Abstract
A method for the detection and quantification of hydroxyl and epoxy arachidonic acid (AA1) metabolites in human plasma was developed using liquid-liquid extraction, phospholipid saponification followed by derivatization of the acid moiety and liquid chromatographic tandem mass spectrometric detection. Derivatization with a pyridinium analog allowed for detection in the positive ion mode, greatly improving sensitivity and the stability of the more labile AA metabolites. The entire method utilizes a 96-well plate format, increasing sample throughput, and was optimized to measure 5-, 8-, 9-, 11-, 12-, 15-, 19-, and 20- hydroxyeicosatetraenoic acid (HETE), 5,6-, 8,9-, 11,12-, and 14,15- dihydroxyeicosatrienoic acid (DHET), and the regio- and cis-/ trans- isomers of 5,6-, 8,9-, 11,12-, and 14,15-epoxyeicosatrienoic acid (EET). The method was validated for its applicability over the FA concentration range found in human plasma. Using 100 μL aliquots of pooled human plasma, EET levels, particularly 5,6-EET, were observed to be higher than previously reported, with measured concentrations of 23.6 ng/ml for 5,6-EET, 5.6 ng/mL for 5,6-trans-EET, 8.0 ng/mL for 8,9-EET, 1.9 ng/mL for 8,9-trans-EET, 8.8 ng/mL for 11,12-EET, 3.4 ng/mL for 11,12-trans-EET, 10.7 ng/mL for 14,15-EET, and 1.7 ng/mL 14,15-trans- EET. This method is suitable for larger population studies to elucidate the complex interactions between the eicosanoids and various disease states and may be used for quantitation of a wide variety of fatty acids beyond eicosanoids from small volumes of human plasma.
Keywords: epoxyeicosatrienoic acid, hydroxyeicosatetraenoic acid, dihydroxyeicosatrienoic acid, arachidonic acid, eicosanoids, liquid chromatography tandem mass spectrometry
1. INTRODUCTION
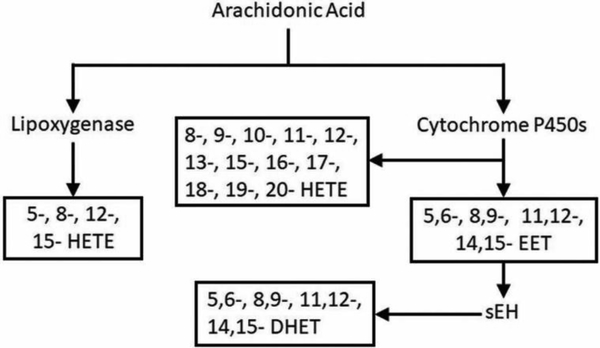
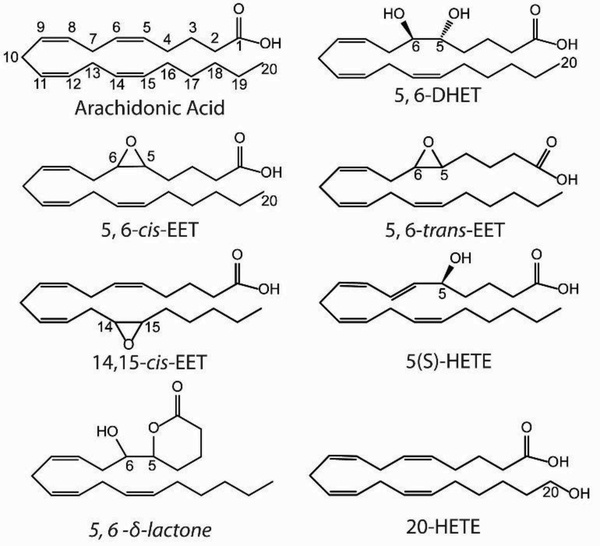
The polyunsaturated ω−6 fatty AA is the precursor to a wide variety of eicosanoids; it contains four cis- double bonds and can constitute up to 10–20% of total phospholipid in human plasma [1]. AA is esterified at the Sn2 position of phospholipids and can be freed by membrane phospholipases, primarily members of the phospholipase A2 (PLA2) family [2]. The three major enzymatic pathways of AA metabolism are by cyclooxygenases (COX) which convert AA to prostaglandins, lipoxygenases (LOX) which convert AA to leukotrienes as well as 5-, 8-, 12-, and 15- HETEs, and cytochrome P450s (CYP) which convert AA to HETEs and EETs [3]. The epoxide moiety of EETs can be hydrolyzed by soluble epoxide hydrolase (sEH) into a threo- diol [4], producing DHET. Here we focus on the EETs, HETEs, and DHETs, whose metabolic pathways are shown in Figure 1. Each of the four different AA double bonds can be metabolized, producing four EET and four DHET regioisomers [5]. Additionally, the cis- double bonds of AA can be converted to trans- by oxidative stress or introduced by the consumption of dietary trans- AA, producing trans EET isomers [6–10]. Representative molecules from each class of eicosanoid of interest are shown in Figure 2.
Figure 1.
Metabolic pathways producing EET, HETE, and DHET fatty acids from arachidonic acid.
Figure 2.
Representative structures of the eicosanoids of interest: AA, DHET, cis-EET, trans-EET, and HETE. 5,6-δ-lactone is a product of an intra-molecular reaction of 5,6-EET.
EETs, HETEs, and DHETs are pleiotropic signaling molecules associated with wide ranging and often opposing effects [11–13], demonstrating the necessity for a method to comprehensively quantify all eicosanoids within a single sample. 12-HETE has been shown to promote carcinogenesis and tumor angiogenesis, while 15-HETE has been shown to promote cell apoptosis [14]. EETs facilitate vasodilation by Ca2+ activated K+ channels, while 20-HETE induces vasorestriction and K+ channel inhibition [11,15].
EETs are cardioprotective, anti-inflammatory, anti-hypertensive, stimulate renal endothelial cell proliferation, promote angiogenesis, induce platelet aggregation, protect against ischemia reperfusion injury, and promote tumor malignancy [16–20]. Greater activity of sEH, converting EETs to DHETs, thereby attenuating the effects of EETs, is associated with hypertension and diabetes [21]. Low levels of EETs, high HETE levels, and high DHET levels have also been shown to be risk factors for ischemic stroke [22]. The four EET regioisomers have overlapping, yet distinct effects and enzyme-substrate activities [23,24], and it has been shown that EET cis- and trans- isomers can have different levels of activity as well [25,26]. Research into the varying effects of EET cis-trans isomers along with an expanded panel of AA metabolites is limited to small sample sizes, possibly due to the difficulty of handling a large number of samples and analyzing them reproducibly.
