Abstract
Objectives
Osteoporosis is a metabolic disease resulting in progressive loss of bone mass as measured by bone mineral density (BMD). Physical exercise has a positive effect on increasing or maintaining BMD in postmenopausal women. The contribution of exercise to the regulation of osteogenesis in osteoblasts remains unclear. We therefore investigated the effect of exercise on osteoblasts in ovariectomized mice.
Methods
We compared the activity of differentially expressed genes of osteoblasts in ovariectomized mice that undertook exercise (OVX+T) with those that did not (OVX), using microarray and bioinformatics.
Results
Many inflammatory pathways were significantly downregulated in the osteoblasts after exercise. Meanwhile, IBSP and SLc13A5 gene expressions were upregulated in the OVX+T group. Furthermore, in in vitro assay, IBSP and SLc13A5 mRNAs were also upregulated during the osteogenic differentiation of MC3T3-E1 and 7F2 cells.
Conclusion
These findings suggest that exercise may not only reduce the inflammatory environment in ovariectomized mice, indirectly suppressing the overactivated osteoclasts, but may also directly activate osteogenesis-related genes in osteoblasts. Exercise may thus prevent the bone loss caused by oestrogen deficiency through mediating the imbalance between the bone resorptive activity of osteoclasts and the bone formation activity of osteoblasts.
Cite this article: W-B. Hsu, W-H. Hsu, J-S. Hung, W-J. Shen, R. W-W. Hsu. Transcriptome analysis of osteoblasts in an ovariectomized mouse model in response to physical exercise. Bone Joint Res 2018;7:601–608. DOI: 10.1302/2046-3758.711.BJR-2018-0075.R2.
Keywords: Microarray, Osteoblast, Ovariectomized mice, Treadmill
Article focus
To explore the effect of exercise on the osteoblast in ovariectomized mice by transcriptome analysis.
Key messages
Exercise has a positive effect on increasing or maintaining bone mineral density in both pre-menopausal and postmenopausal women.
In micro-CT analysis, exercise prevented bone loss in ovariectomized mice.
After exercise, the inflammatory microenvironment of the osteoblast in the ovariectomized mice was reduced.
IBSP and SLc13A5 genes, contributing to bone formation, were upregulated in the osteoblast after exercise.
Strengths and limitations
The study showed that exercise prevented bone loss by stimulating the expression of IBSP and SLc13A5 genes, beneficial for bone formation, in the osteoblast and reducing the inflammatory microenvironment induced by oestrogen deficiency, to inhibit osteoclast formation.
We were unable to determine which pathway linked exercise with the upregulation of IBSP and SLc13A5 genes in the osteoblast.
Introduction
Bone mass is determined by a balance between osteoblastic and osteoclastic activity. Osteoporosis is a silent and progressive metabolic disease causing progressive loss of bone as measured by bone mineral density (BMD). In postmenopausal women, oestrogen deficiency disrupts the metabolic balance owing to large increases in bone resorption through the enhanced formation and reduced apoptosis of osteoclasts.1,2 The continuous demolition of bone increases its fragility. Patients with osteoporotic fractures have high rates of mortality and morbidity, and chronic pain reduces the quality of life and increases the cost of social care.3,4 With the ageing population, osteoporosis has become a major global health problem.3
Previous studies have shown that mechanical stimuli can trigger the formation of bone by activating signal pathways and the expression of genes that contribute to its anabolism.5-7 During physical exercise, bone has mechanical stimuli from ground reaction forces and the contraction of muscles.8,9 Exercise increases or maintains BMD in both pre- and postmenopausal women,10-12 and has been recommended as a preventive strategy to manage osteoporosis.13 In 1998, Oursler found that the gene expression profiles in the osteoblast vary according to the presence or absence of oestrogen.14 The underlying mechanisms of the effect of exercise on the osteoblasts in oestrogen deficiency are not well understood. The primary aim of this study was to analyze the transcriptome of osteoblasts in ovariectomized mice, with or without the intervention of exercise, by microarray. Comparative analysis was performed using bioinformatics tools for clustering and grouping genes.
Materials and Methods
Six-week-old C57BL/6J female mice were purchased from a commercial supplier (BioLASCO, Ilan, Taiwan). A mouse model of osteoporosis was set up as described by Ferguson et al.15 The surgical procedures in six mice in a sham group were the same as those used in the ovariectomized mice (OVX), with the exception of leaving the ovaries intact. The sham group served as controls for micro-CT and quantitative reverse transcription-polymerase chain reaction (RT-qPCR) analysis.
After a two-week postoperative recovery period, the ovariectomized mice were randomly divided into an exercise group (OVX+T, n = 6) and a non-exercise group (OVX, n = 6). As described previously,16,17 we used a treadmill, with modifications, for the exercise. The OVX+T mice used the treadmill five days a week for eight weeks, with each session lasting 60 minutes. The treadmill was run at 10 m/min with an angle of inclination of 10°. The mice were then killed by CO2 asphyxiation and their femurs and tibias were harvested. Serum was also collected for assay of bone metabolism biomarkers.
All procedures were approved by the Institutional Animal Care and Use Committee, and all complied with the Guide for the Care and Use of Laboratory Animals available through the National Academy of Sciences (IACUC number: 2015122311).
The harvested femurs were immediately put into Dulbecco’s modified Eagle’s medium (DMEM) with 10% foetal bovine serum (FBS) (Gibco, Waltham, Massachusetts) and 1% ammonium persulphate (APS) (Gibco) and residual muscle and connective tissue were meticulously removed. All cleaned femurs were stored in 70% alcohol at -4°C until scanning. The µCT data were acquired by the SkyScan 1076 system (Bruker Microct, Kontich, Belgium) with at 48 kV, 200 μA, and a voxel resolution of 11.54 μm. The images were imported into CTAn software (Bruker) to measure four parameters of the architecture of trabecular bone: bone volume / total volume (BV/TV); trabecular thickness (Tb.Th); trabecular separation (Tb.Sp) and the trabecular number (Tb.N) of the femurs in each group.
