Abstract
2,4-Dimethylbenzoylhydrazones 1–30 were synthesized by condensation reactions of 2,4-dimethylbenzoylhydrazide with various aromatic aldehydes and characterized. The assigned structures of compounds 10, 15 and 22 were further supported by single-crystal X-ray diffraction data. The synthesized compounds were evaluated for their in vitro DPPH radical scavenging activity. They exerted varying degree of scavenging activity toward DPPH radical with IC50 values between 25.6–190 µM. Compounds 1, 4, 2, 3, 7, and 6 have IC50 values of 25.6, 28.1, 29.3, 29.8, 30.0 and 30.1 µM respectively, showing better activity than an n-propyl gallate standard (IC50 value = 30.30 µM). For super oxide anion scavenging activity compounds 1, 2 and 3 with IC50 values of 98.3, 102.6, and 105.6, respectively, also showed better activity than the n-propyl gallate standard (IC50 value = 106.34 µM).
Keywords: 2,4-dimethylbenzoylhydrazones; DPPH scavenging; superoxide ion scavenger; crystal structure
1. Introduction
Hydrazone Schiff bases are a versatile set of compounds having unique properties. Hydrazones are the bimolecular condensation product of an aryl or alkyl hydrazine and a carbonyl compound (aldehyde or ketone). Due to their synthetic simplicity and active pharmacophore group, i.e., the C=N moiety, hydrazones are found to display biological and catalytic activities. One of the most prevalent in vivo biochemical processes involving the formation of a Schiff base is the condensation of lysine residues with the carbonyl group of methylglyoxal [1]. In this respect the Schiff base derived from pyrodoxal and amino acids has been thoroughly studied [2]. A broad range of biological activities of hydrazones such as anticancer [3] antibacterial [4] antifungal, herbicidal [5,6], anti-convalescent [7], anti-oxidant [8], and diuretic properties [9,10] have been reported. Several hetrocyclic hydrazones have been found to be potent cytotoxic [11], antimalarial [12], antiproliferative [13], anticancer and antifungal agents [14]. Hydrazones are also well known for their applications in foods and dyes as well as the agrochemical industry [15,16]. Among the various hydrazones, hydrazide-hydrazones merit special attention due to their distinct structural features. They have been reported as antioxidant [17,18,19] antifungal [20], anti-depressant [21], vasodilating [22], and anthelmintic agents [23]. Nifuroxazide, a hydrazide hydrazone has been patented as an intestinal antiseptic. Some of the more effective anti-tuberculosis drugs like iproniazide and isocarboxazide also contain hydrazide-hydrazone moieties [24]. A hydrazide hydazone derived from safrole was found to be s potent antiinflammatory/antinociceptive agent [25], while benzylidene 10H-phenothiazine-1-carbohydrazide has also been reported as an important anti-platelet agent [26]. The active pharmacophore (-CONH-N=CH-) of hydrazide hydrazones is mainly responsible for the significant biological activities although the attached neighboring groups may also be responsible [27]. Metal complexes of hydrazide hydrazones are also biologically active, besides having an important role in catalytic chemistry [28,29,30,31]. These metal complexes showed remarkable anti-cancer, antibacterial and antifungal activities [32].
Free radicals are highly reactive compounds formed in the body during biochemical reactions. They are oxidized the other metabolites in the body and can causes diseases such as Alzheimer’s disease (AD). The main reason for AD is oxidative stress, so it is necessary to inhibit or stop the formation of free radicals. Anti-oxidant compounds either natural of synthetic can be used to prevent the formation of free radicals. The major role of anti-oxidant compounds is to inhibit or neutralize the free radicals as well as repair the damages caused by free radicals [33].
In the view of the above, a series of structurally similar 2,4-dimethylbenzoylhydrazones were synthesized and evaluated for their antioxidant potential. This study has revealed some potential leads for possible pharmaceutical applications and further investigation may help in the development of new anti-oxidative agents for important metabolic functions.
2. Results and Discussion
Chemistry
In the continuation of our work on benzoylhydrazide [34,35,36], 2,4-dimethylbenzoylhydrazones 1–30 were synthesized from 2,4-dimethylbenzoylhydrazide, which in turn was synthesized from methyl 2,4-dimethylbenzoate by refluxing with hydrazine hydrate for 4 h. The 2,4-dimethylbenzoylhydrazide obtained was recrystallized from methanol to afford good yields of product. Next, 2,4-dimethylbenzoylhydrazones were prepared through the condensation reactions of 2,4-dimethylbenzoylhydrazide with different aromatic aldehydes in the presence of acetic acid (Scheme 1). The reaction mixture was refluxed in ethanol for 3 to 4 h. The crude products were recrystallized from methanol to give needle-like crystal in most cases (all yields are reported as crude product). Compounds 1–3, 7, 12, 14–16, 20, and 29 are new, while compounds 4–6, 8–11, 13, 17, 18, 21, 23–28, and 30 showed only CAS registry number with zero reference. Only compound 19 and 22 reported in the literature.
Scheme 1.
Synthesis of 2,4-dimethylbenzoylhydrazones.
The structural confirmation of 2,4-dimethylbenzoylhydrazones 1–30 was accomplished by several spectroscopic techniques, including 1H-NMR and mass spectroscopic. All compounds have an E-configuration as recently reported for some similar products [37,38,39,40,41,42]. We are also reporting three new crystal structures of compounds 10, 15 and 22.
3. Antioxidant Activity
3.1. DPPH Scavenging Activity
2,4-Dimethylbenzoylhydrazones 1–30 were evaluated for their in vitro DPPH radical scavenging activity and showed varying degree of activity, with IC50 values between 25.6–190 µM. Compounds 1, 4, 2, 3, 7, and 6 have IC50 values of 25.6, 28.1, 29.3, 29.8, 30.0 and 30.1 µM, respectively, showing better activity than standard n-propyl gallate IC50 value = 30.30 µM. Compound 5 (IC50 = 34.1 µM), 8 (IC50 = 34.3 µM), 12, (IC50 = 34.2 µM), and 9 (IC50 = 40.1 µM) exhibited good activity comparable to the standard. On the other hand, compounds 14 and 11, with IC50 values of 52.2 and 60.1 µM, respectively, showed only moderate activities. Compounds 20, 29 and 27 were found to be weakly active. Compounds 10, 13, 15–19, 21–26, 28 and 30, showed less than 50% activity; and thus their IC50 values were not evaluated (Table 1).
Table 1.
In vitro DDPH Radical Scavenging Activity of Compounds 1–30.
| R | Comp. No. | R | |||||
|---|---|---|---|---|---|---|---|
| No. | (%) | (µM ± SEM a) | (%) | (µM ± SEM a) | |||
| 1 | 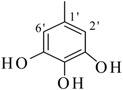 |
84 | 25.6 ± 0.5 | 10 | 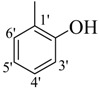 |
88 | NA b |
| 2 |  |
82 | 29.3 ± 1.0 | 11 |  |
90 | 60.1 ± 2.2 |
| 3 |  |
78 | 29.8 ± 1.1 | 12 |  |
87 | 34.2 ± 2.1 |
| 4 | 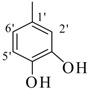 |
84 | 28.14 ± 0.8 | 13 | 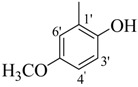 |
90 | NA b |
| 5 |  |
85 | 34.1 ± 1.0 | 14 |  |
87 | 52.2 ± 2.8 |
| 6 |  |
86 | 30.1 ± 1.3 | 15 | 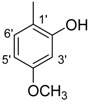 |
82 | NA b |
| 7 |  |
81 | 30.0 ± 1.2 | 16 | 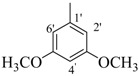 |
82 | NA b |
| 8 |  |
83 | 34.3 ± 1.5 | 17 |  |
84 | NA b |
| 9 | 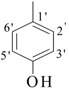 |
92 | 40.0 ± 1.8 | 18 | 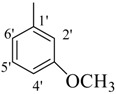 |
83 | NA b |
| 19 | 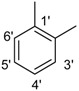 |
85 | NA b | 25 | 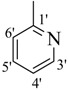 |
82 | NA b |
| 20 | 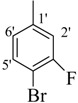 |
87 | 130.0 ± 4.8 | 26 | 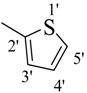 |
88 | NA b |
| 21 |  |
88 | NA b | 27 |  |
90 | 190.0 ± 5.1 |
| 22 |  |
90 | NA b | 28 |  |
92 | NA b |
| 23 |  |
92 | NAb | 29 |  |
91 | 180.0 ± 4.6 |
| 24 |  |
90 | NAb | 30 |  |
84 | NAb |
| Standard drug n-propyl gallate c | 30.30 ± 0.2 | ||||||
a SEM is the standard error of the mean; b NA = Not active; c n-propyl gallate standard used for the DDPH radical scavenging activity assays.
