Abstract
Despite the availability of several therapeutic options, a safer and more effective modality is urgently needed for treatment of bladder cancer. Costunolide, a member of sesquiterpene lactone family, possesses potent anticancer properties. In this study, for the first time we investigated the effects of costunolide on the cell viability and apoptosis in human bladder cancer T24 cells. Treatment of T24 cells with costunolide resulted in a dose-dependent inhibition of cell viability and induction of apoptosis which was associated with the generation of ROS and disruption of mitochondrial membrane potential (Δψm). These effects were significantly blocked when the cells were pretreated with N-acetyl- cysteine (NAC), a specific ROS inhibitor. Exposure of T24 cells to costunolide was also associated with increased expression of Bax, down-regulation of Bcl-2, survivin and significant activation of caspase-3, and its downstream target PARP. These findings provide the rationale for further in vivo and clinical investigation of costunolide against human bladder cancer.
Keywords: bladder cancer, T24 cells, costunolide, apoptosis, reactive oxygen species
1. Introduction
Urinary bladder cancer is one of the most common urological malignancies worldwide. More than 12 million new cases of cancer occur annually worldwide. Of those 5.4 million occur in developed countries and 6.7 million in developing countries [1]. In 2012, approximately 37,510 new urinary bladder cancer cases will be diagnosed and 14,880 will die in the United States [2]. In recent years, bladder cancer has been usually cured with surgery, chemotherapy, and combinations of chemotherapy and radiotherapy, but they all have associated limitations [1]. Prevailing treatment options have limited therapeutic success in human bladder cancer. Hence, the current therapy for bladder cancer is not satisfactory and better therapeutic options are immediately required to develop a more effective therapy for bladder cancer that can reduce the recurrence rate, decrease side effects, and increase overall survival.
Over the last decade, many reports revealed that phytochemicals targeting ROS metabolism can selectively kill cancer cells by raising the level of ROS above a toxic threshold. Since cancer cells show higher levels of endogenous ROS compared with their normal cells, the toxic threshold can be easily achieved in cancer cells [3,4]. In the current study, we carried out high throughput screening of compound library from Chinese herbs, using the bladder cancer cell line T24, in the presence or absence of NAC, a specific ROS inhibitor. This screening strategy helped us to identify natural anticancer compounds targeting ROS mediated apoptosis in bladder cancer cells. Costunolide, a natural compound that belongs to the sesquiterpene lactone family, was identified as a potent growth inhibitor of bladder cancer cells during screening. Sesquiterpene lactones, due to their anti-neoplastic and anti-inflammatory activity, have attracted considerable attention in pharmacological research [5,6]. As a medicine, costunolide is a well known sesquiterpene lactone, which is used as popular herbal remedies, with anti-ulcer [7], anti-inflammatory [8], anti-fungal [9,10], anti-viral properties [11], and inhibitory effects against cellular production of melanin [12]. It has also been documented that costunolide is involved to inhibit the expression of inducible nitric oxide synthase [13] and the DNA-binding activity of NF-κB [14]. Moreover, costunolide potentiated 1,25-(OH)2D3-induced differentiation in HL-60 promyelocytic leukemia cells [15,16,17] via interference with NF-κB activation. Further studies demonstrated that costunolide has anti-tumor potential by inhibiting proliferation, inducing apoptosis and reducing invasion and metastasis of a wide variety of tumor cells as we reviewed recently [18]. However, the effects of costunolide on human bladder cancer T24 cells were still unknown. Therefore, the objectives of present study were two-fold; to explore the effects of costunolide on the proliferation of T24 cells and to determine the role ROS in costunolide-induced apoptosis in bladder cancer cells with a therapeutic potential. Results showed that costunolide effectively inhibited the proliferation of T24 cells through inducing the apoptosis, which is mediated through ROS generation, mitochondrial dysfunction and activation of caspase-3 and its downstream target Poly (ADP-ribose) polymerase (PARP).
2. Results and Discussion
2.1. Costunolide Exerted Anti-Proliferation Activity in T24 Cells
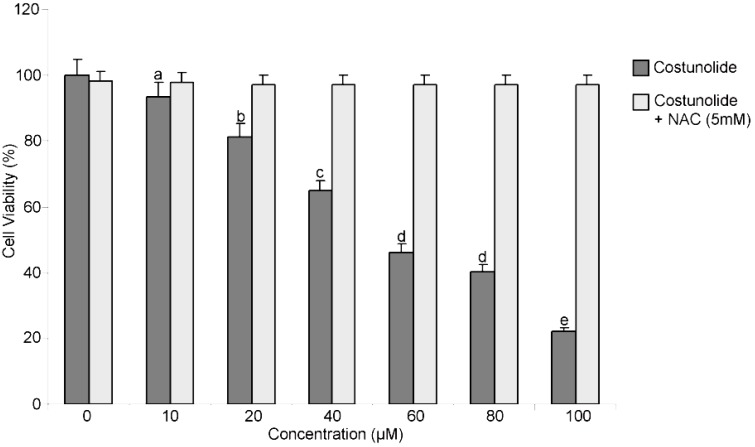
To identify a novel and specific inducer of ROS mediated apoptosis in bladder cancer cells, natural compounds were screened in the presence or absence of NAC, a specific ROS scavenger, using the MTT assay. Costunolide, isolated from the roots of Saussurea lappa (Mu Xiang), was identified as a potent growth inhibitor of bladder cancer cells. The structure of costunolide is shown in Figure 1. The treatment with costunolide for 24 h inhibited the proliferation of T24 cells in a dose-dependent manner. Pretreatment with 5mM NAC restored the viability of cells indicating that costunolide exerts cytotoxic effect on cell viability through ROS generation (Figure 2).
Figure 1.
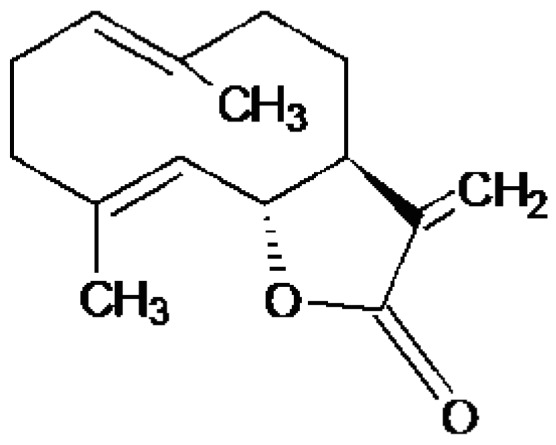
The chemical structure of costunolide.
