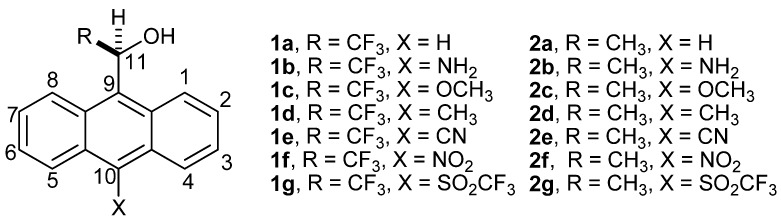Abstract
The nucleophilic character and stability of the carbanions vs. alkoxides derived from 2,2,2-trifluoro-1-(9-anthryl)ethanol and 1-(9-anthryl)ethanol containing X electron-releasing and X electron-acceptor substituents attached to C-10, have been studied at the B3LYP/6-31+G(d,p) level of theory. Results analyzed in terms of the absolute gas-phase acidity, Fukui function, the local hard and soft acids and bases principle, and the molecular electrostatic potential, show that the central ring of the 9-anthryl group confers an ambident nucleophilic character and stabilizes the conjugated carbanion by electron-acceptor delocalization.
Keywords: absolute gas phase acidity, alkoxide, carbanion, Fukui function, nucleophile
1. Introduction
Pure enantiomers of 2,2,2-trifluoro-1-(9-anthryl)ethanol (1a, Figure 1), are mainly used as chiral solvating agents [1,2] and chiral selectors [1,2,3,4] due to their particular hydroxyl (OH) and methine (CH) acidity [5,6,7,8]. The calculated gas phase acidities for 1a, 1-(9-anthryl)ethanol (2a) and 2,2,2-trifluoroethanol have shown that the influence of 9-anthryl ring is more significant than the trifluoromethyl group in modifying the CH rather than the OH acidity of 1a; the trifluoromethyl group increases 6.0 kcal mol−1 the OH acidity more than the CH acidity, while the 9-anthryl group increases 17.0 kcal mol−1 the CH acidity more than the OH acidity [6].
Figure 1.
1-(9-anthryl)ethanol and derivatives.
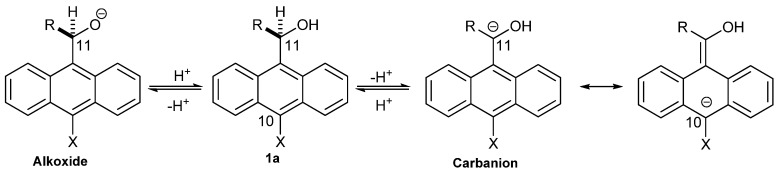
These results imply that the anthryl ring stabilizes more efficiently the negative charge in the carbanion than the alkoxide (Scheme 1). The resonance effects on the alkoxide are absent; while the resonance effects on the carbanion are present and the negative charge ought to be delocalized mainly on the C-11 and C-10 carbon atoms. Therefore, an ambident nucleophilic character should be observed for the carbanion, and there would be an electrophilic attack on the C-11 and C-10 carbon atoms.
Scheme 1.
The resonance effects on the OH and CH ionization and the ambident nucleophilic character for the carbanion.
The proposal is consistent with the ambident nucleophilic character experimentally observed for the 9-anthrylmethyl carbanion and related intermediates [9]. Also, it has been reported that the electrophilic attack on (9-anthryl)phenylmethyl and related anions gave the mixture of the two expected products (electrophilic addition on the C-11 and C-10 carbon atoms), whose composition depended of the substituent attached to the phenyl ring of the substrate [10]. However, experimental studies concerning the acidity of OH and CH of 1a and 2a, the ambident nucleophilic character and the origin of the stability for their carbanions vs. alkoxides have not been carried out so far. Therefore, we take 1a, 2a, and their derivatives containing X electron-releasing 1b–1d and 2b–2d, and X electron-acceptor 1e–1g and 2e–2g substituent attached to C-10 (Figure 1) as a set of alcohols. The Fukui function, a density functional theory (DFT) descriptor [11,12], the local hard and soft acids and bases principle (HSAB) [13,14], and the molecular electrostatic potential (MEP) [15,16] were used to demonstrate, in terms of the electron density, that the resonance effects in the carbanions are responsible for the ambident nucleophilic character and stability with respect to the alkoxides, due to the delocalization of the electron density on the C-11 and C-10 carbon atoms.
