Abstract
Pseudomonas aeruginosa is a human pathogen associated with a variety of life-threatening nosocomial infections. This organism produces a range of virulence factors which actively cause damage to host tissues. One such virulence factor is pyocyanin, known to play a crucial role in the pathogenesis of P. aeruginosa infections. Previous studies had identified a novel compound capable of strongly inhibiting the production of pyocyanin. It was postulated that this inhibition results from modulation of an intercellular communication system termed quorum sensing, via direct binding of the compound with the LasR protein receptor. This raised the possibility that the compound could be an antagonist of quorum sensing in P. aeruginosa, which could have important implications as this intercellular signaling mechanism is known to regulate many additional facets of P. aeruginosa pathogenicity. However, there was no direct evidence for the binding of the active compound to LasR (or any other targets). Herein we describe the design and synthesis of a biotin-tagged version of the active compound. This could potentially be used as an affinity-based chemical probe to ascertain, in a direct fashion, the active compound’s macromolecular biological targets, and thus better delineate the mechanism by which it reduces the level of pyocyanin production.
Keywords: quorum sensing, Pseudomonas aeruginosa, anti-bacterial, target identification, virulence factor
1. Introduction
Pseudomonas aeruginosa is an opportunist Gram-negative human pathogen responsible for a variety of nosocomical infections and life-threatening diseases in immunocompromised and debilitated patients [1,2]. P. aeruginosa infections are notoriously difficult to eradicate, which has been attributed to the predilection of P. aeruginosa cells to form antibiotic-resistant biofilms, and high levels of intrinsic antibiotic resistance [1,2,3]. Indeed, multi-drug resistance P. aeruginosa nosocomical infections are increasingly being detected across the globe [4]. Thus the exploration of new strategies for tackling infections caused by this notorious pathogen is urgently warranted [1,2,5]. The ability of P. aeruginosa to cause disease is dependent upon the production of agents called ‘virulence factors’ that actively cause damage to host tissues [1,6,7,8]. The targeting of virulence factors (for example, inhibition of their production) has been identified as a potential new therapeutic approach to treating P. aeruginosa infections; in principle, this would attenuate the pathogenicity of the bacterium, increasing the likelihood that the host immune system can clear the infection before too much tissue damage is caused [1,7,9,10,11]. One of the many virulence factors produced by P. aeruginosa is pyocyanin (Figure 1) [12]. There is a large body of evidence that this low molecular weight redox-active phenazine dye is important to the pathogenesis of P. aeruginosa infections [1,5,12,13,14]. Unsurprisingly therefore, the inhibition of pyocyanin production has been identified as a potential antivirulence strategy against this organism [1,5,14,15]. Indeed, there has been much interest in the discovery of compounds with the ability to inhibit pyocyanin biosynthesis in recent years [1,16,17,18,19,20].
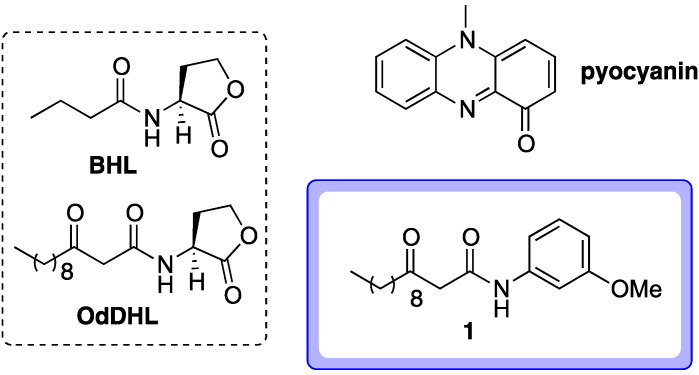
Figure 1.
BHL and OdDHL are two natural AHL autoinducers used by P. aeruginosa in quorum sensing. Pyocyanin is a virulence factor produced by P. aeruginosa. Compound 1, an abiotic OdDHL-mimic, is capable of strongly inhibiting the production of pyocyanin in cultures of wild type P. aeruginosa [1].
Pyocyanin production in P. aeruginosa is regulated by an intercellular signaling process known as quorum sensing [21,22]. Many species of bacteria use quorum sensing systems, which allows for concerted interactions between the cells comprising a population [9]. This communication process is mediated by small diffusible signaling molecules termed autoinducers [9,20,23]. In the majority of Gram-negative species, N-acylated-L-homoserines (AHLs) serve as the autoinducers [20,23]. These are produced by LuxI-type synthase enzymes and bind to cyctoplasmic LuxR-type receptors to initiate the expression of genes associated with bacterial group processes [1,20,23,24,25]. In general, each bacterial species responds specifically to its own unique AHL(s), and uses different LuxI-type synthases and LuxR-type receptors [23,26]. Two AHL-based quorum sensing systems are present in P. aeruginosa. One employs N-butanoyl-L-homoserine lactone (BHL, Figure 1) as the signaling molecule (generated by RhlI and detected by RhlR) and the other uses N-(3-oxododecanoyl)-l-homoserine lactone (OdDHL, Figure 1, generated by LasI with LasR as the cognate receptor). There is a third quorum sensing system in P. aeruginosa which employs a chemically distinct autoinducer (termed the Pseudomonas quinolone signal, PQS). The PQS system is interlinked with the two AHL-based systems, forming an intricate hierarchical quorum sensing network, with the las system generally regarded as standing at the apex [1,23,27,28]. The production of pyocyanin is regulated by RhlR and transcription of the rhlR gene itself is regulated by LasR [1,21]. Thus, inhibitors of LasR would be expected to attenuate the biosynthesis of pyocyanin [1,20,23,29,30,31]. The structure of OdDHL, the natural LasR agonist, has often been used as a template to guide the design and synthesis of abiotic LasR antagonists [1,11,16,20,23]. We recently reported the synthesis of OdDHL analogues containing non-native head groups in place of the natural homoserine lactone moiety [1]. These compounds were evaluated for their ability to inhibit the production of pyocyanin in cultures of wild type P. aeruginosa, with 1 (Figure 1) found to be the most potent (note that compound 1 was not screened in any LasR-based reporter systems).
Given that 1 is closely related in structure to OdDHL (which is known to interact with the LasR receptor) and the fact that pyocyanin production is generally considered to be regulated by LasR-based quorum sensing, it was postulated that 1 reduces the level of pyocyanin production by disrupting OdDHL-dependent activation of LasR [1]. Experimental evidence suggested that 1 is capable of binding to LasR and it was inferred that 1 might be an antagonist of the LasR receptor and an inhibitor of LasR-based quorum sensing in P. aeruginosa. This could have important implications; quorum sensing is known to regulate many additional facets of P. aeruginosa pathogenicity [15,32,33,34] and there is tremendous interest in finding small molecules that can disrupt AHL-mediated signaling in this organism [9,20,23]. However, our previous studies did not provide any direct evidence for an interaction between compound 1 and the LasR receptor, or indeed any other molecular targets. We were therefore interested in examining the molecular basis for the activity of 1 in more detail. Such information should assist in the design of next-generation agents with improved potency. Towards this end, we envisaged the design and synthesis of an affinity-based (“pull down”) chemical probe incorporating 1. This could potentially be employed in affinity-based (“pull down”) proteomic assays in order to directly detect the biological target(s) of 1 and thus better delineate the mechanism by which it reduces the level of pycocyanin production in P. aeruginosa [35,36,37,38,39,40,41].
