Abstract
Herein, we report the design, synthesis and trypanocidal activity of some novel trisubstituted imidazole derivatives. These heterocyclic derivatives were structurally planned by exploring the concept of molecular hybridisation between two arylhydrazones derived from megazol, which has potent trypanocidal activity. The trypanocidal activity of these triarylimidazole derivatives was evaluated against infective trypomastigote forms of T. cruzi and the derivative 2'-(4-bromophenyl)-1-methyl-5'-phenyl-1H,3'H-2,4'-biimidazol-3'-ol showed moderate biological activity (IC50 = 23.9 µM) when compared to benznidazole, a standard trypanocidal drug. These compounds did not present cytotoxic effects at concentrations near the trypanocidal IC50, being considered a good starting point for the development of new anti-Chagas drug candidates.
Keywords: 2,4,5-triaryl-N-hydroxyimidazole; Trypanosoma cruzi; Chagas disease; trypanocidal activity
1. Introduction
Chagas disease, also known as American trypanosomiasis or South American trypanosomiasis, is a protozoan disease caused by the haemoflagellate parasite Trypanosoma cruzi [1]. It is a chronic and debilitating parasitic infection that affects millions of people in Mexico, Central America, and South America. Approximately 25% of the population of Latin America is at risk for acquiring the infection [2]. Currently, the available drug for the clinical treatment of Chagas disease is the nitroheterocyclic drug benznidazole [3]. This drug is effective against the circulating form of the parasite (trypomastigotes) in the acute phase of the disease, but its efficacy during the chronic stage is debatable [4].
Megazol [1-methyl-2-(5-amino-1,3,4-thiadiazole)-5-nitroimidazole] is a nitroheterocyclic derivative shown to be highly active against T. cruzi in vitro and in vivo, including strains that are resistant to benznidazole [5,6,7]; thus, it has become a core structure for the design of new drugs for the treatment of Chagas disease. Megazol has been described as a scavenger of trypanothione, the cofactor for trypanothione reductase [8,9]. Despite its noteworthy trypanocidal activity, megazol development was discontinued due to reports of its in vitro mutagenic and genotoxic effects [10,11,12]. To circumvent this undesirable profile, there have been numerous efforts to obtain megazol analogues [13,14,15,16,17].
The imidazole ring is commonly found in highly significant endogenous biomolecules including biotin, the essential amino acid histidine and the autacoid histamine [18]. Several bioactive compounds with this heterocyclic unit have valuable pharmacological properties such as antiparasitic [19], antifungal [20], antimicrobial [21,22] and antidepressant [23] activity, among others. In this context, 2,4,5-triarylimidazole compounds have gained remarkable importance due to their widespread biological activities and their applicability in synthetic organic chemistry. Moreover, N-hydroxyimidazoles have been reported to possess fungicidal and bacteriostatic activities [24].
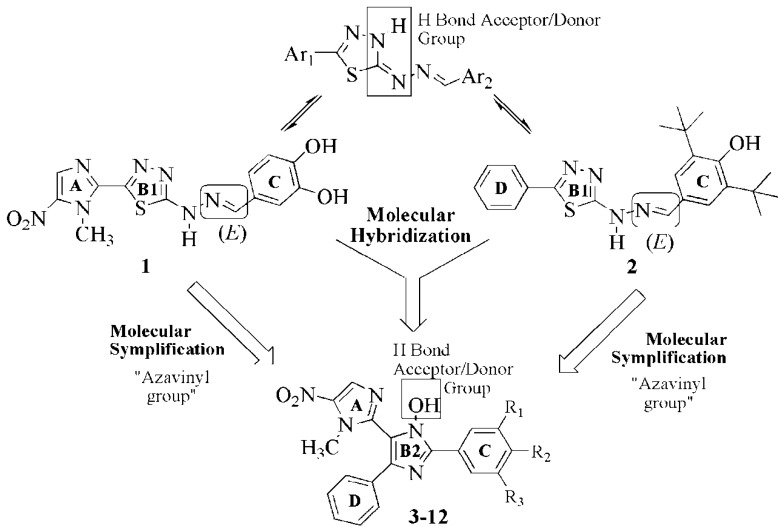
In our continuous effort to develop potent trypanocidal compounds, we decided to construct a new class of 2,4,5-triaryl-N-hydroxyimidazole (TAI) derivatives 3–12 based on the molecular hybridisation [25] of 1,3,4-thiadiazole prototypes 1 and 2 (Figure 1) [14]. In the design concept, the nitroimidazole moiety (A) was preserved due to the pharmacophoric contribution of this group to the mechanism of action against T. cruzi [14]. The 1,3,4-thiadiazole group (B1) of 1 and 2 was isosterically substituted by an imidazole ring (B2) containing a hydroxyl group that mimics the proton donor/accepting behaviour of the tautomeric N-H bond of the hydrazone group attached to B1 (Figure 1).
Figure 1.
Design concept of the new triaryl-N-hydroxyimidazole derivatives 3–12.
2. Results and Discussion
2.1. Chemistry
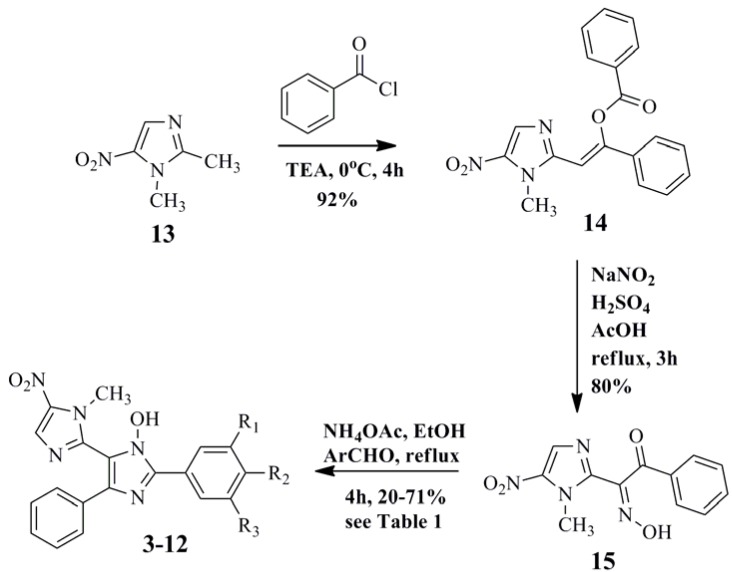
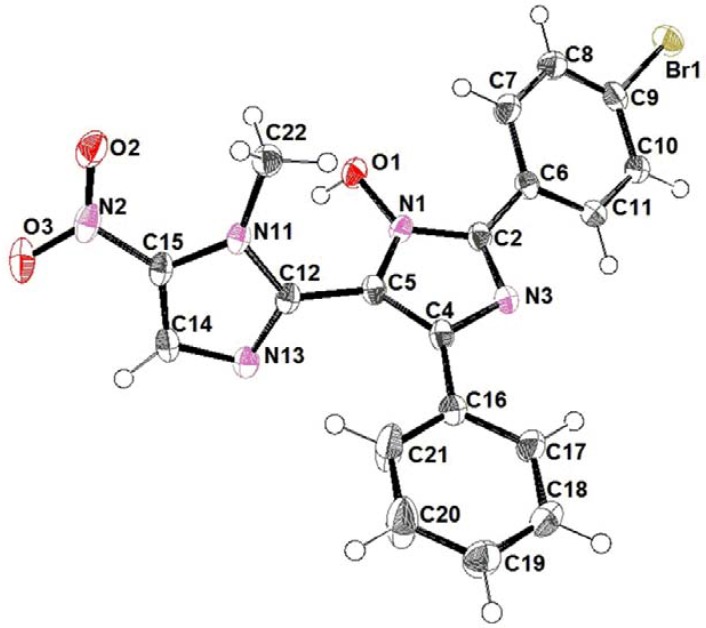
The synthetic route used for the preparation of the title compounds 3–12 is outlined in Scheme 1. 1,2-Dimethyl-5-nitro-1H-imidazole (13) was converted into the corresponding phenylvinyl benzoate 14 in 92% yield through its base-catalysed condensation with benzoyl chloride [26]. Next, the nitrosation of 14 furnished the key ketoxime intermediate 15 in 80% yield [26]. Finally, the desired TAI derivatives 3–12 were obtained, in yields varying from 20–70%, after condensation of 15 with the corresponding benzaldehydes in the presence of ammonium acetate [27]. The 1H- and 13C-NMR spectra and mass spectra of the synthesised compounds 3–12 were consistent with the proposed structures, which was corroborated by X-ray crystallography of the p-bromo-TAI derivative 6 (CCDC 928948 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html) as illustrated in Figure 2.