2. Results and Discussion
Table 1 shows the ΔacidG°(CH) and ΔacidG°(OH) values obtained for the ionization reactions shown in Scheme 1 for 1b–1g and 2b–2g. The calculated acidity values are consistent with the substituent electronic effect, being the alcohol with X = NH2 (compounds 1b, 2b) the least acidic and with X = SO2CF3 (compounds 1g, 2g) the most acidic; they correspond to the two extremes for which ΔacidG°(CH) and ΔacidG°(OH) values decrease over 40 and 18 kcal/mol, respectively. The CH acidity is higher than the OH acidity for 1e–1g and 2b–2g (positive δΔacidG° values), which implies that the carbanions 1e–1g and 2b–2g are more stable than the alkoxides by 3.1–12.4 and 3.8–26.1 kcal mol−1 respectively.
Table 1.
Absolute gas phase acidities for alcohols 1b–2g. Values are reported in kcal mol−1. δΔacidG° = ΔacidG°(OH) − ΔacidG°(CH).
| Alcohol | ΔacidG°(CH) | ΔacidG°(OH) | δΔacidG° |
|---|---|---|---|
| 1b (2b) | 349.8 (352.3) | 342.1 (356.2) | −7.7 (3.9) |
| 1c (2c) | 346.3 (347.8) | 339.3 (353.4) | −7.0 (5.6) |
| 1d (2d) | 345.1 (348.0) | 339.9 (353.3) | −5.2 (5.3) |
| 1e (2e) | 327.2 (328.5) | 330.3 (344.1) | 3.1 (15.6) |
| 1f (2f) | 319.9 (318.6) | 330.5 (343.7) | 10.6 (25.1) |
| 1g (2g) | 313.1 (311.9) | 325.6 (338.0) | 12.5 (26.1) |
Negative δΔacidG° values for 1b–1d indicate that the electron-releasing substituent is not efficient enough to delocalize the negative charge into the aromatic ring; the carbanions of 1b–1d are less stable than the alkoxides by 7.7–5.2 kcal mol−1 respectively. Therefore, the stability of the carbanions of 1 and 2 increases as the electron-acceptor capacity of the substituent increases [17]. The correlation between the electronic effect of X and the ionization reactions of Scheme 1, is evident from the good linear regression analysis obtained for ΔacidG°(CH) or ΔacidG°(OH) and the Hammett substituent constants in the gas σp− (g) or aqueous σp− (aq) phase (see Table 2) [18]. The slope values are quite large and negative for ΔacidG°(CH) as would be expected for the generation of a large amount of negative charge upon the ring. On the other hand the lower slope values for ΔacidG°(OH) indicates localized negative charge on oxygen atom, but not transmittable to the ring [19].
Table 2.
Linear correlation equation between ΔacidG°(CH) or ΔacidG°(OH) and σp−(g), σp−(aq) or 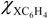 for 1b–1g. The linear correlation equation for 2b–2g is given in parenthesis. ΔacidG° = b + mY(values in kcal mol−1).
for 1b–1g. The linear correlation equation for 2b–2g is given in parenthesis. ΔacidG° = b + mY(values in kcal mol−1).
| Y | Δacid G° | b | M | R2 |
|---|---|---|---|---|
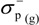 |
CH | 344.9 | −23.0 | 0.99 |
| (347.1) | (−25.2) | (0.98) | ||
| OH | 339.2 | −9.7 | 0.96 | |
| (353.3) | (−1.0) | (0.97) | ||
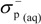 |
CH | 345.6 | −20.1 | 0.97 |
| (343.6) | (−18.2) | (0.98) | ||
| OH | 338.9 | −7.7 | 0.95 | |
| (352.7) | (−8.3) | (0.95) | ||
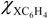 |
CH | 472.5 | −24.7 | 0.98 |
| (488.0) | (−27.3) | (0.97) | ||
| OH | 393.3 | −10.4 | 0.98 | |
| (409.2) | (−10.8) | (0.99) |
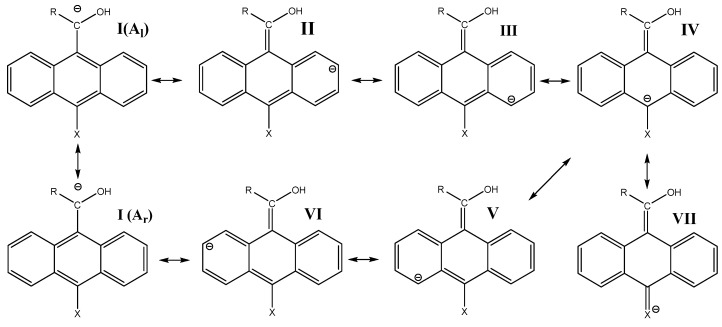
While, the resonance effects on the CH ionization are present, the delocalization of the negative charge of the carbanions into the ring can be illustrated in terms of resonance theory [20]. The Kekulé resonance structures I–VII on Scheme 2 show that the negative charge is localized on the C-2 (II), C-4 (III), C-10 (IV), C-5 (V), C-7 (VI), C-11(I) carbon atoms, and on the substituent X (VII). The classical Kekulé structures Al and Ar are structures in which double bond alternation extends over the whole periphery of the molecule [21].
