Abstract
Aims
Risks of catheter ablation for atrial fibrillation and flutter assessed in retrospective studies, registries, and controlled trials may underestimate ‘real world’ conditions.
Methods and results
To assess complications in a nationwide approach, we included all cases undergoing catheter ablation for atrial fibrillation and atrial flutter in Germany in 2014, using ICD-10-GM-based German Diagnosis Related Group (G-DRG) codes and the well differentiated German Operation and Procedure Classification (OPS) analysing 33 353 in-hospital cases. For left atrial ablations (19 514 cases), the overall complication rate ranged from a mean of 11.7% to 13.8% depending on type and site of applied energy, including major complications ranging from 3.8% to 7.2%. Whereas overall complication rates were lower for atrial flutter ablations (13 871 cases, 10.5%; P < 0.001), interestingly, major complications occurred more frequently (7.4%; P < 0.001). Particularly, in-hospital death was four-times more common following right than following left atrial ablations (47 vs. 18 cases, 0.34% vs. 0.09%; P < 0.001). Stratified by centre ablation volume, significantly fewer overall complications occurred in centres performing >100 vs. ≤100 left atrial ablations annually (12.7% vs. 16.4%; P < 0.002).
Conclusion
Administrative data of all atrial fibrillation ablations in Germany in 2014 revealed higher overall and major complication rates than previously reported. Few patients were treated in low volume centres, but were exposed to a higher overall complication risk. Atrial flutter ablations were associated with surprisingly high rates of life-threatening complications. Advanced age combined with highly prevalent cardiac, pulmonary and, vascular comorbidities likely play a major role. In addition, individual-level clinical studies need to address the safety and benefits of catheter ablation in an elderly, diseased population.
Keywords: Atrial fibrillation , Atrial flutter , Catheter ablation , Complication , Administrative data
Introduction
Within a few years after the first report,1 catheter ablation of atrial fibrillation by pulmonary vein (PV) isolation has emerged as one of the most frequently performed invasive interventions in cardiology world-wide. While it is believed that the benefits of this therapy generally outweigh the risks in properly selected patients,2 these risks so far have been assessed mostly in retrospective, non-comparative studies,3–9 voluntary registries,10–12 and controlled trials.13–15 All these analyses are biased by patient selection and may underestimate the true risk in a ‘real world’ situation.
What is needed is complication assessment in a large, unselected, population-based patient cohort. There are few nationwide cohort studies of administrative data on complications associated with catheter ablation of atrial fibrillation in the United States. Yet, they are either based on procedures in the years 2000–2010, representing early clinical experience with this then new invasive treatment strategy,16 or reflect a nation-wide subgroup of patients only as in more recent analyses of Medicare beneficiaries.17,18 Atrial flutter often coexists with or precedes atrial fibrillation.19 The risk of catheter ablation for atrial flutter, generally assumed to be low, has been reported for cavo-tricuspid isthmus dependent atrial flutter in a meta-analysis of 158 studies in 10 719 patients.20 Far less is known about atypical flutter, which may account for 21% of all flutter cases.21
With the introduction of a diagnosis and procedure related remuneration system in Germany in 2004, known as the German Diagnosis Related Groups (G-DRG) system, it has become mandatory for all German hospitals to transfer patient data on diagnoses, coexisting conditions, and procedures of all consecutive cases to the Institute for the Hospital Remuneration System (InEK, Siegburg, Germany). All diagnoses are coded according to the ICD-10-GM (German modification), and procedures are coded according to the German Operation and Procedure Classification (OPS). Coding of ablation of atrial fibrillation and/or atrial flutter is well differentiated with respect to energy source and location of the cardiac structure targeted for ablation. It has thus become possible to track all kinds of complications until hospital discharge in a differential fashion applying a nationwide approach.
This current report is based on the population of all patients with a German health insurance undergoing atrial fibrillation and/or atrial flutter ablation in Germany in 2014, well-established procedures by that time. As provider experience markedly influenced the rate of complications in a prior report,16 we also assessed the relation between the centre ablation volume and the associated complication rates.
Methods
Data source
Analyses were performed on our behalf by the Research Data Center (RDC) of the Federal Statistical Office and the Statistical Offices of the Federal States in Wiesbaden, Germany. Analysis requests based on DRG Statistics 2014 were submitted to this institute on the basis of SAS codes (SAS software, version 9.2; SAS Institute) for own calculations, and results were reported back to the investigators from the institute. In case of reported frequencies unequal to zero but too low to maintain data anonymity (typically <3 counts per table cell), these data were blinded by the RDC. In such cases, blinded data were considered as zero for analysis purposes, except where stated otherwise.
Study oversight and support
There was no commercial support for conduction of the research or the preparation of this report. Given the strictly anonymized data analysis based on aggregate administrative data, ethics committee approval was determined not to be required in accordance with German law.
Diagnoses and procedural codes
Diagnoses, patient characteristics, comorbidities, and complications of catheter ablation are uniformly coded according to the International Statistical Classification of Diseases and Related Health Problems, 10th revision, German modification (ICD-10-GM). Similarly, endovascular procedures are uniformly coded according to the German Operation and Procedure Classification (OPS). Coding guidelines and annual adaptation by the German Institute for Medical Documentation and Information (Cologne, Germany) ensure uniform documentation (Supplementary material online, Tables S1, S2, S3).
Using the distinctly differentiating OPS codes, we created four separate, disjunct groups of cases for left atrial ablation, stratified by the source and location of energy application, and one group for isolated right atrial ablations. The groups were defined as follows (detailed OPS codes used for patient selection are provided in Supplementary material online, Table S1):
Group 1: Pulmonary vein isolation and/or left atrial ablation using cryo energy (Cryoablation).
Group 2: Isolated pulmonary vein isolation with any kind of energy source except for cryo energy: conventional radiofrequency (RF) with or without irrigated tip catheters, RF applied by multi-electrode catheters, or three-dimensional mapping with pressure monitoring during energy application, bipolar phased RF; other energies (ultrasound, microwave, and laser with endovascular endoscopic guidance). Ablation sites other than pulmonary veins are excluded (PV isolation).
Group 3: Ablation in the left atrium using any kind of energy source except for cryo energy (for details see Group 2). Additional pulmonary vein isolation is included (LA ablation).
Group 4: Ablation of pulmonary veins and/or in the left atrium in combination with ablation in the right atrium using any kind of energy source except cryo energy (LA + RA ablation).
Group 5: Isolated right atrial ablation using any kind of energy source (RA ablation).
Statistical analysis
For each pre-specified group of patients, or each pre-specified stratum thereof, we obtained aggregate data on the presence of comorbidities and any occurrence of complications from the RDC, averaged across the respective group. All discrete data are expressed as absolute and relative frequencies. All continuous data are presented as mean ± standard deviation. Partly, individual groups or strata were merged; thereby, continuous average variables were weighted for the sizes of the underlying groups. We compared differences between groups by Fisher’s exact tests or Student’s t-test, as appropriate.
Of particular note, the aggregate data structure prevented us from analysing individual level information. We were thus not able to calculate models accounting for the possibly confounding distribution of covariates as done in prior work.16,17 All P-values are two-sided and P-values <0.05 were considered significant. Analyses were conducted using STATA 12.0 (Stata Corp, College Station, TX, USA).
