Abstract
A new bidentate phosphoramidite (N-Me-BIPAM) based on Shibasaki’s N-linked BINOL was synthesized. This ligand appears to be highly effective for rhodium-catalyzed asymmetric conjugated addition of arylboronic acids to α,β-unsaturated enones. The reaction of ortho-substituted arylboronic acid with acyclic and cyclic enones provides the corresponding products in good yields and enantioselectivities.
Keywords: asymmetric conjugate addition, rhodium catalyst, bidentate phosphoramidite ligand
1. Introduction
Metal-catalyzed conjugated addition reactions of carbon nucleophiles to α,β-unsaturated compounds are the most widely used reactions for asymmetric carbon-carbon bond formation [1,2,3]. Much interest has recently been shown in rhodium-catalyzed conjugate addition of arylboronic acids to α,β-unsaturated carbonyl compounds [1,2,3,4,5,6,7,8] using various ligands [9,10,11,12,13,14,15,16,17,18,19,20,21] such as biaryl bisphosphines [4,5,6,7,8], phosphoramidites [22,23,24,25,26,27,28], diphosphonite [29], amidomonophosphines [30,31], N-heterocyclic carbenes [32,33], P-chiral phosphine [34], and dienes [35,36,37,38,39,40,41,42,43]. Although many chiral ligands give adducts with good selectivity for cyclic enones, there are few ligands that give good results for both acyclic and cyclic enones. In addition, though conjugate addition of ortho-substituted arylboronic acids to α,β-unsaturated cyclic enones has been achieved in high enantioselectivities [9,10,11,12,13,14,15,16,17,18,19,20,21,44,45,46,47,48,49,50,21,44], there have been few reports on this reaction for acyclic enones [38,51,52]. On the other hand, we have already reported that a new bidentate phosphoramidite ligand (Me-BIPAM) based on O-linked-BINOL was synthesized and that a rhodium/Me-BIPAM complex was a better catalyst than several monodentate phosphoramidites for conjugate addition of arylboronic acids to α,β-unsaturated cyclic and acyclic carbonyl compounds [53,54]. However, we were not satisfied with the enantioselectivities for acyclic enones such as (E)-3-nonene-2-one using Me-BIPAM. Therefore, we reported that the bidentate phosphoramidite N-Me-BIPAM, which was newly synthesized on the basis of N-linked-BINOL [55], was highly efficient for rhodium-catalyzed asymmetric arylation of N-sulfonyl aldimine with arylboronic acids [56].
Herein we report that rhodium/N-Me-BIPAM catalyzed 1,4-addition of arylboronic acids to α,β-unsaturated enones. N-Me-BIPAM was found to be highly effective for rhodium-catalyzed conjugate addition of arylboronic acids to α,β-unsaturated acyclic and cyclic enones. Furthermore, the use of N-Me-BIPAM was necessary to achieve high enantioselectivity for the reaction of ortho-substituted arylboronic acids to acyclic and cyclic enones.
2. Results and Discussion
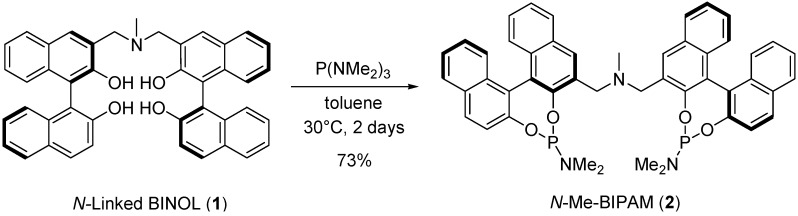
N-linked bidentate phosphoramidite was easily synthesized from Shibasaki’s N-linked BINOL and P(NMe2)3 in good yield (Scheme 1). 31P-NMR of the mixture of Rh(acac)(C2H4)2 and N-Me-BIPAM exhibited a single signal at 160.0 ppm (d, JRh-P = 292.3 Hz), suggesting the intramolecular complexation of two phosphorous atoms to a rhodium metal center. The formation of a 1:1 complex was also confirmed by mass spectroscopy (ESI), which showed a molecular weight of 976.2152 (M+H).
Scheme 1.
Synthesis of bisphosphoramidite (N-Me-BIPAM).
The efficiency of N-Me-BIPAM was investigated in rhodium-catalyzed conjugate addition of arylboronic acids to enones. We first examined the addition of phenylboronic acid to (E)-3-nonene-2-one in an aqueous solvent (Table 1). Preparation of the rhodium catalyst from [Rh(coe)2Cl]2, N-Me-BIPAM and a base in 1,4-dioxane or in DME resulted in low selectivity (entries 1–5). Among the representative inorganic bases employed, K2CO3 was found to be the best, giving 87% ee (entry 4). However, the use of triethylamine for a combination of [Rh(nbd)2]BF4 and N-Me-BIPAM resulted in a quantitative yield with 92% ee (entry 7).
Table 1.
Optimization of the reaction conditions.
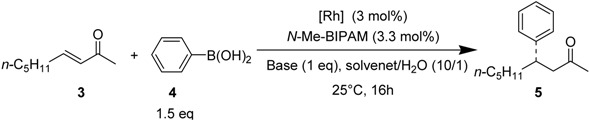 |
| Entry | [Rh] | Solvent | Base | Yield (%) a | ee (%) b |
|---|---|---|---|---|---|
| 1 | [Rh(coe)2Cl]2 | dioxane | KOH | 96 | 78 |
| 2 | [Rh(coe)2Cl]2 | DME | KOH | 99 | 81 |
| 3 | [Rh(coe)2Cl]2 | DME | K3PO4 | 59 | 73 |
| 4 | [Rh(coe)2Cl]2 | DME | K2CO3 | 94 | 87 |
| 5 | [Rh(coe)2Cl]2 | DME | NEt3 | trace | - |
| 6 | [Rh(nbd)2]BF4 | DME | K2CO3 | 57 | 87 |
| 7 | [Rh(nbd)2]BF4 | DME | NEt3 | 99 | 92 |
a Isolated yield; b Determined by HPLC.
With these optimized conditions, 1,4-addition of arylboronic acids to representative α,β-unsaturated acyclic and cyclic enones was carried out in the presence of a [Rh(nbd)2]BF4/N-Me-BIPAM catalyst (Table 2). N-Me-BIPAM achieved high enantioselectivities in the range of 92–95% ee for (E)-3-nonen-2-one (entries 1–3). The selectivities were in the range of 82–90% ee for (E)-5-methyl-3-hexen-2-one (entries 4–6). However, this ligand was not effective for substrates having a phenyl group at the carbonyl carbon or the β-carbon due to steric hindrance of the aryl ring (entries 7 and 8).
Table 2.
Asymmetric conjugated addition of arylboronic acids to α,β-unsaturated enones.
