Abstract
Introduction. Spontaneous bacterial peritonitis (SBP) represents the most severe and common infectious complication in patients suffering from liver cirrhosis. The objectives of the study were the evaluation of the risk factors responsible for the occurrence of the spontaneous bacterial peritonitis in a group of patients suffering from liver cirrhosis, the identification of the bacterial spectrum and assessing the response to antibiotic therapy. Material and method - The studied group included 64 patients suffering from liver cirrhosis, with an episode of SBP, who were admitted to the IInd Medical Clinic of the County Hospital of Craiova, within a period of 24 months. The control group included 61 patients with liver cirrhosis with an episode of decompensation of liver disease. The diagnosis of liver cirrhosis was established by using clinical, biological and imagistic criteria, and the SBP’s diagnosis was based on cytological and bacteriological analysis of the ascites fluid. Patients suffering from hepatocellular carcinoma, portal vein thrombosis and other infectious conditions were excluded. The anamnesis, the duration of the disease, the alcohol intake, the complete clinical examination, the clinical, biological and imagistic evaluation were monitored. Results and conclusions - The most frequent etiology of SBP is represented in 67% of the cases by Gram negative germs, and thus, the antibiotic therapy will be orientated against this etiological segment. In what antibiotic sensitivity is concerned, most of the germs were sensitive to third generation cephalosporins, quinolones, carbapenems and vancomycin.
Keywords: liver cirrhosis, spontaneous bacterial peritonitis, antibiotherapy
Introduction
Spontaneous bacterial peritonitis, a severe complication of liver cirrhosis with portal decompensation, caused by ascites fluid’s infection without a starting detectable, correctable abdominal point (ex. intra-abdominal abscesses), represents the most frequent and severe infectious complication in patients suffering from liver cirrhosis, being encountered in about 25% of the cases, and followed by urinary and respiratory infections – about 20% and, 15% of the cases [1, 2, 3].
The key factor in reducing the mortality in SBP is the prompt diagnosis, which together with completing the antibiotherapy and also with the empirical use of cephalosporins have significantly improved prognosis in the short term for these patients [4].
However, mortality remains high, survival at one year is about 30%, a factor which contributes to the increasing mortality is the tendency to recurrence; the recurrence’s rate after an episode treated with antibiotics (ATB) varies between 50% and 70% at one year.
The incidence of SBP in patients admitted to the hospital is estimated between 10% and 30% [5, 6, 7]. Certain studies show a downward trend of the incidence of SBP, a multicentre study recently highlighted an incidence of SBP of 5,5% [8,9). In what the etiology is concerned, Escherichia Coli, streptococci (especially peneumococci) and Klebsiella cause most of the episodes of SBP in patients with selective intestinal decontamination (5, 10). On the other hand, in patients in whom antibiotic prophylaxis was conducted with quinolones, the incidence of Gram positive bacteria increased [11,12].
The study’s aims were the evaluation of the risk factors responsible for the occurrence of spontaneous bacterial peritonitis (SBP) in a group of patients with liver cirrhosis, and also the study of the bacterial spectrum responsible for SBP.
Material and methods
It was a prospective case-control study, which included 64 patients with liver cirrhosis and with an episode of SBP, who were admitted to the IInd Medical Clinic of the County Hospital of Craiova, within a period of 24 months. The control group included 61 patients with liver cirrhosis with an episode of decompensation of liver disease, who were admitted to the same clinic in the same period of time.
The diagnosis of liver cirrhosis was established by using clinical, biological and imagistic criteria, and the SBP’s diagnosis was based on cytological and bacteriological analysis of the ascites fluid obtained by paracentesis.
The inclusion criteria in the study were the diagnostic criteria for liver cirrhosis with portal decompensation, suggestive clinical elements for SBP (fever, alteration of the mental state, abdominal pain, ileus, vomiting, diarrhea, abdomen with distended volume and sensitive to touch, muscle defenceless and movable dullness in abdominal flancs) and confirmation of SBP by positive cultures.