Results
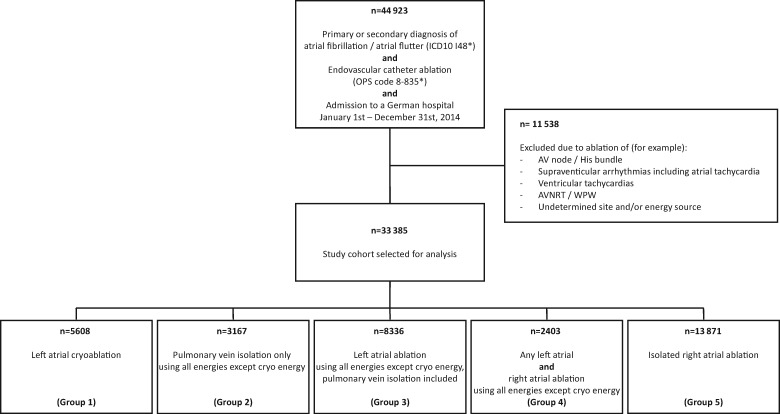
Between 1 January and 31 December 2014, a total of 44 923 cases with a primary or secondary diagnosis of atrial fibrillation/atrial flutter undergoing endovascular catheter ablation were admitted to a German hospital (see flow chart in Figure 1). After exclusion of cases with other sites of ablation or undetermined site or energy source, 33 385 cases were selected as study cohort, divided into five groups as described in the Methods section.
Figure 1.
Study overview. A code marked by an asterisk indicates that all subheadings of this code are included. Detailed group definitions are provided in the Methods section and the Supplementary material online.
These cases were coded as defined in Germany in 2014 and were analysed for the occurrence of the following eight distinct complications defined according to the ICD-10-GM (Supplementary material online, Table S2) accumulating to the overall complication rate: in-hospital death, pericardial effusion, stroke, pneumonia, phrenic nerve injury, access site complications, cardiac arrest, and atrioventricular (AV) block III°. For calculation of major complications only, pericardial effusion was exchanged for the need of pericardial drainage, and access site complications were exchanged for the need of transfusion or vascular intervention/surgery.
The rates of these eight overall in-hospital complications are provided in Table 1 for the total of 33 385 cases stratified by five pre-specified subgroups. For ablation of atrial fibrillation by pulmonary vein isolation and/or ablation of left atrial substrate, complications varied between a subgroup mean of 11.7% and 13.8%, with an average rate of 12.6% (Table 1, left four columns). Individual complications were a mean of 7.1% for access site complications defined as bleeding, haematoma, shock, infection, and vascular complications. Pericardial effusion occurred in 3.5%, pneumonia in 0.8%, stroke in 0.6%, and AV block III° in 0.3%. In-hospital death occurred in 18 cases (0.09%). Considering major complications only, the mean rate of adjudicated complications amounted to 5.1% (Table 1). Oesophageal perforation, pulmonary embolism, and endocarditis occurred too rarely for analysis. Cryoablation and RF ablation for PV isolation (Groups 1 and 2) was associated with a very similar rate of both overall (12.3% vs. 11.7%, P = 0.45) and major complications (3.8% vs. 4.2%, P = 0.43).
Table 1.
Incidence of procedure related complications, stratified by patient group
| Complications, n (%) | Cryoablation | PV isolation | LA ablation | LA + RA ablation | RA ablation |
|---|---|---|---|---|---|
| Group 1 (n = 5608), n (95% CI) (%) | Group 2 (n = 3167), n (95% CI) (%) | Group 3 (n = 8336), n (95% CI) (%) | Group 4 (n = 2403), n (95% CI) (%) | Group 5 (n = 13 871), n (95% CI) (%) | |
| In-hospital death* | 8 (3–17) (0.1) | 0 (0–5) (0.0) | 7 (3–16) (0.1) | 3 (0–10) (0.1) | 47 (34–64) (0.3) |
| Stroke* | 27 (18–40) (0.5) | 17 (10–28) (0.5) | 48 (35–65) (0.6) | 15 (8–26) (0.6) | 70 (54–89) (0.5) |
| Pneumonia* | 30 (20–44) (0.5) | 23 (14–36) (0.7) | 88 (71–109) (1.1) | 25 (16–38) (1.0) | 282 (250–317) (2.0) |
| Phrenic nerve injury* | 21 (13–33) (0.4) | n.a. | n.a. | n.a. | 8 (3–17) (0.1) |
| Cardiac arrest* | 13 (7–23) (0.2) | 3 (0–10) (0.1) | 17 (10–28) (0.2) | 7 (3–16) (0.3) | 49 (36–66) (0.4) |
| AV Block III°* | 11 (5–21) (0.2) | 9 (4–18) (0.3) | 29 (19–43) (0.3) | 8 (3–17) (0.3) | 223 (195–255) (1.6) |
| Pericardial effusion | 167 (143–195) (3.0) | 100 (81–122) (3.2) | 305 (272–341) (3.7) | 98 (80–120) (4.1) | 237 (208–270) (1.7) |
| Pericardial drainage* | 42 (29–55) (0.7) | 26 (16–36) (0.8) | 113 (92–134) (1.4) | 20 (11–29) (0.8) | 47 (34–60) (0.3) |
| Access site complications | 411 (373–452) (7.3) | 219 (192–250) (6.9) | 655 (607–706) (7.9) | 149 (127–175) (6.2) | 536 (492–583) (3.9) |
| Vascular intervention /surgery* | 15 (7–23) (0.3) | 15 (7–23) (0.5) | 97 (78–116) (1.2) | 12 (5–19) (0.5) | 80 (62–98) (0.6) |
| Transfusion* | 48 (34–62) (0.9) | 40 (28–52) (1.3) | 203 (175–231) (2.4) | 35 (23–47) (1.5) | 222 (193–251) (1.6) |
| Total, overall | 688 (637–739) (12.3) | 371 (333–409) (11.7) | 1149 (1083–1215) (13.8) | 305 (271–339) (12.7) | 1452 (1377–1527) (10.5) |
| Total, major | 215 (186–244) (3.8) | 133 (110–156) (4.2) | 602 (554–650) (7.2) | 125 (103–147) (5.2) | 1028 (965–1091) (7.4) |
Major complications are marked by an asterisk (*). Complications are presented as incident cases (percentage), stratified by pre-specified patient groups. Access site complications consist of bleeding, haematoma, shock, infection, and vascular complications. Detailed group definitions are provided in the methods section.
LA, left atrium; n.a., not assessed (indicates table cell with too few observations for display due to data protection policies); RA, right atrium.
Comparison of the four groups of cases undergoing left atrial ablations revealed that structural modification within the left atrium itself, eventually in addition to PV isolation (Group 3), was associated with higher overall and major complication rates than in cases undergoing PV isolation only by cryo- or RF energy (Groups 1, 2, and 4 vs. Group 3; P = 0.001 for overall and P < 0.001 for major complications). Complications in patients undergoing both left and right atrial ablations displayed a complication pattern more similar to other forms of left atrial ablations than to the group of isolated right atrial ablations.
For isolated right atrial ablations (13 871 cases, Table 1, right column), the average overall complication rate (10.5%) was significantly lower than for left atrial ablations (12.6%, P < 0.001). Access site complications (3.9%), pneumonia (2.0%), pericardial effusion (1.7%), AV block III° (1.6%), and stroke (0.5%) were the predominating complications. The rate of major complications was significantly higher in right compared with left atrial ablations (7.4% vs. 5.1%; P < 0.001). Of particular note, in-hospital death occurred in 47 cases of isolated right atrial ablation (0.34%), an incidence four times higher than following left atrial ablation (0.09%, P < 0.001).
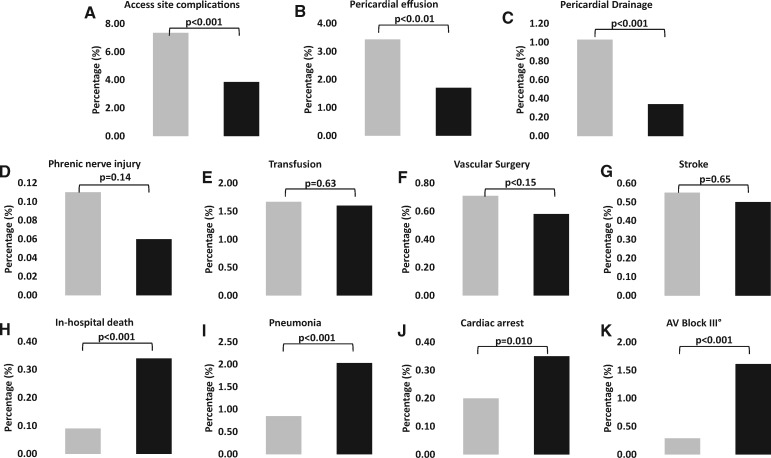
In Figure 2, the incidence of individual complication rates is compared between any isolated left atrial ablation procedure and isolated right atrial ablations: whereas access site complications, pericardial effusions, and pericardial drainage occurred more frequently following left atrial procedures, in-hospital death, pneumonia, cardiac arrest, and AV block III° were more frequently observed with isolated right atrial ablations (P < 0.001 for all mentioned complications).
