Abstract
A new pregnane steroid, 1, and three known analogues 2–4, have been isolated from a gorgonian Carijoa sp. collected from the South China Sea. The planar structure and relative configuration of 1 were elucidated from comprehensive spectroscopic data. Its absolute configuration was determined by application of the modified Mosher method. Compounds 1, 3 and 4 exhibited cytotoxicity against the human hepatoma cell line Bel-7402, with IC50 values of 9.33, 11.02 and 18.68 µM, respectively. Additionally, compound 1 exhibited promising antibacterial activity against Pseudomona puido, with a MIC value of 31 nM, which is approximately 5-fold more potent than ciprofloxacin (MIC = 156 nM).
Keywords: gorgonian coral, Carijoa sp., pregnane steroid, cytotoxicity, antibacterial activity
1. Introduction
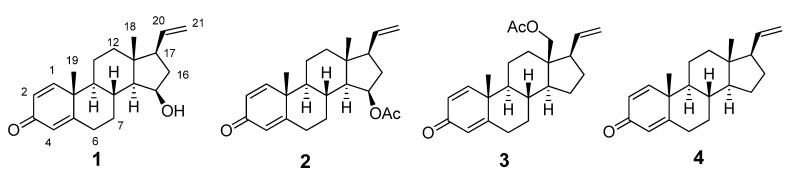
Steroidal compounds from marine organisms possess a wide array of unusual structures. Among them, pregnane steroids characterized by an uncommon vinyl side chain represent a minor group of metabolites, and octocorals appear to be their most prolific source [1,2,3]. Indeed, over 50 such compounds with vinyl side chains have been reported from the marine environment. These compounds have received much attention due to their multiple potent biological properties, including antibacterial [2], antiprotozoan [4,5] anti-inflammatory [6,7] and cytotoxic activities [7,8]. Recently, in the course of our investigation on new bioactive substances from corals collected from the South China Sea [9,10,11,12], the gorgonian Carijoa sp. attracted our attention because the EtOAc crude extract displayed significant toxicity toward the larvae of the brine shrimp Artemia salina, with a mortality rate of 88.6% at a concentration of 25 μg/mL. Chemical investigation on the active extract led to the isolation of four pregnanes with a 3-one framework: 15β-hydroxypregna-1,4,20-trien-3-one (1), 15β-acetoxypregna-1,4,20-trien-3-one (2) [13], 18-acetoxypregna-1,4,20-trien-3-one (3) [13], and pregna-1,4,20-trien-3-one (4) [14,15] (Figure 1). Their structures were elucidated by NMR spectroscopic methods and comparison with data previously reported in the literature. Among these isolated compounds, 1 is a new pregnane steroid.
Figure 1.
Chemical structures of compounds 1–4.
2. Results and Discussion
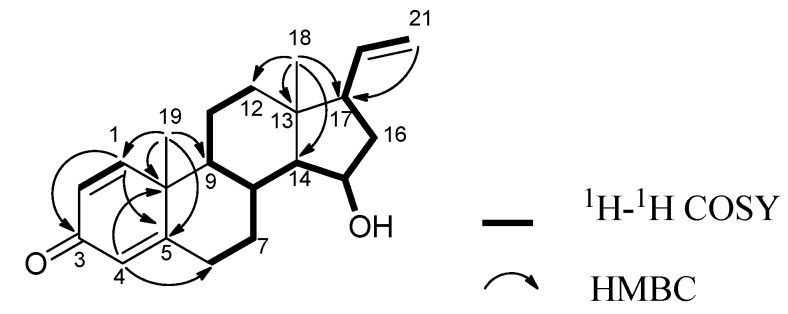
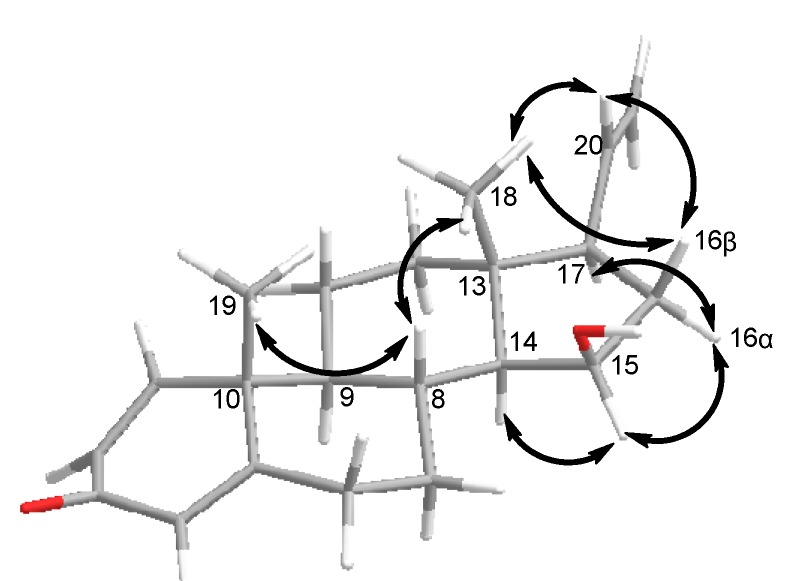
