Abstract
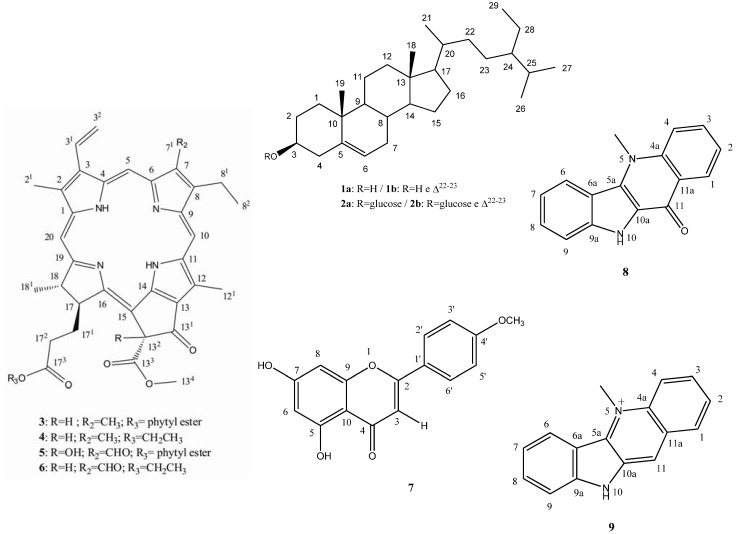
The phytochemical study of Sida rhombifolia L. (Malvaceae) led to the isolation through chromatographic techniques of eleven secondary metabolites: sitosterol (1a) and stigmasterol (1b), sitosterol-3-O-β-d-glucopyranoside (2a) and stigmasterol-3-O-β-d-glucopyranoside (2b), phaeophytin A (3), 173-ethoxypheophorbide A (4), 132-hydroxy phaeophytin B (5), 173-ethoxypheophorbide B (6), 5,7-dihydroxy-4'-methoxyflavone (7), cryptolepinone (8) and a salt of cryptolepine (9). Their structures were identified by 1H- and 13C-NMR using one- and two-dimensional techniques. In addition, the vasorelaxant activity of cryptolepinone in rat mesenteric artery rings is reported herein for the first time.
Keywords: phytochemical study, Sida rhombifolia L., Malvaceae, cryptolepinone, vasorelaxant activity
1. Introduction
The genus Sida has wide neotropical distribution, with several species well represented in the Americas. In Brazil this genus has many representatives in the Northeast and South and, to a lesser extent, in the North, Midwest and Southeast [1]. Sida rhombifolia (Malvaceae) is popularly known in Brazil as “matapasto”, “guanxuma” and “relógio”. It is used in Indian folklore medicine against hypertension, diabetes [2], as well as a traditional medicinal plant for the treatment of gout in Indonesia [3].
A variety of chemical constituents has been isolated from this species, including ecdysteroids [4] quinazoline alkaloids, besides β-phenylethylamines and carboxylated tryptamines [5]. The present study aimed to isolate and identify other classes of secondary metabolites from S. rhombifolia in order to associate them to pharmacological properties. We report the isolation and identification of two mixtures of steroids 1a, 1b and 2a and 2b, four porphyrins 3, 4, 5 and 6, a flavone 7, and two indoquinoline alkaloids 8 and 9, besides showing for the first time the vasorelaxant activity of 8 in rat mesenteric artery with and without functional endothelium.
