Abstract
Mimusops caffra E. Mey. ex A.DC and Mimusops obtusifolia Lam (both members of the Sapotaceae family), and Hypoxis colchicifolia Bak (family Hypoxidaceae) are used by traditional healers in Zululand to manage malaria. Anti-plasmodial investigation of the crude extracts and some triterpenes isolated from the plants showed activity against a chloroquine sensitive (CQS) strain of Plasmodium falciparum (D10). Among the crude extracts the leaves of M. caffra exhibited the highest activity, with an IC50 of 2.14 μg/mL. The pentacyclic tritepenoid ursolic acid (1), isolated from the leaves of M. caffra was the most active compound (IC50 6.8 μg/mL) as compared to taraxerol (2) and sawamilletin (3) isolated from the stem bark of M. obtusifolia (IC50 > 100). Chemical modification of the ursolic acid (1) to 3β-acetylursolic acid (4) greatly enhanced its anti-plasmodial activity. Compound 4 reduced parasitaemia against Plasmodium berghei by 94.01% in in vivo studies in mice. The cytotoxicity of 3β-acetylursolic acid (IC50) to two human cell lines (HEK293 and HepG2) was 366.00 μg/mL and 566.09 μg/mL, respectively. The results validate the use of these plants in folk medicine.
Keywords: Plasmodium falciparum, Mimusops caffra, Mimusops obtusifolia, Hypoxis colchicifolia, ursolic acid
1. Introduction
Malaria is one of the major health problems in tropical Africa, South-east Asia, Central South America and Oceania. Despite the various efforts by governmental and non-governmental organizations aimed at eradicating the disease, malaria is said to kill a child every 30 s [1]. Malaria cases have been reported in other areas of the World that were previously considered eradicated of malaria [2].
In Africa, herbal medicines are an important part of the culture and traditions of its people [3]. Traditional healers use different concoctions prepared from medicinal plants to treat malaria. Given the remarkable anti-malarial properties of Cinchona bark that have been known for more than 300 years, resulting in the discovery of quinine [4] and the more recent development of artemisinin derivatives [5], the potential of plant species to provide effective drugs for the treatment of malaria cannot be overemphasized. Furthermore, the drug resistance of the malaria parasite to chloroquine and sulfadoxine–pyrimethamine, and also the toxicity of the currently available drugs have stimulated the search for alternative medicines which are naturally derived. In addition, modern health care to the rural people is still a far-reaching goal, due to economic constraints [6] and many vulnerable groups depend on plant-based traditional healing. The anti-malarial activity of many plants has been reported [7,8,9,10]. An ethonobotanical survey revealed the extensive utilization of M. caffra, M. obtusifolia and H. colchicifolia for the management of malaria in Zulu traditional medicine.
M. caffra is a small to medium-sized tree that grows up to 15 m high [11]. Its natural habitat is the dune forest from the high tide mark in KwaZulu-Natal and the Eastern Cape Provinces of South Africa and the Mozambique [11]. Medicinal uses of M. caffra include healing properties against sores and wounds [12].
M. obtusifolia has a small to medium-sized tree. Bark is grey to blackish, very rough and fissured in older specimens. Leaves are relatively broader and more rounded, 3.5–10 cm long. It is found in Southern Africa [13]. There is little information in the literature on its pharmacological activities.
H. colchicifolia is a slow-growing plant that often reaches up to 600 mm in height, with erect leaves; it is widespread in southern Africa [14]. It has a large underground tuber that allows it to survive the regular grass fires common to the grassland where it is found. These tubers are used by traditional healers to treat impotency and barrenness. Infusions are also taken as love charm emetics and are administered for hysterical fits [15]. Pharmacological activities of H. colchicifolia include anti-HIV and anti-diabetic properties [16,17]. This study was undertaken to investigate the anti-malarial activity of these plants.