Eicosanoids are most commonly measured by GC-MS after esterification to a pentafluorobenzyl moiety [27–30], LC-MS using negative mode ionization [9,31,32], capillary electrophoresis [33,34], paired ion electrospray ionization [35,36], and LC with fluorescent detection [37]. Our method has several advances over previous measurement strategies. GC-MS requires an HPLC preparatory step and cannot separate all FA cis-trans- and regioisomers. Capillary electrophoresis can have prohibitively long run times; up to several hours per sample. Although LC-MS is more sensitive and selective than other detection strategies, fatty acids are often detected in the electrospray negative ionization mode where carboxylate ions have reduced signal because the negative charge is suppressed by acid typically added to the LC mobile phase. Conjugation of the carboxylic acid moiety through an amide bond to the permanently positively charged group 1-(4-Aminomethyl)phenyl)pyridine-1-ium chloride (AMPP) allows for LC-MS analysis in electrospray positive ionization mode, greatly increasing sensitivity by a factor of up to 60,000 compared to underivatized fatty acids analyzed in the negative ion mode [38,39]. Paired ion electrospray ionization can also allow for detection of negative ions in positive ion mode, but its limit of detection (LOD) of 0.13 μg/mL for fatty acids is not sufficient for the detection of all AA metabolites [35]. Other groups have developed carboxylic acid derivatization and charge reversal strategies for LC-MS that have improved sensitivity by 50–1500 fold [40] and a 2500 fold improvement [41] compared to underivatized, negatively charged fatty acids. 5,6-EET, which forms a 5,6-δ-lactone or hydrolyzes to DHET with a half-life of minutes in aqueous buffer [42], is preserved by AMPP functionalization and its detection is greatly improved. Because AMPP is not specific for a single class of FAs but will add a permanent positively charged moiety to any carboxylic acid, this derivatization method may be suitable for detection of a wide variety of FAs.
EETs are metabolically unstable and rapidly esterified to a phospholipid reservoir [13]; only a small percentage of EETS, roughly 3% in rats [27], are found in plasma in the free form. Phospholipase PLA2 is commonly used to free the FAs, but here we have chosen to use potassium hydroxide. This allows for the measurement of total FAs in plasma in a reproducible manner without the higher cost and potential batch to batch variability in activity of the enzyme PLA2. We developed a method for the extraction and quantification of AA metabolites in an improved throughput 96-well plate format, as well as quantification of all EET regioisomers and cis-trans isomers. Because this method improves sample throughput, sensitivity, and theoretically will work with any compounds containing a carboxylic acid moiety, it is suitable for the quantification of a wide variety of FAs as well as use in larger population studies. This will provide greater statistical “power” to results as well as potentially elucidate complex relationships between various FAs within a single sample and provide guidance for potential future interventions that can improve health outcomes.
2. Material and methods
2.1. Chemicals and Reagents.
Optima grade solvents including methanol, ethyl acetate, acetonitrile (ACN), water, chloroform, 2-propanol and acetic acid, triphenylphosphine (TPP), sodium chloride, hydrochloric acid (HCl), and 10x concentrated phosphate buffered saline (PBS) were purchased from Fisher Scientific (Waltham, MA, USA). Butylated hydroxytoluene (BHT) was purchased from MP Bio (Santa Ana, CA, USA). Potassium hydroxide (KOH), Phospholipase A2 (PLA2) from Naja mossambica mossambica, and 1-Hydroxybenzotriazole (HOBT) was purchased from Sigma-Aldrich (St. Louis, MO, USA). (±)5,6-dihydroxy-8Z,11Z,14Z-eicosatrienoic acid, (±)8,9-dihydroxy-5Z,11Z,14Z-eicosatrienoic acid, (±)11,12-dihydroxy-5Z,8Z,14Z-eicosatrienoic acid, (±) 14,15-dihydroxy-5Z,8Z,11Z-eicosatrienoic acid, (±) 14,15-dihydroxy-5Z,8Z,11Z-eicosatrienoic-d11 acid (±)5,6-epoxy-8Z,11Z,14Z-eicosatrienoic acid, (±)5,6-epoxy-8Z,11Z,14Z-eicosatrienoic-d11 acid, (±)8,9-epoxy-5Z,11Z,14Z-eicosatrienoic acid, (±)8,9-epoxy-5Z,11Z,14Z-eicosatrienoic-d11 acid, (±)11,12-epoxy-5Z,8Z,14Z-eicosatrienoic acid, (±)14,15-epoxy-5Z,8Z,11Z-eicosatrienoic acid, (±)14,15-epoxy-5Z,8Z,11Z-eicosatrienoic-d11 acid, (±)5-hydroxy-6E,8Z,11Z,14Z-eicosatetraenoic acid, (±)8-hydroxy-5Z,9E,11Z,14Z-eicosatetraenoic acid, (±)9-hydroxy-5Z,7E,11Z,14Z-eicosatetraenoic acid, (±)11-hydroxy-5Z,8Z,12E,14Z-eicosatetraenoic acid, (±)12-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid, 12S-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic-d8 acid, (±)15-hydroxy-5Z,8Z,11Z,13E-eicosatetraenoic acid, 19S-hydroxy-5Z,8Z,11Z,14Z-eicosatetraenoic acid, and 20-hydroxy-5Z,8Z,11Z,14Z-eicosatetraenoic acid were purchased from Cayman Chemicals (Ann Arbor, MI, USA). 1-(4-Aminomethyl) phenyl) pyridine-1-ium chloride (AMPP) was purchased from Oxchem Corporation (Wood Dale, IL, USA). 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) was purchased from TCI (Tokyo, Japan). 96-well polypropylene cap mats, 350 μL and 2 mL polypropylene 96-well plates were purchased from NUNC (Rochester, NY, USA). 96-well 0.45 μm hydrophilic PTFE filters from EMD Millipore (Darmstadt, Germany). Plate dryer, positive pressure manifold, and 96-well Evolute Express ABN SPE 30 mg plate from Biotage (Uppsala, Sweden).
2.2. Plasma Sample Collection.
We used anonymous plasma samples from control subjects in the Cardiac Arrest Blood Study Repository. Samples had been collected from study subjects without clinically diagnosed heart disease at the time of an in-person interview and stored at −80°C (PMID: 19303975). Measurement of arachidonic acid and eicosanoids in the repository samples was approved by the University of Washington Human Subject Review Committee. Plasma from several individuals was pooled, homogenized by vortex mixing for 120 seconds, and then aliquoted and stored at −80 °C until used.
2.3. Sample Extraction.
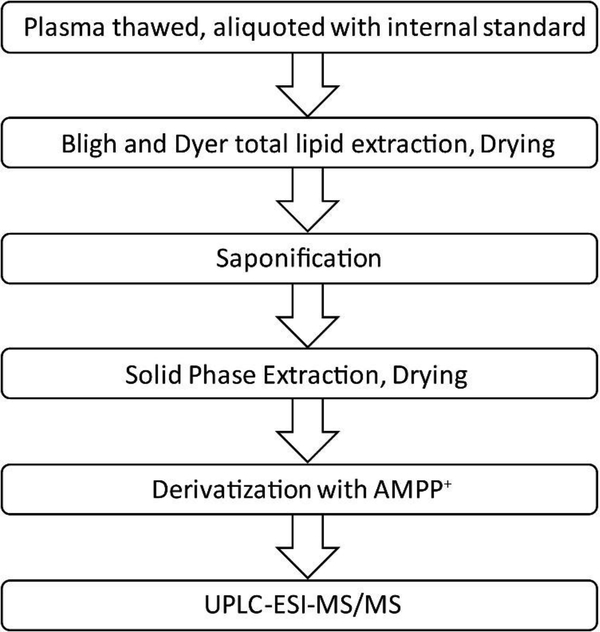
To minimize degradation of the labile epoxy eicosanoids and oxidation of AA, steps 2.3–2.7 were carried out in low light and completed in a single day. Figure 3 summarizes the main steps in the FA quantitation. First, total lipids were extracted from plasma samples using a modified Bligh and Dyer method [43]. Plasma (100 μL aliquots) was thawed overnight on ice at 4 °C, then briefly vortexed and centrifuged at 10,000 X g for 2 min to remove suspended aggregates. In each well of a 2 mL 96-well plate, 1 μL of 50 mM BHT and 5 mM TPP in 2-propanol, 10 μL of 25 ng/mL deuterated internal standard mix was added to 100 μL of plasma or to wells containing the calibration curve. 340 μL of 0.9% NaCl, 0.1% acetic acid solution was then added, along with a mixture of 560 μL 2:1 methanol: chloroform. This was mixed by turbulent mixing by vigorous pipetting with a 1 mL multichannel pipette followed by shaking for 10 min at 500 RPM on a plate shaker. Then, 190 μL of chloroform and another 190 μL of 0.9% NaCl, with 0.1% acetic acid solution was added. The solution was again mixed by pipetting followed by shaking for 10 min at 500 RPM. The 96-well plate was then centrifuged for 30 min at 4 °C, 4500 × g. The lower organic phase was transferred to a clean 96-well plate and dried under nitrogen.