By comparing structural information and antioxidant activity, it was observed that DPPH radical scavenging antioxidant activity depends on two parameters: the functional groups on aromatic ring, and the position of functional groups on the ring. It was observed that the active compounds of the series bear an -OH group on the ring and activity pattern is such that, the more the -OH groups, the activity will be higher. Thus, the tri-OH group-containing derivatives were found to be more active than di- and mono-OH substituted analogues. Compound 1, which is a 3,4,5-trihydroxy derivative was the most active compound of this series, while compound 2 which bears a 2,4,6-trihyroxy group showed only slightly low activity than compound 1. Two factors might thus be involved in this activity pattern, one is the position of -OH groups and second, the extra resonance stability of compound 1 compared to compound 2 [43].
Interesting results are obtained when we compare the dihydroxy derivatives. Five dihydroxy derivatives showed excellent activity and variation in their activity is mainly due to the stability of the resulting radical. Compounds 4, 3, 6, and 7 showed excellent activity, better than standard. Interestingly, compound 5 having a 2,4-dihydroxy group, is the only dihydroxy analogue which has an IC50 value above the standard, indicating less stability of its radical as compared to other analogues [44,45].
Their activities of monohydroxy compounds 8–15 mainly depends upon the position of the hydroxy group. Compounds 8, 9 and 12 showed good activity having a 4-hydroxy group and the variation in their activities is due to the presence of a 3-methoxy as in compound 8 and a bromine in compound 12, which showed almost the same activity, and may be due to the resonance stabilizing potential of the methoxy and bromine substituents. On the other hand compounds 11 and 14 have a 3-hydroxy along with other sustituents but still showed less activity than the para-substituted analogues. It is well established that para-substitution has more stabilizing potential then meta-substitution. Compounds 10, 13 and 15 having 2-hydroxy group are found to be inactive, which may be due to the intramolecular hydrogen bonding. Compounds 20, 27 and 29 have halogen and ester moieties, respectively, which weakly stabilized the radical and showed weak activity. Compounds 10, 13, 15–19, 21–26, 28 and 30, not having any substituent to help stabilize the radical effectively, were found to be inactive as expected.
3.2. Superoxide Scavenging Activity
Compounds 1, 2 and 3 showed IC50 values of 98.3, 102.6 and 105.6 μM, respectively, better than the n-propyl gallate standard (106.3 μM). Compounds 4, 5, 6, 7, 8, 9, 12 and 14 showed moderate activity respectively (145, 170.2, 175.0, 180.1, 190.1, 208.9, 210.1 and 260.3 μM). Compound 20 showed very weak activity (Table 2).
Table 2.
In vitro Superoxide Anion Radical Scavenging Activity of Compounds 1–30.
| Comp. No. | IC50 (μM ± SEM a) | Comp. No. | IC50 (μM ± SEM a) |
|---|---|---|---|
| 1 | 98.3 ± 1.2 | 16 | NA b |
| 2 | 102.6 ± 1.5 | 17 | NA b |
| 3 | 105.6 ± 1.7 | 18 | NA b |
| 4 | 145 ± 2.1 | 19 | NA b |
| 5 | 170.2 ± 3.2 | 20 | 315.1 ± 8.4 |
| 6 | 175.0 ± 3.5 | 21 | NA b |
| 7 | 180.1 ± 3.8 | 22 | NA b |
| 8 | 190.1 ± 3.9 | 23 | NA b |
| 9 | 208.9 ± 5.4 | 24 | NA b |
| 10 | NA b | 25 | NA b |
| 11 | NA b | 26 | NA b |
| 12 | 210.1 ± 4.4 | 27 | NA b |
| 13 | NA b | 28 | NA b |
| 14 | 260.3 ± 6.4 | 29 | NA b |
| 15 | NA b | 30 | NA b |
| n-propyl gallate c | 106.34 ± 1.6 | ||
a SEM is the standard error of the mean, b NA = Not active, c n-propyl gallate was the standard drug for the superoxide anion radical scavenging assays.
Compounds 8, 10, 11, 13, 15–19 and 21–30 showed less than 50% inhibition and therefore the IC50 values were not further evaluated. Compounds 1 and 2 have trihydroxy substitution pattern at ring A, and showed remarkable activity, more active than the standard. Among the five dihydroxy-substituted analogues only compound 3 showed better activity than the standard, while the rest of the dihydroxy derivatives were found to be only moderately active towards superoxide. Compound 9 having a 4-hydroxy at ring A, showed moderate activity. Surprisingly, compound 8 having a 3-methoxy-4-OH substituted showed better activity than compound 9, while replacement of -OCH3 with -Br, decreased the activity. The weak activity of compound 14 might be due to combined effect of iodine and hydroxyl groups. The dihalogenated compound 20 also showed weak activity.
4. Experimental
4.1. General
NMR experiments were performed on an Ultra Shield Bruker FT NMR 500 MHz instrument (Wissembourg Cedex, France). CHN analysis was performed on a Carlo Erba Strumentazion-Mod-1106 (Milan, Italy). Electron impact mass spectra (EI MS) were recorded on a Finnigan MAT-311A unit (Bremen, Germany). Thin layer chromatography (TLC) was performed on pre-coated silica gel aluminum plates (Kieselgel 60, 254, E. Merck, Darmstadt, Germany). Chromatograms were visualized by UV at 254 and 365 nm.
4.2. X-ray Crystallography Studies
The structures of compounds 10, 15 and 22 were further supported by single crystal X-ray diffraction analysis. The 10, 15 and 22 were found to be monoclinic system crystals with space groups P21/c (compounds 10 and 22) and P21/n (compound 15). The ORTEP views of compounds 10 (Figure 1), 15 (Figure 2) and 22 (Figure 3), clearly indicated that structures of all compounds were composed of two planar phenyl rings (C1—C6 and C9—C14) with an E-configuration azomethine double bond. The hydroxyl functionality of the phenyl ring played an important role in stabilizing the E-configuration of the azomethine olefin bond in compounds 10 and 15 via intramolecular interaction. The crystal details of compounds 10, 15 and 22 as well as experimental data are summarized in Table 3. Single crystal X-ray diffraction data was collected on Bruker Smart APEX II, CCD area detector diffractometer [46] followed by data reduction preformed by SAINT program. The structure was solved and expanded by direct method and Fourier transformation techniques, respectively. SHELXL97 program was used to refine the structure by using full-matrix least-square calculation on F2 [47] (Sheldrick 1997). The ORTEP program was used to plot the Figure 1, Figure 2 and Figure 3. Figures were plotted with the aid of ORTEP program [48]. Crystallographic data of compound 10 (CCDC 933686), 15 (CCDC 933687) and 22 (CCDC 933685) can be obtained from Cambridge Crystallographic Data Center without any cost.
Figure 1.
ORTEP view of compound 10 with displacement ellipsoids drawn at 30% probability level. Dashed lines represent the intermolecular interaction; selected bond lengths [Å]: C7-O1 1.229(2), O2-C10 1.351(2), N1-C7 1.348(2), N1-N2 1.382(19), N2-C8 1.276(2).
Figure 2.
ORTEP view of compound 15 with displacement ellipsoids drawn at 30% probability level. Dashed lines represent the intermolecular interaction; selected bond lengths [Å]: C7-O2 1.226(18), O2-C14 1.349(2), O3-C12 1.357(2), N1-C7 1.352(2), N1-N2 1.38(19), N2-C8 1.276(2).
Figure 3.
ORTEP view of compound 22 with displacement ellipsoids drawn at 30% probability level; selected bond lengths [Å]: C7-O1 1.228(2), N1-C7 1.351(3), N1-N2 1.380(2), N2-C8 1.269(3).
Table 3.
The crystal and experimental data of compounds 10, 15 and 22.
