Abstract
In this article the crystal structures of the starting material TZ-5 and the key intermediate TZ-6 of temozolomide (TZ-7), an anticancer therapeutic agent, are presented, together with their spectroscopic and thermal characteristics. Both compounds crystallize in the triclinic P-1 space group. X-ray crystallography studies proved that the compound TZ-6 exists as a monohydrate. A complete structural assignment was obtained for the signals in the 1H-, 13C- and 15N-nuclear magnetic resonance spectra and the structures were confirmed by Fourier-Transform infrared and Raman spectroscopy. The article describes the importance of the high purity of TZ-6 during the small-scale plant production of TZ-7 in a desired polymorphic form III with the purity higher than 99.50%, according to an HPLC method.
Keywords: temozolomide intermediate, X-ray crystallography, nuclear magnetic resonance, high-performance liquid chromatography
1. Introduction
A complete physicochemical characterization has recently become required by both the U.S. Food and Drug Administration (FDA) [1] and by the European Medicine Agency (EMA) [2], not only for active pharmaceutical ingredients (API), but also for all key synthetic intermediates. A well documented impurity profile, the identification and physicochemical characteristic of a starting material and intermediate products are necessary for an API registration.
Temozolomide (TZ-7, 3,4-dihydro-3-methyl-4-oxoimidazo[5,1-d]-as-tetrazine-8-carboxamide) is a cytotoxic antitumor prodrug against malignant brain tumours. At physiological pH temozolomide undergoes decomposition to the active compound MTIC (3-methyl-(triazen-1-yl)imidazole-4-carboxamide) [3,4,5]. The drug (brand names: Temodar, Temodal and Temcad®), originally developed by Schering-Plough Corp., first bacame available in the U.S. in August 1999 [6]. X-ray crystallography studies of temozolomide have been described by Lowe et al. [7]. The crystal structures of temozolomide polymorphs, as well as its cocrystals with 4,4'-bipyridine-N,N'-dioxide have been reported by Jagadeesh Babu et al. [8].
In this article the complete physicochemical characteristics of temozolomide intermediate and starting material have been determined by the following methods: 1H-, 13C- and 15N-nuclear magnetic resonance (NMR), infrared (IR) and Raman spectroscopy, as well as by thermal analyses. Single crystals of both compounds were obtained and their X-ray structures were solved. Furthermore, high-performance liquid chromatography (HPLC) methods for the purity determination of TZ-5 and TZ-6 were evaluated.
2. Results and Discussion
2.1. Structure Elucidation by NMR, IR and Raman Spectroscopy
2.1.1. NMR Spectroscopy
All signals in the 1H-, 13C- and 15N-NMR spectra of temozolomide (TZ-7) and key intermediates TZ-6 and TZ-5 were assigned to the respective atoms using one- and two-dimensional techniques (g-HSQC and g-HMBC (1H-13C and 1H-15N-NMR, Table 1).
Table 1.
Signal assignment for TZ-7, TZ-6 and TZ-5 in 1H- 13C- and 15N-NMR spectra, (in DMSO-d6 solution).
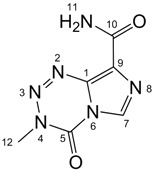 TZ-7 |
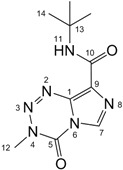 TZ-6 |
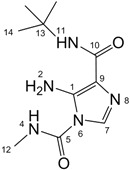 TZ-5 |
||||||
|---|---|---|---|---|---|---|---|---|
| Position | 1H (ppm) | 13C/15N (ppm) | Position | 1H (ppm) | 13C/15N (ppm) | Position | 1H (ppm) | 13C/15N (ppm) |
