Abstract
This study investigates the impact of seasonal variation on the chemical composition of essential oils from the leaves of Porcelia macrocarpa (Annonaceae) obtained over the course of one year (January–December 2011) and the chemical composition of the essential oils obtained from the ripe fruits of the same plant. Furthermore, the essential oils of the leaves were investigated with respect to their antimicrobial activity. The essential oils of the leaves contain a mixture of monoterpenes, one diterpene and several sesquiterpenes. The main components were identified as the sesquiterpenes germacrene D (29%–50%) and bicyclogermacrene (24%–37%). No significant variation was observed for the composition of the essential oil of the leaves over the course ofthe year, except for the month of November, when the ripe fruit were collected. In this month, substantially decreased concentrations of germacrene D (28.8 ± 0.8%) and bicyclogermacrene (23.9 ± 0.6%) were measured and the emergence of spathulenol (10.4 ± 0.2%) was observed. The essential oils extracted from the ripe fruit revealed the presence of a variety of monoterpenes, sesquiterpenes and hydrocarbons. The main constituents of these oils were neryl (8.8 ± 0.2%) and geranyl (27.3 ± 0.7%) formates, γ-muurolene (10.3 ± 0.9%) and dendrolasin (8.23 ± 0.06%). The antimicrobial activity of the essential oil obtained from the leaves of P. macrocarpa towards a range of bacterial and yeast strains was examined. In order to determine the minimum inhibitory concentration (MIC) of essential oils obtained from the January collection of the leaves, broth microdilution assays were carried out, which showed a significant antimicrobial activity towards Cryptococcus neoformans serotypes A and D as well as C. gattii serotypes B and C.
Keywords: Porcelia macrocarpa, essential oils, seasonal variation, antimicrobial activity, C. neoformans
1. Introduction
Porcelia macrocarpa R.E. Fries (Annonaceae) is a tree found in the forest regions on the Atlantic coast and in the interior of Brazil [1]. The chemical composition of various parts of P. macrocarpa has already been the subject of several scientific studies. High contents of acetylene acetogenins were found in the extracts from seeds [2], while several amides, lignanamides, and alkaloids were isolated from branch extracts [3,4,5]. The branches also contained several interesting polar compounds, such as flavonoids, trimethylamonium salts and amino acids [6]. Essential oils extracted from the leaves were analyzed by GC/MS which allowed the identification of nine individual components, of which bicyclogermacrene (27.5%) and germacrene D (37.8%) were the major components [7]. A desirable cytotoxic activity against human tumor cells was observed for these essential oils [8].
Essential oils are not only used in several therapeutic applications of folk medicine, but they also form part of a variety of modern pharmaceutical remedies [9]. However, a meticulous qualitative as well as quantitative analysis of these essential oils is of the utmost importance, since the quality of the macroscopic remedy is highly sensitive towards several factors, e.g., climatic conditions, the chemo- and biotype as well as the phenology of the plant [10]. Important biological activities have already been reported for essential oils of several species of Annonaceae [11] and our group has investigated the composition and biological activity of essential oils obtained from several other Brazilian plants [12,13,14]. The promising results of these studies encouraged us to examine the influence of the seasonal variation on the chemical composition of the essential oils of the leaves of P. macrocarpa. Furthermore, we were interested in a comparison between the chemical composition of these essential oils with the chemical composition of essential oils obtained from the ripe fruits of the same plant and an examination of the antimicrobial activity of leaves oil.
2. Results and Discussion
2.1. Microclimatic Factors
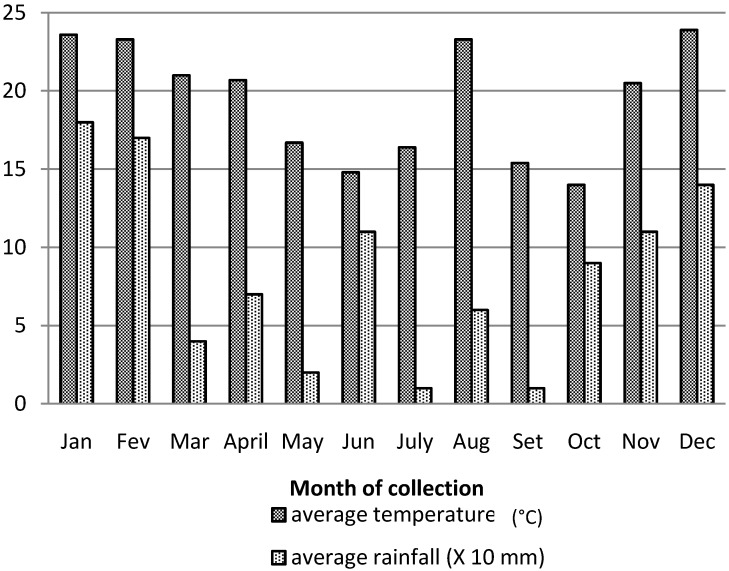
Generally, the temperature pattern for Sao Paulo city can be divided into two periods: a colder period from ca. April–October and a warmer period from November–March. The precipitation pattern is inversely proportional to the temperature profile and minimum rainfall can usually be observed between the months of April–September. The temperature for the collection period of 2011 (see Figure 1) basically follows these temperature/precipitation averages, except for August 2011, which was unusually hot, and June 2011, which showed unusually high precipitation [15].
Figure 1.
Average monthly temperatures (°C) and precipitation (×10 mm) values in 2011 (collection period).
For the collection period of the leaves of P. macrocarpa, the average temperature of the warmer period (January–April and November–December) was 22.2 °C. The average temperature of the colder period from May–October was 16.7 °C. The precipitation pattern showed a maximum around December–February (monthly average of 150 mm) and a minimum around May, July and September (average of 10–20 mm).
