Abstract
Pulsatilla koreana, a species endemic to Korea, is an important herb used in traditional medicine to treat amoebic dysentery and malaria. In the present study, 23 oleanane-type triterpenoid saponins 1–23 and eight lupane-type triterpenoid saponins 24–31 were isolated from the roots of P. koreana. Their structures were elucidated on the basis of spectroscopic data. The methanol extract and isolated compounds were next assessed for nematicidal activity against the root-knot nematode (Meloidogyne incognita). The methanol extract showed strong nematicidal activity after 48 h, with a LC50 value of 92.8 μg/mL. Compounds 2, 5, 9, 20, and 21 showed significant effects, with LC50 values ranging from 70.1 to 94.7 μg/mL after 48 h. These results suggest that triterpenoid saponins from P. koreana should be explored as potential natural nematicides for developing new agents to control root-knot nematode disease.
Keywords: Pulsatilla koreana, triterpene saponin, Meloidogyne incognita, nematicidal activity
1. Introduction
Plant parasitic nematodes inflict serious damage on agricultural crops and plants. The primary pathogen, the root-knot nematode (Meloidogyne incognita), is known to predispose the host to attacks from soil borne fungal pathogens (secondary pathogens), resulting in a synergistic effect. In this phenomenon, M. incognita, by causing wounds in the roots of a plant through penetration of a stylet, provides a way for other fungal pathogens to enter and thus result in disease complex incidences [1,2,3]. In the past, synthetic compounds have mainly been used for plant protection, but many of these pesticides have side effects including residues in plants, contamination of groundwater, the potential for adverse ecological impacts from pesticide use, and the creation of a continuing need for the development of new nematode control strategies and products [4]. Recently, one alternative used has been to screen naturally occurring plant secondary compounds for appropriate nematicidal activity. Various nematicidal substances of plant origin such as triglycerides, sesquiterpenes, alkaloids, steroids, diterpenes, and flavonoids, have been identified in this way [5]. These compounds can be developed for use as nematicides themselves, or can serve as model compounds for the development of chemically synthesized derivatives with enhanced activity and reduced environmental impacts [6].
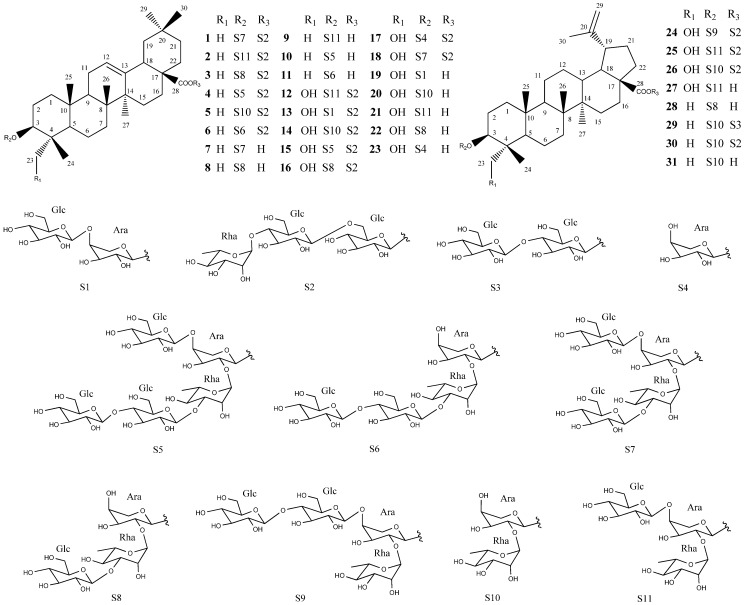
During our search for botanical pesticides from natural plants, we found that the methanol extract of Pulsatilla koreana Nakai (Ranuculaceae) has high nematicidal activity against M. incognita. Pulsatilla koreana, a hairy, tufted, perennial herb that grows in Korea, and is used as a traditional medicine to treat various maladies such as amoebic dysentery and malaria [7]. Several Pulsatilla species, including P. ambigua, P. chinensis, P. dahurica and P. turczaninovii have been employed to treat diarrhea, vaginal trichomoniasis, and bacterial infections. Pharmacological investigations have suggested that triterpene saponins are important bioactive components [8,9,10,11,12]. However, the composition of nematicidally active compounds in this plant has not been previously reported. In this study, 31 triterpenoid saponins were isolated (Figure 1) and their nematicidal activities against the root-knot nematode were evaluated.
Figure 1.
Structures of compounds 1–31 from the roots of P. koreana.
