Abstract
Two bisbenzylisoquinoline alkaloids, two morphine alkaloids, one aporphine alkaloid, syringaresinol and aristolochic acid І were selected as marker compounds and simultaneously analyzed using an ultra-high pressure liquid chromatography-diode array detection (UHPLC-DAD) method. These marker compounds were used for the quality control of Fangchi species of different origins, including Sinomenium acutum, Stephaniatetrandra, Cocculus trilobus and Aristolochia fangchi. A reversed-phase UHPLC-DAD method was developed and validated for the simultaneous quantification of structurally diverse markers in different Fangchi species. In addition, an UHPLC-electrospray ionization tandem mass spectrometry (ESI-MS/MS) method was used for marker identification in Fangchi species, which provided diagnostic MS/MS spectral patterns that were dependent upon the marker structures. The UHPLC-MS/MS data were used to confirm and complement the UHPLC-DAD quality evaluation results. Additionally, magnoflorine and syringaresinol were observed for the first time in S. tetrandra and C. trilobus, respectively. Twenty different Fangchi species samples were analyzed for aristolochic acid I, syringaresinol and the alkaloids using the UHPLC-DAD and MS/MS method. Based on the levels of markers and principal component analysis (PCA), this method allowed for the clear classification of the samples into four different groups representing samples originating from the four species.
Keywords: Fangchi species, alkaloids, aristolochic acid I, lignan, UHPLC-DAD, UHPLC-MS/MS
1. Introduction
Fangchi, one of the most commonly used traditional herbal medicines, is derived from the rhizoma of Sinomenium (S.) acutum [1,2] and the radix of Stephania (S.) tetrandra (Menispermaceae) [3]. S. acutum and S. tetrandra have been widely used for the treatment of rheumatic arthritis [4]. The main bioactive components in S. acutum are alkaloids and lignans such as sinomenine, isosinomenine, magnoflorine and syringaresinol [5]. The main bioactive components in S. tetrandra are tetrandrine and fangchinoline [5]. Additionally, Cocculus (C.) trilobus (Menispermaceae) and Aristolochia (A.) fangchi (Aristolochiaceae) are also referred to as “Mu fangchi” and “Guang fangchi”, respectively. C. trilobus has been used in folk medicine as a diuretic, analgesic and an anti-inflammatory [6]. A. fangchi has been banned for medicinal use because of the presence of aristolochic acids, which are known nephrotoxins and carcinogens [7]. The chemical compositions of Fangchi extracts differ significantly in terms of the species from which they originate. Furthermore, the biological activity of each Fangchi species is closely related to its major chemical components.
Aporphine, morphine and bisbenzylisoquinoline alkaloids, lignans and nitrophenanthrene carboxylic acids are the major components of Fangchi species and have various pharmaceutical effects. It was reported that magnoflorine, an aporphine alkaloid, has antioxidant activity by protecting human high-density lipoprotein against lipid peroxidation [8]. Morphine alkaloids, such as sinomenine and isosinomenine, have been reported to have anti-angiogenic, anti-inflammatory and anti-rheumatic effects [9]. The bisbenzylisoquinoline alkaloids fangchinoline and tetrandrine have shown anti-inflammatory, hypotensive and vasodilating effects [10,11]. Syringaresinol, a furofuran-type lignan, has been shown to inhibit the motility of Helicobacter pylori [12] and to possess anti-proliferative activity [13]. Aristolochic acid I caused rapidly progressive interstitial nephritis in patients who ingested slimming pills derived from herbal medicines containing aristolochic acid I [14].
However, analytical methods have only been narrowly applied for quality evaluations of members of this genus due to the challenge of detecting structurally diverse compounds that vary with Fangchi origin. Moreover, the identification and determination of the bioactive compounds in herbal medicine is a difficult process because it necessitates LC chromatographic separation of structurally diverse compounds and can lead to false positives due to overlapping interferences or structurally similar components present in complicated herbal extracts. Thus, the development of a practical UHPLC method for the identification and simultaneous determination of structurally diverse markers is essential for quality evaluations of Fangchi species of different origins. The UHPLC method had advantages over HPLC in terms of time saving, solvent saving, performance, and efficiency that resulted in higher sample throughput, less solvent consumption, and less sample injection volume than HPLC.
This study demonstrated a rapid and simple UHPLC-DAD method for the simultaneous determination of structurally diverse markers (as shown in Figure 1) in Fangchi species of different origins. The validated UHPLC method was successfully applied for the simultaneous determination of these marker compounds in twenty Fangchi samples. All Fangchi samples were also analyzed using an UHPLC-ESI-MS/MS method to confirm the UHPLC results. The optimized UHPLC methods were used to determine the levels of these marker compounds in twenty Fangchi extracts and to classify the samples into four different Fangchi species through PCA. This study represents the first investigation towards the simultaneous determination of structurally diverse marker compounds in four Fangchi species of different origins. The described methods are applicable for identification and analytical profiling of the four Fangchi species. Even more, they can be used to recognize falsifications and for quality control of medicinal preparations containing Fangchi herbs.
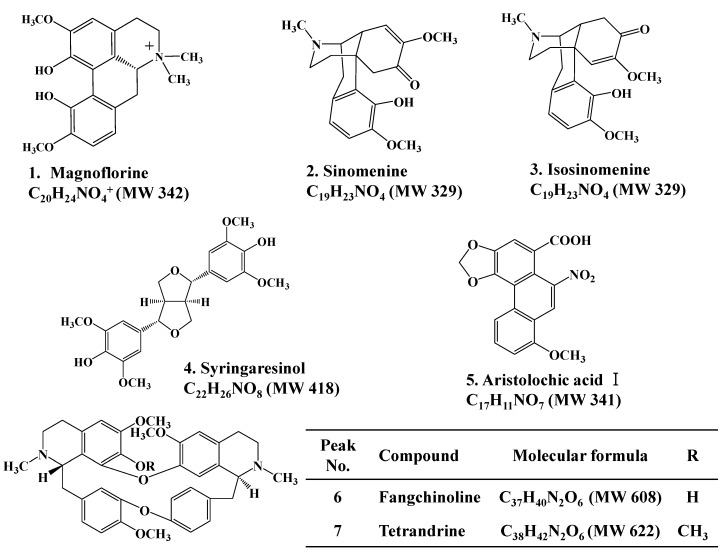
Figure 1.