| 1 | − | 134.58 | 1 | − | 134.21 | 1 | − | 111.67 |
| 2 | − | not obtained | 2 | − | not obtained | 2 | s, 2H, 6.33 | −327.03 |
| 3 | − | 19.59 | 3 | − | 19.42 | − | − | − |
| 4 | − | −180.37 | 4 | − | −180.82 | 4 | q, 1H, 8.44 | −293.78 |
| 5 | − | 139.18 | 5 | − | 139.15 | 5 | − | 150.68 |
| 6 | − | −199.86 | 6 | − | −200.03 | 6 | − | −202.82 |
| 7 | s, 1H, 8.81 | 128.36 | 7 | s, 1H, 8.80 | 128.22 | 7 | s, 1H, 7.61 | 125.90 |
| 8 | − | −106.03 | 8 | − | −107.91 | 8 | − | −125.92 |
| 9 | − | 130.50 | 9 | − | 130.87 | 9 | − | 142.74 |
| 10 | − | 161.51 | 10 | − | 158.93 | 10 | − | 163.84 |
| 11 | s, 1H, 7.67; s, 1H, 7.79 | −274.71 | 11 | s, 1H, 7.55 | −246.00 | 11 | s, 1H, 6.59 | −255.99 |
| 12 | s, 3H, 3.87 | 36.13 | 12 | s, 3H, 3.87 | 36.14 | 12 | s, 3H, 2.79 | 26.56 |
| − | − | − | 13 | − | 50.77 | 13 | − | 49.80 |
| − | − | − | 14 | s, 9H, 1.42 | 28.49 | 14 | s, 9H, 1.36 | 28.94 |
The comparison of TZ-5, TZ-6 and TZ-7 spectra shows that these compounds have a very similar skeletal systems, especially the TZ-7 and TZ-6 pair. Both molecules have the same imidazotetrazine ring in the structure. Their spectra differ only in the region of the amide group. In the imidazole ring the differences between the corresponding values of chemical shifts are usually not more than 0.5 ppm. Only for the nitrogen at position 8 is the difference bigger, i.e., 1.88 ppm. The tert-butyl group substitution in the TZ-7 molecule also increases the shielding of the carbonyl carbon in position 10 (the chemical shift changes from 161.51 ppm to 158.93 ppm). Unfortunately, in our study we did not determine any chemical shift values for the nitrogen atoms in position 2 of the molecules TZ-7 and TZ-6. For the compound TZ-7 this value was measured using an isotopically labeled compound in the work of Wheelhouse et al. [9]. In that article the chemical shift of the nitrogen atom 2 is −33.99 ppm for TZ-7 and a similar value was expected for TZ-6.
2.1.2. IR and Raman Spectroscopy
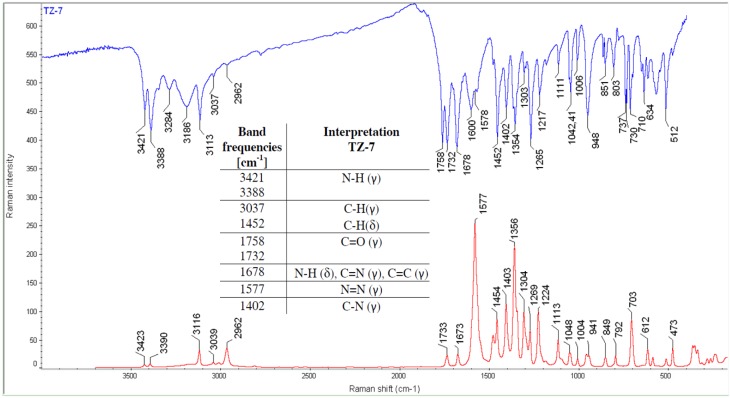
The structures of all the compounds were further confirmed by IR and Raman spectroscopy. Three characteristic bands at: 1678, 730 and 710 cm−1 [10] proved the presence of the form III in the TZ-7 sample (Figure 1). A doublet at 3421 and 3388 cm−1 is presumed to have come from the primary amide N-H stretching vibrations (γ). At 1758 and 1732 cm−1 the first amide band (C=O (γ)) from the primary and tertiary amide is manifested and the band at 1678 cm−1 can come from the second amide band (N-H bending vibrations (δ)) as well as from C=N (γ) and C=C (γ) vibrations. It is supposed that the most intensive Raman band at 1577 cm−1 can come from the N=N (γ) vibration.
Figure 1.
IR and Raman spectra of TZ-7.
The band at 3568 cm−1 is visible only in the IR spectrum of TZ-6 (Figure 2). This band is not visible in the Raman spectrum. It is supposed that this band can indicate the presence of a water molecule incorporated into the crystal structure of TZ-6. The band at 3424 cm−1 can indicate the presence of the secondary amide N-H (γ) vibration. At 1758 and 1710 cm−1 the first amide band from the secondary and tertiary amide is manifested and the band at 1648 cm−1 can come from the second amide band as well as from C=N (γ) and C=C (γ) vibrations. It is supposed that the band at 1569 cm−1 represents the N=N (γ) vibration.
Figure 2.
IR and Raman spectra of TZ-6.
