Abstract
Syzygium jambos and Solanum guaraniticum are both employed in Brazil as medicinal plants, even though their potential toxicity is not well established and they are frequently misused. The aim of this study was investigate the effect of the aqueous leaf extracts of both plants on δ-aminolevulinate dehydratase (δ-ALA-D) and acetylcholinesterase (AChE) activities and the antioxidant action against oxidative damage induced by sodium nitroprusside in rats, using in vitro assays. In addition, the presence of gallic, caffeic and chlorogenic acids, as well as rutin, quercetin and kaempferol as bioactive compounds in the extracts was identified by HPLC and their levels quantified. The antioxidant activities of both extracts were assessed by their capabilities to scavenge nitric oxide and to inhibit lipid peroxidation. Only Syzygium jambos presented thiol-peroxidase-like activity. Although neither extract affected the AChE activity, the aqueous extract of Solanum guaraniticum inhibited brain δ-ALA-D activity, suggesting a possible impairment effect on the central nervous system. Our results showed that both extracts exhibited efficient free radical scavenger activity and are an interesting source of bioactive compounds, justifying their use in folk medicine, although Solanum guaraniticum extract could have neurotoxicity properties and we therefore suggest that its use should be restricted to ensure the health of the population.
Keywords: Solanum guaraniticum, Syzygium jambos, δ-aminolevulinate dehydratase, lipid peroxidation, induced oxidative stress
1. Introduction
Free radicals are accepted as important mediators of tissue injury in several human diseases, such as arthritis inflammation, atherosclerosis, diabetes, cirrhosis and cancer [1]. The efficiency of the antioxidant defense system is altered under pathological conditions and thus, the ineffective scavenging process and/or overproduction of free radicals may play a crucial role in causing tissue damage [2]. Therefore attention has been focused on the search for natural antioxidants with the potential to scavenge free radicals and enhance the antioxidative defense system [3,4].
Medicinal plants have been traditionally used in the treatment of several human diseases and their pharmacological and therapeutic properties have been attributed to the different chemical constituents isolated from their crude extracts [5]. As an example, the leaves of Syzygium jambos (L.) Alston (Myrtaceae) have used as a diuretic, in the treatment of rheumatism, as a febrifuge and present antiviral, anti-inflammatory and digestive properties [6,7,8]. In Brazil this plant is known by the common name “jambolão” and its leaf infusions are also used traditionally in the treatment of diabetes, even thugh some studies have shown its ineffectiveness as a antihiperglycemic agent [9]. Likewise, Solanum guaraniticum A. St.-Hil (Solanaceae) is known by the folk name “falsa-jurubeba” in southern Brazil. Its traditional uses include the treatment of anemia, fevers and liver and gastric dysfunctions such as hepatitis and ulcers [10,11,12]. However, although previous studies have demonstrated the hepatoprotective and antioxidant activities of aqueous extract of Solanum guaraniticum, they also showed that it promoted an increase in the level of serum hepatic enzymes in mice and it is related to cattle intoxications [13,14,15]. Thus, although medicinal plants may have biological activities that are beneficial to humans, the potential toxicity of these bioactive substances has often not been well established [16]. In particular, data on the toxic effects of Brazilian Syzygium jambos and Solanum guaraniticum are scarce and they are often misused in folk medicine.
The enzyme δ-aminolevulinate dehydratase (δ-ALA-D, EC 4.2.1.24) is a sulfhydryl-containing enzyme of heme pathway [17]. Due to its active sulfhydryl groups and the role of heme proteins in many cellular metabolic processes [18], the enzyme presents high sensitivity to pro-oxidant situations and impairment of metabolic processes and has been used to evaluate toxic effects [19,20]. Another important enzyme present in all animals, acetylcholinesterase (AChE, EC 3.1.1.7) is important for function of the cholinergic system, by hydrolysis of the neurotransmitter acetylcholine [21]. The crucial role of cholinesterases in neural transmission makes them a primary target of a large number of cholinesterase-inhibiting drugs and toxins [22], and making them valuable diagnostic tools for verifying exposure to chemical agents [23].
The purpose of this study was to evaluate the effects of aqueous extracts of Solanum guaraniticum and Syzygium jambos on δ-ALA-D and AChE activities. The effects of both extracts on the lipid peroxidation, thiol status and catalase activity on induced oxidative stress in tissue of rats were assessed using in vitro assays.
2. Results and Discussion
2.1. Pytochemical Screening of Aqueous Extract of Syzygium jambos and Solanum guaraniticum
In order to ascertain the phytochemicals responsible for the in vitro biological activities of Syzygium jambos and Solanum guaraniticum, the aqueous leaf extracts of these plants were screened for various compounds. HPLC fingerprinting revealed the presence of gallic and chlorogenic acids, as well as rutin, quercetin and kaempferol in both extracts and the percentage of each compound is shown in Figure 1. Furthermore, considering the chromatographic conditions used, caffeic acid was only detected in the Syzygium jambos extract. The characterization of these aqueous leaf extracts is particularly important since there is scarce data available about this local species and any new studies represent a significant contribution to the knowledge of these plants [7,24].
Figure 1.
HPLC/DAD profile of extracts tested (detection UV at 271 nm) and quantification of phenolic compounds found [Gallic acid (peak 1), chlorogenic acid (peak 2), caffeic acid (peak 3), rutin (peak 4), quercetin (peak 5) and kaempferol (peak 6). Quantification results are expressed as mean ± SEM (n = 3) assessed by one way ANOVA followed by Duncan Multiple Comparison post hoc test. Means marked with different letters are significantly different (p < 0.05). ND = not detected.
The contents of total phenol, flavonoids, and vitamin C are also shown in Table 1. As can be seen, Syzygium jambos aqueous leaf extract has a higher content of total phenolic compounds (p = 0.0002) whereas Solanum guaraniticum extract has higher total flavonoids content levels (p < 0.0001). This result agrees with the proportion of phenolic acids found in the HPLC analysis of the extracts (Figure 1). Along this line, Solanum guaraniticum extract has higher levels of vitamin C (p = 0.0010) than Syzygium jambos extract. As an electron donor, vitamin C is a potent water-soluble antioxidant in humans and an essential dietary nutrient required as a co-factor for several enzymes [25,26]. However, the human body lacks the ability to synthesize this compound, therefore the vegetal species with high content of vitamin C are valuable sources of this nutrient. Furthermore, vitamin C has been reported to contribute to the antioxidant activities of plant food and is present substantially in the extracts. Ascorbic acid is a good reducing agent and exhibits its antioxidant activities by electron donation [27].