2. Results and Discussion
2.1. Structure Elucidation of Compounds 1–31
In the present study, bioassay-guided isolation on the methanol extract of P. koreana roots was carried out to identify 31 triterpenoid saponins, which included 23 oleanane-type triterpenoid saponins 1–23 and eight lupane-type triterpenoid saponins 24–31. Their structures were elucidated as cernuoside A (1) [13], hederacholchiside E (2) [14], beesioside Q (3) [15], 3-O-β-d-glucopyranosyl (1→4)-β-d-glucopyranosyl (1→3)-α-l-rhamnopyranosyl (1→2)[β-d-glucopyranosyl(1→4)]-α-l-arabinopyranosyl oleanolic acid 28-O-α-l-rhamnopyranosyl (1→4)-β-d-glucopyranosyl (1→6)-β-d-glucopyranoside (4) [16], hederacoside B (5) [17], raddeanoside R17 (6) [18], 3-O-β-d-glucopyranosyl (1→3)-α-l-rhamno-pyranosyl (1→2) [β-d-glucopyranosyl (1→4)]-α-l-arabinopyranosyl oleanolic acid (7) [19], 3-O-β-d-glucopyranosyl (1→3)-α-l-rhamnopyranosyl (1→2)-α-l-arabinopyranosyl oleanolic acid (8) [20], raddeanoside R13 (9) [20], 3-O-β-d-glucopyranosyl (1→4)-β-d-glucopyranosyl (1→3)-α-l-rhamno-pyranosyl (1→2)[β-d-glucopyranosyl(1→4)]-α-l-arabinopyranosyl oleanolic acid (10) [21], 3-O-β-d-glucopyranosyl(1→4)-β-d-glucopyranosyl (1→3)-α-l-rhamnopyranosyl (1→2) [β-d-glucopyranosyl-(1→4)]-α-l-arabinopyranosyl oleanolic acid (11) [21], hederacholchiside F (12) [14], fatsiaside G (13) [22], pulsatilla saponin F (14) [23], pulsatilloside F (15) [24], patrinia saponin H3 (16) [25], hederasaponin D (17) [23], cernuoside B (18) [26], scabioside C (19) [20], hederoside C (20) [23], pulsatilla saponin D (21) [27], kalopanaxsaponin H (22) [28], scabioside A (23) [29], 23-hydroxy-3β-[(O-α-l-rhamnopyranosyl-(1→2)-O-[β-d-glucopyranosyl-(1→4)-β-d-glucopyranosyl-(1→4)]-α-l-arabino-pyranosyl)oxy]lup-20(29)-en-28-oic acid 28-O-α-l-rhamnopyranosyl-(1→4)-O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranosyl ester (24) [30], pulsatilloside E (25) [20], anemoside B4 (26) [20], 23-hydroxy-3β-[(O-α-l-rhamnopyranosyl-(1→2)-O-[β-d-glucopyranosyl-(1→4)]-α-l-arabinopyranosyl)-oxy] lup-20(29)-en-28-oic acid (27) [12], 3β-[O-β-d-glucopyranosyl-(1→3)-O-α-l-rhamnopyranosyl-(1→2)-O-α-l-arabinopyranosyl)oxy] lup-20(29)-en-28-oic acid (28) [20], 3β-[(O-α-l-rhamno-pyranosyl-(1→2)-α-l-arabinopyranosyl)oxy] lup-20(29)-en-28-oic acid 28-O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranosyl ester (29) [14], cussosaponin C (30) [30], betulinic acid 3β-O-α-l-rhamnopyranosyl-(1→2)-O-α-l-arabinopyranoside (31) [31] (Figure 1). Their structures were elucidated on the basis of spectroscopic data and comparison of 1D- and 2D-NMR and mass spectral data with reported values.
Among the isolated triterpenoid saponins, compounds 4, 6, 7, 10, 11, 14, 24, and 31 were isolated from P. koreana for the first time. Compounds 1–6, 10–12, 16, 18, 24–26, 30, and 31 were only found previously in P. chinensis and P. cernua among in the genus Pulsatilla, suggesting a genetic relationship between them [11,13,16,21]. Additionally, compounds 27–29 have not been reported in other species of Pulsatilla, they will probably become a chemotaxonomic marker for P. koreana. This is the first integrated chemical investigation of triterpenoid saponins from P. koreana root.
2.2. Nematicidal Activity
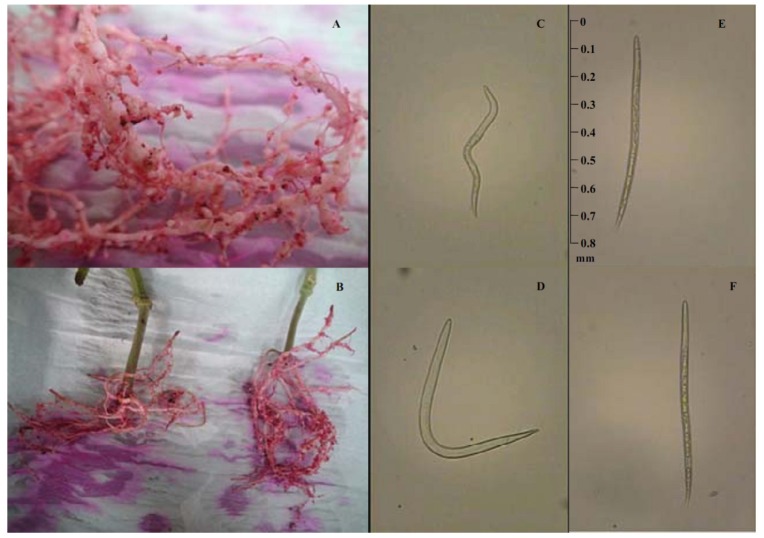
An investigation on the nematicidal activity of the methanol extract and isolated compounds of P. koreana root against M. incognita was conducted. The activity of all samples was also examined separately. The internal structures of the nematodes were disintegrated and many vacuoles formed within their bodies during the experiments. After 72 h, only residual, empty somatocysts of the dead nematodes could be observed (Figure 2E,F). The methanol extract of P. koreana showed strong nematicidal activity after 48 h, with a LC50 value of 92.8 μg/mL compared with positive control (72.7 μg/mL) (Table 1). Moreover, the nematicidal activity of methanol extract dramatically increased 24 h after the treatment.
Figure 2.
Muskmelon roots infested with M. incognita (A and B); Juveniles and adults of M. incognita treated with water and with compounds. A healthy, active nematode (C and D), compounds treated nematode with disrupted internal structures (E and F) and an almost empty somatocysts of the dead nematode after 72 h.
Table 1.