2. Results and Discussion
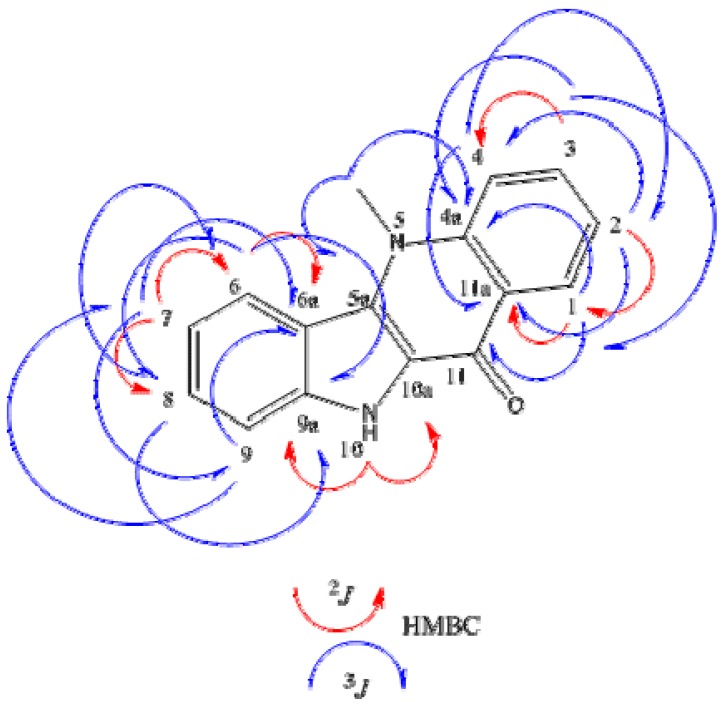
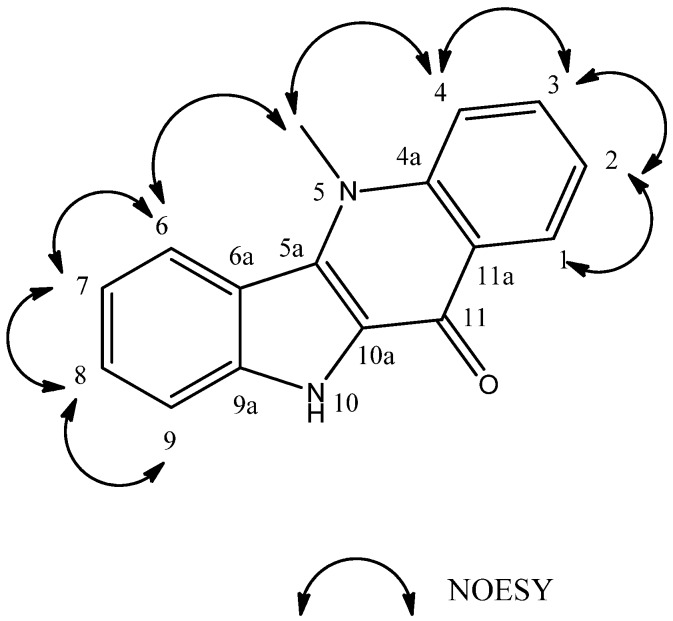
Compound 8 appeared as yellow crystals with melting point >300 °C. The IR spectrum indicated the presence of NH (3431 cm−1) and carbonyl (1622 cm−1) groups. The 1H-NMR spectra of compound 8 (DMSO-d6) showed signals of indoquinoline-like alkaloids consisting of a double doublet [δH 8.44 (1H, dd, J = 8.1 and 1.4 Hz, H-1)], two doublet-doublet-triplets [δH 7.35 (1H, ddt, J = 8.1, 7.3 and 0.8 Hz, H-2)], 7.77 (1H, ddt, J = 8.7, 7.3 and 1.4 Hz, H-3)] and a broad doublet [δH 7.95 (1H, brd, J = 8.7 Hz, H-4)], characterizing the quinoline nucleus, besides two broad doublets [δH 8.38 (1H, brd, J = 8.4 Hz, H-6), 7.57 (1H, brd, J = 8.3 Hz, H-9)] and two other doublet-doublet-triplets [δH 7.47 (1H, ddt, J = 8.3, 7.6 and 1.0 Hz, H-8), 7.20 (1H, ddt, J = 8.4, 7.6 and 1.0 Hz, H-7)] characterizing the indole nucleus, thus leading to conclude that the indoquinoline nucleus was unsubstituted [6,7]. Furthermore, the spectra showed the presence of two singlets [δH 11.89 (1H, s), 4.36 (3H, s)] due to an indole NH and a methyl group bonded to nitrogen, respectively [6]. The 13C BB-NMR spectrum of 8 (DMSO-d6) showed 16 signals, consistent with an unsubstituted indoquinoline nucleus, being 15 aromatic carbons and one sp3-hybridized carbon. The presence of a methyl group bonded to nitrogen [δC 35.41] and a carbonyl [δC 166.45] could be highlighted. Based on the 2D NMR data of HMQC experiment the hydrogenated carbons found in 13C BB-NMR spectrum were established. The 2D NMR data of HMBC experiment showed the position of the nitrogen methyl in the quinoline nucleus due to the 3J correlation signal of the proton at δH 4.36 (s) to C-4a (δC 139.73) and C-5a (δC 129.89). The identity of hydrogen of the indole nucleus at δH 11.89 (s) was further confirmed by its 2J correlations to C-9a (δC 138.25) and C-10a (δC 123.03) and 3J correlations to C-6a (δC 115.70) and C-10a (δC 123.03) (Figure 1). The 2D NMR data of NOESY experiment (Figure 2) confirmed the position of the methyl at N-5 due to the correlations of the protons at δH-51 4.36 (s) with the signals at δH-4 7.96 (d, J = 8.70 Hz) and δH-6 8.39 (d, J = 8.40 Hz) (Table 1). In addition, correlations between the protons from quinoline (H-1/H-2; H-2/H3; H-3/H-4) and indole nuclei (H-6/H-7, H-7/H-8; H-8/H-9) were established (Table 1) (Figure 1). Spectral data compilation and comparison of data in the literature [5] showed that 8 is cryptolepinone, a compound already isolated in the genus Sida [8], but isolated now for the first time from the species Sida rhombifolia.