2.2. Extraction Yields of the Essential Oils from the Leaves of P. macrocarpa
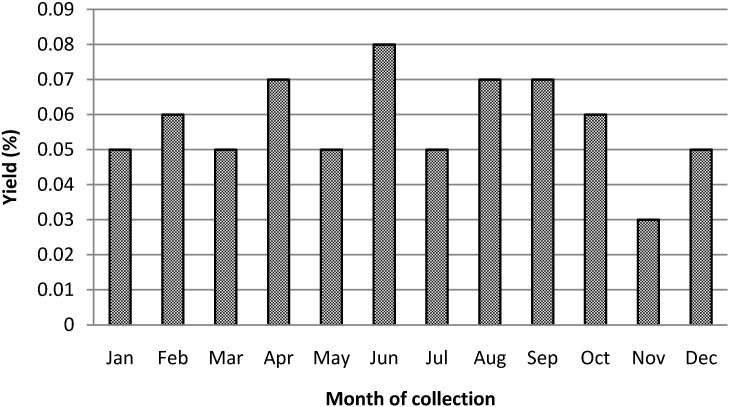
Essential oils were extracted from the leaves by hydro-distillation as described in the Experimental section (Section 3.4) and the extraction yields are summarized in Figure 2. We found that extraction yields of essential oils from the leaves of P. macrocarpa are subject to seasonal changes, reflected in high yields obtained during the months of April (0.07 ± 0.04%), June (0.08 ± 0.02%), August (0.07 ± 0.01%) and September (0.07 ± 0%). Interestingly, the extraction yield decreased in November (0.03 ± 0%), which typically coincides with the month of fructification for this species. An impact of environmental factors on the yields of essential oils was also observed for several species such as Rosmarinus officinalis [16], Mentha suaveolens [17], Satureja horvatii [18], and Baccharis trimera [19]. The influence of several other climatic factors such as temperature and pluviometric index onto the extraction yield also needs to be taken into consideration [10]. For example, the observed precipitation during January and February was significantly higher compared to the other months of 2011. In contrast to that, the temperature remained relatively constant over the course of the study, except for the months of May, June and October. As shown in Figure 2, the maximum oil yields were obtained around June and minimum yields were obtained in December/January. This would suggest an inversely proportional relation between oil extraction yields and the temperature and precipitation pattern. Similar findings have been reported by Gazim et al. [20], who showed that the amount of several volatile compounds produced by Tetradenia riparia (Lamiaceae) were affected by temperature, humidity and rainfall changes over the course of different seasons. Moreover, Lago et al. reported that the relative amounts of essential oils from the leaves of Pittosporium undulatum could be better correlated to microclimatic parameters such as temperature and precipitation index, than to the phenology of the studied species [21]. The yield of essential oils from the fruit of P. macrocarpa, obtained during November 2011 was 0.05 ± 0.01% and is comparable to the average yield (0.06 ± 0.02%) obtained from the leaves (see Figure 2).
Figure 2.
Monthly extraction yields of the essential oils from the leaves of Porcelia macrocarpa (January–December/2011).
2.3. Chemical Composition of the Essential Oils Obtained from the Leaves and the Ripe Fruit of P. macrocarpa
The crude essential oils obtained from the leaves and the ripe fruits of P. macrocarpa were analyzed by GC (DB-5 capillary column) and GC-MS. Individual compounds were assigned according to their Kovats indices in conjunction with a comparison of the experimentally obtained mass spectra to those described in library (NIST 107) and in the literature [22]. In the essential oils of the leaves, nine individual components were observed and identified as verbanyl acetate (monoterpene), α-copaene, iso-longifolene, β-cedrene, α-guainene, germacrene D, bicyclogermacrene, and spathulenol (sesquiterpenes) as well as phytol (diterpene). The main compounds were germacrene D (28.8 ± 0.8%–49.6 ± 0.7%) and bicyclogermacrene (23.9 ± 0.6%–36.8 ± 0.5%). The sum of these nine components accounted for between 66.6 ± 0.1% (November) and 91.1 ± 0.6% (April) of the total oil content (see Table 1). The overall composition was similar to previously described samples [7,8]. A significant decrease (p < 0.05) of the relative amounts of germacrene D (28.8 ± 0.8%) and bicyclo-germacrene (23.9 ± 0.6%) was detected during November 2011, concomitant with the emergence of spathulenol (10.4 ± 0.2%), which could not be detected in any other month. As reported by Bülow and Köning, the presence of spathulenol and the reduced amounts of germacrene D and bicyclogermacrene could be partially explained by biochemical factors, such as the enzymatic oxidation of the latter compounds to form spathulenol [23]. This process could therefore be related directly to the phenology of P. macrocarpa, since it coincides with the end of the fructification period. However, it is worth pointing out here, that during the sterile period, the relative amounts of germacrene D and bicyclogermacrene were also non-constant (67 ± 7% in February to 84 ± 2% in April), which could be attributed to microclimatic factors, e.g., temperature and precipitation [24]. As shown in Table 1 and Figure 2, the sum of relative proportion of both sesquiterpenes was lower in January, February and December (71 ± 7%, 67 ± 7% and 78.9 ± 0.7%, respectively) compared to the other months. For these months, relatively high precipitation values (14–18 mm) and temperatures (23–24 °C) were observed. These observations suggest an influence of these microclimatic factors on the production and/or accumulation of germacrene D and bicyclogermacrene in the crude essential oils.
Table 1.