Chemical structures of compounds identified in four Fangchi species.
2. Results and Discussion
2.1. Analysis of Marker Compounds by UHPLC-DAD
UHPLC separation conditions were optimized to facilitate the simultaneous determination of various markers (alkaloids, aristolochic acid I and a lignan) in Fangchi species in a single LC run. The optimized parameters were pH, NH4OAc concentration and the composition of the mobile phase, as performed in our previous report [15]. In addition, the retention of ionizable compounds using reversed-phase HPLC was strongly dependent on the percentage and strength of the organic solvent used in the mobile phase [16,17,18]. In this study, methanol was selected over acetonitrile as it provided better peak shapes, especially for fangchinoline and tetrandrine.
To obtain good sensitivity and accuracy for the simultaneous quantification of the marker compounds, the DAD detection wavelength was set to 235 nm, which was a compromise between the UV absorption maxima of the marker compounds and interfering components in the extract. Measurement at 235 nm exhibited sufficient sensitivity and a stable chromatographic baseline.
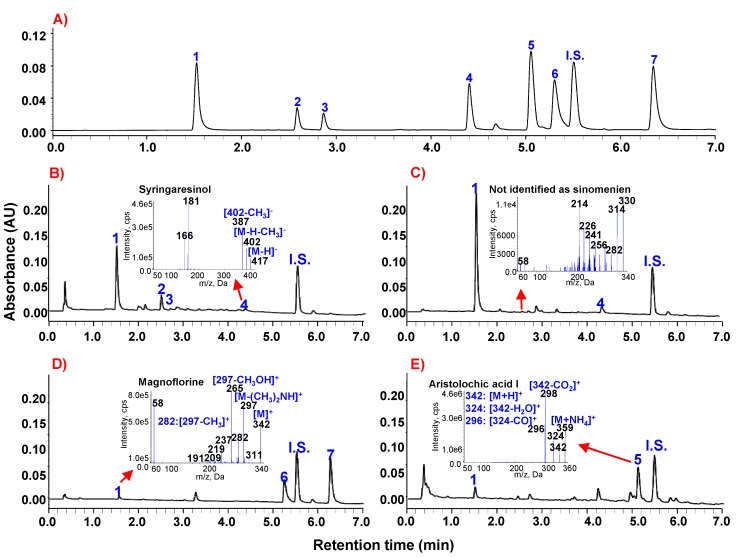
The UHPLC-DAD analysis conditions that produced the best chromatographic separation and detection sensitivities of the seven markers were determined to be a solvent system of 20 mM NH4OAc (adjusted to pH 6.0 with acetic acid)–methanol and detection wavelength at 235 nm. Using the established UHPLC method, diverse marker compounds were successfully separated within a 7 min run time and had resolutions of greater than 2.60, even for the closest peaks, which were aristolochic acid I and fangchinoline (Figure 2A). This UHPLC-DAD method was also successfully applied to determine the level of marker compounds in four typical Fangchi extracts, as shown in Figure 2B~E. Identification of the analytes is based on reference compounds and MS/MS data (see insets of Figure 2B~E). UHPLC chromatograms of extracts of Fangchi species exhibited stable baselines and did not show any significant interferences.
Figure 2.
Representative UHPLC chromatograms of marker compounds: (A) standard mixtures (B) Sinomenium acutum; (C) Cocculus trilobus; (D) Stephania tetrandra; and (E) Aristolochia fangchi. Peak identities: 1: magnoflorine; 2: sinomenine; 3: isosinomenine; 4: syringaresinol; 5: aristolochic acid І; 6: fangchinoline; 7: tetrandrine; and I.S.: propyl-4-hydroxybenzoate.
2.2. UHPLC-ESI-MS/MS Confirmation of UHPLC-DAD Results
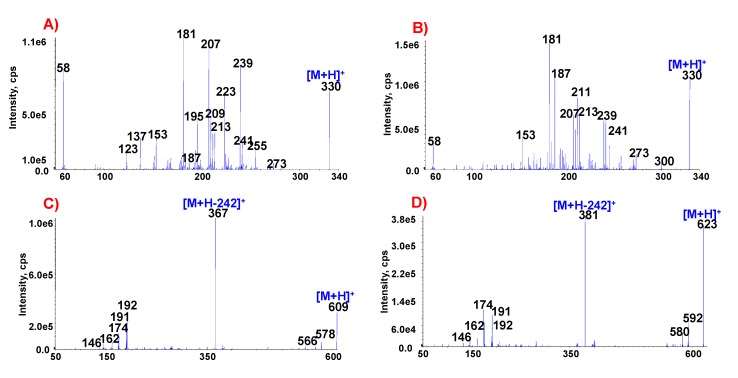
In some cases, it is difficult to accurately identify markers in herbal extracts by LC-DAD due to the presence of interfering compounds with structures that are similar to the markers. For the accurate identification of various types of markers extracted from Fangchi species, authentic markers were preferentially analyzed by ESI-MS/MS to determine the specificity of MS/MS spectral patterns for different molecules. The MS/MS spectra of the seven marker compounds are shown in the insets of Figure 2 and Figure 3. The MS/MS spectral data of the authentic standards are summarized in Table 1, including exact mass measurements of precursor ions and characteristic fragment ions. The exact mass measurements of the markers are indistinguishable from their theoretical values and are within 2.6 mmu of error. The MS/MS spectra of each of the markers exhibited specific patterns.
Figure 3.
ESI-MS/MS spectra of marker compounds found in different Fangchi species. (A) sinomenine; (B) isosinomenine; (C) fangchinoline; (D) tetrandrine.
Table 1.