Figure 1.
HMBC correlations of compound 8.
Figure 2.
NOESY correlations of compound 8.
Table 1.
13C-NMR (125 MHz), 1H-NMR (500 MHz), HMQC, HMBC and NOESY data of cryptolepinone (8) (DMSO-d6, δ ppm).
| HMQC | HMBC | NOESY | |||||
2
J (H C) C) |
3
J (H C) C) |
(H↔H) | |||||
| n° | δC | δH | |||||
| 1 | 124.99 | 8.44 (dd, 1H, J = 8.1 and 1.4 Hz) | C-11a | C-11 | C-4a | H-2 | |
| 2 | 120.04 | 7.35 (ddt, 1H, J = 8.1; 7.3 and 0.8 Hz) | C-1 | C-4 | C-11a | H-1/H-3 | |
| 3 | 130.71 | 7.77 (ddt, 1H, J = 8.7; 7.3 and 1.4 Hz) | C-4 | C-1 | C-4a | H-2/H-4 | |
| 4 | 115.13 | 7.95 (brd, 1H, J = 8.7 Hz) | C-2 | C-11a | H-3 | ||
| 4a | 139.73 | - | |||||
| 5 | - | - | |||||
| 5a | 129.89 | - | |||||
| 6 | 122.45 | 8.38 (brd, 1H, J = 8,4 Hz) | C-6a | C-8 | C-9a | H-7 | |
| 6a | 115.70 | - | |||||
| 7 | 118.62 | 7.20 (ddt, 1H, J = 8.4; 7.6 and 1.0 Hz) | C-6 | C-8 | C-9 | C-6a | H-6/H-8 |
| 8 | 126.54 | 7.47 (ddt, 1H J = 8.3; 7.6 and 1.0 Hz) | C-6 | C-9a | H-7/H-9 | ||
| 9 | 112.36 | 7.57 (brd, 1H J = 8.3 Hz) | C-6a | C-7 | H-8 | ||
| 9a | 138.25 | - | |||||
| 10 (N-H) | - | 11.89 (s, 1H) | C-10a | C-9a | |||
| 10a | 123.03 | - | |||||
| 11 | 166.45 | - | |||||
| 11a | 122.90 | - | |||||
| N-CH3 | 35.41 | 4.36 (s, 3H) | C-4a | C-5a | H-4/H-6 | ||
Using similar methods as described above, compounds 1 to 7 and 9 were identified as sitosterol (1a) and stigmasterol (1b) [7], sitosterol-3-O-β-D-glucopyranoside (2a) and stigmasterol-3-O-β-D-glucopyranoside (2b) [9], phaeophytin A (3) [10], 173-ethoxypheophorbide A (4) [1], 132-hydroxy phaeophytin B (5) [11], 173-ethoxypheophorbide B (6) [12], 5,7-dihydroxy-4'-methoxyflavone (acacetin, 7) [13] and a salt of cryptolepine (9) [7] (Figure 3).
Figure 3.
Chemical constituents isolated and identified from the crude ethanol extract of the aerial parts of S. rhombifolia. (Malvaceae).