Nematicidal activity of isolated compounds and methanol extract of P. koreana root against Meloidogyne incognita.
| Compound | 24 h | 48 h | 72 h |
|---|---|---|---|
| LC50 (μg/mL) * | LC50 (μg/mL) * | LC50 (μg/mL) * | |
| 2 | 136.7 ± 38.6 | 94.7 ± 27.4 | 94.5 ± 25.6 |
| 3 | >200 | >200 | >200 |
| 5 | 126.8 ± 29.7 | 88.9 ± 32.9 | 87.5 ± 19.8 |
| 8 | >200 | >200 | 169.2 ± 38.9 |
| 9 | 88.7 ± 21.9 | 75.8 ± 22.6 | 72.3 ± 24.4 |
| 12 | 177.0 ± 49.2 | 134.7 ± 47.6 | 131.7 ± 28.3 |
| 14 | 186.7 ± 56.4 | 127.5 ± 43.7 | 123.8 ± 36.7 |
| 16 | >200 | >200 | >200 |
| 20 | 92.4 ± 14.6 | 70.1 ± 28.6 | 69.7 ± 18.8 |
| 21 | 103.9 ± 36.2 | 79.9 ± 18.3 | 77.6 ± 22.1 |
| 22 | >200 | >200 | 184.3 ± 36.7 |
| 25 | >200 | >200 | >200 |
| 26 | >200 | >200 | >200 |
| 27 | >200 | >200 | >200 |
| 28 | >200 | >200 | >200 |
| 30 | >200 | >200 | >200 |
| 31 | >200 | >200 | >200 |
| MeOH ext. | 106.7 ± 35.8 | 92.8 ± 27.6 | 86.5 ± 22.8 |
| Fosthiazate ** | 78.6 ± 19.8 | 72.7 ± 21.6 | 71.4 ± 17.6 |
* All data represent the mean ± SD of at least three independent experiments performed in triplicates; ** Positive control.
The isolated compounds were tested for in vitro nematicidal activity against M. incognita. Of these, compounds 9 and 20 exhibited strong activity after 24 h, with LC50 values of 88.7 and 92.4 μg/mL, respectively, compared with the positive control, fosthiazate (78.6 μg/mL). Compounds 2, 5, 12, 14, and 21 showed moderate effects, with LC50 values ranging from 103.9 to 186.7 μg/mL, and compounds 3, 8, 16, and 22–31 exhibited weak activities (LC50 > 200 μg/mL). After 48 h, compounds 2, 5, 9, 20, and 21 showed significant effects, with LC50 values ranging from 70.1 to 94.7 μg/mL. Compounds 9 (2.1 g, 1.05%), 20 (120.0 mg, 0.06%), and 21 (196.0 mg, 0.10%), which are major triterpenoid saponin components, exhibited the same level of mortalities as fosthiazate (Table 1). After 72 h, the nematicidal activity effects of compounds 2, 5, 9, 20, and 21 showed no significant progress, suggesting that the isolated compounds exhibited activity within 48 h after treatment.
A previous study reported that P. koreana showed significant activity against the root-knot nematode, but the active constituents were not determined [32]. To the best of our knowledge, this is the first report of nematicidal active constituents from P. koreana. In the structure-activity relationships of oleanane-type triterpenoid saponins 1–22, compounds 9, 20 and 21 showed the strongest nematicidal activity against M. incognita, and their structures were similar. When the sugar unit at C-3 of the aglycone was linked to a rhamnose-arabinose or rhamnose-(glucose-)arabinose chain, the nematicidal activity increased significantly when compared to other sugar chains. Although compounds 2, 5, 12, and 14 also link a rhamnose-arabinose or rhamnose-(glucose-)arabinose chain, their effects were weaker than those of compounds 9, 20 and 21, suggesting that a carboxyl group at position C-28 of aglycone is a key functional element. This suggestion was supported by comparing with previous work [9,33,34]. These data may be useful to evaluate the structure-activity relationships of other triterpenoid saponins, and to develop nematicidal activity against M. incognita.
3. Experimental
3.1. General Procedures
Optical rotations were determined using a Jasco DIP-370 automatic polarimeter. The FT-IR spectra were measured using a Jasco Report-100 infrared spectrometer. The NMR spectra were recorded using a JEOL ECA 600 spectrometer (1H, 600 MHz; 13C, 150 MHz), and ESI-MS spectra were obtained using a JEOL JMS-T100LC spectrometer. Column chromatography was performed using a silica gel (Kieselgel 60, 70–230, and 230–400 mesh, Merck, Darmstadt, Germany) and YMC RP-18 resins, and thin layer chromatography (TLC) was performed using pre-coated silica-gel 60 F254 and RP-18 F254S plates (both 0.25 mm, Merck).
3.2. Plant Material
Dried roots of P. koreana were purchased from herbal market, Kumsan, Chungnam, Korea in March 2009 and identified by one of the authors (Prof. Young Ho Kim). A voucher specimen (CNU 09106) was deposited at the Herbarium of College of Pharmacy, Chungnam National University, Daejeon, Korea.
3.3. Extraction and Isolation
Dried roots of P. koreana (2.0 kg) were extracted with MeOH under reflux for 10 h (7 L × 3 times) to yield 500.0 g of extract. This extract was suspended in water and partitioned with ethyl acetate to yield the corresponding ethyl acetate (37.0 g) and water (463.0 g) extracts. The water extract was partitioned with n-BuOH to yield the n-BuOH (130.0 g) extract. The ethyl acetate extract was subjected to silica gel column chromatography with a gradient of CHCl3/MeOH (50:1, 20:1,10: 1 and 1:1; 2 L for each step) to give 6 fractions (Fr. E1–E6). The fraction E4 was separated using an YMC column with a MeOH/actone/H2O (0.25:0.3:1–1.3:1.3:1, 1.2 L for each step) elution solvent to give compounds 23 (75.0 mg) and 31 (62.0 mg). The fraction E5 was separated using an YMC column with a MeOH/H2O (3.2:1, 1.4 L) elution solvent to give compound 11 (19.0 mg). The fraction E6 was separated using an YMC column with a MeOH/H2O (2.7:1, 1.5 L) elution solvent to give compound 27 (28.0 mg).
