Abstract
Four new sinapyl alcohol derivatives dichrocephols A–D (compounds 1–4) were isolated from the lipo-soluble part of the whole herb of Dichrocephala benthamii C. B. Clarke, together with the known compound syringenin isovalerate (5). Their structures were elucidated on the basis of spectroscopic analysis. Their absolute configurations were established by the method of alkaline hydrohysis. Compounds 1–3 showed moderate cytotoxity against HeLa cells, with IC50 values of 14.8 μM, 51.6 μM and 81.6 μM, respectively. This is the first time that sinapyl alcohol derivatives were isolated from the genus Dichrocephala.
Keywords: Dichrocephala benthamii C. B. Clarke, Asteraceae, sinapyl alcohol derivatives, absolute configuration determination, cytotoxicity
1. Introduction
The Asteraceae plant Dichrocephala benthamii C. B. Clarke is an annual herb which is only distributed in China and India [1]. Its whole herb has commonly been used as a fork medicine among the Dai nationality of China for the treatment of indigestion, common cold and fever in children, pneumonia and hepatitis [2,3]. However, to the best of our knowledge, there have been few previous reports on phytochemical investigations of this species [4]. In our search for new bioactive constituents from the medicinal plants used by the Dai nationality in China, we initiated chemical studies of the whole herb of D. benthamii.
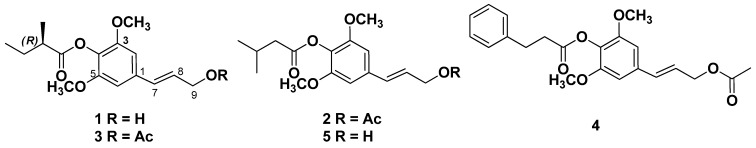
The air-dried and chopped whole herbs of D. benthamii were extracted with 95% EtOH. After removal of solvent, the residue was subject to the diatomaceous silica and bathed successively with petroleum ether, CH2Cl2, EtOAc and MeOH. The petroleum ether part led to the isolation of four new sinapyl alcohol derivatives 1–4 and the known compound syringenin isovalerate (5) (Figure 1) [5].
Figure 1.
Structures of compounds 1–5.
2. Results and Discussion
Compound 1, a colorless oil, had the molecular formula of C16H22O5, as determined by analysis of its HRESIMS (m/z 317.1358 [M+Na]+). The IR spectrum of 1 showed absorption bands at 1757 (C=O), 1596, 1507, 1463 (-phenyl) cm−1. Analysis of the 1H-, 13C-, DEPT, and HSQC NMR data revealed that there was a 1,3,4,5-tetrasubstituted phenyl in 1. The signals at δC 56.6 and δH 3.84 (6H, s) indicated that there were two overlapped methoxy groups, which were symmetrically located on the phenyl ring. Interpretation of the 1H-1H COSY NMR data of 1 led to the identification of two isolated proton spin-systems corresponding to C-7−C-9, C-2′−C-5′ units. The remaining fragment connections were determined by HMBC data, and established a skeleton similar to that of sinapyl alcohols [5,6]. The HMBC correlations from δH 6.79 (H-2) to δC 137.2 (C-1), 131.4 (C-7), δH 6.61 (H-7) to δC 137.2 (C-1), 104.3 (C-2, 6), δH 6.39 (H-8) to δC 137.2 (C-1) indicated that C-1 was connected to C-7. The HMBC correlations from δH 1.64 (1H, m, H-3′a), 1.80 (1H, m, H-3′b), 1.30 (3H, d, J = 7.2 Hz, H-5′) to δC 176.5 (C-1′) indicated that there was a 2-methylbutyryl group in 1. Due to absence of HMBC correlation between δH 4.27 (H-9) and δC 176.5 (C-1′) together with consideration of the chemical shift values of C-9, and compared with its analogues [5,6], 2-methylbutyryloxy was suggested to be connected with C-4. Thus the planar structure of 1 was established as (E)-3,5-dimethoxy-4-(2-methylbutyryloxy) phenylpropanol.
The absolute configuration at C-4 in the 2-methylbutyryl group was established by the alkaline hydrolysis method. According to the αD values of (−14.3) for (R)-2-methylbutyric acid and (+19.3) for (S)-2-methylbutyric acid and compared with the data reported in the literature [7,8,9,10,11], the absolute configuration of the 2-methylbutyryl group in 1 was supposed as R by the negative αD value {[α −4.6 (c 0.04, MeOH)} of the 2-methylbutyric acid. Therefore the structure of 1 was established as (E)-3, 5-dimethoxy-4-(2R-methylbutyryloxy)phenylpropanol, which was named dichrocephol A.