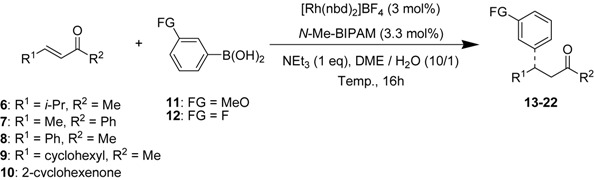 |
| Entry | Enones | FG | Temp. (°C) | Product | Yield (%) a | ee (%) b |
|---|---|---|---|---|---|---|
| 1 | (E)-C5H11CH=CHCOCH3 | H | 25 | 13 | 99 | 92 (S) |
| 2 c | (E)-C5H11CH=CHCOCH3 | MeO | 25 | 14 | 96 | 94 (S) |
| 3 | (E)-C5H11CH=CHCOCH3 | F | 50 | 15 | 83 | 95 (+) |
| 4 | (E)-(CH3)2CHCH=CHCOCH3 | H | 50 | 16 | 83 | 87 (R) |
| 5 | (E)-(CH3)2CHCH=CHCOCH3 | MeO | 25 | 17 | 60 | 90 (R) |
| 6 | (E)-(CH3)2CHCH=CHCOCH3 | F | 50 | 18 | 42 | 82 (+) |
| 7 | (E)-CH3CH=CHCOPh | MeO | 50 | 19 | 87 | 77 (+) |
| 8 | (E)-PhCH=CHCOCH3 | MeO | 50 | 20 | 99 | 77 (+) |
| 9 c | (E)-cyclo-C6H11CH=CHCOCH3 | MeO | 50 | 21 | 94 | 88 (+) |
| 10 c | 2-Cyclohexenone | H | 50 | 22 | 99 | 99 (R) |
a Isolated yield; b Determined by HPLC; c Used [Rh(coe)2Cl]2, KOH and dioxane/H2O instead of optimized condition.
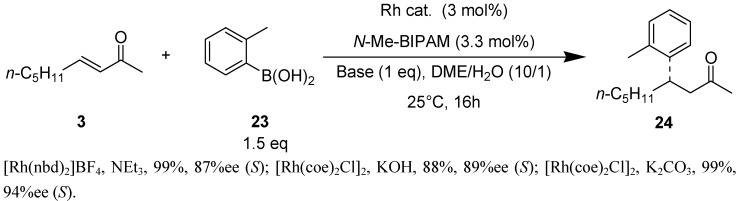
The steric hindrance of an ortho-substituent on the arylboronic acids slows down the reaction rate significantly and decreases the enantioselectivity. It was interesting that N-Me-BIPAM exhibited good performance for such boronic acids. The results of 1,4-addition of 2-tolylboronic acid to (E)-3-nonen-2-one are shown in Scheme 2. The use of neutral [Rh(coe)2Cl]2 and K2CO3 for N-Me-BIPAM resulted in a quantitative yield and the best selectivity (94% ee).
Scheme 2.
Asymmetric 1,4-addition of 2-tolylboronic acids to 3-nonene-2-one.
Table 3 shows the results of 1,4-addition of arylboronic acids possessing a methyl, fluoro or methoxy group at the ortho-carbon or 1-naphthylboronic acid to the representative acyclic and cyclic enones. The use of a series of arylboronic acids for (E)-3-nonen-2-one showed enantioselectivities decreasing in the order of F > Me > 1-naphthyl > MeO (entries 1–5). On the other hand, the effect of substituents was F > MeO > 1-naphthyl > Me for (E)-CH3CH=CHCOPh (entries 6–9). Thus, the selectivities were greatly dependent on the bulkiness and electronic property of substituted arylboronic acids and enone substrates. Among them, 1,4-addition of ortho-tolylboronic acid to (E)-3-nonen-2-one resulted in 92% yield and 97% ee with 0.1 mol% catalyst loading (entry 2). When benzylideneacetone which have the bulky substitution on the β-position was used as a substrate, the product was obtained in good enantioselectivity, but reactivity was lower (entry 10). There was no difficulty in achieving high enantioselectivities for 5- and 6- and 7-membered enones at room temperature (entries 11–16). Most of the reactions resulted in more than 94% ee for these cyclic substrates.
Table 3.
Asymmetric conjugated addition of o-substituted arylboronic acids to α,β-unsaturated enones.
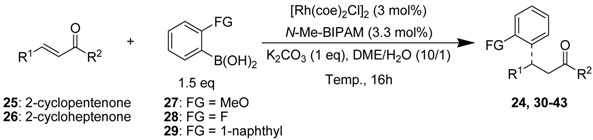 |
| Entry | Enones | FG | Temp. (°C) | Product | Yield (%) a | ee (%) b |
|---|---|---|---|---|---|---|
| 1 | (E)-n-C5H11CH=CHCOCH3 | Me | 25 | 24 | 99 | 94 (S) |
| 2 | (E)-n-C5H11CH=CHCOCH3 | Me | 50 | 24 | 92 | 97 c (S) |
| 3 | (E)-n-C5H11CH=CHCOCH3 | MeO | 50 | 30 | 86 | 80 (+) |
| 4 | (E)-n-C5H11CH=CHCOCH3 | F | 50 | 31 | 90 | 99 (+) |
| 5 | (E)-n-C5H11CH=CHCOCH3 | 1-naphtyl | 80 | 32 | 77 | 86 (+) |
| 6 | (E)-CH3CH=CHCOPh | Me | 50 | 33 | 80 | 81 (−) |
| 7 | (E)-CH3CH=CHCOPh | MeO | 50 | 34 | 88 | 92 (+) |
| 8 | (E)-CH3CH=CHCOPh | F | 50 | 35 | 86 | 99 (−) |
| 9 | (E)-CH3CH=CHCOPh | 1-naphtyl | 50 | 36 | 87 | 83 (−) |
| 10 | (E)-PhCH=CHCOCH3 | Me | 50 | 37 | 49 | 90 (R) |
| 11 | 2-Cyclopentenone | Me | 25 | 38 | 90 | 97 (R) |
| 12 | 2-Cyclopentenone | MeO | 25 | 39 | 67 | 94 (R) |
| 13 | 2-Cyclopentenone | F | 25 | 40 | 32 | 97 (R) |
| 14 | 2-Cyclopentenone | 1-naphtyl | 25 | 41 | 77 | 99 (R) |
| 15 | 2-Cyclopentenone | Me | 25 | 42 | 94 | 87 (R) |
| 16 | 2-Cycloheptenone | Me | 25 | 43 | 92 | 97 (R) |
a Isolated yield; b Determined by HPLC; c Used 0.1 mol% of rhodium catalyst.