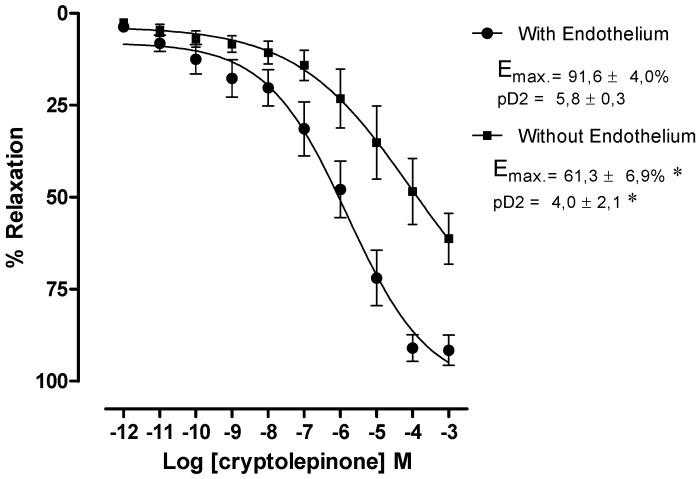
The vasorelaxant activity of 8 was evaluated by increasing cumulative addition of 10−12 to 10−3 M in rat cranial mesenteric artery rings pre-contracted with phenylephrine (PHE) at the concentration of 1 μM. It was observed that 8 caused a vasorelaxation in rings with functional endothelium (Emax = 91.6 ± 4.0%, n = 6) and after removal of endothelium, the vasorelaxant effect of 8 was significantly changed (61.3 ± 6.9%, n = 7) (Figure 4).
Figure 4.
Concentration-response curve of 8 (10−12–10−3 M) in cranial mesenteric artery rings isolated from rats with functional endothelium (•) and without functional endothelium (■) pre-contracted with PHE (1 μM). Values are expressed as mean ± standard error of mean (SEM), * p < 0.05 compared to the ring with functional endothelium.
3. Experimental
3.1. General
Silica gel 60 (Merck) 7734 (0.063–0.2 mm particle, 70–230 mesh), flash silica (0.04–0.063 mm particles, 230–400 mesh), neutral alumina and Sephadex LH-20 were used for the fractionation and isolation of the secondary metabolites from S. rhombifolia. The melting point of the constituents was recorded on a MQAPF-302 apparatus (Microquímica Equipamentos Ltda., Palhoça-SC, Brazil). IR spectra were recorded on a FT-IR-1750 Perkin-Elmer spectrometer. 1H- and 13C-NMR spectra were recorded on a Varian Oxford 200 NMR spectrometer (200/50 MHz) and on a Varian 500 NMR spectrometer (500/125 MHz).
3.2. Collection, Extraction and Isolation
Sida rhombifolia was collected in the municipality of Santa Rita-PB in September 2008 and identified by Dr. Maria de Fátima Agra. A voucher specimen is kept at the herbarium Professor Lauro Pires Xavier (CCEN/UFPB) under the code Agra 7045. The dried and powdered aerial parts of the plant (5.5 kg) were extracted with 95% EtOH (10 L, room temperature) for 72 h. The EtOH extract was concentrated under reduced pressure at 40 °C, providing 570 g of crude ethanol extract (CEE). Part of the CEE (200.0 g) was subjected to filtration under reduced pressure using silica gel 60 as stationary phase and eluted with hexane (Hex.), ethyl acetate (EtOAc) and methanol (MeOH) alone or in binary mixtures following an increasing gradient polarity. The fractions obtained were concentrated under reduced pressure. Fraction Hex.–AcOEt (9:1) (6.0 g) was subjected to silica gel 60 column chromatography (CC) eluted with Hex., dichloromethane (CH2Cl2) and MeOH alone or in binary mixtures following an increasing gradient polarity, yielding 35 mg of compound 1. Fraction Hex.–AcOEt (7:3) (6.11 g) was subjected to the same methodology, but using as eluents Hex., EtOAc and MeOH. A fraction of this column was subjected to preparative TLC in Hex.–EtOAc (9:1) to give 35 mg of 3. Then, the initial TLC band was rechromatographed in Hex.–EtOAc (8:2) to give 5.0 mg of compound 5. Compound 2 (33.0 mg) was obtained from the fraction EtOAc (1.23 g) using the previous technique for column chromatography. Fraction EtOAc–MeOH (9:1) yielded a precipitate and a supernatant (1.24 g). The latter was subjected to CC on Sephadex LH-20 using methanol as eluent. This procedure yielded 10.0 mg of compound 7. Another part of the CEE (200.0 g) was dissolved in EtOH–H2O (9:1) and subjected to liquid-liquid chromatography using Hex., CH2Cl2, EtOAc and n-butanol, obtaining the respective phases, besides the hydroalcoholic phase. The CH2Cl2 phase (26.0 g) was subjected to filtration under reduced pressure with the solvents Hex., CH2Cl2 and MeOH. The fractions Hex.–CH2Cl2 (3:7) (17.4 mg) and CH2Cl2 (200.0 mg) were combined and subjected to CC using flash silica as adsorbent and the eluents Hex., EtOAc and MeOH alone or in binary mixtures following an increasing gradient polarity. Compounds 4 (25.0 mg) and 6 (15.0 mg) were thus obtained. The CEE of Sida rhombifolia reacted positively with Dragendorff, Bouchadart and Mayer reagents, as expected according to literature. The classic alkaloid extraction was performed with 100 g of the CEE. The total alkaloid content (800 mg) was subjected to CC on neutral alumina and the elution system was the same of the previous methodology. Compounds 8 (28.0 mg) and 9 (18.0 mg) were isolated.