The serum level of osteocalcin (SEA471Mu), a marker of bone formation, and cross-linked C-telopeptide of type I collagen (CTX-1) (CEA665Mu), a marker of bone resorption, were measured using commercial enzyme-linked immunosorbent assay (ELISA) kits (Wuhan USCN Business Co. Ltd, Wuhan, Hubei, China) following the manufacturer's instructions.
Working under a tissue culture hood, adherent soft tissue was removed from the harvested tibias in each group. The epiphysis was removed and the bone marrow cells flushed out using a 27-gauge needle syringe with phosphate-buffered saline (PBS). The hollowed-out bones were milled into bone chips of 1 mm2 to 2 mm2 and washed three times with PBS. The chips underwent two sequential 30-minute digestion runs using a mixture (DMEM with 20 ng/ml type II collagenase (Gibco)) at 37°C in an oscillating water bath. After digestion, the chips were washed three times with PBS and transferred into a 10 cm Petri dish containing 20% FBS DMEM (2 mM L-glutamine and 1% penicillin/streptomycin) at a density of about 20 to 30 chips per dish. The primary osteoblast starts to migrate from bone chips. The cells were incubated in a humidified incubator containing 5% CO2 at 37°C. Fresh medium was replaced three times per week.18
RNA extraction and cDNA synthesis were performed as described previously.19 The quality of total RNA was evaluated by the Agilent system (Agilent Technologies, Inc., Santa Clara, California) according to the manufacturer’s protocol. Complementary DNA was synthesized using random hexamers as a primer, as described in the SuperScript IV Reverse Transcriptase Kit protocol for RT (Invitrogen, Waltham, Massachusetts). The cDNA was hybridized to Affymetrix GeneChip Mouse Exon 1.0 ST Array (Affymetrix, Santa Clara, California, and the arrays were normalized (Robust Multi-array Average transcript cluster level) in the Transcriptome Analysis Console (TAC) (Affymetrix, Thermo Fisher Scientific Inc., Santa Clara, California) according to the manufacturer’s instructions. The microarray data were deposited at the Gene Expression Omnibus (GEO) website (GEO accession: GSE111628). These data were filtered by a set of absolute fold change cutoff of 1.5 and then analyzed using Ingenuity Pathway Analysis (IPA), a programme interpreting large-scale transcriptome data across species. (Ingenuity Systems, Redwood City, California). The IPA diseases and functions analysis and canonical pathway tool were used to investigate phenotypes and top canonical pathways associated with the differentially expressed molecules, respectively. According to IPA protocol, data sets containing Affymetrix probeset ID identifiers and corresponding expression values were uploaded into the IPA website. Each probeset ID was mapped to a mouse splicing variant in the Ingenuity Knowledge Base.
MC3T3-E1 (CRL-2593) and 7F2 (CRL-12557) were grown in alpha Minimum Essential Medium (αMEM) (2 mM L-glutamine and 1 mM sodium pyruvate), supplemented with 10% FBS, without ribonucleosides and deoxyribonucleosides. Two cell lines were incubated in a humidified incubator containing 5% CO2 at 37°C. For osteogenic differentiation, MC3T3E1 and 7F2 cells were plated into 48-well plates at about 80% confluence (~3X103) and the growth medium was replaced by differentiation medium (growth medium supplemented with 50 µg/ml L-Ascorbic acid (Sigma-Aldrich, St. Louis, Missouri) and 10 mM β–Glycerophosphate disodium salt hydrate (Sigma-Aldrich)). The mineralization of MC3T3-E1 becomes evident at about 21 days after treatment with differentiation medium and 7F2 at about seven days.20 The medium was refreshed every three to four days.
The RT-qPCR was performed as described previously.19 A total of 500 ng cDNA was used with SYBR green PCR master mix (Bio-Rad Laboratories, Hercules, California) and 10 nM of sequence-specific primers (Supplementary Table ii). Amplification of the target sequences was detected with Bio-Rad CFX96 Real-time PCR System. The expression of Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) was used to normalize the abundance of the test RNAs. All RT-qPCR reactions were performed in triplicate. The dentin matrix protein 1 (DMP1) is a highly expressed and critical factor during osteoblast differentiation.21,22 Alkaline phosphatase (ALP)23 and collagen I (Col I) are also well known markers of this differentiation. Therefore, DMP1, ALP, and Col I served as a positive control for osteoblast differentiation. As time goes by, the density of cells will increase owing to their growth, and the density will enhance differentiation. Thus, the relative expression was first normalized to that at each timepoint without differentiation medium and then the RNA level at day 0 was set equal to 1.
Statistical analysis
Data are presented as the mean and standard deviation. One-way analysis of variance (ANOVA) was used for determining the differences between the groups. A p-value of < 0.05 was considered significant. All analyses were performed using Prism (6.0; GraphPad Software Inc., La Jolla, California).