Compound 2, a colorless oil, was assigned the molecular formula C18H24O6 by HRESIMS analysis (m/z 359.1510 [M+Na]+). The 1H- and 13C-NMR data of 2 was similar to that of 1 except the substituent at C-4 and C-9. The signals at δH 2.04 (3H, s, H-2′′) and δC 170.9 (C-1′′), 20.8 (C-2′′) indicated the existence of the acetyl group. In addition, the signals at δH 1.06 (6H, d, J = 6.6 Hz, H-4′, 5′), 2.24 (1H, m, J = 7.2, 6.6 Hz, H-3′), 2.41 (2H, d, J = 7.2 Hz, H-2′) together with the HMBC correlations shown in Figure 2 revealed that isopentanoyl had replaced the 2-methylbutyryl group in 1. The overlapped signals of C-1′′and C-1′ in 13C-NMR led to the complexity that the two substituents could not be determined to connect with C-4 or C-9 by HMBC correlations. Detailed analysis of the chemical shift values of 2, and comparison of its analogues implied that isopentanoyloxy should be anchored at C-4 [5,6]. The EIMS fragment ions of 2 also supported the above-mentioned deduction.
Figure 2.
1H–1H COSY, HMBC for compounds 1–4.
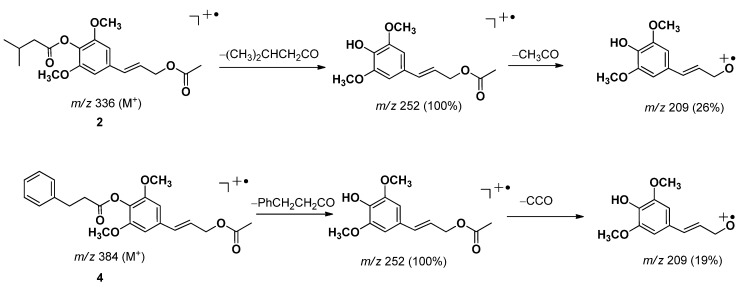
The abundance the peak of m/z 252 was 100%, which implied that the isopentanoyl moiety was repelled by the two methoxy units and lost easily (Figure 3). Therefore 2 was established as (E)-3,5-dimethoxy-4-isopentanoyloxy phenylpropanol acetate, which was named dichrocephol B.
Figure 3.
EIMS fragment ions of 2 and 4.
Compound 3, a colorless oil, was isolated together with 2. The mole ratio of 3 to 2 was determined to be 1:0.6 according to their 1H-NMR integration. The 1H and 13C-NMR data of 3 was very similar to that of 2, except that isopentanoyl in 2 was replaced by the 2-methylbutyl group, which was confirmed by HMBC correlations. The absolute configuration at C-4 in the 2-methylbutyl group was also established by alkaline hydrolysis method, and was suggested as R by the negative αD value {[α −8.0 (c 0.05, MeOH)} of the 2-methylbutyric acid. Thus the structure of 3 was established as (E)-3, 5-dimethoxy-4-(2R-methylbutyryloxy)phenylpropanol acelate, which was named dichrocephol C.
Compound 4, a colorless oil, had the molecular formula C22H24O6, as determined by analysis of its HRESIMS (m/z 385.1640 [M+H]+). Analysis of the 1H and 13C-NMR data revealed the same structural of (E)-3, 5-dimethoxyphenylpropanol acelate as those in compound 2 and 3. The 1H-NMR signals at δH 2.90 (2H, t, J = 6.5 Hz, H-2′), 3.05 (2H, t, J = 6.5 Hz, H-3′), 7.23 (1H, m, J = 7.0, 1.5 Hz, H-4′′′), 7.36 (4H, m, H-2′′′, 3′′′, 5′′′, 6′′′), and the HMBC correlations from δH 3.05 (H-3′) to δC 170.8 (C-1′), 141.7 (C-1′′′), 129.3 (C-2′′′, 6′′′) both indicated the existence of the phenylpropionyl group [12,13]. The HMBC correlations from δH 4.71 (H-9) to δC 170.8 (C-1′′ and C-1′ were overlapped) could not determine that the phenylpropionyloxy and acetyloxy groups was connected with C-4 or C-9, whereas taking account for the chemical shift values and compared with NMR data of compounds 1–3, the phenylpropionyloxy group was supposed to connect to C-4. This hypothesis was also supported by the EIMS spectra the same way as those of 2 (Figure 3). Therefore, compound 4 was characterized as (E)-3, 5-dimethoxy-4-phenylpropionyloxyphenylpropanol acetate, which was named dichrocephol D.