The n-BuOH extract was subjected to silica gel column chromatography with a gradient of CHCl3/MeOH/H2O (5:1:0.1, 2:1:0.1 and 0:1:0; 3 L for each step) to give 6 fractions (Fr. B1–B6). The fraction B3 was separated using a silica gel column with CHCl3/MeOH/H2O (5:1:0.1, 4:1:0.1 and 3:1:0.1, 1 L for each step) to give four sub-fractions (Fr. B3.1–B3.4). Fraction B3.1 was separated using an YMC column with a MeOH/H2O (4.5:1, 1.1 L) elution solvent to give compound 20 (120.0 mg). Fraction B3.2 was separated using an YMC column with a MeOH/acetone/H2O (2.5:0.7:1, 2.5 L) elution solvent to give compounds 8 (78.0 mg), 19 (30.0 mg) and 28 (12.0 mg). Fraction B3.3 was separated using an YMC column with a MeOH/acetone/H2O (1.5:0.7:1, 750 mL) elution solvent to give compound 22 (180.0 mg). Fraction B3.4 was separated using an YMC column with a MeOH/acetone/H2O (2:0.5:1, 2.5 L) elution solvent to give compounds 9 (2.1 g) and 25 (12.0 mg). The fraction B4 was separated using a silica gel column with CHCl3/MeOH/H2O (3.5:1:0.1, 2:1:0.1 and 1:1:0.2, 1 L for each step) to give four sub-fractions (Fr. B4.1–B4.4). Fraction B4.2 was further chromatographed on RP chromatography column with acetone/MeOH/H2O (0.5:1:1.8, 1.5 L) to yield compound 29 (130.0 mg). Fraction B4.3 was separated using an YMC column with a MeOH/acetone/H2O (10.5:1:1–0.85:2:1, each 550 mL) elution solvent to give compounds 10 (25.0 mg), 26 (7.0 mg) and 30 (20.0 mg).
The water extract was chromatographed on a column of highly porous polymer (Diaion HP-20) and eluted with H2O and MeOH, successively, to give four fractions (Fr. W1–W4). Fraction W3 was subjected to silica gel column chromatography with a gradient of CHCl3/MeOH/H2O (6:1:0.1, 4:1:0.1, 2:1:0.1 and 0:1:0; 4 L for each step) to give six fractions (Fr. W3.1–W3.6). Fraction W3.3 using an YMC column with a MeOH/acetone/H2O (1:0.3:1–1:0.4:1.4, 650 mL for each step) elution solvent to give compounds 2 (50.0 mg), 3 (44.0 mg), 13 (17.0 mg), 14 (77.0 mg), and 16 (38.0 mg). Fraction W3.4 was separated using an YMC column with a MeOH/H2O (1.3:1–2.5:1, 750 mL for each step) elution solvent to give compounds 1 (44.0 mg) and 12 (110.0 mg). Fraction W3.5 was separated using an YMC column with an acetone/MeOH/H2O (0.25:1:1–0.32:1:1, 600 mL for each step) elution solvent to give compounds 4 (78.0 mg) and 18 (460.0 mg). Fraction W3.6 was separated using a silica gel column with CHCl3/MeOH/H2O (1.2:1:0.15, 1.5 L) to give compounds 15 (130.0 mg) and 24 (4.0 mg). Fraction W4 was subjected to silica gel column chromatography with a gradient of CHCl3/MeOH/H2O (2.5:1:0.1, 1.5:1:0.15 and 0:1:0; 3 L for each step) to give 3 fractions (Fr. W4.1–W4.3). Fraction W4.1 was further chromatographed on RP chromatography column with acetone/MeOH/H2O (0.7:1.5:1–1:2:1, 1 L for each step) to yield compounds 7 (12.0 mg) and 21 (196.0 mg). Fraction W4.2 was further chromatographed on RP chromatography column with acetone-MeOH-H2O (0.6:1:1–1:1.7:1, 750 mL for each step) to yield compounds 5 (300.0 mg) and 17 (12.0 mg). Compound 6 (36.0 mg) was isolated from W4.3 using RP chromatography column with acetone/MeOH/H2O (0.3:1.7:1).