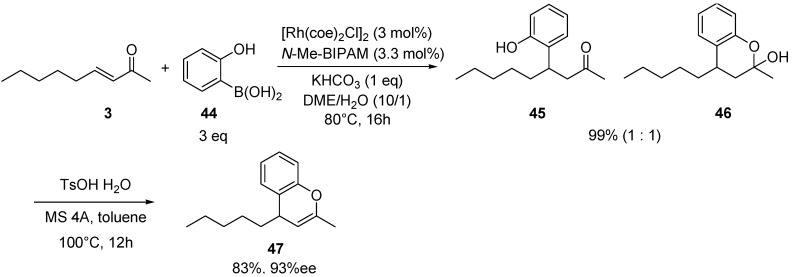
The good performance of N-Me-BIPAM for ortho-substituted arylboronic acids was applied to the synthesis of optically active 4-alkyl-4H-chromenes (Scheme 3). Under conditions optimized in Scheme 3 using K2CO3 as the base, 1,4-addition of 2-hydroxyphenylboronic acid resulted in very low yield due to hydrolytic B-C bond cleavage with water. However, the use of 3 equivalents of boronic acid and KHCO3 at 80 °C afforded an 1,4-adduct in quantitative yield. The 1,4-adduct thus obtained was a 1:1 mixture of ketone and hemiacetal. It was then treated with TsOH·H2O and MS 4A in toluene at 100 °C [57] to give optically active 2-methyl-4-pentyl-4H-chromene in 78% yield with 98% ee [58].
Scheme 3.
Synthesis of 2-methyl-4-pentyl-4H-chromene.
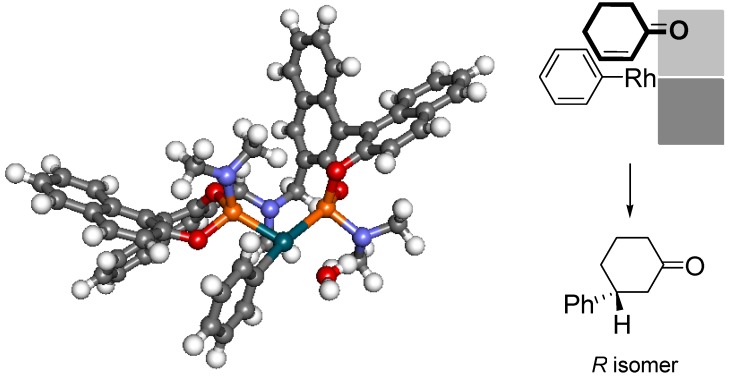
Finally, a stable conformation of the [Rh(Ph)(H2O)((R,R)-N-Me-BIPAM)] intermediate generated by transmetalation of phenylboronic acid to [Rh(OH)(H2O)((R,R)-N-Me-BIPAM)] was calculated on the basis of a theoretical method (B3LYP/6-31G++(d)/B3LYP/LANL2DZ level). There is a completely planar coordination space in the upper and lower left areas consisting of a phenyl group on a rhodium atom. A naphthoxy group in the upper right area occupies a pseudo-axial position and an NMe2 group in the lower right area occupies a pseudo-equatorial position, thus suggesting that the space is accessible to reactants in the upper right quadrant and two quadrants in the left area. On the basis of this calculation, a mode of coordination of an enone to the phenyl rhodium(I) intermediate is proposed in Figure 1. The re-coordination of a substrate can be preferred without significant steric interaction for giving the experimentally observed R enantiomer by parallel coordination of the C-O double bond to the Ph-Rh bond for the next insertion process. On the other hand, the coordination of an enone from its opposite si face is blocked by the equatorial NMe2 group.
Figure 1.
Optimized structure of [Rh(Ph)(H2O)((R,R)-N-Me-BIPAM)].
3. Experimental
3.1. General
1H-NMR spectra were recorded on a JEOL ECX-400 (400 MHz) in CDCl3 with tetramethylsilane as an internal standard. Chemical shifts are reported in part per million (ppm), and signal are expressed as singlet (s), doublet (d), triplet (t), quartet (q), multiplet (m), and broad (br). 13C-NMR spectra were recorded on a JEOL ECX-400 (100 MHz) in CDCl3 (δC = 77.0) with tetramethylsilane as an internal standard. Chemical shifts are reported in part per million (ppm). HPLC analysis was directly performed with chiral stationary phase column, Chiralpak AD-H, IB or Chiralcel OD-H, OB-H purchased from DAICEL Co., Ltd. (Osaka, Japan). High resolution mass spectra (HRMS) were recorded on a JEOL JMS 700TZ mass spectrometer at the Center for Instrumental Analysis, Hokkaido University, Japan. Optical rotations were measured on a HORIBA SEPA-300 digital polarimeter. Kanto Chemical silica gel 60N (particle size 0.063-0.210 mm) was used for flash column chromatography.
3.2. Synthesis of N-Me-BIPAM (2)
(R,R)-3,3'-[Methyliminobis(methylene)]bis(1,1'-binaphtylene-2,2'-diol) (1, 2.4 mmol) and P(NMe2)3 (6 mmol) in dry toluene were stirred for 2 days at 30 °C under nitrogen. The crude solid obtained by evaporation of the solvent was purified by column chromatography to give N-Me-BIPAM (2) as a white solid (73%). [α]22D = −578.3 (c 0.56, CHCl3),1H-NMR (400 MHz, CD2Cl2) δ = 8.32 (s, 2H), 7.93 (q, J = 9.1 Hz, 6H), 7.36–7.41 (m, 4H), 7.18–7.29 (m, 8H), 4.09 (d, J = 15.4 Hz, 2H), 3.76 (d, J = 15.4 Hz, 2H), 2.58 (s, 3H), 2.32 (s, 6H), 2.30 (s, 6H), 13C-NMR (CD2Cl2) δ = 149.5, 149.4, 148.6, 132.9, 131.5, 130.9, 129.8, 129.3, 128.2, 127.6, 127.3, 126.9, 126.4, 125.5, 124.8, 124.0, 122.7, 122.6, 121.1, 57.3, 44.5, 36.1, 34.8 31P-NMR (100 MHz, CD2Cl2) 149.5 HRMS (FAB) calcd for C47H42N3O4P2 (M+H) 774.2651, Found 774.2667.
3.3. General Procedure for Asymmetric 1,4-Addition
A flask charged with rhodium catalyst (0.015 mmol) and N-Me-BIPAM (0.033 mmol) was flushed with nitrogen. Solvent (3 mL) was then added. After being stirred for 1 h at room temperature, aryl boronic acid (1.5 mmol), enone (1 mmol), base (1 mmol) and water (0.3 mL) were added. The resulting mixture was stirred for 16 h at 25 °C, 50 °C or 80 °C. The mixture was extracted with ethyl acetate, washed with brine, and dried over MgSO4. After concentration, the residue was purified by column chromatography on silica gel with hexane/ethyl acetate to give the product as a clear liquid.