Table 1.
Total phenols, total flavonoids and vitamin C contents in aqueous extracts of Syzygium jambos and Solanum guaratiticum.
| Extract content | Syzygium jambos | Solanum guaraniticum | ||
|---|---|---|---|---|
| Total phenolic (mg GAE/g) | 108.2 ± 3.34 | 58.76 ± 1.72 ** | ||
| Total flavonoid (mg QE/g) | 85.55 ± 2.54 | 237.90 ± 7.12 *** | ||
| Vitamin C (mg VIT C/g) | 21.07 ± 0.64 | 58.01 ± 4.21 ** | ||
Data are reported as mean ± SEM (n = 3). GAE: gallic acid equivalents; QE: quercitin equivalents; VIT C: vitamin C. Statistically significant differences as determined by Student’s t-test. (**) and (***) denotes p < 0.001 and p < 0.0001.
Polyphenols are considered to be strong antioxidants due to the redox properties of their hydroxyl groups [28]. Phenolic compounds are capable of removing free radicals, chelating metal catalysts, activating antioxidant enzymes, reducing a-tocopherol radicals, and inhibiting oxidases [29].
2.2. Thiol Peroxidase-Like Activity of Both Extracts Evaluated
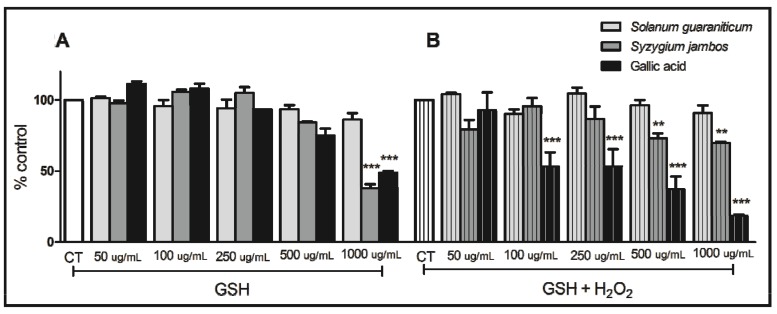
In Figure 2B, we can observe a decrease in SH groups when Syzygium jambos, at 500 µg/mL and 1,000 µg/mL, was incubated with hydrogen peroxide (H2O2). Considering this effect, Syzygium jambos seems to catalyze the reduction of H2O2 with the consumption of glutathione (GSH), thus mimicking the properties of glutathione peroxidase (GPx). GPx is an antioxidant selenoenzyme that protects various organisms from oxidative damage by catalyzing the reduction of H2O2 and other organic peroxides with the help of GSH as the reducing agent [30]. Taking into account that mimetic activities of this enzyme have been investigating in synthetic compounds to explore their antioxidant capacity [20], this results give us an insight into the possible mechanisms by which Syzygium jambos extract exerts its effects, which are under investigation in our laboratory.
Figure 2.
Thiol peroxidase-like activity of aqueous extracts tested in the absence of H2O2 (A), and in the presence of H2O2 (B). Data are reported as mean ± SEM (n = 3) and assessed by one way ANOVA followed by Duncan Multiple Comparison post hoc test. (**) and (***) denotes p < 0.001 and p < 0.0001, compared to respective controls (CT).
However, we also noticed that Syzygium jambos extract at 1,000 µg/mL was able to promote the consumption of GSH even in the absence of H2O2 (Figure 2A), which might have occurred due to a possible reaction between thiols and extract components or GSH oxidation. In fact, plant extracts can present a pro-oxidant or antioxidant behavior depending on the dose [31]. However, in the presence of H2O2, due to a likely competition of this reactive species and GSH as well as due to the extract components, the polyphenol extracts may be used to detoxify H2O2. Moreover, Solanum guaraniticum extract did not cause any changes on SH groups level, neither in the presence nor in the absence of H2O2 suggesting that this extracts, at the concentrations tested, did not present thiol-peroxidase like activity such as antioxidant capacity.
2.3. Nitric Oxide-Scavenging Activity Assay of Extracts
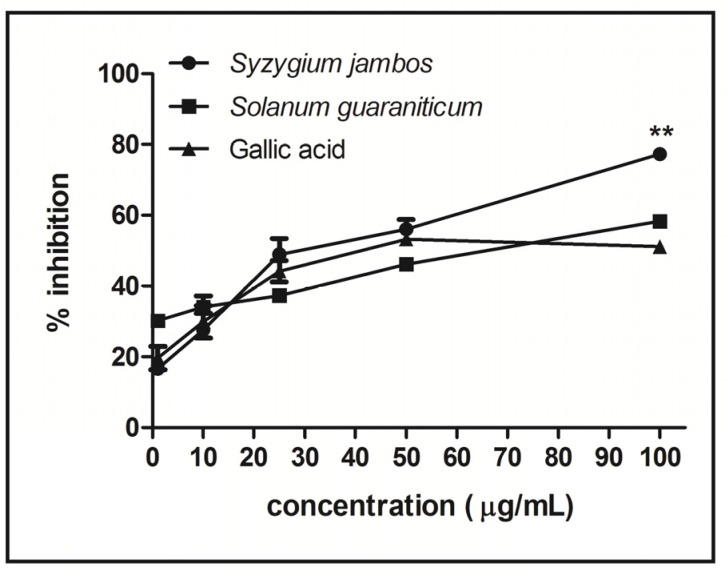
Figure 3 shows that both aqueous extracts studied can scavenge nitric oxide (NO) radical, but Syzygium jambos had a significantly higher scavenging ability than Solanum guaraniticum. At the higher concentration tested (100 µg/mL), the scavenging capacity of Syzygium jambos extract was around 77%, while Solanum guaraniticum demonstrated a scavenging power around 58% (p < 0.001).
Figure 3.
NO scavenging activity of aqueous extracts tested. Data are reported as mean ± SEM (n = 3) and assessed by one way ANOVA followed by Duncan Multiple Comparison post hoc test. ** denotes p < 0.001 compared to Solanum guaraniticum and gallic acid (100 µg/mL).