Figure 2.
Costunolide inhibited the cell growth and induced cell death. T24 Cells were treated with indicated doses of costunolide in the presence or absence of NAC for 24 h and cell viability was measured by MTT assay. Data are expressed as Mean ± SD (n = 3). Columns not sharing the same superscript letter differ significantly (p < 0.05).
2.2. Costunolide Induced Morphological Changes and Cell Death in T24 Cells
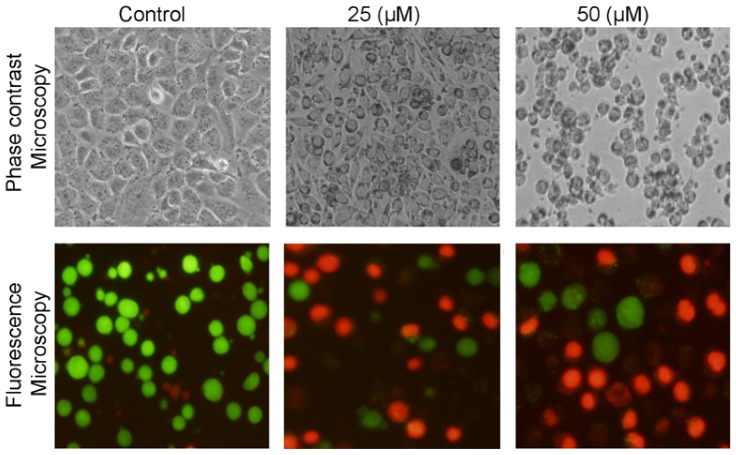
Morphological changes were observed under microscopy after treating cells with costunolide resulting in the decreased number of cells as compared to control group and cells became rounded and shrinked, which were polygonal in untreated cells (Figure 3). Furthermore, the antiproliferative effect of costunolide on T24 cells was confirmed by live/dead assay.
Figure 3.
Morphological changes in human bladder cancer T24 cells were observed under phase contrast and fluorescence microscopy after treatment with 0, 25 and 50 μM of costunolide for 24.
For this purpose, bladder cancer T24 cells were treated with 25 and 50 µM of costunolide for 24 h and live and dead cells were observed using fluorescent probes calcein AM/PI and photographs were taken under fluorescence microscopy (Figure 3). In addition, dead cells were further quantified using fluorescent probes calcein AM/PI and flow cytometry. The results demonstrated that treatment of cells with costunolide decreased the viability of T24 cells in a dose-dependent manner (Figure 4A,B). Costunolide induced growth inhibition of T24 cells in addition to other type of cancer cells previously reported including leukemia [15,19], intestinal carcinoma cells [20], and breast carcinoma cells [21,22].
Figure 4.
Determination of cell viability by calcein/PI staining and flow cytometry. (A) T24 cells were treated with 25 and 50 µM of costunolide in the presence or absence of NAC for 24 h. Histograms show number of PI positive cells (y-axis) vs. calcein positive cells (x-axis). The data shown are representative of three independent experiments with the similar results. (B) Data are expressed as Mean ± SD (n = 3). Columns not sharing the same superscript letter differ significantly (p < 0.05).
2.3. Costunolide Induced G2/M Cell Cycle Arrest in T24 Cells
Recent insights related to cell cycle regulation indicated that cell cycle progression is tightly controlled by various checkpoints in normal cells while alterations the checkpoints of cell cycle progression lead to aberrant cell proliferation and development of cancer [23].
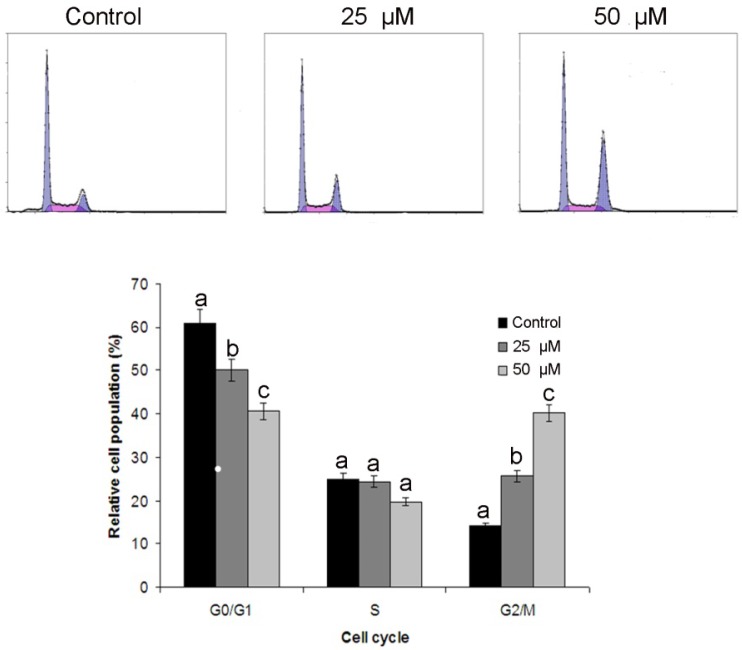
As tumor cells frequently acquire defects in the checkpoints resulting in the deregulation of cell cycle, which lead to unrestrained proliferation. Pharmacological correction of these check points and proper progression of cell cycle is a proficient strategy to control the growth and proliferation of cancer cells [24,25,26]. Next, we analyzed effects of costunolide on cell cycle progression of T24 cells. It was observed that costunolide arrested cell cycle at G2/M phase and the percentage of accumulation of cells in the G2/M phase was increased from 13.78 ± 1.26% in control group to 25.64 ± 2.16% and 41.32 ± 2.66% in the cells treated with 25 and 50 µM of costunolide for 24 h respectively. This increase was coupled with the decreased percentage of cells in G0/G1 phase (Figure 5). These findings are also in line with reported results in other type of cancer cells such as costunolide induced G1-phase cell cycle arrest in human prostate cancer cells [27] and G2/M phase arrest in human hepatocellular carcinoma cells [28].
Figure 5.