Seasonal variation of the chemical composition of essential oils obtained from the leaves of Porcelia macrocarpa (monthly collection from January to December 2011).
| Relative amount (%) b | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KI | January | February | March | April | May | June | July | August | September | October | November | December | |
| verbanyl acetate | 1340 | 0.39 ± 0.04 a | 0.43 ± 0.01 a | 0.40 ± 0.01 a | 0.46 ± 0.06 a | 0.51 ± 0.02 a | 0.42 ± 0.01 a | 0.42 ± 0.04 a | 0.41 ± 0.03 a | 1.76 ± 0.96 a | 0.34 ± 0.01 a | 0.31 ± 0.19 a | 0.38 ± 0.01 a |
| α-copaene | 1376 | 2.0 ± 0.3 a | 1.6 ± 0.9 a | 2.05 ± 0.07 a | 2.2 ± 0.2 a | 1.9 ± 0.8 a | 1.95 ± 0.02 a | 2.01 ± 0.09 a | 1.8 ± 0.2 a | 2.4 ± 0.9 a | 1.71 ± 0.01 a | 0.4 ± 0.2 b | 2.07 ± 0.05 a |
| iso-longifolene | 1387 | 1.4 ± 0.1 a | 1.5 ± 0.1 a | 1.9 ± 0.1 a | 2.0 ± 0.9 a | 1.8 ± 0.9 a | 0.9 ± 0.8 b | 0.50 ± 0.02 b | 0.53 ± 0.02 b | 1.8 ± 0.9 a | 1.2 ± 0.7 b | 0.6 ± 0.2 b | 0.23 ± 0.01 b |
| β-cedrene | 1418 | 0.72 ± 0.06 a | 0.80 ± 0.07 a | 0.75 ± 0.01 a | 0.9 ± 0.1 a | 1.3 ± 0.4 b | 1.4 ± 0.4 b | 1.08 ± 0.02 b | 1.1 ± 0.1 b | 0.96 ± 0.01 a | 0.85 ± 0.09 a | 0.6 ± 0.3 a | 0.85 ± 0.02 a |
| α-guaiene | 1439 | 0.9 ± 0.3 a | 1.1 ± 0.3 a | 0.71 ± 0.01 a | 0.56 ± 0.03 b | 0.52 ± 0.07 b | 0.61 ± 0.08 b | 0.43 ± 0.01 b | 0.61 ± 0.02 b | 0.61 ± 0.01 b | 0.4 ± 0.2 b | 0.41 ± 0.07 b | 0.89 ± 0.01 a |
| germacrene D | 1480 | 40 ± 7 a | 39 ± 7 a | 46.3 ± 0.4 a | 47 ± 1 a | 49.6 ± 0.7 b | 47.1 ± 0.9 a | 49 ± 2 b | 45 ± 1 a | 46.6 ± 0.1 a | 43 ± 3 a | 28.8 ± 0.8 b | 46.2 ± 0.7 a |
| bicyclogermacrene | 1494 | 31 ± 3 a | 28 ± 7 a | 34.2 ± 0.3 a | 37 ± 1 a | 34 ± 2 a | 36.8 ± 0.5 a | 32 ± 2 a | 30.8 ± 0.2 a | 32.5 ± 0.1 a | 35 ± 3 a | 23.9 ± 0.6 b | 32.7 ± 0.3 a |
| spathulenol | 1576 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10.4 ± 0.2 | 0 |
| phytol | 1955 | 7.3 ± 0.9 a | 18 ± 2 b | 3.2 ± 0.2 c | 1.2 ± 0.3 d | 0.55 ± 0.05 d | 0.45 ± 0.04 d | 0.6 ± 0.2 d | 0 | 0.7 ± 0.5 d | 0.6 ± 0.2 d | 1.24 ± 0.04 d | 0.55 ± 0.01 d |
| monoterpenes | 0.39 ± 0.04 | 0.43 ± 0.01 | 0.40 ± 0.01 | 0.46 ± 0.06 | 0.51 ± 0.02 | 0.42 ± 0.01 | 0.42 ± 0.04 | 0.41 ± 0.03 | 1.76 ± 0.96 | 0.34 ± 0.01 | 0.31 ± 0.19 | 0.38 ± 0.01 | |
| sesquiterpenes | 76 ± 3 | 72 ± 3 | 85.9 ± 0.2 | 89.4 ± 0.7 | 88.6 ± 0.6 | 88.7 ± 0.4 | 85 ± 1 | 79.5 ± 0.6 | 84.8 ± 0.5 | 82 ± 1 | 65.0 ± 0.3 | 80.7 ± 0.5 | |
| diterpene | 7.3 ± 0.9 | 18 ± 2 | 3.2 ± 0.2 | 1.2 ± 0.3 | 0.55 ± 0.05 | 0.45 ± 0.04 | 0.6 ± 0.2 | 0 | 0.7 ± 0.5 | 0.6 ± 0.2 | 1.24 ± 0.04 | 0.55 ± 0.01 | |
| TOTAL | 84 ± 2 | 90 ± 3 | 89.6 ± 0.1 | 91.1 ± 0.6 | 90 ± 1 | 89.6 ± 0.4 | 89 ± 1 | 79.9 ± 0.4 | 86.8 ± 0.3 | 83.7 ± 0.7 | 66.6 ± 0.1 | 82.1 ± 0.3 | |
a Individual compounds were assigned according to their Kovats indices in conjunction with a comparison of the experimentally obtained mass spectra to those described in library (NIST 107) and in the literature [22]; b Values in the same line with different subscript (a, b, c, and d) are significantly different within months of collection; t: (p < 0.05). All values displayed represent the mean value ± standard deviation of three independent experiments. Statistical analyses were performed by analysis of variance (ANOVA) using the BIOESTAT 5.0 (Stat Soft Inc., Tulsa, OK, USA) software package. A probability value of p < 0.05 was considered statistically significant.
The essential oils from the ripe fruit consisted of 65 individual components (Table 2), which accounted for 99.6 ± 0.9% of the volatile components. The dominant compounds were monoterpenes (ca. 45%), especially neryl (8.8 ± 0.2%) and geranyl (27.3 ± 0.7%) formates. Other major fractions consisted of sesquiterpenes, such as γ-muurolene (10.3 ± 0.9%), δ-cadinene (2.44 ± 0.03%) and dendrolasin (8.23 ± 0.06%) as well as hydrocarbons such as hexacosane (6.02 ± 0.06%) and heptacosane (2.13 ± 0.06%).