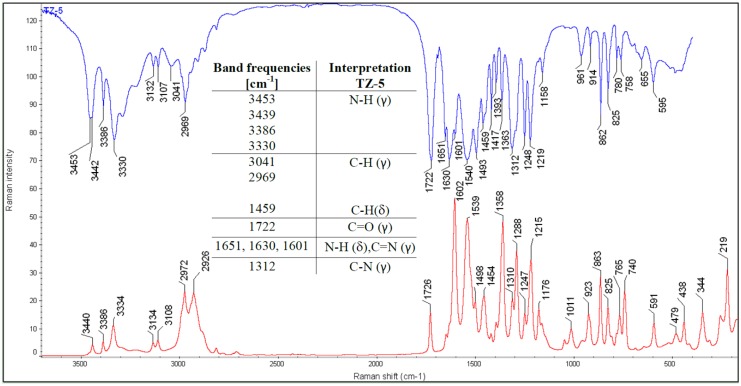
Presumably, in TZ-5 (Figure 3) the bands from 3453 to 3330 cm−1 manifest the presence of the N-H (γ) vibration form the amide and amine groups as well. The most intensive band in the IR spectra, at 1721 cm−1 indicates the C=O (γ) vibration. The bands at 1651, 1630 and 1601 cm−1 can come from the N-H (δ) and C=N (γ) vibrations.
Figure 3.
IR and Raman spectra of TZ-5.
2.1.3. X-Ray Cyrstallography Studies
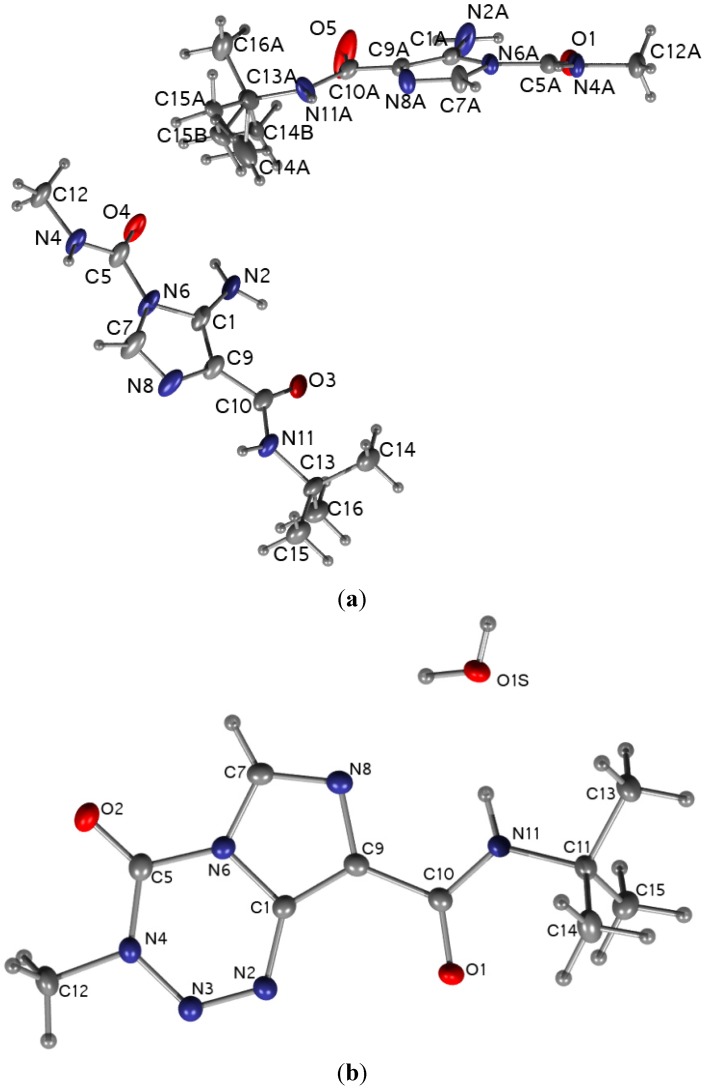
The crystal structure of temozolomide has been previously reported [7,8]. TZ-5 crystallises in the triclinic P-1 space group with two molecules in the asymmetric unit, whereas TZ-6 crystallises as a hydrate with one molecule of water. The molecular structures of TZ-5 and TZ-6 are depicted with the numbering scheme in Figure 4.
Figure 4.
Molecular structure and atomic labelling of TZ-5 (a) and TZ-6 (b). Thermal displacement ellipsoids are drawn at the 50% probability level.
The differences in the corresponding structural parameters of the two molecules in the asymmetric unit of the TZ-5 crystal structure are within the level of errors (less than 3σ). The only significant difference between these molecules is the disorder of the side chain present in one of them. Therefore, the numerical values of interatomic distances will be reported only for one molecule. The TZ-5 molecules are planar and form an intramolecular hydrogen bond between the amine group and the carboxylic oxygen atom. The distances between the acceptor and donor of the hydrogen bond are equal to: 2.91(1)Å and 2.90(1)Å for O(4)…N2 and O(3)…N2, respectively. The planarity of the ring and amide bond is reserved in the TZ-6 molecules where the intramolecular hydrogen bonds are impossible. However, TZ-6 crystallises with one molecule of water per molecule of TZ-6 and the water forms four hydrogen bonds with the precursor (N11…O1S 2.903(1)Å, O1S…N8 2.887(1)Å, O1S…N2x,y+1,z 3.067(1)Å, O1S…O1x,y+1,z 2.719(1)Å).