| Compound 10 | Compound 15 | Compound 22 | |
|---|---|---|---|
| Empirical formula | C16H16N2O2 | C17H18N2O3 | C15H15N1O3 |
| Formula weight | 268.31 | 298.33 | 253.30 |
| Temperature | 273(2) | 273(2) | 273(2) |
| Wavelength | 0.71073 Å | 0.71073 Å | 0.71073 Å |
| Crystal system | Monoclinic | Monoclinic | Monoclinic |
| Space group | P21/c | P21/n | P21/c |
| A | 12.7293(17) Å | 11.1984(9) Å | 14.0960(14) Å |
| B | 13.0700(17) Å | 10.0703(9) Å | 11.8440(12) A |
| C | 8.8762(12) Å | 13.8665(12) Å | 8.2813(8) Å |
| β | 94.845(3)° | 102.535(2)° | 100.889(2)° |
| Volume | 1,463.2(3) A3 | 1,526.5(2) A3 | 1,357.7(2) A3 |
| Z | 4 | 4 | 4 |
| Calculated density | 1.218 mg/m3 | 1.298 mg/m3 | 1.239 mg/m3 |
| Absorption coefficient | 0.082 mm−1 | 0.090 mm−1 | 0.080 mm−1 |
| F(000) | 568 | 632 | 536 |
| Crystal size | 0.52 × 0.12 × 0.11 mm | 0.56 × 0.43 × 0.12 mm | 0.56 × 0.18 × 0.13 mm |
| θ range | 2.24 to 25.49° | 2.13 to 25.49° | 1.47 to 25.40° |
| Reflections Collected | 8546 | 8849 | 7827 |
| Reflections Unique | 2713 | 2758 | 2522 |
| (Rint) | 0.0275 | 0.0212 | 0.0313 |
| R1 with I > 2σ(I) | 0.0463 | 0.0438 | 0.0536 |
| R2 with I > 2σ(I) | 0.1124 | 0.1248 | 0.1508 |
| R1 for all data | 0.0774 | 0.0520 | 0.0702 |
| R2 for all data | 0.1323 | 0.1337 | 0.1607 |
| Goodness of fit | 1.030 | 1.057 | 1.014 |
| max/min ρ eA˚−3 | 0.140 and −0.110 | 0.232 and −0.018 | 0.265 and −0.258 |
4.3. DPPH (1,1-Diphenyl-2-picryl hydrazyl) Free Radical Scavenging Activity
The free radical scavenging activity was measured by 1,1-diphenyl-2-picrylhydrazyl (DPPH) using literature protocols. The reaction mixture contained test sample (5 μL, 1 mM in DMSO) and DPPH (Sigma, 95 μL, 300 μM) in ethanol. The reaction mixture was taken into a 96-well microtiter plate and incubated at 37 °C for 30 min. The absorbance was measured at 515 nm using microtiter plate reader (Molecular Devices, Sunnyvale, CA, USA). Percent radical scavenging activity was determined in comparison with DMSO containing control (Table 1). IC50 values represent the concentration of compounds able to scavenge 50% of DPPH radicals. Propyl gallate was used as positive control. All chemicals used were of analytical grade (Sigma, Ronkonkoma, NY, USA).
4.4. In Vitro Assay for Superoxide Anion Radical Scavenging Activity
The superoxide producing system was set up by mixing phenazinemethosulfate (PMS), NADH, and oxygen (air), and the production of superoxide was estimated by the nitroblue tetrazolium method. Measurement of superoxide radical scavenging activity was carried out on the basis of the method described by the modified method used by Ferda. In aerobic reaction mixtures containing NADH, phenazine methosulphate and nitro blue tetrazolium, PMS is reduced by NADH and then gave rise to O2−, which in turn reduced NBT. On the basis of this PMS has frequently been used to mediate O2−.
The reaction mixture comprised 100 µΜ β-nicotinamide adenine dinucleotide reduced form (NADH, 40 µL), 80 µM of nitro blue tetrazolium (NBT, 40 µL), 8 µM phenazine methosulphate (PMS, 20 µL), 1 mM sample (10 µL), and 0.1 M phosphate buffer (pH 7.4, 90 µL). The reagents were prepared in buffer and sample in DMSO. The reaction was performed in 96-well microtitre plate at room temperature and absorbance was measured at 560 nm. The formation of superoxide was monitored by measuring the formation of water soluble blue formosan dye. A lower absorbance of reaction mixture indicated a higher scavenging activity of the sample. Percent radical scavenging activity (% RSA) by samples was determined in comparison with a control using the following equation:
| %RSA = 100 − {(OD test compound/OD control) × 100 |
4.5. General Procedure for the Synthesis of 2,4-Dimethylbenzohydrazide
Methyl 2,4-dimethylbenzoate (40 mmol) was refluxed with hydrazine hydrate (10 mL) in methanol (50 mL) for 6 h. Excess hydrazine and methanol was evaporated to obtain crude product which was recrystallized from methanol to give pure 2,4-dimethylbenzohydrazide (92% yield).
4.6. General Procedure for the Synthesis of 2,4-Dimethylbenzohydrazones
The 2,4-dimethylbenzohydrazones were synthesized by refluxing in methanol (15mL) for 3 h each pure 2,4-dimethylbenzohydrazide (2 mmol) and various aryl aldehydes (2 mmol) in the presence of a catalytical amount of acetic acid. The progress of reaction was monitored by TLC. After completion of reaction, the solvent was evaporated by vacuum to afford crude products which were further recrystallized from methanol and got needle like pure product in good to excellent yields.
(E)-N'-(3,4,5-Trihydroxybenzylidene)-2,4-dimethylbenzohydrazide (1). Yield: 0.504 g (84%); 1H-NMR (DMSO-d6): δ 11.34 (s, 1H, NH), 9.07 (br. s, 3H, 3× OH), 8.02 (s, 1H, ArCH=N-R), 7.33 (d, 1H, J6,5 = 7.5 Hz, H-6), 7.11 (s, 1H, H-3), 7.01 (d, 1H, J5,6 = 7.5 Hz, H-5), 6.66 (s, 2H, H-2', H-6'), 2.37 (s, 3H, CH3), 2.32 (s, 3H, CH3); IR (KBr, cm−1): 3460 (OH), 3320 (N-H), 1652 (C=O), 1628 (C=N), 1248 (C-N); Anal. Calcd for C16H16N2O4, C = 63.99, H = 5.37, N = 9.33, O = 21.31, Found C = 64.01, H =5.37, N = 9.34, O = 21.32; EI MS m/z (% rel. abund.): 300.
(E)-N'-(2,4,6-Trihydroxybenzylidene)-2,4-dimethylbenzohydrazide (2). Yield: 0.492 g (82%); 1H-NMR (DMSO-d6): δ 11.10 (s, 1H, NH), 9.46 (br. s, 3H, 3× OH), 8.30 (s, 1H, ArCH=N-R), 7.28 (d, 1H, J6,5 = 8.0 Hz, H-6), 7.09 (s, 1H, H-3), 6.98 (d, 1H, J5,6 = 8.0 Hz, H-5), 6.78 (s, 2H, H-3', H-5'), 2.36 (s, 3H, CH3), 2.30 (s, 3H, CH3); IR (KBr, cm−1): 3480 (OH), 3370 (N-H), 1658 (C=O), 1632 (C=N), 1253 (C-N); Anal. Calcd for C16H16N2O4, C = 63.99, H = 5.37, N = 9.33, O = 21.31, Found C = 64.02, H = 5.35, N = 9.32, O = 21.33; EI MS m/z (% rel. abund.): 300.
(E)-N'-(2,5-Dihydroxybenzylidene)-2,4-dimethylbenzohydrazide (3). Yield: 0.443 g (78%); 1H-NMR (DMSO-d6): δ 11.78 (s, 1H, NH), 10.40 (s, 1H, OH), 8.96 (s, 1H, OH), 8.41 (s, 1H, ArCH=N-R), 7.39 (d, 1H, J6,5 = 8.5 Hz, H-6), 7.14 (s, 1H, H-3), 7.10 (d, 1H, J5,6 = 8.5 Hz, H-5), 6.93 (s, 1H, H-6'), 6.74–6.70 (m, 2H, H-3', H-4'), 2.37 (s, 3H, CH3), 2.32 (s, 3H, CH3); IR (KBr, cm−1): 3410 (OH), 3330 (N-H), 1656 (C=O), 1636 (C=N), 1256 (C-N); Anal. Calcd for C16H16N2O3, C = 67.59, H = 5.37, N = 9.85, O = 16.88, Found C = 67.58, H = 5.68, N = 9.84, O = 16.89; EI MS m/z (% rel. abund.): 284.
(E)-N'-(3,4-Dihydroxybenzylidene)-2,4-dimethylbenzohydrazide (4) [37]. Yield: 0.477 g (84%); 1H-NMR (DMSO-d6): δ 11.20 (s, 1H, NH), 9.10 (s, 2H, 2× OH), 8.10 (s, 1H, ArCH=N-R), 7.33 (d, 1H, J6,5 = 7.5 Hz, H-6), 7.21 (s, 1H, H-2') 7.11 (s, 1H, H-3), 7.09 (d, 1H, J5,6 = 7.5 Hz, H-5), 6.09 (d, 1H, J5',6' = 8.5 Hz, H-5'), 6.78 (d, 1H, J6',5' = 8.5 Hz, H-6'), 2.35 (s, 3H, CH3), 2.32 (s, 3H, CH3); IR (KBr, cm−1): 3405 (OH), 3330 (N-H), 1658 (C=O), 1634 (C=N), 1250 (C-N); Anal. Calcd for C16H16N2O3, C = 67.59, H = 5.37, N = 9.85, O = 16.88, Found C = 67.58, H = 5.68, N = 9.84, O = 16.89; EI MS m/z (% rel. abund.): 284.