Flow cytometry analysis of cell cycle phase distribution in T24 cells treated with 25 and 50 µM costunolide for 24 h. Data are expressed as Mean ± SD (n = 3). Columns not sharing the same superscript letter differ significantly (p < 0.05).
2.4. Costunolide Induced Apoptotic Cell Death in T24 Cells
Apoptosis, autophagy, and necrosis are the major types of cell death [29]. Among the three major pathways of cell death, apoptosis is most well planned and orderly mode of cell death [30,31]. More than 50% of neoplasms undergo aberrations in the apoptotic machinery which leads to abnormal cell proliferation [32,33]. The regulation of apoptosis is, therefore, the most important in the treatment of cancer [34,35,36]. Accumulated evidences indicated that the most of chemotherapeutic agents halt tumor cells proliferation via induction of apoptosis [37,38,39,40].
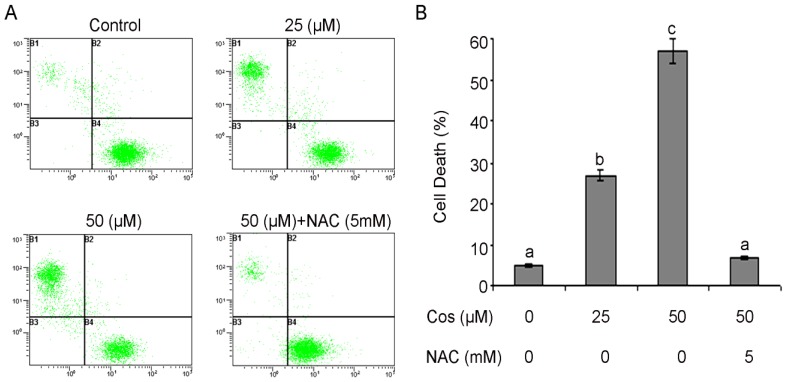
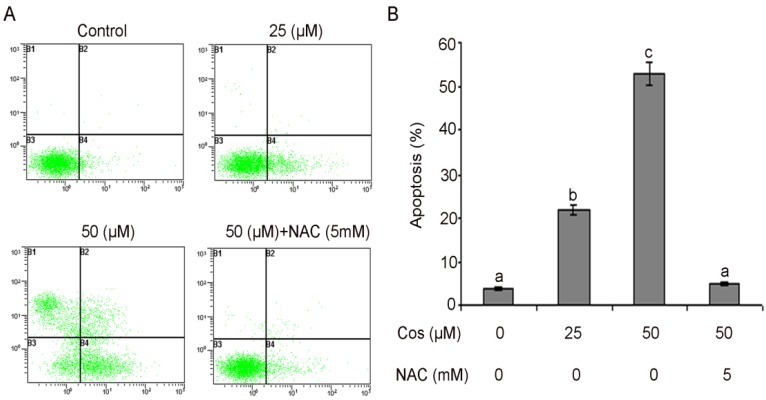
We examined whether costunolide inhibited cell growth of T24 cells through the induction of apoptosis. Costunolide-induced apoptosis was determined by flow cytometric analysis. Cells were seeded in 12 well plates. After incubation of cells with 25 and 50 µM or without costunolide for 24 h, cells were collected in centrifuge tubes and stained with annexin V-FITC and PI double staining as described in the Experimental section. The results of flow cytometric analysis showed that the rates of apoptosis were 21.43 ± 1.36% and 52.87 ± 1.53% in the cells treated with 25 and 50 µM of costunolide respectively for 24 h as compared to the 4.41 ± 0.42% in control cells. Pretreatment with NAC completely blocked the apoptotic effect of costunolide indicating that induction of apoptosis is a ROS-dependent manner (Figure 6A,B). Costunolide-induced apoptosis in T24 cells was compatible with previously reported studies [8,15,19,41,42].
Figure 6.
Apoptosis induced by costunolide in T24 cells. (A) T24 cells were treated with 25 and 50 µM of costunolide for 24 h in the presence or absence of NAC. Then cells were stained with FITC-conjugated Annexin V and PI for flow cytometric analysis. The flow cytometry profile represents Annexin V-FITC staining in x axis and PI in y axis. (B) Data are expressed as Mean ± SD (n = 3). Columns not sharing the same superscript letter differ significantly (p < 0.05).
2.5. Costunolide Increased Generation of ROS in T24 Cells
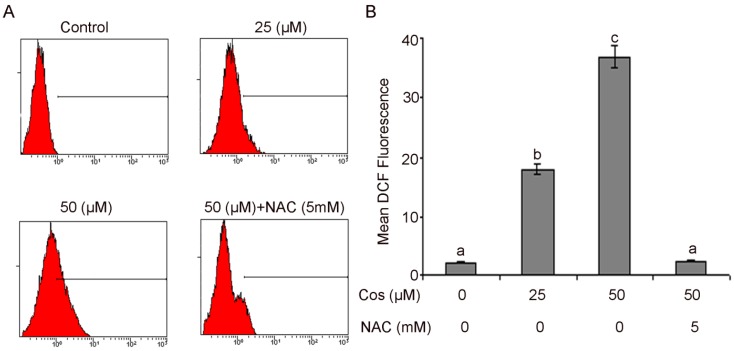
ROS are well known mediators of intracellular signaling of cascades. The excessive generation of ROS can induce oxidative stress, loss of cell functioning, and apoptosis [43]. ROS can also be involved in the process of lipid peroxidation and or the cross linking of thiol groups in proteins; both of these processes can induce the opening of the mitochondrial permeability transition pore (PTP) [44,45]. In the present study, we assumed that costunolide might arouse ROS level, which could be involved in costunolide-induced apoptosis. Therefore, the intracellular ROS level was measured using the ROS-detecting fluorescence dye 2,7-dichlorofluorescein diacetate (DCF-DA) because the DCF assay is highly sensitive, linear, and precise for measuring oxidative stress in irradiated cells [46]. The level of ROS was significantly increased in a dose-dependent manner after treating the cells with costunolide.
As shown in Figure 7A,B, the ratio of DCF-positive cells, treated with 25 and 50 µM costunolide was significantly higher (18.73 ± 1.65 and 36.80 ± 1.83 vs. 1.07 ± 0.53 in control group, p < 0.05). The findings evidenced that costunolide had enhanced the generation of ROS in T24 cells. The chemotherapeutic agents causing enhancement in oxidative stress are likely to be toxic to the cancer cells because they are found to be involved in the biological processes like cell cycle arrest, DNA repair, and apoptosis [47].