2. Results and Discussion
2.1. Probe Design
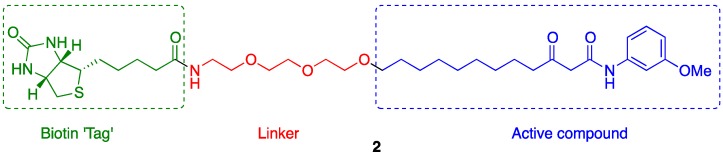
Typically, affinity-based probes are composed of the biologically active molecule of interest tethered via a chemical linker to an insoluble support [38]. Usually, the probe is then incubated with the cell lysate of the relevant organism [42]. The small molecule’s macromolecular targets can then be extracted by virtue of specific binding; washing steps are used to remove non-binding proteins, and the remaining high affinity binders can be eluted from the support, separated using polyacrylaminde gel electrophoresis and identified using various mass spectrometry techniques [35,36,37,42]. Biotin is often used as an equivalent of an insoluble support (“tag”) in affinity probes, since immobilization on streptavidin beads (either before or after incubation with the biological system) is possible by virtue of the strong non-covalent biotin-streptavidin interaction [35,37,42]. Indeed, biotinylated probes have been widely used for the identification of many small molecule biological targets [38]. An advantage of biotinylated probes over solid-phase supports in that they are often cell permeable. Thus in addition to carrying out experiments using cell lysates, it is also possible for such probes to be incubated with live cells and interact with target protein(s) in their native environment inside a living cell or organism [37]. After cell lysis the probe can be pulled out of solution with streptavidin resin, which will also pull out any bound protein(s) [37]. Based on these considerations, we targeted the synthesis of 2 (Figure 2), a biotinylated affinity probe that could potentially be used for detecting the molecular targets of the active compound 1.
Figure 2.
The target biotinylated affinity probe.
It was decided to use a polyethylene glycol (PEG)-based chain as the linker. PEG chains are commonly employed in this regard; they are usually long enough to mitigate undesired steric interactions steric hindrance between the support and small molecule-biomolecule interactions [38] and flexible enough to allow the target molecule to adopt multiple orientations in three-dimensions (and so access a favorable macromolecular binding pose). Furthermore, PEG linkers are also hydrophilic, increasing the solubility of molecules in aqueous solution [38]. A crucial consideration when preparing an affinity-based probe is where on the molecule of interest the linker should be introduced, as it is important that its’ biological activity is not affected significantly [38]. Our previous studies [1] indicated that further substitution of the aromatic ring portion would not be appropriate, as the nature of the aromatic head group was found to have a profound effect upon the ability of OdDHL-mimics of the type of 1 to inhibit pyocyanin production (with strongest inhibition associated with the meta-methoxy aromatic ring of 1). There exists a subtle interplay between the structural and electronic properties of the aromatic ring group governing compound activity, meaning that the impact of further substitution could not be reliably predicted. Furthermore, evidence suggests that the natural 3-oxo-dodecanoyl tail group of OdDHL is important for the inhibition of pyocyanin production by compounds which mimic the structure of AHLs. We therefore decided to retain the dicarbonyl unit and the nine-carbon alkyl chain, and attach the linker at the end of the alkyl chain.
2.2. Probe Synthesis
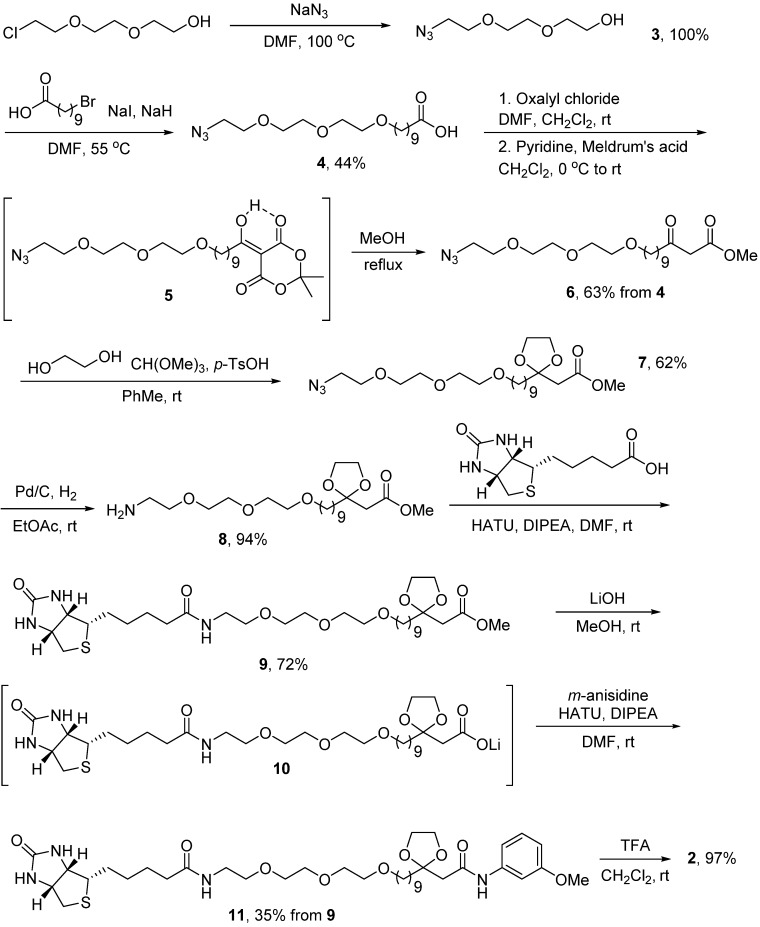
The synthesis of compound 2 (Scheme 1) began with the reaction of 2-(2-(2-chloroethoxy)ethoxy)-ethanol and sodium azide, which furnished azide 3 in quantitative yield. The desired nine-carbon alkyl chain was installed by reaction deprotonation of the hydroxyl group and treatment with 10-bromodecanoic acid.
Scheme 1.
The synthesis of biotin-tagged affinity probe 2. rt = room temperature.
The resulting acid 4 was converted to the corresponding acid chloride and reacted with Meldrum’s acid to generate adduct 5. Subsequent treatment of this crude material with methanol yielded β-ketoester 6. Acetal protection to form 7 was followed by reduction of the azide group to yield 8. Subsequent HATU-mediated coupling with D-biotin proceeded smoothly to generate the protected ester 9. Hydrolysis using lithium hydroxide provided the carboxylate 10. HATU-mediated coupling of 10 with m-anisidine furnished the protected precursor 11 in reasonable yield. Finally, acid-catalyzed deprotection provided the desired compound 2.
Typical affinity-based pull down assays involve the use of one-dimensional (1D) gel electrophoresis to separate proteins binding to the bioactive molecule under investigation. However, this method can suffer from a lack of sensitivity. We have previously proposed a strategy to address this issue based around combining biotin-mediated affinity capture from cell lysates with 2D difference gel electrophoresis (DIGE) [42]. This method of electrophoresis should allow a greater sensitivity compared to 1D techniques and thus facilitate the identification of weak binding-protein targets [42]. Conceivably, such an approach could be used with the affinity probe 2 in order to elucidate molecular targets for the anti-pyocyanin compound 1.