Exact mass measurements of precursor ions (m/z) and characteristic fragment ions (m/z) for seven marker compounds.
| Compound | Precursor ion formula | Precursor ion (m/z) | Exact mass (m/z) | Characteristic ions (m/z) | ||
|---|---|---|---|---|---|---|
| Theoretical | Observed a | Difference (mmu) | ||||
| Magnoflorine | C20H24NO4+ | 342 | 342.1705 | 342.1724 | 1.9 | 58, 191, 209, 219, 237, 265, 282, 297, 311 |
| Sinomenine | C19H24NO4+ | 330 | 330.1705 | 330.1716 | 1.1 | 58, 123, 137, 153, 181, 187, 195, 207, 209, 213, 223, 239, 241, 255, 273 |
| Isosinomenine | C19H24NO4+ | 330 | 330.1705 | 330.1721 | 1.6 | 58, 153, 181, 187, 207, 211, 213, 239, 241, 273, 300 |
| Syringaresinol | C22H25O8− | 417 | 417.1549 | 417.1545 | 0.4 | 166, 181, 387, 402 |
| Fangchinoline | C37H41N2O6+ | 609 | 609.2965 | 609.2991 | 2.6 | 146, 162, 174, 191, 192, 367, 566, 578 |
| Aristolochic acid I | C17H15N2O7+ | 359 | 359.0879 | 359.0884 | 0.5 | 296, 298, 324, 342 |
| Tetrandrine | C38H43N2O6+ | 623 | 623.3121 | 623.3142 | 2.1 | 146, 162, 174, 191, 192, 381, 580, 592 |
a obtained by high-resolution fast atom bombardment mass spectrometry.
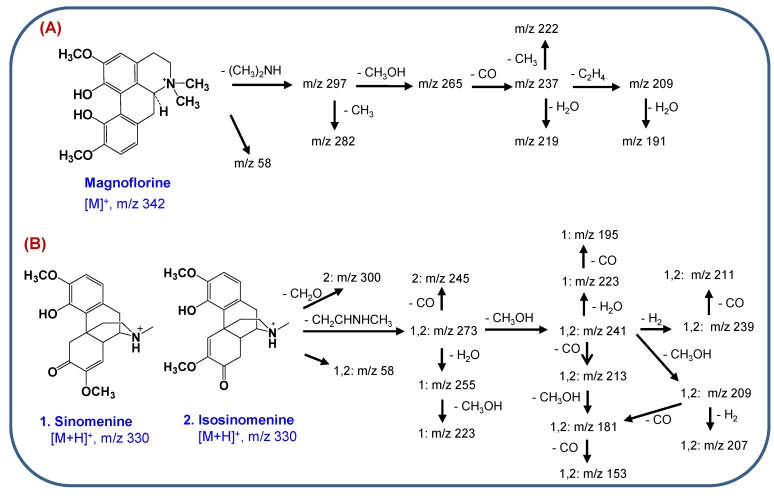
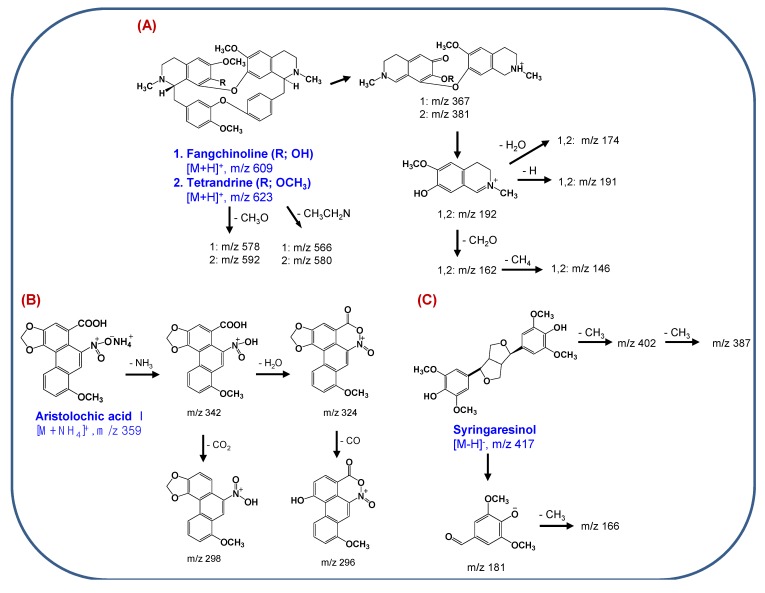
Magnoflorine, an aporphine alkaloid, showed a molecular ion [M]+ at m/z 342 and the initial fragmentation was due to the loss of dimethylamine [(CH3)2NH]+, as shown in the inset of Figure 2C. Other characteristic ions were produced by the successive losses of substituted CH3 groups or CH3OH molecules from the [M-(CH3)2NH]+ ion [19,20]. The proposed MS/MS fragmentation pathways for magnoflorine are shown in Scheme 1A.
Scheme 1.
Proposed MS/MS fragmentation pathways of (A) magnoflorine and (B) the morphine alkaloids.
The MS/MS spectra of sinomenine and isosinomenine, which are structural isomers, exhibited different spectral patterns due to the different positions of the double bonds in the C-ring, as shown in Figure 3A,B. The MS/MS spectral patterns of these isomers can be characterized by the presence of various fragment ions due mainly to cleavage of the piperidine ring. Initial MS fragmentations are a loss of an amine moiety (CH2CHNHCH3) from [M+H]+ induced by successive cleavage of the piperidine ring [21]. The other characteristic fragment ions at m/z 181, 207 and 58 were formed by losses of CH3OH and CO molecules from the [(M+H)-CH2CHNHCH3]+ ion [21]. MS/MS fragmentation pathways of the morphine alkaloids are proposed in Scheme 1B.
The MS/MS spectra of the bisbenzylisoquinoline alkaloids exhibited [M+H]+ ions and characteristic diagnostic ions at m/z 367 and 381 for fangchinoline and tetrandrine, respectively, due to the preferred cleavage of the carbon-carbon bond that is β both to the nitrogen and to the two aromatic systems, followed by O-demethylation of an aromatic methoxyl group (Figure 3C,D). The product ion at m/z 192 most likely corresponds to a single isoquinoline moiety that can lose a H2O molecule, resulting in the formation of an ion at m/z 174 [22]. MS/MS fragmentation pathways of the bisbenzylisoquinoline alkaloids are suggested in Scheme 2A.
Scheme 2.
Proposed MS/MS fragmentation pathways of (A) bisbenzylisoquinoline alkaloids, (B) aristolochic acid I and (C) syringaresinol.