Figure 7.
Flow cytometry analysis of ROS generation. (A) T24 cells were treated with 25 and 50 µM costunolide in the presence or absence of 5 mM NAC for 24 h. (B) Data are expressed as mean ± SD (n = 3). Columns not sharing the same superscript letter differ significantly (p < 0.05).
2.6. Costunolide Decreased Mitochondrial Membrane Potential in T24 cells
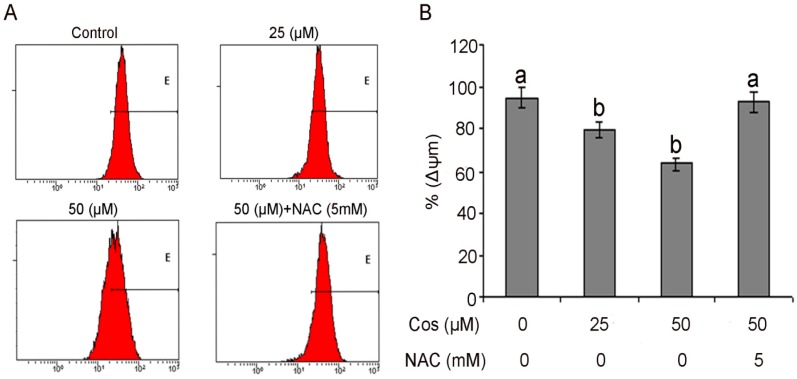
Mitochondria have become an important component of the apoptosis execution machinery, which contain pro-apoptotic proteins (e.g., cytochrome c) [30]. It has been elucidated that upon the depolarization of the mitochondrial membrane potential results in the mitochondrial swelling and subsequent release of cytochrome c from the intermitochondrial membrane space into the cytosol [48]. It is becoming increasingly apparent that the mitochondria play a fundamental role in the processes leading to cell death [49]. The effects of costunolide on the mitochondrial membrane potential of T24 cells were determined by flow cytometry using rhodamine 123 staining. The rates of depletion of mitochondrial membrane potential were 79.7 ± 1.23% and 66.27 ± 1.42% in the cells treated with 25 and 50 µM of costunolide, respectively, for 24 h as compared to 94.90 ± 0.47% in the control group. To further confirm the involvement of ROS in disruption of mitochondrial membrane potential, cells were treated with 5 mM NAC. Pretreatment with NAC completely prevented dissipation of mitochondrial membrane potential, indicating that this was ROS-dependent (Figure 8A,B).
Figure 8.
The effects of costunolide on mitochondrial transmembrane potential of T24 cells were determined by flow cytometry. (A) The values indicate the percentages of rhodamine 123 fluorescence in the T24 cells treated without and with 25 and 50 µM of costunolide for 24 h in the presence or absence of NAC. (B) Data are expressed as Mean ± SD (n = 3). Columns not sharing the same superscript letter differ significantly (p < 0.05).
2.7. Costunolide Regulated Apoptosis-Related Proteins in T24 Cells
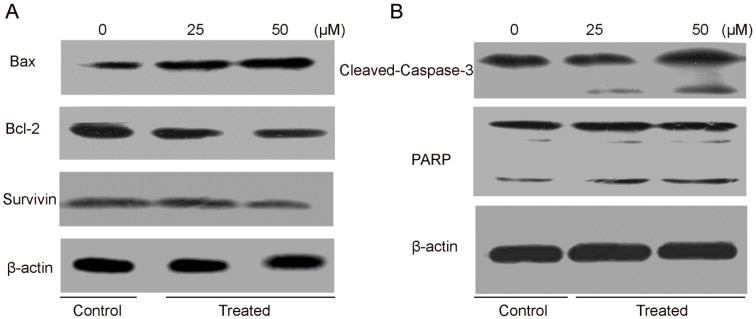
Our data corroborate with the previously reported results that costunolide induced dissipation of mitochondrial membrane potential, which provide the evidence for direct contribution of mitochondria in the costunolide-induced apoptosis [41,42]. Interplay between pro-apoptotic (Bax) and anti-apoptotic (Bcl-2) members of the Bcl-2 family drives the mitochondrial apoptotic pathway [50]. Bcl-2 family proteins are pivotal for increasing the permeability of mitochondrial membranes and the release of cytochrome c, which activates caspases and in turn mobilizes apoptotic cell death [51,52,53]. To investigate the effect of costunolide on expression of Bcl-2, western blotting was done. It was observed that costunolide involved in the down regulation of Bcl-2 in a dose-dependent manner (Figure 9A). These results are similar with previously reported studies [15,41,54]. In addition, we also examined the effect of costunolide on survivin, anti-apoptotic protein. Our results demonstrated that costunolide involved in the down regulation of survivin in a dose-dependent manner (Figure 9A).
Figure 9.
The effect of costunolide on the expression of major apoptosis regulatory proteins. T24 cells were exposed to 25 and 50 µM of costunolide for specified time intervals. Equal amounts of lysate protein were subjected to gel electrophoresis. (A,B) Expression levels of Bax, Bcl-2, survivin, caspase-3 and PARP were monitored by western blot assay. β-actin was used as loading control. Data are representative of three independent experiments with similar results.
The caspases are a family of proteins related to cysteine proteases that are one of the focal executors of the apoptotic process via triggering of the death receptors and mitochondrial pathways to accomplish the programmed cell death [55]. Caspases are present in the form of inactive zymogens those are activated during apoptosis. Among them, caspase-3 is a frequently activated death protease, catalyzing the specific cleavage of many key cellular proteins [56,57]. In order to reveal effects of costunolide on expression of caspase-3 and its downstream target, PARP, western blotting was done. The results showed that procaspase-3 was cleaved to yield 17 and 20 KDa fragments and activation of PARP in treated cells with 25 and 50 µM of costunolide after 24 h as compared to that of control cells (Figure 9B). These findings are supported by previous studies [19,42,54]. These results markedly showed that costunolide induced caspase-dependent cell death in T24 cells.