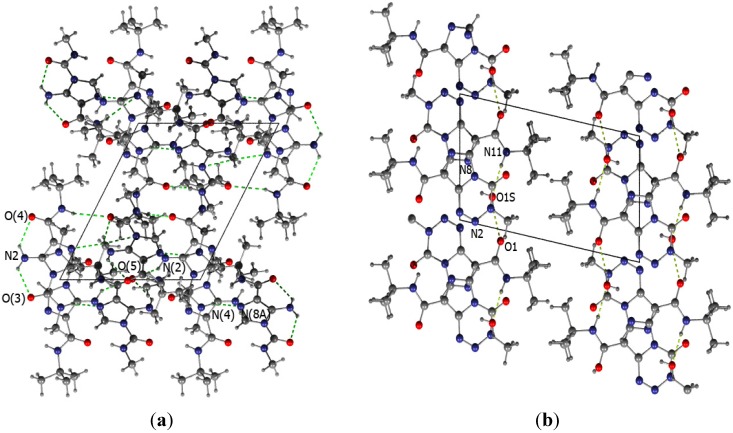
Both structures reveal a similar layered packing scheme with easily distinguished hydrophilic and hydrophobic parts with the supremacy of hydrogen bonds and dispersive interaction, correspondingly (see Figure 5).
Figure 5.
The view of crystal packing of TZ-5 along the y axis (a) and crystal packing of TZ-6 along the x axis (b). The hydrogen bond is marked by a green, dashed line.
The packing of TZ-5 molecules is based on four hydrogen bonds, i.e., two for one molecule from the asymmetric unit (N4…N8A-x,-y+1,-z 2.89(1)Å, N2…O5-x+1,-y+1,-z 2.91(1)Å). The hydrogen bonds between the TZ-6 molecule and water are responsible for the formation of the layered structure with alternating TZ-6 moieties and water molecules (see Figure 5). In the second direction, the molecules are hold together by π…π stacking between the heteroaromatic rings. For both reported structures, the interactions between layers are dispersive. These are mostly the H…H interactions between side chains of molecules. The TZ-7 molecule [7,8] does not have a bulky tert-butyl substituent, and its crystal structure exhibits a more effective packing. In this case, the hydrogen bonds govern the packing in the crystal lattice in all directions.
2.1.4. Thermoanalitycal Studies
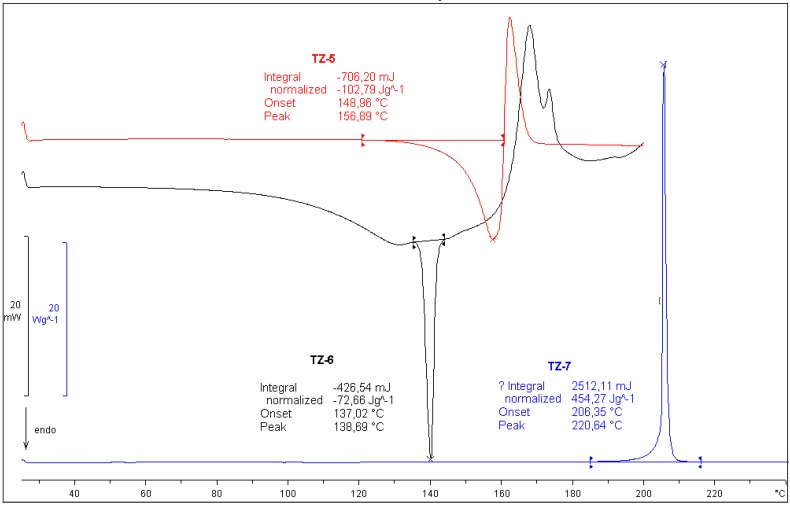
Figure 6 shows the DSC curves of TZ-5, TZ-6 and TZ-7. The DSC curve of TZ-5 is characterized by one exothermal signal from the substance decomposition, followed by an endothermic melting peak at 148.96 °C.
Figure 6.
DSC curves of TZ-5, TZ-6 and TZ-7.