2. Results and Discussion
Traditional medicines are a potential rich source of new drugs against malaria and other infectious diseases. The literature abounds with descriptions of the bioactivity of many antimalarial plants [7,8,9,10]. The observed anti-plasmodial activity of the crude extracts of M. caffra, M. obtusifolia, and H. colchicifolia is presented in Table 1. While the Mimusops species exhibited anti-plasmodial activity, it is apparent that our results do not support the traditional use of the bulb of H. colchicifolia in treating malaria. It is however worth mentioning that we observed antipyretic properties (data not included) of the extract of H. colchicifolia. Fever is the early symptom of malaria, and it is thus likely that the plant is used traditionally to treat the symptoms rather than the disease.
Table 1.
Anti-plasmodial activity against Plasmodium falcipurum (CQS) D10 strain (in vitro) and Plasmodium berghei (in vivo).
| Sample | a IC50 (µg/mL) | b Average % parasitemia | b Average % suppression | a Cytotoxicity (μg/mL) | |
|---|---|---|---|---|---|
| HEK293 | HepG2 | ||||
| M. caffra (leaves) | 2.14 | NT | NT | ||
| M. obtusifolia (bark) | 32.5 | NT | NT | ||
| H. colchicifolia (bulb) | NA | NT | NT | ||
| Ursolic acid | 6.8 | NT | NT | ||
| Ursolic acid acetate | 1.9 | 0.07 | 94.01 | 366.00 | 566.09 |
| 3-oxo-ursolic acid | 7.3 | NT | NT | ||
| Taraxerol | >100 | NT | NT | ||
| Sawamilletin | >100 | NT | NT | ||
| Artesunate | 5.1 * | NT | NT | ||
| Chloroquine | 14.1 * | 0.07 | 83.43 | ||
* = ng/mL, a IC50 = Inhibitory concentration, b Average percentage parasitemia/suppression (in vivo), NT = not tested, NA = not active.
Several triterpenes have been reported to possess both in vitro and in vivo anti-plasmodial activity [18,19,20]. Of the three triterpenes that were isolated from M. caffra and M. obtusifolia (ursolic acid, taraxerol and sawamilletin), only ursolic acid showed any appreciable anti-plasmodial activity (IC50 6.8 μg/mL) at the concentration tested. Ursolic acid has been previously reported to possess anti-plasmodial activity [21], and its presence in M. caffra could have contributed to the observed bioactivity of the plant. The lower activity of the extracted ursolic acid (compared to the crude M. caffra extract) could indicate a synergistic effect with other compounds, decomposition during fractionation, or removal of a protective matrix. Chemical modification of ursolic acid to its acetate derivative however resulted in a 72% increase in the in vitro anti-malarial activity (IC50 1.9 μg/mL). Chemical modification of drugs has been known to improve the potency of the drugs [22,23]; it is noteworthy that the usoric acid acetate exhibits a 94.01% suppression of parasitemia in infected mice (in vivo).
In cytotoxic evaluations a compound is only considered significantly active with an IC50 of less than 30 μg/mL [24]. The cytotoxicity of ursolic acid acetate to HEK293 and HepG2 cell lines is presented in Table 1. It is apparent that, when dilution in the bloodstream is taken into account, ursolic acid acetate should be regarded as non-toxic.
3. Experimental
3.1. Plant Collection
Fresh plant materials of M. obtusifolia and H. colchicifolia were collected from the Manguzi area, KwaZulu-Natal Province, South Africa, at the flowering stage in April, 2011 and M. caffra was collected in May 2012 from Durban, KwaZulu-Natal Province, South Africa. The plants were identified by Mrs. N. R. Ntuli, Department of Botany, University of Zululand, KwaDlangezwa. Voucher specimens were deposited at the University Herbarium [Simelane, MBC/02 (ZULU); Simelane, MBC/03 (ZULU); Simelane, MBC/04 (ZULU)].