The sinapyl alcohol derivatives exhibited notable cytotoxicity against KB, BEL-7404 cancer cell lines [14] and HIV-1 replication [15]. Therefore, compounds 1–5 were tested for their cytotoxicity against HeLa cell lines [16] and inhibitory effect against COX-2 [17] by applying MTT and initial velocity method respectively. The results showed that compounds 1–3 showed moderate cytotoxic activities against HeLa cells with IC50 values of 14.8 μM, 51.6 μM, and 81.6 μM respectively. All of these compounds had no significant effect against COX-2 enzyme unfortunately.
3. Experimental
3.1. General
Optical rotations were measured on a Perkin-Elmer 241 polarimeter (Waltham, MA, USA), and UV data were recorded on Beckman Coulter DU 800 spectrometer (Tokyo, Japan). IR data were recorded using a Shimadzu FTIR-8400S spectrophotometer (Tokyo, Japan). 1H and 13C-NMR data were acquired with a Bruker 600 (Munich, Germany) and Bruker 500 instruments using the solvent signals (CD3OD: δH 3.30/δC 49.0, CD3COCD3: δH 2.05/δC 29.9, 206.7) as references. HRESIMS data were acquired using a LTQ Orbitrap XL mass spectrometer (Santa Clara, CA, USA). EIMS data were recorded on a GCMS-QP 2010 Shimadzu spectrometer (Tokyo, Japan). Sephadex LH-20 (Pharmacia Biotech AB, Uppsala, Sweden), diatomaceous silica, silica gel (80–100, 100–200, 200–300 mesh) and silica gel GF254 sheets (0.20–0.25 mm) (Qingdao Marine Chemical Plant, Qingdao, China) were used for column chromatography and TLC, respectively.
3.2. Plant Material
The whole herb of D. benthamii was collected from Xishuangbanna county, Yunnan Province of People’s Republic of China in October 2008. The sample was identified by one of the authors C. Z. Peng and the voucher specimen (No. DB2008) has been deposited in the Herbarium of the Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences, Beijing.
3.3. Extraction and Isolation
The air-dried and chopped whole herbs of D. benthamii (20 kg) were extracted with 95% EtOH (3 × 80 L) at 70 °C for 1 h/two times. The 95% EtOH extracts were concentrated under reduced pressure at 50 °C. The residue (1.7 kg) was subject to the diatomaceous silica (45 × 18 cm, 1.8 kg) and bathed successively with petroleum ether, CH2Cl2, EtOAc and MeOH. The petroleum ether part (275 g) was subject to silica gel column chromatography (CC) (45 × 12 cm, 80–100 mesh, 2.0 kg), using petroleum ether–Me2CO gradient elution (1:0–0:1) to afford 6 corresponding fractions (A–F). Fraction C (43 g) was fractionated over silica gel CC (50 × 12.5 cm, 100–200 mesh, 1.0 kg) using petroleum ether-Me2CO elution (30:1) to give seven fractions (C-1–C-7). The mixture of 2 and 3 (290 mg) was isolated from fraction C-4 (4.4 g) by further chromatography over silica gel CC (60 × 4.5 cm, 200–300 mesh, 220 g), using petroleum ether-Me2CO (30:1) elution. Fraction C-5 (1.6 g) was further fractionated by silica gel CC (50 × 3.5 cm, 200–300 mesh, 80 g) using petroleum ether-Me2CO (10:1) elution to give five fractions (C-5a–C-5e). Fraction C-5c (50 mg) was purified by semipreparative HPLC (70% aqueous MeOH, 2 mL/min, 210 nm) to afford 2 (tR 34.4 min; 33.0 mg). Fraction C-5d (100 mg) was purified by semipreparative HPLC (80% aqueous MeOH, 2 mL/min, 210 nm) to afford 4 (tR 20.0 min; 9.0 mg). Fraction E (26 g) was fractionated by silica gel CC (50 × 3.5 cm, 100–200 mesh, 0.5 kg) using petroleum ether-Me2CO elution (5:1) to give four fractions (E-1–E-4). Fraction E-4 (3.8 g) was fractionated over silica gel CC (60 × 4.0 cm, 200–300 mesh, 200 g) using petroleum ether-Me2CO elution (4:1) to give three fractions (E-4a–E-4c). Fraction E-4b (150 mg) was purified by semipreparative HPLC (65% aqueous MeOH, 2 mL/min, 210 nm) to afford 5 (tR 27.8 min; 4.9 mg) and 1 (tR 29.2 min; 17.9 mg).