Results
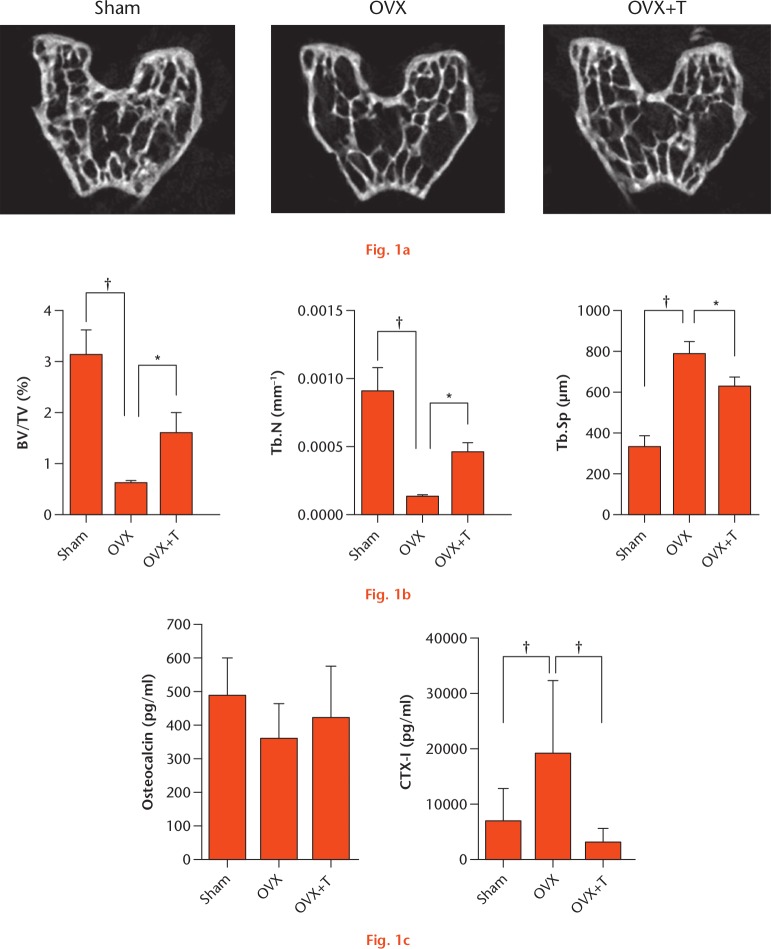
The µCT data (Fig. 1) showed that a decrease in BV/TV and Tb.N, and the increase in Tb.Sp, in the OVX group was significantly diminished after exercise. The level of serum CTX-1 was significantly lower in the OVX+T group than in the OVX group, but the level of osteocalcin was not lower. These data indicate that exercise can help maintain bone mass and microarchitecture and protect against bone loss resulting from oestrogen deficiency.
Physical exercise diminished bone loss in ovariectomized (OVX) mice. a) Images of the distal femoral epiphysis and b) quantification of bone volume fraction (BV/TV), trabecular number (Tb.N), and trabecular spacing (Tb.Sp). c) Serum bone markers osteocalcin and CTX-1 were measured by specific enzyme-linked immunosorbent assay (ELISA). *p < 0.05; †p < 0.01. OVX+T, ovariectomized mice that undertook exercise.
We next compared the gene expression profiles of osteoblasts between OVX+T and OVX groups, by microarray. After normalization of the gene expression, the log2 fold-change between the samples in the two groups was calculated as altered expression values. Scatter plot and hierarchical clustering was performed to visualize the differential expression of genes in the two groups with a set of absolute fold change cutoff of 1.5 (Supplementary Figs aa and ab). Overall, there were 7640 filtered genes; 3267 (42.8%) were downregulated and 4373 (57.2%) were upregulated in the OVX+T group compared with the OVX group (Supplementary Fig. ac).
In order to examine further the biological significance, the filtered genes were subjected to IPA to search the networks for the cellular functions of the differentially expressed genes. Top diseases and functions, determined by IPA, are summarized in Table I. We noted that the differentially expressed genes are primarily involved in metabolic disease and inflammatory response. In order to determine the particular pathway, analysis of the canonical pathways was performed and the results are summarized in Table II. Among these pathways, the role of osteoblasts, osteoclasts and chondrocytes in the rheumatoid arthritis pathway was intuitively relevant to our target call, the osteoblast. So, we further analyzed the molecules in the role of these cells in this pathway and the results are shown in Table III.
Table I.
Top diseases and analysis of the functions of differentially expressed genes predicted by Ingenuity Pathway Analysis (IPA)
| Top diseases and functions | Molecules in network | Score |
|---|---|---|
| Metabolic disease, inflammatory response, organismal injury and abnormalities | ARG1, Bst2, C3, CCL2, Ccl6, Ccl9, CD300LD, Collagen type I, Collagen(s), CREB3L1, CSF2RB, CXCL12, CXCR4, GZMH, ID1, IFIT1B, Ifnar, Ige, IL33, IL12 (complex), IL1R1, IL1RL1, IL1RN, Ly6a (includes others), mir-135, mir-320, MMP9, NFkB (complex), NQO1, OAS2, P38 MAPK, PI3K (complex), PTGS1, RNF213, Wfdc17 | 47 |
| Metabolic disease, endocrine system disorders, gastrointestinal disease | Acp5, AKR1C3, Akt, Alpha catenin, ANTXR2, CCK, COL3A1, CTSK, CTSV, ERK, ERK1/2, F2R, GPNMB, HAS2, HDL-cholesterol, IGFBP7, IRS2, JAK1, Jnk, let-7, LIPA, LPL, p85 (pik3r), PAK1, PDGFRA, PIK3R5, Pka, RGS2, SERPINE1, Tgf beta, THBS4, TNC, TNN, VCAN, XDH | 44 |
| Inflammatory response, infectious diseases, cellular movement | AHR, CACHD1, CELA1, CLEC12A, COX8A, CXCL6, CYP1B1, EDN1, EMP2, F13A1, FADS3, FOLR2, GADD45A, GBP2, GLIS2, Gpr137bps, GSTM2, IKBKE, IL6, IL10RA, ITGA5, KEAP1, LBP, LTBP2, MGP, mir-32, miR-191 5p, Pcp4l1, PPIF, PTAFR, Rpl29, SASH1, SDC1, TLR3, TNC | 20 |
Table II.