3.2. Extraction and Isolation
The air-dried leaves of M. caffra (500 g) and the bark of M. obtusifolia (1.1 kg) were extracted with dichloromethane (DCM) and ethyl acetate (EtOAc) respectively (1:5 w/v). The resultant filtered extracts were concentrated to dryness under reduced pressure in a rotary evaporator (40 ± 2 °C). Dried extracts (5 g) were separately subjected to column chromatograph (20 × 500 mm) using silica gel 60 (300 g; 0.063 to 0.2 mm; 70 to 230 mesh ASTM supplied by Merck (Darmstadt, Germany). The crude extracts were chromatographed using gradient elution of hexane-ethyl acetate in a 5% stepwise increase at a speed of 100 mL per min and collecting 20 mL fractions. The collected fractions were combined based on their TLC (20 × 20 F254—Merck, Whitehouse Station, NJ, USA) profile to yield combined fractions. Visualization was achieved by UV light (254 nm) and by heating with 20% H2SO4 acid in MeOH. The single spot fractions were recrystallized in methanol and hexane to obtain 270 mg of MBCF93 from the n-hexane/EtOAc 7:3 eluate of the M. caffra extract; 310 mg of MBCF45 and 240 mg of MBCF15 were obtained from the n-hexane/EtOAc 9:1 fractions from the bark of M. obtusifolia. No attempt was made to isolate any compounds from H. colchicifolia because the initial crude extracts indicated no observable anti-plasmodial activity.
3.3. Structural Elucidation
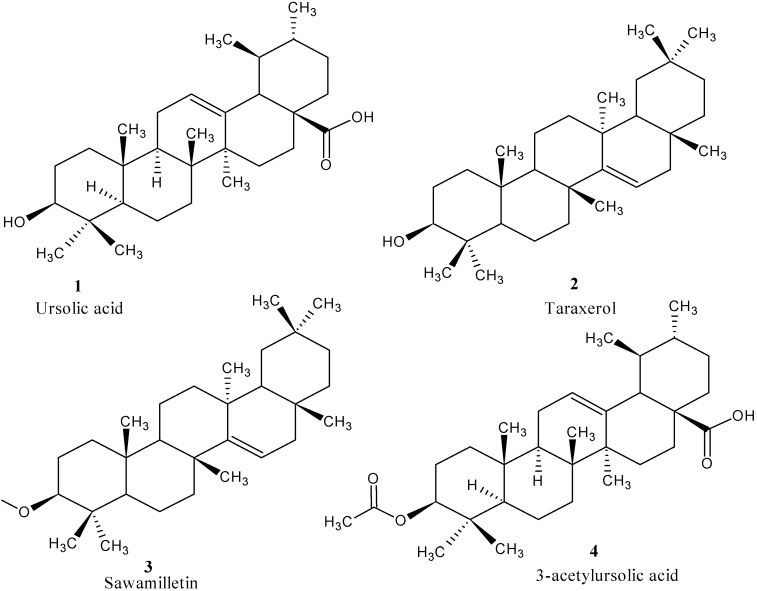
Compound MBCF93 was a white powder with a melting point (mp) of 279–283 °C, MBCF45 was also a white powder with mp 280–287 °C, MBCF15 also came out as a white powder with mp 272–285 °C. The structure of compounds were established using Nuclear Magnetic Resonance (NMR) techniques with the application of 2D-NMR (1H-1H, 13C-13C, DEPT, COSY, HMQC, HMBC and NOESY), infrared (IR) spectra and liquid chromatography mass spectrometry (LC-MS); molecular weights were identified by ESI-MS (positive mode). The compounds were confirmed by spectra comparison of their spectra (1H and 13C-NMR) with reported literature (Table 2). MBCF93 was confirmed as ursolic acid (1), MBCF45 as taraxerol (2), and MBCF15 as sawamilletin (3) (Figure 1).
Table 2.