Scheme 1.
Synthesis of the new triaryl-N-hydroxyimidazole derivatives 3–12
Figure 2.
Atom arrangements for 2'-(4-bromophenyl)-1-methyl-5-nitro-5'-phenyl-1N,3'N-2,4'-biimidazol-3'-ol (6).
2.2. Trypanocidal Activity
The trypanocide profiles of the new TAI derivatives 3–12 was evaluated in vitro using bloodstream trypomastigote forms of T. cruzi (Y strain) isolated from infected Swiss mice and are summarised in Table 1.
Table 1.
Physical and biological properties of 2,4,5-triaryl-N-hydroxyimidazole derivatives 3–12.
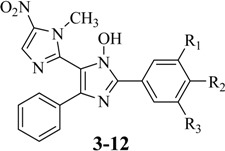
| Cpn | R1 | R2 | R3 | Molecular Formula | M.W. | Yield (%) | M.P. (°C) | IC50 (µM) a | log P values b |
|---|---|---|---|---|---|---|---|---|---|
| 3 | H | H | H | C19H15N5O3 | 361.35 | 69 | 217–218 | 108.2 ± 5.65 | 2.92 ± 0.98 |
| 4 | H | F | H | C19H14FN5O3 | 379.38 | 48 | 248–249 | 67.8 ± 8.39 | 3.14 ± 1.02 |
| 5 | H | Cl | H | C19H14ClN5O3 | 395.80 | 20 | 249–250 | 34.6 ± 2.43 | 3.69 ± 0.99 |
| 6 | H | Br | H | C19H14BrN5O3 | 440.25 | 69 | 218–219 | 23.9 ± 4.88 | 3.86 ± 1.02 |
| 7 | H | NO2 | H | C19H14N6O5 | 406.35 | 62 | 361–362 | 241.6 ± 37.6 | 2.88 ± 0.99 |
| 8 | H | OCH3 | H | C20H17N5O4 | 391.38 | 62 | 218–219 | 191.8 ± 25.3 | 3.09 ± 0.99 |
| 9 | H | OH | H | C19H15N5O4 | 377.35 | 71 | 145–146 | 294.1 ± 27.98 | 2.56 ± 0.99 |
| 10 | OH | OH | H | C20H15N5O5 | 393.35 | 42 | 224–225 | 360.5 ± 24.67 | 2.34 ± 0.99 |
| 11 | OH | OCH3 | H | C20H17FN5O5 | 407.38 | 45 | 213–214 | 199.9 ± 2.02 | 2.53 ± 1.00 |
| 12 | OCH3 | OH | NO2 | C20H16N6O7 | 452.38 | 43 | 245–247 | 241.8 ± 7.54 | 3.22 ± 1.01 |
| Bzn | - | - | - | - | - | - | - | 10.8 ± 0.4 | 0.91 ± 1.00 |
a Mean ± standard deviation of at least four separate experiments, performed with trypomastigote forms of T. cruzi; b Theoretical values calculated using the program ACDLABS.
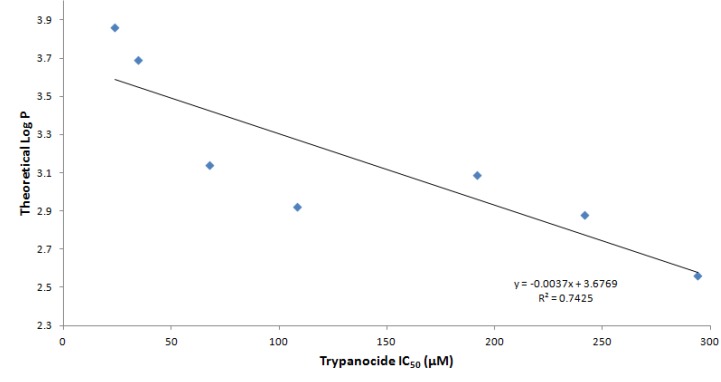
The screening of the TAI derivatives 3–12 showed that compound 6, with a p-bromophenyl group attached to the core imidazole ring, was the most active, with an IC50 = 23.9 µM, which is slightly inferior to that displayed by the standard drug benznidazole (IC50 = 10.8 µM). The superior trypanocidal profile of 6, compared with the other monosubstituted TAI analogues 3–5 and 7–9, could be explained by the remarkable hydrophobic contribution of its bromo group (Hantzsch’s π = 0.86), increasing the log P of this compound to 3.86 (Table 1). We did not find a clear correlation among the stereoelectronic character of para-substituents in TAI compounds 3–9 and their trypanocidal activity. In fact, the introduction of para-substituents from different halogens, independently of any electron-withdrawing or electron-releasing properties, prominently reduced the trypanocidal profile when compared with an unsubstituted TAI derivative 3. Nevertheless, we have found a good correlation (r2 = 0.74) between theoretical log P value and the trypanocide profile of monosubstituted TAI derivatives 3–9, indicating the importance of their relative lipophilicity for the displayed biological activity (Figure 3).
Figure 3.
Correlation between the theoretical log P value of TAI derivatives 3–9 and their corresponding trypanocidal activity (see Table 1).