Figure 2.
Complication rates comparing any pulmonary vein isolation and/or left atrial ablation (Groups 1–4) vs. isolated right atrial ablation (Group 5). In each panel, cases with left atrial ablation procedures are displayed by grey bars and right atrial ablations are displayed by black bars. (A–C) Upper row shows complications with significantly higher rates in left vs. right atrial ablations. (D–G) Middle row shows complications with no significant differences between left vs. right atrial ablations. (H–K) Lower row shows complications with significantly higher rates in right vs. left atrial ablations. Groups are defined as in Table 1. Complication rates are expressed as percentage per group and compared by Fisher’s exact tests.
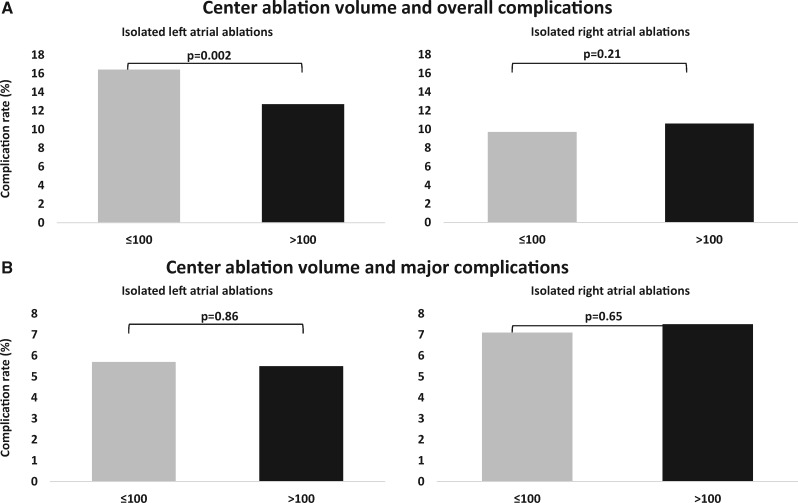
To assess the impact of ablation centre experience, complications were stratified with respect to the number of annually performed cases per institution for isolated left and isolated right atrial ablations (Table 2). Only 0.2%, 1.5%, and 3.7% of cases, respectively, were treated in hospitals with a left atrial ablation volume of ≤25 cases, 26–50 cases, and 51–100 cases, respectively. The overall complication rates for these strata ranged between 13.2% and 18.2%. In centres performing >100 left atrial procedures per year, there was a significant drop in overall complication rates from 16.4% to 12.7% (P = 0.002; Figure 3, left upper panel; Table 2), whereas no such drop was noted for major complications (P = 0.86; Figure 3, left lower panel; Table 2). For isolated right atrial ablations, no impact of centre ablation volume on both overall and major complication rate was apparent (Figure 3, right upper and lower panels; Table 2).
Table 2.
Incidence of procedure related complications of left vs. right atrial ablations, stratified by centre ablation volume
| Complications, n (%) | Isolated pulmonary vein isolation and/or left atrial ablation |
Isolated right atrial ablation |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Centre ablation volume |
Centre ablation volume |
|||||||||
| ≤25 (n = 33), n (95% CI) (%) | 26–50 (n = 258), n (95% CI) (%) | 51–100 (n = 642), n (95% CI) (%) | 101–300 (n = 5636), n (95% CI) (%) | >300 (n = 10 780), n (95% CI) (%) | ≤25 (n = 261), n (95% CI) (%) | 26–50 (n = 667), n (95% CI) (%) | 51–100 (n = 1158), n (95% CI) (%) | 101–300 (n = 4996), n (95% CI) (%) | >300 (n = 6789), n (95% CI) (%) | |
| In-hospital death* | 0 (0–5) (0) | 0 (0–5) (0) | 0 (0–5) (0) | 8 (3–17) (0.1) | 8 (3–17) (0.1) | 0 (0–5) (0) | n.a. | n.a. | 20 (12–32) (0.4) | 20 (12–32) (0.3) |
| Stroke* | 0 (0–5) (0) | n.a. | n.a. | 29 (19–43) (0.5) | 60 (46–78) (0.6) | n.a. | n.a. | 7 (3–16) (0.6) | 33 (23–47) (0.7) | 26 (17–39) (0.4) |
| Pneumonia* | n.a. | 3 (0–10) (1.2) | 11 (5–21) (1.7) | 62 (47–80) (1.1) | 65 (50–84) (0.6) | 7 (3–15) (2.7) | 10 (5–19) (1.5) | 28 (19–41) (2.4) | 141 (120–165) (2.8) | 96 (78–118) (1.4) |
| Phrenic nerve injury* | 0 (0–5) (0) | 0 (0–5) (0) | n.a. | 9 (4–18) (0.2) | 14 (7–25) (0.1) | 0 (0–5) (0) | 0 (0–5) (0) | n.a. | n.a. | 6 (2–14) (0.1) |
| Cardiac arrest* | 0 (0–5) (0) | n.a. | 3 (0–10) (0.5) | 16 (9–27) (0.3) | 13 (7–23) (0.1) | 0 (0–5) (0) | n.a. | n.a. | 21 (13–33) (0.4) | 25 (16–38) (0.4) |
| AV block III°* | 0 (0–5) (0) | 0 (0–5) (0) | n.a. | 18 (11–30) (0.3) | 31 (21–45) (0.3) | 4 (1–11) (1.5) | 9 (4–18) (1.3) | 13 (7–23) (1.1) | 87 (70–107) (1.7) | 110 (90–133) (1.6) |
| Pericardial effusion | n.a. | 7 (3–15) (2.7) | 18 (11–29) (2.8) | 184 (159–213) (3.3) | 370 (334–410) (3.4) | 5 (1–13) (1.9) | 8 (3–17) (1.2) | 13 (7–23) (1.1) | 101 (83–122) (2.0) | 110 (90–133) (1.6) |
| Pericardial drainage* | 0 (0–5) (0) | n.a. | 6 (1–11) (0.9) | 67 (51–83) (1.2) | 110 (89–131) (1.0) | 0 (0–5) (0) | n.a. | n.a. | 21 (12–30) (0.4) | 24 (14–34) (0.4) |
| Access site complications | 6 (2–12) (18.2) | 21 (13–32) (8.1) | 75 (60–93) (11.7) | 434 (395–476) (7.7) | 762 (710–817) (7.1) | 10 (5–19) (3.8) | 27 (18–40) (4.0) | 46 (34–62) (4.0) | 183 (159–209) (3.7) | 270 (239–304) (4.0) |
| Vascular intervention/surgery* | 0 (0–5) (0) | 5 (1–9) (1.9) | n.a. | 69 (53–85) (1.2) | 53 (39–67) (0.5) | n.a. | n.a. | 8 (2–14) (0.7) | 29 (18–40) (0.6) | 34 (23–45) (0.5) |
| Transfusion* | n.a. | 4 (0–8) (1.6) | 10 (4–16) (1.6) | 127 (105–149) (2.3) | 150 (126–174) (1.4) | n.a. | n.a. | 24 (14–34) (2.1) | 81 (63–99) (1.6) | 104 (84–124) (1.5) |
| Total, overall | 6 (2–12) (18.2) | 31 (20–42) (13.2) | 108 (88–128) (17.4) | 760 (706–814) (14.1) | 1323 (1252–1394) (12.8) | 26 (16–36) (10.0) | 54 (40–68) (8.1) | 107 (87–127) (11.2) | 586 (539–633) (13.2) | 663 (613–713) (10.7) |
| Total, major | 0 (0–5) (0) | 12 (5–19) (5.8) | 30 (19–91) (5.5) | 405 (366–444) (7.8) | 504 (460–548) (5.2) | 11 (4–18) (4.2) | 27 (17–37) (4.0) | 80 (62–98) (8.9) | 433 (392–474) (10.1) | 445 (404–486) (7.5) |
Major complications are marked by an asterisk (*). Complications are presented as incidence (percentage), stratified by centre ablation volume and by any pulmonary vein isolation and/or left atrial ablation or isolated right atrial ablation. Centre ablation volume was categorized by centres performing ≤25, 26–50, 51–100, 101–300, or >300 of the respective procedures.