The spectral data of compounds 13 [59], 14 [59], 15 [54], 16 [59], 17 [59], 18 [54], 20 [54], 21 [54], 22 [59], 24 [38], 38 [29], 39 [44], 40 [25], 41 [45], 42 [47], 43 [32] was previously reported. The specific rotations of these compounds were (S)-13 ([α]23D = +15.2 (c 0.11, CHCl3)), (S)-14 [([α]23D = +4.83 (c 0.30, CHCl3)], 15 ([α]23D = +12.6 (c 0.70, CHCl3)), (R)-16 [([α]23D = +27.4 (c 0.55, CHCl3)], (R)-17 ([α]23D = +24.7 (c 0.33, CHCl3)), 18 ([α]23D = +20.1 (c 0.53, CHCl3)), 20 ([α]23D = +1.04 (c 0.60, CHCl3)), 21 ([α]23D = +26.8 (c 0.41, CHCl3)), (R)-22 ([α]23D = +17.9 (c 0.78, CHCl3)), (S)-24 ([α]22D = +12.2 (c 0.51, CHCl3)), (R)-38 ([α]23D = +39.4 (c 0.48, CHCl3)), (R)-39 ([α]23D = +13.8 (c 0.38, CHCl3)), (R)-40 ([α]23D = +11.5 (c 0.31, CHCl3)), (R)-41 ([α]23D = +8.70 (c 0.25, CHCl3)), (R)-42 ([α]23D = +19.4 (c 0.26, CHCl3)), (R)-43 ([α]23D = +20.6 (c 0.30, CHCl3)).
3-(3-Methoxyphenyl)-1-phenylbutan-1-one (19). [α]24D = +0.41 (c 0.48, CHCl3), 77% ee [HPLC conditions: Chiralcel OD-H column, hexane/ethanol = 9:1, flow = 0.5 mL/min, wavelength = 254 nm, tR = 29.4 min and 32.8 min]; 1H-NMR (CDCl3) δ = 1.33 (d, J = 7.25 Hz, 3H), 3.18 (dd, J = 8.2, 16.8 Hz, 1H), 3.31 (dd, J = 5.4, 16.8 Hz, 1H), 3.45–3.54 (m, 1H), 3.79 (s, 3H), 6.74–6.76 (m, 1H), 6.84 (t, J = 1.81 Hz, 1H), 6.88 (d, J = 7.7 Hz, 1H), 7.23 (t, J = 7.7 Hz, 1H), 7.42–7.46 (m, 2H), 7.53–7.56 (m, 1H), 7.94 (dd, J = 1.36, 7.25 Hz, 2H); 13C-NMR (CDCl3) δ = 21.9, 35.7, 47.0, 55.3, 112.1, 112.2, 119.3, 127.4, 127.9, 129.0, 129.5, 130.4, 133.1, 137.2, 148.4, 159.8, 199.1; exact mass calcd for C17H18O2: 254.1307; Found 254.1290.
4-(2-Methoxyphenyl)nonan-2-one (30). [α]24D = +3.13 (c 0.40, CHCl3), 80% ee [HPLC conditions: Chiralcel OJ-H column, hexane/2-propanol = 100:1, flow = 0.3 mL/min, wavelength = 254 nm, tR = 20.5 min and 23.0 min]; 1H-NMR (CDCl3) δ = 0.80–0.84 (m, 3H), 1.10–1.25 (m, 6H), 1.53–1.64 (m, 2H), 2.04 (s, 3H), 2.64–2.75 (m, 2H), 3.52–3.59 (m, 1H), 3.81 (s, 3H), 6.83–6.89 (m, 2H), 7.10–7.16 (m, 2H); 13C-NMR (CDCl3) δ = 13.9, 22.4, 27.0, 30.0, 31.7, 34.6, 34.8, 49.8, 55.2, 110.5, 120.5, 127.0, 127.7, 132.4, 157.1, 208.5; exact mass calcd for C15H24O2: 248.1776; Found 248.1786.
4-(2-Fluorophenyl)nonan-2-one (31). [α]22D = −3.32 (c 0.56, CHCl3), 99% ee [HPLC conditions: Chiralpak IA column, hexane/2-propanol = 200:1, flow = 0.8 mL/min, wavelength = 254 nm, tR = 21.7 min and 24.7 min]; 1H-NMR (CDCl3) δ =0.82 (d, J = 6.8 Hz, 3H), 1.08–1.32 (m, 6H), 1.57–1.62 (m, 2H), 2.05 (s, 3H), 2.76 (d, J = 7.3 Hz, 2H), 3.37–3.44 (m, 1H), 6.95–7.17 (m, 4H); 13C-NMR (CDCl3) δ =14.1, 22.6, 27.2, 30.4, 31.7, 35.2, 49.5, 115.6, 124.2, 127.0, 128.6, 130.0, 131.2, 207.8, 161.5; exact mass calcd for C15H21FO: 236.1576; Found 236.1576.
4-Naphthalen-1-yl-nonan-2-one (32). [α]22D = +18.6 (c 0.54, CHCl3), 81% ee [HPLC conditions: Chiralpak IB column, hexane/2-propanol = 100:1, flow = 0.5 mL/min, wavelength = 254 nm, tR = 17.7 min and 18.9 min]; 1H-NMR (CDCl3) δ =0.80 (t, J = 6.7 Hz, 3H), 1.10–1.30 (m, 6H), 1.70–1.83 (m, 2H), 2.04 (s, 3H), 2.84 (d, J = 6.7 Hz, 2H), 4.02–4.17 (m, 1H), 7.35 (d, J = 7.0 Hz, 1H), 7.41–7.53 (m, 3H), 7.71 (d, J = 7.9 Hz, 1H), 7.85 (d, J = 7.9 Hz, 1H), 8.19 (d, J = 8.2 Hz, 1H); 13C-NMR (CDCl3) δ = 14.1, 22.6, 27.2, 30.7, 32.1, 36.2, 50.9, 123.3, 125.6, 126.1, 126.9, 129.1, 132.0, 134.2, 141.1, 208.1; exact mass calcd for C19H24O: 268.1827; Found 268.1836.
1-Phenyl-3-o-tolylbutan-1-one (33). [α]24D = −20.1 (c 0.42, CHCl3), 81% ee [HPLC conditions: Chiralpak IB column, hexane/2-propanol = 99.8:0.2, flow = 0.5 mL/min, wavelength = 254 nm, tR = 22.4 min and 24.2 min]; 1H-NMR (CDCl3) δ = 1.31 (d, J = 6.8 Hz, 3H), 2.41 (s, 3H), 3.21 (dd, J = 8.2, 16.7 Hz, 1H), 3.30 (dd, J = 5.4, 16.7 Hz, 1H), 3.74–3.82 (m, 1H), 7.10–7.29 (m, 3H), 7.28 (d, J = 6.8 Hz, 1H), 7.46 (t, J = 7.7 Hz, 2H), 7.53–7.58 (m, 1H), 7.96 (d, J = 7.2 Hz, 2H); 13C-NMR (CDCl3) δ = 19.7, 21.5, 30.5, 46.4, 125.3, 125.4, 127.1, 127.9, 128.2, 129.5, 130.6, 132.3, 133.9, 135.4, 137.3, 144.9, 199.3; exact mass calcd for C17H18O: 238.1358; Found 238.1358.