3. Experimental
3.1. Chemicals and Reagents
Cell culture medium reagents and MTT [3′-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide], propidium iodide (PI), and dimethyl sulfoxide (DMSO) were purchased from Sigma. Fetal bovine serum (FBS) was purchased from the Hangzhou Sijiqing Biological Engineering Materials Co., Ltd. An annexin V-FITC apoptosis detection kit was purchased from Beyotime Institute of Biotechnology (Shanghai, China). Rabbit polyclonal anti-human Bcl-2, Bax, survivin, cleaved caspase-3 and PARP antibodies were purchased from Wuhan Boster Biological Technology Co., Ltd. (Wuhan, China). Mouse anti-β-actin and anti-rabbit antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Ponceou and cell lysis buffer for western blots and IP were purchased from Bio SS Beijing (Beijing, China). Rhodamine 123 was purchased from Invitrogen (Eugene, OR, USA).
3.2. Extraction, Isolation, and Identification of Costunolide
Costunolide was isolated from the roots of Chinese herb Saussurea lappa (Chinese name: Mu Xiang) via fractionation of the extract as we described previously [58,59].
3.3. Cell Culture
Human bladder cancer T24 cells were propagated in DMEM nutrients mixture supplemented with 10% FBS and antibiotics at 37 °C in a humidified atmosphere with 5% CO2 and 95% air. Cells were seeded in 10 cm culture dish and allowed to grow to approximately 70% confluence before experimentation.
3.4. Cell Proliferation Assay
The cytotoxic effects of the costunolide on the cells were determined by the MTT assay. Briefly, T24 cells were seeded at a density of 1 × 104 cells per well in 96-well plates and were allowed to grow overnight. Cells were incubated with 100 µL of complete culture medium containing 10, 20, 40, 60, 80 and 100 µM of costunolide. After incubation for 24 h, growth of cells was determined by adding 10 µL MTT (5 mg/mL in phosphate buffered saline) to each well and incubated for 4 h. After removal of the medium, 150 µL DMSO was added to each well and shaken gently and carefully. The absorbance was read at a wavelength of 490 nm in a plate reader (ELX 800, BIO-TEK Instruments Inc., Winooski, VT, USA).
3.5. Morphological Observation under Phase Contrast and Fluorescence Microscope
T24 cells were seeded in 12-well flat bottom microtiter plates and then treated with costunolide at the concentration of 0 25, and 50 µM, respectively. After 24 h of treatment, the morphology of T24 cells was observed under a phase contrast microscope. Furthermore, cells were stained with calcein acetoxymethylester (calcein AM)/PI in the dark for 20 min at room temperature and were observed under fluorescence microscope (Olympus, Tokyo, Japan).
3.6. Live/Dead Assay
In order to quantify the live and dead cells, T24 cells were with the fluorescent probes, calcein AM and PI. Calcein AM is cell membrane permeable and stains only viable cells whereas PI is cell membrane impermeable and stains only dead cells. To determine the effect of costunolide, T24 cells were treated with 25 and 50 µM of costunolide in the presence or absence of NAC for 24 h. Subsequently, treated and untreated cells were collected, washed with phosphate buffered saline (PBS) and incubated with PBS solution containing 2 µM calcein AM and 4 µM PI in the dark for 20 min at room temperature. After washing, cells were resuspended in PBS and analyzed for the fluorescence of calcein and PI by flow cytometry (Beckman Coulter, Epics XL, Miami, FL, USA).
3.7. Flow Cytometric Analysis of Cell Cycle
For cell cycle analysis, T24 cells were seeded in 12-well plates and then treated with 25 and 50 µM of costunolide for 24 h. After treatments, the percentages of cells in the different phases of cell cycle were evaluated by determining the DNA content after propidium iodide (PI) staining as we described previously [28,60].
3.8. Flow Cytometric Determination of Apoptosis
The rate of apoptosis of T24 cells was examined by flow cytometry using annexin V-FITC/PI staining. Briefly, T24 cells were cultured in 6-well plates and allowed to attach overnight. Cells were treated with 25 and 50 µM of costunolide for 24 h. Then cells were collected, washed and resuspended in PBS. Apoptotic cell death was measured by double staining annexin V-FITC and PI using the annexin V-FITC apoptosis detection kit (Beyotime Biotechnology, Shanghai, China) according to the manufacturer’s instructions. Flow cytometric analysis was performed immediately after staining. Data acquisition and analysis were performed by flow cytometry using the Cell Quest software.
3.9. Flow Cytometric Determination of Reactive Oxygen Species (ROS) in T24 Cells
In order to determine the intracellular changes in ROS generation, T24 cells were stained with 2',7'-dichlorofluorescein-diacetate (DCFH-DA). The fluorescent dye DCFH-DA is cell membrane permeable and is converted into the cell membrane impermeable nonfluorescent compound DCFH by intracellular esterases. Oxidation of DCFH by reactive oxygen species produces highly fluorescent DCF. The fluorescence intensity of DCF inside the cells is proportional to the amount of peroxide produced. Briefly, T24 cells were treated with 25 and 50 µM costunolide for 24 h. After treatment, cells were further incubated with 10 μM DCFH-DA at 37 °C for 30 min. Subsequently, cells were harvested, rinsed, re-suspended in PBS, filtered with 300 apertures and analyzed for 2',7'-dichlorofluorescein (DCF) fluorescence by flow cytometry (Beckman Coulter, Epics XL).
3.10. Flow Cytometric Determination of Mitochondrial Membrane Potential (ΔΨm)
To probe the changes in ΔΨm, T24 cells were stained with rhodamine 123 (1 μM) after treatment of 25 and 50 µM of costunolide for 24 h with control group. The fluorescence of rhodamine 123 was measured by flow cytometry with excitation and emission wavelengths of 488 and 530 nm.
3.11. Western Blotting
To reveal the mechanism of the apoptotic effect of costunolide, western blotting was done for apoptotic related proteins as previously described [41]. Briefly, T24 cells were incubated with 25 and 50 µM of costunolide for indicated time. Cells were trypsinized, collected in 1.5 mL centrifuge tube and washed with PBS. The cell pellets were resuspended in lysis buffer and were lysed on ice for 30 min. After centrifugation for 15 min, the supernatant fluids were collected and the protein content of the supernatant was measured by the NanoDrop 1000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). The protein lysates were separated by electrophoresis on 12% SDS-polyacrylamide gel and transferred to a PVDF membrane (Amersham Biosciences, Piscataway, NJ, USA). The membranes were soaked in blocking buffer (5% skimmed milk) for 2 h. To probe for BAX, Bcl-2, survivin, cleaved caspase-3, PARP, and β-actin; membranes were incubated overnight at 4 °C with relevant antibodies, followed by appropriate HRP conjugated secondary antibodies and ECL detection.