(E)-N'-(2,4-Dihydroxybenzylidene)-2,4-dimethylbenzohydrazide (5). Yield: 0.482 g (85%); 1H-NMR (DMSO-d6): δ 11.7 (s, 1H, NH), 11.45 (s, 1H, OH), 9.91 (s, 1H, OH), 8.36 (s, 1H, ArCH=N-R), 7.38 (d, 1H, J6,5 = 7.5 Hz, H-6), 7.27 (d, 1H, J6',5' = 6.5 Hz, H-6'), 7.11 (s, 1H, H-3), 7.08 (d, 1H, J5,6 = 7.5 Hz, H-5), 6.36 (dd, 1H, J5',3' = 2.0, J5',6' = 6.5 Hz, H-5'), 6.32 (d, 1H, J3',5' = 2.0 Hz, H-3'), 2.37 (s, 3H, CH3), 2.33 (s, 3H, CH3); IR (KBr, cm−1): 3445 (OH), 3325 (N-H), 1657 (C=O), 1631 (C=N), 1251 (C-N); Anal. Calcd for C16H16N2O3, C = 67.59, H = 5.37, N = 9.85, O = 16.88, Found C = 67.58, H = 5.68, N = 9.84, O = 16.89; EI MS m/z (% rel. abund.): 284.
(E)-N'-(2,3-Dihydroxybenzylidene)-2,4-dimethylbenzohydrazide (6). Yield: 0.488 g (86%); 1H-NMR (DMSO-d6): δ 11.2 (s, 1H, NH), 9.60 (s, 2H, 2× OH), 8.45 (s, 1H, ArCH=N-R), 7.41 (d, 1H, J6,5 = 7.5 Hz, H-6), 7.14 (s, 1H, H-3), 7.10 (d, 1H, J5,6 = 7.5 Hz, H-5), 6.94 (d 1H, J6',5' = 6.5 Hz, H-6'), 6.86 (d, 1H, J4',5' = 7.0 Hz, H-4'), 6.36 (dd, 1H, J5',4' = 7.0, J5',6' = 6.5 Hz, H-5') 2.38 (s, 3H, CH3), 2.33 (s, 3H, CH3); IR (KBr, cm−1): 3420 (OH), 3335 (N-H), 1657 (C=O), 1632 (C=N), 1254 (C-N); Anal. Calcd for C16H16N2O3, C = 67.59, H = 5.37, N = 9.85, O = 16.88, Found C = 67.58, H = 5.68, N = 9.84, O = 16.89; EI MS m/z (% rel. abund.): 284.
(E)-N'-(3,5-Dihydroxybenzylidene)-2,4-dimethylbenzohydrazide (7). Yield: 0.460 g (81%); 1H-NMR (DMSO-d6): δ 11.30 (s, 1H, NH), 10.50 (s, H, OH), 9.52 (s, H, OH), 8.40 (s, 1H, ArCH=N-R), 7.40 (d, 1H, J6,5 = 7.5 Hz, H-6), 7.18 (s, 1H, H-3), 7.12 (d, 1H, J5,6 = 7.5 Hz, H-5), 6.95 (s, 2H, H-2', H-6'), 6.72 (s, 1H, H-4'), 2.38 (s, 3H, CH3), 2.34 (s, 3H, CH3); IR (KBr, cm−1): 3435 (OH), 3330 (N-H), 1649 (C=O), 1627 (C=N), 1254 (C-N); Anal. Calcd for C16H16N2O3, C = 67.59, H = 5.37, N = 9.85, O = 16.88, Found C = 67.58, H = 5.68, N = 9.84, O = 16.89; EI MS m/z (% rel. abund.): 284.
(E)-N'-(4-Hydroxy-3-methoxybenzylidene)-2,4-dimethylbenzohydrazide (8). Yield: 0.494 g (83%); 1H-NMR (DMSO-d6): δ 11.45 (s, 1H, NH), 9.50 (s, 1H, OH), 8.19 (s, 1H, ArCH=N-R), 7.35 (d, 1H, J6,5 = 7.5 Hz, H-6), 7.31 (d, 1H, J2',6' = 2.0 Hz, H-2'), 7.12 (s, 1H, H-3), 7.10 (d, 1H, J5,6 = 7.5 Hz, H-5), 7.07 (dd, 1H, J6',2' = 2.0, J6',5' = 8.0 Hz, H-6'), 6.84 (d 1H, J5',6' = 8.0 Hz, H-5'), 2.36 (s, 3H, CH3), 2.32 (s, 3H, CH3), IR (KBr, cm−1): 3405 (OH), 3335 (N-H), 1659 (C=O), 1633 (C=N), 1249 (C-N); Anal. Calcd for C17H18N2O3, C = 68.45, H = 5.37, N = 9.39, O = 16.09, Found C = 68.46, H = 6.08, N = 9.40, O = 16.10; EI MS m/z (% rel. abund.): 298.
(E)-N'-(4-Hydroxybenzylidene)-2,4-dimethylbenzohydrazide (9). Yield: 0.493 g (92%); 1H-NMR (DMSO-d6): δ 11.43 (s, 1H, NH), 9.87 (s, 1H, OH), 8.20 (s, 1H, ArCH=N-R), 7.54 (d, 2H, J2',3' = J6',5' = 8.0 Hz, H-2',H-6'), 7.33 (d, 1H, J6,5 = 7.5 Hz, H-6), 7.17 (s, 1H, H-3), 7.11 (d, 1H, J5,6 = 7.5 Hz, H-5), 6.84 (d, 2H, J3',2' = J5',6' = 8.0 Hz, H-3',H-5'), 2.35 (s, 3H, CH3), 2.32 (s, 3H, CH3), IR (KBr, cm−1): 3410 (OH), 3325 (N-H), 1663 (C=O), 1636 (C=N), 1254 (C-N); Anal. Calcd for C16H16N2O2, C = 71.62, H = 6.01, N = 10.44, O = 11.93, Found C = 71.61, H = 6.02, N = 10.45, O = 11.94; EI MS m/z (% rel. abund.): 268.
(E)-N'-(2-Hydroxybenzylidene)-2,4-dimethylbenzohydrazide (10). Yield: 0.471g (88%); 1H-NMR (DMSO-d6): δ 11.11 (s, 1H, NH), 9.25 (s, 1H, OH), 9.01 (s, 1H, ArCH=N-R), 7.71 (dd, 1H, J3',4' = 6.5, J3',5' = 6.5 Hz, H-3') 7.43–7.39 (m, 2H, H-6',H-6), 7.17 (s, 1H, H-3), 7.11 (d, 1H, J5,6 = 7.5 Hz, H-5), 6.99–6.94 (m, 2H, H-4',H-5'), 2.38 (s, 3H, CH3), 2.33 (s, 3H, CH3); IR (KBr, cm−1): 3415 (OH), 3330 (N-H), 1663 (C=O), 1638 (C=N), 1258 (C-N); Anal. Calcd for C16H16N2O2, C = 71.62, H = 6.01, N = 10.44, O = 11.93, Found C = 71.60, H = 6.03, N = 10.44, O = 11.95; EI MS m/z (% rel. abund.): 268.
(E)-N'-(3-Hydroxy-4-methoxybenzylidene)-2,4-dimethylbenzohydrazide (11). Yield: 0.522 g (90%); 1H-NMR (DMSO-d6): δ 11.45 (s, 1H, NH), 9.26 (s, 1H, OH), 8.15 (s, 1H, ArCH=N-R), 7.34 (d, 1H, J6,5 = 7.5 Hz, H-6), 7.32 (d, 1H, J2',6' = 2.0 Hz, H-2'), 7.17 (s, 1H, H-3), 7.10 (d, 1H, J5,6 = 7.5 Hz, H-5), 7.08 (dd, 1H, J6',2' = 2.0, J6',5' = 7.0, H-6'), 7.04 (d, 1H, J5',6' = 7.0, H-5'), 2.35 (s, 3H, CH3), 2.32 (s, 3H, CH3), IR (KBr, cm−1): 3950 (OH), 3320 (N-H), 1658 (C=O), 1630 (C=N), 1254 (C-N); Anal. Calcd for C17H18N2O3, C = 68.45, H = 5.37, N = 9.39, O = 16.09, Found C = 68.46, H = 6.08, N = 9.40, O = 16.10; EI MS m/z (% rel. abund.): 298.
(E)-N'-(3-Bromo-4-hydroxybenzylidene)-2,4-dimethylbenzohydrazide (12). Yield: 0.602 g (87%); 1H-NMR (DMSO-d6): δ 11.35 (s, 1H, NH), 10.57 (s, 1H, OH), 8.30 (s, 1H, ArCH=N-R), 7.87 (d, 1H, J2',6' = 2.0 Hz, H-2'), 7.58 (dd, 1H, J6',2' = 2.0, J6',5' = 7.0, H-6'), 7.32 (d, 1H, J6,5 = 7.5 Hz, H-6), 7.15 (s, 1H, H-3), 7.09 (d, 1H, J5,6 = 7.5 Hz, H-5), 7.05 (d, 1H, J6',5' = 7.0, H-5'), 2.35 (s, 3H, CH3), 2.32 (s, 3H, CH3); IR (KBr, cm−1): 3954 (OH), 3318 (N-H), 1655 (C=O), 1635 (C=N), 1250 (C-N); Anal. Calcd for C16H15BrN2O2, C = 55.35, H = 4.35, N = 8.07, O = 9.22, Found C = 55.36, H = 4.35, N = 8.08, O = 9.21; EI MS m/z (% rel. abund.): 346.