3. Experimental
3.1. General
Reactions were performed using oven-dried glassware under an atmosphere of nitrogen with anhydrous, freshly distilled solvents unless otherwise stated. Dichloromethane, ethyl acetate, methanol, n-hexane, acetonitrile and toluene were distilled from calcium hydride. Diethyl ether was distilled over a mixture of lithium aluminium hydride and calcium hydride. Petroleum ether was distilled before use and refers to the fraction between 40–60 °C. All other reagents were used as obtained from commercial sources. Room temperature (rt) refers to ambient temperature. Temperatures of 0 °C were maintained using an ice-water bath. Reaction times are given either in hours (h) or minutes (min) or overnight (approximately 12 h) Yields refer to chromatographically and spectroscopically pure compounds unless otherwise stated. All flash chromatography was carried out using slurry-packed Merck 9325 Keiselgel 60 silica gel. Where possible, reactions were monitored by thin layer chromatography (TLC) performed on commercially prepared glass plates pre-coated with Merck silica gel 60 F254 or aluminium oxide 60 F254. Visualisation was by the quenching of UV fluorescence (νmax = 254 nm) or by staining with potassium permanganate. Infrared spectra were recorded neat or as a solution in the designated solvent on a Perkin-Elmer Spectrum One spectrometer with internal referencing. Selected absorption maxima (νmax) are reported in wavenumbers (cm−1). Proton magnetic resonance spectra were recorded using an internal deuterium lock at ambient probe temperatures (unless otherwise stated) on the following instruments: Bruker DPX-400 (400 MHz), Bruker Avance 400 QNP (400 MHz) Bruker Avance 500 BB ATM (500 MHz) and Bruker Avance 500 Cryo Ultrashield (500 MHz). Chemical shifts (δH) are quoted in ppm, to the nearest 0.01 ppm, and are referenced to the residual non-deuterated solvent peak. Coupling constants (J) are reported in Hertz. Data are reported as follows: chemical shift, integration, multiplicity [br, broad; s, singlet; d, doublet; t, triplet; q, quartet; quint, quintet; sext, sextet; sept, septet; m, multiplet; or as a combination of these (e.g., dd, dt, etc.)], coupling constant(s) and assignment. Proton assignments were determined either on the basis of unambiguous chemical shift or coupling pattern, by patterns observed in 2D experiments (1H-1H COSY, HMBC and HMQC) or by analogy to fully interpreted spectra for related compounds. Carbon magnetic resonance spectra were recorded by broadband proton spin decoupling at ambient probe temperatures (unless otherwise stated) using an internal deuterium lock on the following instruments: Bruker DPX-400 (100 MHz), Bruker Avance 400 QNP (100 MHz) and Bruker Avance 500 BB ATM (125 MHz) and Bruker Avance 500 Cryo Ultrashield (125 MHz). Chemical shifts (δC) are quoted in ppm, to the nearest 0.1 ppm, and are referenced to the residual non-deuterated solvent peak. Assignments were supported by DEPT editing and determined either on the basis of unambiguous chemical shift or coupling pattern, by patterns observed in 2D experiments (HMBC and HMQC) or by analogy to fully interpreted spectra for related compounds. The ionisation technique used was electron ionisation (EI). High resolution mass spectroscopy measurements were recorded in-house using a Waters LCT Premier Mass Spectrometer or a Micromass Quadrapole-Time of Flight (Q-ToF) spectrometer. Mass values are reported within the error limits of ±5 ppm mass units. The ionisation technique used was electrospray ionization (ESI). Optical rotations were measured in MeOH on a Perkin Elmer 343 Polarimeter; [α]D values are reported in 10−1 degrees cm2 g−1 at 589 nm.
3.2. Experimental Procedures
2-(2-(2-Azidoethoxy)ethoxy)ethanol (3). To a solution of NaN3 (2.06 g, 32.7 mmol, 1.4 eq.) in DMF (10 mL) was added 2-(2-(2-chloroethoxy)ethoxy)ethanol (4 g, 23.8 mmol 1 eq.). The reaction mixture was stirred at 100 °C for 5 h and evaporated to dryness. The resulting residue was partitioned between CH2Cl2 (20 mL) and water (20 mL). The aqueous phase was extracted twice more with CH2Cl2 (2 × 50 mL), the organic layers combined, dried over MgSO4 and evaporated to dryness to give 3 as a pale yellow oil (4.21 g, 24 mmol, 100%) which was used without further purification. Rf 0.16 (70% EtOAc/bp 40–60 petroleum ether); νmax (neat)/cm−1: 3400 (broad, O-H), 2869 (C-H), 2099 (N3); 1H-NMR (400 MHz, CDCl3) δH 3.73 (2H, t, J = 5.5 Hz, CH2OH), 3.69-3.65 (6H, m, 3 × CH2O), 3.61 (2H, t, J = 4.6 Hz, CH2O), 3.30 (2H, t, J = 5.0 Hz, CH2N3), 2.36 (1H, t, J = 6.2 Hz, CH2OH); 13C-NMR (101 MHz, CDCl3) δC 72.6 (CH2O), 70.7 (CH2O), 70.5 (CH2O), 70.2 (CH2O), 61.9 (CH2OH), 50.8 (CH2N3).
10-(2-(2-(2-Azidoethoxy)ethoxy)ethoxy)decanoic acid (4). A solution of bromodecanoic acid (909 mg, 5.2 mmol, 1 eq.), NaI (78 mg, 0.52 mmol, 0.1 eq.) and compound 3 (1.3 g, 5.2 mmol, 1 eq.) in dry DMF (10 mL) was cooled to 0 °C and NaH (60% dispersion in mineral oil, 520 mg, 13 mmol, 2.5 eq.) was added piecewise. After 20 min the reaction mixture was heated to 55 °C and stirred overnight forming an orange gel. The solvent was removed under reduced pressure and the residue was partitioned between HClaq (100 mL) and Et2O (100 mL). The aqueous phase was extracted with Et2O (2 × 100 mL), the combined organic layers dried over MgSO4 and evaporated to dryness. The resulting residue was purified by column chromatography (stepwise gradient, 0%–40% Et2O/bp 40–60 petroleum ether with 1% acetic acid) to yield 4 (0.789 g, 1.78 mmol, 44%) as a colourless oil. Rf: 0.34 (40% EtOAc/bp 40–60 petroleum ether, 1% acetic acid); νmax (neat)/cm−1: 3400 (O-H), 2927 (C-H), 2856 (C-H), 2100 (N3), 1708 (C=O); 1H-NMR (500 MHz, CDCl3) δH 3.70–3.64 (8H, m, 4 × OCH2), 3.59 (2H, app dd, J = 6.0, 3.8 Hz, OCH2), 3.45 (2H, t, J = 6.8 Hz, OCH2), 3.39 (2H, t, J = 5.1 Hz, CH2N3), 2.34 (2H, t, J = 7.5 Hz, HOOCCH2), 1.80–1.50 (4H, m, HOOCCH2CH2 and CH2CH2CH2O), 1.31 (10H, d, J = 18.8 Hz, 5 × CH2); 13C-NMR (126 MHz, CDCl3) δC 178.3 (HOOCCH2) 71.7 (CH2O), 70.9 (CH2O), 70.9 (CH2O), 70.8 (CH2O), 70.2 (CH2O), 70.2(CH2O), 50.9 (CH2N3), 33.9 (HOOCCH2), 29.7 (CH2), 29.5 (CH2), 29.4 (CH2), 29.2 (CH2), 29.1 (CH2), 26.2 (CH2), 24.8 (HOOCCH2CH2); HRMS (ESI+) m/z found [M+H]+ 346.2354, [C16H32N3O5]+ calculated 345.2342.