1H- and 13C-NMR chemical shifts (δ, ppm) of compounds 1, 4 and 2.
| Carbon Position | UA(1) | δ 1H (ppm) | DEPT | UAA(4) | DEPT | ||
|---|---|---|---|---|---|---|---|
| δ 13C (ppm) | δ 13C (ppm) | ||||||
| 1 | 38.7 | CH2 | 38.3 | CH2 | |||
| 2 | 23.5 | CH2 | 24.1 | CH2 | |||
| 3 | 79 | 3.43 (1H, brs) | CH | 80.9 | CH | ||
| 4 | 39.6 | C | 37.7 | C | |||
| 5 | 52.7 | CH | 55.3 | CH | |||
| 6 | 18.3 | CH2 | 18.2 | CH2 | |||
| 7 | 33 | CH2 | 32.9 | CH2 | |||
| 8 | 39.1 | C | 39.5 | C | |||
| 9 | 47.6 | CH | 47.9 | CH | |||
| 10 | 36.7 | C | 36.7 | C | |||
| 11 | 23.7 | CH | 23.3 | CH | |||
| 12 | 125.8 | 5.50 (1H, brs) | CH | 125.8 | CH | ||
| 13 | 138 | C | 138 | C | |||
| 14 | 42 | C | 41.9 | C | |||
| 15 | 29.4 | CH2 | 30.6 | CH2 | |||
| 16 | 23.3 | CH2 | 23.6 | CH2 | |||
| 17 | 47.9 | C | 47.5 | C | |||
| 18 | 55.3 | 2.52 (1H, d, J = 11.0 Hz) | CH | 52.6 | CH | ||
| 19 | 30.6 | CH | 39 | CH | |||
| 20 | 30.4 | CH | 38.8 | CH | |||
| 21 | 27.3 | CH2 | 30.6 | CH2 | |||
| 22 | 37 | CH2 | 36.9 | CH2 | |||
| 23 | 23.4 | 1.24 (3H, s) | CH3 | 23.6 | CH3 | ||
| 24 | 17 | 1.02 (3H, s) | CH3 | 17.1 | CH3 | ||
| 25 | 17 | 0.93 (3H, s) | CH3 | 16.7 | CH3 | ||
| 26 | 15.5 | 1.05 (3H, s) | CH3 | 17.1 | CH3 | ||
| 27 | 24.2 | 1.22 (3H, s) | CH3 | 21.3 | CH3 | ||
| 28 | 176 | C | 182.6 | C | |||
| 29 | 21.1 | 0.97 (3H, s) | CH3 | 15.5 | CH3 | ||
| 30 | 23.4 | 0.99 (3H, d, J = 6.1 Hz) | CH3 | 21.2 | CH3 | ||
| -COCH3 | 28.1 | ||||||
| -COCH3 | 171 | ||||||
| Carbon Position | Taraxerol (2) δ 13C (ppm) | DEPT | δ 1H (ppm) | ||||
| 1 | 38 | CH2 | |||||
| 2 | 27.2 | CH2 | |||||
| 3 | 79.1 | CH | |||||
| 4 | 39 | C | |||||
| 5 | 55.6 | CH | |||||
| 6 | 18.8 | CH2 | |||||
| 7 | 35.1 | CH2 | 2.0 (1H, dt, J = 3.1, 12.6 Hz, H-7a) | ||||
| 8 | 38.8 | C | |||||
| 9 | 48.8 | CH | |||||
| 10 | 37.6 | C | |||||
| 11 | 17.5 | CH2 | |||||
| 12 | 35.8 | CH2 | |||||
| 13 | 37.6 | C | |||||
| 14 | 158.1 | C | |||||
| 15 | 116.9 | CH | 5.5 (1H, dd, J = 3.2, 8.2 Hz) | ||||
| 16 | 36.7 | CH2 | 1.9 (1H, dd, J = 3.0, 14.6 Hz, H-16a) | ||||
| 17 | 37.7 | C | |||||
| 18 | 49.3 | CH | |||||
| 19 | 41.3 | CH2 | |||||
| 20 | 28.8 | C | |||||
| 21 | 33.7 | CH2 | |||||
| 22 | 33.1 | CH2 | |||||
| 23 | 28 | CH3 | 0.98 (3H, s, H-23) | ||||
| 24 | 15.4 | CH3 | 0.80 (3H, s, H-24) | ||||
| 25 | 15.5 | CH3 | 0.93 (3H, s, H-25) | ||||
| 26 | 29.8 | CH3 | 1.09 (3H, s, H-26) | ||||
| 27 | 25.9 | CH3 | 0.91 (3H, s, H-27) | ||||
| 28 | 29.9 | CH3 | 0.82 (3H, s, H-28) | ||||
| 29 | 33.4 | CH3 | 0.95 (3H, s, H-29) | ||||
| 30 | 21.3 | CH3 | 0.90 (3H, s, H-30) | ||||
Figure 1.