Top canonical pathways and percentage of regulated genes analyzed by Ingenuity Pathway Analysis (IPA)
| Pathway | Log (p-value) | Downregulated gene, n (%) | No change, n (%) | Upregulated gene, n (%) |
|---|---|---|---|---|
| Atherosclerosis signalling | 5.47 | 24/114 (21) | 80/114 (70) | 6/114 (5) |
| Th2 pathway | 4.01 | 26/135 (19) | 104/135 (77) | 1/135 (1) |
| IL-6 signalling | 3.34 | 31/124 (25) | 89/124 (72) | 4/124 (3) |
| IL-10 signalling | 3.79 | 21/65 (32) | 42/65 (65) | 2/65 (3) |
| PPAR signalling | 3.11 | 25/91 (27) | 61/91 (67) | 3/91 (3) |
| Role of osteoblasts, osteoclasts, and chondrocytes in rheumatoid arthritis | 2.67 | 51/226 (23) | 163/226 (72) | 12/226 (5) |
| NF-κB signalling | 2.61 | 42/172 (24) | 123/172 (72) | 3/172 (2) |
| HMGB1 signalling | 2.49 | 32/126 (25) | 89/126 (71) | 5/126 (4) |
| IL-17 signalling | 2.45 | 18/79 (23) | 58/79 (73) | 3/79 (4) |
| PDGF signalling | 2.25 | 26/90 (29) | 61/90 (68) | 3/90 (3) |
| IGF-1 signalling | 2.05 | 27/103 (26) | 72/103 (70) | 4/103 (4) |
| CXCR4 signalling | 2.03 | 36/162 (22) | 121/162 (75) | 5/162 (3) |
Table III.
The differentially expressed genes in role of osteoblasts, osteoclasts, and chondrocytes in rheumatoid arthritis pathway
| Gene symbol | Fold change | Full name |
|---|---|---|
| Downregulated | ||
| Il1r1 | -3.29 | Interleukin 1 receptor, type I |
| Ctsk | -3.04 | Cathepsin K |
| Il33 | -2.73 | Interleukin 33 |
| Il1rl1(Il33 receptor) | -2.55 | Interleukin 1 receptor-like 1 |
| Pik3r5 | -2.1 | Phosphoinositide-3-kinase, regulatory subunit 5, p101 |
| Irs2 | -2.02 | Insulin receptor substrate 2 |
| Lrp6 | -1.85 | Low-density lipoprotein receptor-related protein 6 |
| Nfkbia | -1.81 | Nuclear factor of kappa light polypeptide gene enhancer in B cells inhibitor, alpha |
| Il1rn | -1.79 | Interleukin 1 receptor antagonist (IL1Ra) |
| Il6 | -1.77 | Interleukin 6 |
| Igf1 | -1.73 | Insulin-like growth factor 1; insulin-like growth factor 1 (Igf1), transcript variant 2, mRNA. |
| Il1rl2 | -1.67 | Interleukin 1 receptor-like 2 |
| Adam17 | -1.65 | A disintegrin and metallopeptidase domain 17 (Adam17). |
| Mmp3 | -1.63 | Matrix metallopeptidase 3 |
| Tnfrsf11a | -1.62 | Tumour necrosis factor receptor superfamily, member 11a, NFKB activator; tumour necrosis factor receptor superfamily, member 11a (RANK) |
| Pik3r1 | -1.6 | Phosphatidylinositol 3-kinase, regulatory subunit, polypeptide 1 (p85 alpha) |
| Birc2 | -1.6 | Baculoviral IAP repeat-containing 2 |
| Pik3cb | -1.59 | Phosphatidylinositol 3-kinase, catalytic, beta polypeptide |
| Il1b | -1.58 | Interleukin 1 beta; interleukin 1 beta (Il1b) |
| Cbl | -1.57 | Casitas B-lineage lymphoma |
| Tnfrsf1b | -1.57 | Tumour necrosis factor receptor superfamily, member 1b |
| Nfat5 | -1.57 | Nuclear factor of activated T cells 5 |
| Pik3ca | -1.57 | Phosphatidylinositol 3-kinase, catalytic, alpha polypeptide |
| Bmpr2 | -1.56 | Bone morphogenetic protein receptor, type II |
| Nfatc1 | -1.56 | Nuclear factor of activated T cells, cytoplasmic, calcineurin dependent 1 |
| Sfrp4 | -1.55 | Secreted frizzled-related protein 4 |
| Csf1r | -1.54 | Colony stimulating factor 1 receptor |
| Map2k3 | -1.52 | Mitogen-activated protein kinase kinase 3 |
| Upregulated | ||
| Dkk4 | 1.62 | Dickkopf homolog 4 (Xenopus laevis) |
| Il1f10 | 1.5 | Interleukin 1 family, member 10 |
IPA identified the differentially expressed genes between the OVX+T and OVX groups and validated by RT-qPCR. Analysis-Ready Molecule analysis was performed to identify key genes further that are directly stimulated by exercise in the osteoblast. A list of significantly regulated genes with the highest score according to IPA is shown in Table IV. The MMP9, PI15, LPL, THBS4, and ACP5 genes were downregulated in OVX+T compared with the OVX, while SLc13A5 and IBSP genes were upregulated. In order to further validate the gene expression profiles identified by IPA, we measured the levels of expression of these genes in the two groups using a RT-qPCR assay with the sham group as a control. The results are shown in Figure 2 and are consistent with those in the microarray.
Table IV.