Scheme 2.
The Kekulé resonance structures for the carbanion.
We have shown previously that the resonance hybrid structure can be described in terms of the Fukui function [22]. The Fukui function f(r) represents the change of the electronic density ρ(r) in a given point with respect to the change in the number of electrons N, f(r) = (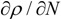 ), and the stability of the ion can be achieved from the link between f(r) and the energy E of the system f(r) =
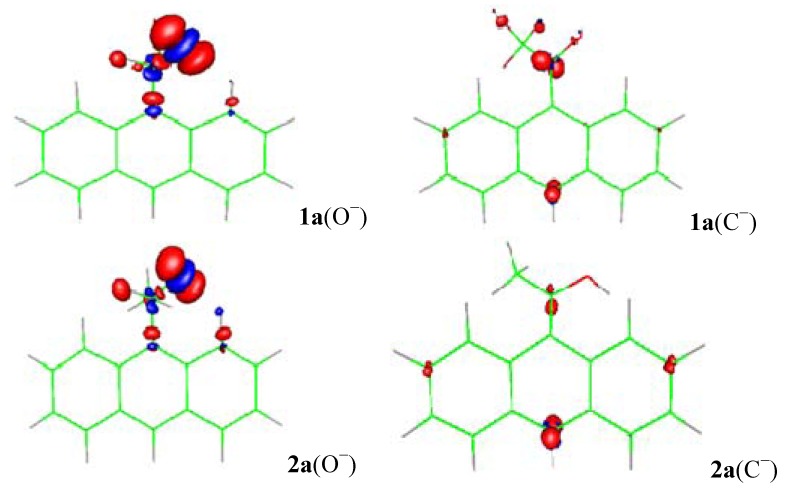
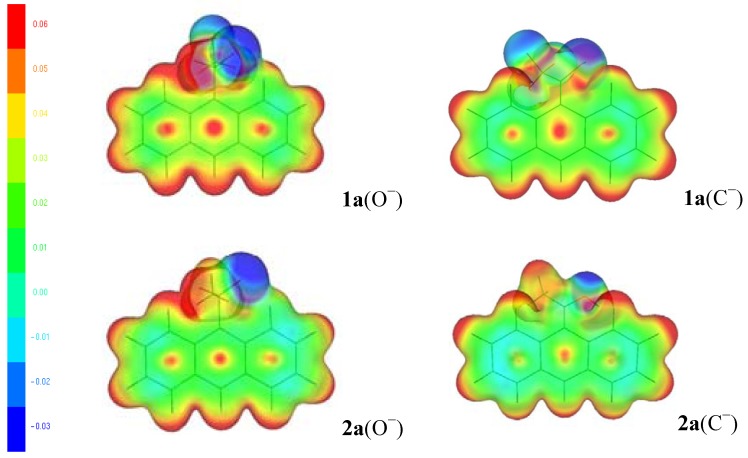
), and the stability of the ion can be achieved from the link between f(r) and the energy E of the system f(r) = 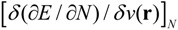 [11,12], where v(r) is the external potential associated to the nucleus. The amount of charge in the carbanions and alkoxides was examined specifically from the Fukui function for electrophilic attack f−(r) (Figure 2, Figure 3 and Figure 4) and the local HSAB principle [13,14]. Figure 2 shows that the largest values of f−(r) for 1a and 2a are located on the O and the C-11/C-10 atoms for the alkoxides and carbanions respectively. They are associated with softer nucleophilic regions (s−(r) = Sf−(r)), by giving up electronic charge and are especially reactive toward soft electrophiles [13]. Therefore, charge localization occurs on the strongly electronegative oxygen atom for the alkoxides; whereas, the charge is located on the C-11/C-10 carbon atoms indicating an ambident soft nucleophilic character for the carbanions and the distribution of the anionic charge into the ring is represented in terms of the resonance structures I and IV.
[11,12], where v(r) is the external potential associated to the nucleus. The amount of charge in the carbanions and alkoxides was examined specifically from the Fukui function for electrophilic attack f−(r) (Figure 2, Figure 3 and Figure 4) and the local HSAB principle [13,14]. Figure 2 shows that the largest values of f−(r) for 1a and 2a are located on the O and the C-11/C-10 atoms for the alkoxides and carbanions respectively. They are associated with softer nucleophilic regions (s−(r) = Sf−(r)), by giving up electronic charge and are especially reactive toward soft electrophiles [13]. Therefore, charge localization occurs on the strongly electronegative oxygen atom for the alkoxides; whereas, the charge is located on the C-11/C-10 carbon atoms indicating an ambident soft nucleophilic character for the carbanions and the distribution of the anionic charge into the ring is represented in terms of the resonance structures I and IV.