The ESI-MS spectrum of aristolochic acid I exhibited an ammoniated ion [M+NH4]+ at m/z 359, due to the easy formation of an adduct of an ammonium ion with the nitro group in positive ion mode. The ammonium is derived from the ammonium acetate in the mobile phase. As shown in Figure 2D, specific product ions at m/z 342 and 298 resulting from successive losses of NH3 and CO2 from the parent [(M+NH4)]+ ion were observed. Two additional specific fragment ions (m/z 324 and m/z 296) were observed, which correspond to [(M+NH4)-NH3-H2O]+ and [[(M+NH4)-NH3-H2O-CO]+ ions. Notably, the loss of a H2O molecule from [(M+NH4)-NH3]+ ion might induce cyclization between the carboxylic acid and nitro groups [23,24]. The MS/MS fragmentation pathways of aristolochic acid I are proposed in Scheme 2B.
Syringaresinol, a furofuranolignan containing two phenol groups, was observed with more sensitivity in negative-ion mode ESI-MS than in positive-ion mode, producing an [M−H]− ion (m/z 417) (inset of Figure 2A). The collision-induced dissociation (CID) spectrum of syringaresinol contained characteristic fragment ions at m/z 402 and m/z 387, resulting from the successive losses of two CH3 groups from the [M−H]− ion [25]. The most abundant ion at m/z 181 might be formed by cleavage of a tetrahydrofuran ring, as shown in Scheme 2C.
Based on these results, the presence of the marker compounds in extracts of Fangchi species was further confirmed, although the marker compound peaks were small. As shown Figure 2B, the small peak at 2.3 min in the C. trilobus extract was erroneously identified as sinomenine based on the UHPLC retention time and ESI-MS results. However, based on its MS/MS spectrum, it was determined that the peak was not sinomenine. The peak at 2.3 min is hypothesized to be a morphine alkaloid based on its MS/MS spectral pattern (inset of Figure 2C) and retention time. At the present time, the identity of this unknown compound is ambiguous and warrants further investigation.
All herbal extracts were analyzed using the UHPLC-MS/MS system to accurately identify markers compounds. Magnoflorine and syringaresinol were observed for the first time in S. tetrandra and C. trilobus, respectively, in this study. The MS/MS fragmentation patterns of these markers are useful for identifying other bioactive components and related compounds in Fangchi species and other herbal medicines.
2.3. Method Validation
The sample used for studies to determine the recovery and precision of the method was an S. acutum extract containing measurable levels of the markers compounds. Calibration curves were constructed in the concentration range of 0.2–100 μg/mL for each of the markers. Each of the seven standard solutions was analyzed in triplicate. Multi-point calibration curves were constructed by linear regression analysis of the peak ratios of each analyte to the internal standard, versus concentration. The calibration equations, linear correlation coefficients, limit of detection (LOD) and limit of quantitation (LOQ) of markers are summarized in Table 2. The correlation coefficients were all greater than 0.9981, indicating good linearity. Calibration curves prepared in the absence and presence of the sample matrix were almost identical within experimental error. Calibration in the absence of the sample matrix is recommended for screening because it is simpler than calibration in the presence of the matrix [26,27]. The LODs and LOQs of the markers were measured as the lowest concentrations that corresponded to signal-to-noise ratios of 3 and 10, respectively. The LODs and LOQs of the marker compounds were in the ranges of 0.01–0.05 μg/mL and 0.05–0.2 μg/mL, respectively.
Table 2.
Calibration curve equations, linearity correlation coefficients, LOD values and LOQ values for the marker compounds.
| Compound | Range (μg/mL) | Linear equation | Correlation coefficient | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|---|
| Magnoflorine | 0.2–100 | y = 0.0402x − 0.0161 | 0.9990 | 0.01 | 0.05 |
| Sinomenine | 0.2–100 | y = 0.0090x − 0.0024 | 0.9989 | 0.05 | 0.17 |
| Isosinomenine | 0.2–100 | y = 0.0056x − 0.0036 | 0.9995 | 0.05 | 0.20 |
| Syringaresinol | 0.2–100 | y = 0.0221x + 0.0021 | 0.9981 | 0.03 | 0.10 |
| Fangchinoline | 0.2–100 | y = 0.0885x − 0.0411 | 0.9998 | 0.03 | 0.10 |
| Aristolochic acid І | 0.2–100 | y = 0.0311x − 0.014 | 0.9982 | 0.01 | 0.05 |
| Tetrandrine | 0.2–100 | y = 0.0511x + 0.0786 | 0.9982 | 0.02 | 0.07 |
To test the accuracy and precision of the analytical method, known amounts of the seven markers (5, 25 and 50 μg/mL) were added to S. acutum extract. The intra- and inter-day variations for the seven marker compounds in the S. acutum extract were determined as described in the Experimental section, and the results are summarized in Table 3. The precision of the method for the simultaneous determination of the seven marker compounds was acceptable because the RSD did not exceed 7.78% at concentrations of 5, 25 and 50 μg/mL. At the same concentrations, the intra-day accuracies ranged from 94.49% to 100.84%, while the inter-day accuracies ranged from 93.81% to 100.83%, indicating good accuracy of the method. These good results indicate that the marker compounds were completely extracted from the Fangchi sample by sonication extraction using 70% MeOH. The reproducibility of retention times was evaluated over 36 injections using the standard solution, and the RSD was 0.14%–1.18%. The repeatability of the area ratio was calibrated using the peak area of each compound divided by the peak area of the internal standard, and the RSD was 1.62%–3.85% (n = 9). These results indicated that the UHPLC method had suitable repeatability with respect to both retention time and peak area.
Table 3.