Top analysis-ready molecules predicted by Ingenuity Pathway Analysis (IPA)
| Gene symbol | Experimental value | Full name |
|---|---|---|
| Downregulated | ||
| MMP9 | -7.90 | Matrix metallopeptidase 9 |
| PI15 | -3.66 | Peptidase inhibitor 15 |
| LPL | -3.24 | Lipoprotein lipase |
| THSB4 | -3.15 | Thrombospondin-4 |
| ACP5 | -2.76 | Tartrate-resistant acid phosphatase type 5 |
| Upregulated | ||
| SLc13A5 | 2.87 | Solute carrier family 13 member 5 |
| IBSP | 2.77 | Integrin binding sialoprotein |
Fig. 2.
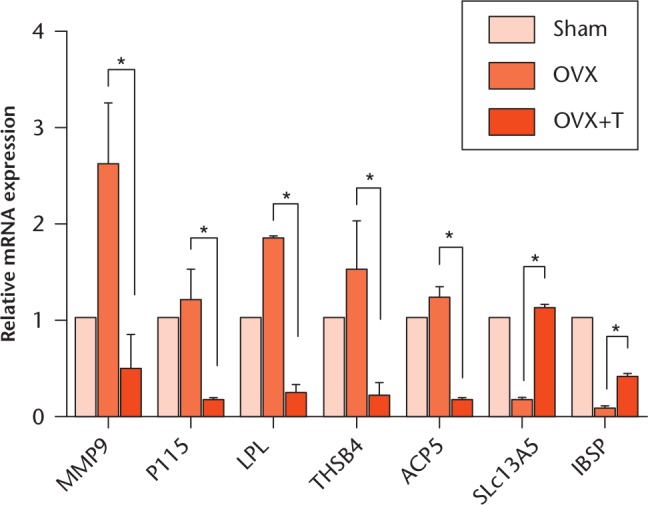
The validation of microarray by quantitative reverse transcription-polymerase chain reaction (RT-qPCR). Total RNAs of primary osteoblasts from sham, ovariectomized (OVX) and ovariectomized plus exercise (OVX+T) groups were collected and subjected to RT-qPCR analysis. The relative expression of indicated genes was calculated by setting the sham RNA level equal to 1. *p < 0.05.
The IBSP and SLc13A5 genes were upregulated in the osteoblasts after exercise and SLc13A5 was expressed during osteogenesis.
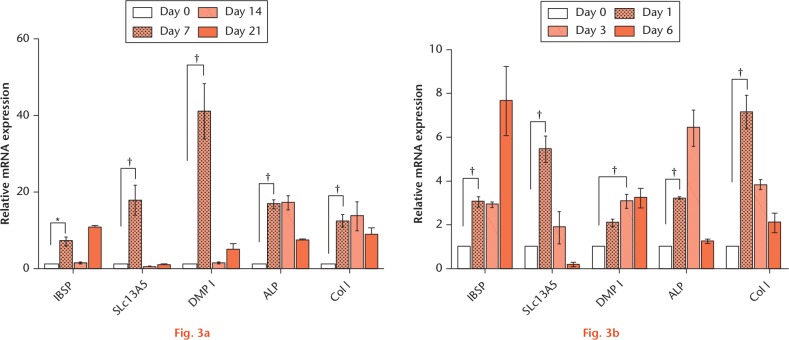
In the upregulated genes, IBSP is known as bone sialoprotein (BSP) and contributes to bone formation.24,25 It has recently been shown that SLc13A5 deficiency leads to a low BMD and impairs bone formation in the young mouse but not in the older mouse. This suggested that SLc13A5 may play a critical role in the development and function of bone.26 Likewise, we found a significantly upregulated expression of IBSP and SLc13A5 genes in the osteoblast after exercise. However, the role of SLc13A5 in bone formation is still unclear. Thus, we next examined the mRNA expression of the SLc13A5 gene during osteogenic differentiation. As shown in Figure 3, the mRNA of IBSP and SLc13A5 was markedly upregulated during both MC3T3-E1 and 7F2 osteogenic differentiation.
IBSP and SLc13A5 mRNA expressed during osteogenesis in vitro. a) MC3T3-E1 and b) 7F2 were cultured with and without differentiation medium. The total RNA was isolated at the indicated timepoint and subjected to RT-qPCR analysis. The relative expression was first normalized to that at each timepoint without differentiation medium and then the RNA level at day 0 was set equal to 1. The expression of DMP-1, ALP, and Col I were as positive controls for osteoblast (OB) differentiation. *p < 0.05. †p < 0.01.
Discussion
Mechanical stimuli can trigger the osteoblasts to enhance the anabolism of bone.5-7,27 Although exercise is effective in increasing or maintaining BMD, its effect on the oestrogen-deficient osteoblast has not been defined. We used a genome-wide screen and compared the transcriptomes of osteoblasts in OVX+T and OVX mice. In the WikiPathway analysis, the number of downregulated genes was markedly more than the number of upregulated genes in the inflammatory cytokines pathway. Similarly, in the IPA analysis, the results of biological network and function and significant canonical pathways showed that the most differentially expressed genes were involved in the inflammatory response. The percentage of downregulated genes was also higher than that of upregulated genes in significant canonical pathways analysis, suggesting that the inflammatory stimulation might be reduced. Previous authors have reported that oestrogen deficiency induced an inflammatory microenvironment,28 and proinflammatory cytokines such as tumour necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), IL-6, and IL-17 stimulate the differentiation of osteoclasts by synergizing with RANKL and upregulating RANKL expression in osteoblasts, thereby expanding the pool of osteoclast precursors.29-31 Among these pathways, IL-1 is identified as an osteoclast-activating factor and participates in various steps of the development of osteoclasts including differentiation, multinucleation and survival in physiological and pathological conditions.32,33 Moreover, it has been shown that excess activation of the IL-1 signalling pathway leads to the resorption of bone.34- 37 Interleukin-6 can stimulate bone marrow cells into osteoclastic differentiation,38 and the bone loss induced by oestrogen deficiency is decreased in IL-6-deficient mice.39 Interleukin-7 leads to bone loss by stimulating the production of osteoclastogenic cytokines in T cells to induce the formation of osteoclasts and by suppressing the production of osteoprotegerin in osteoblasts to inhibit bone formation. Neutralizing IL-7 can protect against bone loss caused by oestrogen deficiency.40,41 Likewise, proinflammatory cytokines can result in aberrant osteoblast activity by disturbing the Wnt and bone morphogenetic protein (BMP) signalling pathways.42 Additionally, regular exercise induces anti-inflammatory activity.43-45 Taken together, we propose that the inflammatory microenvironment caused by oestrogen deficiency might be reduced by exercise, resulting in a decrease in the formation of osteoclasts.