(E)-N'-(2-Hydroxy-5-methoxybenzylidene)-2,4-dimethylbenzohydrazide (13). Yield: 0.536 g (90%); 1H-NMR (DMSO-d6): δ 11.88 (s, 1H, NH), 10.66 (s, 1H, OH), 8.48 (s, 1H, ArCH=N-R), 7.40 (d, 1H, J6,5 = 7.5 Hz, H-6), 7.14–7.09 (m, 1H, H-5, H-3, H-6'), 6.92 (d, 1H, J3',4' = 7.0, H-3'), 6.87 (d, 1H, J4',3' = 7.0, H-4'), 2.38 (s, 3H, CH3), 2.33 (s, 3H, CH3); IR (KBr, cm−1): 3350 (OH), 3310 (N-H), 1650 (C=O), 1632 (C=N), 1248 (C-N); Anal. Calcd for C17H18N2O3, C = 68.45, H = 5.37, N = 9.39, O = 16.09, Found C = 68.46, H = 6.08, N = 9.40, O = 16.10; EI MS m/z (% rel. abund.): 298.
(E)-N'-(3-Hydroxy-2-iodo-4-methoxybenzylidene)-2,4-dimethylbenzohydrazide (14). Yield: 0.738 g (87%); 1H-NMR (DMSO-d6): δ 11.88 (s, 1H, NH), 9.21 (s, 1H, OH), 8.48 (s, 1H, ArCH=N-R), 7.36 (d, 1H, J6,5 = 7.5 Hz, H-6), 7.16 (s, 1H, H-3), 7.10 (d, 1H, J5,6 = 7.5 Hz, H-5), 6.85 (d, 1H, J6',5' = 7.0, H-6'), 6.68 (d, 1H, J5',6' = 7.0, H-5'), 3.84 (s, 3H, OCH3), 2.35 (s, 3H, CH3), 2.32 (s, 3H, CH3); IR (KBr, cm−1): 3350 (OH), 3310 (N-H), 1650 (C=O), 1632 (C=N), 1248 (C-N); Anal. Calcd for C17H17IN2O3, C = 48.13, H = 4.04, N = 6.60, O = 11.31, Found C = 48.14, H = 4.05, N = 6.62, O = 11.33; EI MS m/z (% rel. abund.): 424.
(E)-N'-(2-Hydroxy-4-methoxybenzylidene)-2,4-dimethylbenzohydrazide (15). Yield: 0.488 g (82%); 1H-NMR (DMSO-d6): δ 11.78 (s, 1H, NH), 11.60 (s, 1H, OH), 8.41 (s, 1H, ArCH=N-R), 7.40 (d, 1H, J6,5 = 7.5 Hz, H-6), 7.38 (d, 1H, J3',5' = 2.0 Hz, H-3'), 7.14 (s, 1H, H-3), 7.11 (d, 1H, J5,6 = 7.5 Hz, H-5), 6.53 (d, 1H, J5',6' = 7.5 Hz, H-5'), 6.50 (d, 1H, J6',5' = 7.5 Hz, H-6'), 2.37 (s, 3H, CH3), 2.33 (s, 3H, CH3); IR (KBr, cm−1): 3390 (OH), 3300 (N-H), 1655 (C=O), 1630 (C=N), 1250 (C-N); Anal. Calcd for C17H18N2O3, C = 68.45, H = 5.37, N = 9.39, O = 16.09, Found C = 68.46, H = 6.08, N = 9.40, O = 16.10; EI MS m/z (% rel. abund.): 298.
(E)-N'-(3,5-Dimethoxybenzylidene)-2,4-dimethylbenzohydrazide (16). Yield: 0.511 g (82%); 1H-NMR (DMSO-d6): δ 11.67 (s, 1H, NH), 8.23 (s, 1H, ArCH=N-R), 7.36 (d, 1H, J6,5 = 7.5 Hz, H-6), 7.14 (s, 1H, H-3), 7.11 (d, 1H, J5,6 = 7.5 Hz, H-5), 6.86 (s, 2H, H-2', H-6'), 6.57 (s, 1H, H-4'), 3.86 (s, 6H, 2× OCH3), 2.36 (s, 3H, CH3), 2.33 (s, 3H, CH3); IR (KBr, cm−1): 3310 (N-H), 1650 (C=O), 1628 (C=N), 1252 (C-N); Anal. Calcd for C18H20N2O3, C = 69.21, H = 6.45, N = 8.97, O = 15.37, Found C = 69.20, H = 6.44, N = 8.98, O = 15.38; EI MS m/z (% rel. abund.): 312.
(E)-N'-(3,4-Dimethoxybenzylidene)-2,4-dimethylbenzohydrazide (17). Yield: 0.524 g (84%); 1H-NMR (DMSO-d6): δ 11.40 (s, 1H, NH), 8.20 (s, 1H, ArCH=N-R), 7.35 (d, 1H, J6,5 = 7.5 Hz, H-6), 7.18 (s, 1H, H-2′) 7.12 (s, 1H, H-3), 7.10 (d, 1H, J5,6 = 7.5 Hz, H-5), 6.11 (d, 1H, J5',6' = 8.0 Hz, H-5'), 6.81 (d, 1H, J6',5' = 8.0 Hz, H-6'), 3.90 (s, 3H, OCH3), 3.85 (s, 3H, OCH3), 2.38 (s, 3H, CH3), 2.34 (s, 3H, CH3); IR (KBr, cm−1): 3340 (N-H), 1653 (C=O), 1634 (C=N), 1256 (C-N); Anal. Calcd for C18H20N2O3, C = 69.21, H = 6.45, N = 8.97, O = 15.37, Found C = 69.20, H = 6.44, N = 8.98, O = 15.38; EI MS m/z (% rel. abund.): 312.
(E)-N'-(3-Methoxybenzylidene)-2,4-dimethylbenzohydrazide (18). Yield: 0.468 g (83%); 1H-NMR (DMSO-d6): δ 11.66 (s, 1H, NH), 8.28 (s, 1H, ArCH=N-R), 7.45-7.38 (m, 4H, H-4', H-5', H-6', H-6), 7.13 (s, 1H, H-3), 7.11 (d, 1H, J5,6 = 7.5 Hz, H-5), 7.08 (d, 1H, J2',6' = 2.0 Hz, H-2'), 3.88 (s, 3H, OCH3), 2.36 (s, 3H, CH3), 2.33 (s, 3H, CH3); IR (KBr, cm−1): 3324 (N-H), 1658 (C=O), 1638 (C=N), 1251 (C-N); Anal. Calcd for C17H18N2O2, C = 72.32, H = 6.43, N = 9.92, O = 11.33, Found C = 72.33, H = 6.44, N = 9.91, O = 11.34; EI MS m/z (% rel. abund.): 282.
(E)-N'-(2-Methylbenzylidene)-2,4-dimethylbenzohydrazide (19) [40]; Yield: 0.452 g (85%); 1H-NMR (DMSO-d6): δ 11.66 (s, 1H, NH), 8.28 (s, 1H, ArCH=N-R), 7.35-7.32 (m, 4H, H-3', H-4', H-5', H-6), 7.12 (s, 1H, H-3), 7.09 (d, 1H, J5,6 = 7.5 Hz, H-5), 7.01 (d, 1H, J6',5' = 8.0 Hz, H-6'), 2.38 (s, 3H, CH3), 2.35 (s, 3H, CH3), 2.32 (s, 3H, CH3); IR (KBr, cm−1): 3330 (N-H), 1652 (C=O), 1642 (C=N), 1255 (C-N); Anal. Calcd for C17H18N2O, C = 76.66, H = 6.81, N = 10.52, O = 6.01, Found C = 76.68, H = 6.82, N = 10.50, O = 6.02; EI MS m/z (% rel. abund.): 266.
(E)-N'-(4-Bromo-3-florobenzylidene)-2,4-dimethylbenzohydrazide (20). Yield: 0.605 g (87%); 1H-NMR (DMSO-d6): δ 11.85 (s, 1H, NH), 8.82 (s, 1H, ArCH=N-R), 7.45–7.38 (m, 3H, H-5', H-6', H-6), 7.21 (d, 1H, J2',6' = 2.0 Hz, H-2'), 7.11 (s, 1H, H-3), 7.08 (d, 1H, J5,6 = 7.5 Hz, H-5), 2.38 (s, 3H, CH3), 2.34 (s, 3H, CH3); IR (KBr, cm−1): 3310 (N-H), 1651 (C=O), 1632 (C=N), 1247 (C-N); Anal. Calcd for C16H14BrFN2O, C = 55.03, H = 4.04, N = 8.02, O = 4.59, Found C = 55.03, H = 4.03, N = 8.04, O = 4.59; EI MS m/z (% rel. abund.): 348.