2.4. Effect of Extracts on δ-ALA-D and AChE Activity in Rat Tissues
In this study, Solanum guaraniticum extract inhibited brain δ-ALA-D activity at 1,000 µg/mL (p < 0.05). Taking into account that: (i) some studies have related Solanum guaraniticum, under its synonym S. fastigiatum, to bovine intoxications affecting the central nervous system of the animals and causing neuronal degeneration and sporadic seizures [13,14,32]; and (ii) δ-ALA-D activity can be inhibited during seizures [33]; this finding may be considered an indication of the toxic properties of this plant. Furthermore, the inhibition of the δ-ALA-D causes an accumulation of its substrate 5-aminolevulinic acid (ALA), which has already demonstrated neurotoxicity by inducing seizures and death after intracerebroventricular administration in rodents [34]. ALA may also rapidly oxidize to generate reactive oxygen species [35], which could intensify the toxicological process. Free radicals are one of the main causes of cellular dysfunction in the brain [36], and seizures, oxidative stress and δ-ALA-D activity have already been suggested as linked events [37]. On the other hand, δ-ALA-D activity of liver and kidney tissues was not affected by Solanum guaraniticum extract. Syzygium jambos was not able to inhibit δ-ALA-D activity of any tissue tested (Table 2). Furthermore, none of the concentrations of any extracts tested was able to alter enzymatic activity of AChE (Table 2).
Table 2.
Effect of extracts on tissue δ-ALA-D and AChE activity in rat homogenates.
| Treatment | δ-ALA-D | AChE | ||
|---|---|---|---|---|
| Liver | Brain | Kidney | ||
| Control (PBS) | 4.33 ± 0.48 | 1.23 ± 0.18 | 1.49 ± 0.43 | 2.78 ± 0.14 |
| Lead acetate 10 µM | 2.92 ± 0.23 | 0.95 ± 0.02 | 0.99 ± 0.43 | - |
| Paraoxon 1 µM | - | - | - | 1.26 ± 0.07 *** |
| Syzygium jambos 100 µg/ mL | 4.85 ± 0.71 | 1.10 ± 0.06 | 1.89 ± 0.49 | 2.65 ± 0.14 |
| Syzygium jambos 250 µg/mL | 4.09 ± 0.46 | 1.38 ± 0.28 | 1.97 ± 0.53 | 2.75 ± 0.14 |
| Solanum guaraniticum 500 µg/mL | 4.24 ± 0.52 | 0.91 ± 0.11 | 1.66 ± 0.32 | 2.73 ± 0.14 |
| Solanum guaraniticum 1000 µg/mL | 4.31 ± 0.72 | 0.66 ± 0.14 * | 1.35 ± 0.18 | 2.79 ± 0.14 |
Data are reported as mean ± SEM (n = 6) and assessed by one way ANOVA followed by Duncan Multiple Comparison post hoc test. δ-aminolevulinate dehydratase activity (δ-ALA-D) results are presented as nmol porphobilinogen (PBG)/mg protein/h. Acetylcholinesterase actitivty (AChE) results are presented as µmol AcSCh/h/mg of protein. (*) and (***) denotes p < 0.05 and p < 0.0001 as compared to the respective control samples.
2.5. Effects of Syzygium jambos and Solanum guaraniticum on Lipid Peroxidation, NPSH Content and Catalase Activity of Sodium Nitroprusside (SNP)-Induced Tissues
The ability of both studied aqueous extracts to inhibit SNP-induced lipid peroxidation was measured by the production of thiobarbituric acid reactive substances (TBARS) and the results are presented in Table 3. The data revealed that the incubation of the tested homogenates in the presence of SNP caused a significant (p < 0.05) increase in TBARS content (484.75% to liver, 911.01% to brain and 295.06% to kidney) when compared with the basal value (100%). However, the presence of aqueous extract of Syzygium jambos or Solanum guaraniticum at all concentrations tested inhibited TBARS production, with a more pronounced effect in the brain tissue.
Table 3.
Effect of aqueous extracts of Syzygium jambos and Solanum guaraniticum on lipid peroxidation level before and after sodium nitroprusside (SNP) incubation of rat homogenates.
| Treatments | Liver | Inhibition (%) | Brain | Inhibition (%) | Kidney | Inhibition (%) |
|---|---|---|---|---|---|---|
| Control | 2.23 ± 0.54 | ------- | 3.36 ± 0.93 | ------- | 1.62 ± 0.57 | ------- |
| Gallic acid 25 µg/mL | 1.26 ± 0.42 | 43.49 | 1.57 ± 0.51 | 53.27 | 1.29 ± 0.13 | 20.37 |
| Syzygium jambos 100 µg/mL | 1.98 ± 0.52 | 11.21 | 1.53 ± 0.18 * | 54.46 | 0.93 ± 0.25 | 42.59 |
| Syzygium jambos 250 µg/mL | 2.01 ± 0.51 | 9.86 | 1.19 ± 0.26 ** | 64.58 | 0.73 ± 0.18 | 54.93 |
| Solanum guaraniticum 500 µg/mL | 2.36 ± 0.53 | ------- | 1.49 ± 0.26 * | 55.65 | 0.81 ± 0.15 | 50.00 |
| Solanum guaraniticum 1000 µg/mL | 2.41 ± 0.40 | ------- | 1.89 ± 0.35 * | 43.75 | 0.84 ± 0.14 | 48.14 |
| Induced (SNP) | 10.81 ± 1.45 *** | ------- | 30.61 ± 3.41 *** | ------- | 4.78 ± 1.47 * | ------- |
| Gallic acid 25 µg/mL | 1.08 ± 0.09 ### | 90.00 | 27.88 ± 1.15 | 8.91 | 1.30 ± 0.22 ### | 74.26 |
| Syzygium jambos 100 µg/mL + SNP | 2.39 ± 0.51 ### | 77.89 | 2.97 ± 0.61 ### | 90.29 | 1.09 ± 0.30 ## | 77.19 |
| Syzygium jambos 250 µg/mL + SNP | 2.33 ± 0.47 ### | 78.44 | 1.95 ± 0.78 ### | 93.62 | 0.86 ± 0.10 ## | 82.00 |
| Solanum guaraniticum 500 µg/mL + SNP | 2.58 ± 0.65 ### | 76.13 | 1.86 ± 0.80 ### | 93.92 | 1.00 ± 0.20 ## | 79.07 |
| Solanum guaraniticum 1000 µg/mL + SNP | 2.75 ± 0.59 ## | 74.56 | 2.77 ± 0.74 ### | 90.95 | 1.18 ± 0.17 ## | 75.31 |
Data are reported as mean ± SEM (n = 6) and assessed by one way ANOVA followed by Duncan Multiple Comparison post hoc test. Results are presented as nmol MDA (malondialdehyde)/mg protein. (*), (**) and (***) denotes p < 0.05, p < 0.001 and p < 0.0001, respectively, as compared to the respective control samples. (#), (##) and (###) denotes p < 0.05, p < 0.001 and p < 0.0001, respectively, as compared to the respective induced samples.