The endothermic melting effect of TZ-6 is at 137.02 °C, in the range of decreasing baseline from 100 to 130 °C. A thermogravimetric analysis proved a mass loss of 6.70% in this range. This value agrees well with water content in the sample and theoretical water content for a monohydrate (6.71%). Endothermic effects visible in the temperature range from 160 to 180 °C come from the decomposition of the substance. The DSC curve of temozolomide only exhibits the exothermic decomposition effect at 206.35 °C. The mass loss value calculated from the thermogravimetric curve in the range of this effect is above 50%.
3. Experimental
3.1. Synthesis
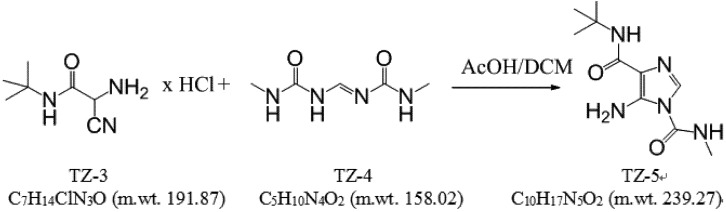
3.1.1. Synthesis of 5-amino-N-(tert-butyl)-3-(methylcarbamoyl)imidazole-4-carboxamide (TZ-5) [11] (Scheme 1).
Scheme 1.
The snthesis of TZ-5.
The mixture of the compounds TZ-3 [12] (96.0 g, 0.5 mol) and TZ-4 [13]: (95.0 g, 0.6 mol) was diluted with dichloromethane (1.0 L), then acetic acid (100%, 5 mL) was added. The whole mixture was stirred at ambient temperature for 1 h, refluxed for an 1 h and then cooled to ambient temperature. The mixture was neutralized with aqueous potassium carbonate (pH 4–5), the organic layer was separated and the aqueous one extracted with dichloromethane (2.0 L). The combined extracts were evaporated in vacuo to approx. 20% of their volume, the precipitate collected and washed with dichloromethane (75 mL). The filtrate was evaporated to approx. 50% of volume and refrigerated at −10 °C for 16 h. The precipitate was collected and washed with dichloromethane (25 mL). The combined precipitates were dried in vacuo at 30 °C to afford 87 g (73%) of the compound TZ-5 in the form of yellow crystals. Elementary analysis: 50.07% (C), 7.18% (H), 29.06% (N). ESI-MS: calcd. for (M+H)+: 239.14, found 239.14.
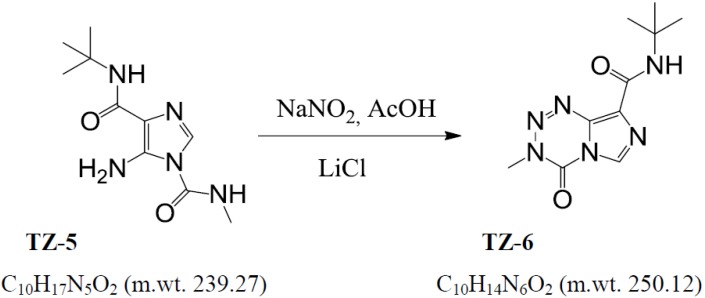
3.1.2. Synthesis of N-(tert-butyl)-3-methyl-4-oxo-3,4-dihydroimidazo[5,1-d]-1,2,3,5-tetrazine-8-carboxamide (TZ-6) [11] (Scheme 2).
Scheme 2.
The synthesis of TZ-6.
The compound TZ-5 (50.0 g, 0.21 mol) was added at 10 °C to the stirred solution of lithium chloride hydrate (378 g) and acetic acid (24 mL) in water (1 L). The mixture was stirred for 30 min and cooled to 4 °C, then sodium nitrite (22 g, 0.32 mol) was added in one portion. The whole mixture was stirred for 1 h at 4–5 °C and for next 18 h at ambient temperature, then diluted with dichloromethane (1.0 L) and stirred vigorously for 10 min. The organic layer was separated and the aqueous one extracted twice with 500 mL and 250 mL portions of dichloromethane.
The combined extracts were then washed with 10% aqueous sodium dithionite (1.0 L) and evaporated to dryness in vacuo at temperature not exceeding 25 °C. The crude product was suspended in the mixture of 2-propanol–water–36% hydrochloric acid (1,000 mL–225 mL–25 mL) and refluxed for 20 min. After filtration the solution was left at ambient temperature for 24 hrs, the precipitate collected, washed with 80% aqueous 2-propanol (125 mL) and dried in vacuo at ambient temperature to afford the compound TZ-6 of HPLC 99.9% + purity in the yield 28.5 g (54%). Elementary analysis: 44.77% (C), 6.02% (H), 31.23% (N). ESI-MS: calcd. for (M+H)+: 250.12, found 250.12.
