Abstract
OxymaPure (ethyl 2-cyano-2-(hydroxyimino)acetate) was tested as an additive for use in the carbodiimide (DIC) approach for the synthesis of a novel series of α-ketoamide derivatives (4-[2-(2-acetylaminophenyl)-2-oxo-acetylamino]benzoyl amino acid ester derivatives). OxymaPure showed clear superiority to HOBt/DIC or carbodiimide alone in terms of purity and yield. The title compounds were synthesized via the ring opening of N-acylisatin. First, N-acetylisatin was reacted with 4-aminobenzoic acid under conventional heating as well as microwave irradiation to afford 4-(2-(2-acetamidophenyl)-2-oxoacetamido)benzoic acid. This α-ketoamide was coupled to different amino acid esters using OxymaPure/DIC as a coupling reagent to afford 4-[2-(2-acetylaminophenyl)-2-oxo-acetylamino]benzoyl amino acid ester derivatives in excellent yield and purity. The synthesized compounds were characterized using FT-IR, NMR, and elemental analysis.
Keywords: N-acetylisatin, 4-aminobenzoic acid, amino acid esters, DIC, OxymaPure, α-ketoamide
1. Introduction
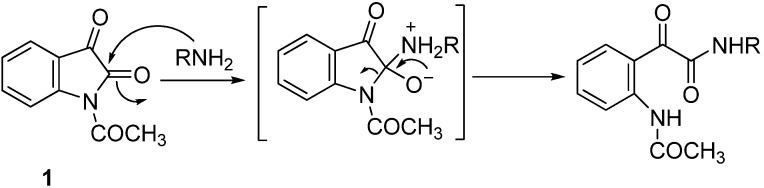
α-Ketoamides are compounds of interest in organic chemistry and are present in many active pharmaceutical compounds [1,2,3,4,5,6]. Parallel with the application of the α-ketoamide moiety in medicinal chemistry, numerous synthetic methods have been described [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21]. Several authors have demonstrated that the synthesis of the α-ketoamide fragment can be achieved by ring opening of N-acetylisatin (1) by the attack of an amine at C2-carbonyl group of N-acetylisatin (Scheme 1) [22,23,24,25,26,27]. Recently, Cheah et al. [28,29] reported the reaction of N-acetylisatin with L-α-amino acid esters as a novel class of N-glyoxylamide peptide mimics.
Scheme 1.
General mechanism for the reaction of N-acylisatin (1) with amines.
Here we present the use of OxymaPure/DIC as a coupling reagent for the synthesis of a novel class of α-ketoamide derivatives (4-[2-(2-acetylaminophenyl)-2-oxo-acetylamino]benzoyl amino acid esters).
2. Results and Discussion
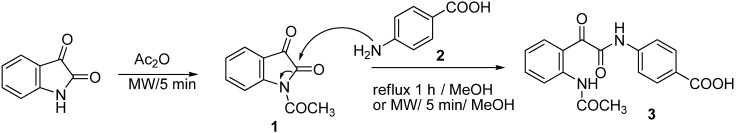
1-Acetylindoline-2,3-dione (N-acetylisatin, 1) was initially prepared by reaction of isatin with acetic anhydride, using conventional heating under the same conditions as those described in the literature [30,31]. However, we demonstrate that the use of a microwave irradiation, using a multimode reactor (Synthos 3000, Anton Paar GmbH, Graz, Austria, 1,400 W maximum magnetron, method B; Experimental section), renders 1 from isatin and acetic anhydride (Scheme 2) in excellent yield in less reaction time and higher purity than the conventional method, as observed from spectral data. This observation is consistent with data in the literature [30].
Scheme 2.
Synthesis and reaction of N-acylisatin (1) with 4-aminobenzoic acid (2).
N-Acetylisatin (1) was then reacted with the poor nucleophile, 4-aminobenzoic acid (2) using conventional heating for 1 h in methanol as a solvent to afford the product 3 (Scheme 2). The IR and NMR spectral analysis of the product revealed that the reaction proceeded through the ring opening to afford the α-ketoamide derivative 3 and not the Schiff base derivative, in contrast to what was reported in the reaction of bromo-N-acetylisatin with aminobenzoic acid. [32,33] The IR spectra of 3 showed three characteristic peaks at 3270, 1679, and 1601 cm−1, corresponding to the COOH, the α-ketoamide (COCONH), and NHCOCH3, respectively. The 1H-NMR of 3 agreed well with the structure, showing eight distinct resonance peaks located at δ 1.99 (s, 3H, COCH3), 7.27–7.730 (m, 2H, Ar), 7.62–7.68 (m, 2H, Ar), 7.90 (d, 2H, Ar), 7.90 (d, 2H, Ar), 10.55 (s, 1H, NH), 10.99 (s, 1H, NH), and 13.00 (brs, 1H, COOH). These peaks were assigned to the acetyl group, aromatic proton (isatin), aromatic proton (4-aminobenzoic acid), two NHs, and COOH respectively. The 13C-NMR of 3 also confirmed the structure, showing the characteristic signals at δ 161.0, 166.1, 168.2, and 188.1 corresponding to the two amide groups, one carbonyl of the carboxyl group, and the α-ketoamide group respectively, along with the rest of the expected carbon signals of the compound.
The same reaction was repeated under microwave irradiation using a multimode reactor (Anton Paar GmbH Synthos 3000, 1,400 W maximum magnetron, method B in the Experimental section) to afford product 3 (Scheme 2) in less reaction time and high purity as shown by its spectral data. The IR and NMR spectra of 3 proved its structure and were in agreement with the product obtained by conventional heating.