Chemical structures of the isolated triterpenes.
3.4. Acetylation of Ursolic Acid
The plant derived ursolic acid (283 mg) was treated with acetic anhydride (10 mL) and pyridine (20 mL). The mixture was stirred at room temperature overnight after which water (10 mL) was added and stirred for at least 30 min. The resultant solid was then filtered and washed thoroughly with dilute HCl solution under vacuum. The white powder obtained (300 mg, mp 280–282 °C) was identified, by its spectral properties, as 3β-acetylursolic acid (4).
3.5. Preparation of 3-Oxoursolic Acid (5)
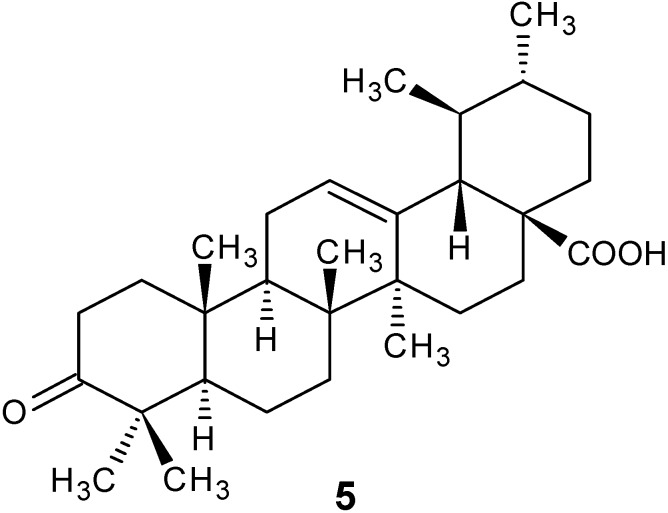
To a solution of compound 4 (100 mg) in acetone (1.5 mL) Jones’ reagent (0.4 mL) was added dropwise in an ice-salt bath. The reaction mixture was allowed to warm up to room temperature and stirred for 1 h. After cooling to 0 °C, 2-propanol (5 mL) was added and the solution stirred at room temperature for 30 min. The green precipitate was collected and washed well with acetone. The acetone solution from the combined filtrates were concentrated and dried. By purification on a silica gel column compound 5 was obtained as a white solid (86 mg) and identified as 3-oxo-ursolic acid (Figure 2).
Figure 2.
Chemical structure of 3-oxourosolic acid.
3.6. Drug Sensitivity Assay
3.6.1. In Vitro Antiplasmodial Activity
The test samples (crude extracts, isolates, and chemical derivatives) were tested in triplicate against a chloroquine sensitive (CQS) strain of Plasmodium falciparum (D10). Continuous in vitro cultures of asexual erythrocyte stages of P. falciparum were maintained using modified method of Trager and Jensen [25]. Quantitative assessment of anti-plasmodial activity in vitro was determined via the parasite lactate dehydrogenase assay using a modified method described by Makler [26]. The test samples were prepared to a 20 mg/mL stock solution in 100% DMSO and sonicated to enhance solubility. Stock solutions were stored at −20 °C. Further dilutions were prepared on the day of the experiment. Chloroquine and artesunate were used as the reference drugs for experiments. A full dose-response was performed for all active compounds to determine the concentration inhibiting 50% of parasite growth (IC50-value). Test samples were tested at a starting concentration of 100 μg/mL, which was then serially diluted 2-fold in complete medium to give 10 preparations of variable concentrations; with the lowest concentration being 0.2 μg/mL. The same dilution technique was used for all samples. CQ was tested at a starting concentration of 1,000 ng/mL. The highest concentration of solvent to which the parasite-infected erythrocytes were exposed to had no measurable effect on the parasite viability (data not shown). The IC50-values were obtained using a non-linear dose-response curve fitting analysis via Graph Pad Prism v.4.0 software (Graph Pad Prism, Inc: San Diego, CA, USA, 1994–2003).