Figure 3.
Process for FA purification and quantitation of EETs, HETEs, and DHETs from plasma.
2.4. Saponification.
Phospholipids were hydrolyzed by adding 150 μL of 2-propanol and 150 μL of 1.0 M KOH. A cap mat was placed on the 96-well plate. The plate was vortexed for 3 seconds and placed in a 37 °C water bath, with shaking at 30 rpm, for 30 min. The solution was then neutralized with 150 μL of 1.0 M HCl and diluted to a final volume of 1 mL with 550 μL PBS.
2.5. Solid phase extraction.
Lipids were extracted using 96-well solid phase extraction cartridges sequentially conditioned with 1 mL each of ethyl acetate, methanol, and then PBS. The FAs were loaded onto the column and a positive pressure manifold was used to maintain a rate ≤ 1 drop/second. The cartridges were then washed with 3 mL of 95% water 5% ACN and dried with a stream of nitrogen gas for 20 min. FAs were eluted with 500 μL methanol, then with 1 mL ethyl acetate and the eluent was dried under nitrogen gas.
2.6. Derivatization.
The FAs were derivatized using a modified version of a method developed by Bollinger et al., producing positively charged molecules with >95% derivatization efficiency [38]. Dried lipids were solubilized in 30 μL of ACN, then derivatized by sequentially adding 10 μL of cold 640 mM EDC dissolved in water, 20 μL of 5 mM HOBT dissolved in ACN, and 20 μL of 10 mM AMPP dissolved in 1:1 ACN: water. Then the 96-well plate was capped, vortexed, and incubated in a water bath at 60 °C for 30 min. The 96-well plate was cooled to room temperature and Optima water was added to bring the final volume to 160 μL per sample. Then, the lipids were filtered using a 96-well Millipore centrifugal filter at 2,500 × g for 3 minutes.
2.7. UPLC-ESI-MS/MS.
Eicosanoid quantification was carried out using a Waters Xevo TQ-s ESI mass spectrometer operating in positive ion mode coupled to a Waters Acquity I-class UPLC. Several UPLC columns were tested and initially, an Ascentis Express RP-amide column (2. 0 μm, 2.1 X 150 mm, Supelco, Bellefonte, PA) was used and found to produce acceptable chromatographic separation, however, it produced large variations in retention times between injections. The best separation and most consistent chromatography was achieved using a Waters Acquity UPLC BEH shield C18 column (1.7 μm, 2.1 ×150 mm). Each sample was run using a 25 μL injection volume, 0.325 mL/min flow rate, with the LC column heated to 60 °C. Mobile phases (A) water and (B) ACN each contained 10 mM formic acid. The chromatographic separation was 18 min long with the following mobile phase gradient: 0–1 min 70% A, 1–4 min 79–69% A, 4–14.5 min 69–65% A, 14.5–16 min 10% A, 16–18 min 70% A. The MS conditions were as follows: positive ionization, capillary voltage 3.1 kV, cone voltage 25 V, collision voltage 35 V, source temperature 150 °C, desolvation temperature 400 °C. The quadrupole MS was run in Selected Reaction Monitoring (SRM) mode to maximize sensitivity, with FAs quantified using the mass transitions shown in Table 1. The mass transitions used for the detection of internal standards were: 516 > 183.1 for 14,15 DHET-d11, 495 > 183.1 for 12S HETE-d8, and 498 > 183.1 for 14,15– 8,9- and 5,6- EET-d11. Data was collected using MassLynx and peak areas were integrated using QuanLynx software.
Table 1.
Calibration curve range, FA retention time, selected MS ion fragment, limit of detection (LOD) in femtograms (fg) on-column (OC), and lower limit of quantitation (LLOQ) in picograms (pg) for each of the quantified FAs. Trans- FAs found in plasma were assumed to have the same instrumental response as their commercially available cis- counterparts.
| Fatty Acid | Calibration Curve Range in pg on column; (in ng/mL) | Retention Time (min) | SRM Mass Transition | LOD in fg OC; | LLOQ in pg OC; (in ng/mL) |