An in-depth analysis of the role of osteoblasts, osteoclasts, and chondrocytes in rheumatoid arthritis pathways showed that a group of phosphatidylinositol 3-kinase (PI3K) family genes (Pik3r1, Pik3r5, Pik3ca, and Pik3cb) was downregulated. Gámez et al,46 in 2016, showed that the p110α (Pik3ca) and p100β (Pik3cb), PI3K catalytic subunits, are critical for osteoblast differentiation and bone formation in vitro and in vivo. The administration of BEZ235 (a pan-class I PI3K and mTOR kinase inhibitor) or PIK75 (a p110α-specific inhibitor) in mice both resulted in loss of bone mass and strength.47 These results conflict with our data that the Pik3ca and Pik3cb gene expressions were downregulated in osteoblasts after exercise. The disparity between their data and ours may be due to the presence or absence of oestrogen. What functional role Pik3ca and Pik3cb play in oestrogen-deficient osteoblasts requires further study.
MMP9 is predominantly expressed in osteoclasts and is required for them to invade ossification centres and remove hypertrophic cartilage or to widen the medullary cavity. It also stimulates angiogenesis. The MMP9 chemical inhibitor can block osteoclastic resorption, and similar results were shown in MMP9 antisense oligos treatment or MMP9 gene knockout.48-50 In our data, MMP9 changes the most in the list (Table IV). The function of MMP9 in osteoblasts is not well defined and what role it plays in oestrogen-deficient osteoblasts also requires further study.
Integrin-binding sialoprotein enhances osteoblast differentiation and matrix mineralization through BSP-type I collagen interaction51,52 and is a key factor in bone formation.24,25 We found that exercise enhances IBSP gene expression in the oestrogen-deficient osteoblast to increase bone formation. It is similar to another significantly upregulated gene, the SLc13A5. Irizarry et al26 reported that impaired development in teeth and bone was seen in SLc13A5-deficient mice, and SLc13A5 mRNA was strongly expressed in primary osteoblasts, osteoclasts, and dental cells, suggesting that it may play an important role in bone formation. Likewise, Mantila Roosa et al53 showed that mechanical loads stimulated IBSP and SLc13A5 gene expression, and the SLc13A5 mRNA level was elevated during bone matrix formation in rats. Based on these findings and ours, we postulated that the upregulation of IBSP and SLc13A5 gene by exercise might be due to mechanical stimulation. Interestingly, the presence of oestrogen is the distinct difference between their findings and ours. Their findings were in the presence of oestrogen while we found that the expression of IBSP and SLc13A5 gene in osteoblasts was still upregulated by exercise in the absence of oestrogen. This suggests that oestrogen has little effect on IBSP and SLc13A5 gene expression stimulated by mechanical load in the osteoblast.
The crosslink peptide sequence of type I collagen, CTX-1, is cleaved by osteoclasts during bone resorption. The serum level of CTX-1 is recommended as a biomarker of the rate of bone turnover.54 However, its level is also influenced by the circadian variation55 and food intake.56 We found the serum level of CTX-1 to be lower in the exercise group than in the sham group. This suggests that exercise might result in interactions between CTX-1 metabolism, circadian variation, and food intake. Despite this further suppression of CTX-1, other parameters of µCT data directly revealed the structure of bone.
Hormone replacement therapy (HRT) is approved for both the prevention and treatment of postmenopausal osteoporosis. Further study is required to confirm the comparison between the effects of HRT and those of exercise.
In conclusion, an overall transcriptomic comparison suggested that the inflammatory environment in the OVX group was reduced by exercise, leading to indirect suppression of overactivated osteoclasts. We also provide evidence that the expression of IBSP and SLc13A5 genes, beneficial for the differentiation of osteoblasts and bone formation, was upregulated in the osteoblast by exercise through a direct mechanical stimulus. Exercise might indirectly suppress the overactivated osteoclast and directly stimulate the osteoblast to mediate the imbalance between osteoclastic bone resorption osteoblastic bone formation.
Footnotes
Author Contributions: W-B. Hsu and W-H. Hsu contributed equally to the work.
W-B. Hsu: Drafted, critically revised, and approved the manuscript, Analyzed and interpreted the data.
W-H. Hsu: Drafted, critically revised, and approved the manuscript, Analyzed and interpreted the data.
J-S. Hung: Acquired the data, Performed statistical analysis, Approved the manuscript.
W-J. Shen: Designed the study, Approved the manuscript.
R. W-W. Hsu: Designed the study, Approved the manuscript.
Conflict of Interest Statement: None declared
Follow us @BoneJointRes
Supplementary material
Transcriptomic comparison of osteoblasts between ovariectomized mice that undertook exercise and those that did not; mouse strain and training protocol; WikiPathway analysis of differentially expressed genes; and primer sequence.
Funding Statement
This work was supported by Ministry of Science and Technology, Taiwan (grant number: NMRPG6F0101) and Chang Gung Memorial Hospital Grant (grant number: CMRPG6G0431 and CORPG6G0301).