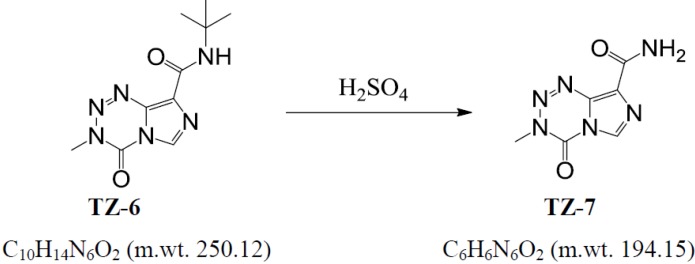
3.1.3. Synthesis of 3,4-dihydro-3-methyl-4-oxoimidazo[5,1-d]-as-tetrazine-8-carboxamide (TZ-7) [11] (Scheme 3).
Scheme 3.
The synthesis of TZ-7.
The compound TZ-6 (25,0 g, 0,10 mol) was added portionwise to stirred sulfuric acid (d 1.8, 70 mL) at 15–25 °C. The whole mixture was stirred at ambient temperature for 3 h and then poured into 96% ethanol (800 mL) at 0 °C. The suspension was stirred for 30 min. at 0 °C, the precipitate collected, washed with cold ethanol (50 mL) and dried on air for 16 h. The obtained crude TZ-7 was dissolved in the refluxed mixture of acetone (450 mL), water (150 mL) and acetic acid (38 mL), the solution was filtered, the filtrate refluxed again, cooled to ambient temperature and stirred for 4 h. The precipitate was then collected, washed with acetone/water 3:1 (80 mL) and dried in vacuo for 16 h at 25 °C to afford 10.1 g (52%) of pharmaceutically pure TZ-7. Elementary analysis: 36.99% (C), 3.00% (H), 43.02% (N). ESI-MS: calcd. for (M+H)+: 194.06, found 194.06.
3.2. Techniques
3.2.1. X-Ray Crystallography
The deposition numbers CCDC 806024 (TZ-5) and CCDC 805454 (TZ-6) contain the supplementary crystallographic data. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.htmL (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk).
The data of TZ-5 and TZ-6 were collected using the Bruker KAPPA APEXII ULTRA diffractometer controlled by the APEXII software [14], equipped with the MoKα rotating anode X-ray source (λ = 0.71073 Å, 50.0 kV, 22.0 mA) monochromatized by multi-layer optics and APEX-II CCD detector. The data collection was carried out at 100 K using the Oxford Cryostream cooling device. Indexing, integration and initial scaling were performed with the SAINT [15] and SADABS [16] software (Bruker AXS, Madison, WI, USA, 2008). The structures were solved by direct methods using the SHELXS-97 [17] program and refined with the SHELXL-97 [15]. A multi-scan absorption correction has been applied in the scaling procedure. Crystal structure parameters are reported in Table 2 for the corresponding structures.
Table 2.
Crystal structure parameters for TZ-5 and TZ-6.
| Crystal Data | TZ-5 | TZ-6 |
|---|---|---|
| Chemical formula | C10H17N5O2 | C10H14N6O2·H2O |
| Mr | 239.29 | 268.29 |
| Crystal system, space group | Triclinic, P-1 | Triclinic, P-1 |
| Temperature (K) | 100 | 100 |
| a, b, c (Å) | 10.4130 (11), 11.425 (2), 12.2354 (12) | 6.5042 (3), 7.5629 (4), 13.1645 (7) |
| α, β, γ, (°) | 99.703 (7), 114.649 (5), 102.470 (7) | 78.729 (3), 85.280 (3), 87.848 (3) |
| V (Å3) | 1,235.7 (3) | 632.77 (6) |
| Z | 4 | 2 |
3.2.2. Nuclear Magnetic Resonance (NMR)
The 1H- and 13C-NMR Heteronuclear Single Quantum Coherence (g-HSQC) and Heteronuclear Multiple Bond Correlation (g-HMBC) (1H-13C i 1H-15N) spectra were acquired on the Varian vnmrs 600 spectrometer at 599.94 MHz transmitter frequency for 1H at the Institute of Organic Chemistry Polish Academy of Science in Warsaw. The spectra were measured in the DMSO-d6 solution at room temperature. The chemical shift standards were as follows: liquid TMS (0.00 ppm for 1H-NMR), residual signal of DMSO (39.50 ppm for 13C-NMR) and liquid nitromethane (0.00 ppm for 15N-NMR).
3.2.3. Infrared Spectroscopy (IR)
The infrared spectra were obtained in the range from 4,000 to 400 cm−1 with the spectral resolution of 4 cm−1 using a Nicolet iS10 FT-IR spectrometer (Thermo Scientific, Waltham, MA, USA) and KBr pellets.