3.4. Spectral Data
Dichrocephol A (1): colorless oil; [α −2.55 (c 0.19, MeOH); UV (MeOH) λmax (log ε) 219 (2.4); IR (KBr) νmax 2967, 1757, 1596, 1507, 1463, 1244, 1147, 845 cm−1; EIMS m/z (%): 294 [M]+ (3), 210 (88), 182 (28), 167 (23), 77 (13), 57 (100); HRESIMS m/z: 317.1358 [M+Na]+ (calcd for C16H22O5Na, 317.1365); 1H-NMR (600 MHz, CD3OD) and 13C-NMR (150 MHz, CD3OD) data see Table 1.
Table 1.
1H and 13C-NMR Spectroscopic Data for dichrocephols A–D (1–4).
| Pos | 1 a (J in Hz) | 2 b (J in Hz) | 3 b (J in Hz) | 4 b (J in Hz) | ||||
|---|---|---|---|---|---|---|---|---|
| δC | δH | δC | δH | δC | δH | δC | δH | |
| 1 | 137.2 C | 135.6 C | 134.6 C | 135.8 C | ||||
| 2 | 104.3 CH | 6.79 s | 104.2 CH | 6.85 s | 103.5 CH | 6.62 s | 104.3 CH | 6.87 s |
| 3 | 153.7 C | 153.4 C | 152.4 C | 153.5 C | ||||
| 4 | 130.7 C | 135.6 C | 134.6 C | 135.8 C | ||||
| 5 | 153.8 C | 153.4 C | 152.4 C | 153.5 C | ||||
| 6 | 104.3 CH | 6.79 s | 104.2 CH | 6.85 s | 103.5 CH | 6.62 s | 104.3 CH | 6.87 s |
| 7 | 131.4 CH | 6.61 d (16.2) | 134.1 CH | 6.65 d (16.2) | 134.2 CH | 6.57 d (15.6) | 134.2 CH | 6.68 d (16.0) |
| 8 | 130.7 CH | 6.39 dt (16.2, 5.4) |
125.0 CH | 6.36 dt (16.2, 6.6) |
123.6 CH | 6.20 dt (15.6, 6.6) |
125.1 CH | 6.39 dt (16.0, 6.5) |
| 9 | 63.6 CH2 | 4.27 dd (5.4, 1.8) |
65.2 CH2 | 4.70 dd (6.0, 0.6) |
65.0 CH2 | 4.71 dd (6.6, 0.6) |
65.2 CH2 | 4.71 dd (6.5, 1.5) |
| OCH3 | 56.6 CH3 | 3.84 s | 56.4 CH3 | 3.83 s | 56.2 CH3 | 3.81 s | 56.6 CH3 | 3.83 s |
| 1′ | 176.5 C | 170.9 C | 174.5 C | 170.8 C | ||||
| 2′ | 42.4 CH | 2.66 m | 43.4 CH2 | 2.41 d (7.2) | 41.1 CH | 2.68 m | 35.8 CH2 | 2.90 t (6.5) |
| 3′ | 28.1 CH2 | 1.64 m, 1.80 m | 26.7 CH | 2.24 m | 27.1 CH2 | 1.61 m, 1.83 m | 31.6 CH2 | 3.05 t (6.5) |
| 4′ | 11.9 CH3 | 1.07 t (7.2) | 22.5 CH3 | 1.06 d (6.6) | 11.6 CH3 | 1.03 t (7.8, 7.2) | ||
| 5′ | 17.4 CH3 | 1.30 d (7.2) | 22.5 CH3 | 1.06 d (6.6) | 16.9 CH3 | 1.29 d (6.6) | ||
| 1′′ | 170.9 C | 170.9 C | 170.8 C | |||||
| 2′′ | 20.8 CH3 | 2.04 s | 21.1 CH3 | 2.10 s | 20.9 CH3 | 2.04 s | ||
| 1′′′ | 141.7 C | |||||||
| 2′′′-6′′′ | 129.3 C | 7.36 m | ||||||
| 4′′′ | 127.1 C | 7.23 tt (7.0, 1.5) |
||||||
a recorded in CD3OD; b recorded in CD3COCD3.