For Table 2, Group 4 (additional right atrial ablation) is eliminated from the analysis on the left of the table, but the sum (17 349 procedures) is more than the sum of Groups 1–3 (17 111 procedures), as in 238 cases both cryo-energy and RF energy were applied, which are not represented in Table 1.
n.a., not assessed (indicates table cell with too few observations for display due to data protection policies).
Figure 3.
Complication rates by centre ablation volume. Bars represent the cumulative incidences of complications occurring for isolated left atrial ablations and isolated right atrial ablation procedure, respectively, stratified by a centre ablation volume of ≤100 ablation procedures per year (grey bars) or >100 ablation procedures per year (black bars), respectively. The constituents of overall and major complications are provided in Tables 1 and 2. Table cell values individually blinded by the Research Data Center (RDC) for reasons of data protection (‘not assessed’) tend to underestimate the true occurrence of complications. For the aggregate presentation of data in the figure, we, therefore, inferred blinded cell values from all available data in Tables 1 and 2. Only for values not exactly inferable, we assumed a value of ‘1’ as the minimum possible count. Comparisons calculated by Fisher’s exact tests.
To inform about possible explanations for the unexpectedly high rate of serious complications following isolated right atrial ablation including death, aggregate patient characteristics were compared between patients receiving any left vs. any right atrial ablation procedure (Table 3).
Table 3.
Aggregate patient characteristics
| Cryoablation (Group 1) (n = 5608) | PV isolation (Group 2) (n = 3167) | LA ablation (Group 3) (n = 8336) | LA + RA ablation (Group 4) (n = 2403) | Any left atrial ablation procedure (Groups 1–4) (n = 19 514) | Isolated right atrial ablation procedure (Group 5) (n = 13 871) | P-value (1–4 vs. 5) | |
|---|---|---|---|---|---|---|---|
| Age (years), mean ± SD | 62.9 ± 10.7 | 62.7 ± 10.6 | 63.7 ± 10.8 | 63.8 ± 10.9 | 63.3 ± 10.8 | 67.2 ± 11.9 | <0.001 |
| ≤50 years, n (%) | 741 (13.2) | 427 (13.5) | 997 (12.0) | 274 (11.4) | 2439 (12.5) | 1210 (8.7) | <0.001 |
| 51–60 years, n (%) | 1382 (24.6) | 816 (25.8) | 1959 (23.5) | 553 (23.0) | 4710 (24.1) | 2298 (16.6) | <0.001 |
| 61–70 years, n (%) | 1906 (34.0) | 1011 (31.9) | 2686 (32.2) | 773 (32.2) | 6376 (32.7) | 3870 (27.9) | <0.001 |
| 71–80 years, n (%) | 1497 (26.7) | 873 (27.6) | 2540 (30.5) | 760 (31.6) | 5670 (29.1) | 5226 (37.7) | <0.001 |
| >80 years, n (%) | 82 (1.5) | 40 (1.3) | 154 (1.8) | 43 (1.8) | 319 (1.6) | 1267 (9.1) | <0.001 |
| Males, n (%) | 3270 (58.3) | 1941 (61.3) | 5069 (60.8) | 1483 (61.7) | 11769 (60.3) | 9964 (71.8) | <0.001 |
| Paroxysmal atrial fibrillation, n (%) | 3893 (69.4) | 1907 (60.2) | 4428 (53.1) | 1048 (43.6) | 11276 (57.8) | 3850 (27.8) | <0.001 |
| Persistent atrial fibrillation, n (%) | 1755 (31.3) | 1226 (38.7) | 3508 (42.1) | 1133 (47.1) | 7622 (39.1) | 1602 (11.5) | <0.001 |
| Permanent atrial fibrillation, n (%) | 96 (1.7) | 37 (1.2) | 220 (2.6) | 36 (1.5) | 389 (2.0) | 314 (2.3) | 0.10 |
| Atrial flutter, n (%) | 312 (5.6) | 324 (10.2) | 1517 (18.2) | 1200 (49.9) | 3353 (17.2) | 12333 (88.9) | <0.001 |
| Hypertension, n (%) | 3484 (62.1) | 2062 (65.1) | 5230 (62.7) | 1461 (60.8) | 12238 (62.7) | 8894 (64.1) | 0.009 |
| Chronic obstructive pulmonary disease, n (%) | 152 (2.7) | 94 (3.0) | 279 (3.3) | 53 (2.2) | 578 (3.0) | 990 (7.1) | <0.001 |
| Pulmonary hypertension, n (%) | 105 (1.9) | 91 (2.9) | 252 (3.0) | 74 (3.1) | 522 (2.7) | 517 (3.7) | <0.001 |
| Obstructive sleep apnoea, n (%) | 184 (3.3) | 111 (3.5) | 285 (3.4) | 78 (3.2) | 658 (3.4) | 496 (3.6) | 0.32 |
| Hyperthyroidism, n (%) | 113 (2.0) | 93 (2.9) | 219 (2.6) | 40 (1.7) | 465 (2.4) | 302 (2.2) | 0.22 |
| Diabetes mellitus, n (%) | 562 (10.0) | 348 (11.0) | 973 (11.7) | 259 (10.8) | 2142 (11.0) | 2754 (19.9) | <0.001 |
| Chronic kidney disease, n (%) | 323 (5.8) | 174 (5.5) | 837 (10.0) | 193 (8.0) | 1527 (7.8) | 2066 (14.9) | <0.001 |
| Coronary artery disease, n (%) | 1108 (19.8) | 666 (21.0) | 182 (2.2) | 446 (18.6) | 4041 (20.7) | 4452 (32.1) | <0.001 |
| Dilative cardiomyopathy, n (%) | 146 (2.6) | 148 (4.7) | 402 (4.8) | 132 (5.5) | 828 (4.2) | 829 (6.0) | <0.001 |
| Heart failure, n (%) | 642 (11.4) | 395 (12.5) | 1418 (17) | 455 (18.9) | 2910 (14.9) | 3470 (25.0) | <0.001 |
| Aortic stenosis, n (%) | 88 (1.6) | 82 (2.6) | 177 (2.1) | 51 (2.1) | 398 (2.0) | 440 (3.2) | <0.001 |
| Mitral valve disease, n (%) | 313 (5.6) | 172 (5.4) | 549 (6.6) | 142 (5.9) | 1176 (6.0) | 936 (6.7) | 0.008 |
| Peripheral artery disease, n (%) | 55 (1.0) | 43 (1.4) | 82 (1.0) | 20 (0.8) | 200 (1.0) | 408 (2.9) | <0.001 |
| Duration of hospital stay (days), mean ± SD | 4.2 ± 3.7 | 4.1 ± 3.7 | 4.7 ± 4.4 | 4.6 ± 4.3 | 4.4 ± 4.1 | 5.1 ± 6.7 | <0.001 |
Statistical comparisons are calculated for two columns on the right-hand side. Among them, the left column includes any left atrial ablation procedure using any source of energy, the right column includes isolated right atrial ablation procedures using any source of energy. Comparisons using Fisher’s exact test or Student’s t-test as appropriate.