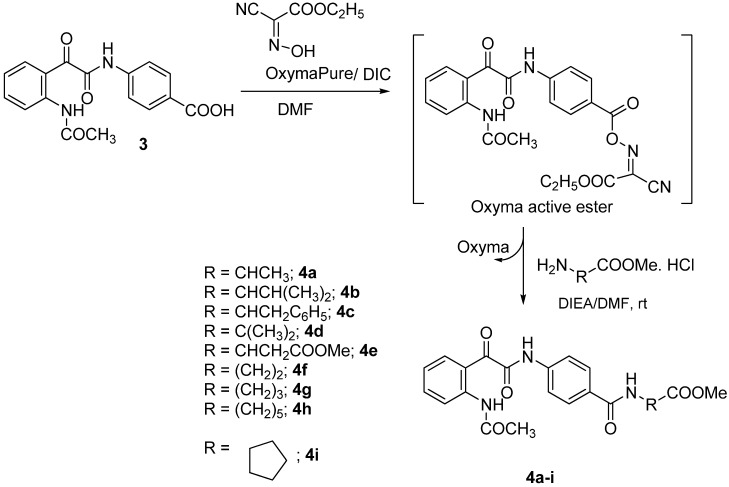
Recently, OxymaPure (ethyl 2-cyano-2-(hydroxyimino)acetate, Scheme 3) was used as an additive for peptide synthesis in combination with carbodiimides [34]. It displayed an appropriate balance of availability and ease of handling. In addition it was safer than HOBt and showed clear superiority in terms of coupling efficiency [34,35,36,37,38,39,40,41,42].
Scheme 3.
Synthesis 4-[2-(2-acetylaminophenyl)-2-oxo-acetylamino]benzoyl amino acid ester derivatives 4a–i.
Here we tested OxymaPure as an additive for the coupling of compound 3 to H-Ala-OMe·HCl. In a general experiment, 3 was preactivated with OxymaPure/DIC for 5 min in DMF to generate the corresponding active ester (Scheme 3), which reacted directly with H-Ala-OMe·HCl in the presence of 1 equiv. of DIEA at 0 °C for 1 h and then at r.t overnight. After workup and removal of the solvent, product 4a was obtained as a white solid in 88% yield (Scheme 3).
The reaction of 3 with H-Ala-OMe was then repeated using various coupling reagents (Table 1). The best results were obtained using OxymaPure/DIC; OxymaPure showed clear superiority to HOBt/DIC and carbodiimide alone in terms of yield and purity. DCC consistently showed some impurities from dicyclohexylurea (DCU) as observed from NMR spectra.
Table 1.
Reaction of 3 with H-Ala-OMe.HCl using various coupling conditions.
| Coupling Condition | Yield (%) | Mp (°C) |
|---|---|---|
| DIC/Oxyma | 88 | 174–176 |
| DCC/Oxyma * | 82 | 168–172 |
| DIC/HOBt | 72 | 172–175 |
| DCC/HOBt * | 70 | 170–174 |
| DIC | 60 | 170–173 |
| DCC * | 60 | 168–173 |
* NMR showed impurities corresponding to the dicyclohexylurea byproduct.
The IR spectrum of 4a showed four characteristic peaks at 3288, 1741, 1672, and 1607 cm−1, corresponding to the NH, CO-ester, α-ketoamide and CO-amide, respectively. The 1H-NMR showed a doublet peak at δ 1.41, which was related to the CH3 for the alanine unit, two singlet peaks at δ 2.00 and 3.65 for the acetyl and the methyl ester, and three singlet peaks at δ 8.73, 10.55, 10.92 for three NHs, respectively. The 13C-NMR also confirmed the structure of 4a, showing signals at δ 17.3 (CHCH3), 24.2 (NCOCH3), 49.9 (COOCH3), 52.1 (CH-NH), 162.3, 166.2, and 169.5 (for three CONHs), 173.8 (COOCH3), and 190.0 (COCO), along with the remaining carbon residues related to 4a.
Several amino acid esters were prepared following the reported method [43,44] and coupled with 3 using OxymaPure/DIC under the same conditions used for the coupling of H-Ala-OMe. This approach afforded products 4a–i in excellent yield and purity (Scheme 3, Table 2). The structures of all the compounds synthesized were confirmed by IR, NMR (1H-NMR and 13C-NMR) and elemental analysis.
Table 2.
Yield (%), Mp (°C), and Elemental analysis of 4a–i.
| Compd. No | Yield (%) | Mp (°C) | Elemental Analysis Calcd. (Found) | ||
|---|---|---|---|---|---|
| C | H | N | |||
| 4a | 88 | 174–176 | 61.31 (61.60) | 5.14 (5.34) | 10.21 (10.00) |
| 4b | 81 | 178–180 | 62.86 (63.02) | 5.73 (5.96) | 9.56 (9.581) |
| 4c | 83 | 168–170 | 66.52 (66.67) | 5.17 (5.24) | 8.62 (8.88) |
| 4d | 76 | 216–218 | 62.11 (62.38) | 5.45 (5.66) | 9.88 (10.07) |
| 4e | 82 | 154–156 | 66.52 (66.33) | 5.17 (5.26) | 8.62( 8.90) |
| 4f | 88 | 154–156 | 61.31 (61.09) | 5.14 (5.23) | 10.21 (10.48) |
| 4g | 86 | 118–120 | 62.11 (62.37) | 5.45 (5.67) | 9.88 (10.04) |
| 4h | 83 | 180–182 | 63.56 (63.38) | 6.00 (6.13) | 9.27 (9.53) |
| 4i | 78 | 238–240 | 63.85 (64.06) | 5.58 (5.65) | 9.31 (9.04) |
3. Experimental
3.1. General
The solvents used were of HPLC reagent grade. Melting points were determined with a Mel-Temp apparatus and are uncorrected. Fourier transform infrared spectroscopy (FTIR) spectra was recorded on Nicolet 560 spectrometer. Nuclear magnetic resonance spectra (1H-NMR and 13C-NMR spectra) were recorded on a JOEL 400 MHz spectrometer with chemical shift values reported in δ units (ppm) relative to an internal standard. The microwave irradiation used a multimode reactor (Synthos 3000, Anton Paar GmbH, and 1,400 W maximum magnetron). Elemental analyses were performed on Perkin-Elmer 2400 elemental analyzer, and the values found were within ±0.3% of the theoretical values. Follow-up of the reactions and checks of the purity of the compounds were done by TLC on silica gel-protected aluminum sheets (Type 60 GF254, Merck) and the spots were detected by exposure to a UV-lamp at λ 254 nm for a few seconds. The compounds were named using ChemDraw Ultra version 11, Cambridge Soft Corporation (Cambridge, MA, USA).