Patients suffering from hepatocellular carcinoma, portal vein thrombosis and other infectious conditions (pulmonary, urinary, cutaneous) were excluded from the study.
The monitored parameters for patients included in the study were the anamnesis, duration of the disease, alcohol intake, complete clinical examination, clinical, biological and imagistic examination.
Biological examination was performed in the laboratory of the County Hospital of Craiova and included: complete blood count (hemoglobin, number of leukocytes, platelets), hepatic cytolysis syndrome, cholestasis syndrome, level of albumin in the blood, time of prothrombin, viral markers (HBs antigen, antibodies anti HCV, antibodies anti-HD).
By using ultrasound abdominal evaluation, size, contour and liver structure, portal-spleen axis in the ascites fluid were examined. By using upper gastrointestinal endoscopy, the presence and the aspect of gastric and esophageal varices and portal-hypertensive gastropathy were examined.
Included in the imagistic exploration, a chest radiograph was performed in order to exclude active pulmonary lesions, and also a simple abdominal radiograph was performed in order to exclude some causes of secondary peritonitis.
For all the patients included in the study a careful analysis of the ascites fluid, including leukocytes’ counting and leukocyte formula, level of glucose, cultures from the liquid, including Lowenstein medium.
Inoculation of the culture medium was made directly to bedside, using 10-20 ml of the ascites fluid on 100 ml of culture medium (bottles for blood cultures).
From all the forms of SBP those with positive cultures were chosen, precisely for following the study’s objective of identifying the etiological spectrum (Table 1).
Table 1.
Forms of presentation of spontaneous bacterial peritonitis
| SPONTANEUS BACTERIAL PERITONITIS | ||
| VARIANTS | Polymorphonuclear count | Culture |
| SBP culture positive | >250 cells/mm3 | Positive |
| Neutrocytic ascites | >250 cells/mm3 | Negative |
| Bacterascites | <250 cells/mm3 | Positive |
Statistical analysis - Database management was performed with Excel program from the Microsoft Office, and the statistical analysis was performed by using MedCalc and Epi Info 2000. The results are expressed as average values ± standard deviation (SD).
Statistical indicators - from the central tendency indicators the following were analyzed: arithmetic mean, median, module, and from the scattering data indicators, the following including standard deviation, standard error of the mean and the 95% trust interval of the mean were analyzed.
Results
Comparing the main demographic and anthropometric parameters between the two groups, one does not notice significant differences in what the average age is concerned 46,78±10,91 years in the study group and 44,16±11,23 years in the control group, gender distribution (M/F) was 42/44 in the study group and 39/22 in the control group and the average duration of the disease’s progression was 8,79±4,33 years in the study group and 8,09±4,04 in the control group (Table 2).
Table 2.
The main demographic, anthropometric and hematological parameters studied in comparison between the two groups
| PARAMETERS | Patients with SBP (n=64) | Patient without SBP (n=61) |
| sex ratio (B/F) | 42/24 | 39/22 |
| age | 46,78±10,91 | 44,16±11,23 |
| duration of disease progression | 8,79±4,33 | 8,09± 4,04 |
| hemoglobin (g/dl) | 8,87±2,1 | 10,88±2,26 |
| number of leukocytes | 10921±4083 | 4681,90+/2293,95 |
| trombocytes | 80500±3560 | 139245,9+/100034.2 |
The results of the hematological evaluation reveal a mean hemoglobin of 8,79±4,33g/dl in patients with SBP and 10,88±2,26g/dl in patients with liver cirrhosis without SBP, the number of leukocytes from the serum and from the ascites fluid was 10921±4083/mmc, and 332,04±61,22/mmc in patients with SBP compared with 4681,9±2293,95mmc, and 65,55±38,18/mmc in patients without SBP. The number of platelets was 80500±3560 in the study group and 139245,9±100034,2/mmc in the control group (Table 2).