Intra- and inter-day precision and accuracy for the quantification of marker compounds in spiked S. acutum extracts.
| Compound | Fortified conc. (μg/mL) | Intra-day (n = 5) | Inter-day (n = 5) | |||||
|---|---|---|---|---|---|---|---|---|
| Observed Conc. (μg/mL) | Precision (%) | Accuracy (%) | Observed Conc. (μg/mL) | Precision (%) | Accuracy (%) | |||
| Magnoflorine | 5 | 4.72 | 5.19 | 94.49 | 4.69 | 5.27 | 93.81 | |
| 25 | 24.78 | 0.63 | 99.14 | 24.69 | 2.22 | 98.78 | ||
| 50 | 49.13 | 1.37 | 98.26 | 49.45 | 2.97 | 98.90 | ||
| Sinomenine | 5 | 4.90 | 6.54 | 98.05 | 4.97 | 4.10 | 99.49 | |
| 25 | 24.81 | 1.68 | 99.23 | 25.19 | 3.97 | 100.76 | ||
| 50 | 48.35 | 0.11 | 96.70 | 48.66 | 1.25 | 97.31 | ||
| Isosinomenine | 5 | 5.04 | 7.09 | 100.84 | 4.98 | 4.34 | 99.63 | |
| 25 | 23.86 | 7.78 | 95.46 | 24.53 | 5.16 | 98.11 | ||
| 50 | 49.93 | 1.44 | 99.86 | 50.27 | 3.08 | 100.53 | ||
| Syringaresinol | 5 | 4.80 | 4.03 | 96.02 | 4.86 | 1.99 | 97.15 | |
| 25 | 24.74 | 0.16 | 98.95 | 24.75 | 1.82 | 98.99 | ||
| 50 | 49.87 | 0.66 | 99.75 | 49.56 | 1.30 | 99.11 | ||
| Fangchinoline | 5 | 4.80 | 2.83 | 95.97 | 4.74 | 2.12 | 94.84 | |
| 25 | 24.53 | 5.02 | 98.11 | 24.54 | 2.09 | 98.17 | ||
| 50 | 50.36 | 0.15 | 100.72 | 48.79 | 4.67 | 97.58 | ||
| Aristolochic acid I | 5 | 4.96 | 6.15 | 99.26 | 5.04 | 3.66 | 100.83 | |
| 25 | 24.82 | 0.62 | 99.29 | 25.12 | 1.36 | 100.47 | ||
| 50 | 50.41 | 0.50 | 100.82 | 49.29 | 4.69 | 98.52 | ||
| Tetrandrine | 5 | 4.85 | 0.66 | 97.08 | 4.97 | 3.37 | 99.48 | |
| 25 | 24.55 | 1.59 | 98.18 | 24.72 | 2.44 | 98.86 | ||
| 50 | 50.36 | 0.27 | 100.72 | 49.58 | 2.67 | 99.16 | ||
2.4. Method Application
Twenty Fangchi samples of different origins (A. fangchi, S. acutum, S. tetrandra and C. trilobus) were successfully analyzed using the established method for quality control purposes. Typical UHPLC chromatograms of extracts from four Fangchi species that showed different patterns based on their origins are shown in Figure 2. As shown in this Figure, the established method was successfully applied for the determination of marker compounds in different Fangchi species and there were substantial differences in the constituents of the samples from different species.
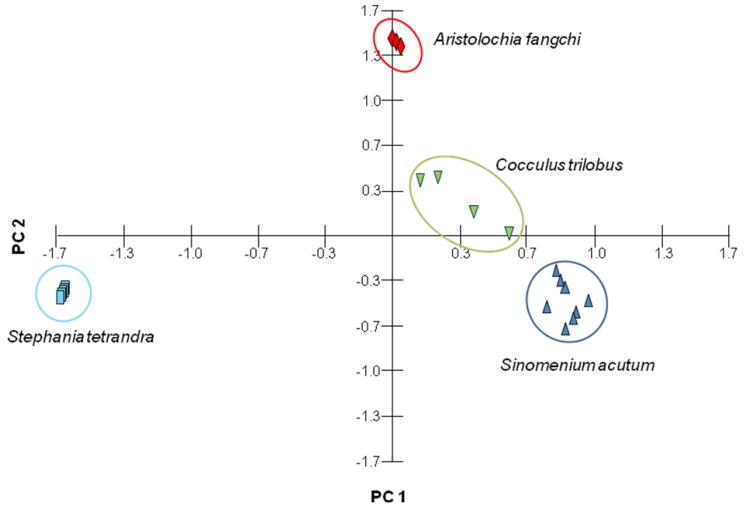
The amounts of each compound in the various Fangchi species extracts are listed in Table 4. Seven compounds were observed with markedly different abundances among the Fangchi species under our experimental conditions. The RSDs for the marker compounds ranged from 0.02%–5.91%. Magnoflorine was observed in all samples, but the amount varied significantly depending on the sample origin. In this study, magnoflorine was observed for the first time in S. tetrandra, as shown in the inset of Figure 2D. The morphine alkaloids were observed in all of the S. acutum samples and the bisbenzylisoquinolines were observed in all S. tetrandra samples. The lignan, syringaresinol, was detected as a minor component of the S. acutum and C. trilobus samples. Aristolochic acid I could prove to be an important marker, although it was only observed in small quantities in A. fangchi. These results indicate UHPLC-DAD is a useful tool for the determination of the seven characteristic marker compounds used for the quality control of four Fangchi species and for classification of the origins of herbal material by PCA (Figure 4).
Table 4.
The amounts of marker compounds in extracts of Fangchi species of different origins.