3.2. Synthesis of 1-Acetyl-1H-indole-2,3-dione (N-acetylisatin) (1)
Conventional method (A): A mixture of isatin (0.01 M) and acetic anhydride (5 mL) was refluxed for 5 h. After cooling to r. t., it was left to stand overnight. The precipitate was collected, washed with 96% ethanol and air-dried.
Microwave method (B): A multimode reactor (Anton Paar GmbH Synthos 3000, 1,400 W maximum magnetron) was used. The initial step was conducted with a 2-Teflon vessels rotor (MF 100). Isatin (5 mmol) was suspended in acetic anhydride (10 mL) and the reaction was processed by heating the vessels for 5 min. at 80 °C and holding it at the same temperature for 5 min (under 0.2/s bar pressure, 400 W). Cooling was accomplished by a fan (for 5 min) and the desired product was obtained as a yellow needle in excellent yield without further recrystallization. The spectral data were in accordance with the data reported in the literature [30].
1-Acetyl-1H-indole-2,3-dione (N-acetylisatin) (1) The product was obtained as green crystals, mp: 146–148 °C; yields: 79% (method A); 95% (method B) (lit. [23] 141 °C, yield 97%; lit. [30] 137–139 °C, yield 70%). 1H-NMR (CDCl3) δ (ppm): 2.69 (s, 3H, COCH3), 7.31 (t, J = 8.04 Hz, 1H), 7.69 (t, J = 7.32 Hz,1H), 7.73 (d, J = 7.32 Hz, 1H), 8.36 (d, J = 8.08 Hz,1H); 13C-NMR (CDCl3) δ (ppm): 26.5 (COCH3), 118.3, 119.2, 125.3, 126.2, 139.0, 148.6, 158.0 (CO), 169.8 (CO), 180.2 (CO).
3.3. Synthesis of 4-(2-(2-Acetamidophenyl)-2-oxoacetamido)benzoic Acid (3)
Conventional method (A): A mixture of N-Acetyl isatin (0.01 M) and 4-amino-benzoic acid (0.01 M) in absolute methanol (20 mL) was refluxed for 1 h in the presence of 2–3 drops of glacial acetic acid. After cooling, was filtered and recrystallized from ethanol to afford the product in 96% yield.
Microwave method (B): A multimode reactor (Synthos 3000 Aton Paar, GmbH, 1400 W maximum magnetron) was used. The initial step was conducted with 2-Teflon vessels rotor (MF 100) that allows the reaction to be processed under the same conditions. N-acetylisatin and 4-aminobenzoic acid were mixed in methanol as a solvent in the presence or absence of glacial acetic acid (2–3 drops). The individual vessels were placed in the corresponding rotor, and finally the rotor was closed with a protective hood. The vessels were heated for 2 min. at 80 °C and held at the same temperature for another 2 min (~2 bar pressure, 400 W). Cooling was accomplished by a fan (5 min). The final product was washed with cold methanol, and then dried under vacuum to afford the product in a pure state as observed from spectral analysis.
The product was obtained as a pale yellow powder, mp: 238–240 °C; yield (57% method A); (86% method B). 1H-NMR (DMSO-d6) δ (ppm): 1.99 (s, 3H, COCH3), 7.25–7.30 (m, 2H), 7.62–7.68 (m, 2H), 7.90 (d, J = 8.79 Hz, 2H), 7.95 (d, J = 8.43 Hz, 2H), 10.55(s, 1H, NH), 10.99 (s, 1H, NH), 13.00 (brs, 1H, COOH); 13C-NMR (DMSO-d6) δ (ppm): 22.8 (NHCOCH3), 118.7, 121.0, 123.1, 124.3, 125.4, 129.6, 130.3, 132.9, 136.8, 141.2, 161.00 (CONH), 166.1(CONH), 168.2(COOH), 188.1 (COCO). IR (cm−1): 3270 (COOH), 1679, 1601 (C=O). Anal. Calcd. for C17H14N2O5: C, 62.57; H, 4.32; N, 8.59. Found: C, 62.80; H, 4.21; N, 8.33.
3.4. General Method for the Synthesis of Amino Acid Esters
Thionyl chloride (10 mL) was slowly added to a cold suspension solution of the appropriate amino acid (50 mmol) in methanol (50 mL) at 0 °C. The reaction mixture was stirred for 8–10 h and then concentrated on a rotary evaporator. The white precipitate formed was washed with anhydrous ether and then dried under vacuum. All data agreed with the reported data [43,44].
Methyl 1-aminocyclopentane carboxylate HCl. White powder, mp: 204–206 °C, yield 87%. 1H-NMR (DMSO-d6) δ (ppm): 1.62–1.72 (m, 2H, CH2CH2CH2), 1.81–1.91 (m, 2H, HNCH2CH2CH2), 1.94–1.96 (m, 2H, CH2CH2CO), 2.07–2.14 (m, 2H, CH2CH2NH), 3.73 (s, 3H, COOCH3), 8.81(brs, 2H, NH2). 13C-NMR (DMSO-d6) δ (ppm): 24.3, 35.2, 52.4 (COOCH3), 63.4, 171.8(COOCH3).
Methyl 3-aminopropionate HCl. White powder, mp: 90–92 °C, yield 85%. 1H-NMR (DMSO-d6) δ (ppm): 2.72 (t, J = 7.35 Hz, 2H, NH2CH2CH2CO), 2.98 (m, 2H, NH2CH2CH2CO), 3.62 (s, 3H, COOCH3), 8.22 (brs, 2H, NH2).
Methyl 4-aminobutanoate HCl. White powder, mp: 104–106 °C, yield 87%. 1H-NMR (DMSO-d6) δ (ppm): 1.80 (m, 2H, NH2CH2CH2CH2CO), 2.43 (t, J = 7.0 Hz, 2H, NH2CH2CH2CH2CO), 2.78 (m, 2H, NH2CH2CH2CH2CO), 3.59 (s, 3H, COOCH3), 8.16 (brs, 2H, NH2).
Methyl 6-aminohexanoate HCl. White powder, mp: 104–106 °C, yield 90%. 1H-NMR (DMSO-d6) δ (ppm): 1.26–1.31 (m, 2H, NH2CH2CH2CH2CH2CH2CO), 1.49–1.57 (m, 4H, NH2CH2CH2CH2CH2CH2CO), 2.29 (t, J = 7.30 Hz, 2H, NH2CH2CH2CH2CH2CH2CO), 2.71 (m, 2H, NH2CH2CH2CH2CH2CH2CO), 3.57 (s, 3H, COOCH3), 8.08 (brs, 2H, NH2).