From the biochemical exams, for the study group with SBP versus the control group, one can notice an average total bilirubin of 9,90±6,53 mg/dl versus 3,24±1,33, an average value of serum albumin of 3,03±1,14 g/dl versus 5,03±2,03 g/dl, an average value of the ascites fluid’s albumin of 0,46±0,26 g/dl versus 1,4±0,59 g/dl and a prothrombin index of 37,73±13,27% versus 65,93±20,27% (Table 3).
Table 3.
The main biochemical parameters studied in comparison between the two groups
| PARAMETERS | Patients with SBP (n=64) | Patient without SBP (n=61) |
| ASAT (U/L) | 86,68±30,07 | 78,73±30,76 |
| ALAT (U/L) | 96±28,91 | 81,90±21,44 |
| total bilirubin (mg/dl) | 9,90±6,53 | 3,24±1,33 |
| level of albumin in the blood (g/dl) | 3,03±1,14 | 5,03±2,03 |
| level of albumin in the ascites (g/dl) | 0,46±0,26 | 1,4±0,59 |
| PMN ascites | 332,04±61,22 | 65,55±38,18 |
| prothrombin-time (%) | 37,73±13,27 | 65,93±20.27 |
| creatinin (mg/dl) | 1,45±0,73 | 1,1±0,73 |
| glucose | 144,12±37,44 | 90,91±14,37 |
| Na (mEq/l) | 126,82±10,46 | 130,44±10,44 |
Also, patients suffering from SBP had higher values for serum creatinine 1,45±0,73 mg/dl and for serum level of glucose 144,12±37,44 mg/dl in comparison with patients without SBP 1,1±0,73 mg/dl, and 90,91±14,37 mg/dl. Serum sodium had higher values in the study group 126,82±10,46 mEq/l and 130,44±10,44 mEq/l in the control group (Table 3).
From the 64 patients included in the study group, 44 had alcoholic etiology, the rest including 10 cases with B viral etiology, 2 cases with B+D viral etiology, 7 cases with C viral etiology and 1 case with alpha-1-antitrypsine deficiency (Fig.1).
Figure 1.
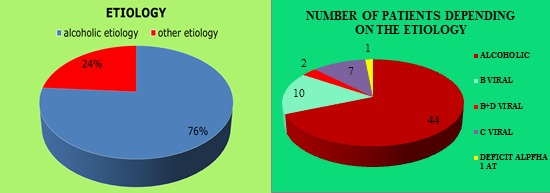
Patients’ distribution in the study group depending on the etiology
By making a comparative analysis of the groups in what the distribution depending on the Child-Pugh classification is concerned, one can notice that in the study group with a percentage of 62,5% (40 cases) C class was in majority, followed by B class with a percentage of 25% (16 cases) and A class with a percentage of 12,5% (8 cases), in comparison with the control group where the main percentage is represented by A class, 63,93% (39 patients), followed by B class 22,95% (14 patients) and C class, 13,11% (8 patients) (Fig.2).
Figure 2.
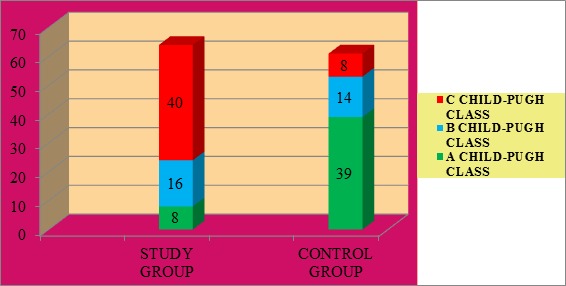
Patients’ distribution depending on the severity class
The etiology of SBP was represented by Gram negative bacilli 67%, Gram positive bacilli 30% and anaerobic bacilli 3%, the etiological spectrum contains 27 cases (42%) Escherichia Coli, 9 cases (14%) Klebsiella, 7 cases (11%) enterobacter, 6 cases (9%) streptococcal pneumonia, 5 cases (8%) streptococcus viridans, 8 cases (13%) enterococcus and 2 cases (3%) bacteroides (Fig.3).