References
- 1. Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 2000;21:115-137. [DOI] [PubMed] [Google Scholar]
- 2. Riggs BL. The mechanisms of estrogen regulation of bone resorption. J Clin Invest 2000;106:1203-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cauley JA. Public health impact of osteoporosis. J Gerontol A Biol Sci Med Sci 2013;68:1243-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006;17:1726-1733. [DOI] [PubMed] [Google Scholar]
- 5. Turner CH, Pavalko FM. Mechanotransduction and functional response of the skeleton to physical stress: the mechanisms and mechanics of bone adaptation. J Orthop Sci 1998;3:346-355. [DOI] [PubMed] [Google Scholar]
- 6. Ingber DE. Mechanobiology and diseases of mechanotransduction. Ann Med 2003;35:564-577. [DOI] [PubMed] [Google Scholar]
- 7. Qin YX, Hu M. Mechanotransduction in musculoskeletal tissue regeneration: effects of fluid flow, loading, and cellular-molecular pathways. Biomed Res Int 2014;2014:863421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lanyon LE, Hampson WG, Goodship AE, Shah JS. Bone deformation recorded in vivo from strain gauges attached to the human tibial shaft. Acta Orthop Scand 1975;46:256-268. [DOI] [PubMed] [Google Scholar]
- 9. Usui T, Maki K, Toki Y, et al. Measurement of mechanical strain on mandibular surface with mastication robot: influence of muscle loading direction and magnitude. Orthod Craniofac Res 2003;6(Suppl 1):163-167. [DOI] [PubMed] [Google Scholar]
- 10. Martyn-St James M, Carroll S. A meta-analysis of impact exercise on postmenopausal bone loss: the case for mixed loading exercise programmes. Br J Sports Med 2009;43:898-908. [DOI] [PubMed] [Google Scholar]
- 11. Kelley GA, Kelley KS, Tran ZV. Resistance training and bone mineral density in women: a meta-analysis of controlled trials. Am J Phys Med Rehabil 2001;80:65-77. [DOI] [PubMed] [Google Scholar]
- 12. Watson SL, Weeks BK, Weis LJ, et al. High-Intensity Resistance and Impact Training Improves Bone Mineral Density and Physical Function in Postmenopausal Women With Osteopenia and Osteoporosis: The LIFTMOR Randomized Controlled Trial. J Bone Miner Res 2018;33:211-220. [DOI] [PubMed] [Google Scholar]
- 13. Howe TE, Shea B, Dawson LJ, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev 2011;7:CD000333. [DOI] [PubMed] [Google Scholar]
- 14. Oursler MJ. Estrogen regulation of gene expression in osteoblasts and osteoclasts. Crit Rev Eukaryot Gene Expr 1998;8:125-140. [DOI] [PubMed] [Google Scholar]
- 15. Ferguson VL, Ayers RA, Bateman TA, Simske SJ. Bone development and age-related bone loss in male C57BL/6J mice. Bone 2003;33:387-398. [DOI] [PubMed] [Google Scholar]
- 16. Wu J, Wang XX, Higuchi M, Yamada K, Ishimi Y. High bone mass gained by exercise in growing male mice is increased by subsequent reduced exercise. J Appl Physiol (1985) 2004;97:806-810. [DOI] [PubMed] [Google Scholar]
- 17. Chen X, Li L, Guo J, et al. Treadmill running exercise prevents senile osteoporosis and upregulates the Wnt signaling pathway in SAMP6 mice. Oncotarget 2016;7:71072-71086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bakker AD, Klein-Nulend J. Osteoblast isolation from murine calvaria and long bones. Methods Mol Biol 2012;816:19-29. [DOI] [PubMed] [Google Scholar]
- 19. Hsu WB, Shih JL, Shih JR, et al. Cellular protein HAX1 interacts with the influenza A virus PA polymerase subunit and impedes its nuclear translocation. J Virol 2013;87:110-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gencheva M, Hare I, Kurian S, et al. Bone marrow osteoblast vulnerability to chemotherapy. Eur J Haematol 2013;90:469-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kalajzic I, Braut A, Guo D, et al. Dentin matrix protein 1 expression during osteoblastic differentiation, generation of an osteocyte GFP-transgene. Bone 2004;35:74-82. [DOI] [PubMed] [Google Scholar]
- 22. Feng JQ, Ward LM, Liu S, et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet 2006;38:1310-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu J, Li X, Zhang H, et al. Ubiquitin E3 ligase Itch negatively regulates osteoblast function by promoting proteasome degradation of osteogenic proteins. Bone Joint Res 2017;6:154-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Malaval L, Wade-Guéye NM, Boudiffa M, et al. Bone sialoprotein plays a functional role in bone formation and osteoclastogenesis. J Exp Med 2008;205:1145-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bouet G, Bouleftour W, Juignet L, et al. The impairment of osteogenesis in bone sialoprotein (BSP) knockout calvaria cell cultures is cell density dependent. PLoS One 2015;10:e0117402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Irizarry AR, Yan G, Zeng Q, et al. Defective enamel and bone development in sodium-dependent citrate transporter (NaCT) Slc13a5 deficient mice. PLoS One 2017;12:e0175465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ziebart J, Fan S, Schulze C, et al. Effects of interfacial micromotions on vitality and differentiation of human osteoblasts. Bone Joint Res 2018;7:187-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Straub RH. The complex role of estrogens in inflammation. Endocr Rev 2007;28:521-574. [DOI] [PubMed] [Google Scholar]
- 29. Kotake S, Udagawa N, Takahashi N, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest 1999;103:1345-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lam J, Takeshita S, Barker JE, et al. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest 2000;106:1481-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schett G, Teitelbaum SL. Osteoclasts and Arthritis. J Bone Miner Res 2009;24:1142-1146. [DOI] [PubMed] [Google Scholar]