Phaeophytin A (3) (without phytyl ester): Green powder. IR (KBr) vmax: 3435, 2926, 2854, 1735, 1701, 1618, 1377 cm−1. 1H-NMR (500.00 MHz) (CDCl3) δ: 3.37 (s, 3H, H-21), 7.92 (1H, dd, J = 17.3 and 11.2 Hz, H-31), 6.25 and 6.15 (2H, d and d, J = 17.3 and 11.2 Hz, H-32), 9.28 (s, 1H, H-5), 3.15 (s, 1H, H-71), 3.60 (m, 2H, H-81), 1.66 (m, 3H, H-82), 9.44 (s, 1H, H-5), 3.68 (s, 3H, H-121), 6.29 (s, 1H, H-132), 3.91 (s, 3H, H-134), 4.04 (m, 1H, H-17), 1.14 (m, 2H, 171), 4.30 (m, 1H, H-18), 1.85 (3H, d, J = 7.3 Hz, H-181), 8.57 (s, 1H, H-20). 13C-NMR (125.00 MHz) (CDCl3) δ: 142.26 (C-1), 131.08 (C-2), 12.26 (C-21), 136.69 (C-3), 129.21 (C-31), 122.95 (C-32), 136.69 (C-4), 97.65 (C-5), 155.58 (C-6), 136.34 (C-7), 11.33 (C-71), 145.30 (C-8), 19.57 (C-81), 17.54 (C-82), 149.93 (C-9), 104.72 (C-10), 138.11 (C-11), 128.46 (C-12), 12.29 (C-121), 128.12 (C-13), 189.83 (C-131), 64.93 (C-132), 173.14 (C-133), 53.20 (C-134), 149.93 (C-14), 105.48 (C-15), 161.17 (C-16), 51.55 (C-17), 29.91 (C-171), 31.40 (C-172), 173.23 (C-173), 50.33 (C-18), 23.38 (C-181), 172.46 (C-19), 93.41 (C-20).
173-Ethoxypheophorbide A (4): Bluish green powder with a metallic luster. 1H-NMR (500.00 MHz) (CDCl3) δ: 3.31 (s, 3H, H-21), 7.77 (1H, dd, J = 17.5 and 12.5 Hz, H-31), 6.14 and 6.05 (2H, d and d, J = 17.5 and 12.5Hz, H-32), 9.07 (s, 1H, H-5), 2.98 (s, 3H, H-71), 3.41 (2H, q, J = 15.0 Hz, H-81), 1.58 (3H, t, J = 10.0 Hz, H-82), 9.28 (s, 1H, H-10), 3.63 (s, 3H, H-121), 6.30 (s, 1H, H-132), 3.93 (s, 3H, H-134), 4.24 (1H, brd, J = 10.0 Hz, H-17), 1.27 (brs, 2H, H-171), 2.22–2.39 (2H, m, H-172), 4.07 (2H, q, J = 12.5 Hz, H-174), 1.15 (3H, t, J = 5.0 Hz, H-175), 4.48 (m, 1H, H-18), 1.85 (3H, d, J = 5.0 Hz, H-181), 8,53 (s, 1H, H-20). 13C-NMR (125.00 MHz) (CDCl3) δ: 141.93 (C-1), 131.66 (C-2), 11.93 (C-21), 135.90 (C-3), 128.83 (C-31), 122.47 (C-32), 135.94 (C-4), 97.24 (C-5), 155.37 (C-6), 135.90 (C-7), 10.89 (C-71), 144.88 (C-8), 19.14 (C-81), 17.22 (C-82), 150.74 (C-9), 104.12 (C-10), 137.78 (C-11), 128.80 (C-12), 11.98 (C-121), 128.80 (C-13), 189.58 (C-131), 64.72 (C-132), 172.89 (C-133), 52.80 (C-134), 149.60 (C-14), 105.14 (C-15), 161.17 (C-16), 51.16 (C-17), 29.82 (C-171), 31.26 (C-172), 172.11 (C-173), 60.48 (C-174), 14.06 (C-175), 50.10 (C-18), 23.04 (C-181), 169.61 (C-19), 92.98 (C-20).