Figure 3.
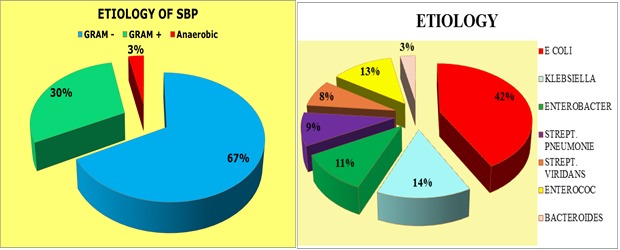
Etiological spectrum of SBP
In what antibiotic sensitivity is concerned, most germs were sensitive to third generation cephalosporins, qiunolones, carbapenems and vancomycine (Table 4).
Table 4.
Sensitivity to antibiotics
| BACTERIES | Gram negative | Gram positive | Anaerobic | ||||
| E COLI | KLEBSIELLA | ENTEROBACTER | STREPT. PNEUMONIAE | STREPT. VIRIDANS | ENTEROCOCCUS | BACTEROIDES | |
| AMPICILLIN | 2 | 1 | 6 | ||||
| AMOXI-CLAVULANIC ACID | 6 | 2 | 8 | 1 | |||
| CEPHALOSPORINS –CEFOTAXIME/ CEFTRIAXONE/ CEFEPIME | 19/17/15 | 6/5/3 | 3/4/2 | 5/6/5 | 4/4/5 | 5/7/7 | 1/1/1 |
| FLUOROQUINOLONES – CIPROFLOXACIN/ LEVOFLOXACIN/ MOXIFLOXACIN | 21/24/23 | 6/8/7 | 5/6/4 | 2/3/5 | 2/2/3 | 1/3/2 | -/1/1 |
| CARBAPENEMS – IMIPENEM/ ERTAPENEM | 25/26 | 8/9 | 5/6 | 6/6 | 5/5 | 7/8 | 1/2 |
| METRONIDAZOL | 3 | 2 | |||||
| CLINDAMYCIN | 1 | 2 | 1 | 2 | |||
| VANCOMYCIN | 5 | 1 | 3 | 4 | 4 | 6 | 1 |
Assessing the response to the initial empirical antibiotic therapy, until getting the cultures with the corresponding antibiogram, by the evolution of PMN in the ascites fluid at 48 hours after the initiation of the treatment (quinolones± third generation cephalosporins), showed a favorable evolution in most of the cases (Fig.4).
Figure 4.
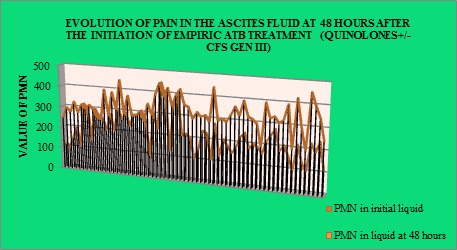
Assessing the response to empiric antibiotic treatment
Discussion
After making a comparison of the mean values in patients with SBP and also in patients without SBP, bilirubin and creatinine had higher mean values in patients with SBP, and total proteins, albumins and also the prothrombin time had lower mean values in patients with SBP, these biochemical parameters are correlated with the degree of liver failure, and they can be considered risk factors of SBP.
Leukocytosis, both peripheral and from the ascites fluid, a low level of hemoglobin, trombocytopenia, Child-Pugh score, a low level of proteins in the ascites fluid can be considered predictive factors of SBP in patients with liver cirrhosis.
Patients with liver cirrhosis are more prone to infections, having a high risk for bacteraemia and SBP, and bacterial infections are frequent, especially in the alcoholic form. Patients with alcoholic liver cirrhosis have a decreased ability of neutrophil’s phagocytosis, the consequence being the alteration of the means of defense against infections.