Raddeanoside R13 (9): White powder; ESI-MS m/z 895 [M−H]−; 1H-NMR of aglycone (600 MHz, pyridine-d5) δ: 1.05 (1H, m, H-1a), 1.52 (1H, m, H-1b), 2.00 (1H, m, H-2a), 2.23 (1H, m, H-2b), 4.29 (1H, dd, J = 3.6, 11.0 Hz, H-3), 0.72 (1H, d, J = 12.0 Hz, H-5), 1.60 (2H, m, H-6), 1.29 (1H, m, H-7a), 1.40 (1H, m, H-7b), 1.61 (1H, m, H-9), 0.88 (2H, m, H-11), 5.35 (1H, br s, H-12), 1.16 (1H, m, H-15a), 2.27 (1H, m, H-15b), 1.90 (1H, m, H-16a), 2.05 (1H, m, H-16b), 3.31 (1H, d, J = 12.0 Hz, H-18), 1.21 (1H, m, H-19a), 1.67 (1H, m, H-19b), 0.90 (1H, m, H-21a), 1.09 (1H, m, H-21b), 1.75 (1H, m, H-22a), 1.85 (1H, m, H-22b), 1.26 (3H, s, H-23), 1.12 (3H, s, H-24), 0.84 (3H, s, H-25), 1.00 (3H, s, H-26), 1.32 (3H, s, H-27), 0.97 (3H, s, H-29), 0.85 (3H, s, H-30); 13C-NMR of aglycone (150 MHz, pyridine-d5) δ: 39.2 (C-1), 27.0 (C-2), 89.1 (C-3), 39.9 (C-4), 56.3 (C-5), 18.7 (C-6), 33.5 (C-7), 40.1 (C-8), 48.4 (C-9), 37.4 (C-10), 24.0 (C-11), 123.0 (C-12), 145.3 (C-13), 42.5 (C-14), 28.6 (C-15), 24.0 (C-16), 47.0 (C-17), 42.3 (C-18), 46.8 (C-19), 31.3 (C-20), 34.6 (C-21), 33.5 (C-22), 28.5 (C-23), 17.5 (C-24), 15.9 (C-25), 17.7 (C-26), 26.5 (C-27), 180.8 (C-28), 33.6 (C-29), 24.1 (C-30); 1H-NMR of sugar moieties (600 MHz, pyridine-d5) δ: 4.85 (1H, d, J = 6.5 Hz, Ara-1), 4.15 (1H, m, Ara-2), 4.35 (1H, m, Ara-3), 4.24 (1H, m, Ara-4), 3.96 (1H, m, Ara-5a), 4.40 (1H, m, Ara-5b), 6.23 (1H, br s, Rha-1), 4.90 (1H, m, Rha-2), 4.74 (1H, m, Rha-3), 4.50 (1H, m, Rha-4), 4.95 (1H, m, Rha-5), 1.57 (1H, d, J = 6.0 Hz, Rha-6), 5.48 (1H, d, J = 7.7 Hz, Glc-1), 3.92 (1H, m, Glc-2), 4.28 (1H, m, Glc-3), 4.31 (1H, m, Glc-4), 4.08 (1H, m, Glc-5), 3.82 (1H, m, Glc-6a), 4.44 (1H, m, Glc-6b); 13C-NMR of sugar moieties (150 MHz, pyridine-d5) δ: 105.9 (Ara-1), 76.7 (Ara-2), 74.5 (Ara-3), 80.2 (Ara-4), 65.0 (Ara-5), 102.1 (Rha-1), 72.0 (Rha-2), 72.9 (Rha-3), 74.5 (Rha-4), 70.2 (Rha-5), 18.8 (Rha-6), 106.9 (Glc-1), 75.1 (Glc-2), 78.9 (Glc-3), 71.6 (Glc-4), 79.2 (Glc-5), 62.9 (Glc-6).
Hederoside C (20): White powder; ESI-MS m/z 749 [M−H]−; 1H-NMR of aglycone (600 MHz, pyridine-d5) δ: 1.05 (1H, m, H-1a), 1.52 (1H, m, H-1b), 2.05 (1H, m, H-2a), 2.44 (1H, m, H-2b), 4.35 (1H, dd, J = 3.6, 11.0 Hz, H-3), 1.75 (1H, d, J = 12.0 Hz, H-5), 1.53 (2H, m, H-6), 1.26 (1H, m, H-7a), 1.55 (1H, m, H-7b), 1.77 (1H, m, H-9), 1.96 (2H, m, H-11), 5.50 (1H, br s, H-12), 1.15 (1H, m, H-15a), 2.27 (1H, m, H-15b), 1.90 (1H, m, H-16a), 2.09 (1H, m, H-16b), 3.28 (1H, d, J = 12.0 Hz, H-18), 1.20 (1H, m, H-19a), 1.69 (1H, m, H-19b), 0.91 (1H, m, H-21a), 1.08 (1H, m, H-21b), 1.73 (1H, m, H-22a), 1.87 (1H, m, H-22b), 4.02 (1H, d, J = 12.0 Hz, H-23a), 4.50 (1H, d, J = 12.0 Hz, H-23b), 1.04 (3H, s, H-24), 0.96 (3H, s, H-25), 1.01 (3H, s, H-26), 1.25 (3H, s, H-27), 0.93 (3H, s, H-29), 0.94 (3H, s, H-30); 13C-NMR of aglycone (150 MHz, pyridine-d5) δ: 39.3 (C-1), 26.6 (C-2), 81.4 (C-3), 43.8 (C-4), 48.5 (C-5), 18.5 (C-6), 33.6 (C-7), 40.1 (C-8), 48.1 (C-9), 37.2 (C-10), 24.2 (C-11), 123.0 (C-12), 145.3 (C-13), 42.5 (C-14), 28.7 (C-15), 24.0 (C-16), 47.0 (C-17), 42.3 (C-18), 46.8 (C-19), 31.3 (C-20), 34.5 (C-21), 33.2 (C-22), 64.3 (C-23), 14.3 (C-24), 16.4 (C-25), 17.8 (C-26), 26.5 (C-27), 180.8 (C-28), 33.6 (C-29), 24.1 (C-30); 1H-NMR of sugar moieties (600 MHz, pyridine-d5) δ: 5.22 (1H, d, J = 6.5 Hz, Ara-1), 4.25 (1H, m, Ara-2), 4.35 (1H, m, Ara-3), 4.34 (1H, m, Ara-4), 4.00 (1H, m, Ara-5a), 4.40 (1H, m, Ara-5b), 6.29 (1H, br s, Rha-1), 4.72 (1H, m, Rha-2), 4.55 (1H, m, Rha-3), 4.28 (1H, m, Rha-4), 4.37 (1H, m, Rha-5), 1.56 (1H, d, J = 6.0 Hz, Rha-6); 13C-NMR of sugar moieties (150 MHz, pyridine-d5) δ: 104.9 (Ara-1), 76.2 (Ara-2), 75.2 (Ara-3), 69.8 (Ara-4), 66.2 (Ara-5), 102.1 (Rha-1), 72.8 (Rha-2), 72.9 (Rha-3), 74.5 (Rha-4), 70.1 (Rha-5), 18.9 (Rha-6).
