisms though GSH elevation and blockage of lipid peroxidation.
Interestingly, other studies proved that nicotinamide adenine dinucleotide phosphate (NADPH) oxidases were known as major sources of reactive oxygen species in the vasculature (21). These enzymes were found to be responsible for the oxidative stress in AIA (27). Ang II activates the NADPH oxidase via AT1receptor activation through stimulation of intracellular signaling pathways such as arachidonic acid metabolites (28). Furthermore, Ang II induces a rapid translocation of the small GTPase rac1 to the cellular membrane, a prerequisite of NAD PH oxidase activation (26). These findings suggest that the antioxidant action of losartan is mediated at least in part through inhibition of lipid peroxidation and AT1 receptor blockade.
In addition, this antioxidant role resulted in significant influence on lipid profile changes through nearly normalization of HDL, LDL, TG, and cholesterol. This hypolipidemic effect contributes to the ability of losartan to reduce the susceptibility of LDL to oxidative modification, as a result of significant reduction in oxidative stress levels (29).
Interestingly, Inflammation induced by AIA was associated with increased oxidative stress levels and antioxidant inhibitory action resulting in dramatic hematological changes expressed as massive WBCs production along with decrease in RBCs, Hb, Hct and platelet values (30). Inflammation results in hepcidin production, the key mediator of anemia related to inflammation and a bridge between innate immunity and iron metabolism (31). On the other hand, oxidative stress production and activation of reactive oxygen species (ROS) result in massive destruction in RBCs (32). Losartan treatment showed beneficial modulatory effects on hematological parameters where WBCs count was significantly inhibited and other values of RBCs, Hb, Hct, and platelets were significantly increased, providing a promising role of losartan in RA treatment.
Conclusion
The results of the present study conclude that losartan was able to alleviate the changes associated with adjuvant- induced arthritis. This could be attributed at least in part to its anti-arthritic and anti-anemic effects.
Acknowledgment
The authors wish to thank Dr Alsamraa Hassan, Assistant Professor of Histology, Faculty of Medicine, Beni-Suef University, Egypt for helping in histopathological examination.
References
- 1.Abdin AA, Abd El-Halim MS, Hedeya SE, El-Saadany AA. Effect of atorvastatin with or without prednisolone on Freund’s adjuvant induced-arthritis in rats. Eur. J. Pharmacol. 2012;676:34–40. doi: 10.1016/j.ejphar.2011.11.052. [DOI] [PubMed] [Google Scholar]
- 2.llán-Gómez F1, Gonzálvez-Ortega M, Orea-Soler I, Alcaraz-Tafalla MS, Aragón-Alonso A, Pascual-Díaz M, Pérez-Paredes M, Lozano-Almela ML. Obesity and inflammation: change in adiponectin, C-reactive protein, tumour necrosis factor-alpha and interleukin-6 after bariatric surgery. Obes Surg. 2012;22:950–5. doi: 10.1007/s11695-012-0643-y. [DOI] [PubMed] [Google Scholar]
- 3.Gibofsky A. Epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis: A Synopsis. Am. J. Manag. Care . 2014;(7 Suppl):S128–S135. [PubMed] [Google Scholar]
- 4.Refaat R, Salama M, Abdel ME, El SA, Gowayed M. Evaluation of the effect of losartan and methotrexate combined therapy in adjuvant-induced arthritis in rats. Eur. J. Pharmacol. 2013;698:421–8. doi: 10.1016/j.ejphar.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 5.Dixon WG, Abrahamowicz M, Beauchamp ME, Ray DW, Bernatsky S, Suissa S, Sylvestre MP. Immediate and delayed impact of oral glucocorticoid therapy on risk of serious infection in older patients with rheumatoid arthritis: a nested case–control analysis. Ann. Rheumatic Dis . 2012;71:1128–33. doi: 10.1136/annrheumdis-2011-200702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi Y1, Satoh M, Minami Y, Tabuchi T, Itoh T, Nakamura M. Expression of miR-146a/b is associated with the Toll-like receptor 4 signal in coronary artery disease: effect of renin–angiotensin system blockade and statins on miRNA-146a/b and Toll-like receptor 4 levels. Clin. Sci. 2010;119:395–405. doi: 10.1042/CS20100003. [DOI] [PubMed] [Google Scholar]