Table 2.
The chemical composition of essential oils obtained from the ripe fruit of Porcelia macrocarpa (November 2011).
| Compound a | KI | Relative amount (%) b |
|---|---|---|
| o-cymene | 1026 | 0.09 ± 0.01 |
| benzene acetaldehyde | 1042 | 0.15 ± 0.02 |
| γ-terpinene | 1059 | 0.18 ± 0.06 |
| oct-2E-en-1-ol | 1066 | 0.12 ± 0.02 |
| dehydrolinalool | 1090 | 0.06 ± 0.01 |
| non-3Z-en-1-ol | 1157 | 0.06 ± 0.01 |
| terpinen-4-ol | 1177 | 0.09 ± 0.02 |
| methyl salicylate | 1191 | 0.12 ± 0.03 |
| dec-2E-enal | 1263 | 0.09 ± 0.02 |
| geranial | 1267 | 0.18 ± 0.01 |
| neryl formate | 1282 | 8.8 ± 0.2 |
| geranyl formate | 1298 | 27.3 ± 0.7 |
| dimethoxy-Z-citral | 1318 | 0.13 ± 0.05 |
| dimethoxy-E-citral | 1341 | 1.26 ± 0.03 |
| ethyl nerolate | 1354 | 0.99 ± 0.01 |
| Z-α-damascone | 1358 | 0.66 ± 0.02 |
| neryl acetate | 1361 | 0.84 ± 0.01 |
| α-ylangene | 1375 | 1.14 ± 0.01 |
| geranyl acetate | 1381 | 0.51 ± 0.02 |
| β-bourbonene | 1388 | 1.12 ± 0.02 |
| E-α-damascone | 1393 | 0.75 ± 0.03 |
| ethyl geranate | 1395 | 0.21 ± 0.01 |
| E-caryophyllene | 1419 | 1.14 ± 0.01 |
| β-duprezianene | 1422 | 0.56 ± 0.06 |
| neryl acetone | 1436 | 0.50 ± 0.03 |
| E-β-farnesene | 1456 | 2.8 ± 0.7 |
| γ-muurolene | 1479 | 10.3 ± 0.9 |
| α-amorphene | 1484 | 0.63 ± 0.03 |
| cis-eudesma-6,11-diene | 1489 | 1.17 ± 0.03 |
| α-muurolene | 1500 | 0.69 ± 0.01 |
| butylated hydroxytoluene | 1515 | 0.96 ± 0.01 |
| δ-cadinene | 1523 | 2.44 ± 0.03 |
| trans-cadina-1,4-diene | 1534 | 0.3 ± 0.02 |
| α-cadinene | 1538 | 0.40 ± 0.02 |
| α-calacorene | 1545 | 0.27 ± 0.01 |
| E-nerolidol | 1563 | 1.03 ± 0.05 |
| dendrolasin | 1571 | 8.23 ± 0.06 |
| globulol | 1590 | 1.08 ± 0.03 |
| viridiflorol | 1592 | 0.33 ± 0.01 |
| cubeban-11-ol | 1595 | 0.21 ± 0.01 |
| geranyl 2-methylbutanoate | 1601 | 0.48 ± 0.01 |
| geranyl isovalerate | 1607 | 0.30 ± 0.03 |
| 5-epi-7-epi-α-eudesmol | 1607 | 0.48 ± 0.02 |
| himachalol | 1653 | 0.78 ± 0.01 |
| α-cadinol | 1654 | 0.92 ± 0.01 |
| E-bisabol-11-ol | 1667 | 0.45 ± 0.02 |
| γ-dodelactone | 1677 | 0.8 ± 0.1 |
| Z-nerolidyl acetate | 1677 | 0.45 ± 0.01 |
| α-bisabolol | 1685 | 0.99 ± 0.07 |
| davanol acetate | 1689 | 0.30 ± 0.01 |
| 2E,6E-farnesol | 1743 | 0.15 ± 0.02 |
| β-bisabolenal | 1769 | 0.15 ± 0.01 |
| 1-octadecene | 1790 | 1.6 ± 0.7 |
| n-hexadecanol | 1875 | 1.38 ± 0.01 |
| 5E,9E-farnesyl acetone | 1913 | 0.18 ± 0.03 |
| isophytol | 1947 | 0.30 ± 0.03 |
| 3Z-cembrene A | 1966 | 0.63 ± 0.01 |
| ethyl hexadecanoate | 1993 | 0.54 ± 0.02 |
| E,E-geranyl linalool | 2027 | 0.21 ± 0.06 |
| manool | 2057 | 0.48 ± 0.04 |
| n-octadecanol | 2077 | 0.39 ± 0.02 |
| E-phytol acetate | 2218 | 1.14 ± 0.02 |
| pentacosane | 2500 | 1.86 ± 0.03 |
| hexacosane | 2600 | 6.02 ± 0.06 |
| heptacosane | 2700 | 2.13 ± 0.06 |
| monoterpenes | 44.8 ± 0.9 | |
| sesquiterpenes | 37.1 ± 0.9 | |
| diterpenes | 0.51 ± 0.06 | |
| hydrocarbons | 10.49 ± 0.06 | |
| other compounds | 6.7 ± 0.1 | |
| TOTAL | 99.6 ± 0.9 | |
a Individual compounds were assigned according to their Kovats indices in conjunction with a comparison of the experimentally obtained mass spectra to those described in library (NIST 107) and in the literature [22]; b Values displayed represent the mean value ± standard deviation of three independent experiments.