|---|---|---|---|---|---|
| 0–469; | 4.3; | ||||
| 5,6-DHET | (0–20) | 5.8 | 505→239 | 25 | (0.185) |
| 0–469; | 4.3; | ||||
| 8,9-DHET | (0–20) | 4.8 | 505→239 | 100 | (0.185) |
| 0–469; | 4.3; | ||||
| 11,12-DHET | (0–20) | 4.0 | 505→239 | 100 | (0.185) |
| 0–469; | 4.3; | ||||
| 14,15-DHET | (0–20) | 3.4 | 505→239 | 50 | (0.185) |
| 0–2340 | 26; | ||||
| 5-HETE | (0–100) | 9.8 | 487→283 | 50 | (1.11) |
| 0–2340; | 26; | ||||
| 9-HETE | (0–100) | 8.7 | 487→307 | 50 | (1.11) |
| 0–2340; | 26; | ||||
| 11-HETE | (0–100) | 7.6 | 487→335 | 50 | (1.11) |
| 0–2340; | 26; | ||||
| 12-HETE | (0–100) | 7.9 | 487→347 | 50 | (1.11) |
| 0–2340; | 26; | ||||
| 15-HETE | (0–100) | 6.9 | 487→183 | 10 | (1.11) |
| 0–350;s | 1.7; | ||||
| 19-HETE | (0–15) | 4.8 | 487→183 | 50 | (0.074) |
| 0–350; | 1.7; | ||||
| 20-HETE | (0–15) | 5.2 | 487→183 | 50 | (0.074) |
| 0–700; | 4.0; | ||||
| 5,6-EET | (0–30) | 11.6 | 487→283 | 25 | (0.185) |
| 0–700; | 4.0; | ||||
| 8,9-EET | (0–30) | 10.8 | 487→307 | 25 | (0.185) |
| 0–700; | 4.0; | ||||
| 11,12-EET | (0–30) | 10.3 | 487→333 | 25 | (0.185) |
| 0–700; | 4.0; | ||||
| 14, 15-EET | (0–30) | 9.0 | 487→333 | 75 | (0.185) |
2.8. Calibration curve.
4 μg/mL stock solutions of each fatty acid were prepared in ethyl acetate and stored at −80 °C in Teflon septum screwcap vials. An eight-point calibration curve in ethyl acetate with 0.1 mM BHT and 0.1 mM TPP was generated; the calibration curve range, retention time, SRM fragment used for quantitation, LOD, and lower limit of quantitation (LLOQ) for each fatty acid are listed in Table 1. The LOD was defined as three times the signal to noise ratio, while the LLOQ was the lowest concentration of analyte in the calibration curve. Concentrations of unknowns were determined from least squares linear regression of the peak area ratio of FAs to that of their corresponding internal standards. The calibration curve was split into two-the lowest five points and the highest three points-to reduce bias imparted from the points with highest concentrations. Fatty acids were identified by unique fragmentation patterns. Trans-EETs, lacking commercial standards, were identified by retention time as well as fragmentation pattern [7]. Prior to LC-MS analysis, the calibration curve underwent the same lipid extraction process as the plasma samples.
3. RESULTS and DISCUSSION
3.1. Validation.
Selectivity of each of the FAs was first determined using commercial standards to verify molecular weight, fragmentation patterns, adequate chromatographic resolution, as well as linear instrumental response over the FA concentration ranges found in plasma. The calibration curve and quality control concentrations, relative errors (RE), and coefficients of variation (CV) for each FA, as well as calibration curve R2 values are shown in Table S1. These tabulated values fall within the ranges deemed acceptable by the FDA Bioanalytical Method Validation Draft Guidance [44]. The recovery for each of the fatty acid internal standards that have been subjected to the entire extraction process are shown below in Table 2. 8-HETE was not included in our assay initially but was identified later. Since 8-HETE was not part of our original calibration curve it was quantified using the 9-HETE calibration curve. We have since found that the difference in instrumental response between 8-HETE and 9-HETE is 5–6%; less than the inter-day or intra-day coefficient of variation (CV) of either 8-HETE or 9-HETE.
Table 2.
Recovery and coefficient of variation for internal standards from 8 pooled plasma samples vs. 8 neat standards.
| % Recovery | % CV | |
|---|---|---|
| 14, 15-DHETd11 | 58.2 | 12.0 |
| 12-HETE-d8 | 54.0 | 10.9 |
| 14,15-EET-d11 | 63.5 | 8.9 |
| 8,9-EET-d11 | 62.7 | 10.0 |
| 5,6-EET-d11 | 47.6 | 9.6 |
3.2. EET loss from TPP.
Our extraction protocol initially added TPP to the plasma at a concentration of 0.1 mM, rather than the 0.005 mM currently used, and was added at a concentration of 0.1 mM with BHT a second time after the solid phase extraction step and before FA derivatization. We have found that the two 0.1 mM additions of TPP strongly decreased the detected amount of EETs. This may be due to a nucleophilic attack on the epoxide’s carbon atoms by the phosphate’s lone electron pair aided by the elevated temperature used for derivatization and saponification, although the mechanism was not investigated. prevented by optimizing the amount of TPP added and removing the second addition of antioxidant.
3.3. Liquid-Liquid Extraction and Saponification.
In plasma, a large portion of the phospholipids are bound to lipoproteins or serum albumin [45]. Bligh and Dyer liquid-liquid extraction is usually accomplished by sample agitation, and in addition to that our method used turbulent mixing from pipetting a solution through the small tip aperture to mechanically facilitate desorption of phospholipids from serum albumin and lipoproteins. Following extraction, we chose to hydrolyze esterified FAs using potassium hydroxide rather than the commonly used PLA2. Using base reduces the cost for large studies, as well as alleviates concerns of potential PLA2 batch to batch variability. We compared KOH and PLA2 hydrolysis side by side using 8 pooled plasma samples for each treatment. Plasma, after liquidliquid extraction, was treated either with the KOH method described previously or with 10 units/mL PLA2 diluted into 300 μL of 50 mM Tris-HCL, 100 mM NaCl, 1 mM CaCl2 buffer, pH 9.2 for 30 minutes at 37 °C. The hydrolysis efficiency and sample to sample variability are shown in Table 3. Table 3 also shows the observed effect of the method of hydrolysis on the internal standard peak area. The amounts of 14,15-DHET-d11 and 12-HETE-d8 remain the same, while PLA2 treatment yielded slightly less 5,6-EET-d11 and more 8,9-EET-d11 and 14,15-EET-d11.
Table 3.
Comparing hydrolysis efficiency and reproducibility of FAs in pooled plasma using PLA2 to KOH. Column 1 compares the peak area ratios of the FAs when hydrolyzed by PLA2, divided by the peak area ratio of that fatty acid hydrolyzed by KOH (PLA2 treated plasma FA Peak area/IS Peak area)/(KOH treated plasma FA peak area/IS Peak Area). Numbers below 100% signify more FA was observed using KOH, while numbers above 100% signify more FA was observed by PLA2 hydrolysis. For all FAs with the exception of 19-HETE, more FAs were observed with KOH. The greater quantity of observed FAs from KOH may be due to the less specific nature of KOH hydrolysis. Columns 2 and 3 are the CV for PLA2 and KOH hydrolysis, respectively.