3.2.4. Raman Spectroscopy
FT Raman spectra were recorded on a Nicolet NXR 9650 instrument (Thermo Scientific), using 1,064 nm excitation from Nd:YVO4 laser, in the range from 3,700 to 150 cm−1 with a spectral resolution of 8 cm−1.
3.2.5. Thermal Analysis (DSC, TGA)
Thermal analyses were carried out on a DSC 822 differential scanning calorimeter equipped with IntraCooler and on a TGA/SDTA 851cells thermogravimeter (Mettler Toledo GmbH, Schwerzenbach, Switzerland) under nitrogen atmosphere. Accurately weighed samples (4–7 mg) were packed in aluminum pans with pierced lids. TGA measurements were blank curve corrected.
3.2.6. Water Content
Water content determination was done by Karl Fischer volumetric titration using the Methrohm 701 KF Titrino apparatus (Methrohm AG, Herisau, Switzerland).
3.2.7. High-Performance Liquid Chromatography (HPLC)
The purity of temozolomide produced in PRI according to an HPLC method [18] is not less than 99.50%. It has been proved experimentally, in a small-plant scale, that in order to obtain such high purity of TZ-7 in a desired polymorph form III [10], the purity of the intermediate TZ-6 and the starting material TZ-5 should not be less than 99.7% and 97.5%, respectively. Lower purity of TZ-6 leads to a higher level of impurities in TZ-7 which are difficult to remove during crystallisation. The purification of TZ-7 leads to its degradation to 5-amino-4-imidazolecarboxamide (AIC) or to the partial transformation, mainly into polymorph IX [10].
The determination of the chemical purity of TZ-7, TZ-6 and TZ-5 was carried out using Waters Alliance 2695e HPLC systems (Waters, Milford, MA, USA) equipped with gradient pumps, column heaters, autosamplers and either a UV-Vis 2487 or a PDA 2998 detector. The separations during the determination of TZ-7 purity were performed using Aqua C18, 250 × 4.6 mm, 5 µm column (Phenomenex, Torrance, CA, USA). Temozolomide-related substances were separated using water with 0.5% acetic acid and acetonitrile as Eluent A and Eluent B, respectively. The following gradient elution program was applied, t (min)/%B: 0/8; 8/8; 28/40; 31/8; 36/8. Other chromatographic parameters were as follows: column temperature: 25 °C; autosampler temperature: 5 ± 2 °C; flow rate: 1 mL min−1; injection volume: 20 µL; UV detection wavelength: λ = 254 nm; diluent: water with 0.5% HAc:ACN (4:1, v/v); the concentration of temozolomide in the investigated sample solutions: 1 mg mL−1. The chemical purity of TZ-6 was determined with the use of a XTerra C18, 250 × 4.6 mm, 5 µm column (Waters). 0.1% H3PO4 and methanol were used as Eluent A and Eluent B, respectively. The following gradient elution program was applied, t (min)/%B: 0/5; 20/50; 30/50; 32/5; 40/5. Other chromatographic parameters were as follows: column temperature: 25 °C; autosampler temperature: 15 °C; flow rate: 1 mL min−1; injection volume: 20 µL; UV detection wavelength: λ1 = 254 nm, λ2 = 201 nm; diluent: water with 0.1% H3PO4-MeOH (9:1, v/v); the concentration of TZ-6 in the investigated sample solutions: 0.5 mg mL−1. The chemical purity of TZ-5 was investigated using Aqua C18, 250 × 4.6 mm, 5 µm column. The separations were carried out using 0.1% H3PO4 and methanol as Eluent A and Eluent B, respectively. The following gradient elution program was applied, t (min)/%B: 0/0; 4/0; 15/50; 30/50; 32/0; 40/0. Other chromatographic parameters were as follows: column temperature: 25 °C; autosampler temperature: 5 ± 2 °C; flow rate: 1 mL min−1; injection volume: 10 µL; UV detection wavelength: λ1 = 254 nm, λ2 = 201 nm; diluent: ACN; the concentration of TZ-5 in the investigated sample solutions: 0.5 mg mL−1.
4. Conclusions
The structures of TZ-5 and TZ-6 were confirmed by X-ray diffraction and spectroscopic methods. The compounds were compared to temozolomide in the structural aspect. The thermal characteristics of the compounds were also presented. TZ-5 and TZ-6 were successfully obtained on a validated small-plant scale. The process is well documented as sets of operational instructions, batch reports and validation protocols. Two methods for the purity determination of both compounds were developed. The high purity of TZ-6 is crucial for obtaining temozolomide in polymorph form III with purity higher than 99.50%.