2.4. Antimicrobial Activity
Several species of the Annonaceae are known to produce essential oils which display antimicrobial activity, e.g., Annona vepretorum [25], Duguetia lanceolata [26], Guatteriopsis blepharophylla, G. friesiana, and G. hispida [27]. In order to determine the antimicrobial activity of the essential oils obtained from the leaves and the ripe fruit of P. macrocarpa, the minimum inhibitory concentration (MIC concentration range: 0.003–1.0 mg/mL) was ascertained for the prokaryote and eukaryote microbes. No biological activity could be observed for the essential oils obtained from the fruit, since no inhibition was detected at 1.0 mg/mL. The essential oils from the leaves (collected in January 2011) however, displayed significant biological activity towards all four Cryptococcus strands tested. No biological activity was detected for the prokaryotes tested as well as for the several Candida spp. or S. cerevisiae. The most sensitive strand is C. neoformans serotype D, which exhibited a growth inhibition rate of 85% at a dose of 0.06 mg/mL essential oil (see Table 3). Another clinically very important strain is C. neoformans serotype A, which accounts for most of the cases of cryptococosis found among immunosuppressed patients. The essential oils of the leaves showed an inhibition rate of 80% against this strain for a dose of 0.5 mg/mL essential oil. The remaining strains of C. gattii serotypes B and C are pathogens, which usually affect immunocompetent patients, and required higher concentrations of around 1.0 mg/mL essential oil in order to cause inhibition rates of 98% and 61%, respectively. C. gattii presented a lower inhibition at the maximum concentration tested and not the traditional 80% inhibition, however, we found important to report this result. The observed MICs for all four strains were in accordance with literature values [28,29], suggesting a high accuracy of the assay. Fluconazole was used as positive control.
Table 3.
Minimum inhibitory concentrations (MICs) obtained from broth microdilution assays for essential oils from the leaves of Porcelia macrocarpa (January 2011).
| Microorganism | Essential oil dosage (mg/mL) | Positive Control |
|---|---|---|
| Fluconazole (mg/mL) | ||
| C. neoformans (serotype A) | 0.50 (80 ± 18%) | 0.013 |
| C. neoformans (serotype D) | 0.06 (95 ± 8%) | 0.006 |
| C. gattii (serotype B) | 1.00 (98 ± 6%) | 0.025 |
| C. gattii (serotype C) | 1.00 (61 ± 1%) * | 0.006 |
Numbers in parenthesis represent the mean percentage inhibition at each MIC ± standard deviation. * 61% inhibition does not represent MIC80 in this case.
In addition to the desirable antimicrobial activity, previous reports have also reported a beneficial antifungal activity for some monoterpenes, diterpenes and sesquiterpenes (especially germacrene D and bicyclogermacrene), which were found in the essential oils of other plants [30,31,32,33]. For example, Cabral et al. showed that germacrene D, the main compound in Vitex rivularis oil, displayed a significant activity against dermatophytes [34]. Bicyclogermacrene and α-copaene have moreover been associated with an antifungal activity, mainly in studies involving the genus Candida and dermatophytes [31,34,35,36,37]. To the best of our knowledge, there are no reports in the scientific literature describing the activity of the essential oils (or their main components) of the leaves of P. macrocarpa against C. neoformans and C. gattii, which cause fungal meningitisin immunocompromised as well as immunocompetent patients [38,39].
3. Experimental
3.1. Chemical Reagents
All solvents used were of analytical grade and purchased from CAAL (São Paulo, Brazil). Linear n-alkane (C8–C20) reference standards, as well as all culture media and standard antibiotic discs of fluconazole and chloramphenicol were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). All other chemicals were purchased from Merck (Darmstadt, Germany), except for hygromycin B, which was obtained from Invitrogen (Carlsbad, CA, USA).
3.2. Microclimatic Factors
Temperatures were measured in situ with a digital Pocket Weather Meter Kestrel 3000 (Nielsen-Kellerman, Boothwyn, PA, USA). Precipitation values (in mm) were measured during each period of collection (from 12th to 18th day) using a pluviometer, which was custom-made in our laboratory.
3.3. Plant Material
Leaves of P. macrocarpa R.E. Fries (Annonaceae) were collected randomly from three individual trees in the Jardim Botânico de São Paulo (São Paulo, SP, Brazil) at 12 a.m. on the 15th day of each month (January to December 2011). Ripe fruit samples were collected from the very same trees on 15th November, 2011 at 12 a.m. Reference specimen were deposited at the herbarium of the Instituto de Botânica (São Paulo, Brazil) and compared with those under reference SP76791. Samples of the crude oils are available from the authors.
3.4. Hydro-Distillation of the Essential Oils
Each batch of fresh leaves or fruitsamples from P. macrocarpa was hydro-distilled for four hours in a Clevenger type apparatus [21]. The essential oils were extracted from the aqueous fraction using CH2Cl2 (3 × 5 mL). The combined organic fractions were subsequently dried over anhydrous Na2SO4, before the solvent was evaporated and the oil was stored at 4 °C in the absence of light.
3.5. Gas Chromatography Analysis (GC)
The crude essential oils were analyzed by GC, using a Shimadzu GC-2010 gas chromatograph, equipped with an FID-detector and an automatic injector (Shimadzu AOC-20i). As the stationary phase, an RtX-5 capillary column (5% phenyl, 95% polydimethylsiloxane, 30 m × 0.32 mm × 0.25 μm film thickness; Restek, Bellefonte, PA, USA) was used with helium as the carrier gas (flow rate: 1 mL/min). The oven temperature was raised from 60 °C to 280 °C at a rate of 3 °C/min and subsequently kept at 280 °C for further ten minutes. The injector temperature was 220 °C and the detector (FID) was kept at 280 °C. Composition percentages were obtained from electronic integration of the FID output and a series of linear n-alkanes (C8–C20), which were used as reference points for the determination of the Kovats indices (KI).