Despite the use of antibiotics with a large spectrum, bacterial infections are responsible for 25% of the deaths of the patients with liver disease. Infectious complications can worsen the evolution of liver cirrhosis, causing the occurrence of hepatic encephalopathy, of hepatocellular failure or of hepato-renal syndrome.
Bacterial infections are common and frequent in patients suffering from liver cirrhosis. That is why identifying the patients with a high risk is important in order to intervene in a timely manner and to prevent their unfavorable evolution.
The use of supportive antibiotic therapy decreased both morbidity and mortality among the years. However, the use of antibiotics must be judicious, otherwise their unjustified use may lead to antibiotic resistance with potentially disastrous consequences.
In patients with liver cirrhosis and infectious complications, the early use of antibiotics and the correction of the main parameters’ changes (intravenous administration of albumin) decrease the risk of kidney failure (mortality predictor) and improve survival.
The most common etiology of SBP is represented by Gram negative germs (67%), similar to previous studies’ results, which showed that the most common microorganisms isolated from patients with SBP were Escherichia coli (∼70%), Klebsiella species (∼10%), Proteus species and Pseudomonas species (∼2%) [13, 14, 15], being in contradiction with the studies that suggested the predominance of Gram positive germs [16].
A study led by Javier Fernández et al identified Gram-positive cocci as being responsible for about 53% of the cases. Patients admitted in the intensive care units, who had undergone invasive procedures had, in about 77% of the cases, high rates of infections with Gram-positive cocci; 50% of the positive cultures belonging to patients who were administrated norfloxacine on long term and for 16% of the patients who did not receive this therapy the cultures were positive for Gram-negative bacilli qiunolones-resistant. The rate of positive cultures with Gram-negative bacilli trimethoprim-sulfamethoxazole-resistant was also very high (44%) (5). Although the use of antibiotics in the primary prophylaxis of SBP in patients suffering from liver cirrhosis is controversial, Rohit Loomba et al wanted to determine the beneficial effects of flouroqiunolones compared to placebo, and they demonstrated that the oral use of fluoroquinolones reduces the risk for the first episode of SBP and also the mortality both of patients with liver cirrhosis and of those with a low level of proteins in the ascites fluid, so they can be used for SBP’s primary prophylaxis at-risk patients (17).
Conclusions
-The main factors associated with SBP in patients with liver cirrhosis were leukocytosis both peripheral and in the ascites fluid, low level of hemoglobin, trombocytopenia, Child-Pugh score, low level of proteins in serum and in ascites fluid, prothrombin-time, high serum values of bilirubin and creatinine.
-The most common etiology of SBP is represented by Gram-negative germs (67%), and antibiotherapy will be orientated towards this etiological segment.
-SBP’s empirical therapy with qiunolones± third generation cephalosporines led to the decrease of the number of PMN in the ascites fluid at 48 hours in most of the cases from the study group.
-Knowing the etiological spectrum influences the choice of antibiotics until getting the bacteriological result.
Acknowledgments
All authors had equal contribution.
Conflict of interests
The authors declare that they have no conflict of interests.