Di- and tri-substituted TAI derivatives 10–12 have shown poor trypanocidal activity, distinct from the corresponding brazilizone derivatives, e.g., 1 [14,15].
The cellular viability in the presence of most active TAI derivatives 4–6 was determined by microplate Alamar Blue assay (Invitrogen) at three different concentrations, 100, 10 and 1 μM. The results displayed in Table 2 were expressed in percentage cell viability. Excepting the p-bromo TAI derivative 6, that weakly reduced cell viability at 100 μM, the other tested compounds were not cytotoxic to the host cells at concentrations near the IC50 for trypanocidal action (Table 1).
Table 2.
Data of cytotoxic effects of 2,4,5-triaryl-N-hydroxyimidazole derivatives (4–6) on murine macrophages cells 21 h after the treatment.
| TAI Compound | % Cell Viability/concentration (μM) | ||
|---|---|---|---|
| 100 | 10 | 1 | |
| 4 | 97.6 | 98.9 | 94.2 |
| 5 | 93.1 | 97.6 | 95.7 |
| 6 | 78.7 | 97.6 | 97.3 |
3. Experimental
3.1. General Procedures
Melting points were determined on a Buchi apparatus and are uncorrected. Infrared spectra were recorded on a Thermo Nicolet Nexus 670 spectrometer in potassium bromide pellets and frequencies are expressed in cm−1. 1H-NMR spectra were recorded at room temperature on Bruker Avance 500 and Bruker Avance 400 spectrometers operating at 500/125 and 400/100 MHz (1H/13C), respectively. Chemical shifts are reported in ppm (δ) downfield from tetramethylsilane, which was used as an internal standard. Low resolution mass spectra (MS) were obtained by electron-spray ionisation in a micromass ZQ 4000. Microanalysis data were obtained using a Perkin–Elmer 240 analyser, using a Perkin–Elmer AD-4 balance. The progress of all reactions was monitored by TLC, which was performed on 2.0 X 6.0 cm aluminium sheets that were precoated with silica gel 60 (HF-254, Merck) to a thickness of 0.25 mm. The developed chromatograms were viewed under ultraviolet light (254–265 nm).
3.2. Procedure for the Synthesis of (Z)-2-(1-Methyl-5-nitro-1H-imidazol-2-yl)-1-phenylvinyl benzoate (14) [26]
In a 250 mL flask, cooled in an ice bath, acetone (34 mL) and 1,2-dimethyl-5-nitroimidazole (13, 10 g, 70.85 mmol) were added while stirring. Next, triethylamine (50 mL, 70.85 mmol) and then benzoyl chloride (24.4 mL, 210.0 mmol, 3 equiv.) were added slowly. The temperature was adjusted to 20 °C, and acetone (46 mL) was then added. The reaction was stirred for 4 hours. Next, water (40 mL) was added, and the suspension was filtered on a Buchner funnel and washed with acetone, affording 25 g (92% yield) of 14. Light green solid; mp 245–275 °C; 1H-NMR (500 MHz, DMSO-d6) δ 4.05 (s, 3H, 5-NO2-imidazole-N-CH3), 7.28 (s, 1H, 5-NO2-imidazole-CH=C-), 7.49 (m, 3H, Ar(C3)H, Ar(C4)H and Ar(C5)H), 7.61 (t, 2H, benzoate(C3)H and benzoate(C5)H), 7.75 (t, 1H, benzoate(C4)H), 7.83 (m, 2H, Ar(C2)H and Ar(C6)H), 7.99 (s, 1H, 5-NO2-imidazole-(C4)H), 8.13 (d, 2H, benzoate(C2)H and benzoate(C6)H): 13C-NMR (125 MHz, DMSO-d6) δ 33.16 (5-NO2-imidazole-N-CH3), 101.90 (5-NO2-imidazole-(C2)-CH=C-), 125.39 (benzoate(C3)H and benzoate(C5)H), 128.49 (benzoate(C1)), 128.97 (Ar(C3)H and Ar(C5)H), 130.00 (Ar(C2)H and Ar(C6)H), 130.29 (benzoate(C2)H and benzoate(C6)H), 132.59 (Ar(C1)), 133.74 (5-NO2-imidazole-(C4)H and Ar(C4)H), 133.90 benzoate-(C4)H), 138.76 (5-NO2-imidazole-(C2)CH=C-), 146.44 (5-NO2-imidazole-(C5)NO2), 152.24 (5-NO2-imidazole-(C2)CH=C-), 163.71(Ar-COO-),: MS (ESI) m/z: 372.0 (M+.[+Na]) (100%). Anal. Calcd. for C19H15N3O4: C: 65.32; H: 4.33; N: 12.03. Found: C: 65.31; H: 4.33; N: 12.03.
3.3. Procedure for the Synthesis of (1E)-1-(1-Methyl-5-nitro-1H-imidazol-2-yl)-2-phenylethane-1,2-dione 1-oxime (15) [26]
In small portions, NaNO2 (3.82 g, 55.36 mmol) were added to a 250 mL flask that was seeded with H2SO4 (56.6 mL) and cooled in an ice bath while stirring. After 30 min, glacial acetic acid (20.6 mL) and then phenylvinyl benzoate 14 (10.0 g, 28.62 mmol) were added. The reaction was maintained at 65 °C for 3 h and the temperature was then adjusted to 20 °C. Next, water (166 mL) was added, and this mixture was subsequently filtered in a Buchner funnel. Compound 15 was suspended in ethanol and filtered through a Buchner funnel, resulting in a yellow solid with a yield of 80%. mp 180–182 °C; 1H-NMR (500 MHz, DMSO-d6) δ 3.78 (s, 3H, 5-NO2-imidazole-N-CH3), 7.58 (m, 2H, Ar(C3)H and Ar(C5)H), 7.71 (t, 1H, Ar(C4)H), 7.98 (m, 2H, Ar(C2)H and Ar(C6)H), 8.21 (s, 1H, 5-NO2-imidazole-(C4)H), 13.85 (s, 1H, C=N-OH): 13C-NMR (125 MHz, DMSO-d6) δ 34.51 (5-NO2-imidazole-N-CH3), 128.47 (Ar(C3)H and Ar(C5)H), 130.16 (Ar(C2)H and Ar(C6)H), 132.24 (5-NO2-imidazole-(C4)H), 133.53 (Ar(C4)H), 135.79 (5-NO2-imidazole-(C5)NO2), 139.45 (5-NO2-imidazole-(C2)-C=N-OH), 142.46 (Ar(C1)), 146.04 (C=N-OH), 189.07 (C=O): MS (ESI) m/z: 273.1 (100%). Anal. Calcd. for C12H10N4O4: C: 52.56; H: 3.68; N: 20.43. Found: C: 52.54; H: 3.68; N: 20.42.