3.6.2. In Vivo Antiplasmodial Activity
This study was carried out after the approval from the Ethical Committee on Animal Use and Care of the University of Zululand (UZREC 171110-030 PGD 2013/26). Swiss mice (20–25 g each) of both sexes were obtained from biomedical research unit (BRU) in the University of KwaZulu Natal, Durban. They were kept in plastic cages, and given standard laboratory diet and water ad libitum and maintained under laboratory conditions of temperature and 12 h light and 12 h dark cycle. The animals were allowed to acclimatize to the laboratory at a controlled temperature of 22 °C for 15 days before being subjected to the experiments. The animals were divided into seven groups (eight in each group) consisting of control, chloroquine (5 mg/kg/day), and the extract treated group. The extract treated group was sub divided into five sub groups that received 50, 100, 200, 300 and 400 mg/kg/day body weight respectively.
3.6.2.1. Parasite Inoculation
A single donor mouse infected with Plasmodium berghei parasites was bled into sterile heparinized culture medium and the blood was diluted with RPMI 1640 medium. The healthy experimental mice were infected intravenously via a tail vein with 0.2 mL of the diluted blood containing 1 × 107 parasitized (Plasmodium berghei) red blood cells on day one.
3.6.2.2. Evaluation of Antimalarial Activity
The antimalarial activity tests were performed using the four-day suppressive tests described by Peters et al. [27]. The extracts dissolved in 0.5% carboxymethyl cellulose (CMC) were administered orally using cannula (equivalent to 0.2 mL solution per mouse) for four consecutive days. Parallel tests with chloroquine were conducted for reference purposes at the same dose in one group and with an equivalent volume of 0.5% CMC (0.2 mL/mouse/day) in the control group. Thin smears were obtained from the tail vein of each mouse on day five after infection. The smears were fixed with methanol and stained with Giemsa stain. The percent parasitemia suppression was determined by counting the number of parasitized erythrocytes out of 500 red blood cells on random fields under the microscope. The average percentage suppression of parasitemia was calculated using the following formula:
| % suppression = A – B/C | (1) |
where A = % parasitemia in untreated controls, B = % parasitemia in treated groups, and C = % parasitemia in untreated controls. The data were analyzed using the F-test. A P-value < 0.05 was considered significant.
3.7. MTT Cell Proliferation Assay
Human embryonic kidney (HEK293) and human hepatocellular carcinoma (HepG2) cells were all grown to confluenecy in 25 cm2 flasks. This was then trypsinized and plated into 48 well plates at specific seeding densities. Cells were incubated overnight at 37 °C. Medium was then removed and fresh medium (MEM + Glutmax + antibiotics) was added. Extracts (50–350 μL/mL) were then added in triplicate and incubated for 4 h. Thereafter medium was removed and replaced by complete medium (MEM + Glutmax + antibiotics + 10% Fetal bovine serum). After 48 h cells were subjected to the MTT assay [28]. Data were evaluated through regression analysis using QED statistics program and from the linear equation the IC50 values representing the lethal concentration for 50% mortality was calculated.
3.8. Statistical Analyses
The mean and standard error mean of three experiments were determined. Statistical analysis of the differences between mean values obtained for experimental groups were calculated using Microsoft Excel Program, 2010 and Graph Pad Prism v.4.0 software (Graph Pad Prism, Inc: San Diego, CA, USA, 1994–2003) for IC50. Data were subjected to one way analysis of variance (ANOVA). P values < 0.05 were regarded as significant and P values < 0.01 as very significant.
4. Conclusion
This study was conducted to investigate the anti-malarial activity of some indigenous plants used by Zulu traditional healers to treat malaria. Even though the observed activity of the crude plant extracts, the isolated triterpenes, and the chemical derivative may not be as high as those reported for the standards (chloroquine and artesunate), the activity of the extracts were dose dependent, and with low toxicity levels, encourages the use of M. caffra in managing malaria in traditional medicine.