Dimethyl 2-aminosuccinate HCl. White powder, mp: 110–112 °C, yield 82%. 1H-NMR (DMSO-d6) δ (ppm): 3.03 (q, J = 4.0 Hz, 2H, COCH2CHNH2), 3.64 (s, 3H, COOCH3), 3.72 (s, 3H, COOCH3), 4.33 (m, 1H, CH2CHNH2), 8.75 (brs, 2H, NH2).
3.5. General Procedure for the Synthesis of 4a–i
Acid 3 (1 mmol), Oxyma (1 mmol), and DIC (1 mmol) were mixed in DMF (5 mL) at 0 °C. The reaction mixture was stirred for 5 min at 0 °C to preactivate the acid and generate the active ester, and then DIEA (1 mmol) followed by amino acid ester (1 mmol) were added. The reaction mixture was stirred at 0 °C for 1 h and at room temperature overnight. The mixture was then diluted with ethyl acetate (50 mL) and then extracted with 1 N HCl (2 × 10 mL), 10% NaHCO3 (2 × 10 mL), and saturated NaCl (2 × 10 mL). The organic phase was dried over anhydrous MgSO4, filtered, and the solvent was removed under vacuum. The residue was recrystallized from dichloromethane-hexane to afford the pure product.
Methyl 2-(4-(2-(2-acetamidophenyl)-2-oxoacetamido)benzamido)propanoate (4a). White powder, mp: 174–176 °C, yield 88%. 1H-NMR (DMSO-d6) δ (ppm): 1.41 (d, J = 7.32 Hz, 3H, CHCH3), 2.00 (s, 3H, COCH3), 3.65 (s, 3H, COOCH3), 4.48 (m, 1H, NHCHCH3), 7.45 (t, J = 7.2 Hz, 1H), 7.64 (d, J = 4.4 Hz, 1H), 7.68 (d, J = 7.36 Hz, 2H), 7.89 (m, 4H, Ar), 8.73(s, 1H, NH), 10.55(s, 1H, NH), 10.92 (s, 1H, NH); 13C-NMR (DMSO-d6) δ (ppm): 17.3 (NHCHCH3), 24.2 (COCH3), 49.9 (COOCH3), 52.1 (NHCHCH3), 120.0, 122.4, 124.4, 126.4, 128.9, 129.0, 131.6, 134.21, 139.4, 142.2, 162.3 (CONH), 166.2 (CONH), 169.5 (CONH), 173.8 (COOCH3), 190.0 (COCO). IR (cm−1): 3288, 3124 (NH), 1741, 1672, 1607, 1536 (C=O). Anal. Cacld for C21H21N3O6: C, 61.31; H, 5.14; N, 10.21. Found: C, 61.60; H, 5.34; N, 10.00.
Methyl 2-(4-(2-(2-acetamidophenyl)-2-oxoacetamido)benzamido)-3-methylbutanoate (4b). White powder, mp: 178–180 °C, yield 81%. 1H-NMR (CDCl3) δ (ppm): 1.00 (t, J = 8.60 Hz, 6H, CH (CH3)2), 1.59 (m, 1H, CHCH(CH3)2), 2.27 (s, 3H, COCH3), 3.78 (s, 3H, COOCH3), 4.78 (m, 1H, CHCHNH), 6.61 (d, J = 8.08 Hz, 1H), 7.17 (t, J = 8.08 Hz, 1H), 7.64 (t, J = 8.08 Hz, 1H), 7.78 (d, J = 8.08 Hz, 1H), 7.86 (d, J = 8.08 Hz, 2H), 8.50 (d, J = 8.08 Hz, 1H) 8.64 (d, J = 8.08 Hz, 1H), 8.99 (s, 1H, NH), 10.79 (s, 1H, NH); 13C-NMR (CDCl3) δ (ppm): 18.1, 19.1(CHCH(CH3)2), 25.0 (COCH3), 31.0 (CHCH(CH3)2), 52.4 (COOCH3),57.6 (CHCH(CH3)2), 119.4, 119.8, 120.9, 122.8, 128.4, 130.6, 134.3, 136.8, 140.0, 142.1,160.6 (CONH), 166.5 (CONH), 169.4 (CONH), 172.8 (COOCH3), 190.8 (COCO). IR (cm−1): 3293 (NH), 1747, 1679, 1634, 1608, 1526 (C=O). Anal. Cacld. for C23H25N3O63: C, 62.86; H, 5.73; N, 9.56. Found: C, 63.02; H, 5.96; N, 9.81.
Methyl 2-(4-(2-(2-acetamidophenyl)-2-oxoacetamido)benzamido)-3-phenylpropanoate (4c). White powder, mp: 168–170 °C, yield 83%. 1H-NMR (CDCl3) δ (ppm): 2.24 (s, 3H, COCH3), 3.25 (m, 2H, CHCH2C6H5), 3.80 (s, 3H, COOCH3), 5.08 (m, 1H, CHCH2C6H5), 6.56 (d, J = 7.36 Hz, 1H), 7.12 (d, J = 6.6 Hz, 1H), 7.15–7.29 (m, 5H, CHCH2C6H5), 7.65 (t, J = 6. 6 Hz, 1H), 7.76 (m, 4H, NHC6H4CO), 8.47 (d, J = 8.08 Hz, 1H), 8.63 (d, J = 8.08 Hz, 1H), 9.01(s, 1H, NH), 10.79 (s, 1H, NH); 13C-NMR (CDCl3) δ (ppm): 25.0 (COCH3), 38.0 (CHCH2C6H5), 49.2 (COOCH3), 52.6 (CHCH2C6H5), 118.8, 119.8, 121.0, 122.8, 127.3, 128.39, 128.7, 129.4, 130.5, 134.4, 135.9, 136.9, 139.9, 142.1, 160.3 (CONH), 166.0 (CONH), 169.4 (CONH), 172.1 (COOCH3), 190.6 (COCO). IR (cm−1): 3250, 3116 (NH), 1750, 1679, 1635, 1608, 1523 (C=O). Anal. Calcd for C27H25N3O6: C, 66.52; H, 5.17; N, 8.62. Found: C, 66.67; H, 5.24; N, 8.88.