3.6. Gas Chromatography- Mass Spectrometry (GC-MS) Analysis
GC-MS analysis was carried out using a Shimadzu GC-17A chromatograph connected to a MS-QP-5050A mass spectrometer. The GC analysis was carried out with an RtX-5 capillary column (5% phenyl, 95% polydimethylsiloxane, 30 m × 0.32 mm × 0.25 μm film thickness; Restek, Bellefonte, PA, USA) and the operating conditions were identical with those described in the previous section. Retention indices for all compounds were determined according to the Kovats indices (KI), as described in the previous section. The EI-MS analysis was carried out under an ionization voltage of 70 eV and an ion source temperature of 230 °C. The identification of individual compounds was achieved by a comparison of the KI values in conjunction with matching mass spectrometric fragmentation patterns to those of mass spectra library (NIST 107), published MS fragmentation patterns [22] and/or MS spectra of authentic compounds.
3.7. Microbial Strain Media, Antibiotics and Growth Conditions
In order to test the antimicrobial activity of the essential oils obtained from the leaves and fruits of P. macrocarpa, Gram-positive (Streptococcus equi—CBMAI 264, Staphylococcus epidermidis—CBMAI 604, and Enterococcus fecalis), Gram-negative (Escherichia coli, Serratia marcescens—CBMAI 469, and Pseudomonas aeruginosa—CBMAI 602) and yeast (Candida dubliniensis—ATCC 7978, C. tropicalis—ATCC 13803, C. albicans—ATCC 18804/CBMAI 560, C. glabrata—ATCC 90030, C. parapsilosis—clinical isolate 68, C. krusei—clinical isolate 9602, Cryptococcus neoformans—KN99α serotype A/JEC21 serotype D, C. gattii—NIH312 serotype C/R265 serotype B, and Saccharomyces cerevisiae—BY4742) strains were subjectedto a broth microdilution assays. Microbial strains were kept as criostocks at −80 °C, cultivated onYEPD (2% peptone, 2% dextrose and 2% agar) platesfor yeasts (1% yeast extract) or LB (1% tryptone, 1% NaCl and 2% agar) for bacterial strains (0.5% bacterial strains). Fluconazole and chloramphenicol were used as positive controls for yeast and bacteria, respectively. Essential oils were diluted to 10% with dimethylsulfoxide (DMSO).
3.8. Broth Microdilution Assay for the Determination of Minimum Inhibitory Concentrations (MIC)
Micro titer plates (96 wells) were used for broth microdilution assays in order to ascertain the MIC for each tested strain. Two independent assays were conducted according to the guidelines of the National Committee for Clinical Laboratory Standards (CLSI, M100-S9). The following minor modifications were implemented: the target microorganisms were grown in test tubes overnight in 3 mL of the respective medium (RPMI 1640 for yeast and BHI for bacteria) at 30 °C and agitated in a rotary shaker (150 rpm). The cellular concentration was adjusted to 1× 102 – 2 × 102 CFU (yeast) and 1 × 104 – 2 × 104 CFU (bacteria) per well. The concentration was confirmed by viability counts on YEPD and LB plates. Nine dilutions of essential oils and reference standards were used (two-fold serial dilutions). A negative sterilization control, containing medium only and a positive growth control, containing cells and 10 μL DMSO (100% growth) were also included. Microtiter plates were subsequently incubated at 30 °C for 24 or 48 h, respectively. Finally, the absorbance at 530 nm was measured in a plate reader (Epoch, Bio-Tek, Winooski, VT, USA). The threshold for the MIC was set at a minimum of an 80% growth inhibition. All tests were performed in triplicate in 100 µL of reaction volume. The concentration range for the essential oils of the leaves ranged from 0.003–1.0 mg/mL. Fluconazole and chloramphenicol concentrations ranged between 0.0007 and 0.05 mg/mL and 0.00312 and 0.400 mg/mL, respectively.
4. Conclusions
A great benefit of this study is the characterization of the composition of the essential oils of the leaves and ripe fruits of P. macrocarpa. The biological activity documented in this study should be of great pharmacological interest, since the major components of the essential oils may now be tested individually against both species of the Cryptococcus genus, which continue to cause life-threatening diseases and demand new potent antifungal drugs in order to allow effective treatment.
Acknowledgments
The authors would like to thank the CNPq (300546/2012-2) and the FAPESP (2011/51739-0) for providing financial support and fellowships.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of essential oils from leaves and fruits of P. macrocarpa are available from the authors.