First, among patients undergoing left atrial ablations, no major differences were noted between Groups 1 through 4. As a trend however, cases with more complex left atrial ablation procedures (Groups 3 and 4) compared with pulmonary vein isolation procedures only (Groups 1 and 2), less likely presented with paroxysmal atrial fibrillation, but presented more likely with persistent and permanent atrial fibrillation or atrial flutter, and more likely had chronic kidney disease and heart failure. In addition, their hospital stays tended to be longer.
Second, patients receiving isolated right atrial procedures for atrial flutter were 3.9 years older on average, and were more frequently males. A significantly higher prevalence (P < 0.001 for all mentioned comparisons) of chronic obstructive pulmonary disease, pulmonary hypertension, diabetes mellitus, chronic kidney disease, coronary artery disease, dilative cardiomyopathy, heart failure, aortic stenosis, mitral valve disease, and peripheral artery disease were noted for those undergoing right atrial ablations. In addition, patients receiving right atrial ablations were characterized by significantly longer hospital stays.
Finally, we compared the aggregate characteristics of cases experiencing the major complications of death, pneumonia, cardiac arrest, and AV block III° to those without, following right atrial ablation (Table 4).
Table 4.
Aggregate characteristics of patients with and without in-hospital death, pneumonia, cardiac arrest, and AV block III° following isolated right atrial ablation
| Isolated right atrial ablation procedure |
P-value | Isolated right atrial ablation procedure |
P-value | Isolated right atrial ablation procedure |
P-value | Isolated right atrial ablation procedure |
P-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| With in-hospital death | Without in-hospital death | With pneumonia | Without pneumonia | With cardiac arrest | Without cardiac arrest | With AV block III° | Without AV block III° | |||||
| n = 47 | n = 13 824 | n = 282 | n = 13 589 | n = 49 | n = 13 822 | n = 223 | n = 13 648 | |||||
| Age (years), mean ± SD | 71.7 ± 9.6 | 67.3 ± 11.9 | 0.011 | 71.3 ± 9.6 | 67.2 ± 11.9 | <0.001 | 69.7 ± 9.9 | 67.3 ± 11.9 | 0.16 | 71.4 ± 12.1 | 67.2 ± 11.8 | <0.001 |
| ≤50 years, n (%) | 0 (0.0) | 1210 (8.8) | 0.032 | 7 (2.5) | 1203 (8.9) | <0.001 | n.a. | 1209 (8.7) | n.a. | 15 (6.7) | 1195 (8.8) | 0.34 |
| 51–60 years, n(%) | 4 (8.5) | 2294 (16.6) | 0.17 | 30 (10.6) | 2268 (16.7) | 0.006 | 5 (10.2) | 2293 (16.6) | 0.33 | 17 (7.6) | 2281 (16.7) | <0.001 |
| 61–70 years, n (%) | 17 (36.2) | 3853 (27.9) | 0.25 | 76 (27.0) | 3794 (27.9) | 0.79 | 17 (34.7) | 3853 (27.9) | 0.34 | 45 (20.2) | 3825 (28.0) | 0.01 |
| 71–80 years, n (%) | 16 (34.0) | 5210 (37.7) | 0.65 | 128 (45.4) | 5098 (37.5) | 0.008 | 19 (38.8) | 5207 (37.7) | 0.88 | 100 (44.8) | 5126 (37.6) | 0.031 |
| >80 years, n (%) | 10 (21.3) | 1257 (9.1) | 0.009 | 41 (14.5) | 1226 (9.0) | 0.003 | 6 (12.2) | 1261 (9.1) | 0.45 | 46 (20.6) | 1221 (8.9) | <0.001 |
| Males, n (%) | 36 (76.6) | 9828 (71.1) | 0.52 | 212 (75.2) | 9652 (71.0) | 0.14 | 36 (73.5) | 9828 (71.1) | 0.88 | 159 (71.3) | 9705 (71.1) | 1.00 |
| Paroxysmal atrial fibrillation, n (%) | 16 (34.0) | 3834 (27.7) | 0.33 | 97 (34.4) | 3753 (27.6) | 0.013 | 19 (38.8) | 3831 (27.7) | 0.11 | 59 (26.5) | 3791 (27.8) | 0.71 |
| Persistent atrial fibrillation, n (%) | 3 (6.4) | 1599 (11.6) | 0.36 | 51 (18.1) | 1551 (11.4) | 0.001 | 12 (24.5) | 1590 (11.5) | 0.011 | 34 (15.2) | 1568 (11.5) | 0.09 |
| Permanent atrial fibrillation, n (%) | 7 (14.9) | 307 (2.2) | <0.001 | 28 (9.9) | 286 (2.1) | <0.001 | 4 (8.2) | 310 (2.2) | 0.025 | 14 (6.3) | 300 (2.2) | 0.001 |
| Atrial flutter, n (%) | 36 (76.6) | 12 297 (89.0) | 0.016 | 231 (81.9) | 12 102 (89.1) | <0.001 | 36 (73.5) | 12 297 (89) | 0.002 | 181 (81.2) | 12 152 (89.0) | 0.001 |
| Hypertension, n (%) | 27 (57.4) | 8867 (64.1) | 0.36 | 184 (65.2) | 8710 (64.1) | 0.71 | 35 (71.4) | 8859 (64.1) | 0.37 | 159 (71.3) | 8735 (64.0) | 0.024 |
| Chronic obstructive pulmonary disease, n (%) | 10 (21.3) | 980 (7.1) | 0.001 | 66 (23.4) | 924 (6.8) | <0.001 | 9 (18.4) | 981 (7.1) | 0.007 | 19 (8.5) | 971 (7.1) | 0.43 |
| Pulmonary hypertension, n (%) | 5 (10.6) | 512 (3.7) | 0.03 | 33 (11.7) | 484 (3.6) | <0.001 | 6 (12.2) | 511 (3.7) | 0.009 | 18 (8.1) | 499 (3.7) | 0.002 |
| Obstructive sleep apnoea, n (%) | n.a. | 495 (3.6) | n.a. | 18 (6.4) | 478 (3.5) | 0.021 | 3 (6.1) | 493 (3.6) | 0.26 | 20 (9.0) | 476 (3.5) | <0.001 |
| Hyperthyroidism, n (%) | 3 (6.4) | 299 (2.2) | 0.08 | 11 (3.9) | 291 (2.1) | 0.059 | n.a. | 301 (2.2) | n.a. | 5 (2.2) | 297 (2.2) | 0.82 |
| Diabetes mellitus, n (%) | 21 (44.7) | 2733 (19.8) | <0.001 | 81 (28.7) | 2673 (19.7) | <0.001 | 20 (40.8) | 2734 (19.8) | 0.001 | 57 (25.6) | 2697 (19.8) | 0.034 |
| Chronic kidney disease, n (%) | 23 (48.9) | 2043 (14.8) | <0.001 | 109 (38.7) | 1957 (14.4) | <0.001 | 21 (42.9) | 2045 (14.8) | <0.001 | 59 (26.5) | 2007 (14.7) | <0.001 |
| Coronary artery disease, n (%) | 27 (57.4) | 4425 (32.0) | <0.001 | 145 (51.4) | 4307 (31.7) | <0.001 | 33 (67.3) | 4419 (32) | <0.001 | 99 (44.4) | 4353 (31.9) | <0.001 |
| Dilative cardiomyopathy, n (%) | 5 (10.6) | 824 (6.0) | 0.20 | 36 (12.8) | 793 (5.8) | <0.001 | 5 (10.2) | 824 (6.0) | 0.22 | 26 (11.7) | 803 (5.9) | 0.001 |
| Heart failure, n (%) | 31 (66.0) | 3439 (24.9) | <0.001 | 182 (64.5) | 3288 (24.2) | <0.001 | 33 (67.3) | 3437 (24.9) | <0.001 | 87 (39.0) | 3383 (24.8) | <0.001 |
| Aortic stenosis, n (%) | 3 (6.4) | 437 (3.2) | 0.19 | 19 (6.7) | 421 (3.1) | 0.003 | 3 (6.1) | 437 (3.2) | 0.20 | 13 (5.8) | 427 (3.1) | 0.032 |
| Mitral valve disease, n (%) | 8 (17.0) | 928 (6.7) | 0.012 | 39 (13.8) | 897 (6.6) | <0.001 | 12 (24.5) | 924 (6.7) | <0.001 | 17 (7.6) | 919 (6.7) | 0.59 |
| Peripheral artery disease, n (%) | 6 (12.8) | 402 (2.9) | 0.002 | 22 (7.8) | 386 (2.8) | <0.001 | 10 (20.4) | 398 (2.9) | <0.001 | 10 (4.5) | 398 (2.9) | 0.16 |
| Duration of hospital stay (days), mean ± SD | 26.4 ± 19.1 | 5.1 ± 5.8 | <0.001 | 21.7 ± 17.7 | 4.8 ± 5.6 | <0.001 | 29.5 ± 30.9 | 5.1 ± 5.8 | <0.001 | 9.6 ± 12.8 | 5.1 ± 5.8 | <0.001 |
Statistical comparisons are calculated for the respective subgroups of patients with or without the complication of interest. Comparisons using Fisher’s exact test or Student’s t-test as appropriate.