3.4. General Procedure for the Synthesis of 2,4,5-Trisubstituted Imidazole Derivatives (3–12) [27]
In a 100 mL flask, ketoxime 15 (0.5 g, 1.82 mmol, 1 eq.), NH4OAc (0.850 g, 11.02 mmol, 6 eq.), the corresponding benzaldehyde (1.82 mmol, 1 eq.) and ethanol (12 mL) were added while stirring. The temperature was maintained at reflux for 5 h. The reaction was cooled 20 °C and then placed on crushed ice. The precipitate was filtered on a Buchner funnel and washed with water. All new 2,4,5-triaryl-N-hydroxyimidazole derivatives 3–12 were purified by recrystallization from a 9:1 solution of ethanol/water.
1-Methyl-5-nitro-2',5'-diphenyl-1H,3'H-2,4'-biimidazol-3'-ol (3). Yellow solid; mp 217–218 °C; yield 69%; 1H-NMR (500 MHz, DMSO-d6) δ 3.72 (5-NO2-imidazole-N-CH3), 7.28 (t, 1H, Ar(C4)H), 7.34 (t, 2H, Ar(C3)H and Ar(C5)H), 7.47 (t, 1H, Ar(C4’)H), 7.54 (t, 2H, Ar(C3’)H and Ar(C5’)H), 7.57 (d, 2H, Ar(C2)H and Ar(C6)H), 8.16 (d, 2H, Ar(C2’)H and Ar(C6’)H), 8.35 (s, 1H, 5-NO2-imidazole-(C4)H), 12.48 (s, 1H, N-OH-imidazole-N-OH): 13C-NMR (125 MHz, DMSO-d6) δ 34.57 (5-NO2-imidazole-N-CH3), 115.87 (N-OH-imidazole-C4), 125.89 (Ar(C2)H and Ar(C6)H), 127.39 (Ar(C2’)H and Ar(C6’)H), 127.71 (Ar(C4)H), 128.42 (Ar(C1’)), 128.61 (Ar(C3)H and Ar(C5)H), 128.70 (Ar(C3’)H and Ar(C5’)H), 129.33 (Ar(C4’)H), 132.75 (5-NO2-imidazole-(C4)H), 133.10 (N-OH-imidazole-C2), 137.56 (Ar(C1)), 139.97 (5-NO2-imidazole-(C2)), 141.28 (5-NO2-imidazole-(C5)NO2), 142.25 (N-OH-imidazole-C5): 13C-NMR DEPT (100 MHz, DMSO-d6)δ 34.57 (5-NO2-imidazole-N-CH3), 125.89 (Ar(C2)H and Ar(C6)H), 127.39 (Ar(C2’)H and Ar(C6’)H), 127.71 (Ar(C4)H), 128.61 (Ar(C3)H and Ar(C5)H), 128.70 (Ar(C3’)H and Ar(C5’)H), 129.33 (Ar(C4’)H), 132.75 (5-NO2-imidazole-(C4)H): IR (KBr) νmax cm−1: 3284 (ν N-O-H), 3130–3064 (ν C-H(aromatic), 1526–1470 (ν C=C(aromatic), 1368 (ν N=O2): MS (ESI) m/z: 360.5 (100%). Anal. Calcd. for C19H15N5O3: C: 63.15; H: 4.18; N: 19.38. Found: C: 63.17; H: 4.18; N: 19.40.
2'-(4-Fluorophenyl)-1-methyl-5-nitro-5'-phenyl-1H,3'H-2,4'-biimidazol-3'-ol (4). Yellow solid; mp 148–149 °C; yield 48%; 1H-NMR (400 MHz, DMSO-d6) δ 3.87 (5-NO2-imidazole-N-CH3), 7.27 (t, 1H, Ar(C4)H), 7.34 (t, 2H, Ar(C3’)H and Ar(C5’)H), 7.39 (d, 2H, Ar(C2’)H and Ar(C6’)H), 7.56 (d, 2H, Ar(C2)H and Ar(C6)H), 8.19 (t, 2H, Ar(C3’)H and Ar(C5’)H), 8.34 (s, 1H, 5-NO2-imidazole-(C4)H), 12.49 (s, 1H, N-OH-imidazole-N-OH): 13C-NMR (100 MHz, DMSO-d6) δ 34.51 (5-NO2-imidazole-N-CH3), 115.72 (d, J = 21.8 Hz, Ar(C3’)H and Ar(C5’)H), 124.95 (N-OH-imidazole-(C4)), 125.85 (Ar(C2)H and Ar(C6)H), 127.70 (Ar(C4)H), 128.57 (Ar(C3)H and Ar(C5)H), 129.63 (d, J = 8.7 Hz, Ar(C2’)H and Ar(C6’)H), 132.70 (5-NO2-imidazole-(C4)H), 132.97 (N-OH-imidazole-(C2)), 137.49 (Ar(C1)), 139.95 (5-NO2-imidazole-(C2)), 141.17 (5-NO2-imidazole-(C5)NO2), 141.42 (N-OH-imidazole-(C5)), 162.52 (d, J = 245.8 Hz, Ar(C4’)F): IR (KBr) νmax cm−1: 3284 (ν N-O-H), 3130–3066 (ν C-H(aromatic), 1534–1468 (ν C=C(aromatic), 1365 (ν N=O2), 1225 (ν C-F): MS (ESI) m/z: 378.2 (100%). Anal. Calcd. for C19H14FN5O3: C: 60.16; H: 3.72; N: 18.46. Found: C: 60.18; H: 3.72; N: 18.47.
2'-(4-Chlorophenyl)-1-methyl-5-nitro-5'-phenyl-1H,3'H-2,4'-biimidazol-3'-ol (5). green solid; mp 149–250 °C; yield 20%; 1H-NMR (500 MHz, DMSO-d6) δ 3.71 (5-NO2-imidazole-N-CH3), 7.27 (t, 1H, Ar(C4)H), 7.33 (t, 2H, Ar(C3)H and Ar(C5)H), 7.55 (d, 2H, J = 7.5 Hz, Ar(C2)H and Ar(C6)H), 7.60 (d, 2H, J = 7.5 Hz, Ar(C2’)H and Ar(C6’)H), 8.17 (d, 2H, J = 7.5 Hz, Ar(C3’)H and Ar(C5’)H), 8.33 (s, 1H, 5-NO2-imidazole-(C4)H), 12.58 (s, 1H, N-OH-imidazole-N-OH): 13C-NMR (125 MHz, DMSO-d6) δ 34.54 (5-NO2-imidazole-N-CH3), 116.14 (N-OH-imidazole-(C4)), 125.86 (Ar(C2)H and Ar(C6)H), 127.72 (Ar(C4)H), 128.58 (Ar(C3)H and Ar(C5)H), 128.77 (Ar(C2’)H and Ar(C6’)H), 128.86 (Ar(C3’)H and Ar(C5’)H), 132.72 (5-NO2-imidazole-(C4)H), 133.86 (N-OH-imidazole-(C2)), 137.60 (Ar(C1)), 139.94 (5-NO2-imidazole-(C2)), 141.10 (5-NO2-imidazole-(C5)NO2): IR (KBr) νmax cm−1: 3437 (ν N-O-H), 3144–3058 (ν C-H(aromatic), 1533–1470 (ν C=C(aromatic), 1364 (ν N=O2): MS (ESI) m/z: 394.5 (100%). Anal. Calcd. for C19H14ClN5O3: C: 57.66; H: 3.57; N: 17.69. Found: C: 57.68; H: 3.57; N: 17.69.