| PLA2/KOH (%) | PLA2 CV (%) | KOH CV (%) | |
|---|---|---|---|
| 5,6-DHET | 14.6 | 32.8 | 18.9 |
| 8,9-DHET | 44.7 | 18.5 | 17.5 |
| 11,12-DHET | 22.4 | 16.7 | 29.6 |
| 14,15-DHET | 51.0 | 60.0 | 37.7 |
| 5-HETE | 18.3 | 18.0 | 14.5 |
| 8-HETE | 37.7 | 9.0 | 5.5 |
| 9-HETE | 31.1 | 7.4 | 6.9 |
| 11-HETE | 34.1 | 9.9 | 8.3 |
| 12-HETE | 65.3 | 5.7 | 4.5 |
| 15-HETE | 45.6 | 18.5 | 11.7 |
| 19-HETE | 142.4 | 26.1 | 10.6 |
| 20-HETE | 89.4 | 8.7 | 9.8 |
| 5,6-cis-EET | 80.1 | 38.6 | 21.2 |
| 5,6-trans-EET | 68.9 | 59.8 | 24.2 |
| 8,9-cis-EET | 51.7 | 31.6 | 21.0 |
| 8,9-trans-EET | 39.3 | 61.0 | 16.9 |
| 11,12-cis-EET | 37.0 | 33.0 | 21.4 |
| 11,12-trans-EET | 37.4 | 56.8 | 11.4 |
| 14,15-cis-EET | 41.4 | 22.2 | 33.6 |
| 14,15-trans-EET | 36.7 | 50.6 | 14.3 |
| 14,15-DHET-d11 | 95.9 | 8.5 | 6.5 |
| 12-HETE-d8 | 97.3 | 11.4 | 5.3 |
| 5,6-EET-d8 | 88.1 | 6.3 | 27.3 |
| 8,9-EET-d8 | 116.3 | 5.6 | 9.4 |
| 14,15-EET-d8 | 143.8 | 6.1 | 11.2 |
3.4. Derivatization.
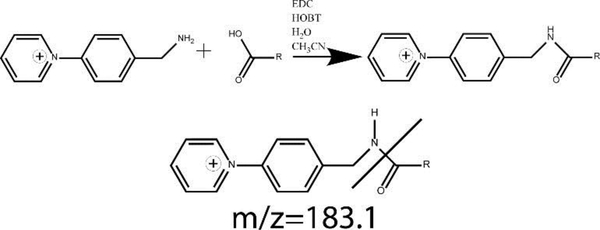
The amide coupling of AMPP to the carboxylic acid, shown in Figure 4, provided a highly sensitive, pH independent charge for the electrospray positive ion mode. Parent ion masses are 168 Daltons heavier than the base FAs, giving parent ion masses of 487 for EETs and HETEs and 505 for DHETs. Daughter ion fragments were selected by scanning daughter ion fragments from perfused standards introduced to the tandem MS and selecting fragments with suitable selectivity and maximum signal for the FAs of interest. The most common daughter ion fragment at m/z 183.1 was the cleavage of the AMPP group as shown in Figure 4. This fragment was not selective enough to achieve baseline separation of all EET regio- and cis-trans isomers. The DHETs were baseline separated without selecting specific fragment ions for each regioisomers, and an optimal signal to noise ratio using a m/z 239 fragment. Fatty acids were analyzed at several collision voltages and 35 V was found to have the best combination of high signal and characteristic ion fragmentation for FA identification.
Figure 4.
Derivatization of FAs with AMPP by reaction of primary amine to carboxylic acid using EDC. The most populous MRM daughter ion fragment of all FAs, formed by the cleavage of the AMPP moiety, is also shown.
3.5. Resolution of isomers.
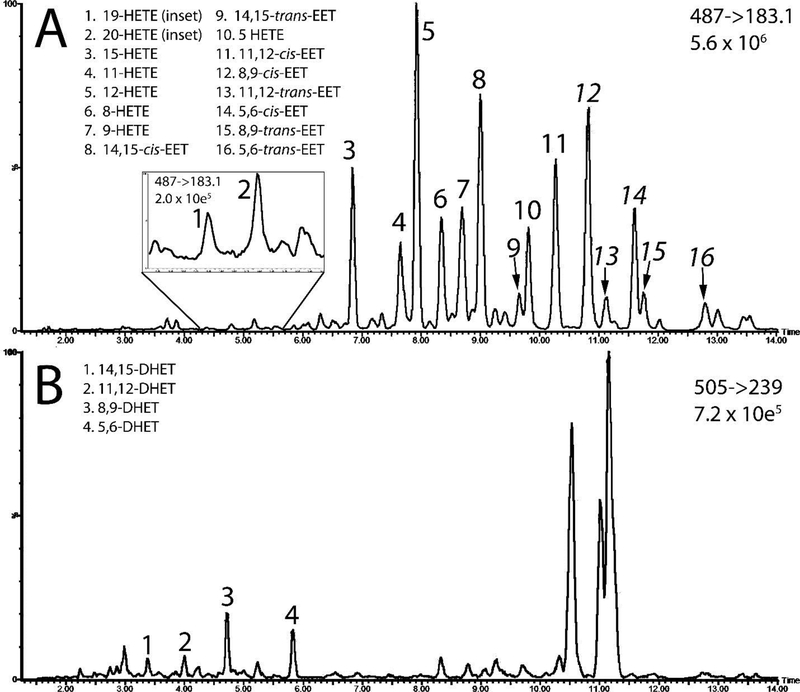
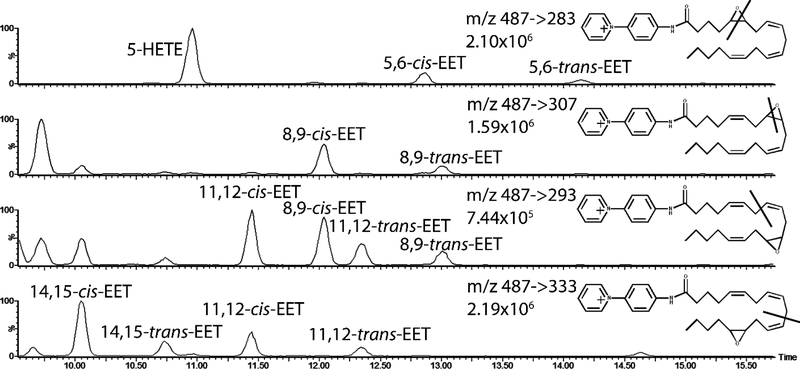
Through optimization of the HPLC method and selection of specific molecular fragments, we were able to separate each of the EET, HETE, and DHET regioisomers as well as the cis-trans EET isomers. Representative chromatograms of one plasma sample is shown in Figure 5. The m/z 183.1 daughter ion channel did not produce baseline resolution for all FAs, so FA mass fragments listed in Table 1 are used for quantification. Representative chromatograms of EETs extracted from human plasma and the putative molecular fragmentation location for the EET regioisomers are shown in figure 6.
Figure 5.
Representative chromatograms of FAs after extraction from plasma. Daughter/fragment ions and the maximum instrument response are shown for each chromatogram. LC-MS channel containing the HETEs and EETs are shown in 5A, with an inset showing an expanded view of 19- and 20- HETE. The channel containing DHETs is shown in 5B.
Figure 6:
Chromatograms of selected mass fragments used to achieve baseline resolution as well as maximum signal of cis-trans isomers and regioisomers of EETs extracted from human plasma. 5,6 EET and 8,9 EET produced characteristic ion fragments from the cleavage of their epoxide moiety while 11,12 EET and 14,15 EET produced fragments with a higher signal along the carbon backbone before the epoxide moiety.
3.6. Quantification of DHETs, HETEs, and EETs in Human Plasma.
We quantified plasma extracted from 24 (100 μL aliquots) of pooled plasma to determine inter-sample variability. Inter-day variability was determined from pooled plasma extracted and quantified during extractions on six different days. The FA calibration curve and quality controls are injected before and after the plasma samples and pass during both injections, suggesting that the derivatized FAs remain stable over the course of LC-MS analysis. The concentrations of the DHETs, HETEs, and EETs from pooled plasma are shown in Table 4. Because trans- EETs are not commercially available, their concentrations were determined using the calibration curve of their cis- counterparts and they were assumed to have the same instrumental response. The higher inter-day CV for the trans-EETs requires further exploration with more samples.
Table 4.