Acknowledgments
The authors are grateful to National Research Fund (SA) and University of Zululand Research committee for financial support.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds (1–5) are available from the authors.
References
- 1.World Health Organization. World malaria situation in 1994. WHO Wkly. Epidemiol. Rec. 2012;22:161–167. [Google Scholar]
- 2.World Health Organization. World Health Organization Malaria Fact Sheet No. 94. 2010. [(accessed on 13 July 2013)]. Available online: http://www.who.int/mediacentre/factsheets/fs094/en/print.html/
- 3.Mugabe J. Biodiversity and sustainable development in Africa. In: Mugabe J., Clark N., editors. National Systems of Conservation and Innovation in Africa. African Centre for Technology Studies (ACTS); Nairobi, Kenya: 1998. [Google Scholar]
- 4.Camacho M.D.R., Croft S.L., Phillipson J.D. Natural products as sources of antiprotozoal drugs. Curr. Opin. AntiInfect. Investig. Drugs. 2000;2:47–62. [Google Scholar]
- 5.Ploypradith P. Development of artemisinin and its structurally simplified trioxane derivatives as antimalarial drugs. Acta Trop. 2004;89:329–342. doi: 10.1016/j.actatropica.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Grover J.K., Yadav S., Vats V. Medicinal plants of India with antidiabetic potential. J. Ethnopharmacol. 2002;81:81–100. doi: 10.1016/S0378-8741(02)00059-4. [DOI] [PubMed] [Google Scholar]
- 7.Nethengwe M.F., Opoku A.R., Dludla P.V., Madida K.T., Shonhai A., Smith P., Singh M. Larvicidal, antipyretic and antiplasmodial activity of some Zulu medicinal plants. J. Med. Plants Res. 2012;6:1255–1262. [Google Scholar]
- 8.Kaou A.M., Valérie M., Cécile C., Laurent D., Kujala T.S., Loponen J.M., Klika K.D., Pihlaja K. Phenolics and betacyanins in red beetroot (Beta vulgaris) root: Distribution and effect of cold storage on the content of total phenolics and three individual Compounds. J. Agric. Food Chem. 2000;48:5388–5342. doi: 10.1021/jf000523q. [DOI] [PubMed] [Google Scholar]
- 9.Salawu O.A., Tijani A.Y., Babayi H., Nwaeze A.C., Anagbogu R.A., Agbakwuru V.A. Anti-malarial activity of ethanolic stem bark extract of Faidherbia Albida (Del) a. Chev (Mimosoidae) in mice. Arch. Appl. Sci. Res. 2012;2:261–268. [Google Scholar]
- 10.Gathirwa J.W., Rukunga G.M., Njagi S.A., Omara P.G., Mwitaria A.N., Guantai F.M., Tolo C.W., Kimani C.N., Muthaura P.G., Kirira T.N., et al. The in vitro anti-plasmodial and in vivo anti-malarial efficacy of combinations of some medicinal plants used traditionally for treatment of malaria by the Meru community in Kenya. J. Ethnopharmacol. 2008;115:223–231. doi: 10.1016/j.jep.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 11.Pooley E. The Complete Field Guide to Trees of Natal, Zululand and Transkei. Natal Flora Publications Trust; Durban, South Africa: 1993. [Google Scholar]
- 12.Kupicha F.K. Sapotaceae. In: Launert E., editor. Flora Zambesiaca. Volume 7. Flora Zambesiaca Managing Committee; London, UK: 1983. pp. 210–247. [Google Scholar]
- 13.Raimondo D., von Staden L., Foden W., Victor J.E., Helme N.A., Turner R.C., Kamundi D.A., Manyama P.A. Red List of South African Plants. Strelitzia 25. South African National Biodiversity Institute; Pretoria, South Africa: 2009. [Google Scholar]
- 14.Hutchings A. Zulu Medicinal Plants: An Inventory. University of Natal; Pietermaritzburg, South Africa: 1996. [Google Scholar]