- 7.Patil MB, Bhise SD, Patil MV, Kandhare AD. Anti-arthritic and anti-inflammatory activity of Xanthium srtumarium L ethanolic extract in Freund’s complete adjuvant induced arthritis. Biomed. Aging Patholo. 2012;2:6–15. [Google Scholar]
- 8.Paine SK, Sen A, Choudhuri S, Mondal LK, Chowdhury IH, Basu A, Mukherjee A, Bhattacharya B. Association of tumor necrosis factor α, interleukin 6, and interleukin 10 promoter polymorphism with proliferative diabetic retinopathy in type 2 diabetic subjects. Retina . 2012;32:1197–203. doi: 10.1097/IAE.0b013e31822f55f3. [DOI] [PubMed] [Google Scholar]
- 9.Rysz J, Banach M, Cialkowska-Rysz A, Stolarek R, Barylski M, Drozdz J, Okonski P. Blood serum levels of IL-2, IL-6, IL-8, TNF-alpha and IL-1beta in patients on maintenance hemodialysis. Cell Mol. Immunol. 2006;3:151–4. [PubMed] [Google Scholar]
- 10.Lee WS, Kim TY. Relation between red blood cell distribution width and inflammatory biomarkers in rheumatoid arthritis. Archi. Pathol. Lab. Med. 2010;134:505–6. doi: 10.5858/134.4.505.c. [DOI] [PubMed] [Google Scholar]
- 11.Todd DJ, Knowlton N, Amato M, Frank MB, Schur PH, Izmailova ES, Roubenoff R, Shadick NA, Weinblatt ME, Centola M, Lee DM. Erroneous augmentation of multiplex assay measurements in patients with rheumatoid arthritis due to heterophilic binding by serum rheumatoid factor. Arthritis Rheum. 2011;63:894–903. doi: 10.1002/art.30213. [DOI] [PubMed] [Google Scholar]
- 12.Pedrini M, Massuda R, Fries GR, de Bittencourt Pasquali MA, Schnorr CE, Moreira JC, Teixeira AL, Labato MI, Walz JC, Belmonte -de- Abreu PS, Kapczinski F, Gama CS. Similarities in serum oxidative stress markers and inflammatory cytokinesaauer -Sant Anna M,Ka in patients with overt schizophrenia at early and late stages of chronicity. J. psychiatr. Res. 2012;46:819–24. doi: 10.1016/j.jpsychires.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Padurariu M, Ciobica A, Hritcu L, Stoica B, Bild W, Stefanescu C. Changes of some oxidative stress markers in the serum of patients with mild cognitive impairment and Alzheimer’s disease. Neurosci. Lett. 2010;469:6–10. doi: 10.1016/j.neulet.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 14.Akbas HS, Basyigit S, Suleymanlar I, Kemaloglu D, Koc S, Davran F, Demir I, Suleymanlar G. The assessment of carotid intima media thickness and serum paraoxonase-1 activity in Helicobacter pylori positive subjects. Lipids Health Dis. 2010;9:1–9. doi: 10.1186/1476-511X-9-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng XX, Xu YL, Li SH, Liu XX Hui R, Huang XH. Green tea intake lowers fasting serum total and LDL cholesterol in adults: a meta-analysis of 14 randomized controlled trials. Am. J. Clin. Nutr. 2011;94:601–10. doi: 10.3945/ajcn.110.010926. [DOI] [PubMed] [Google Scholar]
- 16.Desai NR, Kholi P, Guigliano RP, O’Donoghue ML, Somaratne R, Zhou J, Hoffman EB, Huang F, Rogers WJ, Wasserman SM, Scott R, Sabatine MS. AMG145, a Monoclonal Antibody Against Proprotein Convertase Subtilisin Kexin Type 9, Significantly Reduces Lipoprotein (a) in Hypercholesterolemic Patients Receiving Statin Therapy An Analysis From the LDL-C Assessment With Proprotein Convertase Subtilisin Kexin Type 9 Monoclonal Antibody Inhibition Combined With Statin Therapy (LAPLACE)–Thrombolysis in Myocardial Infarction (TIMI) 57 Trial. Circulation . 2013;128:962–9. doi: 10.1161/CIRCULATIONAHA.113.001969. [DOI] [PubMed] [Google Scholar]
- 17.Flouris AD, Poulianiti KP, Chorti MS, Jamurtas AZ, Kouretas D, Owolabi EO, Tzatzarakis MN, Tsatsakis AM, Koutedakis Y. Acute effects of electronic and tobacco cigarette smoking on complete blood count. Food Chem. Toxicol. 2012;50:3600–3. doi: 10.1016/j.fct.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 18.Elsam J. Histological and Histochemical Methods: Theory and Practice. Biotech. Histochem. 2016;91:145. [Google Scholar]