References
- 1.Murray N.A. Revision of Cymbopetalum and Porcelia (Annonaceae) Syst. Bot. Monogr. 1993;40:121–140. doi: 10.2307/25027830. [DOI] [Google Scholar]
- 2.Chaves M.H., Roque N.F. Acetogenins from Porcelia macrocarpa: Stereochemical determination of 2-alkyl-3-hydroxy-4-methyl γ-lactones by 13C-NMR spectroscopy. Phytochemistry. 1997;44:523–528. doi: 10.1016/S0031-9422(96)00542-0. [DOI] [Google Scholar]
- 3.Chaves M.H., Roque N.F. Amides and lignanamides from Porcelia macrocarpa. Phytochemistry. 1997;46:879–881. doi: 10.1016/S0031-9422(97)00364-6. [DOI] [Google Scholar]
- 4.Chaves M.H., Santos L.A., Lago J.H.G., Roque N.F. Alkaloids from Porcelia macrocarpa. J. Nat. Prod. 2001;64:240–242. doi: 10.1021/np000373d. [DOI] [PubMed] [Google Scholar]
- 5.Lago J.H.G., Chaves M.H., Ayres M.C.C., Agripino D.G., Young M.C.M. Evaluation of antifungal and DNA-damaging activities of alkaloids from branches of Porcelia macrocarpa. Planta Med. 2007;73:292–295. doi: 10.1055/s-2007-967108. [DOI] [PubMed] [Google Scholar]
- 6.Chaves M.H., Freitas A., Roque N.F., Cavalheiro A.J. Separação e identificação de constituintes químicos polares dos galhos de Porcelia macrocarpa. Quím. Nova. 2000;23:307–309. doi: 10.1590/S0100-40422000000300004. [DOI] [Google Scholar]
- 7.Chaves M.H., Lago J.H.G., Roque N.F. Macrocarpane, a new sesquiterpene skeleton from the leaves of Porcelia macrocarpa. J. Braz. Chem. Soc. 2003;14:16–19. [Google Scholar]
- 8.Silva E.B.P., Matsuo A.L., Figueiredo C.R., Chaves M.H., Sartorelli P., Lago J.H.G. Chemical constituents and cytotoxic evaluation of essential oils from leaves of Porcelia macrocarpa (Annonaceae) Nat. Prod. Commun. 2013;8:277–279. [PubMed] [Google Scholar]
- 9.Nakatsuo T., Lupo A.T., Jr., Chinn J.W., Jr., Kang R.K.L. Biological activity of essential oils and their constituents. Bioact. Nat. Prod. 2000;21:571–631. [Google Scholar]
- 10.Gobo-Neto L., Lopes N.P. Plantas medicinais: Fatores de influência no conteúdo de metabólitos secundários. Quím. Nova. 2007;30:374–381. doi: 10.1590/S0100-40422007000200026. [DOI] [Google Scholar]
- 11.Silva A.A.C.A., Souza E.A., Matsuo A.L., Lago J.H.G., Chaves M.H. Intraspecific variation and cytotoxic evaluation of the essential oils from Oxandra sessiliflora R. E. Fries. J. Med. Plant Res. 2013;7:504–508. [Google Scholar]
- 12.Sartorelli P., Santana J.S., Guadagnin R.C., Lago J.H.G., Pinto E.G., Tempone A.G., Stefani H.A., Soares M.G., Silva A.M. In vitro trypanocidal evaluation of pinane derivatives from essential oils of ripe fruits from Schinus terebinthifolius Raddi (Anacardiaceae) Quím. Nova. 2012;35:743–747. doi: 10.1590/S0100-40422012000400017. [DOI] [Google Scholar]
- 13.Bou D.D., Lago J.H.G., Figueiredo C.R., Matsuo A.L., Guadagnin R.C., Soares M.G., Sartorelli P. Chemical composition and cytotoxicity evaluation of essential oil from leaves of Casearia sylvestris, its main compound α-zingiberene and derivatives. Molecules. 2013;18:9477–9887. doi: 10.3390/molecules18089477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lago J.H.G., Carvalho L.A.C., da Silva F.S., Toyama D.D., Favero O.A., Romoff P. Chemical composition and anti-inflammatory evaluation of essential oils from leaves and stem barks from Drimys brasiliensis Miers (Winteraceae) J. Braz. Chem. Soc. 2010;21:1760–1765. doi: 10.1590/S0103-50532010000900024. [DOI] [Google Scholar]
- 15.INMET (Brazilian National Institute of Meteorology) Database. [(accessed on 15th September 2012)]. Available online: http://www.inmet.gov.br.
- 16.Lakusić D., Ristić M., Slavkovska V., Lakusić B. Seasonal variations in the composition of the essential oils of rosemary (Rosmarinus officinalis, Lamiaceae) Nat. Prod. Commun. 2013;8:131–134. [PubMed] [Google Scholar]
- 17.El-Kashoury S.A., El-Askary H.I., Kandil Z.A., Salem M.A., Sleem A.A. Chemical composition and biological activities of the essential oil of Mentha suaveolens Ehrh. Z. Naturforsch. C. 2012;67:571–579. doi: 10.5560/ZNC.2012.67c0571. [DOI] [PubMed] [Google Scholar]
- 18.Lakušić B., Ristić M., Slavkovska V., Milenković M., Lakušić D. Environmental and seasonal impacts on the chemical composition of Satureja horvatii Šilić (Lamiaceae) essential oils. Chem. Biodivers. 2011;8:483–493. doi: 10.1002/cbdv.201000169. [DOI] [PubMed] [Google Scholar]
- 19.Silva F.G., Oliveira C.B.A., Pinto J.E.B.P., Nascimento V.E., Santos S.C., Seraphin J.C., Ferri P.H. Seasonal variability in the essential oils of wild and cultivated Baccharis trimera. J. Braz. Chem. Soc. 2007;18:990–997. doi: 10.1590/S0103-50532007000500017. [DOI] [Google Scholar]
- 20.Gazim Z.C., Amorim A.C.L., Hovell A.M.C., Rezende C.M., Nascimento I.A., Ferreira G.A., Cortez D.A.G. Seasonal variation, chemical composition, and analgesic and antimicrobial activities of the essential oils from leaves of Tetradenia riparia (Hochst.) Coddin Southern Brazil. Molecules. 2010;15:5509–5524. doi: 10.3390/molecules15085509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lago J.H.G., Fávero O.A., Romoff P. Microclimatic factors and phenology influences in the chemical composition of the essential oils from Pittosporum undulatum Vent leaves. J. Braz. Chem. Soc. 2006;17:1334–1338. doi: 10.1590/S0103-50532006000700021. [DOI] [Google Scholar]