Methyl 12-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)-3-oxododecanoate (6) To a solution of compound 4 (242 mg, 0.70 mmol, 1 eq) in dry CH2Cl2 (1.5 mL) at rt was added oxalyl chloride (100 μL, 1.19 mmol, 1.7 eq.) and DMF (10 μL). After stirring at rt for 1 h TLC indicated complete turnover (small samples of the reaction mixture were quenched with MeOH), the solvent was removed under reduced pressure and the acid chloride was used without further purification. A solution of Meldrum’s acid (101 mg, 0.7 mmol, 1 eq.) in dry CH2Cl2 (1.6 mL) was cooled to 0 °C and pyridine (114 μL, 1.4 mmol, 2 eq.) was added dropwise over 20 min. The acid chloride in CH2Cl2 (1 mL) was then added and the mixture was stirred at 0 °C for a further 2 h. The reaction mixture was allowed to warm to rt diluted with CH2Cl2 (10 mL) and poured into ice HCl (2N, 15 mL). The organic layer was washed with NaClaq (25 mL), dried over MgSO4 and evaporated to dryness. The resultant crude product material was dissolved in methanol (2.5 mL) and heated to reflux with stirring for 5 h. The solvent was removed under reduced pressure and the resulting residue was purified by column chromatography (40% Et2O/bp 40–60 petroleum ether) to yield 6 (176 mg, 0.416 mmol, 63%) as a pale yellow oil. Rf 0.22 (40% Et2O/bp 40–60 petroleum ether); νmax (neat)/cm−1: 2925 (C-H), 2857 (C-H), 2101 (N3), 1747 (C=O), 1717 (C=O); 1H-NMR (400 MHz, CDCl3) δH 3.74 (3H, s, CH3), 3.70–3.62 (8H, m, 4 × OCH2), 3.60–3.55 (2H, m, OCH2CH2N3), 3.45 (2H, t, J = 6.77 Hz, CH2CH2CH2O), 3.44 (2H, s, COCH2CO) 3.39 (2H, t, J = 5.10 Hz, CH2N3), 2.52 (2H, t, J = 7.37 Hz, COCH2CH2), 1.65–1.51 (4H, app. m (also H2O), COCH2CH2CH2 and CH2CH2CH2O), 1.39–1.23 (10H, m, 5 × CH2); 13C-NMR (101 MHz, CDCl3) δC 203.0 (CH2COCH2), 167.8 (H3CCO), 71.6 (CH2CH2CH2O), 70.9 (CH2O), 70.9 (CH2O), 70.8 (CH2O), 70.2 (CH2O), 70.2 (CH2O), 52.5 (H3C), 50.8 (COCH2CO), 49.2 (CH2N3), 43.2 (COCH2CH2), 29.7 (CH2), 29.5 (CH2), 29.5 (CH2), 29.4 (CH2), 29.1 (CH2), 26.2 (CH2), 23.6 (COCH2CH2CH2); HRMS (ESI+) m/z found [M+H]+ 424.2411 [C19H35N3O623Na1]+ calculated 424.2418.
Methyl 2-(2-(9-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)nonyl)-1,3-dioxolan-2-yl)acetate (7). To a solution of the β keto ester 6 (100 mg, 0.24 mmol, 1 eq.) in toulene (5 mL) at rt was added tosic acid (12.2 mg, 0.1 eq.), trimethylorthoformate (0.21 mL, 1.2 mmol, 5 eq.) and ethylene glycol (0.119 mL, 2.13 mmol, 8.9 eq.) sequentially with stirring. The reaction was left to stir overnight at rt. The toluene was removed under reduced pressure and the resulting reside was disolved in CH2Cl2 (10 mL), washed with saturated aqueous sodium bicarbonate solution (3 × 10 mL), dried over MgSO4 and evaporated to dryness. The resulting crude product was purrified by column chromatography (stepwise gradient, 10%–40% Et2O/bp 40-60 petroleum ether) to yield 7 (69 mg, 0.148 mmol, 62%) as a colourless oil. Rf: 0.42 (70% Et2O/bp 40-60 petroleum ether); νmax (neat)/cm−1: 2924 (C-H), 2855 (C-H), 2103 (N3), 1738 (C=O); 1H-NMR (500 MHz, CDCl3) δH 4.01–3.94 (4H, m, OCH2CH2O (actetal)), 3.69 (3H, s, OCH3), 3.68–3.65 (8H, m, 4 × CH2O), 3.58 (2H, app dd, J = 3.60, 5.90 Hz, OCH2), 3.45 (2H, t, J = 6.82 Hz, CH2CH2CH2O), 3.39 (2H, t, J = 5.12 Hz, CH2N3), 2.66 (2H, s, CH3OOCCH2), 1.85–1.74 (2 H, app. M, CCH2), 1.55 (2H, dt, J = 22.0, 11.2 Hz, CH2CH2CH2O), 1.45–1.18 (12H, m, 6 × CH2); 13C (126 MHz, CDCl3) δC 170.2 (CH3OOC), 109.6 (CH3OOCCH2CO), 71.7 (CH2CH2CH2O), 70.9 (OCH2), 70.9 (OCH2), 70.8 (OCH2), 70.2 (OCH2), 70.2 (OCH2), 65.3 (OCH2CH2O acetal), 51.9 (CH3O), 50.9 (CH2N3), 42.6 (CH3OOCCH2), 37.9 (CCH2), 29.8 (CH2), 29.8 (CH2), 29.6 (CH2), 29.6 (CH2), 29.6 (CH2), 26.2 (CH2), 23.6 (CCH2CH2). HRMS (ESI+) m/z found [M+H]+ 468.2675 [C21H39N3O723Na1]+ calculated 468.2680.
Methyl 2-(2-(9-(2-(2-(2-aminoethoxy)ethoxy)ethoxy)nonyl)-1,3-dioxolan-2-yl)acetate (8) To a solution of compound 7 (62.0 mg, 0.133 mmol, 1 eq.) in degassed EtOAc (2 mL) under an inert N2 atmosphere was added Pd (5% on carbon, 7.8 mg, 0.0037 mmol, 0.028 eq.). The reaction vessel was then purged with H2 before being stirred under an atmosphere of H2 for overnight at rt. The reaction mixture was filtered through celite (washed with EtOAc) and evaporated to dryness to give compound 8 (52.2 mg, 0.124 mmol, 94%) as a yellow oil which was used without further purification. νmax (neat)/cm−1: 3373 (N-H), 2925 (C-H), 2855 (C-H), 1738 (C=O), 1739 (C=O), 1672; 1H-NMR (500 MHz, CDCl3) δH 4.02–3.93 (4H, m, OCH2CH2O (acetal)), 3.69 (3H, s, OCH3), 3.68–3.53 (10H, m, 5 × CH2O), 3.45 (2H, t, J = 6.84 Hz, CH2CH2CH2O), 2.91 (2H, dt, J = 10.57, 5.16 Hz, CH2NH2), 2.66 (2H, s, CH3OOCCH2), 2.36 (2H, br s, CH2NH2), 1.81–1.72 (2 H, app. m, CCH2), 1.55 (2H, m, CH2CH2CH2O), 1.41–1.23 (12H, m, 6 × CH2); 13C (126 MHz, CDCl3) δC 170.2 (CH3OOC), 109.6 (CH3OOCH2C), 72.4 (OCH2CH2NH2), 71.7 (CH2CH2CH2O), 70.8 (OCH2), 70.7 (OCH2), 70.4 (OCH2), 70.2 (OCH2), 65.3 (OCH2CH2O acetal), 51.9 (CH3O), 42.6 (CH3OOCCH2), 41.5 (CH2NH2), 37.9 (CCH2), 29.8 (CH2), 29.7 (CH2), 29.6 (CH2), 29.6 (CH2), 29.6 (CH2), 26.2 (CH2), 23.6 (CCH2CH2); HRMS (ESI+) m/z found [M+H]+ 420.2947 [C21H42N3O7]+ calculated 420.2956.