3.12. Statistical Analysis of Data
For the statistical analysis of data, comparisons between results from different groups were analyzed with SPSS for Window Version 15.0. Student’s t-test was employed to determine the statistical significance of the difference between different experimental groups and control group. p < 0.05 value was defined as statistically significant. All experiments were repeated at least three times. Data were presented as mean ± standard deviation (S.D).
4. Conclusions
Although it has been reported that costunolide-induced apoptosis involves the generation of ROS in various cancer cells, our study is the first to describe the role of ROS in the induction of apoptosis in bladder cancer cells. In addition, this study and the pathway that we have described herein is novel and has not been elucidated before. To conclude, costunolide induces cell death in T24 bladder cancer cells via induction of ROS-mediated apoptosis. Analysis of apoptosis-related proteins in T24 cells revealed that costunolide induced the upregulation of Bax and the parallel downregulation of Bcl-2 and survivin expression. This ultimately led to dissipation of mitochondrial membrane potential (ΔΨm) and the sequential activation of caspase-3 and its downstream substrate, PARP, leading to apoptosis. Based on our previous and present studies, we suggest that use of costunolide may represent a new therapeutic strategy for the treatment of human cancers. Further studies are required to support our observations of the anti-tumor potential of this compound, which may represent a promising candidate for in vivo studies of mono-therapies as well as combined anti-tumor therapies.
Acknowledgments
This work was Supported by Ministry of Science and Technology (No. 2010DFA31430), Ministry of Education of China (NCET-10-0316; 101020031), Jilin Provincial Science & Technology Department (No. YYZX201241, 20070719, 200905116), Changchun Science & Technology Department (No. 2011114-11GH29). The authors also would like to acknowledge Wang Xiu-Li from Northeast Normal University for technical assistance during the flow cytometry experiments.
Footnotes
Sample Availability: Sample of the compound is available from the authors.
References
- 1.Ploeg M., Aben K.K., Kiemeney L.A. The present and future burden of urinary bladder cancer in the world. World J. Urol. 2009;27:289–293. doi: 10.1007/s00345-009-0383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R., Naishadham D., Jemal A. Cancer statistics, 2012. CA Cancer J. Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Fruehauf J.P., Meyskens F.L., Jr. Reactive oxygen species: A breath of life or death? Clin. Cancer Res. 2007;13:789–794. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]
- 4.Schumacker P.T. Reactive oxygen species in cancer cells: Live by the sword, die by the sword. Cancer Cell. 2006;10:175–176. doi: 10.1016/j.ccr.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Gu J.Q., Gills J.J., Park E.J., Mata-Greenwood E., Hawthorne M.E., Axelrod F., Chavez P.I., Fong H.H., Mehta R.G., Pezzuto J.M., et al. Sesquiterpenoids from Tithonia diversifolia with potential cancer chemopreventive activity. J. Nat. Prod. 2002;65:532–536. doi: 10.1021/np010545m. [DOI] [PubMed] [Google Scholar]
- 6.Koch E., Klaas C.A., Rungeler P., Castro V., Mora G., Vichnewski W., Merfort I. Inhibition of inflammatory cytokine production and lymphocyte proliferation by structurally different sesquiterpene lactones correlates with their effect on activation of NF-kappaB. Biochem. Pharmacol. 2001;62:795–801. doi: 10.1016/S0006-2952(01)00714-6. [DOI] [PubMed] [Google Scholar]
- 7.Robles M., Aregullin M., West J., Rodriguez E. Recent studies on the zoopharmacognosy, pharmacology and neurotoxicology of sesquiterpene lactones. Planta Med. 1995;61:199–203. doi: 10.1055/s-2006-958055. [DOI] [PubMed] [Google Scholar]
- 8.Park H.J., Jung W.T., Basnet P., Kadota S., Namba T. Syringin 4-O-beta-glucoside, a new phenylpropanoid glycoside, and costunolide, a nitric oxide synthase inhibitor, from the stem bark of Magnolia sieboldii. J. Nat. Prod. 1996;59:1128–1130. doi: 10.1021/np960452i. [DOI] [PubMed] [Google Scholar]
- 9.Barrero A.F., Oltra J.E., Alvarez M., Raslan D.S., Saude D.A., Akssira M. New sources and antifungal activity of sesquiterpene lactones. Fitoterapia. 2000;71:60–64. doi: 10.1016/S0367-326X(99)00122-7. [DOI] [PubMed] [Google Scholar]
- 10.Wedge D.E., Galindo J.C., Macias F.A. Fungicidal activity of natural and synthetic sesquiterpene lactone analogs. Phytochemistry. 2000;53:747–757. doi: 10.1016/S0031-9422(00)00008-X. [DOI] [PubMed] [Google Scholar]
- 11.Chen H.C., Chou C.K., Lee S.D., Wang J.C., Yeh S.F. Active compounds from Saussurea lappa Clarks that suppress hepatitis B virus surface antigen gene expression in human hepatoma cells. Antiviral Res. 1995;27:99–109. doi: 10.1016/0166-3542(94)00083-K. [DOI] [PubMed] [Google Scholar]
- 12.Choi J.Y., Choi E.H., Jung H.W., Oh J.S., Lee W.H., Lee J.G., Son J.K., Kim Y., Lee S.H. Melanogenesis inhibitory compounds from Saussureae Radix. Arch. Pharm. Res. 2008;31:294–299. doi: 10.1007/s12272-001-1154-0. [DOI] [PubMed] [Google Scholar]