Methyl 2-(4-(2-(2-acetamidophenyl)-2-oxoacetamido)benzamido)-2-methylpropanoate (4d). White powder, mp: 212–218 °C, yield 76%. 1H-NMR (DMSO-d6) δ (ppm): 1.46 (s, 6H, HNC(CH3)2), 1.99 (s, 3H, COCH3), 3.58 (s, 3H, COOCH3), 7.63–7.87 (m, 8H, NHC6H4COCONHC6H4CO), 8.54 (s, 1H, NH), 10.56 (s, 1H, NH), 10.90 (s, 1H, NH); 13C-NMR (DMSO-d6) δ (ppm): 25.6 (COCH3), 40.5 (HNC(CH3)2), 52.4 (COOCH3), 56.1 HNC(CH3)2, 119.8, 121.3, 124.5, 126.4, 129.0, 130.0, 131.5, 134.2, 138.1, 142.20,158.0 (CONH), 165.9 (CONH), 169.5(CONH), 175.1 (COOCH3), 189.5 (COCO). IR (cm−1): 3428 (NH), 1749, 1680, 1637, 1525 (C=O). Anal. Calcd. for C22H23N3O6: C, 62.11; H, 5.45; N,9.88. Found: C, 62.38; H, 5.66; N, 10.07.
Dimethyl 2-(4-(2-(2-acetamidophenyl)-2-oxoacetamido)benzamido)succinate (4e). White powder, mp: 154–156 °C, yield 82%. 1H-NMR (DMSO-d6) δ (ppm): 1.99 (s, 3H, COCH3), 2.85–2.98 (m, 2H, CHCH2COOCH3), 3.62 (s, 3H, COOCH3), 3.65 (s, 3H, COOCH3), 4.83 (m, 1H, HNCHCH2), 7.28–7.87 (m, 8H, NHC6H4COCONHC6H4CO), 8.86 (d, 1H, NH), 10.54 (s, 1H, NH), 10.93 (s, 1H, NH); 13C-NMR (DMSO-d6) δ (ppm): 23.9 (COCH3), 36.0 (NHCHCH2CO), 49.8 (NHCHCH2CO), 52.3 (COOCH3), 52.8 (COOCH3), 119.9, 122.4, 124.4, 125.7, 128.9, 129.5, 131.55, 134.2, 138.1, 141.5, 162.3 (CONH), 166.2 (CONH), 169.5 (CONH), 171.1(COOCH3), 171.9 (COOCH3), 189.5 (COCO). IR (cm−1): 3295 (NH), 1748, 1667, 1608, 1526 (C=O). Anal. Calcd. for C27H25N3O6: C, 66.52; H, 5.17; N, 8.62. Found: C, 66.33; H, 5.26; N, 8.90.
Methyl 3-(4-(2-(2-acetamidophenyl)-2-oxoacetamido)benzamido)propanoate (4f). White powder, mp: 154–156 °C, yield 88%. 1H-NMR (DMSO-d6) δ (ppm): 1.99 (s, 3H, COCH3), 2.60 (t, J = 6.60 Hz, 2H, NHCH2CH2CO), 3.49 (q, 2H, NHCH2CH2CO), 3.61 (s, 3H, COOCH3), 7.29–7.85 (m, 8H, NHC6H4COCONH C6H4CO), 8.51 (s, 1H, NH), 10.55 (s, 1H, NH), 10.90 (s, 1H, NH); 13C-NMR (DMSO-d6) δ (ppm): 23.9 (COCH3), 34.1 (NHCH2CH2CO), 36.1(NHCH2CH2CO), 52.0 (COOCH3), 119.9, 122.4, 124.4, 125.6, 128.6, 130.4, 131.6, 134.3, 138.2, 141.1, 162.4 (CONH), 166.3 (CONH), 169.5 (CONH), 172.4 (COOCH3), 189.6 (COCO). IR (cm−1): 3295 (NH), 1742, 1666, 1635, 1608, 1527 (C=O). Anal. Calcd. for C21H21N3O6: C, 61.31; H, 5.14; N, 10.21. Found: C, 61.09; H, 5.23; N, 10.48.
Methyl 4-(4-(2-(2-acetamidophenyl)-2-oxoacetamido)benzamido)butanoate (4g). White powder, mp: 118–120 °C, yield 86%. 1H-NMR (DMSO-d6) δ (ppm): 1.77 (m, 2H, NHCH2CH2CH2CO), 1.99 (s, 3H, COCH3), 2.37 (t, J = 7.32 Hz, 2H, NHCH2CH2CH2CO), 3.27 (q, 2H, NHCH2CH2CH2CO), 3.58 (s, 3H, COOCH3), 7.28 (t, J = 7.3, 2.2 Hz, 1H), 7.64–7.85 (m, 7H, Ar), 8.42 (brs, 1H, NH), 10.55 (s, 1H, NH), 10.89 (s, 1H, NH); 13C-NMR (DMSO-d6) δ (ppm): 23.9 (NHCH2CH2CH2CO), 24.2 (COCH3), 31.4 (NHCH2CH2CH2CO), 41.6 (NHCH2CH2CH2CO), 51.8 (COOCH3), 119.9, 122.4, 124.4, 125.6, 128.6, 130.7, 131.6, 134.3, 138.7, 141.0, 162.3 (CONH), 166.2 (CONH), 169.5 (CONH), 173.7 (COOCH3), 189.7 (COCO). IR (cm−1): 3343 (NH), 1738, 1661, 1630, 1600, 1527 (C=O). Anal. Calcd. for C22H23N3O6: C, 62.11; H, 5.45; N, 9.88. Found: C, 61.37; H, 5.67; N, 10.04.