(E)-N'-(4-Methoxybenzylidene)-2,4-dimethylbenzohydrazide (21). Yield: 0.496 g (88%); 1H-NMR (DMSO-d6): δ 11.30 (s, 1H, NH), 8.10 (s, 1H, ArCH=N-R), 7.62 (d, 2H, J2',3' = J6',5' = 8.0 Hz, H-2', H-6'), 7.34 (d, 1H, J6,5 = 7.0 Hz, H-6), 7.11 (s, 1H, H-3), 7.08 (d, 1H, J5,6 = 7.0 Hz, H-5), 6.92 (d, 2H, J3',2' = J5',6' = 8.0 Hz, H-3', H-5'), 3.84 (s, 3H, OCH3), 2.36 (s, 3H, CH3), 2.33 (s, 3H, CH3); IR (KBr, cm−1): 3342 (N-H), 1661 (C=O), 1640 (C=N), 1254 (C-N); Anal. Calcd for C17H18N2O2, C = 72.32, H = 6.43, N = 9.92, O = 11.33, Found C = 72.33, H = 6.45, N = 9.92, O = 11.32; EI MS m/z (% rel. abund.): 282.
(E)-2,4-Dimethyl-N'-((pyridin-3-yl)methylene)benzohydrazide (22). Yield: 0.455 g (90%); 1H-NMR (DMSO-d6): δ 11.82 (s, 1H, NH), 8.84 (s, 1H, ArCH=N-R), 8.61 (s, 1H, H-2'), 8.31 (s, 1H, H-6'), 8.13 (d, 1H, J4',5' = 7.0 Hz, H-4'), 7.49 (d, 1H, J5',4' = 7.0 Hz, H-5'), 7.38 (d, 1H, J6,5 = 7.0 Hz, H-6), 7.14 (s, 1H, H-3), 7.12 (d, 1H, J5,6 = 7.5 Hz, H-5), 2.37 (s, 3H, CH3), 2.33 (s, 3H, CH3); IR (KBr, cm−1): 3360 (N-H), 1670 (C=O), 1645 (C=N), 1258 (C-N); Anal. Calcd for C15H15N3O, C = 71.13, H = 5.97, N = 16.59, O = 6.32, Found C = 71.14, H = 5.98, N = 16.61, O = 6.33; EI MS m/z (% rel. abund.): 253.
(E)-N'-(4-Methylbenzylidene)-2,4-dimethylbenzohydrazide (23). Yield: 0.490 g (92%); 1H-NMR (DMSO-d6): δ 11.58 (s, 1H, NH), 8.27 (s, 1H, ArCH=N-R), 7.61 (d, 2H, J2',3' = J6',5' = 7.5 Hz, H-2', H-6'), 7.35 (d, 1H, J6,5 = 7.5 Hz, H-6), 7.32 (d, 2H, J3',2' = J5',6' = 7.5 Hz, H-3', H-5'), 7.17 (s, 1H, H-3), 7.13 (d, 1H, J5,6 = 7.5 Hz, H-5), 2.37 (s, 3H, CH3), 2.33 (s, 6H, 2× CH3), IR (KBr, cm−1): 3345 (N-H), 1658 (C=O), 1632 (C=N), 1250 (C-N); Anal. Calcd for C17H18N2O, C = 76.66, H = 6.81, N = 10.52, O = 6.01, Found C = 76.67, H = 6.82, N = 10.50, O = 6.02; EI MS m/z (% rel. abund.): 266.
(E)-2,4-Dimethyl-N'-((pyridin-4-yl)methylene)benzohydrazide (24). Yield: 0.455 g (90%); 1H-NMR (DMSO-d6): δ 11.99 (s, 1H, NH), 8.65 (d, 2H, J2',3' = J6',5' = 6.0 Hz, H-2', H-6'), 8.30 (s, 1H, ArCH=N-R), 7.66 (d, 2H, J3',2' = J5',6' = 6.0 Hz, H-3', H-5'), 7.40 (d, 1H, J6,5 = 7.5 Hz, H-6), 7.15 (s, 1H, H-3), 7.12 (d, 1H, J5,6 = 7.5 Hz, H-5), 2.38 (s, 3H, CH3), 2.32 (s, 3H, CH3), IR (KBr, cm−1): 3350 (N-H), 1654 (C=O), 1638 (C=N), 1254 (C-N); Anal. Calcd for C15H15N3O, C = 71.13, H = 5.97, N = 16.59, O = 6.32, Found C = 71.13, H = 6.00, N = 16.62, O = 6.32; EI MS m/z (% rel. abund.): 253.
(E)-2,4-Dimethyl-N'-((pyridin-2-yl)methylene)benzohydrazide (25). Yield: 0.414 g (82%); 1H-NMR (DMSO-d6): δ 11.87 (s, 1H, NH), 8.55 (d, 2H, J6',5' = 6.0 Hz, H-6'), 8.25 (s, 1H, ArCH=N-R), 7.90–7.86 (m, 2H, H-4', H-5'), 7.38 (d, 1H, J6,5 = 7.0 Hz, H-6), 7.13 (s, 1H, H-3), 7.10 (d, 1H, J5,6 = 7.0 Hz, H-5), 6.95 (d, 2H, J3',4' = 6.5 Hz, H-3'), 2.37 (s, 3H, CH3), 2.33 (s, 3H, CH3), IR (KBr, cm−1): 3342 (N-H), 1656 (C=O), 1636 (C=N), 1253 (C-N); Anal. Calcd for C15H15N3O, C = 71.13, H = 5.97, N = 16.59, O = 6.32, Found C = 71.15, H = 6.00, N = 16.62, O = 6.32; EI MS m/z (% rel. abund.): 253.
(E)-2,4-Dimethyl-N'-((thiophen-2-yl)methylene)benzohydrazide (26). Yield: 0.454 g (88%); 1H-NMR (DMSO-d6): δ 11.63 (s, 1H, NH), 8.51 (s, 1H, ArCH=N-R), 7.66 (s, 1H, H-5'), 7.43 (s, 1H, H-2'), 7.35 (d, 1H, J6,5 = 7.5 Hz, H-6), 7.11–7.08 (m, 3H, H-3, H-5, H-4'), 2.36 (s, 3H, CH3), 2.33 (s, 3H, CH3), IR (KBr, cm−1): 3350 (N-H), 1654 (C=O), 1638 (C=N), 1254 (C-N); Anal. Calcd for C14H14N2OS, C = 65.09, H = 5.46, N = 10.84, O = 6.19, Found C = 65.08, H = 5.48, N = 10.83, O = 6.18; EI MS m/z (% rel. abund.): 258.
Methyl 4-((E)-(2,4-dimethylbenzoylimino)methyl)benzoate (27). Yield: 0.558 g (90%); 1H-NMR (DMSO-d6): δ 11.83 (s, 1H, NH), 8.36 (s, 1H, ArCH=N-R), 8.04 (d, 2H, J2',3' = J6',5' = 7.5 Hz, H-2', H-6'), 7.86 (d, 2H, J3',2' = J5',6' = 7.5 Hz, H-3', H-5'), 7.38 (d, 1H, J6,5 = 7.5 Hz, H-6), 7.14 (s, 1H, H-3), 7.11 (d, 1H, J5,6 = 7.5 Hz, H-5), 3.88 (s, 3H, OCH3), 2.36 (s, 3H, CH3), 2.33 (s, 3H, CH3), IR (KBr, cm−1): 3345 (N-H), 1658 (C=O), 1632 (C=N), 1250 (C-N); Anal. Calcd for C18H18N2O3, C = 69.66, H = 5.85, N = 9.03, O = 15.47, Found C = 69.67, H = 5.86, N = 9.04, O = 15.48; EI MS m/z (% rel. abund.): 310.
(E)-N'-(4-Chlorobenzylidene)-2,4-dimethylbenzohydrazide (28). Yield: 0.526 g (92%); 1H-NMR (DMSO-d6): δ 11.72 (s, 1H, NH), 8.30 (s, 1H, ArCH=N-R), 7.74 (d, 2H, J2',3' = J6',5' = 7.5 Hz, H-2', H-6'), 7.54 (d, 2H, J3',2' = J5',6' = 7.5 Hz, H-3', H-5'), 7.37 (d, 1H, J6,5 = 7.5 Hz, H-6), 7.13 (s, 1H, H-3), 7.10 (d, 1H, J5,6 = 7.5 Hz, H-5), 2.36 (s, 3H, CH3), 2.33 (s, 3H, CH3), IR (KBr, cm−1): 3345 (N-H), 1658 (C=O), 1632 (C=N), 1250 (C-N); Anal. Calcd for C16H15ClN2O, C = 67.02, H = 5.27, N = 9.77, O = 5.58, Found C = 67.03, H = 5.28, N = 9.78, O = 5.57; EI MS m/z (% rel. abund.): 286.