- 32. Kim JH, Jin HM, Kim K, et al. The mechanism of osteoclast differentiation induced by IL-1. J Immunol 2009;183:1862-1870. [DOI] [PubMed] [Google Scholar]
- 33. Lee YM, Fujikado N, Manaka H, Yasuda H, Iwakura Y. IL-1 plays an important role in the bone metabolism under physiological conditions. Int Immunol 2010;22:805-816. [DOI] [PubMed] [Google Scholar]
- 34. Kitazawa R, Kimble RB, Vannice JL, Kung VT, Pacifici R. Interleukin-1 receptor antagonist and tumor necrosis factor binding protein decrease osteoclast formation and bone resorption in ovariectomized mice. J Clin Invest 1994;94:2397-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kimble RB, Vannice JL, Bloedow DC, et al. Interleukin-1 receptor antagonist decreases bone loss and bone resorption in ovariectomized rats. J Clin Invest 1994;93:1959-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lorenzo JA, Naprta A, Rao Y, et al. Mice lacking the type I interleukin-1 receptor do not lose bone mass after ovariectomy. Endocrinology 1998;139:3022-3025. [DOI] [PubMed] [Google Scholar]
- 37. Pacifici R, Vannice JL, Rifas L, Kimble RB. Monocytic secretion of interleukin-1 receptor antagonist in normal and osteoporotic women: effects of menopause and estrogen/progesterone therapy. J Clin Endocrinol Metab 1993;77:1135-1141. [DOI] [PubMed] [Google Scholar]
- 38. Ishimi Y, Miyaura C, Jin CH, et al. IL-6 is produced by osteoblasts and induces bone resorption. J Immunol 1990;145:3297-3303. [PubMed] [Google Scholar]
- 39. Poli V, Balena R, Fattori E, et al. Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. EMBO J 1994;13:1189-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weitzmann MN, Cenci S, Rifas L, Brown C, Pacifici R. Interleukin-7 stimulates osteoclast formation by up-regulating the T-cell production of soluble osteoclastogenic cytokines. Blood 2000;96:1873-1878. [PubMed] [Google Scholar]
- 41. Weitzmann MN, Roggia C, Toraldo G, Weitzmann L, Pacifici R. Increased production of IL-7 uncouples bone formation from bone resorption during estrogen deficiency. J Clin Invest 2002;110:1643-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Walsh NC, Gravallese EM. Bone remodeling in rheumatic disease: a question of balance. Immunol Rev 2010;233:301-312. [DOI] [PubMed] [Google Scholar]
- 43. Nimmo MA, Leggate M, Viana JL, King JA. The effect of physical activity on mediators of inflammation. Diabetes Obes Metab 2013;15(Suppl 3):51-60. [DOI] [PubMed] [Google Scholar]
- 44. Ertek S, Cicero A. Impact of physical activity on inflammation: effects on cardiovascular disease risk and other inflammatory conditions. Arch Med Sci 2012;8:794-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Papenberg G, Ferencz B, Mangialasche F, et al. Physical activity and inflammation: effects on gray-matter volume and cognitive decline in aging. Hum Brain Mapp 2016;37:3462-3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gámez B, Rodríguez-Carballo E, Graupera M, Rosa JL, Ventura F. Class I PI-3-Kinase Signaling Is Critical for Bone Formation Through Regulation of SMAD1 Activity in Osteoblasts. J Bone Miner Res 2016;31:1617-1630. [DOI] [PubMed] [Google Scholar]
- 47. Smith GC, Ong WK, Costa JL, et al. Extended treatment with selective phosphatidylinositol 3-kinase and mTOR inhibitors has effects on metabolism, growth, behaviour and bone strength. FEBS J 2013;280:5337-5349. [DOI] [PubMed] [Google Scholar]
- 48. Engsig MT, Chen QJ, Vu TH, et al. Matrix metalloproteinase 9 and vascular endothelial growth factor are essential for osteoclast recruitment into developing long bones. J Cell Biol 2000;151:879-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Delaissé JM, Andersen TL, Engsig MT, et al. Matrix metalloproteinases (MMP) and cathepsin K contribute differently to osteoclastic activities. Microsc Res Tech 2003;61:504-513. [DOI] [PubMed] [Google Scholar]
- 50. Colnot C, Thompson Z, Miclau T, Werb Z, Helms JA. Altered fracture repair in the absence of MMP9. Development 2003;130:4123-4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gordon JA, Tye CE, Sampaio AV, et al. Bone sialoprotein expression enhances osteoblast differentiation and matrix mineralization in vitro. Bone 2007;41:462-473. [DOI] [PubMed] [Google Scholar]
- 52. Mizuno M, Imai T, Fujisawa R, Tani H, Kuboki Y. Bone sialoprotein (BSP) is a crucial factor for the expression of osteoblastic phenotypes of bone marrow cells cultured on type I collagen matrix. Calcif Tissue Int 2000;66:388-396. [DOI] [PubMed] [Google Scholar]
- 53. Mantila Roosa SM, Liu Y, Turner CH. Gene expression patterns in bone following mechanical loading. J Bone Miner Res 2011;26:100-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kuo TR, Chen CH. Bone biomarker for the clinical assessment of osteoporosis: recent developments and future perspectives. Biomark Res 2017;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Qvist P, Christgau S, Pedersen BJ, Schlemmer A, Christiansen C. Circadian variation in the serum concentration of C-terminal telopeptide of type I collagen (serum CTx): effects of gender, age, menopausal status, posture, daylight, serum cortisol, and fasting. Bone 2002;31:57-61. [DOI] [PubMed] [Google Scholar]
- 56. Clowes JA, Hannon RA, Yap TS, et al. Effect of feeding on bone turnover markers and its impact on biological variability of measurements. Bone 2002;30:886-890. [DOI] [PubMed] [Google Scholar]