Methyl 6-(4-(2-(2-acetamidophenyl)-2-oxoacetamido)benzamido)hexanoate (4h). White powder, mp: 180–182 °C, yield 83%. 1H-NMR (DMSO-d6) δ (ppm): 1.32–1.36 (m, 2H, NHCH2CH2CH2CH2CH2CO), 1.49–1.56 (m, 4H, NHCH2CH2CH2CH2CH2CO), 2.00 (s, 3H, COCH3), 2.31 (t, J = 7.32 Hz, 2H, NHCH2CH2CH2CH2CH2CO), 3.25 (t, J = 5.88 Hz, 2H, NHCH2CH2CH2 CH2 CH2CO), 3.63 (s, 3H, COOCH3), 7.28 (t, J = 5.2 Hz, 1H), 7.64–7.85 (m, 7H, Ar), 8.38 (brs, 1H, NH), 10.55 (s, 1H, NH), 10.88 (s, 1H, NH); 13C-NMR (DMSO-d6) δ (ppm): 24.76 (COCH3), 26.5 (NHCH2CH2CH2CH2CH2CO), 29.4 (NHCH2CH2CH2CH2CH2CO, 33.8 (NHCH2CH2CH2CH2CH2CO), 41.5 (NHCH2CH2CH2CH2CH2CO), 51.7 (COOCH3), 119.9, 122.4, 124.4, 126.5, 128.5, 130.8, 131.6, 134.3, 138.2, 140.9, 162.3 (CONH), 166.0 (CONH), 169.5 (CONH), 173.9 (COOCH3), 189.7(COCO). IR (cm−1): 3290 (NH), 1738, 1664, 1637, 1608, 1528 (C=O). Anal. Calcd. for C24H27N3O6: C, 63.56; H, 6.00; N, 9.27. Found: C, 63.38; H, 6.13; N, 9.53.
Methyl 1-(4-(2-(2-acetamidophenyl)-2-oxoacetamido)benzamido)cyclopentanecarboxylate (4i). White powder, mp: 238–240 °C, yield 78%. 1H-NMR (DMSO-d6) δ (ppm): 1.69–1.71 (m, 4H, CH2CH2CH2CH2), 1.99 (s, 3H, COCH3), 2.00–2.13 (m, 4H, CH2CH2CH2CH2), 3.58 (s, 3H, COOCH3), 7.28 (t, J = 6.16 Hz, 1H,), 7.63–7.87 (m, 7H, Ar), 8.50(s, 1H, NH), 10.55(s, 1H, NH), 10.90 (s, 1H, NH); 13C-NMR (DMSO-d6) δ (ppm): 24.1 (CH2CH2CH2CH2), 24.7 (COCH3), 39.8(CH2CH2CH2CH2), 52.5 (COOCH3), 66.2 (C), 119.8, 122.5, 124.5, 125.9, 129.0, 130.0, 131.5, 134.2, 138.0, 141.3, 162.2 (CONH), 166.4 (CONH), 169.5 (CONH), 174.9 (COOCH3), 189.5 (COCO). IR (cm−1): 3291, 3114 (NH), 1746, 1679, 1633, 1607, 1524 (C=O). Anal. Calcd. for C24H25N3O6 (451.47): C, 63.85; H, 5.58; N, 9.31. Found: C, 64.06; H, 5.65; N, 9.04.
4. Conclusions
In conclusion, we have demonstrated that reaction of N-acetylisatin (1) with 4-aminobenzoic acid (2), either using conventional heating or microwave irradiation affords the α-ketoamide 4-(2-(2-acetamidophenyl)-2-oxoacetamido)benzoic acid (3) in good yield. All the spectral data proved the ring opening structure in which the 4-aminobenzoic acid attacks C2 rather than C3. Reaction of the 4-aminobenzoic acid derivative 3 with different amino acid esters using Oxyma/DIC afforded a novel series of 4-[2-(2-acetylaminophenyl)-2-oxo-acetylamino]benzoyl amino acid esters 4a–i. OxymaPure/DIC showed clear superiority to HOBt/DIC and carbodiimide alone in terms of yield and purity.
Acknowledgments
The authors thank the Deanship of Scientific Research at King Saud University for funding this work through research group “RGP-VPP-234”. The work in Barcelona was partially supported by CICYT (CTQ2012-30930) and the Generalitat de Catalunya (2009SGR 1024).
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/18/12/14747/s1.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of compounds are available from authors.
References
- 1.Li Z., Ortega-Vilain A.C., Patil G.S., Chu D.L., Foreman J.E., Eveleth D.D., Powers J.C. Novel peptidyl α-Keto amide inhibitors of calpains and other cysteine proteases. J. Med. Chem. 1996;39:4089–4098. doi: 10.1021/jm950541c. [DOI] [PubMed] [Google Scholar]
- 2.James D.A., Koya K., Li H., Liang G., Xia Z., Ying W., Wu Y., Sun L. Indole- and indolizine-glyoxylamides displaying cytotoxicity against multidrug resistant cancer cell lines. Bioorg. Med. Chem. Lett. 2008;18:1784–1787. doi: 10.1016/j.bmcl.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 3.Montalban A.G., Boman E., Chang C.D., Ceide S.C., Dahl R., Dalesandro D., Delaet N.G.J., Erb E., Ernst J.T., Gibbs A., et al. The design and synthesis of novel α-ketoamide-based p38 MAP kinase inhibitors. J. Bioorg. Med. Chem. Lett. 2008;18:1772–1777. doi: 10.1016/j.bmcl.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 4.Yu P.-F., Chen H., Wang J., He C.-X., Cao B., Li M., Yang N., Lei Z.Y., Cheng M.-S. Design, synthesis and cytotoxicity of novel podophyllotoxin derivatives. Chem. Pharm. Bull. 2008;56:831–834. doi: 10.1248/cpb.56.831. [DOI] [PubMed] [Google Scholar]
- 5.Perni R.B., Farmer L.J., Cottrell K.M., Court J.J., Courtney L.F., Deininger D.D., Gates C.A., Harbeson S.L., Kim J.L., Lin C., et al. Inhibitors of hepatitis C virus NS3·4A protease. Part 3: P2 proline variants. Bioorg. Med. Chem. Lett. 2004;14:1939–1942. doi: 10.1016/j.bmcl.2004.01.078. [DOI] [PubMed] [Google Scholar]
- 6.Victor F., Lamar J., Snyder N., Yip Y., Guo D., Yumibe N., Johnson R.B., Wang Q.M., Glass J.I., Chen S.H. P1 and P3 optimization of novel bicycloproline P2 bearing tetrapeptidyl α-ketoamide based HCV protease inhibitors. Bioorg. Med. Chem. Lett. 2004;14:257–261. doi: 10.1016/j.bmcl.2003.09.075. [DOI] [PubMed] [Google Scholar]
- 7.Banfi L., Guanti G., Riva R. Passerini multicomponent reaction of protected alpha-aminoaldehydes as a tool for combinatorial synthesis of enzyme inhibitors. J. Chem. Soc. Chem. Commun. 2000:985–986. doi: 10.1039/b002027n. [DOI] [Google Scholar]