Pulsatilla saponin D (21): White powder; ESI-MS m/z 911 [M−H]−; 1H-NMR of aglycone (600 MHz, pyridine-d5) δ: 1.07 (1H, m, H-1a), 1.54 (1H, m, H-1b), 2.05 (1H, m, H-2a), 2.40 (1H, m, H-2b), 4.38 (1H, dd, J = 3.6, 11.0 Hz, H-3), 1.75 (1H, d, J = 12.0 Hz, H-5), 1.53 (2H, m, H-6), 1.26 (1H, m, H-7a), 1.55 (1H, m, H-7b), 1.77 (1H, m, H-9), 1.96 (2H, m, H-11), 5.50 (1H, br s, H-12), 1.15 (1H, m, H-15a), 2.27 (1H, m, H-15b), 1.90 (1H, m, H-16a), 2.09 (1H, m, H-16b), 3.30 (1H, d, J = 12.0 Hz, H-18), 1.20 (1H, m, H-19a), 1.69 (1H, m, H-19b), 0.90 (1H, m, H-21a), 1.08 (1H, m, H-21b), 1.73 (1H, m, H-22a), 1.87 (1H, m, H-22b), 4.05 (1H, d, J = 12.0 Hz, H-23a), 4.52 (1H, d, J = 12.0 Hz, H-23b), 1.04 (3H, s, H-24), 0.96 (3H, s, H-25), 1.01 (3H, s, H-26), 1.25 (3H, s, H-27), 0.93 (3H, s, H-29), 0.94 (3H, s, H-30); 13C-NMR of aglycone (150 MHz, pyridine-d5) δ: 39.1 (C-1), 26.4 (C-2), 82.4 (C-3), 43.8 (C-4), 47.9 (C-5), 18.5 (C-6), 33.6 (C-7), 40.1 (C-8), 47.9 (C-9), 37.3 (C-10), 24.2 (C-11), 123.0 (C-12), 145.3 (C-13), 42.5 (C-14), 28.7 (C-15), 24.0 (C-16), 47.0 (C-17), 42.3 (C-18), 46.8 (C-19), 31.3 (C-20), 34.5 (C-21), 33.2 (C-22), 64.8 (C-23), 13.9 (C-24), 16.4 (C-25), 17.8 (C-26), 26.5 (C-27), 180.8 (C-28), 33.6 (C-29), 24.1 (C-30); 1H-NMR of sugar moieties (600 MHz, pyridine-d5) δ: 4.93 (1H, d, J = 6.5 Hz, Ara-1), 4.15 (1H, m, Ara-2), 4.35 (1H, m, Ara-3), 4.24 (1H, m, Ara-4), 3.96 (1H, m, Ara-5a), 4.40 (1H, m, Ara-5b), 5.82 (1H, br s, Rha-1), 4.72 (1H, m, Rha-2), 4.55 (1H, m, Rha-3), 4.28 (1H, m, Rha-4), 4.37 (1H, m, Rha-5), 1.64 (1H, d, J = 6.0 Hz, Rha-6), 5.26 (1H, d, J = 7.0 Hz, Glc-1), 3.95 (1H, m, Glc-2), 4.20 (1H, m, Glc-3), 4.89 (1H, m, Glc-4), 4.10 (1H, m, Glc-5), 4.27 (1H, m, Glc-6a), 4.52 (1H, m, Glc-6b); 13C-NMR of sugar moieties (150 MHz, pyridine-d5) δ: 104.2 (Ara-1), 76.0 (Ara-2), 74.9 (Ara-3), 80.3 (Ara-4), 65.3 (Ara-5), 101.5 (Rha-1), 72.1 (Rha-2), 72.3 (Rha-3), 73.9 (Rha-4), 69.5 (Rha-5), 18.4 (Rha-6), 106.6 (Glc-1), 75.3 (Glc-2), 78.3 (Glc-3), 71.0 (Glc-4), 78.6 (Glc-5), 62.2 (Glc-6).
3.4. Nematicidal Assay
A soil sample was collected from a pure culture of M. incognita and maintained on muskmelon roots in a greenhouse in Gimcheon (Gyeongbuk, Korea). Emerged larvae were collected from the soil using the Baermann funnel technique. Larvae were placed in a cavity block with water for a bioassay after they had been counted in a counting chamber. MeOH extract and the compounds isolated from P. koreana (5.0 mg) were prepared in 0.1 mL dimethyl sulfoxide (DMSO) and then diluted in water to obtain various concentration preparations (50–200 μg/mL). The standard nematicide fosthiazate was used for the comparison [35]. As a negative control of nematicidal activity, 5% DMSO was used. Approximately 40–60 freshly hatched second-stage juveniles in 450 μL of water and 50 μL of each of the 12 compounds at different concentrations were introduced into 24-well plates with three replicates performed. Plates were kept at room temperature (23–25 °C) under laboratory conditions. Inactive nematodes (dead nematodes) counted after 24, 48, and 72 h [36]. After the last count, inactive juveniles were maintained in distilled water for 72 h to observe their revival (Figure 2). Five repetitions for each treatment were performed using water as a control.