Acknowledgments
The support from European and Regional Funds under the project UDA-POIG.01.03.01-14-069/08-00 grant is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds TZ-5 and TZ-6 are available from the authors.
References
- 1.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research . ANDAs: Impurities in Drug Substances. Silver Spring, MD, USA: 2009. [Google Scholar]
- 2.ICH Harmonised Tripartite Guideline: Impurities in New Drug Substances Q3A (R2) London, UK: 2006. The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. [Google Scholar]
- 3.Newlands E.S., Stevens M.F.G., Wedge S.R., Wheelhouse R.T., Brock C. Temozolomide: A review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat. Rev. 1997;23:35–61. doi: 10.1016/S0305-7372(97)90019-0. [DOI] [PubMed] [Google Scholar]
- 4.Darkes M.J.M., Plosker G.L., Jarvis B. Temozolomide a review of its use in the treatment of malignant gliomas, malignant melanoma and other advanced cancers. Am. J. Cancer. 2002;1:55–80. doi: 10.2165/00024669-200201010-00006. [DOI] [Google Scholar]
- 5.Stupp R., Gander M., Leyvraz S., Newlands E. Current and future developments in the use of temozolomide for the treatment of brain tumours. Lancet Oncol. 2001;2:552–560. doi: 10.1016/S1470-2045(01)00489-2. [DOI] [PubMed] [Google Scholar]
- 6.FDA Approval for Temozolomide. Product information. TEMODAR® (temozolomide) CAPSULES. [(accessed on 15 March 2005)]. Available online: http://www.cancer.gov/cancertopics/druginfo/fda-temozolomide.
- 7.Lowe P.R., Sansom C.E., Schwalbe C.H., Stevens M.F.G., Clark A.S. Antitumor Imidazotetrazines. Crystal structure of 8-carbamoyl-3-methylimidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one (Temozolomide) and ctructural comparisons with the related drugs mitozolomide and DTIC. J. Med. Chem. 1992;35:3377–3382. doi: 10.1021/jm00096a013. [DOI] [PubMed] [Google Scholar]
- 8.Babu N.J., Reddy L.S., Aitipamula S., Nangia A. Polymorphs and polymorphic cocrystals of temozolomide. Chem. Asian J. 2008;3:1122–1133. doi: 10.1002/asia.200800070. [DOI] [PubMed] [Google Scholar]
- 9.Wheelhouse R.T., Wilman D.E.V., Thomson W., Stevens M.F.G. Antitumour imidazotetrazines. Part 31. The synthesis of isotopically labelled temozolomide and a multinuclear (1H, 13C, 15N) magnetic resonance investigation of temozolomide and mitozolomide. J. Chem. Soc. Perkin Trans. 1995;1:249–252. [Google Scholar]
- 10.Adin I., Iustain C. Novel Crystalline Forms of Temozolomide. 2005/063757. Patent WO. 2004 Dec 30;
- 11.Kuo S.-C., Mas J.L., Hou D. Synthesis of Temozolomide and Analogs. 2002/057269 (EP 135392 A1) Patent WO. 2002 Jan 16;
- 12.Cichy B., Gabarski K., Lipner G., Kaczmarek L., Rasinska M. An Improved Process for the Preparation of 2-amino-N-tert-butyl-2-cyanoamide Hydrochloride. Application No. P-395 425 A1. Polish Patent. 2011 Jun 24;
- 13.Whitehead C.W. The reactions of orthoesters with ureas. A new synthesis of pyrimidines. J. Am. Chem. Soc. 1953;75:671–675. doi: 10.1021/ja01099a047. [DOI] [Google Scholar]
- 14.APEXII-2008. Bruker Nonius; Madison, WI, USA: 2007. v 1.0. [Google Scholar]
- 15.SAINT V7.34A. Bruker Nonius; Madison, WI, USA: 2007. [Google Scholar]
- 16.SADABS-2008/1. Bruker AXS; Madison, WI, USA: 2008. SADABS-2008/1, Bruker AXS area detector scaling and absorption correction. [Google Scholar]
- 17.Sheldrick G.M. A short history of SHELX. Acta Cryst. 2008;A64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 18.Jedynak Ł., Puchalska M., Zezula M., Łaszcz M., Łuniewski W., Zagrodzka J. Stability of sample solution as a crucial point during HPLC determination of chemical purity of temozolomide drug substance. J. Pharmaceut. Biomed. Anal. 2013;83:19–27. doi: 10.1016/j.jpba.2013.04.032. [DOI] [PubMed] [Google Scholar]