2'-(4-Bromophenyl)-1-methyl-5-nitro-5'-phenyl-1H,3'H-2,4'-biimidazol-3'-ol (6). Yellow solid; mp 218–219 °C; yield 69%; 1H-NMR (500 MHz, DMSO-d6) δ 3.71 (s, 3H, 5-NO2-imidazole-N-CH3), 7.28 (t, 1H, Ar(C4)H), 7.33 (t, 2H, Ar(C3)H and Ar(C5)H), 7.55 (d, 2H, J = 8.0 Hz, Ar(C2)H and Ar(C6)H), 7.75 (d, 2H, J = 8.0 Hz, Ar(C2’)H and Ar(C6’)H), 8.10 (d, 2H, J = 8.0 Hz, Ar(C3’)H and Ar(C5’)H), 8.34 (s, 1H, 5-NO2-imidazole-(C4)H), 12.51 (s, 1H, N-OH-imidazole-N-OH): 13C-NMR (125 MHz, DMSO-d6) δ 34.54 (5-NO2-imidazole-N-CH3), 116.15 (N-OH-imidazole-(C4)), 122.68 (Ar(C4’)Br), 125.84 (Ar(C2)H and Ar(C6)H), 127.54 (Ar(C1’)), 127.75 (Ar(C4)H), 128.60 (Ar(C3)H and Ar(C5)H), 129.12 (Ar(C2’)H and Ar(C6’)H), 131.73 (Ar(C3’)H and Ar(C5’)H), 132.71 (5-NO2-imidazole-(C4)H), 132.89 (N-OH-imidazole-(C2)), 137.66 (Ar(C1)), 139.97 (5-NO2-imidazole-(C2)), 141.00 (5-NO2-imidazole-(C5)NO2), 141.15 (N-OH-imidazole-(C5)): IR (KBr) νmax cm−1: 3140–3056 (ν C-H(aromatic), 1533–1470 (ν C=C(aromatic), 1364 (ν N=O2), 828 (ν C-N (Ar-NO2),: MS (ESI) m/z: 440.3 (100%). Anal. Calcd. for C19H14BrN5O3: C: 51.83; H: 3.21; N: 15.91. Found: C: 51.85; H: 3.21; N: 15.91.
1-Methyl-5-nitro-2'-(4-nitrophenyl)-5'-phenyl-1H,3'H-2,4'-biimidazol-3'-ol (7). Yellow solid; mp 361–362 °C; yield 62%; 1H-NMR (500 MHz, DMSO-d6) δ 3.73 (s, 3H, 5-NO2-imidazole-N-CH3), 7.27 (t, 1H, Ar(C4)H), 7.33 (t, 2H, Ar(C3)H and Ar(C5)H), 7.57 (d, 2H, Ar(C2)H and Ar(C6)H), 7.80 (d, 1H, Ar(C2’)H, Ar(C3’)H, Ar(C5’)H, or Ar(C6’)H), 7.92 (t, 1H, Ar(C2’)H, Ar(C3’)H, Ar(C5’)H, or Ar(C6’)H), 7.97 (t, 1H, Ar(C2’)H, Ar(C3’)H, Ar(C5’)H, or Ar(C6’)H), 8.15(d, 1H, Ar(C2’)H, Ar(C3’)H, Ar(C5’)H, or Ar(C6’)H), 8.33 (s, 1H, 5-NO2-imidazole-(C4)H), 12.40 (s, 1H, N-OH-imidazole-N-OH): 13C-NMR (125 MHz, DMSO-d6) δ 34.39 (5-NO2-imidazole-N-CH3), 115.50 (N-OH-imidazole-(C4)), 124.63 (Ar(C3’)H and Ar(C5’)H), 125.94 (Ar(C2)H and Ar(C6)H), 127.78 (Ar(C4)H), 128.56 (Ar(C3)H and Ar(C5)H), 131.15 (Ar(C2’)H and Ar(C6’)H), 132.60 (5-NO2-imidazole-(C4)H), 132.79 (N-OH-imidazole-(C2)), 133.64 (Ar(C1)), 139.49 (N-OH-imidazole-(C5)), 140.05 (5-NO2-imidazole-(C2)NO2), 140.72 (5-NO2-imidazole-(C5)NO2), 148.48 (Ar(C4’)NO2): IR (KBr) νmax cm−1: 3120–3033 (ν C-H(aromatic), 1524–1465 (ν C=C(aromatic), 1349–1365 (ν N=O2), 826 (ν C-N (Ar-NO2),: MS (ESI) m/z: 405.3 (100%). Anal. Calcd. for C19H14N6O5: C: 56.16; H: 3.47; N: 20.68. Found: C: 56.15; H: 3.47; N: 20.67.
2'-(4-Methoxyphenyl)-1-methyl-5-nitro-5'-phenyl-1H,3'H-2,4'-biimidazol-3'-ol (8). Yellow solid; mp 218–219 °C; yield 62%; 1H-NMR (400 MHz, DMSO-d6) δ 3.73 (s, 3H, 5-NO2-imidazole-N-CH3), 3.81 (s, 3H, Ar(C4’)OCH3), 7.08 (t, 1H, J = 8.4 Hz, Ar(C2’)H, Ar(C3’)H, Ar(C5’)H or Ar(C6’)H), 7.17 (d, 1H, J = 8.4 Hz, Ar(C2’)H, Ar(C3’)H, Ar(C5’)H or Ar(C6’)H), 7.25 (t, 1H, Ar(C4)H), 7.32 (t, 2H, Ar(C2)H, Ar(C3)H, Ar(C5)H or Ar(C6)H), 7.51 (t, 2H, Ar(C3’)H and Ar(C5’)H), 7.56 (d, 2H, J = 7.2 Hz, Ar(C2)H and Ar(C6)H), 8.34 (s, 1H, 5-NO2-imidazole-(C4)H), 11.72 (s, 1H, N-OH-imidazole-N-OH): 13C-NMR (100 MHz, DMSO-d6) δ 34.43 (5-NO2-imidazole-N-CH3), 55.72 (Ar(C4’)OCH3), 111.77 and 120.18 (Ar(C3’)H and Ar(C5’)H), 114.71 (N-OH-imidazole-(C4)), 117.77 (Ar(C1’)), 125.82 (Ar(C2)H and Ar(C6)H), 127.38 (Ar(C4)H), 128.43 (Ar(C3)H and Ar(C5)H), 131.37 and 131.54 (Ar(C2’)H and Ar(C6’)H), 132.63 (5-NO2-imidazole-(C4)H), 133.35 (N-OH-imidazole-(C2)), 137.48 (Ar(C1)), 139.85 (5-NO2-imidazole-(C2)), 141.58 (5-NO2-imidazole-(C5)NO2), 141.76 (N-OH-imidazole-(C5)), 157.70 (Ar(C4’)OCH3): IR (KBr) νmax cm−1: 3448 (ν N-O-H), 3070–3054 (ν C-H(aromatic), 1471–1488 (ν C=C(aromatic), 1363 (ν N=O2), 825 (ν C-N (Ar-NO2),: MS (ESI) m/z: 390.4 (100%). Anal. Calcd. for C20H17N5O4: C: 56.16; H: 3.47; N: 20.68. Found: C: 56.15; H: 3.47; N: 20.67.