Figure 2.
The surface plot of the electrophilic Fukui function for the alkoxides (O−) and carbanions (C−) of 1a and 2a.
Figure 3.
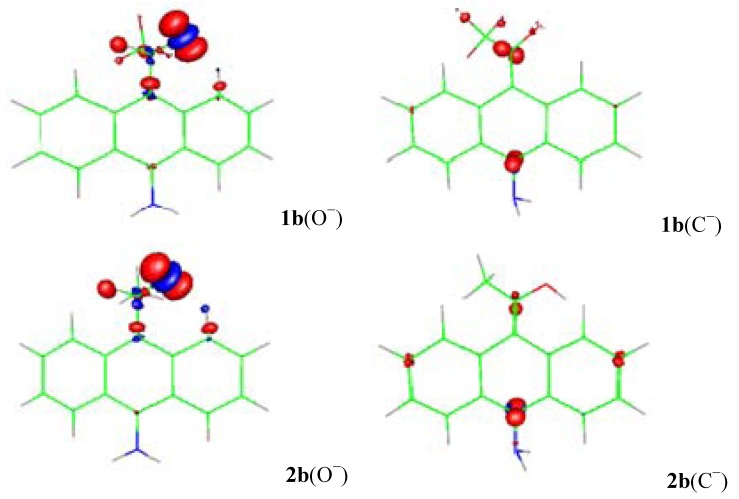
The surface plot of the electrophilic Fukui function for the alkoxides (O−) and carbanions (C−) of 1b and 2b.
Figure 4.
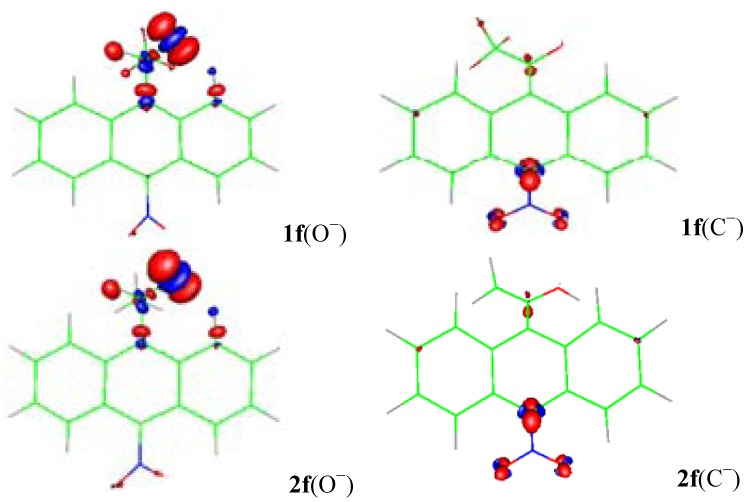
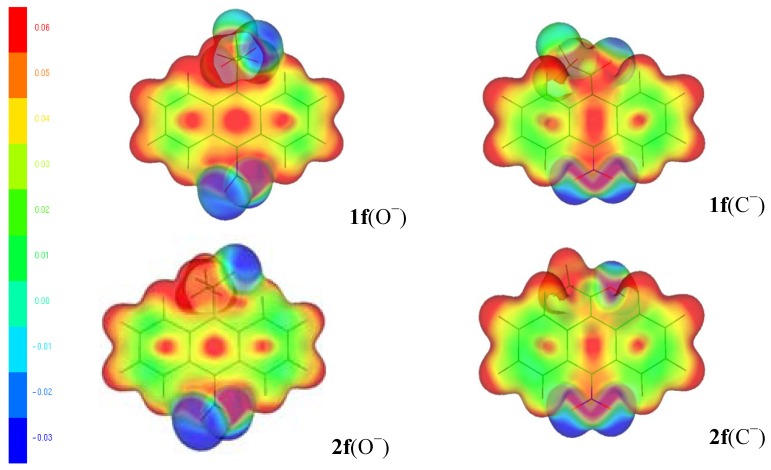
The surface plot of the electrophilic Fukui function for the alkoxides (O−) and carbanions (C−) of 1f and 2f.