Methyl 2-(2-(5-oxo-1-((3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)-9,12,15-trioxa-6-azatetracosan-24-yl)-1,3-dioxolan-2-yl)acetate (9) To a solution of D-biotin (31.2 mg, 0.128 mmol, 1.16 eq.) in DIPEA (1.77 mL) at rt was added HATU (41.7 mg, 0.110 mmol, 1 eq.) with stirring. The solution was stirred for a further 15 min before the addition of compound 8 (46 mg, 0.110 mmol, 1 eq.). This was stirred at rt overnight. The reaction was quenched by the addition of MeOH (1 mL), the solvent removed under reduced pressure and the resulting residue purified by column chromatography (stepwise gradient, 1%–10% MeOH/CH2Cl2) to give 9 (50.9 mg, 0.079 mmol, 72%) as a sticky pink syrup. Rf: 0.21 (5% MeOH/CH2Cl2); νmax (neat)/cm−1: 3294 (N-H amide), 2926 (C-H), 2854 (C-H), 1739 (C=O), 1700 (C=O), 1645 (C=O); 1H-NMR (500 MHz, CDCl3) δH 6.56 (1H, br s, OCH2CH2NHCO), 5.99 (1H, br s, NHCONH), 5.17 (1H, br s, NHCONH), 4.49–4.43 (1H, m, CONHCHCH2S), 4.30–4.23 (1H, m, NHCHCHS), 3.94–3.86 (4H, m, OCH2CH2O acetal), 3.62 (3H, s, CH3O), 3.59–3.54 (6H, m, 3 × OCH2), 3.53–3.47 (4H, m, OCH2 and OCH2CH2NH), 3.37 (4H, m, CH2CH2CH2O and OCH2CH2NH), 3.09 (1H, dd, J = 11.10, 6.89 Hz, NHCHCHS), 2.85 (1H, dd, J = 12.75, 4.47 Hz, CONHCHCHHS), 2.85 (1H, d, J = 12.77 Hz, CONHCHCHHS), 2.59 (2H, s, CH3OOCCH2), 2.22–2.11 (2H, m, NHCOCH2CH2CH2), 1.74–1.68 (2H, app. m, CCH2), 1.67–1.54 (4H, m, NHCOCH2CH2CH2), 1.50 (2H, dt, J = 13.94, 6.83 Hz, CH2CH2CH2CH2O), 1.38 (2H, dt, J = 14.93, 7.52 Hz, NHCOCH2CH2CH2), 1.34–1.27 (2H, m, CCH2CH2), 1.27–1.16 (10H, m, 5 × CH2); 13C-NMR (126 MHz, CDCl3) δC 173.4 (CH2NHCOCH2), 170.2 (CH3OOC), 163.6 (NHCONH), 109.6 (CH2CCH2), 71.7 (CH2CH2CH2O), 70.7, (OCH2), 70.6 (OCH2), 70.3 (OCH2), 70.2 (OCH2), 70.0 (OCH2), 65.3 (OCH2CH2O acetal), 62.0 (NHCHCHS), 60.4 (NHCHCH2S), 55.4 (NHCHCHS), 51.9 (CH3O), 42.6 (COCH2C), 40.7 (NHCHCH2S), 39.4 (OCH2CH2NH), 37.9 (COCH2CCH2), 35.9 (NHCOCH2CH2CH2), 29.8 (CH2), 29.8 (CH2), 29.7 (CH2), 29.7 (CH2), 29.6 (CH2), 28.2 (CH2), 28.1 (CH2), 26.2 (CH2), 25.6 (CH2), 23.6 (CCH2CH2); HRMS (ESI+) m/z found [M+H]+ 646.3278 [C31H56N3O9S]+ calculated 646.3732; +28 (c = 0.917 mmol in MeOH).
Lithium 2-(2-(5-oxo-1-((3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)-9,12,15-trioxa-6-azatetracosan-24-yl)-1,3-dioxolan-2-yl)acetate (10) Compound 9 (48 mg, 0.074 mmol, 1 eq.) was dissolved in 66% aqueous MeOH (5 mL), lithium hydroxide monohydrate (21 mg, 0.5 mmol, 6.8 eq.) was added and the reaction stirred overnight at rt. The solvent was removed under reduced pressure to give a mixture of compound 10 and LiOH (69 mg) which was used without further purification. νmax (neat)/cm−1: 3277 (N-H amide), 2924 (C-H), 2854 (C-H), 1686 (C=O), 1593 (C=O); 1H-NMR (500 MHz, CD3OD) δH 4.46 (1H, ddd, J = 7.85, 4.94, 0.73 Hz, NHCHCH2S), 4.27 (1H, dd, J = 7.88, 4.47 Hz, NHCHCHS)), 4.03–3.80 (4H, m, OCH2CH2O acetal), 3.65–3.57 (6H, m, 3 × OCH2), 3.55 (2H, m, OCH2), 3.51 (2H, t, J = 5.46 Hz, OCH2CH2NH), 3.44 (2H, t, J = 6.65 Hz, CH2CH2CH2O), 3.33 (2H, t, J = 5.33 Hz, OCH2CH2NH), 3.20–3.14 (1H, m, NHCHCHS), 2.90 (1H, dd, J = 12.75, 5.00 Hz, CONHCHCHHS), 2.67 (1H, d, J = 12.72 Hz, CONHCHCHHS), 2.43 (2H, s, CH3OOCCH2), 2.19 (2H, t, J = 7.40 Hz, NHCOCH2CH2CH2), 1.82–1.76 (2H, app. m, CCH2), 1.75–1.49 (6H, m, 3 × CH2), 1.45–1.34 (m, 4H, 2 × CH2), 1.34–1.19 (10H, m, 5 × CH2); 13C-NMR (126 MHz, CD3OD) δC 178.3 (CO), 176.2 (CO), 166.1 (NHCONH), 111.6 (CH2CCH2), 72.4 (CH2CH2CH2O), 71.6 (OCH2), 71.6 (OCH2), 71.3 (OCH2), 71.1 (OCH2), 70.6 (OCH2), 65.8 (OCH2CH2O acetal), 63.4 (NHCHCHS), 61.6 (NHCHCH2S), 57.0 (NHCHCHS), 47.0 (COCH2CO), 41.1 (NHCHCH2S), 40.4 (OCH2CH2NH), 38.7 (COCH2CCH2), 36.7 (NHCOCH2CH2CH2), 31.0 (CH2), 30.7 (CH2), 30.7 (CH2), 30.7 (CH2), 30.6 (CH2), 29.8 (CH2), 29.5 (CH2), 27.2 (CH2), 26.9 (CH2), 24.6 (CCH2CH2); HRMS (ESI+) m/z found [M+H]+ 632.3584 [C30H59N3O9S]+ calculated 632.3575.