132-Hydroxy phaeophytin B (5) (without phytyl ester): Yellow green powder. IR (KBr) vmax: 3435, 1161 cm−1. 1H-NMR (200.00 MHz) (CDCl3) δ: 3.59 (s, 3H, H-21), 8.02 (1H, dd, J = 17.5 and 11.6 Hz, H-31), 6.37 and 6.22 (2H, d and d, J = 17.5 and 11.6 Hz, H-32), 9.76 (s, 1H, H-5), 11.17 (s, 1H, H-71), 4.10 (2H, brd, J = 7.8 Hz, H-81), 1.83 (3H, t, J = 7.5 Hz, H-82), 10.47 (s, 1H, H-10), 3.71 (s, 1H, H-121), 3.85 (s, 3H, H-134), 5.31 (1H, brd, J = 3.4 Hz, H-17), 4.41 (m, 1H, H-18), 1.57 (3H, d, J = 7.6 Hz, H-181), 8.58 (s, 1H, H-20).
173-Ethoxypheophorbide B (6): Yellow green powder with a metallic luster. IR (KBr) vmax: 2729 cm−1. 1H-NMR (500.00 MHz) (CDCl3) δ: 3.36 (s, 3H, H-21), 7.97 (1H, dd, J = 15.0 and 10.0 Hz, H-31), 6.20 and 6.23 (2H, d and d, J = 15.0 and 10.0 Hz, H-32), 10.33 (s, 1H, H-5), 11.12 (s, 1H, H-71), 4.00 (m, 2H, H-81), 1.62 (m, 3H, H-82), 9.61 (s, 1H, H-10), 3.66 (s, 3H, H-121), 6.21 (s, 1H, H-132), 3.88 (s, 3H, H-134), 4.44 (1H, brd, J = 10.0 Hz, H-17), 2.46–2.61 (m, 2H, H-171), 2.20–2.33 (m, 2H, H-172), 4.00 (m, 2H, H-174), 1.10 (3H, t, J = 7.5 Hz, H-175), 4.18 (1H, brd, J = 10.0 Hz, H-18), 1.80 (3H, d, J = 5.0 Hz, H-181), 8.52 (s, 1H, H-20). 13C-NMR (125.00 MHz) (CDCl3) δ: 143.57 (C-1), 132.44 (C-2), 12.05 (C-21), 135.54 (C-3), 128.65 (C-31), 123.57 (C-32), 137.19 (C-4), 101.60 (C-5), 159.38 (C-6), 137.19 (C-7), 187.72 (C-71), 147.19 (C-8), 19.13 (C-81), 19.36 (C-82), 150.73 (C-9), 105.01 (C-10), 138.04 (C-11), 129.75 (C-12), 12.23 (C-121), 132.20 (C-13), 189.49 (C-131), 64.58 (C-132), 172.76 (C-133), 52.95 (C-134), 151.25 (C-14), 104.96 (C-15), 164.03 (C-16), 51.35 (C-17), 31.25 (C-171), 29.70 (C-172), 174.00 (C-173), 60.55 (C-174), 14.07 (C-175), 50.12 (C-18), 23.04 (C-181), 169.25 (C-19), 93.55 (C-20).
5,7-Dihydroxy-4'-methoxyflavone (7): Yellow crystals. 1H-NMR (500.00 MHz) (CD3OD) δ: 6.63 (s, 1H, H-3), 6.21 (1H, d, J = 1.75 Hz, H-6), 6.46 (1H, d, J = 1.75 Hz, H-8), 7.94 (2H, d, J = 9.0 Hz, H-2’/H-6’), 7.08 (2H, d, J = 9.0 Hz, H-3'/H-6'), 3.88 (s, 3H, OCH3 H-4'). 13C-NMR (125.00 MHz) (DMSO-d6) δ: 163.73 (C-2), 102.99 (C-3), 181.21 (C-4), 160.87 (C-5), 98.37 (C-6), 162.75 (C-7), 93.49 (C-8), 156.79 (C-9), 103.19 (C-10), 122.30 (C-1’), 127.74 (C-2'/C-6'), 114.04 (C-3'/C-5'), 161.76 (C-4'), 55.02 (OCH3 C-4').
Cryptolepinone (8): Yellow crystals. IR (KBr) vmax: 3,431, 1,622 cm−1. 1H-NMR and 13C-NMR: See Table 1.