When the NH2 electron-releasing substituent is included (Figure 3), the behavior of the f−(r) for 1b and 2b is almost similar to that of 1a and 2a; the largest values of f−(r) are located on the O and the C-11/C-10 atoms. The NH2 substituent has no contribution to the charge dispersal. For the alkoxide charge localization occurs on the strongly electronegative oxygen atom; whereas, for the carbanion the charge dispersion involved the C-11/C-10 carbon atoms indicating an ambident soft nucleophilic character that is well represented by resonance structures I and IV (Scheme 2). The generation of a small amount of negative charge upon the ring makes the carbanion of 1b 7.7 kcal mol−1 less stable than the corresponding alkoxide. The carbanion of 2b delocalizes the negative charge into the aromatic ring more efficiently than that of 1b due the f−(r) trends for 1b and 2b are C-11 > C-10 and C-11 < C-10 respectively; the carbanion of 2b is 3.9 kcal mol−1 more stable than the corresponding alkoxide (See Table 1). The results are similar for X = OCH3 and CH3 (See Figures in the Electronic Supplementary Information).
When the NO2 substituent is included (Figure 4), f−(r) for the alkoxides of 1f and 2f shows localized negative charge on oxygen atom, but not transmittable to the ring. For the carbanions of 1f and 2f, f−(r) indicates generation of a large amount of negative charge localized on C-10 and on the NO2 substituent (with most of the negative charge residing on the two oxygen atoms) [23,24,25], making the carbanions of 1f and 2f 10.6 and 25.1 kcal mol−1 more stable than their alkoxides respectively (see Table 1). Therefore, the distribution of the anionic charge into the ring is represented in terms of the resonance structures IV and VII (Scheme 2). The results are similar for X = CN and SO2CF3 (see figures in the Supplementary Information).
As we can observe, the Fukui function for electrophilic attack shows that delocalization of the anionic charge mainly occurs in the central ring of the 9-anthryl group, making the central ring more reactive than the edge rings for soft-soft interactions. This important result can be supported by the electronegativity of the central ring effect of the 9-anthryl group on the ΔacidG°(CH) or ΔacidG°(OH).
The electronegativity of the central ring can be approximated by the XC6H4 fragment electronegativity 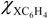 calculated previously for a set of p-substituted phenols XC6H4OH [7]. From Table 2 we can observe the negative slope values; the acidity increases (ΔacidG° decreases) when
calculated previously for a set of p-substituted phenols XC6H4OH [7]. From Table 2 we can observe the negative slope values; the acidity increases (ΔacidG° decreases) when 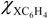 increases, validating the proposal that the central ring exhibits more contribution to ΔacidG° than the edge rings and it behaves as a rather separated benzenoid ring or localized entity. This is a nice coincidence with the ambident nucleophilic character experimentally observed for the 9-anthrylmethyl carbanion and related intermediates [26], and the calculated aromaticity of the central ring of anthracene [27,28,29].
increases, validating the proposal that the central ring exhibits more contribution to ΔacidG° than the edge rings and it behaves as a rather separated benzenoid ring or localized entity. This is a nice coincidence with the ambident nucleophilic character experimentally observed for the 9-anthrylmethyl carbanion and related intermediates [26], and the calculated aromaticity of the central ring of anthracene [27,28,29].
Figure 5, Figure 6 and Figure 7 show the molecular electrostatic potential (MEP) [15,16] mapped onto an isosurface of the total electron density for the alkoxides and carbanions of 1a–2a, and 1f–2f respectively. The MEP can be appropriate for analyzing hard-hard interactions because the proton (a hard electrophile) prefers a site with maximum net charge [13,30]. In Figure 5, we observe the MEP for the alkoxides and carbanions of 1a and 2a, in these anions, the blue area, towards the oxygen (1a and 2a) and fluorine atoms (1a), indicates higher negative charge; the red color, located near the hydrogen atoms and methyl group indicate more positive charge and lower electron density.
Figure 5.
MEP mapped onto an isosurface of the total electron density for the alkoxides (O−) and carbanions (C−) of 1a and 2a.
Figure 6.
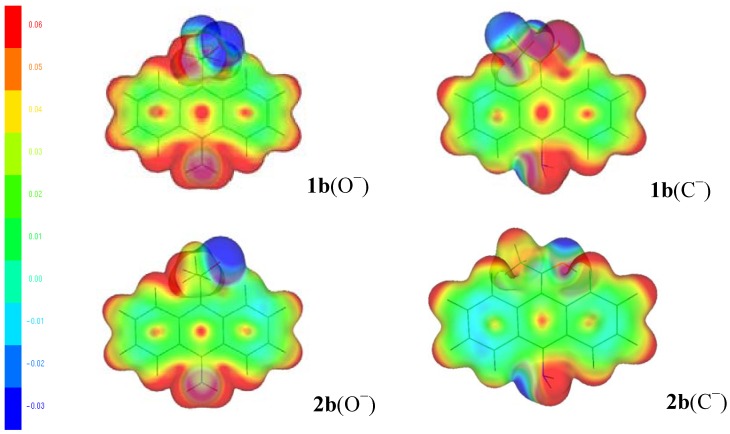
MEP mapped onto an isosurface of the total electron density for the alkoxides (O−) and carbanions (C−) of 1b and 2b.