- 22.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th ed. Allured Publishing Corporation; Carol Stream, IL, USA: 2008. [Google Scholar]
- 23.Bülow N., König W.A. The role of germacrene D as a precursor in sesquiterpene biosynthesis: Investigations of acid catalyzed, photochemically and thermally induced rearrangements. Phytochemistry. 2000;55:141–168. doi: 10.1016/S0031-9422(00)00266-1. [DOI] [PubMed] [Google Scholar]
- 24.Pala-Paul J., Perez-Alonso M.J., Velasco-Negueruela A., Pala-Paul R., Sanz J., Conejero F. Seasonal variation in chemical constituents of Santolina rosmarinifolia L. ssp. rosmarinifolia. Biochem. Syst. Ecol. 2001;29:663–672. doi: 10.1016/S0305-1978(01)00032-1. [DOI] [PubMed] [Google Scholar]
- 25.Costa E.V., Dutra L.M., Nogueira P.C., Moraes V.R., Salvador M.J., Ribeiro L.H., Gadelha F.R. Essential oil from the leaves of Annona vepretorum: Chemical composition and bioactivity. Nat. Prod. Commun. 2012;7:265–266. [PubMed] [Google Scholar]
- 26.Sousa O.V., Del-Vechio-Vieira G., Alves M.S., Araújo A.A., Pinto M.A., Amaral M.P., Rodarte M.P., Kaplan M.A. Chemical composition and biological activities of the essential oils from Duguetia lanceolata St. Hil. barks. Molecules. 2012;17:11056–11066. doi: 10.3390/molecules170911056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costa E.V., Teixeira S.D., Marques F.A., Duarte M.C., Delarmelina C., Pinheiro M.L., Trigo J.R., Sales-Maia B.H. Chemical composition and antimicrobial activity of the essential oils of the Amazon Guatteriopsis species. Phytochemistry. 2008;69:1895–1899. doi: 10.1016/j.phytochem.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Espinel-Ingroff A. In vitro antifungal activities of anidulafungin and micafungin, licensed agents and the investigational triazole posaconazole as determined by NCCLS methods for 12,052 fungal isolates: review of the literature. Rev. Iberoam. Micol. 2003;20:121–136. [PubMed] [Google Scholar]
- 29.Lago J.H.G., Souza E.D., Mariane B., Pascon R., Vallim M.A., Martins R.C., Baroli A.A., Carvalho B.A., Soares M.G., dos Santos R.T., et al. Chemical and biological evaluation of essential oils from two species of Myrtaceae—Eugenia uniflora L. and Plinia trunciflora (O. Berg) Kausel. Molecules. 2011;16:9827–9837. doi: 10.3390/molecules16129827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodrigues F.F., Oliveira L.G., Rodrigues F.F., Saraiva M.E., Almeida S.C., Cabral M.E., Campos A.R., Costa J.G. Chemical composition, antibacterial and antifungal activities of essential oil from Cordia verbenacea DC leaves. Pharmacogn. Res. 2012;4:161–165. doi: 10.4103/0974-8490.99080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verma R.S., Padalia R.C., Chauhan A. Volatile constituents of Origanum vulgare L., ‘thymol’ chemotype: Variability in North India during plant ontogeny. Nat. Prod. Res. 2012;26:1358–1362. doi: 10.1080/14786419.2011.602017. [DOI] [PubMed] [Google Scholar]
- 32.Cordeiro R.A., Nogueira G.C., Brilhante R.S., Teixeira C.E., Mourão C.I., Castelo-Branco Dde S., Paiva M.A., Ribeiro J.F., Monteiro A.J., Sidrim J.J., et al. Farnesol inhibits in vitro growth of the Cryptococcus neoformans species complex with no significant changes in virulence-related exoenzymes. Vet. Microbiol. 2012;159:375–380. doi: 10.1016/j.vetmic.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 33.Marongiu B., Piras A., Porcedda S., Falconieri D., Gonçalves M.J., Salgueiro L., Maxia A., Lai R. Extraction, separation and isolation of volatiles from Vitex agnus-castus L. (Verbenaceae) wild species of Sardinia, Italy, by supercritical CO2. Nat. Prod. Res. 2010;24:569–579. doi: 10.1080/14786410902899915. [DOI] [PubMed] [Google Scholar]
- 34.Cabral C., Gonçalves M., Cavaleiro C., Sales F., Boyom F., Salgueiro L. Composition and anti-fungal activity of the essential oil from Cameroonian Vitex rivularis Gurke. Nat. Prod. Res. 2009;23:1478–1484. doi: 10.1080/14786410802390106. [DOI] [PubMed] [Google Scholar]
- 35.Fontenelle R.O., Morais S.M., Brito E.H., Brilhante R.S., Cordeiro R.A., Nascimento N.R., Kerntopf M.R., Sidrim J.J., Rocha M.F. Antifungal activity of essential oils of Croton species from the Brazilian Caatinga biome. J. Appl. Microbiol. 2008;104:1383–1390. doi: 10.1111/j.1365-2672.2007.03707.x. [DOI] [PubMed] [Google Scholar]
- 36.Gallori S., Bilia A.R., Mulinacci N., Bicchi C., Rubiolo P., Vincieri F.F. Identification of volatile constituents of Tambourissa leptophylla. Planta Med. 2001;67:290–292. doi: 10.1055/s-2001-12001. [DOI] [PubMed] [Google Scholar]
- 37.Costa E.V., Dutra L.M., de Jesus H.C., Nogueira P.C., Moraes V.R., Salvador M.J., Cavalcanti S.C., dos Santos R.L., Prata A.P. Chemical composition and antioxidant, antimicrobial, and larvicidal activities of the essential oils of Annona salzmannii and A. pickelii (Annonaceae) Nat. Prod. Commun. 2011;6:907–912. [PubMed] [Google Scholar]
- 38.Li S.S., Mody C.H. Cryptococcus. Proc. Am. Thorac. Soc. 2010;7:186–196. doi: 10.1513/pats.200907-063AL. [DOI] [PubMed] [Google Scholar]
- 39.Lin X., Heitman J. The biology of the Cryptococcus neoformans species complex. Ann. Rev. Microbiol. 2006;60:69–105. doi: 10.1146/annurev.micro.60.080805.142102. [DOI] [PubMed] [Google Scholar]