Dichrocephol B (2): colorless oil; UV (MeOH) λmax (log ε) 220 (2.6); IR (KBr) νmax 2959, 1738, 1597, 1507, 1456, 1245, 1132 cm−1; EIMS m/z (%): 336 [M]+ (3), 252 (100), 209 (26), 149 (8), 57 (41); HRESIMS m/z 359.1510 [M+Na]+ (calcd for C18H24O6Na, 359.1471); 1H-NMR (600 MHz, CD3COCD3) and 13C-NMR (150 MHz, CD3COCD3) see Table 1.
Dichrocephol C (3): colorless oil; [α −4.80 (c 0.06, acetone); UV (acetone) λmax (log ε) 230 (3.7); IR (KBr) νmax 2964, 1732, 1597, 1244, 1130, 848 cm−1; EIMS m/z (%): 336 [M]+ (3), 252 (100), 209 (26), 149 (8), 105 (5), 77 (5), 57 (41); HRESIMS m/z 359.1510 [M + Na]+ (calcd for C18H24O6Na, 359.1471); 1H-NMR (600 MHz, CD3COCD3) and 13C-NMR (150 MHz, CD3COCD3) data see Table 1.
Dichrocephol D (4): colorless oil; UV (MeOH) λmax (log ε) 218 (2.4); IR (KBr) νmax 2936, 1738, 1597, 1506, 1455, 1245, 1131, 965, 847 cm−1; EIMS m/z (%): 384 [M]+ (4), 327 (8), 267 (13), 252 (100), 209 (19), 134 (41), 105 (61), 91 (85), 77 (35), 55 (100); HRESIMS m/z: 385.1640 [M+H]+ (calcd for C22H25O6, 385.1651); 1H-NMR (500 MHz, CD3COCD3) and 13C-NMR (125 MHz, CD3COCD3) see Table 1.
3.5. Absolute Configurations at C-4 in the 2-Methylbutyryl Group of Compounds 1 and 3
A mixture of 2 and 3 (20.0 mg) was dissolved in EtOH (2.0 mL) and treated with 5% KOH in H2O (4.0 mL). After stirring at room temperature for 24 h, the reaction mixture was concentrated and then partitioned between EtOAc and H2O. After extraction with EtOAc three times, the aqueous layer was acidified with HCl to pH = 3.0 and then extracted with CH2Cl2 three times. The CH2Cl2 layer was combined and subjected to Sephadex LH-20 CC (CH2Cl2–MeOH, 1:1) to yield a mixture of 2-methybutyric acid and isopentoic acid (1.5 mg). Since isopentoic acid is optically inactive, the absolute configuration at C-4 of 2-methybutyric acid was identified as R by the αD value of {[α −8.0 (c 0.05, MeOH)}. In the same way, the absolute configuration of C-4 in the 2-methylbutyryl group of 1 was also established as R by its αD value of {[α −4.6 (c 0.04, MeOH)}.
3.6. Bioassays
The cytotoxic activities of the isolated compounds were evaluated against HeLa cell line by MTT colorimetric method with 5-fluorouracil as positive control (IC50 value 5.9 μM). The inhibitory effect of compounds 1−5 on COX-2 (sheep) enzyme (Gayman 760111) were tested by initial velocity method.
4. Conclusions
Four new sinapyl alcohol derivatives dichrocephols A–D 1–4 were isolated from the lipo-soluble part of the whole herb of Dichrocephala benthamii C. B. Clarke, together with the known syringenin isovalerate (5). Compounds 1–3 showed moderate cytotoxities against HeLa cells with IC50 values of 14.8 μM, 51.6 μM and 81.6 μM respectively. This is the first time that sinapyl alcohol derivatives were isolated from the genus Dichrocephala.
Acknowledgments
We gratefully acknowledge financial support from the Chinese National S&T Special Project on Major New Drug Innovation (2011ZX09307-002-01, 2012ZX09301002-001).
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/18/2/1720/s1.
Footnotes
Sample Availability: Samples of dichrocephols A–D are available from the authors.