Figure 7.
MEP mapped onto an isosurface of the total electron density for the alkoxides (O−) and carbanions (C−) of 1f and 2f.
We notice an uneven distribution of electrons with positive charge in the 9-anthryl ring, the central ring exhibits more positive charge than the edge rings. That is, in the alkoxides and carbanions of 1a and 2a, the hydrogen atoms are more positive (red color) than the carbon atoms of the 9-anthryl ring and the C-11 carbon atom, and the oxygen and fluorine atoms are more negative (blue colors). When the NH2 electron-releasing substituent is included (Figure 6), the behavior of the MEP for the 2b, the hydrogen atoms are more positive (red color) than the 9-anthryl ring and the C-11 carbon atom. The oxygen, nitrogen and fluorine atoms are more negative (blue colors). The results are similar for X = OCH3 and CH3 (see figures in the Supplementary Information).
In Figure 7, we observe the MEP for the alkoxides and carbanions of 1f and 2f, in these anions, the blue negative area is located towards the oxygen, nitrogen (compounds 1f and 2f) and fluorine atoms (1f); the red color is located near the hydrogen atoms, methyl group, C-11 carbon atom and the 9-anthryl ring. The uneven distribution of electrons with positive charge in the 9-anthryl ring is observed, the central ring exhibits more positive charge than the edge rings, indicating lower electron density than in the anions of 1a–2a and 1b–2b. The results are similar for X = CN and SO2CF3 (see figures in the Supplementary Information). Therefore, the proton (a hard electrophile) prefers the oxygen (alkoxides 1 and 2) and fluorine atoms (alkoxide 1) with maximum MEP (the blue area with higher negative charge).
3. Theoretical Methodology
The ground state structures and energies of alcohols 1a–2g were calculated at the B3LYP/6-31+G(d,p) level of theory using GAUSSIAN03 [31]. The thermodynamic stability of the carbanions and alkoxides of 1a–2g was obtained from the calculated CH and OH absolute gas-phase acidities [32]. Gas phase studies are required to separate intrinsic molecular properties from interfering solvation effects [33]. The absolute gas-phase acidity ΔacidG° is given by ΔacidG° = G°(anion) + G°(H+) − G°(alcohol) [34]. The Gibbs free energies G° (anion), G°(alcohol) and G°(H+) were obtained by means of partition functions using statistical thermodynamic relationships [35]. The electrophilic Fukui function and the electrostatic potential of the molecules were visualized throughout by the gOpenmol software [36].
4. Conclusions
The nucleophilic character and the relative stability of the carbanions vs. the corresponding alkoxides of 1-(9-anthryl)ethanol and its derivatives has been studied in terms of the absolute gas phase acidity, Fukui function, the local HSAB bases principle and the molecular electrostatic potential. The central ring of the 9-anthryl group stabilizes more efficiently the conjugated carbanion than the oxygen atom of the alkoxide by electron-acceptor delocalization; as a consequence the negative charge is delocalized on the C-11 and C-10 carbon atoms that is well represented for the resonance structures I and IV. The C-11 and C-10 carbon atoms with maximum Fukui function give an ambident soft nucleophilic character to the carbanions 1 and 2. The X substituent has an important contribution to the negative charge dispersal. Therefore, the negative charge is localized on the oxygen atom of the alkoxide, but nor transmittable to the ring; the proton (a hard electrophile) prefers the oxygen (alkoxides 1 and 2) and fluorine atoms (alkoxide 1) with maximum MEP (the blue area with higher negative charge) [37]. The results obtained in this work open the possibility to analyze the stability of anions as a result of the delocalization of the anionic charge in terms of the electron density. Studies related to the experimental reactivity of 1a–1f are underway and will be reported in due course.
Acknowledgments
This work has been supported by research grants 180523, 163234, 61626 and 32261E from Consejo Nacional de Ciencia y Tecnología de México (CONACyT), who also supported Ramsés. E. Ramírez with a Ph.D. scholarship 134559. The authors acknowledge the insightful comments of Hugo A. Jiménez-Vázquez.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/18/9/10254/s1.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 1a and 2a are available from the authors.