These findings are in accordance with the NO scavenging effect of Syzygium jambos and Solanum guaraniticum verified in Figure 3. SNP cause cytotoxicity through the release of cyanide and/or nitric oxide (NO) [38]. Therefore, a plausible mechanism by which these extracts are conferring protective action against SNP-induced lipid peroxidation could be the ability of the extract phytochemicals to scavenge NO radicals produced by SNP. The phenolic compounds in the extracts can also quench free radicals which may have been resulted from lipid peroxidation chain reaction [39]. In fact, phenolics present in plant sources have received considerable attention over the past decade because of their potential to prevent lipid peroxidation and diseases associated with it [40]. In line with this, we can associate the antioxidant activity of extracts studied here with their folk medicinal use. Syzygium jambos leaves are often used to manage diabetes mellitus, a disease for which the involvement of lipid peroxidation is well known [41]. Solanum guaraniticum is also used mainly to treat liver diseases and the lipid peroxidation process can contribute to the initiation and progress of liver damage [42].
The non-protein thiol (NPSH) status of the tested homogenates was also verified. Table 4 shows that the extracts did not affect thiol status of tissues alone and that the presence of SNP decreased thiol levels (56.63% to liver and 65.13% to brain tissue) when compared with the basal value, except for kidney tissue. However, both Syzygium jambos and Solanum guaraniticum extracts, at all concentrations tested, were capable of maintaining thiol level at the brain homogenate when they were present in the SNP incubation.
Table 4.
Effect of aqueous extracts of Syzygium jambos and Solanum guaraniticum on non-protein thiol (NPSH) content and catalase activity in the liver, brain and kidney of rats.
| Treatments | Thiol content | CAT | ||||
|---|---|---|---|---|---|---|
| Liver | Brain | Kidney | Liver | Brain | Kidney | |
| Data are reported as mean ± SEM (n = 6) and assessed by one way ANOVA followed by Duncan Multiple Comparison post hoc test. Results are presented as mmol NPSH (non-protein thiol)/mg protein and units of catalase (CAT)/mg protein. (*) and (**) denotes p < 0.05 and p < 0.001, respectively, as compared to the respective control samples. (#), (##) and (###) denotes p < 0.05, p < 0.001 and p < 0.0001, respectively, as compared to the respective induced samples. | ||||||
| Control | 11.16 ± 2.16 | 7.57 ± 1.01 | 5.66 ± 1.19 | 38.14 ± 6.49 | 6.36 ± 1,49 | 17.71 ± 2.22 |
| Gallic acid 25 µg/mL | 11.44 ± 2.6 | 9.85 ± 0.29 | 7.31 ± 0.78 | 40.28 ± 9.87 | 6.34 ± 0.34 | 26.53 ± 1.53 * |
| Syzygium jambos 100 µg/mL | 7.41 ± 0.84 | 9.12 ± 0.50 | 6.21 ± 1.81 | 37.65 ± 7.81 | 4.16 ± 1.25 | 15.41 ± 2.75 |
| Syzygium jambos 250 µg/mL | 7.32 ± 1.89 | 10.66 ± 0.73 | 9.73 ± 1.36 | 28.72 ± 6.54 | 2.80 ± 1.10 | 13.84 ± 2.33 |
| Solanum guaraniticum 500 µg/mL | 6.42 ± 1.82 | 9.20 ± 1.00 | 10.52 ± 1.76 * | 41.98 ± 6.18 | 3.43 ± 1.07 | 20.80 ± 2.03 |
| Solanum guaraniticum 1000 µg/mL | 10.94 ± 2.35 | 10.91 ± 1.69 | 8.84 ± 0.20 | 49.10 ± 6.45 | 3.37 ± 1.17 | 18.82 ± 1.65 |
| Induced (SNP) | 4.84 ± 1.69 * | 2.64 ± 0.57 ** | 5.42 ± 1.70 | 46.78 ± 9.98 | 4.04 ± 1.17 | 16.74 ± 1.01 |
| Gallic acid 25 µg/mL | 5.51 ± 0.57 | 3,01 ± 0.20 | 5.43 ± 0.54 | 53.47 ± 10.44 | 2.95 ± 0.56 | 26.52 ± 3.08 # |
| Syzygium jambos 100 µg/mL + SNP | 5.80 ± 1.46 | 7.52 ± 0.79 ## | 6.13 ± 1.00 | 48.04 ± 7.70 | 4.13 ± 1.42 | 17.92 ± 1.52 |
| Syzygium jambos 250 µg/mL + SNP | 6.66 ± 0.90 | 9.40 ± 0.65 ### | 10.56 ± 1.96 # | 40.04 ± 9.82 | 2.37 ± 1.37 | 19.47 ± 3.23 |
| Solanum guaraniticum 500 µg/mL + SNP | 8.45 ± 1.87 | 7.30 ± 0.52 ## | 6.95 ± 0.62 | 47.69 ± 13.39 | 4.17 ± 1.01 | 19.18 ± 1.17 |
| Solanum guaraniticum 1000 µg/mL + SNP | 7.96 ± 1.67 | 9.03 ± 0.86 ### | 9.50 ± 1.51 | 41.60 ± 11.14 | 3.52 ± 1.06 | 19.18 ± 1.69 |
The brain and nervous system are particularly vulnerable to oxidative stress due to their limited antioxidant capacity [43]. In this context, the thiol redox state is an essential parameter associated with major biologic processes such as oxidative stress and intracellular redox homeostasis [44]. In fact, the results observed in Table 4 demonstrated that brain NP-SH level was more sensitive to SNP and more responsive to both extracts tested than the liver and kidney. This effect could be linked to the phytochemical compounds identified on the extracts since several studies of polyphenol rich foods or individual flavonoids have demonstrated their neuroprotective properties against oxidative and inflammatory stressors [45].
In relation to enzymatic antioxidants, the SNP treatment was not able to alter significantly the CAT activity, even the extracts alone, in any of the tissue tested. Similar results were observed in previous studies [20,46]. Catalase is a heme protein that catalyzes the reduction of H2O2 and protects tissue from highly reactive hydroxyl radicals [47]. Thus, the effect observed here may have occurred because this enzyme is not directly effective towards the reactive species formed on the in vitro system.