| Sample | Mean concentration (mg/g) ± standard deviation (relative standard deviation) | ||||||
|---|---|---|---|---|---|---|---|
| Magnoflorine | Sinomenine | Isosinomenine | Syringaresinol | Fangchinoline | Tetrandrine | Aristolochic acid І | |
| S. acutum 1 | 21.08 ± 0.03 (0.03) | 1.70 ± 0.26 (3.10) | 2.35 ± 0.27 (2.27) | 2.44 ± 0.07 (0.61) | - | - | - |
| S. acutum 2 | 9.61 ± 0.01 (0.03) | 1.69 ± 0.21 (2.44) | 1.34 ± 0.20 (2.95) | 0.24 ± 0.01 (1.22) | - | - | - |
| S. acutum 3 | 7.46 ± 0.02 (0.05) | 3.68 ± 0.03 (0.16) | 1.73 ± 0.02 (0.27) | 0.64 ± 0.02 (0.76) | - | - | - |
| S. acutum 4 | 8.22 ± 2.07 (5.04) | 3.79 ± 1.12 (5.91) | 0.30 ± 0.02 (1.48) | 0.38 ± 0.09 (4.90) | - | - | - |
| S. acutum 5 | 8.53 ± 0.55 (1.30) | 6.30 ± 0.51 (1.62) | 3.02 ± 0.24 (1.59) | 0.08 ± 0.01 (3.23) | - | - | - |
| S. acutum 6 | 25.06 ± 0.03 (0.03) | 24.35 ± 0.02 (0.02) | 14.10 ± 0.05 (0.07) | 0.14 ± 0.02 (2.90) | - | - | - |
| S. acutum 7 | 6.65 ± 0.03 (0.10) | 13.28 ± 0.04 (0.06) | 4.86 ± 0.01 (0.04) | 0.02 ± 0.01 (5.75) | - | - | - |
| S. acutum 8 | 5.69 ± 1.14 (4.02) | 11.03 ± 0.83 (1.50) | 0.55 ± 0.10 (3.65) | 0.03 ± 0.004 (2.57) | - | - | - |
| C. trilobus 1 | 9.38 ± 0.78 (1.67) | - | - | 0.51 ± 0.10 (3.82) | - | - | - |
| C. trilobus 2 | 17.21 ± 0.04 (0.04) | - | - | 0.01 ± 0.001 (2.90) | - | - | - |
| C. trilobus 3 | 3.14 ± 0.22 (1.40) | - | - | 0.21 ± 0.05 (4.32) | - | - | - |
| C. trilobus 4 | 18.85 ± 0.03 (0.03) | - | - | 0.31 ± 0.02 (1.27) | - | - | - |
| S. tetrandra 1 | 0.14 ± 0.01 (0.78) | - | - | - | 3.14 ± 0.01 (0.07) | 2.80 ± 0.02 (0.12) | - |
| S. tetrandra 2 | 0.37 ± 0.01 (0.50) | - | - | - | 5.49 ± 0.01 (0.05) | 6.35 ± 0.02 (0.06) | - |
| S. tetrandra 3 | 0.33 ± 0.06 (3.83) | - | - | - | 5.26 ± 0.57 (2.16) | 5.86 ± 1.13 (3.87) | - |
| S. tetrandra 4 | 0.58 ± 0.02 (0.60) | - | - | - | 8.08 ± 0.02 (0.04) | 10.31 ± 0.02 (0.05) | - |
| S. tetrandra 5 | 0.37 ± 0.03 (1.73) | - | - | - | 6.41 ± 0.64 (1.99) | 9.31 ± 0.41 (0.89) | - |
| A. fangchi 1 | 0.17 ± 0.004 (0.47) | - | - | - | - | - | 0.10 ± 0.001 (0.28) |
| A. fangchi 2 | 0.27 ± 0.01 (0.38) | - | - | - | - | - | 0.40 ± 0.01 (0.62) |
| A. fangchi 3 | 0.20 ± 0.01 (0.52) | - | - | - | - | - | 0.20 ± 0.003 (0.29) |
Figure 4.
PCA plot of different Fangchi species combined with 20 chromatographic data.
3. Experimental
3.1. Materials and Reagents
All reagents and organic solvents were analytical grade. Acetonitrile, methanol, ethanol and acetone were purchased from J.T. Baker (Phillipsburg, NJ, USA). Sinomenine, isosinomenine, magnoflorine and syringaresinol were isolated from Fangchi species using a previously reported method [28]. The purity and identity of these compounds were determined by HPLC-DAD and several spectroscopic methods. Tetrandrine (purity ≥ 90%) was purchased from Sigma-Aldrich (Milwaukee, WI, USA). Fangchinoline and Aristolochic acid І (purity > 98%) were purchased from Chengdu Biopurify Phytochemicals Ltd. (Sichuan, China). Propyl-4-hydroxybenzoate, which was used as an internal standard (I.S.), was purchased from Daejung (Eumseong, Korea, purity > 99%). All standards were kept in a refrigerator. The chemical structures of the standards used in this study are depicted in Figure 1. Samples of Fangchi species of different origins were collected from herbal markets in Korea and China.
3.2. Preparation of Standards
Stock solutions were prepared by dissolving 1 mg of each standard (sinomenine, isosinomenine, magnoflorine, tetrandrine, fangchinoline and syringaresinol) in 1 mL of methanol, and 1 mg of aristolochic acid І in 1 mL of acetone. Each stock solution was diluted to create seven calibration points (0.2, 0.5, 1, 5, 10, 50 and 100 µg/mL) for preparation of the calibration curves. The concentration of propyl-4-hydroxybenzoate (I.S.) was 100 µg/mL for all analytes. All stock solutions were stored at −4 °C until analysis.
3.3. Preparation of Crude Rug Extracts
Dried Fangchi species were pulverized and the resulting powders were screened through 30 mesh sieves. One gram of powder was placed into 20 mL of 70% methanol. The sample mixture was extracted for 30 min in 42 KHz of an ultrasonic bath (Branson 5510, Branson Ultrasonic Corp., Danbury, CT, USA) at room temperature. After extraction, the sample mixture was centrifuged twice at 3,000 rpm for 10 min. The supernatant was collected and filtered through a 0.22 µm membrane filter (GHP membrane filters, 0.20 μm pore size, Woongki Science, Seoul, Korea), and propyl-4-hydroxybenzoate (I.S.) was added to the extract solution to obtain a final solution of 5 mg powder/mL for, S. acutum, S. tetrandra and C. trilobus, 25 mg powder/mL A. fangchi, and 100 µg/mL propyl-4-hydroxybenzoate prior to injection into the UHPLC system. Different extraction methods, including classical ultra-sonication, reflux and immersion, were tested.
3.4. UHPLC-DAD Conditions
UHPLC analysis was performed using a WATERS Acquity UPLC system (Waters, Milford, MA) equipped with a quaternary solvent delivery manager, a column manager, a sample manager and a diode array detector (DAD). The chromatographic separation analysis was carried out on a WATERS (Milford, MA, USA) Acquity UPLC ® BEH C18 column (50 × 2.1 mm, i.d., 1.7 µm) connected to an Acquity UPLC ® BEH C18 VanGuard™ pre-column (5 × 2.1 mm, i.d., 1.7 µm). The mobile phases consisted of solvent A (20 mM NH4OAc at pH 6.0, adjusted with acetic acid) and solvent B (methanol). The gradient elution mode was programmed as follows: 10%–50% B for 0.0–3.5 min and 50%–80% B for 3.5–7.0 min. The UV detection wavelength was 235 nm. The flow rate and injection volume were 0.4 mL/min and 0.5 µL, respectively.