References and Notes
- 1.Wenzel T.J., Wilcox J.D. Chiral reagents for the determination of enantiomeric excess and absolute configuration using NMR spectroscopy. Chirality. 2003;15:256–270. doi: 10.1002/chir.10190. [DOI] [PubMed] [Google Scholar]
- 2.García-Martínez C., Cervantes H., Méndez F., Escalante J. Stoichiometry, association constant, and solvation model of chiral hydroxyfuranones in the presence of Pirkle’s alcohols. J. Spectrosc. Lett. 2011;44:168–175. doi: 10.1080/00387010.2010.489096. [DOI] [Google Scholar]
- 3.Pirkle W.H., Finn J.M. Chiral high-pressure liquid chromatographic stationary phases. 3. General resolution of arylalkylcarbinols. J. Org. Chem. 1981;46:2935–2938. doi: 10.1021/jo00327a019. [DOI] [Google Scholar]
- 4.Pirkle W.H., Hoover D.J. In: Topics in Stereochemistry. Allinger N.L., Eliel E.L., Wilen S.H., editors. Volume 13. John Wiley& Sons; New York, NY, USA: 1982. pp. 263–331. [Google Scholar]
- 5.Stewart R., van der Linden R. The acidity of some aromatic fluoro alcohols and ketones. Can. J. Chem. 1960;38:399–406. doi: 10.1139/v60-056. [DOI] [Google Scholar]
- 6.Ramírez E.R., García-Martínez C., Méndez F. Influence of fluorine atoms and aromatic rings on the acidity of ethanol. J. Phys. Chem. A. 2009;113:10753–10758. doi: 10.1021/jp810475z. [DOI] [PubMed] [Google Scholar]
- 7.Romero M.L., Méndez F. Is the hydrogen atomic charge representative of the acidity of parasubstituted phenols? J. Phys. Chem. A. 2003;107:4526–4530. doi: 10.1021/jp027351f. [DOI] [Google Scholar]
- 8.Romero M.L., Méndez F. The local HSAB principle and bond dissociation energy of p-substituted phenol. J. Phys. Chem. A. 2003;107:5874–5875. doi: 10.1021/jp035159n. [DOI] [Google Scholar]
- 9.Engler T.A., Shechter H. Generation and the ambident character of 9-anthrylmethyl carbanions. Tetrahedron Lett. 1983;24:4645–4648. doi: 10.1016/S0040-4039(00)86216-4. [DOI] [Google Scholar]
- 10.Takagi M., Nojima M., Kusabayashi S. Protonation and alkylation of ambident (9-anthryl)arylmethyl anions. J. Am. Chem. Soc. 1983;105:4676–4684. doi: 10.1021/ja00352a025. [DOI] [Google Scholar]
- 11.Parr R.G., Yang W. Density Functional Theory of Atoms and Molecules. Oxford University Press; New York, NY, USA: 1989. [Google Scholar]
- 12.Lee C., Yang W., Parr R.G. Local softness and chemical reactivity in the molecules CO, SCN− and H2CO. J. Mol. Struct. 1988;163:305–313. doi: 10.1016/0166-1280(88)80397-X. [DOI] [Google Scholar]
- 13.Pearson R.G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963;85:3533–3539. doi: 10.1021/ja00905a001. [DOI] [Google Scholar]
- 14.Méndez F., Gázquez J.L. Chemical reactivity of enolate ions: The local hard and soft acids and bases principle viewpoint. J. Am. Chem. Soc. 1994;116:9298–9301. doi: 10.1021/ja00099a055. [DOI] [Google Scholar]
- 15.Levine I. Quantum Chemistry. 5th ed. Prentice Hall; Upper Saddle River, NJ, USA: 2001. pp. 493–495. [Google Scholar]
- 16.Politzer P., Murray J.S., Lipkowitz K.B., Boyd D.B. Reviews in Computational Chemistry. Volume 2. Wiley-VCH; Hoboken, NJ, USA: 1991. Molecular Electrostatic Potentials and Chemical Reactivity. Chapter 7. [Google Scholar]
- 17.Berger S.T.A., Ofial A.R., Mayr H. Inverse solvent effects in carbocation carbanion combination reactions: The unique behavior of trifluoromethylsulfonyl stabilized carbanions. J. Am. Chem. Soc. 2007;129:9753–9761. doi: 10.1021/ja072135b. [DOI] [PubMed] [Google Scholar]
- 18.Hansch C., Leo A., Taft R.W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 1991;91:165–195. doi: 10.1021/cr00002a004. [DOI] [Google Scholar]
- 19.Taft R.W., Bordwell F.G. Structural and solvent effects evaluated from acidities measured in dimethyl sulfoxide and in the gas phase. Acc. Chem. Res. 1988;21:463–469. doi: 10.1021/ar00156a005. [DOI] [Google Scholar]