Quantified FA concentrations from human pooled plasma given in nanograms per mL of plasma. Intra-day variability is the CV of 24 pooled plasma samples run on the same day. Inter-day variability is the CV from 6 pooled plasma samples quantified during 6 separate extractions.
| FA Concentration in ng/mL | Intra-day CV (%) | Inter-day CV (%) | |
|---|---|---|---|
| 5,6-DHET | 1.6 | 6.8 | 17.5 |
| 8,9-DHET | 6.08 | 7.7 | 9.6 |
| 11,12-DHET | 1.47 | 7.9 | 7.9 |
| 14,15-DHET | 0.87 | 12.1 | 20.0 |
| 5-HETE | 70.91 | 10.3 | 29.9 |
| 8-HETE | 28.37 | 9.5 | 14.5 |
| 9-HETE | 42.99 | 9.1 | 21.5 |
| 11-HETE | 29.82 | 9.9 | 20.6 |
| 12-HETE | 56.84 | 9.5 | 18.4 |
| 15-HETE | 38.96 | 10.4 | 14.6 |
| 19-HETE | 1.03 | 9.1 | 20.2 |
| 20-HETE | 1.86 | 8.3 | 20.4 |
| 5,6-EET | 23.6 | 19.5 | 26.9 |
| 5,6-trans-EET | 5.58 | 1.47 | 51.7 |
| 8,9-EET | 8.04 | 1.97 | 14.1 |
| 8,9-trans-EET | 1.95 | 23.1 | 68.7 |
| 11,12-EET | 8.84 | 19.1 | 19.4 |
| 11,12-trans-EET | 3.44 | 22.8 | 57.2 |
| 14,15-EET | 10.71 | 19.8 | 21.0 |
| 14,15-trans-EET | 1.66 | 19.3 | 60.8 |
3.7. EET Stability and non-enzymatic oxidative product FA formation.
Although the use of strong acids or bases may lead to epoxide hydrolysis and conversion of EETs to their respective DHETs, we only found low concentrations of DHETs in the plasma samples and no DHET peaks during the extraction of DHET-free EET standards, suggesting that our method did not lead to EET hydrolysis and DHET formation. Trans-EETs were not detected in the pure standards of the calibration curve, suggesting there is no cis- to trans- interconversion of EETs.
After extraction and injection, samples were stored for one week at −20°C and then re-injected to determine sample stability. It was found that measured concentrations of FAs in plasma, but not in neat standards, increased after storage. This was possibly due to auto-oxidation of AA in the samples. Trans-EETs, increased the most in the stored samples while cis-EETs concentrations remained relatively similar. This supports a free radical mechanism of formation which preferentially forms trans-EETs [9]. Despite the stability of the cis-EETs, UPLC-MS/MS quantitation occurred immediately followed FA extraction and derivatization to avoid further auto-oxidation.
3.8. FA Saponification; Free vs total FAs.
Only a small percentage of EETs are free in rat plasma [46], and we found this to be the case in human plasma as well. We quantified FA content from 8 non-saponified plasma samples, replacing KOH and HCl with PBS during the saponification portion of the sample preparation to prevent hydrolysis of esterified fatty acids. Free FAs were found to account for only a small percentage of total FAs, except for 12-, 19-, and 20-HETE. This suggests that after AA is metabolized to 12-, 19-, and 20-HETE that they are not re-esterified to phospholipids to the same extent as the other fatty acids. The percentage of FA that is free vs. esterified is shown in table 5.
Table 5.
Percentage of each FA found free in plasma without phospholipid saponification and the standard deviation of 8 plasma samples. % of free FA was determined as a ratio of the FA peak area ratio for free FA divided by total FA.
| Free (%) | CV (%) | |
|---|---|---|
| 5,6-DHET* | 2.2 | 32.8 |
| 8,9-DHET* | 3.6 | 21.9 |
| 11,12-DHET* | 6.8 | 33.0 |
| 14,15-DHET* | 3.2 | 41.0 |
| 5-HETE* | 3.4 | 15.1 |
| 8-HETE* | 3.0 | 9.9 |
| 9-HETE* | 2.3 | 8.3 |
| 11-HETE* | 3.0 | 11.6 |
| 12-HETE | 40.4 | 5.3 |
| 15-HETE* | 4.8 | 8.1 |
| 19-HETE | 70.0 | 13.9 |
| 20-HETE | 82.4 | 7.8 |
| 5,6-cis-EET* | 0.8 | 29.0 |
| 5,6-trans-EET | Below LOD | - |
| 8,9-cis-EET* | 0.9 | 29.1 |
| 8,9-trans-EET* | 0.7 | 32.3 |
| 11,12-cis-EET* | 0.6 | 28.5 |
| 11,12-trans-EET* | 0.6 | 21.3 |
| 14,15-cis-EET* | 0.9 | 25.8 |
| 14,15-trans-EET* | 0.7 | 27.2 |
denotes a FA that was below the LLOQ.
CONCLUSIONS
There is a real need for a better understanding of the complex interactions between various arachidonic acid metabolites and their potential outcomes on human health. The methodology in this publication demonstrates a significant improvement in sensitivity, throughput and stability of the analysis of HETE, EET, and DHET metabolites. Using a small plasma volume in a single analytical run we are able to separate not only the 4 regioisomers of EET, but also all of the cis-trans isomers of plasma samples, a 5-point calibration curve and 3 quality control samples in a 96-well plate format with a UPLC-MS/MS run time of 18 minutes per sample. By conjugating the FAs to a pyridinium analog with a permanent positive charge, the sensitivity is increased by several orders of magnitude over the negatively charged native species. This pyridinium moiety also reduces intramolecular interactions from labile compounds, namely 5,6-EET. We have also shown that most AA metabolites are bound to phospholipids, and our hydrolysis method frees more of the FAs from their ester bond than PLA2 in order to accurately measure total FAs. This methodology will be instrumental in answering questions regarding the complex effects of eicosanoids on human health in larger population studies.
Supplementary Material
Highlights:
A 96-well plate method using ESI positive ionization to measure P450 mediated arachidonic acid metabolites is reported.
Fatty acids are derivatized introducing a permanent positive charge to improve sensitivity.
Method can detect EETs, DHETs and HETEs in a single UPLC-MS/MS run under 20 min.
All regio- and geometic EET isomers are detected allowing for large human studies on these important signaling molecules.
ACKNOWLEDGMENTS
I would like to thank the University of Washington School of Pharmacy’s Mass Spectrometry Center, J. Scott Edgar, Theresa Aliwarga, Julie Denham, Paul Jensen, and Alan Chen for their assistance and helpful advice. This work was supported by NIH grants R01HL128709, R01HL130880, R01HL088456 R01HL128809.