Previous studies have already demonstrated the radical scavenging properties and antioxidant effect of Solanum guaraniticum on ferrous-induced lipid peroxidation [24]. In relation to Syzygium jambos, radical scavenging properties and in vivo antioxidant power have already been demonstrated [48] although this is the first study involving the aqueous extract of this plant, which is its most popular form of medicinal use [9]. In this sense, a deep evaluation of the antioxidant activity by different methods is substantial for a better understanding of biological potential activities of plant extracts, since the distinct antioxidant properties could indicate that they were acting via distinct mechanisms.
3. Experimental
3.1. Chemicals and Apparatus
5'-Aminolevulinic acid (δ-ALA), thiobarbituric acid (TBA), 5,5'-dithiobis(2-nitrobenzoic acid) (DTNB), gallic acid, ascorbic acid and quercitin were purchased from Sigma (St. Louis, MO, USA). Sodium nitroprusside (SNP) was obtained from Merck (Darmstadt, Germany). All other chemicals were of analytical grade and obtained from standard commercial suppliers. High performance liquid chromatography (HPLC-DAD) was performed with a Shimadzu HPLC system (Shimadzu, Kyoto, Japan), comprising a Prominence Auto Sampler (SIL-20A), equipped with Shimadzu LC-20AT reciprocating pumps connected to a DGU 20A5 degasser, CBM 20A integrator and SPD-M20A UV-VIS diode array detector (DAD) and Software LC solution 1.22 SP1. Absorbance measurements were recorded on a Hitachi U-18,000 UV-Visible Reading Spectrophotometer (Hitachi High-Technologies Corporation, Tokyo, Japan) using disposable cuvettes for the visible range, and quartz cuvettes for measurements in the ultraviolet (UV) range.
3.2. Plant Material and Preparation of Extracts
Leaves of Solanum guaraniticum and Syzygium jambos were collected in the cities of Boca do Monte and Tupanciretã, respectively. A voucher specimen was identified and deposited at the herbarium of Federal University of Santa Maria. The leaves were dried in a greenhouse, smashed in a knife mill and submitted to extraction with ethanol 80% in a Soxhlet apparatus until exhaustion. After extraction, the solvent was evaporated on a rotavapor, supplying the crude extract.
3.3. Phytochemical Analysis
3.3.1. High-Performance Liquid Chromatography (HPLC) Characterization
HPLC characterization under gradient conditions using C18 column (4.6 mm × 250 mm) packed with 5 μm diameter particles; the mobile phases were water containing 2% acetic acid (A) and methanol (B), and the composition gradient was: 5% of B until 2 min and then changed to obtain 25%, 40%, 50%, 60%, 70% and 100% B at 10, 20, 30, 40, 50 and 80 min, respectively. The flow rate was 0.7 mL/min, injection volume 40 μL and the detection wavelengths were 271 nm for gallic acid, 325 nm for caffeic and chlorogenic acids, and 365 nm for quercetin, rutin and kaempferol. The chromatography peaks were confirmed by comparing their retention times with those of reference standards and by DAD spectra (200 to 500 nm).
3.3.2. Total Polyphenols Content
Total phenolic content of extracts (1 mg/mL) was determined with Folin-Ciocalteu’s reagent in alkaline medium and expressed as milligram of gallic acid equivalents per gram of extract powder (mg GAE/g) [49].
3.3.3. Total Flavonoid Content
The total flavonoid content, as milligram of quercitin equivalents per gram of extract powder (mg QE/g), was measured on extracts (1 mg/mL) based on aluminum chloride colorimetric method reported by Zhishen and Mengcheng [50].
3.3.4. Determination of Vitamin C Content
The vitamin C content was determined in extracts (1 mg/mL) using the method of Benderitter et al. [51], based on its reaction with 4-dinitrophenylhydrazine (DNPH), calculated using a vitamin C standard curve and expressed as milligram of vitamin C per gram of extract powder (mg VIT C/g).
3.4. Nitric Oxide-Scavenging Assay of Extracts
The scavenging effect of extracts on nitric oxide (NO) was measured according to the method of Sreejayan and Rao [52]. Gallic acid was used as positive control. For the assay, sodium nitroprusside (10mM), was mixed with different gallic acid or extracts concentrations, incubated 150 min and then mixed with 0.5 mL of Griess reagent and measure at 546 nm. In the control, sample extract was substituted by PBS. The capability of scavenging NO was calculated using the following equation:
| Scavenging effect (%) = [1 − (Asample/Acontrol)] × 100 | (1) |
3.5. Thiol Peroxidase-Like Activity of Extracts
The catalytic effect of Solanum guaraniticum and Syzygium jambos on the reduction of hydrogen peroxide (H2O2) by reduced glutathione (GSH) was assessed using the rate of GSH oxidation. Different concentrations of extracts were incubated in the medium containing GSH (1mM) with and without H2O2 (0.3 mM). At 120 min, aliquots of the reaction mixture (200 µL) were checked for the amount SH groups according to Ellman [53]. Gallic acid was used as positive control. The values are expressed in percentage of control [20].
3.6. Animals
Male adult albino Wistar rats (200–250 g) from our own breeding colony, that are maintained at 22 ± 2 °C, on a 12 h light/dark cycle, with water and food were provided ad libitum. The animals were used according to the guidelines of the Committee on Care and Use of Experimental Animal Resources, Federal University of Santa Maria, Brazil (Process number 23081.009003/2012-01). All efforts were made to minimize the number of animals used and their suffering. The rats were euthanized and the brain, liver and kidney tissues were rapidly dissected, weighted and placed on ice. The tissues were immediately homogenate in 10 mM Tris-HCl, pH 7.4 (1/10 w/v). The homogenates were centrifuged at 4,000× g at 4 °C for 10 min to yield a low-speed supernatant (S1) that was used for δ-ALA-D and AChE activity besides TBARS, thiol content and catalase activity assays. Moreover, protein content of S1 was measured by Peterson [54].
3.6.1. δ-ALA-D Activity
δ-ALA-D activity was assayed by the method of Sassa [55] with some modifications. An aliquot of S1 (200 μL) was incubated at 37 °C in the presence or absence of aqueous extracts at different concentrations (100 and 250 µg/mL of Syzygium jambos and 500 and 1,000 µg/mL of Solanum guaraniticum), based on previous studies in our laboratory. Enzymatic reaction was initiated by adding the substrate δ-aminolevulinic acid and the incubation was carried out at 37 °C for 1 h to liver and kidney and for 3 h to brain homogenate. The porphobilinogen which is formed during the incubation period, was mixed with modified Ehrlich’s reagent, and the color developed was measured spectrophotometrically (555 nm) against a blank. A set of tubes was assessed with lead acetate (AcPb, 10 µM) as positive control of inhibition enzymatic activity [56] Results were expressed as nmol porphobilinogen (PBG)/mg protein/h.