2'-(4-Hydroxyphenyl)-1-methyl-5-nitro-5'-phenyl-1H,3'H-2,4'-biimidazol-3'-ol (9). Yellow solid; mp 145–146 °C; yield 71%; 1H-NMR (400 MHz, DMSO-d6) δ 3.70 (s, 3H, 5-NO2-imidazole-N-CH3), 6.90 (d, 2H, J = 9.2 Hz, Ar(C3’)H and Ar(C5’)H), 7.25 (t, 1H, Ar(C4)H), 7.32 (t, 2H, Ar(C3)H and Ar(C5)H), 7.53 (d, 2H, J = 7.6 Hz, Ar(C2)H and Ar(C6)H), 7.97 (d, 2H, J = 8.4 Hz, Ar(C2’)H and Ar(C6’)H), 8.36 (s, 1H, 5-NO2-imidazole-(C4)H), 9.89 (s, 1H, Ar(C4’)OH), 12.21 (s, 1H, N-OH-imidazole-N-OH): 13C-NMR (100 MHz, DMSO-d6) δ 34.52 (5-NO2-imidazole-N-CH3), 115.09 (N-OH-imidazole-(C4)), 115.42 (Ar(C3’)H and Ar(C5’)H), 119.40 (Ar(C1’)), 125.79 (Ar(C2)H and Ar(C6)H), 127.51 (Ar(C4)H), 128.54 (Ar(C3)H and Ar(C5)H), 129.04 (Ar(C2’)H and Ar(C6’)H), 132.75 (5-NO2-imidazole-(C4)H), 133.26 (N-OH-imidazole-(C2)), 137.13 (Ar(C1)), 139.87 (5-NO2-imidazole-(C2)), 141.56 (5-NO2-imidazole-(C5)NO2), 142.74 (N-OH-imidazole-(C5)), 158.50 (Ar(C4’)OH): IR (KBr) νmax cm−1: 3123–3062 (ν C-H(aromatic), 1527–1465 (ν C=C(aromatic), 1283 (ν N=O2), 827 (ν C-N (Ar-NO2),: MS (ESI) m/z: 376.3 (100%). Anal. Calcd. for C19H15N5O4: C: 60.47; H: 4.01; N: 18.56. Found: C: 60.49; H: 4.01; N: 18.56.
4-(3'-Hydroxy-1-methyl-5-nitro-5'-phenyl-1H,3'H-2,4'-biimidazol-2'-yl)benzene-1,2-diol (10). Yellow solid; mp 224–225 °C; yield 42%; 1H-NMR (500 MHz, DMSO-d6) δ 3.70 (s, 3H, 5-NO2-imidazole-N-CH3), 6.85 (d, 1H, J = 8.0 Hz, Ar(C5’)H), 7.25 (t, 1H, Ar(C4)H), 7.32 (t, 2H, Ar(C3)H and Ar(C5)H), 7.49 (dd, 1H, J = 8.5 Hz, Ar(C6’)H), 7.53 (d, 2H, Ar(C2)H and Ar(C6)H), 7.62 (d, 1H, Ar(C2’)H), 8.32 (s, 1H, 5-NO2-imidazole-(C4)H), 9.23 (s, 1H, Ar(C3’)OH or Ar(C4’)OH), 9.33 (s, 1H, Ar(C3’)OH or Ar(C4’)OH), 12.16 (s, 1H, N-OH- midazole-N-OH): 13C-NMR (125 MHz, DMSO-d6) δ 34.54 (5-NO2-imidazole-N-CH3), 114.87 (Ar(C2’)H), 115.09 (N-OH-imidazole-(C4)), 115.63 (Ar(C5’)H), 119.17 (Ar(C6’)H), 119.69 (Ar(C1’)), 125.80 (Ar(C2)H and Ar(C6)H), 127.49 (Ar(C4)H), 128.54 (Ar(C3)H and Ar(C5)H), 132.77 (5-NO2-imidazole-(C4)H), 133.31 (N-OH-imidazole-(C2)), 137.05 (Ar(C1)), 139.86 (5-NO2-imidazole-(C2)), 141.61 (5-NO2-imidazole-(C5)NO2), 142.69 (N-OH-imidazole-(C5)), 145.20 Ar(C3’)OH), 146.82 Ar(C4’)OH): IR (KBr) νmax cm−1: 3367 (ν O-H), 3124–3066 (ν C-H(aromatic), 1528–1468 (ν C=C(aromatic), 1365 (ν N=O2), 827 (ν C-N (Ar-NO2),: MS (ESI) m/z: 392.2 (100%). Anal. Calcd. for C19H15N5O5: C: 58.01; H: 3.84; N: 17.80. Found: C: 57.09; H: 3.84; N: 17.79.
2'-(3-Hydroxy-4-methoxyphenyl)-1-methyl-5-nitro-5'-phenyl-1H,3'H-2,4'-biimidazol-3'-ol (11). Gray solid; mp 213 °C; yield 45%; 1H-NMR (400 MHz, DMSO-d6) δ 3.70 (s, 3H, 5-NO2-imidazole-N-CH3), 3.82 (s, 3H, Ar(C4’)OCH3), 7.05 (d, 1H, Ar(C2)H, Ar(C5)H or Ar(C6)H), 7.26 (t, 1H, Ar(C4)H), 7.33 (t, 2H, Ar(C3)H and Ar(C5)H), 7.53 (d, 2H, Ar(C2)H and Ar(C6)H), 7.61 (d, 1H, Ar(C2)H, Ar(C5)H or Ar(C6)H), 8.32 (s, 1H, 5-NO2-imidazole-(C4)H), 9.28 (s, 1H, N-OH-imidazole-N-OH): 13C-NMR (125 MHz, DMSO-d6) δ 34.46 (5-NO2-imidazole-N-CH3), 56.41 (Ar(C4’)OCH3), 113.63 (Ar(C2’)H, 114.73 (N-OH-imidazole-(C4)), 116.10 (Ar(C6’)H, 118.21 (Ar(C1’), 125.91 (Ar(C2)H and Ar(C6)H), 127.46 (Ar(C4)), 128.48 (Ar(C3)H and Ar(C5)H), 132.70 (5-NO2-imidazole-(C4)H), 137.11 (Ar(C1)), 133.15 (N-OH-imidazole-(C2)), 136.53 (Ar(C5’)NO2, 137.02 (Ar(C1)), 139.74 (5-NO2-imidazole-(C2)), 140.09 (Ar(C4’)OH, 141.59 (5-NO2-imidazole-(C5)NO2), 144.67 (N-OH-imidazole-(C5)), 149.82 (Ar(C3’)OCH3: IR (KBr) νmax cm−1: 3368 (ν N-O-H), 3130–3056 (ν C-H(aromatic), 1533–1503 (ν C=C(aromatic), 1369 (ν N=O2), 827 (ν C-N (Ar-NO2),: MS (ESI) m/z: 406.4 (100%). Anal. Calcd. for C20H17N5O5: C: 58.97; H: 4.21; N: 17.19. Found: C: 58.98; H: 4.21; N: 17.19.