3-(2-Methoxyphenyl)-1-phenylbutan-1-one (34). [α]22D = +6.73 (c 0.54, CHCl3), 92% ee [HPLC conditions: Chiralpak IB column, hexane/2-propanol = 99.8:0.2, flow = 0.5 mL/min, wavelength = 254 nm, tR = 30.4 min and 58.1 min]; 1H-NMR (CDCl3) δ = 1.32 (d, J = 6.8 Hz, 3H), 3.03–3.09 (m, 1H), 3.37 (dd, J = 4.5, 15.9 Hz, 1H), 3.80–3.90 (m, 1H), 3.82 (s, 3H), 6.86 (d, J = 8.2 Hz, 1H), 6.94 (t, J = 7.3 Hz, 1H), 7.26–7.18 (m, 2H), 7.45 (t, J = 7.7 Hz, 2H), 7.55 (t, J = 7.3 Hz, 1H), 7.99 (d, J = 7.7 Hz, 2H); 13C-NMR (CDCl3) δ = 19.9, 29.7, 46.1, 55.4, 110.6, 120.7, 127.3, 127.5, 127.8, 128.1, 129.1, 129.4, 132.2, 133.8, 134.5, 137.3, 156.9, 199.8; exact mass calcd for C17H18O2: 254.1307 ; Found 254.1317.
3-(2-Fluorophenyl)-1-phenylbutan-1-one (35). [α]23D = −2.91 (c 0.53, CHCl3), 95% ee [HPLC conditions: Chiralpak IB column, hexane/2-propanol = 99.8:2, flow = 0.5 mL/min, wavelength = 254 nm, tR = 22.2 min and 24.2 min]; 1H-NMR (CDCl3) δ = 1.35 (d, J = 6.8 Hz, 3H), 3.21 (dd, J = 8.2, 16.8 Hz, 1H), 3.38 (dd, J = 5.9, 16.8 Hz, 1H), 3.71–3.80 (m, 1H), 6.98–7.09 (m, 2H), 7.14–7.20 (m, 1H), 7.27 (ddt, J = 1.36, 1.81, 7.7 Hz, 1H), 7.44 (t, J = 7.7 Hz, 2H), 7.54 (dd, J = 7.3, 7.7 Hz, 1H), 7.95 (dd, J = 1.4, 7.3 Hz, 2H); 13C-NMR (CDCl3) δ = 20.7, 29.9, 45.4, 115.7, 123.5, 125.5, 127.0, 127.4, 127.9, 129.0, 129.5, 132.3, 134.0, 137.1, 160.9, 198.9; exact mass calcd for C16H15FO: 242.1107; Found 242.1119.
3-Naphthalen-1-yl-1-phenylbutan-1-one (36). [α]22D = −56.3 (c 0.51, CHCl3), 83% ee [HPLC conditions: Chiralpak IB column, hexane/2-propanol = 99.8:0.2, flow = 0.5 mL/min, wavelength = 254 nm, tR = 47.3 min and 63.0 min]; 1H-NMR (CDCl3) δ = 1.50 (d, J = 6.8 Hz, 3H), 3.32–3.45 (m, 2H), 4.39–4.48 (m, 1H), 7.44–7.59 (m, 7H), 7.75 (dd, J = 4.5, 5.0 Hz, 1H), 7.89 (d, J = 8.2 Hz, 1H), 7.98 (d, J = 7.7 Hz, 2H), 8.22 (d, J = 8.6 Hz, 1H); 13C-NMR (CDCl3) δ = 21.2, 29.7, 46.8, 122.6, 123.3, 125.2, 125.7, 126.2, 127.1, 127.4, 128.2, 128.7, 129.0, 129.9, 131.2, 132.4, 134.0, 137.3, 142.7, 199.5; exact mass calcd for C20H18O: 274.1358; Found 274.1358.
(R)-4-Phenyl-4-o-tolylbutan-2-one (37). [α]24D = −66.6 (c 0.52, CHCl3), 90% ee [HPLC conditions: Chiralcel OD-H column, hexane/ethanol = 9:1, flow = 0.5 mL/min, wavelength = 254 nm, tR = 14.8 min and 16.8 min]; 1H-NMR (CDCl3) δ = 2.07 (s, 3H), 2.30 (s, 3H), 3.15 (d, J = 7.2 Hz, 2H), 4.78 (t, J = 7.2 Hz, 1H), 7.09–7.13 (m, 2H), 7.13–7.20 (m, 4H), 7.20–7.24 (m, 3H); 13C-NMR (CDCl3) δ = 19.8, 30.7, 41.9, 50.0, 126.0, 126.3, 126.3, 126.4, 127.9, 128.5, 130.8, 136.4, 141.5, 143.5, 206.9; exact mass calcd for C17H18O: 238.1358; Found 238.1373.
2-Methyl-4-pentyl-4H-chromene (47). [α]22D = +282.1 (c 0.70, THF), 98% ee [HPLC conditions: Chiralcel OD-H column, hexane/ethanol = 100:1, flow = 0.5 mL/min, wavelength = 254 nm, tR = 7.5 min and 8.6 min]; 1H-NMR (CD2Cl2) δ = 0.84 (t, J = 6.8 Hz, 3H), 1.17–1.33 (m, 6H), 1.51–1.59 (m, 2H), 1.87 (s, 3H), 3.38 (d, J = 5.4 Hz, 1H), 4.70 (d, J = 4.1 Hz, 1H), 6.81–6.83 (m, 1H), 6.94–6.99 (m, 1H), 7.07–7.11 (m, 1H); 13C-NMR (CD2Cl2) δ = 13.9, 19.1, 23.9, 31.4, 33.9, 39.5, 99.9, 115.8, 122.8, 124.6, 126.9, 128.4, 147.5, 151.9; exact mass calcd for C15H20O: 216.1514; found 216.1517.
4. Conclusions
We have synthesized a new bidentate phosphoramidite, N-Me-BIPAM, based on Shibasaki’s N-linked BINOL. This ligand was found to be an excellent ligand for both cyclic and acyclic enones. Due to its low electron-donating property, the reactions were completed in a shorter time at room temperature than that of traditional BINAP complexes. Furthermore, N-Me-BIPAM allowed the 1,4-addition of ortho-substituted arylboronic acid to acyclic and cyclic enones with high enantioselectivities.
Footnotes
Sample Availability: Samples of the bidentate phosphoramidite are available from the authors.