References
- 1.Editorial Committee of Flora of China . Flora of China. Science Press; Beijing, China: 1977. pp. 76–79. [Google Scholar]
- 2.Wu Z.Y. Southern Yunnan Materia Medica. Yunnan’s Medical Publishing House; Kunming, China: 2000. p. 13. [Google Scholar]
- 3.Compiling Group of the National Assembly in Herbal Medicine . National Assembly in Herbal Medicine. People’s Medical Publishing House; Beijing, China: 1978. [Google Scholar]
- 4.Zhu S.H., Zhang Q.J, Chen Q., Zhou T., Yao R.J. Study on the Chemical Constituents of Dichrocephala integrifolia. J. Chin. Med. Mat. 2010;33:53–55. [PubMed] [Google Scholar]
- 5.Bohlmann F., Gupta R.K., Jakupovic J. An acylpyrrole derivative and further constituents from Jamaican representatives of the tribe Senecioneae. Phytochemistry. 1981;20:831–832. doi: 10.1016/0031-9422(81)85189-8. [DOI] [Google Scholar]
- 6.Zhao Y., Jia Z.J., Li Y. Sinapyl alcohol derivatives and other constituents from Ligularia nelumbifolia. Phytochemistry. 1994;37:1149–1152. [Google Scholar]
- 7.Brechbuhler S., Buchi G., Milne G. The Absolute Configuration of the Aflato xins. J. Org. Chem. 1967;32:2641–2642. doi: 10.1021/jo01283a068. [DOI] [PubMed] [Google Scholar]
- 8.Korver O., Gorkom M.V. Optically active 2-methyl substituted acids and esters: Chiroptical properties, conformational equilibria and NMR with opticallly active shift reagents. Tetrahedron. 1974;30:4041–4048. doi: 10.1016/S0040-4020(01)97381-8. [DOI] [Google Scholar]
- 9.Meyers A.I., Knaus G., Kamata K. Synthesis via 2-Oxazolines. IV. An asymmetric Synthesis of 2-Methylalkanoic Acids from a Chiral Oxazoline. J. Am. Chem. Soc. 1974;96:268–270. doi: 10.1021/ja00808a054. [DOI] [Google Scholar]
- 10.Pan J.Y., Chen S.L., Li M.Y., Li J., Yang M.H., Wu J. Limonoids from the Seeds of a Hainan Mangrove, Xylocarpus granatum. J. Nat. Prod. 2010;73:1672–1679. doi: 10.1021/np100395w. [DOI] [PubMed] [Google Scholar]
- 11.Sarigaputi C., Nuanyai T., Teerawatananond T., Pengpreecha S., Muangsin N., Pudhom K. Xylorumphiins A-D, Mexicanolide Limonoids from the Seed Kernels of Xylocarpus rumphii. J. Nat. Prod. 2010;73:1456–1459. doi: 10.1021/np100423w. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y., Kinoshita K., Koyama K., Takahashi K., Tai K., Nunoura Y., Watanabe K. Two Novel Anti-emetic Principles of Alpinia katsumadai. J. Nat. Prod. 1999;62:1672–1674. doi: 10.1021/np990096e. [DOI] [PubMed] [Google Scholar]
- 13.Shin J.E., Han M., Song M.C., Baek N.I., Kim D.H. 5-Hydroxy-7-(4’-hydroxy-3’-methoxyphenyl)-1-phenyl-3-heptanone: A Pancreatic Lipase Inhibitor Isolated from Alpinia officinarum. Biol. Pharm. Bull. 2004;27:138–140. doi: 10.1248/bpb.27.138. [DOI] [PubMed] [Google Scholar]
- 14.Zou H.B., Dong S.Y., Zhou C.X., Hu L.H., Wu Y.H., Li H.B., Gong J.X., Sun L.L., Wu X.M., Bai H., et al. Design, synthesis, and SAR analysis of cytotoxic sinapyl alcohol derivatives. Bioorg. Med. Chem. 2006;14:2060–2071. doi: 10.1016/j.bmc.2005.10.056. [DOI] [PubMed] [Google Scholar]
- 15.Fan X.N., Zi J.C., Zhu C.G., Shi J.G. Chemical Constituents of Heteroplexis micocephala. J. Nat. Prod. 2009;72:1184–1190. doi: 10.1021/np900213w. [DOI] [PubMed] [Google Scholar]
- 16.Zou G.A., Ding G., Su Z.H., Yang J.S., Zhang H.W., Peng C.Z., Aisa H.A., Zou Z.M. Lactonecembranoids from Croton laeWigatus. J. Nat. Prod. 2010;73:792–795. doi: 10.1021/np100044t. [DOI] [PubMed] [Google Scholar]
- 17.Lü J.X., Shen Q., Jiang J.H., Shen G.L., Yu R.Q. QSAR analysis of cyclooxygenase inhibitor using particle swarm optimization and multiple linear regression. J. Pharm. Biomed. Anal. 2004;35:679–687. doi: 10.1016/j.jpba.2004.02.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.