Methyl 2-((E)-(2,4-dimethylbenzoylimino)methyl)-3,5-dimethoxybenzoate (29). Yield: 0.673 g (91%); 1H-NMR (DMSO-d6): δ 11.62 (s, 1H, NH), 8.41 (s, 1H, ArCH=N-R), 7.36 (d, 1H, J6,5 = 7.0 Hz, H-6), 7.11–7.08 (m, 3H, H-3, H-5, H-6'), 6.72 (d, 1H, J4',6' = 2.0 Hz, H-4'), 3.89 (s, 3H, R-COOCH3), 3.81(s, 6H, 2× OCH3), 2.37 (s, 3H, CH3), 2.34 (s, 3H, CH3), IR (KBr, cm−1): 3345 (N-H), 1658 (C=O), 1632 (C=N), 1250 (C-N); Anal. Calcd for C20H22N2O5, C = 64.85, H = 5.99, N = 7.56, O = 21.60, Found C = 64.87, H = 5.97, N = 7.58, O = 21.62; EI MS m/z (% rel. abund.): 370.
(E)-N'-(4-Nitrobenzylidene)-2,4-dimethylbenzohydrazide (30). Yield: 0.499 g (84%); 1H-NMR (DMSO-d6): δ 11.97 (s, 1H, NH), 8.87 (s, 1H, ArCH=N-R), 8.31 (d, 2H, J2',3' = J6',5' = 8.5 Hz, H-2', H-6'), 7.99 (d, 2H, J3',2' = J5',6' = 8.5 Hz, H-3', H-5'), 7.40 (d, 1H, J6,5 = 7.5 Hz, H-6), 7.15 (s, 1H, H-3), 7.12 (d, 1H, J5,6 = 7.5 Hz, H-5), 2.38 (s, 3H, CH3), 2.34 (s, 3H, CH3), IR (KBr, cm−1): 3345 (N-H), 1658 (C=O), 1632 (C=N), 1250 (C-N); Anal. Calcd for C16H15N3O3, C = 64.64, H = 5.09, N = 14.13, O = 16.14, Found C = 64.65, H = 5.09, N = 14.14, O = 16.15; EI MS m/z (% rel. abund.): 297.
5. Conclusions
In this study we synthesized thirty (30) 2,4-dimethylbenzoylhydrazones derivatives. Out of thirty compounds, six (6) compounds showed better activities than the reference compound (n-propyl gallate) for DPPH activity and three compounds showed better activity than standard toward superoxide anion. Along with these results we also reported three new crystal structures. We believe that these molecules merit further study of their antiaging, antioxidant as well as potential anticancer activities.
Acknowledgments
Authors would like to acknowledge Universiti Teknologi MARA for the financial support under Dana Kecemerlangan Grant Scheme.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Contact authors.
References
- 1.Tsai M.D., Herschel J., Weintraub R., Byrn S.R., Chang C., Floss H.G. Conformation-reactivity relationship for pyridoxal Schiff’s bases. Rates of racemization and alpha-hydrogen exchange of the pyridoxal Schiff’s bases of amino acids. Biochemistry. 1978;17:3183–3188. doi: 10.1021/bi00609a002. [DOI] [PubMed] [Google Scholar]
- 2.Schirch L., Slotter R.A. Spectral properties of Schiff bases of amino acid esters with pyridoxal and pyridoxal N-methochloride in ethanol. Biochemistry. 1966;5:3175–3181. doi: 10.1021/bi00874a015. [DOI] [PubMed] [Google Scholar]
- 3.Popp F.D., Kirsch W.J. Synthesis of potential anticancer agents. V. Schiff bases and related compounds1–2. J. Org. Chem. 1961;26:3858–3860. doi: 10.1021/jo01068a056. [DOI] [Google Scholar]
- 4.Kumar S., Niranjan M.S., Chaluvaraju K.C., Jamakhandi C.M., Kadadevar D. Synthesis and antimicrobial study of some Schiff bases of sulfonamides. J. Curr. Pharm. Res. 2010;1:39–42. [Google Scholar]
- 5.Mallikarjun S.Y., Sangamesh A.P. Synthesis, characterization and biological studies of cobalt(II) and nickel(II) complexes with new Schiff bases. Trans. Met. Chem. 1997;22:220–224. doi: 10.1023/A:1018400121316. [DOI] [Google Scholar]
- 6.Akelah A., Kenawy E.R., Sherrington D.C. Agricultural polymers with herbicide/fertilizer function-III. Polyureas and poly(Schiff base)s based systems. Eur. Polym. J. 1993;29:1041–1045. doi: 10.1016/0014-3057(93)90306-Z. [DOI] [Google Scholar]
- 7.Jain J.S., Srivastava R.S., Aggarwal N., Sinha R. Synthesis and evaluation of Schiff bases for anticonvulsant and behavioral depressant properties. Cent. Nerv. Syst. Agents Med. Chem. 2007;7:200–204. doi: 10.2174/187152407781669143. [DOI] [Google Scholar]
- 8.Fareed G., Versiani M.A., Afza N., Fareed N., Iqbal L., Lateef M. Structure activity relationship: Antioxidant potential of some novel Schiff bases containing benzophenone moiety. Int. J. Curr. Pharm. Res. 2013;5:61–64. [Google Scholar]
- 9.Mishra P., Gupta P.N., Shakya A.K., Shukla R., Srimal R.C. Anti-inflammatory and diuretic activity of a new class of compounds—Schiff bases of 3-amino-2-methylquinazolin 4(3H)-ones. Indian J. Physiol. Pharmacol. 1995;39:169–172. [PubMed] [Google Scholar]
- 10.Supuran C.T., Barboiu M., Luca C., Pop E., Brewster M.E., Dinculescu A. Carbonic anhydrase activators. Part 14. Syntheses of mono and bis pyridinium salt derivatives of 2-amino-5-(2-aminoethyl)- and 2-amino-5-(3-aminopropyl)-1,3,4-thiadiazole and their interaction with isozyme II. Eur. J. Med. Chem. 1996;31:597–606. doi: 10.1016/0223-5234(96)89555-9. [DOI] [Google Scholar]
- 11.Tarafder M.T., Kasbollah A., Saravanan N., Crouse K.A., Ali A.M., OoK T. S-Methyldithiocarbazate and its Schiff bases: Evaluation of bondings and biological properties. Biochem. Mol. Bio. Biophy. 2002;6:85–91. doi: 10.1080/10258140290027207. [DOI] [PubMed] [Google Scholar]
- 12.Walcourt A., Loyevsky M., Lovejoy D.B., Gordeuk V.R., Richardson D.R. Novel aroylhydrazone and thiosemicarbazone iron chelators with anti-malarial activity against chloroquine-resistant and -sensitive parasites. Int. J. Biochem. Cell Biol. 2004;36:401–407. doi: 10.1016/S1357-2725(03)00248-6. [DOI] [PubMed] [Google Scholar]
- 13.Vicini P., Geronikaki A., Incerti M., Busonera B., Poni G., Cabras C.A., Colla P.L. Synthesis and biological evaluation of benzo[d]isothiazole, benzothiazole and thiazole Schiff bases. Bioorg. Med. Chem. 2003;11:4785–4789. doi: 10.1016/S0968-0896(03)00493-0. [DOI] [PubMed] [Google Scholar]
- 14.Andreani A., Rambaldi M., Bonazzi D., Greci L., Andreani F. Study on compounds with potential antitumor activity. III. Hydrazone derivatives of 5-substituted 2-chloro-3-formyl-6-methylindole. Farmaco Sci. 1979;34:132–138. doi: 10.1002/chin.197924211. [DOI] [PubMed] [Google Scholar]
- 15.Gaur S. Physico-chemical and Biological properties of Mn(II), Co(II), Ni(II) and Cu(II) chelates of Schiff Bases. Asian J. Chem. 2003;15:250–254. [Google Scholar]
- 16.Gemi M.J., Biles C., Keiser B.J., Poppe S.M., Swaney S.M., Tarapley W.G., Romeso D.L., Yage Y. Novel 1,5-diphenylpyrazole nonnucleoside HIV-1 reverse transcriptase inhibitors with enhanced activity versus the delavirdine-resistant P236L mutant: Lead identification and SAR of 3- and 4-substituted derivatives. J. Med. Chem. 2000;43:1034–1040. doi: 10.1021/jm990383f. [DOI] [PubMed] [Google Scholar]
- 17.Al-Amiery A., Al-Majedy Y.K., Ibrahim H.H., Al-Tamimi A. Antioxidant, antimicrobial, and theoretical studies of the thiosemicarbazone derivative Schiff base 2-(2-imino-1-methylimidazolidin-4-ylidene)hydrazinecarbothioamide (IMHC) Org. Med. Chem. Lett. 2012;2:4. doi: 10.1186/2191-2858-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corona A.B., Viveros J.P., Flores A.P., Peraza A.C., Martínez J.M., Sumaya M.M., Organillo R. Antioxidant activity of butyl- and phenylstannoxanes derivedfrom 2-, 3- and 4-pyridinecarboxylic acids. Molecules. 2010;15:5445–5459. doi: 10.3390/molecules15085445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dharmaraj N., Viswanathamurthi P., Natarajan K. Ruthenium(II) complexes containing bidentate Schiff bases and their antifungal activity. Trans. Met. Chem. 2001;26:105–109. doi: 10.1023/A:1007132408648. [DOI] [Google Scholar]