References
- 1.Krause N., Hoffmann-Röder A. Recent advances in catalytic enantioselective Michael additions. Synthesis. 2001;2001:171–196. doi: 10.1055/s-2001-10803. [DOI] [Google Scholar]
- 2.Tomioka K., Nagaoka Y. Conjugate Addition of Organometallic Reagents. In: Jacobsen E.N., Pfalts A., Yamamoto H., editors. Comprehensive Asymmetric Catalysis. Vol. 31.1. Springer Verlag; Berlin, Germany: 1999. pp. 1105–1120. [Google Scholar]
- 3.Chan A.S.C., Kwong F.Y., Lu G. C-C Bond Formation through Conjugate Addition of C-M to C=C-C=O and C=C-NO2. In: Crabtree R.H., Mingos S.M.P., editors. Comprehensive Organometallic Chemistry III. 10.08. Vol. 10. Elsevier; Oxford, UK: 2007. pp. 369–491. [Google Scholar]
- 4.Hayashi T. Rhodium-catalyzed asymmetric 1,4-addition of organoboronic acids and their derivatives to electron deficient olefins. Synlett. 2001;2001:879–887. doi: 10.1055/s-2001-14657. [DOI] [Google Scholar]
- 5.Fagnou K., Lautens M. Rhodium-catalyzed carbon-carbon bond forming reactions of organometallic compounds. Chem. Rev. 2003;103:169–196. doi: 10.1021/cr020007u. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi T., Yamazaki K. Rhodium-catalyzed asymmetric 1,4-addition and its related asymmetric reactions. Chem. Rev. 2003;103:2829–2844. doi: 10.1021/cr020022z. [DOI] [PubMed] [Google Scholar]
- 7.Tian P., Dong H.-Q., Lin G.-Q. Rhodium-catalyzed asymmetric arylation. ACS Catal. 2012;2:95–119. doi: 10.1021/cs200562n. [DOI] [Google Scholar]
- 8.Berthon-Gelloz G., Hayashi T. Rhodium-catalyzed asymmetric conjugate addition of organoboronic acids. In: Hall D.G., editor. Boronic Acids Preparation and Applications in Organic Synthesis, Medicine and Materials. 2nd. Vol. 5. Wiley-VHC; Weinheim, Germany: 2011. pp. 263–313. [Google Scholar]
- 9.Narui R., Hayashi S., Oromo H., Shintani R., Hayashi T. (S)-Phenylalanine-derived chiral phosphorus-olefin ligands in rhodium-catalyzed asymmetric 1,4-addition reactions. Tetrahedron Asymmetry. 2012;23:284–293. doi: 10.1016/j.tetasy.2012.02.007. [DOI] [Google Scholar]
- 10.Grugel H., Albrecht F., Minuth T., Boysen M.M. Efficient pseudo-enantiomeric carbohydrate olefin ligands. Org. Lett. 2012;14:3780–3783. doi: 10.1021/ol3015896. [DOI] [PubMed] [Google Scholar]
- 11.Jin S.-S., Wang H., Zhu T.-S., Xu M.-H. Design of N-cinnamyl sulfinamides as new sulfur-containing olefin ligands for asymmetric catalysis: Achieving structural simplicity with a categorical linear framework. Org. Biomol. Chem. 2012;10:1764–1768. doi: 10.1039/c2ob06723d. [DOI] [PubMed] [Google Scholar]
- 12.Xue F., Li X., Wan B. A class of benzene backbone-base olefin-sulfoxide ligands for Rh-catalyzed enantioselective addition of arylboronic acids to enones. J. Org. Chm. 2011;76:7256–7262. doi: 10.1021/jo2011472. [DOI] [PubMed] [Google Scholar]
- 13.Qi W.-Y., Zhu T.-S., Xu M.-H. Design of chiral sulfoxide-olefins as a new class of sulfur-based olefin ligands for asymmetric catalysis. Org. Lett. 2011;13:3410–3413. doi: 10.1021/ol201151r. [DOI] [PubMed] [Google Scholar]
- 14.Jin S.-S., Wang H., Xu M.-H. Design of N-sulfinyl homoallylic amines as novel sulfinamide-olefin hybrid ligands for asymmetric catalysis: Application in Rh-catalyzed enantioselective 1,4-additions. Chem. Commun. 2011;47:7230–7232. doi: 10.1039/c1cc12322j. [DOI] [PubMed] [Google Scholar]
- 15.Thaler T., Guo L.-N., Steibo A.K., Raducan M., Karaghiosoff K., Mayer P., Knochel P. Sulfoxide-alkene hybrids: A new class of chiral ligands for the Hayashi-Miyaura reaction. Org. Lett. 2011;13:3182–3185. doi: 10.1021/ol200841x. [DOI] [PubMed] [Google Scholar]
- 16.Feng X., Wei B., Yang J., Du H. Chiral N-tert-butanesulfinyl a,b-unsaturated ketimine: A simple and highly effective olefin/sulfinimide hybrid ligand for asymmetric 1,4-additions. Org. Biomol. Chem. 2011;9:5927–5929. doi: 10.1039/c1ob05971h. [DOI] [PubMed] [Google Scholar]
- 17.Chen G., Gui J., Li L., Liao J. Chiral sulfoxide-olefin ligands: Completely switchable stereoselective in rhodium-catalyzed asymmetric conjugate additions. Angew. Chem. Int. Ed. Engl. 2011;50:7681–7685. doi: 10.1002/anie.201102586. [DOI] [PubMed] [Google Scholar]
- 18.Hahn B.T., Tewes F., Fröhlich R., Glorius F. Olefin-oxazolines (OlefOx): Highly modular, easoly tunable ligands for asymmetric catalysis. Angew. Chem. Int. Ed. Engl. 2010;49:1143–1146. doi: 10.1002/anie.200905712. [DOI] [PubMed] [Google Scholar]
- 19.Lang F., Li D., Chen J., Chen J., Li L., Cun L., Zhu J., Deng J., Liao J. tert-Butanesulfinylphosphines: Simple chiral ligands in rhodium-catalyzed asymmetric addition of arylboronic acids to electron-deficient olefins. Adv. Synth. Catal. 2010;352:843–846. doi: 10.1002/adsc.200900792. [DOI] [Google Scholar]
- 20.Bürgi J.J., Mariz R., Gatti M., Drinkel E., Luan X., Blumentritt S., Linden A., Dorta R. Unprecedented selectivity via electronic substrate recognition in the 1,4-addition to cyclic olefins using a chiral disulfoxide rhodium catalyst. Angew. Chem. Int. Ed. Engl. 2009;48:2768–2771. doi: 10.1002/anie.200900429. [DOI] [PubMed] [Google Scholar]
- 21.Mariz R., Luan X., Gatti M., Linden A., Dorta R. A chiral bis-sulfoxide ligand in late-transition metal catalysis; rhodium-catalyzed asymmetric addition of arylboronic acids to electron-deficient olefins. J. Am. Chem. Soc. 2008;130:2172–2173. doi: 10.1021/ja710665q. [DOI] [PubMed] [Google Scholar]