Cryptolepine (9): Yellow crystals. 1H-NMR (500.00 MHz) (CD3OD) δ: 8.45 (1H, dd, J = 8.3 and 1.5 Hz, H-1), 7.91 (2H, m, H-2/H-8), 8.16 (1H, ddd, J = 9.1, 6.9 and 1.5 Hz, H-3), 8.63 (1H, brd, J = 9.1 Hz, H-4), 8.71 (1H, brd J = 8.5 Hz, H-6), 7.53 (1H, ddd, J = 8.5 , 7.1 and 1.0 Hz, H-7), 7.77 (1H, dd, J = 8.4 and 1.0 Hz, H-9), 9.11 (s, 1H, H-11), 5.07 (s, 3H, CH3 N-5). 13C-NMR (125.00 MHz) (CD3OD) δ: 131.10 (C-1), 128.50 (C-2), 134.00 (C-3), 118.30 (C-4), 139.80 (C-5a), 126.80 (C-6), 115.30 (C-6a), 123.00 (C-7), 135.40 (C-8), 114.30 (C-9), 147.70 (C-9a), 135.00 (C-10a), 126.10 (C-11), 128.00 (C-11a), 40.7 (CH3 N-5).
3.3. Bioactivity Assay
Experimental procedures were performed as described by França-Silva et al. [14] and were approved by the Federal University of Paraíba Ethical Committee for Animal Use under the protocol CEPA#305/09. After euthanasia by decaptation using a guilliotine, the cranial mesenteric artery was isolated, placed in Tyrode’s solution and dissected in order to make it free of adhering tissue. In endothelium-denuded experiments, endothelium was removed by rubbing the intimal surface of the vessels. Rings with 1–2 mm were obtained and placed in physiological Tyrode’s solution, maintained to 37 °C, gassed with carbogenic mixture (95% O2 and 5% CO2) and kept at pH 7.4. All preparations were stabilized under a resting tension of 0.75 g for 1 h. The solution was replaced every 15 min in order to prevent the accumulation of metabolites. The force of contraction was isometrically recorded by a force transducer (Miobath-4, WPI, Sarasota, FL, USA) coupled to an amplifier-recorder (Miobath-4) and to a computer equipped with an analog–to–digital converter board as described earlier. The presence of functional endothelium was assessed by the ability of acetylcholine (10 µM) to induce more than 80% relaxation of vessels pre-contracted with phenylephrine (10 µM). Less than 10% of relaxation to acetylcholine was taken as evidence that the vessel segments were functionally denuded of endothelium. The preparations were exposed to cumulatively concentrations of cryptolepinone (10−12–10−3 M) added to preparations until a maximum response to the drug accumulation was observed as indicated by a plateau response (approximately 3–5 min).
4. Conclusions
The phytochemical study of the crude ethanol extract from the aerial parts of Sida rhombifolia (Malvaceae) led to the identification of eleven compounds: two mixtures of steroids (1a and 1b; 2a and 2b), four porphyrins (3, 4, 5 and 6), a flavone (7), and two indoquinoline alkaloids (8 and 9).
The compounds 132-hydroxyphaeophytin B (5) and 173-ethoxypheophorbide B (6) are being described hering for the fiorst time in the family Malvaceae, and the flavonoid 5,7-dihydroxy-4'-methoxyflavone (acacetin, 7) is reported herein for the first time in the genus Sida and the other compounds were isolated for the first time in the species S. rhombifolia. In addition, the vasorelaxant activity of cryptolepinone (8) in rat cranial mesenteric artery with and without functional vascular endothelium is herein reported for the first time.
Acknowledgments
The authors are grateful to CNPq for financial support, to UFPB for technical assistance, to the Center of Characterization and Analysis (LMCA-UFPB) for obtaining the spectra.
Footnotes
Sample Availability: Samples of the compounds sitosterol and stigmasterol (mixture), sitosterol-3-O-β-D-glucopyranoside and stigmasterol-3-O-β-D-glucopyranoside (mixture), 173-ethoxypheophorbide A, 173-ethoxypheophorbide B, cryptolepinone and a salt of cryptolepine are available from the authors.