N-(2-(2-(2-((9-(2-(2-((3-Methoxyphenyl)amino)-2-oxoethyl)-1,3-dioxolan-2-yl)nonyl)oxy)ethoxy)ethoxy)ethyl)-5-((3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)pentanamide (11) To a solution of m-anisidine (16 µL, 0.142 mmol, 2 eq.) in a solution of DIPEA (12.2 µL) and DMF (1 mL) at rt was added HATU (27 mg, 0.071 mmol, 1 eq.) with stirring. The solution was stirred for a further 15 min before the addition of the crude compound 10 (65.3 mg, ≈0.071 mmol, 1 eq.). This was stirred at rt overnight. The reaction was quenched by the addition of MeOH (1 mL), the solvent removed under reduced pressure and the resulting residue purified by column chromatography (stepwise gradient, 1%–7% MeOH/CH2Cl2) to give 11 (18.4 mg, 0.025 mmol, 35% over two steps) as a sticky pale orange residue. Rf: 0.5 (10% MeOH/CH2Cl2); νmax (neat)/cm−1: 3647 (N-H amide) 3437 (N-H amide) 2926 (C-H), 2853 (C-H), 1662 (C=O), 1609 (C=O), 1544 (C=O); 1H-NMR (400 MHz, CD3OD) δH 9.53 (1H, br s, CNHCO), 7.30 (1H, dd, J = 4.27, 2.09 Hz, CH3OCCHCNH), 7.23 (1H, t, J = 8.15 Hz, CCHCHCHC), 7.08 (1H, ddd, J = 8.07, 1.76, 0.76 Hz, CCHCHCHC), 6.70 (1H, ddd, J = 8.28, 2.48, 0.73 Hz, CCHCHCHC), 4.52 (1H, dd, J = 7.90, 4.33 Hz, NHCHCH2S), 4.33 (1H, dd, J = 7.90, 4.40 Hz, NHCHCHS), 4.14–3.88 (4H, m, OCH2CH2O acetal), 3.81 (3H, s, CH3O), 3.71–3.59 (8H, m, 4 × OCH2), 3.57 (2H, t, J = 5.45 Hz, OCH2CH2NH), 3.50 (2H, t, J = 6.63 Hz, CH2CH2CH2O), 3.39 (2H, t, J = 5.41 Hz, OCH2CH2NH), 3.22 (1H, dt, J = 27.40, 11.44 Hz, NHCHCHS), 2.96 (1H, dt, J = 12.74, 4.17 Hz, CONHCHCHHS), 2.73 (1H, d, J = 13.8 Hz, CONHCHCHHS), 2.71 (2H, s, NHCOCH2CO), 2.25 (2H, t, J = 7.38 Hz, NHCOCH2CH2CH2), 1.84–1.54 (8H, m, 4 × CH2), 1.54-1.42 (4H, m, 2 × CH2), 1.43–1.28 (10H, m, 5 × CH2); 13C-NMR (126 MHz, CD3OD) δC 176.1 (CO), 170.7 (CO), 167.6 (NHCONH), 166.1 (CH3OC), 140.9 (CHCNH), 130.5 (CH-Ar), 117.0 (COCH2C), 113.4 (CH-Ar), 110.7 (CH-Ar), 107.1 (CH-Ar), 72.4 (CH2CH2CH2O), 71.6 (OCH2), 71.6 (OCH2), 71.3 (OCH2), 71.1 (OCH2), 70.6 (OCH2), 64.3 (OCH2CH2O acetal), 63.4 (NHCHCHS), 61.7 (NHCHCH2S), 57.0 (NHCHCHS), 55.7 (CH3O), 43.9 (NCOCH2CO), 41.0 (CONHCHCH2S), 40.4 (OCH2CH2NH), 39.1 (COCH2CCH2), 36.7 (NHCOCH2CH2CH2), 30.7 (CH2), 30.5 (CH2), 30.5 (CH2), 30.5 (CH2), 30.4 (CH2), 29.8 (CH2), 29.5 (CH2), 27.2 (CH2), 26.9 (CH2), 24.5 (CCH2CH2); HRMS (ESI+) m/z found [M+H]+ 737.4138 [C37H61N4O9S]+ calculated 737.4154; +27 (c = 2.183 mmol in MeOH).
N-(3-Methoxyphenyl)-3-oxo-12-(2-(2-(2-(5-((3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)pentanamido)ethoxy)ethoxy)ethoxy)dodecanamide (2) Compound 11 (11.0 mg, 0.015 mmol, 1 eq.) was dissolved in CH2Cl2 (0.5 mL) with TFA (160 µL, 2.09 mmol, 139 eq.) in a flask open to the air and stirred for 3 h at rt. The solvent was removed under reduced pressure and the resulting residue purified by column chromatography (stepwise gradient, 1%–7% MeOH/CH2Cl2) to yield the final compound 2 (10.1 mg, 0.146 mmol, 97%) as a colourless sticky residue. Rf: 0.51 (10% MeOH/CH2Cl2); νmax (neat)/cm−1: 3641 (N-H amide) 3294 (N-H amide) 2926 (C-H), 2853 (C-H), 1682 (C=O), 1629 (C=O), 16411 (C=O), 1544 (C=O); 1H-NMR (500 MHz, CD3OD) δH 7.30 (1H, t, J = 2.20 Hz, CH3OCHCNH), 7.22 (1H, t, J = 8.16 Hz, CCHCHCHC), 7.07 (1H, ddd, J = 8.04, 1.91, 0.85 Hz, CCHCHCHC), 6.70 (1H, ddd, J = 8.27, 2.49, 0.79 Hz, CCHCHCHC), 4.54–4.46 (1H, m, CONHCHCH2S), 4.32 (1H, dd, J = 7.86, 4.50 Hz, NHCHCHS), 3.80 (3H, s, CH3O), 3.70–3.58 (8H, m, 4 × CH2O), 3.56 (2H, t, J = 5.5 Hz, OCH2CH2NH2), 3.52–3.44 (2H, m,CH2CH2CH2O), 3.42–3.36 (m, 4H, OCH2CH2NH and COCH2CO), 3.24–3.16 (1H, m, NHCHCHS), 2.94 (1H, dt, J = 12.76, 4.97 Hz, CONHCHCHHS), 2.72 (1H, d, J = 12.72 Hz, CONHCHCHHS), 2.63 (2H, t, J = 7.28 Hz, COCH2COCH2), 2.24 (2H, t, J = 7.35 Hz, NHCOCH2CH2), 1.82–1.52 (8H, m, 4 × CH2), 1.53–1.10 (14H, m, 7 × CH2); 13C-NMR (126 MHz, CD3OD) δC 206.8 (NHCOCH2CO), 176.1 (CNHCOCH2CO) 167.6 (OCH2CH2NHCO), 166.1 (NHCONH), 161.6 (CH3OC), 140.7 (CHCNH), 130.6 (CH Ar), 113.31 (CH Ar), 110.9 (CH Ar), 107.0 (CH Ar), 72.4 (CH2CH2CH2O), 71.6 (OCH2), 71.6 (OCH2), 71.3 (OCH2), 71.1 (OCH2), 70.6 (OCH2), 63.4 (NHCHCHS), 61.6 (NHCHCH2S), 57.0 (NHCHCHS), 55.7 (CH3O), 49.9 (COCH2CO) 43.9 (COCH2COCH2), 41.5 (CONHCHCH2S), 40.4 (OCH2CH2NH), 36.7 (NHCOCH2CH2CH2) 30.7 (CH2), 30.5 (CH2), 30.5 (CH2), 30.4 (CH2), 30.10 (CH2), 29.8 (CH2), 29.5 (CH2), 27.2 (CH2), 26.9 (CH2), 24.5 (COCH2CH2); HRMS (ESI+) m/z found [M+H]+ 693.11 [C35H57N4O8S]+ calculated 693.3892; +28 (c = 2.183 mmol in MeOH).