- 15.Leistner O.A. Seed Plants of Southern Africa: Families and Genera. Strelitzia 10. National Botanical Institute; Pretoria, South Africa: 2000. [Google Scholar]
- 16.Klos M., van de Venter M., Milne P.J., Traore H.N., Meyer D., Oosthuizen V. In vitro anti-HIV activity of five selected South African medicinal plant extracts. J. Ethnopharmacol. 2009;124:182–188. doi: 10.1016/j.jep.2009.04.043. [DOI] [PubMed] [Google Scholar]
- 17.Mahop T.M., Mayet M. En route to biopiracy? Ethnobotanical research on anti-diabetic medicinal plants in the Eastern Cape Province, South Africa. Afr. J. Biotechnol. 2007;6:2945–2952. [Google Scholar]
- 18.Christensen S.B., Kharazmi A. Antimalarial natural products. Isolation, characterization and biological properties. In: Tringali C., editor. Bioactive Compounds from Natural Sources: Isolation, Characterization and Biological Properties. Taylor & Francis; London, UK: 2001. pp. 379–432. [Google Scholar]
- 19.Suksamrarn S., Panseeta P., Kunchanawatta S., Distaporn T., Ruktasing S., Suksamrarn A. Ceanothane- and lupane-type triterpenes with antiplasmodial and antimycobacterial activities from Ziziphus cambodiana. Chem. Pharm. Bull. 2006;54:535–537. doi: 10.1248/cpb.54.535. [DOI] [PubMed] [Google Scholar]
- 20.Attioua B., Yeo D., Lagnika L., Harisolo R., Antheaume C., Weniger B., Kaiser M., Lobstein A., Vonthron-Sénécheau C. In vitro antileishmanial, antiplasmodial and cytotoxic activities of a new ventiloquinone and five known triterpenes from Parinari excelsa. Pharm. Biol. 2012;50:801–806. doi: 10.3109/13880209.2011.633270. [DOI] [PubMed] [Google Scholar]
- 21.Amusan O.O., Adesogan E.K., Makinde J.M. Anti-malarial active principles of Spathodea campanulata stem bark. Phytother. Res. 1996;10:692–693. doi: 10.1002/(SICI)1099-1573(199612)10:8<692::AID-PTR928>3.0.CO;2-O. [DOI] [Google Scholar]
- 22.Bai K., Yu Z., Chen F., Li F., Li W., Guo Y. Synthesis and evaluation of ursolic acid derivatives as potent cytotoxic agents. Bioorg. Med. Chem. Lett. 2012;22:2488–2493. doi: 10.1016/j.bmcl.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Leal S.A., Salvador A.R.J., Yongkui J. Novel ursolic acid derivatives with potent antitumor activity. Cancer Res. 2012;72:1931–1940. [Google Scholar]
- 24.Suffness M., Pezzuto J.M. Assays related to cancer drug discovery. In: Hostettman K., editor. Methods in Plant Biochemistry: Assays for Bioactivity. Academic Press; London, UK: 1990. pp. 71–133. [Google Scholar]
- 25.Trager W., Jensen J.B. Human malaria parasite in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 26.Makler M.T., Ries J.M., Williams J.A., Bancroft J.E., Piper R.C., Gibbins B.L., Hinrichs D.J. Parasite lactate dehydrogenase as an assay for Plasmodium failciparum drugs sensitivity. Am. J. Trop. Med. Hyg. 1993;48:739–741. doi: 10.4269/ajtmh.1993.48.739. [DOI] [PubMed] [Google Scholar]
- 27.Peters W., Portus J.H., Robinson B.L. The chemotherapy of rodent malaria, XXV: Anti-malarial activity of WR 122, 455(9-phenanthrenemethanol) in vivo and in vitro. Ann. Trop. Med. Parasitol. 1975;70:262. doi: 10.1080/00034983.1976.11687122. [DOI] [PubMed] [Google Scholar]
- 28.Mosman T. Rapid coloricmetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–65. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]