- 8.Nakamura M., Inoue J., Yamada T. A two-step, one-pot synthesis of diverse N-pyruvoyl amino acid derivatives using the Ugi reaction. Bioorg. Med. Chem. Lett. 2000;10:2807–2810. doi: 10.1016/S0960-894X(00)00577-1. [DOI] [PubMed] [Google Scholar]
- 9.Xu P., Lin W., Zhou X. Synthesis of a peptidomimetic HCMV protease inhibitor library. Synthesis. 2002;8:1017–1026. [Google Scholar]
- 10.Chen J.J., Deshpande V. Rapid synthesis of α-ketoamides using microwave irradiation simultaneous cooling method. Tetrahedron Lett. 2003;44:8873–8876. doi: 10.1016/j.tetlet.2003.09.180. [DOI] [Google Scholar]
- 11.Faggi C., Neo A.G., Marcaccini S., Menchi G., Revuelta J. Ugi four-component condensation with two cleavable components: The easiest synthesis of 2,N-diarylglycines. Tetrahedron Lett. 2008;49:2099–2102. doi: 10.1016/j.tetlet.2008.01.134. [DOI] [Google Scholar]
- 12.Grassot J.M., Masson G., Zhu J. Synthesis of α-ketoamides by a molecular-sieves-promoted formal oxidative coupling of aliphatic aldehydes with isocyanides. Angew. Chem. Int. Ed. 2008;47:947–950. doi: 10.1002/anie.200704840. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y.H., Zhang Y.H., Zhang H.J., Liu D.Z., Gu M., Li J.Y., Wu F., Zhu X.Z., Li J., Nan F.J. Design, synthesis, and biological evaluation of isoquinoline-1,3,4-trione derivatives as potent Caspase-3 inhibitors. J. Med. Chem. 2006;49:1613–1623. doi: 10.1021/jm050896o. [DOI] [PubMed] [Google Scholar]
- 14.Song B., Wang S., Sun C., Deng H., Xu B. Cesium carbonate promoted aerobic oxidation of arylacetamides: An efficient access to N-substituted α-keto amides. Tetrahedon Lett. 2007;48:8982–8986. doi: 10.1016/j.tetlet.2007.10.099. [DOI] [Google Scholar]
- 15.Chen C.T., Bettigeri S., Weng S.S., Pawar V.D., Lin Y.H., Liu C.Y., Lee W.Z. Asymmetric aerobic oxidation of α-hydroxy acid derivatives by C4-Symmetric, Vanadate-Centered, Tetrakisvanadyl(V) clusters derived from N-Salicylidene-α-aminocarboxylates. J. Org. Chem. 2007;72:8175–8185. doi: 10.1021/jo070575f. [DOI] [PubMed] [Google Scholar]
- 16.Lamberth C., Jeanguenat A., Cederbaum F., de Mesmaecker A., Zeller M., Kempf H.J., Zeun R. Multicomponent reactions in fungicide research: The discovery of mandipropamid. Bioorg. Med. Chem. 2008;16:1531–1545. doi: 10.1016/j.bmc.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 17.Sanz R., Castroviejo M.P., Guilarte V., Pérez A., Fañanás F.J. Regioselective synthesis of 4- and 7-Alkoxyindoles from 2,3-Dihalophenols: Application to the preparation of indole inhibitors of phospholipase A2. J. Org. Chem. 2007;72:5113–5118. doi: 10.1021/jo070643y. [DOI] [PubMed] [Google Scholar]
- 18.Maresh J.J., Giddings L.-A., Friedrich A., Loris E.A., Panjikar S., Trout B.L., Stöckigt J., Peters B., O’Connor S.E. Strictosidine synthase: Mechanism of a pictet−spengler catalyzing enzyme. J. Am. Chem. Soc. 2008;130:710–723. doi: 10.1021/ja077190z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hua R., Takeda H.A., Abe Y., Tanaka M. Reactions of a carbamoylstannane with acid chlorides: Highly efficient synthesis of α-Oxo amides. J. Org. Chem. 2004;69:974–976. doi: 10.1021/jo035572r. [DOI] [PubMed] [Google Scholar]
- 20.Arasappan A., Venkatraman S., Padilla A.I., Wu W., Meng T., Jin Y., Wong J., Prongay A., Girijavallabhan V., Njoroge F.G. Practical and efficient method for amino acid derivatives containing β-quaternary center: Application toward synthesis of hepatitis C virus NS3 serine protease inhibitors. Tetrahedron Lett. 2007;48:6343–6347. doi: 10.1016/j.tetlet.2007.07.002. [DOI] [Google Scholar]
- 21.Zhang L., Sun F., Li Y., Sun X., Liu X., Huang Y., Zhang L.-H., Ye X.-S., Xiao J. Rapid synthesis of iminosugar derivatives for cell-based in situ screening: Discovery of “Hit” compounds with anticancer activity. ChemMedChem. 2007;2:1594–1597. doi: 10.1002/cmdc.200700120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.González J.F., de la Cuesta E., Avendaño C. Atom-efficient synthesis of 2,6-diazacyclophane compounds through alcoholysis/reduction of 3-nitroarylmethylene-2,5-piperazinediones. Tetrahedron. 2008;64:2762–2771. doi: 10.1016/j.tet.2008.01.047. [DOI] [Google Scholar]
- 23.Popp F.D., Piccirilli M. The reaction of N-acetylisatin with amines. J. Heterocycl. Chem. 1971;8:473–475. doi: 10.1002/jhet.5570080319. [DOI] [Google Scholar]
- 24.Obafemi C.A., Taiwo F.O., Iwalewai E.O., Akinpelu D.A. Synthesis, antibacterial and anti-inflammatory activities of some 2-phenylglyoxylic acid derivatives. Int. J. Life Sci. 2013;2:22–36. [Google Scholar]