4. Conclusions
We have reported the efficient synthesis of 2, a biotin-tagged derivative of a potent inhibitor 1 of the production of the virulence factor pyocyanin in the pathogenic bacterium P. aeruginosa. Compound 2 could potentially be used as a chemical probe in affinity “pull-down” assays in order to identify the biological target(s) of 1 and thus help to elucidate the mechanism by which it exerts this biological effect. Such information should prove useful in the design of more potent compounds. If 2 is found to bind to LasR, this would strongly suggest that 1 is a direct antagonist of quorum sensing in P. aeruginosa and, as such, it would represent a valuable molecular tool for the study and manipulation of this signaling pathway. The binding studies are currently in progress and the results of these investigations will be reported in due course [43].
Acknowledgments
The authors thank Bayer Schering and GSK, the EU, EPSRC, BBSRC, MRC and Wellcome Trust for funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 1–11 are available from the authors.
References and Notes
- 1.Morkunas B., Galloway W.R.J.D., Wright M., Ibbeson B.M., Hodgkinson J.T., O'Connell K.M., Bartolucci N., Valle M.D., Welch M., Spring D.R. Inhibition of the production of the Pseudomonas aeruginosa virulence factor pyocyanin in wild-type cells by quorum sensing autoinducer-mimics. Org. Biomol. Chem. 2012;10:8452–8464. doi: 10.1039/c2ob26501j. [DOI] [PubMed] [Google Scholar]
- 2.Mesaros N., Nordmann P., Plesiat P., Roussel-Delvallez M., Van Eldere J., Glupczynski Y., van Laethem Y., Jacobs F., Lebecque P., Malfroot A., et al. Pseudomonas aeruginosa: Resistance and therapeutic options at the turn of the new millennium. Clin. Microbiol. Infect. 2007;13:560–578. doi: 10.1111/j.1469-0691.2007.01681.x. [DOI] [PubMed] [Google Scholar]
- 3.Antunes L.C.M., Ferreira R.B.R., Buckner M.M.C., Finlay B.B. Quorum sensing in bacterial virulence. Microbiology. 2010;156:2271–2282. doi: 10.1099/mic.0.038794-0. [DOI] [PubMed] [Google Scholar]
- 4.Zaborina O., Kohler J.E., Wang Y., Bethel C., Shevchenko O., Wu L., Turner J.R., Alverdy J.C. Identification of multi-drug resistant Pseudomonas aeruginosa clinical isolates that are highly disruptive to the intestinal epithelial barrier. Ann. Clin. Microbiol. Antimicrob. 2006;5:14. doi: 10.1186/1476-0711-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kipnis E., Sawa T., Wiener-Kronish J. Targeting mechanisms of Pseudomonas aeruginosa pathogenesis. Med. Maladies Infect. 2006;36:78–91. doi: 10.1016/j.medmal.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Strateva T., Mitov I. Contribution of an arsenal of virulence factors to pathogenesis of Pseudomonas aeruginosa infections. Ann. Microbiol. 2011;61:717–732. doi: 10.1007/s13213-011-0273-y. [DOI] [Google Scholar]
- 7.Clatworthy A.E., Pierson E., Hung D.T. Targeting virulence: A new paradigm for antimicrobial therapy. Nat. Chem. Biol. 2007;3:541–548. doi: 10.1038/nchembio.2007.24. [DOI] [PubMed] [Google Scholar]
- 8.Barczak A.K., Hung D.T. Productive steps toward an antimicrobial targeting virulence. Curr. Opin. Microbiol. 2009;12:490–496. doi: 10.1016/j.mib.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galloway W.R.J.D., Hodgkinson J.T., Bovvden S., Welch M., Spring D.R. Applications of small molecule activators and inhibitors of quorum sensing in Gram-negative bacteria. Trends Microbiol. 2012;20:449–458. doi: 10.1016/j.tim.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Deep A., Chaudhary U., Gupta V. Quorum sensing and Bacterial Pathogenicity: From Molecules to Disease. J. Lab. Physicians. 2011;3:4–11. doi: 10.4103/0974-2727.78553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitehead N.A., Barnard A.M.L., Slater H., Simpson N.J.L., Salmond G.P.C. Quorum-sensing in gram-negative bacteria. FEMS Microbiol. Rev. 2001;25:365–404. doi: 10.1111/j.1574-6976.2001.tb00583.x. [DOI] [PubMed] [Google Scholar]
- 12.O'Malley Y.Q., Reszka K.J., Spitz D.R., Denning G.M., Britigan B.E. Pseudomonas aeruginosa pyocyanin directly oxidizes glutathione and decreases its levels in airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Phys. 2004;287:L94–L103. doi: 10.1152/ajplung.00025.2004. [DOI] [PubMed] [Google Scholar]
- 13.Lau G.W., Ran H.M., Kong F.S., Hassett D.J., Mavrodi D. Pseudomonas aeruginosa pyocyanin is critical for lung infection in mice. Infect. Immun. 2004;72:4275–4278. doi: 10.1128/IAI.72.7.4275-4278.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lau G.W., Hassett D.J., Ran H., Kong F. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol. Med. 2004;10:599–606. doi: 10.1016/j.molmed.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Garner A.L., Struss A.K., Fullagar J.L., Argawal A., Moreno A.Y., Cohen S.M., Janda K.D. 3-Hydroxy-1-alkyl-2-methylpyridine-4(1H)-thiones: Inhibition of the Pseudomonas aeruginosa Virulence Factor LasB. ACS Med. Chem. Lett. 2012;3:668–672. doi: 10.1021/ml300128f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodgkinson J.T., Galloway W.R.J.D., Wright M., Mati I.K., Nicholson R.L., Welch M., Spring D.R. Design, synthesis and biological evaluation of non-natural modulators of quorum sensing in Pseudomonas aeruginosa. Org. Biomol. Chem. 2012;10:6032–6044. doi: 10.1039/c2ob25198a. [DOI] [PubMed] [Google Scholar]
- 17.Chong Y.M., Yin W.F., Ho C.Y., Mustafa M.R., Hadi A.H., Awang K., Narrima P., Koh C.L., Appleton D.R., Chan K.G. Malabaricone C from Myristica cinnamomea exhibits anti-quorum sensing activity. J. Nat. Prod. 2011;74:2261–2264. doi: 10.1021/np100872k. [DOI] [PubMed] [Google Scholar]
- 18.Krishnan T., Yin W.F., Chan K.G. Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa PAO1 by Ayurveda spice clove (Syzygium Aromaticum) Bud Extract. Sensors. 2012;12:4016–4030. doi: 10.3390/s120404016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan L.Y., Yin W.F., Chan K.G. Silencing quorum sensing through extracts of melicope lunu-ankenda. Sensors. 2012;12:4339–4351. doi: 10.3390/s120404339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geske G.D., O'Neill J.C., Blackwell H.E. Expanding dialogues: From natural autoinducers to non-natural analogues that modulate quorum sensing in Gram-negative bacteria. Chem. Soc. Rev. 2008;37:1432–1447. doi: 10.1039/b703021p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dekimpe V., Deziel E. Revisiting the quorum-sensing hierarchy in Pseudomonas aeruginosa: The transcriptional regulator RhlR regulates LasR-specific factors. Microbiology. 2009;155:712–723. doi: 10.1099/mic.0.022764-0. [DOI] [PubMed] [Google Scholar]