n.a., not assessed (indicates table cell with too few observations for display due to data protection policies).
The 47 patients dying in hospital were 4.4 years older on average, including 10 individuals more than 80 years old. Of the 47 patients, 31 had heart failure, 27 had coronary artery disease, 23 had chronic kidney disease, 21 had diabetes mellitus, 10 had chronic obstructive pulmonary disease, 8 had mitral valve disease, 6 had peripheral artery disease, and 5 had pulmonary artery hypertension (see left column of Table 4, all significantly different from controls). A similar pattern of highly prevalent comorbidity was observed in patients experiencing cardiac arrest and pneumonia, and to a somewhat lower degree in patients with AV block III° (see Table 4).
Discussion
We present nationwide data regarding complications of catheter ablation for atrial fibrillation and atrial flutter in a ‘real world scenario’ of all patients undergoing these procedures in Germany. Our results, unbiased by insurance status, geographic substratification, or providing institution, are based on administrative data of all relevant complications in a total of 33 385 cases by analysis of mandatory coding for renumeration of all hospitals for Germany in 2014.
Atrial fibrillation
One major finding of our study is a high overall in-hospital complication rate ranging from a mean of 11.7% to 13.8% depending on the site and energy applied for left atrial ablation procedures, and a major complication rate ranging from a mean of 3.8% to 7.2%. In the literature, periprocedural complication rates are reported to range from 1% to 8%, depending on the type of study and definitions of complications.3,22,23 Retrospective clinical studies conducted at experienced centres and registries focusing on major complications have reported lower rates between 3.9% and 4.5%.4,10 Yet, the incidence of vascular complications alone may vary markedly23 with female sex, age >75 years, and presence of hypertensive heart disease being associated with a higher risk for complications.4,5,24 Interestingly, a nationwide cohort study of administrative data like ours, performed in the earlier years of catheter ablation in the USA, showed a combined vascular complication rate of 3.4%, consisting of haemorrhage, haematoma, vascular complications requiring surgical repair, and accidental arterial puncture.16 Over a time period from 2000 to 2011, the rate of complications increased from 5.3% to 7.5%.16 Even in elderly Medicare beneficiaries, no more than 4.1% of patients experienced serious complications in one study, 17 whereas an overall complication rate of 9.1%, increasing from 6.7% in 2001 to 10.1% in 2006 was noted in another.18 It is tempting to assume that the relatively high rate of access site complications in our study of 2014 also reflect the adverse consequences of current strategies to maintain strict anticoagulation throughout the procedure for prevention of stroke.
Of note, our assessment of both overall and major in-hospital complications did not reveal any relevant differences between cryo-energy and RF-energy used for PV isolation, well in accordance with a recent large randomized clinical trial where the primary safety endpoint, a composite of death, cerebrovascular events, and serious treatment-related adverse events after a mean-follow-up of 1.5 years, was also not different.15
Our report eliminates any patient selection bias through administrative data analysis of a complete data set of a large, unselected, and consecutive patient population in an entire country. It focusses on a recent data basis, and evaluates all complications using a uniform coding system for all hospitals in the country, thereby best representing the ‘real world’ in the year 2014 in Germany.
Our data also indicate that in institutions with an ablation volume of >100 left atrial ablation procedures per year, the overall complication rate is significantly lower than in low volume centres as a surrogate of different levels of experience; however, this reduction was not seen for major complications. These data partially confirm prior work,10,12,16 and further substantiate consensus recommendations to restrict left atrial ablation to centres with a volume of >100 procedures per year.2,25–27
Of note, only a minority of 0.2%, 1.5%, and 3.7% of interventions, respectively, were performed in institutions with a left atrial ablation volume of ≤25, 26–50, and 51–100 procedures per year, respectively. This is in sharp contrast to data from the United States for the years 2000–2010, where 68.2% and 85, 6% of procedures were performed in hospitals with a volume of ≤50 and ≤100 procedures per year.16 Our data do not allow to analyse the effect of ablation volume per individual operator as it has been reported in the US study.16 Nevertheless, from the standpoint of safety it is rewarding to see that the vast majority (>90%) of procedures is actually being performed in high volume centres.
Atrial flutter
Cavo-tricuspid isthmus ablation for the treatment of atrial flutter is a seemingly easy-to-do procedure associated with a low risk of acute complications of 2.6%, based on a meta-analysis of 158 studies with limitations due to patient selection and reporting bias.20 Our study of 13 871 unselected patients for atrial flutter ablation revealed an overall rate of complications slightly lower than for left atrial ablations. However, more frequent serious complications were noted quite unexpectedly (Table 1). Our definition of atrial flutter included typical and atypical flutter, as well as atrial flutter and fibrillation. Sinus tachycardia, atrial tachycardia, AV nodal and AV re-entry tachycardias were excluded. The German OPS system only allows to code ‘right atrial ablation’. Therefore, non-isthmus-dependent right atrial flutter cases are included as one of the possible explanations for the higher complication rates in our atrial flutter cases. Lower rates of pericardial effusions and access site complications compared with longer-lasting left atrial ablation procedures with tight control of anticoagulation regimen appear comprehensible (see Figure 2), and higher rates of AV block III° may be explained by the proximity of the right atrial isthmus and the AV junction. The occurrence of a few cases of phrenic nerve injury, though significantly less frequent compared with left atrial ablation, can be interpreted by our definition of atrial flutter: given the anatomic variability of the phrenic nerve, injury may occur during isolation of the superior vena cava or possibly also during ablation of scar-related, non-isthmus-dependent right atrial flutter.28,29 However, the significantly increased rates of pneumonia, cardiac arrest, and—most importantly—in-hospital death, are less intuitively understood. In contrast to left atrial ablations, where a presumed higher experience accounted for a reduction of at least overall complications in high volume centres, the centre ablation volume for right atrial ablations—generally considered straightforward—appeared not to be a safety factor for both overall and major complications (Figure 3). In fact, 40 of the 47 in-hospital deaths occurred in high volume centres with >100 isolated right atrial ablation cases per year. Based on our data, one may speculate whether the absence of an expected reduction of complication rates in high volume centres—as seen for right atrial ablations and regarding major complications also for left atrial ablations—is in part due to higher work load, heterogeneous training levels among the ablationist staff, more aggressive procedure protocols, and more challenging anticoagulation strategies. However, the most likely explanation for high rates of in-hospital death, pneumonia, cardiac arrest, and AV block III° after isolated right atrial ablations is older patient age combined with advanced cardiac, pulmonary, and vascular comorbidities, as shown in Tables 3 and 4. A meta-analysis in 2009 on 10 719 patients with common atrial flutter and a mean age of 59.8 ± 0.5 years reported an acute complication rate of 2.6%.20 It appears that because of this perceived low risk and the straightforward procedure of cavo-tricuspid isthmus ablation for atrial flutter, also older patients with relevant comorbidities are nowadays accepted in general for ablation. This becomes evident in our study by a mean age of 67.2 ± 11.9 years, compared with a mean age of 63.3 ± 10.8 years for patients undergoing left atrial ablation (Table 3). Patients actually affected by serious complications had an even higher mean age (Table 4). Our study is the first to demonstrate an excess of complications associated with right atrial flutter ablation in the elderly. Hopefully, our results of a complete nationwide set of aggregate, anonymized data will prompt clinical studies investigating the specific causes and circumstances of complications in elderly patients with comorbidities undergoing catheter ablation for atrial flutter.