3.5. UHPLC-ESI-MS Conditions
All ESI-MS experiments were performed using an API 3200 instrument (MDS Sciex, Concord, ON, Canada) equipped with an ESI source. The chromatographic conditions were the same as those used for UHPLC-DAD with the exception of the NH4OAc concentration. To avoid damage to the MS system, 15 mM NH4OAc at pH 6.0 (adjusted with acetic acid) was used as solvent A. All MS parameters were optimized according to the manufacturer’s instructions. In positive-ion mode ESI experiments, the mass spectrometric conditions were as follows: curtain gas, 20 psi; electron voltage, 4500 V; temperature, 400 °C; nebulizing gas, 50 psi; and heating gas, 50 psi; the mass scan range was m/z 50–635 in the full-mass scan. The collision energy was 40% in Q2. A syringe pump was used for direct analysis of the seven reference compounds at a flow rate of 10 µL/min. The ESI-MS/MS experimental conditions were as follows: magnoflorine, declustering potential (DP) 50 V and collision energy (CE) 35%; sinomenine and isosinomenine, DP 50 V and CE 44%; aristolochic acid, DP 25 V and CE 15%; fangchinoline and tetrandrine, DP 81 V and CE 55%. In the negative-ion mode ESI experiments, the mass spectrometric conditions were as follows: curtain gas, 10 psi; electron voltage, −4,500 V; temperature, 400 °C; nebulizing gas, 50 psi; and heating gas, 50 psi; the mass scan range was m/z 50–430 in the full-mass scan. The ESI-MS/MS experimental conditions for syringaresinol were DP −35 V and CE 30%.
3.6. Method Validation
Validation of the analytical method for the seven marker compounds was determined by the linearity, LOD, LOQ, accuracy and precision. The linear calibration curves were performed at least five times for each reference compound and were constructed by plotting the ratios of peak areas (analytes/internal standard) against the concentrations for each compound. Precision, accuracy and intra- and inter-day recovery tests were performed by adding known amounts of standards (5, 25 and 50 µg/mL) to S. acutum (n = 5). Precision and accuracy were expressed as relative standard deviations (RSDs) and recoveries (%), respectively.
4. Conclusions
In this study, a rapid and simple UHPLC-DAD method was developed and validated for the simultaneous determination of syringaresinol, aristolochic acid І and five alkaloids in four Fangchi species of different origins. These marker compounds, comprising morphine, aporphine and bisbenzylisoquinoline alkaloids, a nitrophenanthrene carboxylic acid and a lignan were successfully identified by UHPLC-ESI-MS/MS in four Fangchi species extracts, providing confirmation of the UHPLC-DAD results. Furthermore, in this study, magnoflorine and syringaresinol were observed for the first time in S. tetrandra and C. trilobus, respectively. Four different Fangchi species could be readily distinguished from one another based on the presence and levels of specific marker compounds. UHPLC-DAD combined with MS/MS analysis was found to be a useful and practical tool for the quality control of Fangchi species.
Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF 2011-0012671) and the Basic Science Research Program through the NRF funded by the Ministry of Education, Science and Technology (NRF-2012R1A6A3A01039038).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds (Sinomenine, isosinomenine, magnoflorine and syringaresinol) are available from the authors.
References
- 1.Korea Food and Drug Administration. The Korean Pharmacopoeia IX. 9th. Sinil Books Press Inc.; Seoul, Korea: 2008. [Google Scholar]
- 2.Ministry of Health, Labor and Welfare. The Japanese Pharmacopoeia. 16th. Yakuji Nippo Ltd.; Tokyo, Japan: 2010. [Google Scholar]
- 3.National Committee of Chinese Pharmacopoeia. The Pharmacopoeia of the People’s Republic of China. Chemical Industry Press; Beijing, China: 2010. [Google Scholar]
- 4.Chang D.M., Kuo S.Y., Lai J.H., Chang M.L. Effects of anti-rheumatic herbal medicines on cellular adhesion molecules. Ann. Rheum. Dis. 1999;58:366–371. doi: 10.1136/ard.58.6.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang W.T., Su C.H., Sheu S.J. Separation and identification of the constituents in Fangchi radix of different origins. J. Food Drug Anal. 2006;14:357–367. [Google Scholar]
- 6.Rahman A.U. Bioactive Natural Products (Part E) 1st. Elsevier Science; Amsterdam, The Netherlands: 2001. pp. 294–295. [Google Scholar]
- 7.Debelle F.D., Vanherweghem J.L., Nortier J.L. Aristolochic acid nephropathy: A worldwide problem. Kidney Int. 2008;74:158–169. doi: 10.1038/ki.2008.129. [DOI] [PubMed] [Google Scholar]
- 8.Hung T.M., Lee J.P., Min B.S., Choi J.S., Na M.K., Zhang X.F., Ngoc T.M., Lee I.S., Bae K.H. Magnoflorine from Coptidis Rhizoma protects high density lipoprotein during oxidant stress. Biol. Pharm. Bull. 2007;30:1157–1160. doi: 10.1248/bpb.30.1157. [DOI] [PubMed] [Google Scholar]
- 9.Kok T.W., Yue P.Y.K., Mak N.K., Fan T.P.D., Liu L., Wong R.N.S. The anti-angiogenic effect of sinomenine. Angiogenesis. 2005;8:3–12. doi: 10.1007/s10456-005-2892-z. [DOI] [PubMed] [Google Scholar]
- 10.Kim H.S., Zhang Y.H., Oh K.W., Ahn H.Y. Vasodilating and hypotensive effects of fangchinoline and tetrandrine on the rat aorta and the stroke-prone spontaneously hypertensive rat. J. Ethnopharmacol. 1997;58:117–123. doi: 10.1016/S0378-8741(97)00092-5. [DOI] [PubMed] [Google Scholar]