Footnotes
CONFLICTS OF INTEREST DISLOSURE
No conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbrevietions: AA, Arachidonic Acid; DHET, EET, epoxyeicosatrienoic acid; dihydroxyeicosatrienoic acid; HETE, hydroxyeicosatetraenoic acid; FA, fatty acids; UPLC-MS/MS, Ultra performance liquid chromatography tandem mass spectrometry; SRM, Selected Reaction Monitoring; PLA2, phospholipase A2; COX, cyclooxygenase; LOX, lipoxygenase; sEH, soluble epoxide hydrolase; TPP, triphenylphosphine; BHT, butylated hydroxytoluene; HOBT, 1-Hydroxybenzotriazole; EDC, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride; AMPP, 1-(4-Aminomethyl) phenyl) pyridine-1-ium chloride; RPM, revolutions per minute; ESI, electrospray ionization; ACN, acetonitrile; LOD, limit of detection; fg, femtograms; pg, picograms; ng, nanograms; OC, on-column; LLOQ, lower limit of quantitation; CV, coefficient of variation
REFERENCES
- [1].Nelson GJ, Schmidt PC, Bartolini G, Kelley DS, Phinney SD, Kyle D, Silbermann S, Schaefer EJ, The effect of dietary arachidonic acid on plasma lipoprotein distributions, apoproteins, blood lipid levels, and tissue fatty acid composition in humans, Lipids. 32 (1997) 427–433. doi:10.1007/s11745-997-0056-6. [DOI] [PubMed] [Google Scholar]
- [2].Balsinde J, Winstead MV, Dennis EA, Phospholipase A2 regulation of arachidonic acid mobilization, FEBS Lett. 531 (2002) 2–6. doi:10.1016/S0014-5793(02)03413-0. [DOI] [PubMed] [Google Scholar]
- [3].Buczynski MW, Dumlao DS, Dennis EA, Thematic Review Series: Proteomics. An integrated omics analysis of eicosanoid biology, J. Lipid Res 50 (2009) 1015–1038. doi:10.1194/jlr.R900004-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jiang H, Zhu AG, Mamczur M, Morisseau C, Hammock BD, Falck JR, McGiff JC, Hydrolysis of cis- and trans-Epoxyeicosatrienoic Acids by Rat Red Blood Cells, J. Pharmacol. Exp. Ther 326 (2008) 330–337. doi:10.1124/jpet.107.134858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang D, Dubois RN, Epoxyeicosatrienoic acids: a double-edged sword in cardiovascular disease and cancer, J. Clin. Invest 122 (2012) 19–22. doi:10.1172/JCI61453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jiang H, Kruger N, Lahiri DR, Wang D, Vatèle J-M, Balazy M, Nitrogen Dioxide Inducescistrans-Isomerization of Arachidonic Acid within Cellular Phospholipids DETECTION OF TRANS-ARACHIDONIC ACIDS IN VIVO, J. Biol. Chem 274 (1999) 16235–16241. doi:10.1074/jbc.274.23.16235. [DOI] [PubMed] [Google Scholar]
- [7].Nakamura T, Bratton DL, Murphy RC, Analysis of Epoxyeicosatrienoic and Monohydroxyeicosatetraenoic Acids Esterified to Phospholipids in Human Red Blood Cells by Electrospray Tandem Mass Spectrometry, J. Mass Spectrom 32 (1997) 888–896. doi:10.1002/(SICI)1096-9888(199708)32:8<888::AID-JMS548>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- [8].Roy U, Joshua R, Stark RL, Balazy M, Cytochrome P450/NADPH-dependent biosynthesis of 5,6-trans-epoxyeicosatrienoic acid from 5,6-trans-arachidonic acid, Biochem. J 390 (2005) 719–727. doi:10.1042/BJ20050681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Aliwarga T, Raccor BS, Lemaitre RN, Sotoodehnia N, Gharib SA, Xu L, Totah RA, Enzymatic and Free Radical Formation of Cis- and Trans- Epoxyeicosatrienoic Acids In Vitro and In Vivo, Free Radic. Biol. Med (2017). doi:10.1016/j.freeradbiomed.2017.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zghibeh CM, Raj Gopal V, Poff CD, Falck JR, Balazy M, Determination of trans-arachidonic acid isomers in human blood plasma, Anal. Biochem 332 (2004) 137–144. doi:10.1016/j.ab.2004.04.030. [DOI] [PubMed] [Google Scholar]
- [11].Roman RJ, P-450 Metabolites of Arachidonic Acid in the Control of Cardiovascular Function, Physiol. Rev 82 (2002) 131–185. doi:10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- [12].Axelrod J, Burch RM, Jelsema CL, Receptor-mediated activation of phospholipase A2 via GTP-binding proteins: arachidonic acid and its metabolites as second messengers, Trends Neurosci. 11 (1988) 117–123. doi:10.1016/0166-2236(88)90157-9. [DOI] [PubMed] [Google Scholar]
- [13].Capdevila JH, Falck JR, Harris RC, Cytochrome P450 and arachidonic acid bioactivation. Molecular and functional properties of the arachidonate monooxygenase, J. Lipid Res 41 (2000) 163–181. [PubMed] [Google Scholar]
- [14].Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A, Dietary long-chain n−3 fatty acids for the prevention of cancer: a review of potential mechanisms, Am. J. Clin. Nutr 79 (2004) 935–945. [DOI] [PubMed] [Google Scholar]
- [15].Lange A, Gebremedhin D, Narayanan J, Harder D, 20-Hydroxyeicosatetraenoic Acid-induced Vasoconstriction and Inhibition of Potassium Current in Cerebral Vascular Smooth Muscle Is Dependent on Activation of Protein Kinase C, J. Biol. Chem 272 (1997) 27345–27352. doi:10.1074/jbc.272.43.27345. [DOI] [PubMed] [Google Scholar]
- [16].Theken KN, Schuck RN, Edin ML, Tran B, Ellis K, Bass A, Lih FB, Tomer KB, Poloyac SM, Wu MC, Hinderliter AL, Zeldin DC, Stouffer GA, Lee CR, Evaluation of cytochrome P450-derived eicosanoids in humans with stable atherosclerotic cardiovascular disease, Atherosclerosis. 222 (2012) 530–536. doi:10.1016/j.atherosclerosis.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Deng Y, Theken KN, Lee CR, Cytochrome P450 epoxygenases, soluble epoxide hydrolase, and the regulation of cardiovascular inflammation, J. Mol. Cell. Cardiol 48 (2010) 331–341. doi:10.1016/j.yjmcc.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Spector AA, Norris AW, Action of epoxyeicosatrienoic acids on cellular function, Am. J. Physiol. Cell Physiol 292 (2007) C996–1012. doi:10.1152/ajpcell.00402.2006. [DOI] [PubMed] [Google Scholar]
- [19].Xu X, Zhang XA, Wang DW, The roles of CYP450 epoxygenases and metabolites, epoxyeicosatrienoic acids, in cardiovascular and malignant diseases, Adv. Drug Deliv. Rev 63 (2011) 597–609. doi:10.1016/j.addr.2011.03.006. [DOI] [PubMed] [Google Scholar]
- [20].Kaspera R, Totah RA, Epoxyeicosatrienoic acids: formation, metabolism and potential role in tissue physiology and pathophysiology, Expert Opin. Drug Metab. Toxicol 5 (2009) 757–771. doi:10.1517/17425250902932923. [DOI] [PubMed] [Google Scholar]