Strengths and limitations of this analysis
Analysing all left and right atrial ablation cases in Germany for 2014, the bias of patient selection inherent to retrospective studies, voluntary registries, and controlled trials is avoided, and thus a nationwide ‘real world’ situation is presented. As in previous studies, overall and major complications were calculated as the cumulative sum of individual complications. However, as one single patient may also experience two or more complications, the percentage of affected patients may be somewhat smaller compared with the presented rates of overall and major complications. One limitation is that our analysis of administrative data may be subject to miscoding of ICD codes. However, between 15% and 20% of all annual cases in the cardiovascular field are re-evaluated by independent physicians from health insurance providers to assure appropriate coding and thereby correct reimbursement. Any omition of procedure codes is unlikely since this would have a direct and markedly negative effect on hospital reimbursement. Systematic overcoding should not escape independent physician control, but cannot entirely be excluded. Further, our analysis handles raw aggregate, anonymized data, cannot rely on individual patient information, and precludes any adjustment for covariates. Table cells with too few observations were blinded administratively by the RDC to ensure anonymity, and this process may lead to an underestimation of complication rates in small groups of patients. Finally, only in-hospital complications are considered in our analysis. Thus, any serious complications manifesting after hospital discharge, like atrio-oesophageal fistula, delayed pericardial tamponade, and pulmonary vein stenosis is overlooked. Also, if a patient is not discharged home, but transferred to another in-hospital department, for example for emergency cardiac surgery, the outcome is not reported. Therefore, this analysis might have underestimated some serious complications including death, which might have occurred after hospital discharge or after transferral to another hospital or medical department.
Conclusions
In an administrative data analysis of all patients undergoing ablation of atrial fibrillation in Germany in 2014, both the overall and major complication rates are higher than reported previously. Similar analyses in different European countries are strongly encouraged. In Germany, only a minority of patients were treated in low ablation volume hospitals with a procedure volume ≤100 per year, and vice versa, the vast majority received care in high volume centres. This went along with a reduction of overall complications. Isolated right atrial ablation for atrial flutter when compared with left atrial ablations was associated with a somewhat lower overall complication rate, but was associated with a hitherto unknown high rate of serious or life-threatening complications including cardiac arrest, pneumonia, AV block III°, and an up to four times higher rate of in-hospital death. This is most probably due to the fact that because of the seemingly low risk attributed to right atrial flutter ablations reported in the literature in younger patients, the indications for such ablations of atrial flutter have been extended in Germany to more elderly patients with an advanced comorbidity profile. Hence, a word of caution appears warranted in this respect.
Supplementary Material
Acknowledgements
We thank Melanie Scheller, Research Data Center (RDC) of the Federal Statistical Office and the Statistical Offices of the Federal States in Wiesbaden, Germany, for her continuous and efficient support and advice during the conduct of this study.
Conflict of interest : G.S. was reimbursed for travel expenses and congress fees by Medtronic and Biotronik and is financially compensated for consultation by BMS and for his work in DSMBs by Biotronik. H.R. has received speaker honoraria from BMS, MedUpdate, NephroUpdate, and Pfizer. He has acted as a consultant for BMS, Pfizer and Pluristem receiving in part also financial compensations for this work. He has received research grants from the German Federal Ministry for Education and Research (BMBF). His division within the University Hospital of Muenster has taken or is still taking part in multicentre trials of Bard, Bayer, Biotronik, and Pluristem, receiving patient fees and financial compensation for these efforts. All other authors declared no conflict of interest related to the study.
References
- 1. Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J.. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659–666. [DOI] [PubMed] [Google Scholar]
- 2. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GY, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K.. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 3. Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Packer D, Skanes A.. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation 2005;111:1100–1105. [DOI] [PubMed] [Google Scholar]
- 4. Dagres N, Hindricks G, Kottkamp H, Sommer P, Gaspar T, Bode K, Arya A, Husser D, Rallidis LS, Kremastinos DT, Piorkowski C.. Complications of atrial fibrillation ablation in a high-volume center in 1,000 procedures: still cause for concern?. J Cardiovasc Electrophysiol 2009;20:1014–1019. [DOI] [PubMed] [Google Scholar]
- 5. Shah RU, Freeman JV, Shilane D, Wang PJ, Go AS, Hlatky MA.. Procedural complications, rehospitalizations, and repeat procedures after catheter ablation for atrial fibrillation. J Am Coll Cardiol 2012;59:143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gupta A, Perera T, Ganesan A, Sullivan T, Lau DH, Roberts-Thomson KC, Brooks AG, Sanders P.. Complications of catheter ablation of atrial fibrillation: a systematic review. Circ Arrhythm Electrophysiol 2013;6:1082–1088. [DOI] [PubMed] [Google Scholar]
- 7. Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Natale A, Packer D, Ricci C, Skanes A, Ranucci M.. Delayed cardiac tamponade after radiofrequency catheter ablation of atrial fibrillation: a worldwide report. J Am Coll Cardiol 2011;58:2696–2697. [DOI] [PubMed] [Google Scholar]
- 8. Nair GM, Nery PB, Redpath CJ, Lam BK, Birnie DH.. Atrioesophageal fistula in the era of atrial fibrillation ablation: a review. Can J Cardiol 2014;30:388–395. [DOI] [PubMed] [Google Scholar]
- 9. Michowitz Y, Rahkovich M, Oral H, Zado ES, Tilz R, John S, Denis A, Di Biase L, Winkle RA, Mikhaylov EN, Ruskin JN, Yao Y, Josephson ME, Tanner H, Miller JM, Champagne J, Della Bella P, Kumagai K, Defaye P, Luria D, Lebedev DS, Natale A, Jais P, Hindricks G, Kuck KH, Marchlinski FE, Morady F, Belhassen B.. Effects of sex on the incidence of cardiac tamponade after catheter ablation of atrial fibrillation: results from a worldwide survey in 34 943 atrial fibrillation ablation procedures. Circ Arrhythm Electrophysiol 2014;7:274–280. [DOI] [PubMed] [Google Scholar]
- 10. Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Natale A, Packer D, Skanes A, Ambrogi F, Biganzoli E.. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010;3:32–38. [DOI] [PubMed] [Google Scholar]