- 22.Iguchi Y., Itooka R., Miyaura N. Asymmetric conjugate addition of arylboronic acids to enones catalyzed by rhodium-monodentate phosphoramidite Complexes in the presence of vases. Synlett. 2003;2003:1040–1042. [Google Scholar]
- 23.Boiteau J.-G., Imbos R., Minnerd A.J., Feringa B.L. Rhodium-catalyzed asymmetric conjugate additions of boronic acids using monodentate phosphoramidite ligands. Org. Lett. 2003;5:681–684. doi: 10.1021/ol027465+. [DOI] [PubMed] [Google Scholar]
- 24.Duursma A., Hen J., Schuppan J., Minnerd J., Feringa B.L. First examples of improved catalytic asymmetric C-C bond formation using the monodentate ligand combination approach. Org. Lett. 2003;5:3111–3113. doi: 10.1021/ol035106o. [DOI] [PubMed] [Google Scholar]
- 25.Boiteau J.-G., Minnerd A.J., Feringa B.L. High efficiency and enantioselectivity in the Rh-catalyzed conjugate addition of arylboronic acids using monodentate phosphoramidites. J. Org. Chem. 2003;68:9481–9484. doi: 10.1021/jo035155e. [DOI] [PubMed] [Google Scholar]
- 26.Duursma A., Boiteau J.-G., Lefort L., Boorgers J.A.F., de Vries A.H.M., de Vries J.G., Minnerd A.J., Feringa B.L. Highly enantioselective conjugate additions of potassium organotrifluoroborates to enones by use of monodentate phosphoramidite ligands. J. Org. Chem. 2004;69:8045–8052. doi: 10.1021/jo0487810. [DOI] [PubMed] [Google Scholar]
- 27.Martina S.L.X., Minnerd A.J., Hesssen B., Feringa B.L. Highly enantioselective rhodium-catalyzed conjugate addition of arylboronic acids to enones at room temperature. Tetrahedron Lett. 2005;46:7159–7163. doi: 10.1016/j.tetlet.2005.08.095. [DOI] [Google Scholar]
- 28.Drinkel E., Brieño A., Dorta R., Dorta R. Hemilabile P-alkene ligands in chiral rhodium and copper complexes: Catalytic asymmetric 1,4-additions to enones 2. Organometallics. 2010;29:2503–2514. doi: 10.1021/om100248u. [DOI] [Google Scholar]
- 29.Reetz M.T., Moulin D., Gosberg A. BINOL-based diphosphonites as ligands in the asymmetric Rh-catalyzed conjugate addition of arylboronic acids. Org. Lett. 2001;3:4083–4085. doi: 10.1021/ol010219y. [DOI] [PubMed] [Google Scholar]
- 30.Kuriyama M., Tomioka K. Chiral amidomonophosphine-rhodium(I) catalyst for asymmetric 1,4-addition of arylboronic acids to cycloalkenones. Tetrahedron Lett. 2001;42:921–923. doi: 10.1016/S0040-4039(00)02130-4. [DOI] [Google Scholar]
- 31.Kuriyama M., Nagai K., Yamada K., Miwa Y., Tomioka K. Hemilabile amidomonophosphine ligand-rhodium(I) complex-catalyzed asymmetric 1,4-addition of arylboronic acids to cycloalkenones. J. Am. Chem. Soc. 2002;124:8932–8939. doi: 10.1021/ja0261933. [DOI] [PubMed] [Google Scholar]
- 32.Ma Y., Song C., Ma C., Sun Z., Chai Q., Andrus M.B. asymmetric addition of aryl boron reagents to enones with Rhodium dicyclophane imidazolium carbene catalysis. Angew. Chem. Int. Ed. Engl. 2003;42:5871–5874. doi: 10.1002/anie.200352679. [DOI] [PubMed] [Google Scholar]
- 33.Becht J.-M., Bappert E., Helmchen G. Application of rhodium complexes of chiral diphenylphosphino-functionalized N-heterocyclic carbenes as catalysts in enantioselective conjugate additions of arylboronic acids. Adv. Synth. Catal. 2005;347:1495–1498. doi: 10.1002/adsc.200404327. [DOI] [Google Scholar]
- 34.Imamoto T., Sugita K., Yoshida K. An Air-Stable P-Chiral Phosphine Ligand for Highly enantioselective transition-metal-catalyzed reactions. J. Am. Chem. Soc. 2005;127:11934–11935. doi: 10.1021/ja053458f. [DOI] [PubMed] [Google Scholar]
- 35.Hayashi T., Ueyama K., Tokunaga N., Yoshida K. A chiral chelating diene as a new type of chiral ligand for transition metal catalysts: Its preparation and use for the rhodium-catalyzed asymmetric 1,4-addition. J. Am. Chem. Soc. 2003;125:11508–11509. doi: 10.1021/ja037367z. [DOI] [PubMed] [Google Scholar]
- 36.Tokunaga N., Otomaru Y., Okamoto K., Ueyama K., Shintani R., Hayashi T. C2-symmetric bicyclo[2.2.2]octadienes as chiral ligands: Their high performance in rhodium-catalyzed asymmetric arylation of N-tosylarylimines. J. Am. Chem. Soc. 2004;126:13584–13585. doi: 10.1021/ja044790e. [DOI] [PubMed] [Google Scholar]
- 37.Defieber C., Paquin J.-F., Serna S., Carreira E.M. Chiral [2.2.2] dienes as ligands for Rh(I) in conjugate additions of boronic acids to a wide range of acceptors. Org. Lett. 2004;6:3873–3876. doi: 10.1021/ol048240x. [DOI] [PubMed] [Google Scholar]
- 38.Otomura Y., Okamoto K., Shintani R., Hayashi T. Preparation of C2-symmetric bicyclo [2.2.2]octa-2,5-diene ligands and their use for rhodium-catalyzed asymmetric 1,4-addition of arylboronic acids. J. Org. Chem. 2005;70:2503–2508. doi: 10.1021/jo047831y. [DOI] [PubMed] [Google Scholar]
- 39.Chen F.-X., Kina A., Hayashi T. High performance of a chiral diene-rhodium catalyst for the asymmetric 1,4-addition of arylboroxines to α,β-unsaturated ketones. Org. Lett. 2006;8:341–344. doi: 10.1021/ol052756e. [DOI] [PubMed] [Google Scholar]
- 40.Feng C.-G., Wang Z.-Q., Shao C., Xu M.-H., Lin G.-Q. Highly practical catalytic asymmetric 1,4-addition of arylboronic acids in water using new hydrophilic chiral bicyclo[3.3.0]diene ligands. Org. Lett. 2008;10:4101–4104. doi: 10.1021/ol801665z. [DOI] [PubMed] [Google Scholar]