2'-(3-Hydroxy-4-methoxy-5-nitrophenyl)-1-methyl-5-nitro-5'-phenyl-1H,3'H-2,4'-biimidazol-3'-ol (12). Red solid; mp 245–247 °C; yield 43%; 1H-NMR (400 MHz, DMSO-d6) δ 3.69 (s, 3H, 5-NO2-imidazole-N-CH3), 3.90 (s, 3H, Ar(C3’)OCH3), 7.25 (t, 1H, Ar(C4’)OH), 7.32 (t, 2H, Ar(C3)H and Ar(C5)H), 7.54 (d, 2H, Ar(C2)H and Ar(C6)H), 7.89 (s, 1H, 5-NO2-imidazole-(C4)H), 8.26 (d, 2H, Ar(C2’)H and Ar(C6’)H): 13C-NMR (100 MHz, DMSO-d6) δ 34.43 (5-NO2-imidazole-N-CH3), 55.41 (Ar(C3’)OCH3), 125.90 (Ar(C2)H and Ar(C6)H), 127.46 (Ar(C4)H), 128.44 (Ar(C3)H and Ar(C5)H), 132.70 (5-NO2-imidazole-(C4)H), 137.15 (Ar(C1)), 139.74 (5-NO2-imidazole-(C2)), 140.09 (5-NO2-imidazole-(C5)NO2), 141.59 (Ar(C1’)), 149.82 (Ar(C3’)OCH3): IR (KBr) νmax cm−1: 3530 (ν O-H), 1555–1542 (ν C=C(aromatic), 1365 (ν N=O2), 827 (ν C-N (Ar-NO2),: MS (ESI) m/z: 451.4 (100%). Anal. Calcd. for C20H16N6O7: C: 53.10; H: 3.56; N: 18.58. Found: C: 52.98; H: 3.57; N: 18.55.
3.5. Activity against Bloodstream Trypomastigote Forms [28]
Stock solutions of NAH derivatives were prepared in DMSO. Bloodstream trypomastigote forms of T. cruzi (Y strain) were isolated from infected Swiss mice and re-suspended with Dulbecco’s modified Eagle medium plus 10% foetal calf serum (DMES) to a parasite concentration of 10 × 106 cells/mL. This suspension (100 μL) was added to the same volume of each NAH derivative, previously prepared at twice the desired concentrations in DMES. Stock solutions of the NAH derivatives were prepared in dimethylsulfoxide (DMSO), and they were assayed in the range of 0.5 to 2000 μg/mL. The final concentration of DMSO never exceeded 0.5%, thus, it had no deleterious effect on the parasites [29].
3.6. Cytotoxic Effect on Murine Macrophages
Cellular viability in the presence and absence of TAI derivatives (4–6) was determined using Alamar Blue assay (Invitrogen). The macrophage cell line J774 was seeded into black clear flat-bottomed 96-well plates in a density of 2.5 × 106 cells/well. After 1 h of incubation in controlled atmosphere (5% CO2, 37 °C),cells received fresh medium with or without Tween 20 (3%), DMSO (0.5%) and the TAI compounds (1 to 100 μM) in a quadruplicate assay. After 21 h of incubation in the previous conditions, 20 μL of Alamar Blue solution was added to each well and after 3 h, fluorescence was measured using SpectraMax M5/M5e microplate reader (Molecular Devices; λexc = 555 nm, λem = 585 nm).
4. Conclusions
We have described herein a novel structural triarylheterocyclic template capable of displaying a significant antitrypanosomal profile in vitro. Among these N-hydroxytriarylimidazole (TAI) derivatives, we were able to identify the derivative 2'-(4-bromophenyl)-1-methyl-5'-phenyl-1H,3'H-2,4'-biimidazol-3'-ol (6), which showed moderate trypanocidal activity (IC50 = 23.9 µM) when compared to benznidazole, which is used as the standard drug. This compound did not present cytotoxic effects at concentrations near the IC50, being considered a good starting point in the development of new anti-Chagas drug candidates.
Acknowledgments
The authors thank CAPES (BR), CNPq (BR), FAPERJ (BR) and FIOCRUZ (BR) for financial support and fellowships.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.World Health Organization (WHO) Working to Overcome the Global Impact of Neglected Tropical Diseases: First WHO Report on Neglected Tropical Diseases. WHO; Geneva, Switzerland: 2010. pp. 1–172. [Google Scholar]
- 2.Rassi-Jr A., Rassi A., Marin-Neto J.A. Chagas disease. Lancet. 2010;375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 3.Ministério da Saúde do Brasil. Secretaria Nacional de Vigilância em Saúde. Doença de Chagas Aguda. Manual Prático de Subsídio à Notificação Obrigatória no SINAN. [(accessed on 10 June 2012)]. Available online: http://portal.saude.gov.br/portal/arquivos/pdf/manual_chagas.pdf.