- 11. Arbelo E, Brugada J, Hindricks G, Maggioni AP, Tavazzi L, Vardas P, Laroche C, Anselme F, Inama G, Jais P, Kalarus Z, Kautzner J, Lewalter T, Mairesse GH, Perez-Villacastin J, Riahi S, Taborsky M, Theodorakis G, Trines SA; Atrial Fibrillation Ablation Pilot Study I. The atrial fibrillation ablation pilot study: a European Survey on Methodology and results of catheter ablation for atrial fibrillation conducted by the European Heart Rhythm Association. Eur Heart J 2014;35:1466–1478. [DOI] [PubMed] [Google Scholar]
- 12. Chen J, Dagres N, Hocini M, Fauchier L, Bongiorni MG, Defaye P, Hernandez-Madrid A, Estner H, Sciaraffia E, Blomstrom-Lundqvist C; Scientific Initiatives Committee of the European Heart Rhythm Association EHRA. Catheter ablation for atrial fibrillation: results from the first European Snapshot Survey on Procedural Routines for Atrial Fibrillation Ablation (ESS-PRAFA) Part II. Europace 2015;17:1727–1732. [DOI] [PubMed] [Google Scholar]
- 13. Cosedis Nielsen J, Johannessen A, Raatikainen P, Hindricks G, Walfridsson H, Kongstad O, Pehrson S, Englund A, Hartikainen J, Mortensen LS, Hansen PS.. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med 2012;367:1587–1595. [DOI] [PubMed] [Google Scholar]
- 14. Di Biase L, Burkhardt JD, Santangeli P, Mohanty P, Sanchez JE, Horton R, Gallinghouse GJ, Themistoclakis S, Rossillo A, Lakkireddy D, Reddy M, Hao S, Hongo R, Beheiry S, Zagrodzky J, Rong B, Mohanty S, Elayi CS, Forleo G, Pelargonio G, Narducci ML, Russo AD, Casella M, Fassini G, Tondo C, Schweikert RA, Natale A.. Periprocedural stroke and bleeding complications in patients undergoing catheter ablation of atrial fibrillation with different anticoagulation management: results from the Role of Coumadin in Preventing Thromboembolism in Atrial Fibrillation (AF) Patients Undergoing Catheter Ablation (COMPARE) randomized trial. Circulation 2014;129:2638–2644. [DOI] [PubMed] [Google Scholar]
- 15. Kuck KH, Brugada J, Furnkranz A, Metzner A, Ouyang F, Chun KR, Elvan A, Arentz T, Bestehorn K, Pocock SJ, Albenque JP, Tondo C; FIRE and ICE Investigators. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016;374:2235–2245. [DOI] [PubMed] [Google Scholar]
- 16. Deshmukh A, Patel NJ, Pant S, Shah N, Chothani A, Mehta K, Grover P, Singh V, Vallurupalli S, Savani GT, Badheka A, Tuliani T, Dabhadkar K, Dibu G, Reddy YM, Sewani A, Kowalski M, Mitrani R, Paydak H, Viles-Gonzalez JF.. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: analysis of 93 801 procedures. Circulation 2013;128:2104–2112. [DOI] [PubMed] [Google Scholar]
- 17. Piccini JP, Sinner MF, Greiner MA, Hammill BG, Fontes JD, Daubert JP, Ellinor PT, Hernandez AF, Walkey AJ, Heckbert SR, Benjamin EJ, Curtis LH.. Outcomes of Medicare beneficiaries undergoing catheter ablation for atrial fibrillation. Circulation 2012;126:2200–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ellis ER, Culler SD, Simon AW, Reynolds MR.. Trends in utilization and complications of catheter ablation for atrial fibrillation in Medicare beneficiaries. Heart Rhythm 2009;6:1267–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Halligan SC, Gersh BJ, Brown RD Jr, Rosales AG, Munger TM, Shen WK, Hammill SC, Friedman PA.. The natural history of lone atrial flutter. Ann Intern Med 2004;140:265–268. [DOI] [PubMed] [Google Scholar]
- 20. Perez FJ, Schubert CM, Parvez B, Pathak V, Ellenbogen KA, Wood MA.. Long-term outcomes after catheter ablation of cavo-tricuspid isthmus dependent atrial flutter: a meta-analysis. Circ Arrhythm Electrophysiol 2009;2:393–401. [DOI] [PubMed] [Google Scholar]
- 21. Della BP, Fraticelli A, Tondo C, Riva S, Fassini G, Carbucicchio C.. Atypical atrial flutter: clinical features, electrophysiological characteristics and response to radiofrequency catheter ablation. Europace 2002;4:241–253. [DOI] [PubMed] [Google Scholar]
- 22. Bertaglia E, Zoppo F, Tondo C, Colella A, Mantovan R, Senatore G, Bottoni N, Carreras G, Coro L, Turco P, Mantica M, Stabile G.. Early complications of pulmonary vein catheter ablation for atrial fibrillation: a multicenter prospective registry on procedural safety. Heart Rhythm 2007;4:1265–1271. [DOI] [PubMed] [Google Scholar]
- 23. Calkins H, Hindricks G, Cappato R, Kim Y-H, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, Chen P-S, Chen S-A, Chung MK, Nielsen JC, Curtis AB, Davies DW, Day JD, d’Avila A, de Groot NMSN, Di Biase L, Duytschaever M, Edgerton JR, Ellenbogen KA, Ellinor PT, Ernst S, Fenelon G, Gerstenfeld EP, Haines DE, Haissaguerre M, Helm RH, Hylek E, Jackman WM, Jalife J, Kalman JM, Kautzner J, Kottkamp H, Kuck KH, Kumagai K, Lee R, Lewalter T, Lindsay BD, Macle L, Mansour M, Marchlinski FE, Michaud GF, Nakagawa H, Natale A, Nattel S, Okumura K, Packer D, Pokushalov E, Reynolds MR, Sanders P, Scanavacca M, Schilling R, Tondo C, Tsao H-M, Verma A, Wilber DJ, Yamane T.. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. Europace 2018;20:157–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoffmann BA, Kuck KH, Andresen D, Spitzer SG, Hoffmann E, Schumacher B, Eckardt L, Brachmann J, Becker R, Steven D, Rostock T, Junger C, Senges J, Willems S.. Impact of structural heart disease on the acute complication rate in atrial fibrillation ablation: results from the German Ablation Registry. J Cardiovasc Electrophysiol 2014;25:242–249. [DOI] [PubMed] [Google Scholar]
- 25. Calkins H, Brugada J, Packer DL, Cappato R, Chen S-A, Crijns HJG, Damiano RJ, Davies DW, Haines DE, Haissaguerre M, Iesaka Y, Jackman W, Jais P, Kottkamp H, Kuck KH, Lindsay BD, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Natale A, Pappone C, Prystowsky E, Raviele A, Ruskin JN, Shemin RJ, Calkins H, Brugada J, Chen S-A, Prystowsky EN, Kuck KH, Natale A, Haines DE, Marchlinski FE, Calkins H, Davies DW, Lindsay BD, McCarthy PM, Packer DL, Cappato R, Crijns HJG, Damiano RJ, Haissaguerre M, Jackman WM, Jais P, Iesaka Y, Kottkamp H, Mont L, Morady F, Nademanee K, Pappone C, Raviele A, Ruskin JN, Shemin RJ; Heart Rhythm Society; European Heart Rhythm Association; European Cardiac Arrhythmia Society; American College of Cardiology; American Heart Association; Society of Thoracic Surgeons. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation developed in partnership with the European Heart Rhythm Association (EHRA) and the European Cardiac Arrhythmia Society (ECAS); in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Endorsed and approved by the governing bodies of the American College of Cardiology, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, and the Heart Rhythm Society. Europace 2007;9:335–379. [DOI] [PubMed] [Google Scholar]
- 26. Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P; ESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012;33:2719–2747. [DOI] [PubMed] [Google Scholar]
- 27. Camm AJ, Kirchhof P, Lip GYH, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le Heuzey J-Y, Ponikowski P, Rutten FH.. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369–2429. [DOI] [PubMed] [Google Scholar]
- 28. Sacher F, Monahan KH, Thomas SP, Davidson N, Adragao P, Sanders P, Hocini M, Takahashi Y, Rotter M, Rostock T, Hsu LF, Clementy J, Haissaguerre M, Ross DL, Packer DL, Jais P.. Phrenic nerve injury after atrial fibrillation catheter ablation: characterization and outcome in a multicenter study. J Am Coll Cardiol 2006;47:2498–2503. [DOI] [PubMed] [Google Scholar]
- 29. Garan H. Atypical atrial flutter. Heart Rhythm 2008;5:618–621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.