3.6.2. Acetylcholinesterase Activity Assay for Brain
The AChE enzymatic assay was determined by a modification of the spectrophotometric method of Ellman et al. [57] as previously described [58]. An aliquot of brain homogenate (50 μL) was pre-incubated for 1 h at 37 °C in the presence or absence of aqueous extracts at different concentrations (100 and 250 µg/mL of Syzygium jambos and 500 and 1,000 µg/mL of Solanum guaraniticum). The reaction was initiated by adding 0.8 mM acetylthiocholine iodice (AcSCh) to the reaction mixture (2 mL final volume) contained 100 mM TFK; pH 7.5 and 1 mM 5,5'-dithio-bis-nitrobenzoic acid (DTNB). The method is based on the formation of the yellow anion measured by absorbance at 412 nm during 2-min incubation at 25 °C. Paraoxon (1 µM, an organophosphate inhibitor of AChE), was used as positive control [59]. The enzyme activity was expressed in µmol AcSCh/h/mg of protein.
3.6.3. Sodium Nitroprusside (SNP) induced Oxidative Stress
This assay was carried out to determine if the extracts protect against oxidative stress induced by SNP in rat brain, liver and kidney homogenates in vitro. SNP was used as classical inductor of oxidative stress. The supernatant of each tissue was incubated with or without freshly prepared SNP (50 µM) and different concentrations of the plants extract (100 and 250 µg/mL of Syzygium jambos and 500 and 1,000 µg/mL of Solanum guaraniticum) at 37 °C for 1 h. After the incubation time, lipid peroxidation was measure by TBARS levels according to the method of Niehaus and Samuelsson [60], nonprotein thiols (NPSH) content was determined according to Ellman [53] and catalase activity as described by Aebi [61]. Gallic acid 25 µg/mL was used as positive control in all assays [5].
3.7. Statistical Analysis
The analyses were performed using STATISTICA for Windows, version 6.0 (StatSoft. Inc., Tulsa, OK, USA). All data were analyzed using Student’s t-test or one way ANOVA, followed by Duncan’s multiple range test, when appropriate and presented as mean ± standard error of mean (SEM). A value of p < 0.05 was considered statistically significant for all analyses.
4. Conclusions
The study of medicinal plants is important, in particular because the population generally believes that plant extracts are safe due their natural origin, although studies have shown that even plants used popularly with therapeutic purposes can present different degrees of toxicity. The current study has unequivocally demonstrated the antioxidant effect of both aqueous leaf extracts tested, evidencing properties to justify their popular use, meanwhile, Solanum guaraniticum extract inhibited the brain δ-ALA-D activity, suggesting a possible impairment on the central nervous system. The results presented in this study indicate that Solanum guaraniticum may be neurotoxic and caution must be exxercised in its administration. Therefore, more studies are needed to clarify its mechanisms of action.
Acknowledgments
We are gratefully acknowledge the financial support provided by CNPq (Proc. 477029/2011-6) and FAPERGS (Proc. 11/1167-5) and the offer of doctoral fellowship to Gabriela Bonfanti by Fundação Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil as well as Federal University of Santa Maria (UFSM), RS, Brazil, for support in this study.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Not available.
References
- 1.Nordberg J., Arner E.S.J. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001;3:1287–1312. doi: 10.1016/S0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 2.Halliwell B. Free radicals and antioxidants, and human disease: Curiosity, cause, or consequence. Lancet. 1994;344:721–724. doi: 10.1016/S0140-6736(94)92211-X. [DOI] [PubMed] [Google Scholar]
- 3.Cho E.J., Lee Y.A., Yoo H.H., Yokozawa T. Protective effects of broccoli (Brassica oleracea) against oxidative damage in vitro and in vivo. J. Nutr. Sci. Vitaminol. 2006;52:437–444. doi: 10.3177/jnsv.52.437. [DOI] [PubMed] [Google Scholar]
- 4.Bahramikia S., Yazdanparast R. Antioxidant efficacy of nasturtium officinale extracts using various in vitro assay systems. J. Acupunct. Meridian Stud. 2010;3:283–290. doi: 10.1016/S2005-2901(10)60049-0. [DOI] [PubMed] [Google Scholar]
- 5.Pereira R.P., Fachinetto R., de Souza Prestes A., Puntel R.L., Santos da Silva G.N., Heinzmann B.M., Boschetti T.K., Athayde M.L., Bürger M.E., Morel A.F., et al. Antioxidant effects of different extracts from Melissa officinalis, Matricaria recutita and Cymbopogon citratus. Neurochem. Res. 2009;34:973–983. doi: 10.1007/s11064-008-9861-z. [DOI] [PubMed] [Google Scholar]
- 6.Morton J.F. Rose Apple. USA: Fruits of Warm Climates. Florida Flair Books; Miami, FL, USA: 1987. pp. 383–386. [Google Scholar]
- 7.Slowing K., Carretero E., Villar A. Anti-inflammatory activity of leaf extracts of Eugenia jambos in rats. J. Ethnopharmacol. 1994;43:9–11. doi: 10.1016/0378-8741(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 8.Slowing K., Carretero E., Villar A. Anti-inflammatory compounds of Eugenia jambos. Phytother. Res. 1996;10:8126–8127. [Google Scholar]
- 9.Teixeira C.C., Fuchs F.D., Blotta R.M., Knijnik J., Delcado I.C., Netto M.S., Ferreira E., Costa A.P., Mussnich D.G., Ranquetat G.G., et al. Effect of tea prepared from leaves of Syzygium jambos on glucose tolerance in nondiabetic subjects. Diabetes Care. 1990;13:907–908. doi: 10.2337/diacare.13.8.907. [DOI] [PubMed] [Google Scholar]
- 10.Costa O.A. Jurubeba. Revista Brasileira De Farmácia. 1940;21:404–416. [Google Scholar]
- 11.Costa O.F. Farmacognosia. 2nd ed. Ed Lisboa-Fundacao Calouste-Gulbenkian; Lisboa, Portugal: 1975. [Google Scholar]