- 41.Hu X., Zhuang Z., Cao Z., Du H. Simple chiral chain dienes as ligands for Rh(I)-catalyzed conjugated additions. Org. Lett. 2009;11:4744–4747. doi: 10.1021/ol901949n. [DOI] [PubMed] [Google Scholar]
- 42.Li Q., Dong Z., Yu Z.-X. α,β-Divinyl terahydropyrroles as chiral chain diene ligands in rhodium(I)-catalyzed enantioselective conjugated additions. Org. Lett. 2011;13:1122–1125. doi: 10.1021/ol103152t. [DOI] [PubMed] [Google Scholar]
- 43.Liu C.-C., Janmanchi D., Chen C.-C., Wu H.L. Expanding the C1-symmetric bicylo[2.2.1]heptadiene ligand family: Highly enantioselective synthesis of cyclic β-aryl-substituted carbonyl compounds. Eur. J. Org. Chem. 2012;2012:2503–2507. doi: 10.1002/ejoc.201200143. [DOI] [Google Scholar]
- 44.Shi Q., Xu L., Li X., Jia X., Wang R., Au-Yeung T.T.-L., Chan A.S.C., Hayashi T., Cao R.C., Hong M. Bipyridyl-based diphosphine as an efficient ligand in the rhodium-catalyzed asymmetric conjugate addition of arylboronic acids to α,β-unsaturated ketones. Tetrahedron Lett. 2003;55:6505–6508. [Google Scholar]
- 45.Madec J., Michaud G., Genêt J.-P., Marinetti A. New developments in the synthesis of heterotopic atropisomeric diphosphines via diastereoselective aryl coupling reactions. Tetrahedron: Asymmetry. 2004;15:2253–2261. doi: 10.1016/j.tetasy.2004.05.014. [DOI] [Google Scholar]
- 46.Stemmler R.T., Bolm C. Planar-chiral cyrhetrenes for the Rhodium-catalyzed asymmetric 1,4-addition and the hydrogenation of enamides. J. Org. Chem. 2005;70:9925–9931. doi: 10.1021/jo051676l. [DOI] [PubMed] [Google Scholar]
- 47.Vandyck K., Matthys B., Willen M., Robeyns K., Meervelt L.C., der Eychen J.V. Rhodium-catalyzed asymmetric conjugate additions of boronic acids to enones using DIPHONANE: A novel chiral bisphosphine ligand. Org. Lett. 2006;8:363–366. doi: 10.1021/ol0522788. [DOI] [PubMed] [Google Scholar]
- 48.Duan W.-L., Iwamura H., Shintani R., Hayashi T. Chiral Phosphine-Olefin Ligands in the Rhodium-Catalyzed Asymmetric 1,4-Addition Reactions. J. Am. Chem. Soc. 2007;129:2130–2138. doi: 10.1021/ja0671013. [DOI] [PubMed] [Google Scholar]
- 49.Monti C., Gennari C., Piarulli U. Rh-catalyzed enantioselective conjugate addition of arylboronic acids with a dynamic library of chiral tropos phosphorus ligands. Chem. Eur. J. 2007;13:1547–1558. doi: 10.1002/chem.200600960. [DOI] [PubMed] [Google Scholar]
- 50.Mariz R., Luan X., Gatti M., Linden A., Dorta R. A chiral bis-sulfoxide ligand in late-transition metal catalysis; rhodium-catalyzed asymmetric addition of arylboronic acids to electron-deficient olefins. J. Am. Chem. Soc. 2008;130:2172–2173. doi: 10.1021/ja710665q. [DOI] [PubMed] [Google Scholar]
- 51.Liao Y.-X., Xing C.-H., Israel M., Hu Q.-S. Sequential aldol condensation-transition metal-catalyzed addition reactions of aldehydes, methyl ketones, and arylboronic acids. Org. Lett. 2011;13:2058–2061. doi: 10.1021/ol200457q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wei W.-T., Yeh J.-Y., Kuo T.-S., Wu H.-L. Highly enantioselective rhodium-catalyzed asymmetric 1,4-addition reactions of arylboronic acids to acyclic α,β-unsaturated compounds: The formal synthesis of (−)-Indatraline. Chem. Eur. J. 2011;17:11405–11409. doi: 10.1002/chem.201102073. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto Y., Kurihara K., Sugishita N., Oshita K., Piao D., Miyaura N. Chiral bis-phosphoramidites based on linked-BINOL for rhodium-catalyzed 1,4-addition of arylboronic acids to α,β-unsaturated carbonyl compounds. Chem. Lett. 2005;34:1224–1225. doi: 10.1246/cl.2005.1224. [DOI] [Google Scholar]
- 54.Kurihara K., Sugishita N., Oshita K., Piao D., Yamamoto Y., Miyaura N. Enantioselective 1,4-addition of arylboronic acids to α,β-unsaturated carbonyl compounds catalyzed by rhodium(I)-chiral phosphoramidite complexes. J. Organomet. Chem. 2007;692:428–435. doi: 10.1016/j.jorganchem.2006.04.042. [DOI] [Google Scholar]
- 55.Majima K., Takita R., Okada A., Oshima T., Shibasaki M. Catalytic asymmetric michael reaction of β-keto esters: Effects of the linker heteroatom in linked-BINOL. J. Am. Chem. Soc. 2003;125:15837–15845. doi: 10.1021/ja037635t. [DOI] [PubMed] [Google Scholar]
- 56.Kurihra K., Yamamoto Y., Miyaura N. An N-linked bidentate phosphoramidite ligand (N-Me-BIPAM) for Rhodium-catalyzed asymmetric addition of arylboronic acids to N-sulfonylarylaldimines. Adv. Synth. Catal. 2009;351:260–270. doi: 10.1002/adsc.200800631. [DOI] [Google Scholar]
- 57.Zacheis D., Dhar A., Lu S., Madler M.M., Klucik J., Brown C.W., Liu S., Clement F., Subramanian S., Weerasekare G.M., et al. Heteroarotinoids inhibit head and neck cancer cell lines in vitro and in vivo through both RAR and RXR retinoic acid receptors. J. Med. Chem. 1999;42:4434–4445. doi: 10.1021/jm990292i. [DOI] [PubMed] [Google Scholar]
- 58.Nishikata T., Yamamoto Y., Miyaura N. Palladium(II)-catalyzed 1,4-addition of arylboronic acids to β-arylenones for enantioselective synthesis of 4-aryl-4H-chromenes. Adv. Synth. Catal. 2007;349:1759–1764. doi: 10.1002/adsc.200600622. [DOI] [Google Scholar]
- 59.Itooka R., Iguchi Y., Miyaura N. Rhodium-catalyzed 1,4-addition of arylboronic acids to α,β-unsaturated carbonyl compounds: Large accelerating effects of bases and ligands. J. Org. Chem. 2003;68:6000–6004. doi: 10.1021/jo0207067. [DOI] [PubMed] [Google Scholar]