4. Conclusions
In present study, bioassay-guided chromatographic fractionation and isolation were successfully used to yield 31 triterpenoid saponins from P. koreana roots. Three of the isolated compounds, raddeanoside R13 (9), hederoside C (20), and pulsatilla saponin D (21) may have potential as natural nematicides or as lead molecules for developing new nematicides to control root-knot nematode disease caused by M. incognita.
Acknowledgments
This study was supported by the Priority Research Center Program (2009-0093815) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology, Korea.
Footnotes
Sample Availability: Samples of the compounds 1–31 are available from the authors.
References
- 1.Batten C.K., Powell N.T. Rhizoctonia-Meloidogyne disease complex in flux-cured tobacco. J. Nematol. 1971;3:164–169. [PMC free article] [PubMed] [Google Scholar]
- 2.Chahal P.P.K., Chabra H.K. Interaction of M. incognita with Rhizoctonia solani on tomato. Indian J. Nematol. 1984;14:56–57. [Google Scholar]
- 3.Goswami J., Pandey R.K., Tewari J.P., Goswami B.K. Management of root knot nematode on tomato through application of fungal antagonists, Acremonium strictum and Trichoderma harzianum. J. Environ. Sci. Health B. 2008;43:237–240. doi: 10.1080/03601230701771164. [DOI] [PubMed] [Google Scholar]
- 4.Akhtar M. Current options in integrated management of plantparasitic nematodes. Integ. Pest Manag. Rev. 1997;2:187–197. doi: 10.1023/A:1018409303298. [DOI] [Google Scholar]
- 5.Chitwood D.J. Phytochemical based strategies for nematode control. Annu. Rev. Phytopathol. 2002;40:221–249. doi: 10.1146/annurev.phyto.40.032602.130045. [DOI] [PubMed] [Google Scholar]
- 6.Faizi S., Fayyaz S., Bano S., Iqbal E.Y., Lubna, Siddiqi H., Naz A. Isolation of nematicidal compounds from Tagetes patula L. yellow flowers: Structure-activity relationship studies against cyst nematode Heterodera zeae infective stage larvae. J. Agric. Food Chem. 2011;59:9080–9093. doi: 10.1021/jf201611b. [DOI] [PubMed] [Google Scholar]
- 7.Kim Y., Bang S.C., Lee J.H., Ahn B.Z. Pulsatilla saponin D: The antitumor principle from Pulsatilla koreana. Arch. Pharm. Res. 2004;27:915–918. doi: 10.1007/BF02975843. [DOI] [PubMed] [Google Scholar]
- 8.Guan Y.L., Liu J.Y., Xu Y.N. Research progress in the triterpene saponins and biological activities of the genus Pulsatilla. Shenyang Yaoke Daxue Xuebao. 2009;26:80–84. [Google Scholar]
- 9.Li W., Ding Y., Sun Y.N., Yan X.T., Yang S.Y., Choi C.W., Kim E.J., Kang H.K., Kim Y.H. Oleanane-type triterpenoid saponins from the roots of Pulsatilla koreana and their apoptosis-inducing effects on HL-60 human promyelocytic leukemia cells. Arch. Pharm. Res. 2013 doi: 10.1007/s12272-013-0042-5. [DOI] [PubMed] [Google Scholar]
- 10.Son M.K., Jung K.H., Hong S.W., Lee H.S., Zheng H.M., Choi M.J., Seo J.H., Suh J.K., Hong S.S. SB365, Pulsatilla saponin D suppresses the proliferation of human colon cancer cells and induces apoptosis by modulating the AKT/mTOR signalling pathway. Food Chem. 2013;136:26–33. doi: 10.1016/j.foodchem.2012.07.096. [DOI] [PubMed] [Google Scholar]
- 11.Ye W.C., Ji N.N., Zhao S.X., Liu J.H., Ye T., McKervey M.A., Stevenson P. Triterpenoids from Pulsatilla chinensis. Phytochemistry. 1996;42:799–802. doi: 10.1016/0031-9422(96)00043-x. [DOI] [PubMed] [Google Scholar]
- 12.Ye W.C., Ou B.X., Ji N.N., Zhao S.X., Ye T., McKervey M.A., Stevenson P. Patensin, a saponin from Pulsatilla patens var. multifida. Phytochemistry. 1995;39:937–939. doi: 10.1016/0031-9422(95)00021-x. [DOI] [PubMed] [Google Scholar]
- 13.Fu Y., Chen H., Liu D., Zhang Z. Chemical constituents of Pulsatilla koreana. Zhong Cao Yao. 2008;39:26–29. [Google Scholar]
- 14.Yang H.J., Cho Y.W., Kim S.H., Kim Y.C., Sung S.H. Triterpenoidal saponins of Pulsatilla koreana roots. Phytochemistry. 2010;71:1892–1899. doi: 10.1016/j.phytochem.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Tommasi N., Autore G., Bellino A., Pinto A., Pizza C., Sorrentino R., Venturella P. Antiproliferative triterpene saponins from Trevesia palmata. J. Nat. Prod. 2000;63:308–314. doi: 10.1021/np990231n. [DOI] [PubMed] [Google Scholar]