- 13.Fukuda K., Akao S., Ohno Y., Yamashita K., Fujiwara H. Inhibition by costunolide of phorbol ester-induced transcriptional activation of inducible nitric oxide synthase gene in a human monocyte cell line THP-1. Cancer Lett. 2001;164:7–13. doi: 10.1016/S0304-3835(00)00704-7. [DOI] [PubMed] [Google Scholar]
- 14.Koo T.H., Lee J.H., Park Y.J., Hong Y.S., Kim H.S., Kim K.W., Lee J.J. A sesquiterpene lactone, costunolide, from Magnolia grandiflora inhibits NF-kappa B by targeting I kappa B phosphorylation. Planta Med. 2001;67:103–107. doi: 10.1055/s-2001-11503. [DOI] [PubMed] [Google Scholar]
- 15.Choi J.H., Ha J., Park J.H., Lee J.Y., Lee Y.S., Park H.J., Choi J.W., Masuda Y., Nakaya K., Lee K.T. Costunolide triggers apoptosis in human leukemia U937 cells by depleting intracellular thiols. Jpn. J. Cancer Res. 2002;93:1327–1333. doi: 10.1111/j.1349-7006.2002.tb01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim S.H., Danilenko M., Kim T.S. Differential enhancement of leukaemia cell differentiation without elevation of intracellular calcium by plant-derived sesquiterpene lactone compounds. Br. J. Pharmcol. 2008;155:814–825. doi: 10.1038/bjp.2008.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim S.H., Kang S.N., Kim H.J., Kim T.S. Potentiation of 1,25-dihydroxyvitamin D(3)-induced differentiation of human promyelocytic leukemia cells into monocytes by costunolide, a germacranolide sesquiterpene lactone. Biochem. Pharmacol. 2002;64:1233–1242. doi: 10.1016/S0006-2952(02)01292-3. [DOI] [PubMed] [Google Scholar]
- 18.Rasul A., Parveen S., Ma T. Costunolide: A novel anti-cancer sesquiterpene lactone. Bangladesh J. Pharmacol. 2012;7:6–13. [Google Scholar]
- 19.Kanno S., Kitajima Y., Kakuta M., Osanai Y., Kurauchi K., Ujibe M., Ishikawa M. Costunolide-induced apoptosis is caused by receptor-mediated pathway and inhibition of telomerase activity in NALM-6 cells. Biol. Pharm. Bull. 2008;31:1024–1028. doi: 10.1248/bpb.31.1024. [DOI] [PubMed] [Google Scholar]
- 20.Mori H., Kawamori T., Tanaka T., Ohnishi M., Yamahara J. Chemopreventive effect of costunolide, a constituent of oriental medicine, on azoxymethane-induced intestinal carcinogenesis in rats. Cancer Lett. 1994;83:171–175. doi: 10.1016/0304-3835(94)90315-8. [DOI] [PubMed] [Google Scholar]
- 21.Bocca C., Gabriel L., Bozzo F., Miglietta A. A sesquiterpene lactone, costunolide, interacts with microtubule protein and inhibits the growth of MCF-7 cells. Chem. Biol. Interact. 2004;147:79–86. doi: 10.1016/j.cbi.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Choi S.H., Im E., Kang H.K., Lee J.H., Kwak H.S., Bae Y.T., Park H.J., Kim N.D. Inhibitory effects of costunolide on the telomerase activity in human breast carcinoma cells. Cancer Lett. 2005;227:153–162. doi: 10.1016/j.canlet.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Collins K., Jacks T., Pavletich N.P. The cell cycle and cancer. Proc. Natl. Acad. Sci. USA. 1997;94:2776–2778. doi: 10.1073/pnas.94.7.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grana X., Reddy E.P. Cell cycle control in mammalian cells: Role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs) Oncogene. 1995;11:211–219. [PubMed] [Google Scholar]
- 25.Kastan M.B., Canman C.E., Leonard C.J. P53, cell cycle control and apoptosis: implications for cancer. Cancer Metastasis Rev. 1995;14:3–15. doi: 10.1007/BF00690207. [DOI] [PubMed] [Google Scholar]
- 26.Pavletich N.P. Mechanisms of cyclin-dependent kinase regulation: structures of Cdks, their cyclin activators, and Cip and INK4 inhibitors. J. Mol. Biol. 1999;287:821–828. doi: 10.1006/jmbi.1999.2640. [DOI] [PubMed] [Google Scholar]
- 27.Hsu J.L., Pan S.L., Ho Y.F., Hwang T.L., Kung F.L., Guh J.H. Costunolide induces apoptosis through nuclear calcium2+ overload and DNA damage response in human prostate cancer. J. Urol. 2011;185:1967–1974. doi: 10.1016/j.juro.2010.12.091. [DOI] [PubMed] [Google Scholar]
- 28.Rasul A., Yu B., Yang L., Arshad M., Khan M., Ma T., Yang H. Costunolide, a sesquiterpene lactone induces G2/M phase arrest and mitochondria-mediated apoptosis in human gastric adenocarcinoma SGC-7901 cells. J. Med. Plant. Res. 2012;6:1191–1200. [Google Scholar]
- 29.Leist M., Jaattela M. Four deaths and a funeral: from caspases to alternative mechanisms. Nat. Rev. Mol. Cell. Biol. 2001;2:589–598. doi: 10.1038/35085008. [DOI] [PubMed] [Google Scholar]
- 30.Elmore S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hengartner M.O. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 32.Mashima T., Tsuruo T. Defects of the apoptotic pathway as therapeutic target against cancer. Drug Resist. Updat. 2005;8:339–343. doi: 10.1016/j.drup.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Pommier Y., Sordet O., Antony S., Hayward R.L., Kohn K.W. Apoptosis defects and chemotherapy resistance: Molecular interaction maps and networks. Oncogene. 2004;23:2934–2949. doi: 10.1038/sj.onc.1207515. [DOI] [PubMed] [Google Scholar]
- 34.Fulda S. Evasion of apoptosis as a cellular stress response in cancer. Int. J. Cell Biol. 2010;2010:370835. doi: 10.1155/2010/370835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawen A. Apoptosis-an introduction. Bioessays. 2003;25:888–896. doi: 10.1002/bies.10329. [DOI] [PubMed] [Google Scholar]
- 36.Reed J.C. Apoptosis-based therapies. Nat. Rev. Drug Discov. 2002;1:111–121. doi: 10.1038/nrd726. [DOI] [PubMed] [Google Scholar]