- 20.Ozdemir A., Turan-Zitouni G., Kaplancikli Z.A., Demirci F., Iscan G. Studies on hydrazone derivatives as antifungal agents. J. Enzyme Inhib. Med. Chem. 2008;23:470–475. doi: 10.1080/14756360701709094. [DOI] [PubMed] [Google Scholar]
- 21.Ergenç N., Günay N.S., Demirdamar R. Synthesis and antidepressant evaluation of new 3-phenyl-5-sulfonamideindole derivatives. Eur. J. Med. Chem. 1998;33:143–148. doi: 10.1016/S0223-5234(98)80039-1. [DOI] [Google Scholar]
- 22.Silva A.G., Zapata-Suto G., Kummerle A.E., Fraga C.A.M., Barreiro E.J., Sudo R.T. Synthesis and vasodilatory activity of new N-acylhydrazone derivatives, designed as LASSBio-294 analogues. Bioorg. Med. Chem. 2005;13:3431–3437. doi: 10.1016/j.bmc.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Cavier R., Rips R. Dihydrazides, a new class of anthelmintics. J. Med. Chem. 1965;8:706–708. doi: 10.1021/jm00329a038. [DOI] [Google Scholar]
- 24.Rollas S., Kuçukguzel S.G. Biological activities of hydrazone derivatives. Molecules. 2007;12:1910–1939. doi: 10.3390/12081910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lima P.C., Lima L.M., Silva K.C., Leda P.H., Miranda A.L.P., Fraga C.A.M., Barreiro E.J. Synthesis and analgesic activity of novel N-acylarylhydrazones and isosters, derived from natural safrole. Eur. J. Med. Chem. 2000;35:187–203. doi: 10.1016/S0223-5234(00)00120-3. [DOI] [PubMed] [Google Scholar]
- 26.Fraga A.G.M., Rodrigues C.R., Miranda A.L.P., Barreiro E.J., Fraga C.A.M. Synthesis and pharmacological evaluation of novel heterotricyclic acylhydrazone derivatives, designed as PAF antagonists. Eur. J. Pharm. Sci. 2000;11:285–290. doi: 10.1016/S0928-0987(00)00102-0. [DOI] [PubMed] [Google Scholar]
- 27.Satyanarayana V.S.V., Sivakumar A., Ghosh A.R. Synthesis, characterization of some new five membered heterocycles based on imidazole moiety and their applications on therapeutics. Lett. Drug. Des. Discov. 2011;8:276–283. doi: 10.2174/157018011794578196. [DOI] [Google Scholar]
- 28.Tumer M., Toroglu E.S.A., Kayraldiz A., Donbak L. Synthesis and characterization of Schiff base metal complexes: Their antimicrobial, genotoxicity and electrochemical properties. J. Coord. Chem. 2008;61:2935–2949. doi: 10.1080/00958970801989902. [DOI] [Google Scholar]
- 29.Barton D.H.R., Yadav-Bhatnagar N., Finet J.P., Khamsi J. Phenylation of aromatic and aliphatic amines by phenyllead triacetate using copper catalysis. Tetrahedron Lett. 1987;28:3111–3114. doi: 10.1016/S0040-4039(00)96298-1. [DOI] [Google Scholar]
- 30.Aranha P.E., Dos Santos M.P., Romera S., Dockal E.R. Synthesis, characterization, and spectroscopic studies of tetradentate Schiff base chromium(III) complexes. Polyhedron. 2007;26:1373–1382. doi: 10.1016/j.poly.2006.11.005. [DOI] [Google Scholar]
- 31.Prakash A., Adhikari D. Application of Schiff bases and their metal complexes—A review. Int. J. Chem. Tech. Res. 2011;3:1891–1896. [Google Scholar]
- 32.Capasso R., Evidente A., Tremblay E., Sala A., Santoro C., Cristinzio G. Direct and mediated effects on Bactrocera oleae (Gmelin) (Diptera; Tephritidae) of natural polyphenols and some of related synthetic compounds: Structure-activity relationships. J. Chem. Ecol. 1994;20:1189–1199. doi: 10.1007/BF02059753. [DOI] [PubMed] [Google Scholar]
- 33.Sun-Waterhouse D., Chen J., Chuah C., Wibisono R., Melton L.D., Laing W., Ferguson L.R., Skinner M.A. Kiwifruit-based polyphenols and related antioxidants for functional foods: Mkiwifruit extract-enhabnced gluten-free bread. Int. J. Food Sci. Nutr. 2009;60:251–264. doi: 10.1080/09637480903012355. [DOI] [PubMed] [Google Scholar]
- 34.Taha M., Baharudin M.S., Ismail N.H., Khan K.M., Jaafar F.M., Samreen, Siddiqui S., Choudhary M.I. Synthesis of 2-methoxybenzoylhydrazone and evaluation of their antileishmanial activity. Bioorg. Med. Chem. Lett. 2013;23:3463–3466. doi: 10.1016/j.bmcl.2013.03.051. [DOI] [PubMed] [Google Scholar]
- 35.Khan K.M., Taha M., Rahim F., Fakhri M.I., Jamil W., Khan M., Rasheed S., Karim A., Perveen S., choudhary Acylhydrazide Schiff Bases: Synthesis and Antiglycation Activity. J. Pak. Chem Soc. 2013;35:929–937. [Google Scholar]
- 36.Khan K.M., Rahim F., Ambreen N., Taha M., Khan M., Jahan H., Najeebullah, Shaikh A., Iqbal S., Perveen S., Choudhary M.I. Synthesis of Benzophenonehydrazone Schiff Bases and their In Vitro Antiglycating Activities. Med. Chem. 2013;9:588–595. doi: 10.2174/1573406411309040013. [DOI] [PubMed] [Google Scholar]
- 37.Taha M., Ismail N.H., Jaafar F.M., Aziz A.N., Yousuf S. (E)-N'-(3,4-Dihydroxybenzylidene)-2,4-dimethylbenzohydrazide monohydrate. Acta Cryst. 2013;E69:o490. doi: 10.1107/S1600536813005692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baharudin M.S., Taha M., Ismail N.H., Shah S.A.A., Yousuf S. (E)-N'-(4-Chlorobenzylidene)-2-methoxybenzohydrazide. Acta Cryst. 2013;E69:o276. [Google Scholar]
- 39.Taha M., Baharudin M.S., Ismail N.H., Shah S.A.A., Yousuf S. (E)-2-Methoxy-N'-(2,4,6-trihydroxybenzylidene)Benzohydrazide. Acta Cryst. 2013;E69:o277. doi: 10.1107/S1600536813001748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taha M., Ismail N.H., Jaafar F.M., Khan K.M., Yousuf S. (E)-2,4-Dimethyl-N'-(2-methylbenzylidene) benzohydrazide. Acta Cryst. 2013;E69:o400. doi: 10.1107/S1600536813004388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baharudin M.S., Taha M., Ismail N.H., Shah S.A.A., Yousuf S. N-[(E)-2-Hydroxy-5-methoxybenzylidene]-2-methoxybenzohydra-zide. Acta Cryst. 2012;E68:o3255. [Google Scholar]
- 42.Taha M., Baharudin M.S., Ismail N.H., Shah S.A.A., Yousuf S. N'-[(E)-2,3-Dihydroxybenzylidene]-2-methoxybenzohydrazide. Acta Cryst. 2012;E68:o3256. [Google Scholar]
- 43.Khan K.M., Shah Z., Ahmad V.U., Khan M., Taha M., Ali S., Perveen S., Choudhary M.I., Voelter W. 2,4,6-Trichlorophenylhydrazine Schiff bases as DPPH radical and super oxide anion scavengers. Med. Chem. 2012;8:452–461. doi: 10.2174/1573406411208030452. [DOI] [PubMed] [Google Scholar]
- 44.Khan K.M., Taha M., Naz F., Ali S., Perveen S., Choudhary M.I. Acylhydrazide Schiff bases: DPPH radical and superoxide anion scavengers. Med. Chem. 2012;8:705–710. doi: 10.2174/157340612801216111. [DOI] [PubMed] [Google Scholar]
- 45.Khan K.M., Khan M., Ali M., Taha M., Hameed A., Ali S., Perveen S., Choudhary M.I. Synthesis and DPPH radical scavenging activity of 5-Arylidene-N,NDimethylbarbiturates. Med. Chem. 2011;7:231–236. doi: 10.2174/157340611795564231. [DOI] [PubMed] [Google Scholar]
- 46.Siemens SMART and SAINT. Siemens Analytical X-ray Instruments Inc.; Madison, WI, USA: 1996. [Google Scholar]
- 47.Sheldrick G.M. A Program for Refinement of Crystal Structures. SHELXL 97; Göttingen, Germany: 1997. [Google Scholar]
- 48.Johnson C.K. Report ORNL-5138. Oak Ridge National Laboratory; Tennessee, USA: 1976. ORTEP II. [Google Scholar]