- 12.Penna M. Dicionario Brasileiro de Plantas Medicinais. Kosmos; Rio de Janeiro, Brazil: 1964. [Google Scholar]
- 13.Paulovich F.B., Portiansky E.L., Gimeno E.J., Schild A.L., Mendez M.C., Riet-Correa F. Lectin histochemical study of lipopigments present in the cerebellum of Solanum fastigiatum var. fastigiatum intoxicated cattle. J. Vet. Med. A Physiol. Pathol Clin. Med. 2002;49:473–477. doi: 10.1046/j.1439-0442.2002.00481.x. [DOI] [PubMed] [Google Scholar]
- 14.Rech R.R., Rissi D.R., Rodrigues A., Pierezan F., Piazer J.V.M., Kommers G.D., Barros C.S.L. Intoxicação por Solanum fastigiatum (Solanaceae) em bovinos: epidemiologia, sinais clínicos e morfometria das lesões cerebelares. Pesq. Vet. Bras. 2006;26:183–189. [Google Scholar]
- 15.Sabir S.M., Rocha J.B.T. Antioxidant and hepatoprotective activity of aqueous extract of Solanum fastigiatum (false “Jurubeba”) against paracetamol-induced liver damage in mice. J. Ethnopharmacol. 2008;120:226–232. doi: 10.1016/j.jep.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 16.Rosidah, Yam M.F., Sadikun A., Ahmad M., Akowuah G.A., Asmawi M.Z. Toxicology evaluation of standardized methanol extract of Gynura procumbens. J. Ethnopharmacol. 2009;123:244–249. doi: 10.1016/j.jep.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Bevan D.R., Bodlaender P., Shemin D. Mechanism of porphobilinogen synthase. Requirement of Zn2+ for enzyme activity. J. Biol. Chem. 1980;255:2030–2035. [PubMed] [Google Scholar]
- 18.Brito V.B., Folmer V., Soares J.C.M., Silveira I.D. Long-term sucrose and glucose consumption decreases the δ-aminolevulinate dehydratase activity in mice. Nutrition. 2007;23:818–826. doi: 10.1016/j.nut.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Nogueira C.W., Borges V.C., Zeni G., Rocha J.B.T. Organochalogens effects on δ-aminolevulinate dehydratase activity from human erythrocytic cells in vitro. Toxicology. 2003;191:169–178. doi: 10.1016/S0300-483X(03)00250-6. [DOI] [PubMed] [Google Scholar]
- 20.Souza A.C.G., Luchese C., Santos Neto J.S., Nogueira C.W. Antioxidant effect of a novel class of telluroacetilene compounds: Studies in vitro and in vivo. Life Sci. 2009;84:351–357. doi: 10.1016/j.lfs.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 21.Silver A. The Biology of Cholinesterases. North-Holland Publishing Company; Amsterdam, The Netherlands: 1974. p. 596. [Google Scholar]
- 22.Pohanka M. Cholinesterases, a target of pharmacology and toxicology. Biomed. Papers. 2011;155:219–223. doi: 10.5507/bp.2011.036. [DOI] [PubMed] [Google Scholar]
- 23.Shenouda J., Green P., Sultatos L. An evaluation of the inhibition of human butyrylcholinesterase and acetylcholinesterase by the organophosphate chlorpyrifos oxon. Toxicol Appl. Pharmacol. 2009;241:135–142. doi: 10.1016/j.taap.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zadra M., Piana M., Brum T.F., Boligon A.A., de Freitas R.B., Machado M.M., Stefanello S.T., Soares F.A.A., Athayde M.L. Antioxidant activity and phytochemical composition of the leaves of Solanum guaraniticum A. St.-Hil. Molecules. 2012;17:12560–12574. doi: 10.3390/molecules171112560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banerjee A., Nabasree D., De B. In vitro study of antioxidant activity of Syzygium cumini fruit. Food Chem. 2005;90:727–733. doi: 10.1016/j.foodchem.2004.04.033. [DOI] [Google Scholar]
- 26.Padayatty S.J., Katz A., Wang Y., Eck P., Kwon O., Lee J.H., Chen S., Corpe C., Dutta A., Dutta S.K., et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003;22:18–35. doi: 10.1080/07315724.2003.10719272. [DOI] [PubMed] [Google Scholar]
- 27.Oboh G. Effect of blanching on the antioxidant properties of some tropical green leafy G vegetables. Food Sci. Technol. 2005;38:513–517. [Google Scholar]
- 28.Materska M., Perucka I. Antioxidant activity of the main phenolic compounds isolated from hot pepper fruit (Capsicum annuum L.) J. Agric. Food Chem. 2005;53:1750–1756. doi: 10.1021/jf035331k. [DOI] [PubMed] [Google Scholar]
- 29.Amic D., Davidovic-Amic D., Beslo D., Trinajstic N. Structure-radical scavenging activity relationship of flavonoids. Croat. Chem. Acta. 2003;76:55–61. [Google Scholar]
- 30.Flohe L., Gunzler W.A., Schock H.H. Glutathione peroxidase: A selenoenzyme. FEBS Lett. 1973;32:132–134. doi: 10.1016/0014-5793(73)80755-0. [DOI] [PubMed] [Google Scholar]
- 31.Sakihama Y., Cohen M.F., Grace S.C., Yamasaki H. Plant phenolic antioxidant and prooxidant activities: Phenolics-induced oxidative damage mediated by metals in plants. Toxicology. 2002;177:67–80. doi: 10.1016/S0300-483X(02)00196-8. [DOI] [PubMed] [Google Scholar]
- 32.Riet-Correa F., Méndez M.C., Schild A.L., Summers B.A., Oliveira J.A. Intoxication by Solanum fastigiatum var. fastigiatum as a cause of cerebellar degeneration of cattle. Cornell Vet. 1983;73:240–256. [PubMed] [Google Scholar]
- 33.Prigol M., Wilhelm E.A., Schneider C.C., Rocha J.B.T., Nogueira C.W., Zeni G. Involvement of oxidative stress in seizures induced by diphenyl diselenide in rat pups. Brain Res. 2007;1147:226–232. doi: 10.1016/j.brainres.2007.01.126. [DOI] [PubMed] [Google Scholar]
- 34.Emanuelli T., Prauchner C.A., Dacanal J., Zeni A., Reis E.C., de Mello C.F., de Souza D.O. Intrastriatal administration of 5-aminolevulinic acid induces convulsions and body asymmetry through glutamatergic mechanisms. Brain Res. 2000;868:88–94. doi: 10.1016/S0006-8993(00)02327-1. [DOI] [PubMed] [Google Scholar]
- 35.Hermes-Lima M., Pereira B., Bechara E.J. Are free radicals involved in lead poisoning? Xenobiotica. 1991;21:1085–1090. doi: 10.3109/00498259109039548. [DOI] [PubMed] [Google Scholar]
- 36.Floyd R.A., Zaleska M.M., Harmon J. In: Free Radicals in Molecular Biology, Aging and Disease. Armstrong D., Sohal R.S., Cutler R.G., Slater T.F., editors. Raven Press; New York, NY, USA: 1984. pp. 143–161. [Google Scholar]