- 19.Caporali R, Todoerti M, Sakellariou G, Montecucco C. Glucocorticoids in rheumatoid arthritis. Drugs . 2013;73:31–43. doi: 10.1007/s40265-013-0008-4. [DOI] [PubMed] [Google Scholar]
- 20.da Silveira KD, Coelho FM, Vieira AT, Sachs D, Barroso LC, Costa VV, Bretas TL, Bader M, de Sousa LP, da Silva TA, dos Santos RA, Simões e Silva AC, Teixeira MM. Anti-inflammatory effects of the activation of the angiotensin-(1–7) receptor, MAS, in experimental models of arthritis. J. Immunol. 2010;185:5569–76. doi: 10.4049/jimmunol.1000314. [DOI] [PubMed] [Google Scholar]
- 21.Sakuta T, Morita Y, Satoh M, Fox DA, Kashihara N. Involvement of the renin–angiotensin system in the development of vascular damage in a rat model of arthritis: effect of angiotensin receptor blockers. Arthritis Rheum. 2010;62:1319–128. doi: 10.1002/art.27384. [DOI] [PubMed] [Google Scholar]
- 22.Wang D1, Hu S, Zhu J, Yuan J, Wu J, Zhou A, Wu Y, Zhao W, Huang Q, Chang Y, Wang Q, Sun W, Wei W. Angiotensin II type 2 receptor correlates with therapeutic effects of losartan in rats with adjuvant-induced arthritis. J. Cell Mol. Med. 2013;17:1577–87. doi: 10.1111/jcmm.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Widdop RE, Jones ES, Hannan RE, Gaspari TA. Angiotensin AT2 receptors: cardiovascular hope or hype? Br. J. Pharmacol. 2003;140:809–24. doi: 10.1038/sj.bjp.0705448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karagiozoglou-Lampoudi T, Trachana M, Agakidis C, Pratsidou-Gertsi P, Taparkou A, Lampoudi S, Kanakoudi-Tsakalidou F. Ghrelin levels in patients with juvenile idiopathic arthritis: relation to anti–tumor necrosis factor treatment and disease activity. Metabolism . 2011;60:1359–qx62. doi: 10.1016/j.metabol.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Roustit M1, Blaise S, Allanore Y, Carpentier PH, Caglayan E, Cracowski JL. Phosphodiesterase-5 inhibitors for the treatment of secondary Raynaud’s phenomenon: systematic review and meta-analysis of randomised trials. Ann. Rheum. Dis. 2013;72:1696–9. doi: 10.1136/annrheumdis-2012-202836. [DOI] [PubMed] [Google Scholar]
- 26.Heeba GH. Angiotensin II receptor blocker, losartan, ameliorates gentamicin-induced oxidative stress and nephrotoxicity in rats. Pharmacology . 2011;87:232–40. doi: 10.1159/000325457. [DOI] [PubMed] [Google Scholar]
- 27.Shah D, Ajay W, Archana B. Interaction between oxidative stress and chemokines: possible pathogenic role in systemic lupus erythematosus and rheumatoid arthritis. Immunology . 2011;216:1010–7. doi: 10.1016/j.imbio.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Masoura C, Pitsavos C, Aznaouridis K, Skoumas I, Vlachopoulos C, Stefanadis C. Arterial endothelial function and wall thickness in familial hypercholesterolemia and familial combined hyperlipidemia and the effect of statins A systematic review and meta-analysis. Atherosclerosis . 2011;214:129–38. doi: 10.1016/j.atherosclerosis.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Lorenzen JM, Neunhöffer H, David S, Kielstein JT, Haller H, Fliser D. Angiotensin II receptor blocker and statins lower elevated levels of osteopontin in essential hypertension—results from the EUTOPIA trial. Atherosclerosis . 2010;209:184–8. doi: 10.1016/j.atherosclerosis.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Arakawa M, Uchida N, Kanda N, Kurosawa Y, Odani T, Kanmatsuse K, Endo M, Yamazaki T, Hidaka S. Influence of losartan intake on the circadian rhythm of melatonin secretion in humans. Pharmazie . 2014;69:192–7. [PubMed] [Google Scholar]
- 31.Song SN, Tomosugi N, Kawabata H, Ishikawa T, Nishikawa T, Yoshizaki K. Down-regulation of hepcidin resulting from long-term treatment with an anti–IL-6 receptor antibody (tocilizumab) improves anemia of inflammation in multicentric Castleman disease. Blood . 2010;116:3627–34. doi: 10.1182/blood-2010-03-271791. [DOI] [PubMed] [Google Scholar]
- 32.Iuchi Y1, Kibe N, Tsunoda S, Suzuki S, Mikami T, Okada F, Uchida K, Fujii J. Implication of oxidative stress as a cause of autoimmune hemolytic anemia in NZB mice. Free Radical Biolo. Med. 2010;48TF:935–44. doi: 10.1016/j.freeradbiomed.2010.01.012. [DOI] [PubMed] [Google Scholar]