- 4.Rassi-Jr A., Rassi A., Marcondes de Rezende J. American trypanosomiasis (Chagas disease) Infect. Dis. Clin. North Am. 2012;26:275–291. doi: 10.1016/j.idc.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 5.de Castro S.L., de Meirelles M.N. Effect of drugs on Trypanosoma cruzi and on its interaction with heart muscle cell “in vitro”. Mem. Inst. Oswaldo Cruz. 1987;82:209–218. doi: 10.1590/s0074-02761987000200009. [DOI] [PubMed] [Google Scholar]
- 6.Filard L.S., Brener Z. A nitroimidazole-thiadiazole derivative with curative action in experimental Trypanosoma cruzi infections. Ann. Trop. Med. Parasitol. 1982;76:293–297. doi: 10.1080/00034983.1982.11687544. [DOI] [PubMed] [Google Scholar]
- 7.Lages-Silva E., Filard L.S., Brener Z. Effect of the host specific treatment in the phagocytosis of Trypanosoma cruzi blood forms by mouse peritoneal macrophages. Mem. Inst. Oswaldo Cruz. 1990;85:401–405. doi: 10.1590/S0074-02761990000400003. [DOI] [PubMed] [Google Scholar]
- 8.Maya J.D., Bollo S., Nunez-Vergara L.J., Squella J.A., Repetto Y., Morello A., Périé J., Chauviére G. Trypanosoma cruzi: Effect and mode of action of nitroimidazole and nitrofuran derivatives. Biochem. Pharmacol. 2003;65:999–1006. doi: 10.1016/S0006-2952(02)01663-5. [DOI] [PubMed] [Google Scholar]
- 9.Viodé C., Bettache N., Cenas N., Krauth-Siegel R.L., Chauvaviére G., Bakalara N., Périé J. Enzymatic reduction studies of nitroheterocycles. Biochem. Pharmacol. 1999;57:549–557. doi: 10.1016/S0006-2952(98)00324-4. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira R.C., Ferreira L.C. Mutagenicity of CL 64855, a potent anti-Trypanosoma cruzi drug. Mut. Res. 1986;171:11–15. doi: 10.1016/0165-1218(86)90003-0. [DOI] [PubMed] [Google Scholar]
- 11.Nesslany F., Brugier S., Mouriès M.A., Le Curieux F., Marzin D. In vitro and in vivo in vivo chromosomal aberrations induced by megazol. Mut. Res. 2004;560:147–158. doi: 10.1016/j.mrgentox.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Poli P., Mello M.A., Buschini A., Mortara R.A., Albuquerque C., Silva S., Rossi C., Zucchi T.M. Cytotoxic and genotoxic effects of megazol, an anti-Chagas' disease drug, Assessed by different short-term tests. Biochem. Pharmacol. 2002;64:1617–1627. doi: 10.1016/S0006-2952(02)01390-4. [DOI] [PubMed] [Google Scholar]
- 13.Boechat N., Carvalho A.S., Fernandez-Ferreira E., Soares R.O., Souza A.S., Gibaldi D., Bozza M., Pinto A.C. Novel nitroimidazoles with trypanocidal and cell growth inhibition activities. Cytobios. 2001;105:83–90. [PubMed] [Google Scholar]
- 14.Carvalho S.A., da Silva E.F., Santa-Rita R.M., de Castro S.L., Fraga C.A.M. Synthesis and antitrypanosomal profile of new functionalized 1,3,4-thiadiazole-2-arylhydrazone derivatives, designed as non-mutagenic megazol analogues. Bioorg. Med. Chem. Lett. 2004;14:5967–5970. doi: 10.1016/j.bmcl.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Carvalho S.A., Lopes F.A.S., Salomão K., Romeiro N.C., Wardell S.M.V.S., de Castro S.L., da Silva E.F., Fraga C.A.M. Studies toward the structural optimization of new brazilizone-relatedtrypanocidal 1,3,4-thiadiazole-2-arylhydrazone derivatives. Bioorg. Med. Chem. 2008;16:413–421. doi: 10.1016/j.bmc.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 16.Carvalho A.S., Menna-Barreto R.F.S., Romeiro N.C., de Castro S.L., Boechat N. Design, synthesis and activity against Trypanosoma cruzi of azaheterocyclic analogs of megazol. Med. Chem. 2007;3:460–465. doi: 10.2174/157340607781745519. [DOI] [PubMed] [Google Scholar]
- 17.Salomão K., de Souza E.M., Carvalho S.A., da Silva E.F., Fraga C.A.M., Barbosa H.S., de Castro S.L. In vitro and in vivo activities of 1,3,4-thiadiazole-2-arylhydrazone derivatives of megazol against Trypanosoma cruzi. Antimicrob. Agents Chemother. 2010;54:2023–2031. doi: 10.1128/AAC.01241-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katritzky A.R. Comprehensive Heterocyclic Chemistry. Volume V. Pergamon; Exeter, UK: 1984. pp. 469–498. [Google Scholar]
- 19.Mukherjee A., Kumar S., Seth M., Bhaduri A.P. Synthesis of 1-methyl-4-nitro-5-substituted imidazole and substituted imidazolothiazole derivatives as possible antiparasitic agents. Indian J. Chem. 1989;28B:391–396. [Google Scholar]
- 20.AyhanKilcigil G., Altanlar N. Synthesis and antifungal properties of some benzimidazole derivatives. Turk. J. Chem. 2006;30:223–228. [Google Scholar]
- 21.Norman S.M., Bennett R.D., Poling S.M., Maier V.P., Nelson M.D. Paclobutrazol inhibits abscisic acid biosynthesis in Cercospora rosicola. Plant Physiol. 1986;80:122–125. doi: 10.1104/pp.80.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guven O.O., Erdogan T., Goker H., Yildiz S. Synthesis and antimicrobial activity of some novelphenyl and benzimidazole substituted benzyl ethers. Bioorg. Med. Chem. Lett. 2007;17:2233–2236. doi: 10.1016/j.bmcl.2007.01.061. [DOI] [PubMed] [Google Scholar]
- 23.Hadizadeh F., Hosseinzadeh H., Motamed-Shariaty V.-S., Seifi M., Kazemi S. Synthesis and antidepressant activity of N-substituted imidazole-5-carboxamides in forced swimming test model. Iranian J. Pharm. Res. 2008;7:29–33. [Google Scholar]
- 24.Franchetti P., Stein M.L., Grifantini M., Lucarelli C. Structure-activity relationships in reactivators of acetylcholinesterase inhibited by organophosphorus compounds. 4. N-hydroxyimidazole 3-oxides and their quaternary salts. Farmaco-Ed Sci. 1972;27:46–59. [PubMed] [Google Scholar]
- 25.Viegas-Jr C., Danuello A., Bolzani V.B., Barreiro E.J., Fraga C.A.M. Molecular hybridization: a useful tool in the design of new drug prototypes. Curr. Med. Chem. 2007;14:1829–1852. doi: 10.2174/092986707781058805. [DOI] [PubMed] [Google Scholar]
- 26.Albright J.D., Shepherd R.G. 1,2-disubstituted-5-nitroimidazoles. n 3, 652,555. US Patent. 1972
- 27.Wang M., Gao J., Song Z. A practical and green approach toward synthesis of 2,4,5-trisubstituted imidazoles without adding catalyst. Prep. Biochem. Biotechnol. 2010;40:347–353. doi: 10.1080/10826068.2010.525418. [DOI] [PubMed] [Google Scholar]
- 28.Salomão K., Dantas A.P., Borba C.M., Campos L.C., Machado D.G., Aquino-Neto F.R., de Castro S.L. Chemical composition and microbicidal activity of extracts from Brazilian and Bulgarian propolis. Lett. Applied Microbiol. 2004;38:87–92. doi: 10.1111/j.1472-765X.2003.01458.x. [DOI] [PubMed] [Google Scholar]
- 29.de Castro S.L., Pinto M.C., Pinto A.V. Screening of natural and synthetic drugs against Trypanosoma cruzi: I — Establishing a structure/activity relationship. Microbios. 1994;78:83–90. [PubMed] [Google Scholar]