References
- 1.Castellote J, Girbau A, Maisterra S, et al, et al. Spontaneous bacterial peritonitis and bacterascites prevalence in asymptomatic cirrhotic outpatients undergoing large-volume paracentesis. J Gastroenterol Hepatol. 2008;23:256–259. doi: 10.1111/j.1440-1746.2007.05081.x. [DOI] [PubMed] [Google Scholar]
- 2.Rimola A, Garcia-Tsao G, Navasa M, et al. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. J Hepatol. 2000;32:142–153. doi: 10.1016/s0168-8278(00)80201-9. [DOI] [PubMed] [Google Scholar]
- 3.Christou L, Pappas G, Falagas ME. Bacterial in fection-related morbidity and mortality in cirrhosis. Am J Gastroenterol. 2007;102:1510–1517. doi: 10.1111/j.1572-0241.2007.01286.x. [DOI] [PubMed] [Google Scholar]
- 4.Tarsila CR Ribeiro, 1Julio MF Chebli, 2Mario Kondo, 1Pedro Duarte Gaburri, 3Liliana Andrade Chebli, 2 and Ana Cristina Amaral Feldner. Spontaneous bacterial peritonitis: How to deal with this life-threatening cirrhosis complication? Ther Clin Risk Manag. 2008;4(5):919–925. doi: 10.2147/tcrm.s2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fern�ndez J, Navasa M, Gomez J, et al. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35:140–148. doi: 10.1053/jhep.2002.30082. [DOI] [PubMed] [Google Scholar]
- 6.Thuluvath PJ, Morss S, Thompson R. Spontaneous bacterial peritonitis � In-hospital mortality, predictors of survival, and health care costs from 1988 to 1998. Am J Gastroenterol. 2001;96:1232–1236. doi: 10.1111/j.1572-0241.2001.03708.x. [DOI] [PubMed] [Google Scholar]
- 7.Singh N, Wagener MM, Gayoski T. Changing epidemiology and predictors of mortality in patents with spontaneous bacterial peritonitis at liver transplant unit. Clin Microbiol Infect. 2003;9:531–537. doi: 10.1046/j.1469-0691.2003.00691.x. [DOI] [PubMed] [Google Scholar]
- 8.Sette H, Mies S, Barros MFA, et al. Peritonite bacteriana espon-t�nea. Rev Paul Med. 1996;104:292–297. [PubMed] [Google Scholar]
- 9.Nousbaum JB, Cadranel JF, Nahon P, et al. Diagnostic accuracy of the Multistix 8 SG reagent strip in diagnosis of spontaneous bacterial peritonitis. Hepatology. 2007;45:1275–1281. doi: 10.1002/hep.21588. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Tsao G. Spontaneous bacterial peritonitis. Gastroenterol Clin N Am. 1992;21:257–275. [PubMed] [Google Scholar]
- 11.Llovet J, Rodriguez-Iglesias P, Moitinho E, et al. Spontaneous bacterial peritonitis in patients with cirrhosis undergoing selective intestinal decontamination. J Hepatol. 1997;26:88–95. doi: 10.1016/s0168-8278(97)80014-1. [DOI] [PubMed] [Google Scholar]
- 12.Almeida PR, Camargo NS, Arenz M, et al. Spontaneous bacterial peritonitis: impact of microbiological changes. Arq Gastroenterol. 2007;44:68–72. doi: 10.1590/s0004-28032007000100015. [DOI] [PubMed] [Google Scholar]
- 13.Arroyo V, Bataller R, Gin�s P. O'Grady and Lake's comprehensive clinical hepatology. 1. Barcelona: Mosby; Spontaneous Bacterial Peritonitis; pp. 7–10. [Google Scholar]
- 14.Guarner C, Soriano G. Eur J Gastroenterol Hepatol. 2005. Bacterial translocation and its consequences in patients with cirrhosis; pp. 17–27. [DOI] [PubMed] [Google Scholar]
- 15.Wiest R, Garcia-Tsao G. Bacterial translocation in cirrhosis. Hepatology. 2005;41:422–433. doi: 10.1002/hep.20632. [DOI] [PubMed] [Google Scholar]
- 16.Luke T Evans, W Ray Kim, John J Poterucha, et al. Spontaneous bacterial peritonitis in asymptomatic outpatients with cirrhotic ascites. Hepatology. 2003;37(4):897–901. doi: 10.1053/jhep.2003.50119. [DOI] [PubMed] [Google Scholar]
- 17.Rohit Loomba, Robert Wesley, Andrew Bain, et al. Role of Fluoroquinolones in the Primary Prophylaxis of Spontaneous Bacterial Peritonitis: Meta-Analysis. Clinical Gastroenterology and Hepatology; 2009;7(4):487–493. doi: 10.1016/j.cgh.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]