- 37.Rasul A., Ding C., Li X., Khan M., Yi F., Ali M., Ma T. Dracorhodin perchlorate inhibits PI3K/Akt and NF-kappaB activation, up-regulates the expression of p53, and enhances apoptosis. Apoptosis. 2012;17:1104–1119. doi: 10.1007/s10495-012-0742-1. [DOI] [PubMed] [Google Scholar]
- 38.Rasul A., Ma T. In vitro cytotoxic screening of 300 selected Chinese medicinal herbs against human gastric adenocarcinoma SGC-7901 cells. Afr. J. Pharm. Pharmacol. 2012;6:592–600. [Google Scholar]
- 39.Rasul A., Yu B., Khan M., Zhang K., Iqbal F., Ma T., Yang H. Magnolol, a natural compound, induces apoptosis of SGC-7901 human gastric adenocarcinoma cells via the mitochondrial and PI3K/Akt signaling pathways. Int. J. Oncol. 2012;40:1153–1161. doi: 10.3892/ijo.2011.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rasul A., Yu B., Zhong L., Khan M., Yang H., Ma T. Cytotoxic effect of evodiamine in SGC-7901 human gastric adenocarcinoma cells via simultaneous induction of apoptosis and autophagy. Oncol. Rep. 2012;27:1481–1487. doi: 10.3892/or.2012.1694. [DOI] [PubMed] [Google Scholar]
- 41.Seo J.Y., Park J., Kim H.J., Lee I.A., Lim J.S., Lim S.S., Choi S.J., Park J.H., Kang H.J., Kim J.S. Isoalantolactone from Inula helenium caused Nrf2-mediated induction of detoxifying enzymes. J. Med. Food. 2009;12:1038–1045. doi: 10.1089/jmf.2009.0072. [DOI] [PubMed] [Google Scholar]
- 42.Lee M.G., Lee K.T., Chi S.G., Park J.H. Costunolide induces apoptosis by ROS-mediated mitochondrial permeability transition and cytochrome C release. Biol. Pharm. Bull. 2001;24:303–306. doi: 10.1248/bpb.24.303. [DOI] [PubMed] [Google Scholar]
- 43.Slater A.F., Stefan C., Nobel I., van den Dobbelsteen D.J., Orrenius S. Signalling mechanisms and oxidative stress in apoptosis. Toxicol. Lett. 1995;82-83:149–153. doi: 10.1016/0378-4274(95)03474-9. [DOI] [PubMed] [Google Scholar]
- 44.Lee J.H., Baek N.I., Kim S.H., Park H.W., Yang J.H., Lee J.J., Kim S.J., Jeong S., Oh C.H., Lee K.H., et al. A new cytotoxic prenylated chalcone from Sophora flavescens. Arch. Pharm. Res. 2007;30:408–411. doi: 10.1007/BF02980212. [DOI] [PubMed] [Google Scholar]
- 45.Vercesi A.E., Kowaltowski A.J., Grijalba M.T., Meinicke A.R., Castilho R.F. The role of reactive oxygen species in mitochondrial permeability transition. Biosci. Rep. 1997;17:43–52. doi: 10.1023/A:1027335217774. [DOI] [PubMed] [Google Scholar]
- 46.Wan X.S., Zhou Z., Kennedy A.R. Adaptation of the dichlorofluorescein assay for detection of radiation-induced oxidative stress in cultured cells. Radiat. Res. 2003;160:622–630. doi: 10.1667/3099. [DOI] [PubMed] [Google Scholar]
- 47.Kang N., Zhang J.H., Qiu F., Chen S., Tashiro S., Onodera S., Ikejima T. Induction of G(2)/M phase arrest and apoptosis by oridonin in human laryngeal carcinoma cells. J. Nat. Prod. 2010;73:1058–1063. doi: 10.1021/np9008199. [DOI] [PubMed] [Google Scholar]
- 48.Buytaert E., Dewaele M., Agostinis P. Molecular effectors of multiple cell death pathways initiated by photodynamic therapy. Biochim. Biophys. Acta. 2007;1776:86–107. doi: 10.1016/j.bbcan.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 49.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–2933. [PubMed] [Google Scholar]
- 50.Mallat Z., Tedgui A. Apoptosis in the vasculature: mechanisms and functional importance. Br. J. Pharmacol. 2000;130:947–962. doi: 10.1038/sj.bjp.0703407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adams J.M., Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burlacu A. Regulation of apoptosis by Bcl-2 family proteins. J. Cell. Mol. Med. 2003;7:249–257. doi: 10.1111/j.1582-4934.2003.tb00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Danial N.N. BCL-2 family proteins: Critical checkpoints of apoptotic cell death. Clin. Cancer Res. 2007;13:7254–7263. doi: 10.1158/1078-0432.CCR-07-1598. [DOI] [PubMed] [Google Scholar]
- 54.Park H.J., Kwon S.H., Han Y.N., Choi J.W., Miyamoto K., Lee S.H., Lee K.T. Apoptosis-Inducing costunolide and a novel acyclic monoterpene from the stem bark of Magnolia sieboldii. Arch. Pharm. Res. 2001;24:342–348. doi: 10.1007/BF02975104. [DOI] [PubMed] [Google Scholar]
- 55.Cohen G.M. Caspases: The executioners of apoptosis. Biochem. J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adams J.M. Ways of dying: Multiple pathways to apoptosis. Genes Dev. 2003;17:2481–2495. doi: 10.1101/gad.1126903. [DOI] [PubMed] [Google Scholar]
- 57.Porter A.G., Janicke R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 58.Shawi A.A., Rasul A., Khan M., Iqbal F., Ma T. Eupatilin: A flavonoid compound isolated from the artemisia plant, induces apoptosis and G2/M phase cell cycle arrest in human melanoma A375 cells. Afr. J. Pharm. Pharmacol. 2011;5:582–588. [Google Scholar]
- 59.Rasul A., Khan M., Yu B., Ma T., Yang H. Xanthoxyletin, a Coumarin Induces S Phase Arrest and Apoptosis in Human Gastric Adenocarcinoma SGC-7901 Cells. Asian Pac. J. Cancer Prev. 2011;12:1219–1223. [PubMed] [Google Scholar]
- 60.Rasul A., Yu B., Yang L.F., Ali M., Khan M., Ma T., Yang H. Induction of mitochondria-mediated apoptosis in human gastric adenocarcinoma SGC-7901 cells by kuraridin and Nor-kurarinone isolated from Sophora flavescens. Asian Pac. J. Cancer Prev. 2011;12:2499–2504. [PubMed] [Google Scholar]