- 25.Andreani A., Burnelli S., Granaiola M., Leoni A., Locatelli A., Morigi R., Rambaldi M., Varoli L., Cremonini M.A., Placucci G., et al. New isatin derivatives with antioxidant activity. Eur. J. Med. Chem. 2010;45:1374–1378. doi: 10.1016/j.ejmech.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 26.Boechat N., Kover W.B., Bastos M.M., Pinto A.C., Maciel L.C., Mayer L.M.U., da Silva F.S.Q., Sá P.M., Mendonça J.S., Wardella S.M.S.V., et al. N-Acyl-3,3-difluoro-2-oxoindoles as versatile intermediates for the preparation of different 2,2-difluorophenylacetic derivatives. J. Braz. Chem. Soc. 2008;19:445–457. doi: 10.1590/S0103-50532008000300011. [DOI] [Google Scholar]
- 27.Ghazzali M., El-Faham A., Abdel-Megeed A., Al-Farhan K. Microwave-assisted synthesis, structural elucidation and biological assessment of 2-(2-acetamidophenyl)-2-oxo-N phenyl acetamide and N-(2-(2-oxo-2(phenylamino)acetyl)phenyl)propionamide derivatives. J. Mol. Struct. 2012;1013:163–167. [Google Scholar]
- 28.Cheah W.C., Black D.S., Goh W.K., Kumar N. Synthesis of anti-bacterial peptidomimetics derived from N-acylisatins. Tetrahedron Lett. 2008;49:2965–2968. doi: 10.1016/j.tetlet.2008.03.007. [DOI] [Google Scholar]
- 29.Cheah W.C., Wood K., Black D.S., Kumar N. Facile ring-opening of N-acylisatins for the development of novel peptidomimetics. Tetrahedron. 2011;67:7603–7610. doi: 10.1016/j.tet.2011.07.036. [DOI] [Google Scholar]
- 30.Hossain M.M., Islam R.M., Saha S.K., Islam M.K. An efficient microwave-assisted synthesis of dihydropyrazinones and bis-benzoylketones. Tetrahedron Lett. 2010;51:1155–1157. doi: 10.1016/j.tetlet.2009.12.057. [DOI] [Google Scholar]
- 31.Somogy L. Transformation of Isatin 3-Acylhydrazones under acetylating conditions: Synthesis and structure elucidation of 1,5'-Disubstituted 3'-Acetylspiro[oxindole-3,2'-[1,3,4]oxadiazolines] Bull. Chem. Soc. Jpn. 2001;74:873–881. doi: 10.1246/bcsj.74.873. [DOI] [Google Scholar]
- 32.Pandey S.K. Synthesis and evaluation of anti-inflammatory activity of 3-substituted indole derivatives. J. Pharm. Res. 2010;3:2738–2741. [Google Scholar]
- 33.Verma M., Pandeya S.N., Singh K.N., Stables J. Anticonvulsant activity of Schiff bases of isatin derivatives. Acta Pharm. 2004;54:49–56. [PubMed] [Google Scholar]
- 34.Subirós-Funosas R., Prohens R., Barbas R., El-Faham A., Albericio F. Oxyma: An efficient additive for peptide synthesis to replace benzotriazole-based HOBt and HOAt with a lower risk of explosion. Chem. Eur. J. 2009;15:9394–9403. doi: 10.1002/chem.200900614. [DOI] [PubMed] [Google Scholar]
- 35.Subirós-Funosas R., Khattab S.N., Nieto-Rodriguez L., El-Faham A., Albericio F. Oxyma as cyanooxime building block for the construction of versatile reagents assisting acylation reactions in peptide and organic chemistry. Aldrichimica Acta. 2013;40:21–40. [Google Scholar]
- 36.Jad Y.E., Khattab S.N., El-Faham A., Albericio F. Oxime-based carbonates as useful reagents for both N-protection and peptide coupling. Molecules. 2012;17:14361–14376. doi: 10.3390/molecules171214361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khattab S.N., Subirós-Funosas R., El-Faham A., Albericio F. Screening of N-alkyl-cyanoacetamido oximes as substitutes for N-Hydroxysuccinimide. ChemistryOpen. 2012;1:147–152. doi: 10.1002/open.201200012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Subiros-Funosas R., El-Faham A., Albericio F. Use of Oxyma as pH modulatory agent to be used in the prevention of base-driven side reactions and its effect on 2-chlorotrityl chloride resin. Biopolymer. 2012;98:89–97. doi: 10.1002/bip.21713. [DOI] [PubMed] [Google Scholar]
- 39.El-Faham A., Albericio F. Peptide coupling reagents, more than a letter soup. Chem. Rev. 2011;111:6557–6602. doi: 10.1021/cr100048w. [DOI] [PubMed] [Google Scholar]
- 40.Subiros-Funosas R., El-Faham A., Albericio F. Aspartimide formation in peptide chemistry: Occurrence, prevention strategies and the role of N-hydroxylamines. Tetrahedron. 2011;67:8595–8606. doi: 10.1016/j.tet.2011.08.046. [DOI] [Google Scholar]
- 41.El-Faham A., Subiros-Funosas R., Albericio F. A novel family of onium salts based upon isonitroso Meldrum’s acid proves useful as peptide coupling reagents. Eur. J. Org. Chem. 2010;2010:3641–3649. doi: 10.1002/ejoc.201000314. [DOI] [Google Scholar]
- 42.Subiros-Funosas R., Acosta G.A., El-Faham A., Albericio F. Microwave irradiation and COMU: A superior tool for solid phase peptide synthesis. Tetrahedron Lett. 2009;50:6200–6202. doi: 10.1016/j.tetlet.2009.08.117. [DOI] [Google Scholar]
- 43.Kudelko A., Zieliński W. An efficient synthesis of new 2-aminomethyl-1,3,4-oxadiazoles from enantiomeric phenylglycine hydrazides. Tetrahedron. 2009;65:1200–1206. doi: 10.1016/j.tet.2008.11.096. [DOI] [Google Scholar]
- 44.Li J., Sha Y. A convenient synthesis of amino acid methyl esters. Molecules. 2008;13:1111–1119. doi: 10.3390/molecules13051111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.