References
- 1.Silva D.A., Silva T.M.S., Lins A.C.S., Costa D.A., Cavalcante J.M.S., Matias W.N., Souza M.F.V., Braz Filho R. Constituintes Químicos e Atividade Antioxidante de Sida galheirensis Ulbr. (Malvaceae) Quim. Nova. 2006;29:1250–1256. [Google Scholar]
- 2.Thounaojam M.C., Jadeja R.N., Ansarullah, Patel V.B., Devkar R.V., Ramachandran A.V. Potential of Sida rhomboidea.Roxb Leaf Extract in Controlling Hypertriglyceridemia in Experimental Models. Pharmacognosy Res. 2009;1:208–212. [Google Scholar]
- 3.Iswantini D., Darusman L.K., Hidayat R. Indonesian Sidaguri (Sida rhombifolia L.) as Antigout and Inhibition Kinetics of Flavonoids Crude Extract on the Activity of Xanthine Oxidase. J. Biol. Sci. 2009;9:504–508. [Google Scholar]
- 4.Jadhava A.N., Pawara R.S., Avulaa B., Khan I.A. Ecdysteroid Glycosides from Sida rhombifolia L. Chem. Biodivers. 2007;4:2225–2230. doi: 10.1002/cbdv.200790180. [DOI] [PubMed] [Google Scholar]
- 5.Prakash A., Varma R.K., Ghosal S. Chemical Constituents of the Malvaceae. Part III. Alkaloidal Constituents of Sida acuta, Sida humilis, Sida rhombifolia and Sida spinosa. Planta Med. 1981;43:384–388. doi: 10.1055/s-2007-971529. [DOI] [PubMed] [Google Scholar]
- 6.Martin G.E., Guido J.E., Robins R.H., Sharaf M.H.M., Schiff P.L., Jr., Tackie A.N. Submicro Inverse-Detection Gradient NMR: A Powerful New Way of Conducting Structure Elucidation Studies with <0.05 micromol Samples. J. Nat. Prod. 1998;61:555–559. doi: 10.1021/np980099b. [DOI] [PubMed] [Google Scholar]
- 7.Tousek J., Vanmiert S., Pieters L., Baelen G.V., Hostyn S., Maes B.U.W., Lemiere G., Dommisse R., Marek R. Structural and Solvent Effects on the 13C and 15N NMR Chemical Shifts of Indoloquinoline Alkaloids: Experimental and DFT Study. Magn. Reson. Chem. 2008;46:42–51. doi: 10.1002/mrc.2125. [DOI] [PubMed] [Google Scholar]
- 8.Karou S.D., Nadembega W.M.C., Ilboudo D.P., Ouermi D., Gbeassor M., de Souza C., Simpore J. Sida acuta Burm. f.: A Medicinal Plant with Numerous Potencies. Afr. J. Biotechnol. 2007;6:2953–2959. [Google Scholar]
- 9.Kojima H., Sato N., Hatano A., Ogura H. Sterol Glucosides from Prunella vulgaris. Phytochemistry. 1990;29:2351–2355. doi: 10.1016/0031-9422(90)83073-A. [DOI] [Google Scholar]
- 10.Tomaz A.C.A., Nogueira R.B.S.S., Pinto D.S., Agra M.F., Souza M.F.V., Da-Cunha E.V.L. Chemical Constiuents from Richardia grandiflora (Cham. & Schltdl.) Steud. (Rubiaceae) Rev. Bras. Farmacogn. 2008;18:47–52. [Google Scholar]
- 11.Sakdarat S., Shuyprom A., Ayudhya T.D., Waterman P.G., Karagianis G. Chemical Composition Investigation of the Clinacanthus nutans Lindau Leaves. Thai J. Phytopharm. 2006;13:13–24. [Google Scholar]
- 12.Chee C-F, Lee H.B., Ong H.C., Siong-Hockho A. Photocytotoxic Pheophorbide-Related Compounds from Aglaonema simplex. Chem. Biodivers. 2005;2:1648–1655. doi: 10.1002/cbdv.200590134. [DOI] [PubMed] [Google Scholar]
- 13.Gomes R.A., Ramirez R.R.A., Maciel J.K.S., Falcão-Siva V.F., Siqueira-Junior J.P., Agra M.F., Souza M.F.V. Phenolic compounds from Sidastrum micranthum (A. St.-Hil.) Fryxell and Evaluation of Acacetin and 7,4'-Di-O-Methylisoscutellarein as Modulator of Bacterial Drug Resistance. Quim. Nova. 2011;34:1385–1388. [Google Scholar]
- 14.França-Silva M.S., Luciano M.N., Ribeiro T.P., Silva J.S., Santos A.F., França K.C., Nakao L.S., Athayde-Filho P.F., Braga V.A., Medeiros I.A. The 2-Nitrate-1,3-Dibuthoxypropan, a New Nitric Oxide Donor, Induces Vasorelaxation in Mesenteric Arteries of the Rat. Eur. J. Pharmacol. 2012;690:170–175. doi: 10.1016/j.ejphar.2012.06.043. [DOI] [PubMed] [Google Scholar]