- 20.Smith M.B., March J. March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure. John Wiley & Sons, Inc.; New York, NY, USA: 2001. pp. 32–97. [Google Scholar]
- 21.Shaik S., Zilberg S., Haas Y. A kekulé-crossing model for the “anomalous” behavior of the b2u modes of aromatic hydrocarbons in the lowest excited 1B2u state. Acc. Chem. Res. 1996;29:211–218. doi: 10.1021/ar950206i. [DOI] [Google Scholar]
- 22.López P., Méndez F. Fukui function as a descriptor of the imidazolium protonated cation resonance hybrid structure. Org. Lett. 2004;6:1781–1783. doi: 10.1021/ol049454h. [DOI] [PubMed] [Google Scholar]
- 23.Gázquez J.L., Méndez F. The hard and soft acids and bases principle: An atoms in molecules viewpoint. J. Phys. Chem. 1994;98:4591–4593. doi: 10.1021/j100068a018. [DOI] [Google Scholar]
- 24.Chattaraj P.-K. Chemical reactivity and selectivity: Local HSAB principle versus frontier orbital theory. J. Phys. Chem. A. 2001;105:511–513. doi: 10.1021/jp003786w. [DOI] [Google Scholar]
- 25.Méndez F., Galván M., Garritz A., Vela A., Gázquez J.L. Local softness and chemical reactivity of maleimide: nucleophilic addition. J. Mol. Struct. 1992;277:81–86. doi: 10.1016/0166-1280(92)87131-I. [DOI] [Google Scholar]
- 26.Experimental results suggested the same= effects for NO2 and SO2CF3 in nitro and triflinate stabilized 9-anthrylmethyl carbanions
- 27.Wheland G.W. Resonance in Organic Chemistry. Wiley; New York, NY, USA: 1955. p. 517. [Google Scholar]
- 28.Schleyer P.R., Manoharan M., Jiao H., Stahl F. The acenes: Is there a relationship between aromatic stabilization and reactivity? Org. Lett. 2001;3:3643–3646. doi: 10.1021/ol016553b. [DOI] [PubMed] [Google Scholar]
- 29.Aihara J., Kanno H. Local aromaticities in large polyacene molecules. J. Phys. Chem. A. 2005;109:3717–3721. doi: 10.1021/jp047183m. [DOI] [PubMed] [Google Scholar]
- 30.Chigo-Anota E., Ramírez-Gutierrez R.E., Escobedo-Morales A., Hernández-Cocoletzi G. Influence of point defects on the electronic properties of boron nitride nanosheets. J. Mol. Model. 2012;18:2175–2184. doi: 10.1007/s00894-011-1233-y. [DOI] [PubMed] [Google Scholar]
- 31.M. J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Montgomery J.A., Jr., Vreven T., Kudin K.N., Burant J.C., et al. Gaussian 03, Revision C.02. Frisch, Gaussian, Inc.; Wallingford, CT, USA: 2004. [Google Scholar]
- 32.Although there are no experimental ΔacidG° values for 1a–1g and 2a–2g reported in the literature, the B3LYP/6–31+G(d,p) calculated ΔacidG° values for ethanol, its fluorine derivatives, phenylethanol and p-substituted phenols showed a good agreement with the experimental values reported in the literature
- 33.Solca N., Dopfer O. Spectroscopic identification of oxonium and carbenium ions of protonated phenol in the gas phase: IR spectra of weakly bound C6H7O+-L dimers (L = Ne, Ar, N2) J. Am. Chem. Soc. 2004;126:1716–1725. doi: 10.1021/ja0305858. [DOI] [PubMed] [Google Scholar]
- 34.Fujio M., McIver R.T., Jr., Taft R.W. Effects of the acidities of phenols from specific substituent-solvent interactions. Inherent substituent parameters from gas-phase acidities. J. Am. Chem. Soc. 1981;103:4017–4029. doi: 10.1021/ja00404a008. [DOI] [Google Scholar]
- 35.McQuarrie D.A., Simon J.D. Molecular Thermodynamics. University Science Books; Sausalito, CA, USA: 1999. pp. 273–285. [Google Scholar]
- 36.The g0penMol. [(accessed on 22 February 2013)]. Available online: http://www.csc.fi/english/pages/g0penMol.
- 37.Lum R.C., Grabowski J.J. Intrinsic competition between elimination and substitution mechanisms controlled by nucleophile structure. J. Am. Chem. Soc. 1992;114:9663–9665. doi: 10.1021/ja00050a059. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.