- 16.Liu J.Y., Guan Y.L., Zou L.B., Gong Y.X., Hua H.M., Xu Y.N., Zhang H., Yu Z.G., Fan W.H. Saponins with neuroprotective effects from the roots of Pulsatilla cernua. Molecules. 2012;17:5520–5531. doi: 10.3390/molecules17055520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Majester-Savornin B., Elias R., Diaz-Lanza A.M., Balansard G., Gasquet M., Delmas F. Saponins of the ivy plant, Hedera helix, and their leishmanicidic activity. Planta Med. 1991;57:260–262. doi: 10.1055/s-2006-960086. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y., Li M., Liu J. Haemolytic activities and adjuvant effect of Anemone raddeana saponins (ARS) on the immune responses to ovalbumin in mice. Int. Immunopharmacol. 2008;8:1095–1102. doi: 10.1016/j.intimp.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 19.Schenkel E.P., Werner W., Schulte K.E. Saponins from Thinouia coriaceae. Planta Med. 1991;57:463–467. doi: 10.1055/s-2006-960152. [DOI] [PubMed] [Google Scholar]
- 20.Bang S.C., Kim Y., Lee J.H., Ahn B.Z. Triterpenoid saponins from the roots of Pulsatilla koreana. J. Nat. Prod. 2005;68:268–272. doi: 10.1021/np049813h. [DOI] [PubMed] [Google Scholar]
- 21.Mimaki Y., Kuroda M., Asano T., Sashida Y. Triterpene saponins and lignans from the roots of Pulsatilla chinensis and their cytotoxic activity against HL-60 cells. J. Nat. Prod. 1999;62:1279–1283. doi: 10.1021/np9901837. [DOI] [PubMed] [Google Scholar]
- 22.Li X., Wang D., Wu S., Yang C. Triterpenoid saponins from Pulsatilla campanella. Phytochemistry. 1990;29:595–599. [Google Scholar]
- 23.Tran H.Q., Nguyen T.T.N., Chau V.M., Phan V.K., Nguyen X.N., Bui H.T., Nguyen P.T., Nguyen H.T., Song S.B., Kim Y.H. Anti-inflammatory triterpenoid saponins from the stem bark of Kalopanax pictus. J. Nat. Prod. 2011;74:1908–1915. doi: 10.1021/np200382s. [DOI] [PubMed] [Google Scholar]
- 24.Li W., Ding Y., Sun Y.N., Yan X.T., Yang S.Y., Choi C.W., Cha J.Y., Lee Y.M., Kim Y.H. Triterpenoid saponins of Pulsatilla koreana root have inhibition effects of tumor necrosis factor-α secretion in lipopolysaccharide-induced RAW264.7 Cells. Chem. Pharm. Bull. 2013;61:471–476. doi: 10.1248/cpb.c12-01034. [DOI] [PubMed] [Google Scholar]
- 25.Kang S.S., Kim J.S., Kim Y.H., Choi J.S. A triterpenoid saponin from Patrinia scabiosaefolia. J. Nat. Prod. 1997;60:1060–1062. doi: 10.1021/np970175v. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Q., Ye W., Yan X., Zhu G., Che C.T., Zhao S. Cernuosides A and B, two sucrase inhibitors from Pulsatilla cernua. J. Nat. Prod. 2000;63:276–278. doi: 10.1021/np990207+. [DOI] [PubMed] [Google Scholar]
- 27.Shimizu M., Shingyouchi K., Morita N., Kizu H., Tomimori T. Triterpenoid saponins from Pulsatilla cenuiui spreng. I. Chem. Pharm. Bull. 1978;26:1666–1671. doi: 10.1248/cpb.26.1666. [DOI] [Google Scholar]
- 28.Saito S., Sumita S., Tamura N., Nagamura Y., Nishida K., Ito M., Ishiguro I. Saponins from the leaves of Aralia elata Seem. (Araliaceae) Chem. Pharm. Bull. 1990;38:411–414. doi: 10.1248/cpb.38.411. [DOI] [Google Scholar]
- 29.Baykal T., Panayir T., Sticher O., Calis I. Scabioside A: A new triterpenoid saponoside from Scabiosa rotate. J. Facul. Pharm. Gazi Univ. 1997;14:31–36. [Google Scholar]
- 30.Mimaki Y., Yokosuka A., Kuroda M., Hamanaka M., Sakuma C., Sashida Y. New bisdesmosidic triterpene saponins from the roots of Pulsatilla chinensis. J. Nat. Prod. 2001;64:1226–1229. doi: 10.1021/np010252t. [DOI] [PubMed] [Google Scholar]
- 31.Gauthier C., Legault J., Lavoie S., Rondeau S., Tremblay S., Pichette A. Synthesis of two natural betulinic acid saponins containing α-l-rhamnopyranosyl-(1→2)-α-L-arabinopyranose and their analogs. Tetrahedron. 2008;64:7386–7399. [Google Scholar]
- 32.Kim S.I., Heo J.W., Jung I.H., Yang Y.C., Lee J.G., Choi D.R., Ahn Y.J. Nematicide Composition Comprising Plant Extract. Repub. KR 2009102136 A 20090930. Korean Kongkae Taeho Kongbo. 2009 Sep 30;
- 33.Zhang H., Samadi A.K., Rao K.V., Cohen M.S., Timmermann B.N. Cytotoxic oleanane-type saponins from Albizia inundata. J. Nat. Prod. 2011;74:477–482. doi: 10.1021/np100702p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mimaki Y., Yokosuka A., Hamanaka M., Sakuma C., Yamori T., Sashida Y. Triterpene saponins from the roots of Clematis chinensis. J. Nat. Prod. 2004;67:1511–1516. doi: 10.1021/np040088k. [DOI] [PubMed] [Google Scholar]
- 35.Gao D., Miao J., Liu F. Susceptibilities of cereal cyst nematode from different regions to different types of nematicides. Mailei Zuowu Xuebao. 2012;32:168–172. [Google Scholar]
- 36.Ntalli N.G., Vargiu S., Menkissoglu-Spiroudi U., Caboni P. Nematicidal carboxylic acids and aldehydes from Melia azedarach fruits. J. Agric. Food Chem. 2010;58:11390–11394. doi: 10.1021/jf1025345. [DOI] [PubMed] [Google Scholar]