- 22.Pukatzki S., Kessin R.H., Mekalanos J.J. The human pathogen Pseudomonas aeruginosa utilizes conserved virulence pathways to infect the social amoeba Dictyostelium discoideum. Proc. Natl. Acad. Sci. USA. 2002;99:3159–3164. doi: 10.1073/pnas.052704399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galloway W.R.J.D., Hodgkinson J.T., Bowden S.D., Welch M., Spring D.R. Quorum sensing in gram-negative bacteria: Small-molecule modulation of AHL and Al-2 quorum sensing pathways. Chem. Rev. 2011;111:28–67. doi: 10.1021/cr100109t. [DOI] [PubMed] [Google Scholar]
- 24.Stevens A.M., Queneau Y., Soulere L., von Bodman S., Doutheau A. Mechanisms and synthetic modulators of AHL-dependent gene regulation. Chem. Rev. 2011;111:4–27. doi: 10.1021/cr100064s. [DOI] [PubMed] [Google Scholar]
- 25.Ng W.L., Bassler B.L. Bacterial quorum-sensing network architectures. Annu. Rev. Genet. 2009;43:197–222. doi: 10.1146/annurev-genet-102108-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McInnis C.E., Blackwell H.E. Design, synthesis, and biological evaluation of abiotic, non-lactone modulators of LuxR-type quorum sensing. Bioorg. Med. Chem. 2011;19:4812–4819. doi: 10.1016/j.bmc.2011.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams P., Winzer K., Chan W.C., Camara M. Look who’s talking: Communication and quorum sensing in the bacterial world. Philos. Trans. R. Soc. B Biol. Sci. 2007;362:1119–1134. doi: 10.1098/rstb.2007.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hodgkinson J.T., Galloway W.R.J.D., Saraf S., Baxendale I.R., Ley S.V., Ladlow M., Welch M., Spring D.R. Microwave and flow syntheses of Pseudomonas quinolone signal (PQS) and analogues. Org. Biomol. Chem. 2011;9:57–61. doi: 10.1039/c0ob00652a. [DOI] [PubMed] [Google Scholar]
- 29.Taha M.O., Al-Bakri A.G., Zalloum W.A. Discovery of potent inhibitors of pseudomonal quorum sensing via pharmacophore modeling and in silico screening. Bioorg. Med. Chem. Lett. 2006;16:5902–5906. doi: 10.1016/j.bmcl.2006.08.069. [DOI] [PubMed] [Google Scholar]
- 30.Smith K.M., Bu Y.G., Suga H. Library screening for synthetic agonists and antagonists of a Pseudomonas aeruginosa autoinducer. Chem. Biol. 2003;10:563–571. doi: 10.1016/S1074-5521(03)00107-8. [DOI] [PubMed] [Google Scholar]
- 31.Ishida T., Ikeda T., Takiguchi N., Kuroda A., Ohtake H., Kato J. Inhibition of quorum sensing in Pseudomonas aeruginosa by N-acyl cyclopentylamides. Appl. Environ. Microbiol. 2007;73:3183–3188. doi: 10.1128/AEM.02233-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Popat R., Crusz S.A., Diggle S.P. The social behaviours of bacterial pathogens. Br. Med. Bull. 2008;87:63–75. doi: 10.1093/bmb/ldn030. [DOI] [PubMed] [Google Scholar]
- 33.Skindersoe M.E., Alhede M., Phipps R., Yang L., Jensen P.O., Rasmussen T.B., Bjarnsholt T., Tolker-Nielsen T., Hoiby N., Givskov M. Effects of antibiotics on quorum sensing in pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2008;52:3648–3663. doi: 10.1128/AAC.01230-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brint J.M., Ohman D.E. Synthesis of multiple exoproducts in Pseudomonas-Aeruginosa is under the control of RhlR-RhlI, another set of regulators in strain pao1 with homology to the autoinducer-responsive LuxR-LuxI family. J. Bacteriol. 1995;177:7155–7163. doi: 10.1128/jb.177.24.7155-7163.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ziegler S., Pries V., Hedberg C., Waldmann H. Target identification for small bioactive molecules: Finding the needle in the haystack. Angew. Chem. Int. Ed. 2013;52:2744–2792. doi: 10.1002/anie.201208749. [DOI] [PubMed] [Google Scholar]
- 36.Praneenararat T., Palmer A.G., Blackwell H.E. Chemical methods to interrogate bacterial quorum sensing pathways. Org. Biomol. Chem. 2012;10:8189–8199. doi: 10.1039/c2ob26353j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leslie B.J., Hergenrother P.J. Identification of the cellular targets of bioactive small organic molecules using affinity reagents. Chem. Soc. Rev. 2008;37:1347–1360. doi: 10.1039/b702942j. [DOI] [PubMed] [Google Scholar]
- 38.Matsuyama A., Yashiroda Y., Yoshida M. Chemical Proteomics: A Global Study of Protein-Small Molecule Interactions. In: Fu H., editor. Chemical Genomics. Cambridge University Press; Cambridge, UK: 2012. p. 26. Chapter 3. [Google Scholar]
- 39.Praneenararat T., Beary T.M.J., Breitbach A.S., Blackwell H.E. Synthesis and application of an N-acylated L-homoserine lactone derivatized affinity matrix for thte isolation of quourm sensing signal receptors. Bioorg. Med. Chem. Lett. 2011:5054–5057. doi: 10.1016/j.bmcl.2011.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seabra R., Brown A., Hooi D., Kerkhoff C., Chhabra S.R., Harty C., Williams P., Pritchard D.I. A eukaryotic binding protein for the immune modulatory bacterial quorum sensing molecule N-(3-oxododecanoyl)-L-homoserine lactone. Calcium Bind. Proteins. 2008;3:31–37. [Google Scholar]
- 41.Karlsson T., Turkina M.V., Yakymenko O., Magnusson K., Vikström E. The Pseudomona aeruginosa N-acylhomoserine lactone quorum sensing molecules target IQGAP1 and modulate epithelial cell migrations. PLoS Pathog. 2012;8:e1002953. doi: 10.1371/journal.ppat.1002953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spandl R.J., Nicholson R.L., Marsden D.M., Hodgkinson J.T., Su X.B., Thomas G.L., Salmond G.P.C., Welch M., Spring D.R. Synthesis of a biotin-labeled quorum-sensing molecule: Towards a general method for target identification. Synlett. 2008;2008:2122–2126. doi: 10.1055/s-2008-1077978. [DOI] [Google Scholar]
- 43.Most successful applications of affinity probes involve active parent compounds with nanomolar activities. The active compound 1 can block pyocyanin production by approximately 75% at mid-micromolar levels (approximately 50 μM, see reference 1). This was also the concentration at which this compound was tested in a LasR ligand binding domain production assay, which provided indirect evidence that 1 can directly bind to LasR (see reference 16). Given this activity level, the identification of targets for compound 1 using affinity probe 2 is likely to be quite a challenging (though not implausible) endeavor.