- [21].Imig JD, Hammock BD, Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases, Nat. Rev. Drug Discov 8 (2009) 794–805. doi:10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yi X, Wu L, Liao D, Wang C, Zhang B, Interactions Among CYP2C8, EPHX2, and CYP4A11 Variants and CYP Plasma Metabolite Levels in Ischemic Stroke, J. Atheroscler. Thromb 23 (2016) 1286–1293. doi:10.5551/jat.35279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Falck JR, Reddy LM, Reddy YK, Bondlela M, Krishna UM, Ji Y, Sun J, Liao JK, 11,12-Epoxyeicosatrienoic acid (11,12-EET): structural determinants for inhibition of TNF-α-Induced VCAM-1 expression, Bioorg. Med. Chem. Lett 13 (2003) 4011–4014. doi:10.1016/j.bmcl.2003.08.060. [DOI] [PubMed] [Google Scholar]
- [24].Zeldin DC, Kobayashi J, Falck JR, Winder BS, Hammock BD, Snapper JR, Capdevila JH, Regio- and enantiofacial selectivity of epoxyeicosatrienoic acid hydration by cytosolic epoxide hydrolase., J. Biol. Chem 268 (1993) 6402–6407. [PubMed] [Google Scholar]
- [25].Fitzpatrick FA, Ennis MD, Baze ME, Wynalda MA, McGee JE, Liggett WF, Inhibition of cyclooxygenase activity and platelet aggregation by epoxyeicosatrienoic acids. Influence of stereochemistry, J. Biol. Chem 261 (1986) 15334–15338. [PubMed] [Google Scholar]
- [26].Jiang H, McGiff JC, Quilley J, Sacerdoti D, Reddy LM, Falck JR, Zhang F, Lerea KM, Wong PY-K, Identification of 5,6-trans-Epoxyeicosatrienoic Acid in the Phospholipids of Red Blood Cells, J. Biol. Chem 279 (2004) 36412–36418. doi:10.1074/jbc.M403962200. [DOI] [PubMed] [Google Scholar]
- [27].Karara A, Wei S, Spady D, Swift L, Capdevila JH, Falck JR, Arachidonic acid epoxygenase: Structural characterization and quantification of epoxyeicosatrienoates in plasma, Biochem. Biophys. Res. Commun 182 (1992) 1320–1325. doi:10.1016/0006-291X(92)91877-S. [DOI] [PubMed] [Google Scholar]
- [28].Nithipatikom K, DiCamelli RF, Kohler S, Gumina RJ, Falck JR, Campbell WB, Gross GJ, Determination of Cytochrome P450 Metabolites of Arachidonic Acid in Coronary Venous Plasma during Ischemia and Reperfusion in Dogs, Anal. Biochem 292 (2001) 115–124. doi:10.1006/abio.2001.5044. [DOI] [PubMed] [Google Scholar]
- [29].Campbell WB, Gebremedhin D, Pratt PF, Harder DR, Identification of Epoxyeicosatrienoic Acids as Endothelium-Derived Hyperpolarizing Factors, Circ. Res 78 (1996) 415–423. doi:10.1161/01.RES.78.3.415. [DOI] [PubMed] [Google Scholar]
- [30].Katoh T, Takahashi K, Capdevila J, Karara A, Falck JR, Jacobson HR, Badr KF, Glomerular stereospecific synthesis and hemodynamic actions of 8,9-epoxyeicosatrienoic acid in rat kidney, Am. J. Physiol.-Ren. Physiol 261 (1991) F578–F586. doi:10.1152/ajprenal.1991.261.4.F578. [DOI] [PubMed] [Google Scholar]
- [31].Jiang H, Quilley J, Reddy LM, Falck JR, Wong PY-K, McGiff JC, Red blood cells: reservoirs of cis- and trans-epoxyeicosatrienoic acids, Prostaglandins Other Lipid Mediat 75 (2005) 65–78. doi:10.1016/j.prostaglandins.2004.10.003. [DOI] [PubMed] [Google Scholar]
- [32].Mesaros C, Blair IA, Targeted Chiral Analysis of Bioactive Arachidonic Acid Metabolites Using Liquid-Chromatography-Mass Spectrometry, Metabolites. 2 (2012) 337–365. doi:10.3390/metabo2020337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].VanderNoot VA, VanRollins M, Capillary Electrophoresis of Cytochrome P-450 Epoxygenase Metabolites of Arachidonic Acid. 1. Resolution of Regioisomers, Anal. Chem 74 (2002) 5859–5865. doi:10.1021/ac025909+. [DOI] [PubMed] [Google Scholar]
- [34].VanRollins M, VanderNoot VA, Simultaneous resolution of underivatized regioisomers and stereoisomers of arachidonate epoxides by capillary electrophoresis, Anal. Biochem 313 (2003) 106–116. doi:10.1016/S0003-2697(02)00503-1. [DOI] [PubMed] [Google Scholar]
- [35].Lee J-H, Kim S-J, Lee S, Rhee J-K, Lee SY, Na Y-C, Saturated fatty acid determination method using paired ion electrospray ionization mass spectrometry coupled with capillary electrophoresis, Anal. Chim. Acta 984 (2017) 223–231. doi:http://dx.doi.org/10.1016/j.aca.2017.06.052. [DOI] [PubMed] [Google Scholar]
- [36].Renee J Soukup-Hein JW Remsburg PK Dasgupta DW Armstrong A General, Positive Ion Mode ESI-MS Approach for the Analysis of Singly Charged Inorganic and Organic Anions Using a Dicationic Reagent, Anal. Chem 79 (2007) 7346–7352. doi:10.1021/ac071102b. [DOI] [PubMed] [Google Scholar]
- [37].Yue H, Strauss KI, Borenstein MR, Barbe MF, Rossi LJ, Jansen SA, Determination of bioactive eicosanoids in brain tissue by a sensitive reversed-phase liquid chromatographic method with fluorescence detection, J. Chromatogr. B 803 (2004) 267–277. doi:10.1016/j.jchromb.2003.12.027. [DOI] [PubMed] [Google Scholar]
- [38].Bollinger JG, Thompson W, Lai Y, Oslund RC, Hallstrand TS, Sadilek M, Turecek F, Gelb MH, Improved Sensitivity Mass Spectrometric Detection of Eicosaniods by Charge Reversal Derivitization, Anal. Chem 82 (2010) 6790–6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bollinger JG, Rohan G, Sadilek M, Gelb MH, LC/ESI-MS/MS detection of FAs by charge reversal derivatization with more than four orders of magnitude improvement in sensitivity, J. Lipid Res 54 (2013) 3523–3530. doi:10.1194/jlr.D040782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jiang R, Jiao Y, Zhang P, Liu Y, Wang X, Huang Y, Zhang Z, Xu F, Twin Derivatization Strategy for High-Coverage Quantification of Free Fatty Acids by Liquid Chromatography–Tandem Mass Spectrometry, Anal. Chem 89 (2017) 12223–12230. doi:10.1021/acs.analchem.7b03020. [DOI] [PubMed] [Google Scholar]
- [41].Yang W-C, Adamec J, Regnier FE, Enhancement of the LC/MS Analysis of Fatty Acids through Derivatization and Stable Isotope Coding, Anal. Chem 79 (2007) 5150–5157. doi:10.1021/ac070311t. [DOI] [PubMed] [Google Scholar]
- [42].Fulton D, Falck JR, McGiff JC, Carroll MA, Quilley J, A method for the determination of 5,6-EET using the lactone as an intermediate in the formation of the diol, J. Lipid Res 39 (1998) 1713–1721. [PubMed] [Google Scholar]
- [43].Bligh EG, Dyer WJ, A Rapid Method of Total Lipid Extraction and Purification, Can. J. Biochem. Physiol 37 (1959) 911–917. [DOI] [PubMed] [Google Scholar]
- [44].Draft Guidance for Industry on Bioanalytical Method Validation, (2013). https://www.regulations.gov/document?D=FDA-2013-D-1020-0001 (accessed October 4, 2017).
- [45].Brash AR, Arachidonic acid as a bioactive molecule, J. Clin. Invest 107 (2001) 1339–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Jiang H, Anderson GD, McGiff JC, Red blood cells (RBCs), epoxyeicosatrienoic acids (EETs) and adenosine triphosphate (ATP), Pharmacol. Rep. PR 62 (2010) 468–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.