- 11.Choi H.S., Kim H.S., Min K.R., Kim Y., Lim H.K., Chang Y.K., Chung M.W. Anti-inflammatory effects of fangchinoline and tetrandrine. J. Ethnopharmacol. 2000;69:173–179. doi: 10.1016/S0378-8741(99)00141-5. [DOI] [PubMed] [Google Scholar]
- 12.Miyazawa M., Utsunomiya H., Inada K., Yamada T., Okuno Y., Tanaka H., Tatematsu M. Inhibition of Helicobacter pylori motility by (+)-syringaresinol from unripe Japanese apricot. Biol. Pharm. Bull. 2006;29:172–173. doi: 10.1248/bpb.29.172. [DOI] [PubMed] [Google Scholar]
- 13.Park B.Y., Oh S.R., Ahn K.S., Kwon O.K., Lee H.K. Syringaresinol inhibits proliferation of human promyelocytic HL-60 leukemia cells via G1 arrest and apoptosis. Int. Immunopharm. 2008;8:967–973. doi: 10.1016/j.intimp.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Arlt V.M., Stiborova M., Schmeiser H.H. Aristolochic acid as a probable human cancer hazard in herbal remedies: a review. Mutagenesis. 2002;17:265–277. doi: 10.1093/mutage/17.4.265. [DOI] [PubMed] [Google Scholar]
- 15.Kim J.H., Sim H.J., Lee K.R., Hong J. UHPLC separation of structurally diverse markers in Fangchi Species. Bull. Korean Chem. Soc. 2013;34:1–4. [Google Scholar]
- 16.Pérez-Arribas L.V., Manuel de Villena-Rueda F.J., León-González M.E., Gonzalo-Lumbreras R., Polo-Díez L.M. New approach to optimize HPLC separations of acid-base compounds with elution order involved, by using combined three-band resolution maps. Anal. Bioanal. Chem. 2010;396:2647–2656. doi: 10.1007/s00216-010-3493-2. [DOI] [PubMed] [Google Scholar]
- 17.Subirats X., Bosch E., Rosés M. Retention of ionisable compounds on high-performance liquid chromatography XVI. Estimation of retention with acetonitrile/water mobile phases from aqueous buffer pH and analyte pKa. J. Chromatogr. A. 2006;1121:170–177. doi: 10.1016/j.chroma.2006.03.126. [DOI] [PubMed] [Google Scholar]
- 18.Kim E.K., Jeong E.K., Han S.B., Jung J.H., Hong J. HPLC separation of isoquinoline alkaloids for quality control of Corydalis species. Bull. Korean Chem. Soc. 2011;32:3597–3602. [Google Scholar]
- 19.Zhang Y., Shi Q., Shi P., Zhang W., Cheng Y. Characterization of isoquinoline alkaloids, diterpenoids and steroids in the Chinese herb Jin-Guo-Lan (Tinospora sagittata and Tinospora capillipes) by high-performance liquid chromatography/electrospray ionization with multistage mass spectrometry. Rapid Commun. Mass Spectrom. 2006;20:2328–2342. doi: 10.1002/rcm.2593. [DOI] [PubMed] [Google Scholar]
- 20.Jeong E.K., Lee S.Y., Yu S.M., Park N.H., Lee H.S., Yim Y.H., Hwang G.S., Cheong C., Jung J.H., Hong J. Identification of structurally diverse alkaloids in Corydalis species by liquid chromatography/electrospray ionization tandem mass spectrometry. J. Rapid Commun. Mass Spectrom. 2012;26:1661–1674. doi: 10.1002/rcm.6272. [DOI] [PubMed] [Google Scholar]
- 21.Raith K., Neubert R., Poeaknapo C., Boettcher C., Zenk M.H., Schmidt J. Electrospray tandem mass spectrometric investigations of morphinans. J. Am. Soc. Mass Spectrom. 2003;14:1262–1269. doi: 10.1016/S1044-0305(03)00539-7. [DOI] [PubMed] [Google Scholar]
- 22.Wu W.N., McKown L.A., Gopaul V.S. In-vitro metabolism of isotetrandrine, a bisbenzylisoquinoline alkaloid, in rat hepatic S9 fraction by high-performance liquid chromatography-atmospheric pressure ionization mass spectrometry. J. Pharm. Pharmacol. 2004;56:749–755. doi: 10.1211/0022357023547. [DOI] [PubMed] [Google Scholar]
- 23.Koh H.L., Wang H., Zhoua S., Chan E., Woo S.O. Detection of aristolochic acid I, tetrandrine and fangchinoline in medicinal plants by high performance liquid chromatography and liquid chromatography/mass spectrometry. J. Pharm. Biomed. Anal. 2006;40:653–661. doi: 10.1016/j.jpba.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Chan W., Cui L., Xu G., Cai Z. Study of the phase I and phase II metabolism of nephrotoxin aristolochic acid by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2006;20:1755–1760. doi: 10.1002/rcm.2513. [DOI] [PubMed] [Google Scholar]
- 25.Eklund P.C., Backman M.J., Kronberg L.A., Smeds A.I., Sjoholm R.E. Identification of lignans by liquid chromatography-electrospray ionization ion-trap mass spectrometry. J. Mass Spectrom. 2008;43:97–107. doi: 10.1002/jms.1276. [DOI] [PubMed] [Google Scholar]
- 26.Du G., Zhao H.Y., Zhang Q.W., Li G.H., Yang F.Q., Wang Y., Li Y.C., Wang Y.T. A rapid method for simultaneous determination of 14 phenolic compounds in Radix Puerariae using microwave-assisted extraction and ultra high performance liquid chromatography coupled with diode array detection and time-of-flight mass spectrometry. J. Chromatogr. A. 2010;1217:705–714. doi: 10.1016/j.chroma.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 27.Wang Q., Shi R., Ma Y.M., Jiang P., Zhong J., Cui H.Y., Liu P., Liu C.H. Content determination of the major constituents of Yinchenzhufu decoction via ultra high-performance liquid chromatography coupled with electrospray ionisation tandem mass spectrometry. J. Pharmaceut. Biomed. 2013;77:88–93. doi: 10.1016/j.jpba.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 28.Min Y.D., Choi S.U., Lee K.R. Aporphine alkaloids and their reversal activity of multidrug resistance (MDR) from the stems and rhizomes of Sinomenium acutum. Arch. Pharm. Res. 2006;29:627–632. doi: 10.1007/BF02968246. [DOI] [PubMed] [Google Scholar]