- 37.Freitas R.M. Lipoic acid alters d-aminolevulinic dehydratase, glutathione peroxidase and Na+, K+-ATPase activities and glutathione reduced levels in rat hippocampus after pilocarpineinduced seizures. Cell. Mol. Neurobiol. 2010;30:381–387. doi: 10.1007/s10571-009-9460-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oboh G., Rocha J.B.T. Water extractable phytochemicals from Capsicu pubescens (tree pepper) inhibit lipid peroxidation induced by different pro-oxidant agents in brain: In vitro. Eur. Food Res. Technol. 2008;226:707–713. doi: 10.1007/s00217-007-0580-5. [DOI] [Google Scholar]
- 39.Manian R., Anusuya N., Siddhuraju P., Manian S. The antioxidant activity and free radical scavenging potential of two different solvent extracts of Camellia sinensis (L.) O. Kuntz, Ficus bengalensis L. and Ficus racemosa L. Food Chem. 2008;107:1000–1007. doi: 10.1016/j.foodchem.2007.09.008. [DOI] [Google Scholar]
- 40.Awaha F.M., Uzoegwua P.N., Ifeonua P., Oyugib J.O., Rutherfordb J., Yao X., Fehrmannb F., Fowkeb K.R., Eze M.O. Free radical scavenging activity, phenolic contents and cytotoxicity of selected Nigerian medicinal plants. Food Chem. 2012;131:1279–1286. [Google Scholar]
- 41.Giugliano D., Ceriello A., Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19:257–267. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- 42.Zhu R., Wang Y., Zhang L., Guo Q. Oxidative stress and liver disease. Hepatol. Res. 2012;42:741–749. doi: 10.1111/j.1872-034X.2012.00996.x. [DOI] [PubMed] [Google Scholar]
- 43.Vega-Naredo I., Poeggeler B., Sierra-Sánchez V., Caballero B., Tomás-Zapico C., Alvarez-García O., Tolivia. D., Rodríguez-Colunga M.J., Coto-Montes A. Melatonin neutralizes neurotoxicity induced by quinolinic acid in brain tissue culture. J. Pineal Res. 2005;39:266–275. doi: 10.1111/j.1600-079X.2005.00243.x. [DOI] [PubMed] [Google Scholar]
- 44.Sies H. Glutathione and its role in cellular functions. Free Radic. Biol. Med. 1999;27:916–921. doi: 10.1016/S0891-5849(99)00177-X. [DOI] [PubMed] [Google Scholar]
- 45.Calabrese V., Cornelius C., Dinkova-Kostova A.T., Iavicoli I., di Paola R., Koverech A., Cuzzocrea S., Rizzarelli E., Calabrese E.J. Cellular stress responses, hormetic phytochemicals and vitagenes in aging and longevity. Biochim. Biophys. Acta. 2012;1822:753–783. doi: 10.1016/j.bbadis.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 46.Posser T., Moretto M.B., Dafre A.L., Farina M., da Rocha J.B., Nogueira C.W., Zeni G., Ferreira J.S., Leal R.B., France J.L. Antioxidant effect of diphenyl diselenide against sodium nitroprusside (SNP) induced lipid peroxidation in human platelets and erythrocyte membranes: An in vitro evaluation. Chem. Biol. Interact. 2006;164:126–135. doi: 10.1016/j.cbi.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 47.Li S., Yan T., Yang J.Q., Oberley T.D., Oberley L.W. The role of cellular glutathione peroxidase redox regulation in the suppression of tumor cell growth by manganese superoxide dismutase. Cancer Res. 2000;60:3927–3939. [PubMed] [Google Scholar]
- 48.Islam M.R., Parvin M.S., Islam M.E. Antioxidant and hepatoprotective activity of an ethanol extract of Syzygium jambos (L.) leaves. Drug Discov. Ther. 2012;6:205–211. [PubMed] [Google Scholar]
- 49.Subramanian K.N., Padmanaban G., Sarma P.S. Folin-Ciocalteu reagent for the estimation of siderochromes. Anal. Biochem. 1965;12:106–112. doi: 10.1016/0003-2697(65)90147-8. [DOI] [PubMed] [Google Scholar]
- 50.Zhishen J., Mengcheng T.W.J. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]
- 51.Benderitter M., Maupoil V., Vergely C., Dalloz F., Briot F., Rochette L. Studies by electron paramagnetic resonance of the importance of iron in the hydroxyl scavenging properties of ascorbic acid in plasma: Effects of iron chelators. Fundam. Clin. Pharmacol. 1998;12:510–516. doi: 10.1111/j.1472-8206.1998.tb00979.x. [DOI] [PubMed] [Google Scholar]
- 52.Sreejayan N., Rao M.N.A. Nitric oxide scavenging by curcuminoids. J. Pharm. Pharmacol. 1997;49:105–107. doi: 10.1111/j.2042-7158.1997.tb06761.x. [DOI] [PubMed] [Google Scholar]
- 53.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 54.Peterson G.L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal. Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 55.Sassa S. Delta-aminolevulinic acid dehydratase assay. Enzyme. 1982;28:133–145. doi: 10.1159/000459097. [DOI] [PubMed] [Google Scholar]
- 56.Peixoto N.C., Roza T., Pereira M.E. Sensitivity of d-ALA-D (E.C. 4.2.1.24) of rats to metals in vitro depends on the stage of postnatal growth and tissue. Toxicol. In Vitro. 2004;18:805–809. doi: 10.1016/j.tiv.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 57.Ellman G.L., Courtney K.D., Andres V. JR., Feather-stone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 58.Rocha J.B., Emanuelli T., Pereira M.E. Effects of early undernutrition on kinetic parameters of brain acetylcholinesterase from adult rats. Acta Neurobiol. Exp. 1993;53:431–437. [PubMed] [Google Scholar]
- 59.Herkerta N.M., Freudeb G., Kunzb U., Thiermanna H., Woreka F. Comparative kinetics of organophosphates and oximes with erythrocyte, muscle and brain acetylcholinesterase. Toxicol. Lett. 2012;209:173–178. doi: 10.1016/j.toxlet.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 60.Niehaus W.G.J., Samuelsson B. Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur. J. Biochem. 1968;6:126–130. doi: 10.1111/j.1432-1033.1968.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 61.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–125. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]