Compound 1 was obtained as pale yellow oil. Its molecular formula was determined as C21H28O2 by HRESIMS, suggesting eight degrees of unsaturation. Like 2, the 1H-NMR spectrum of 1 (Table 1) showed the typical signals in the low-field region of a dienone system (H-1, H-2 and H-4) along with vinyl group (H-21a, H-21b and H-20) signals. The most obvious differences were the presence of one signal at δH 4.10 (m H-15) in 1 instead of the corresponding signal δH 5.10 (m, H-15) in 2, and the disappearance of the methyl group signal at δH 2.05 (s, COCH3) in 1. In the 13C-NMR spectra, the methine carbon signal of C-15 was shifted upfield (δC 69.0 in 1 vs. δC 73.7 in 2) and the carbonyl carbon signal at δC 170.5, together with the acetyl methyl carbon signal at δC 21.6, had disappeared in 1, which was the result of a hydroxy substituent at C-15 in 1, instead of the acetoxy group in 2. Furthermore, the position of the hydroxy group was confirmed as C-15 on the basis of the 1H–1H COSY correlation between H-15 (δH 4.10 m) and the hydroxyl proton (δH 4.45 d J = 4.0). The contiguous sequence of correlations from H-6 to H-12, and from H-8 to H-21 in the 1H–1H COSY spectrum (Figure 2) and the HMBC correlations (Figure 2) between H-1/C-3 and C-5, H-4/C-6 and C-10, H-21/C17, as well as from H-18 to C-12, C-13, C-14 and C-17, and from H-19 to C-1, C-5, C-9 and C-10 (Table 1) indicated that 1 has a 3-one pregnane skeleton similar to that of 2, but with a hydroxy group at C-15. Treatment of 2 with NaOH in the presence of ethanol afforded 1 as the major product, which further confirmed the structure of 1. Because the relative configurations of 2 have been previously established, the chemical conversion from 2 allowed the determination of β-orientation of hydroxy group at C-15 in 1, and the NOESY correlations between H-14/H-15, H-15/H-16α and H-16α/H-17, as well as H-18/H-16β, H-16β/H-20 and H-20/H-18, also confirmed the β-stereochemistry of the hydroxy group at C-15. This configuration was also confirmed by the correlations between H-19/H-8 and H-8/H-18 in the NOESY spectrum (Figure 3).
Table 1.
1H and 13C-NMR data for compound 1 (400 and 100 MHz, in DMSO, ppm, J/Hz).
| Positon | δH | δC | HMBC |
|---|---|---|---|
| 1 | 7.21 d (10.1) | 156.9 | C-3, C-5, C-9, C-10, C-19 |
| 2 | 6.11 dd (10.1, 1.9) | 127.1 | C-10 |
| 3 | – | 185.3 | |
| 4 | 6.00 s | 123.3 | C-6, C-10 |
| 5 | – | 170.1 | |
| 6 | 2.48 m2.33 m | 32.4 | C-5 |
| 7 | 2.27 m0.98 m | 32.7 | |
| 8 | 1.94 m | 31.7 | |
| 9 | 1.05 m | 53.1 | C-19 |
| 10 | – | 43.8 | |
| 11 | 1.68 m1.61 m | 22.3 | |
| 12 | 1.57 m1.01 m | 38.3 | C-18 |
| 13 | – | 43.3 | |
| 14 | 0.78 dd (11.1, 5.8) | 59.0 | C-8, C-13, C-18 |
| 15 | 4.10 m | 69.0 | |
| 16 | 2.18 m ( α ) | 39.7 | C-13 |
| 1.51 m ( β ) | |||
| 17 | 1.85 m | 55.0 | |
| 18 | 0.87 s | 15.7 | C-12, C-13, C-14, C-17 |
| 19 | 1.22 s | 18.9 | C-1, C-5, C-9, C-10 |
| 20 | 5.79 ddd(17.3, 10.4, 7.4) | 139.6 | |
| 21 | 4.98 dd (10.4, 1.7) | 115.3 | C-17 |
| 4.96 dd (17.3, 1.7) | |||
| 15-OH | 4.45 d (4.0) |
Figure 2.
Key 1H–1H COSY and HMBC correlations for compound 1.
Figure 3.
Selected NOESY correlations for compound 1.
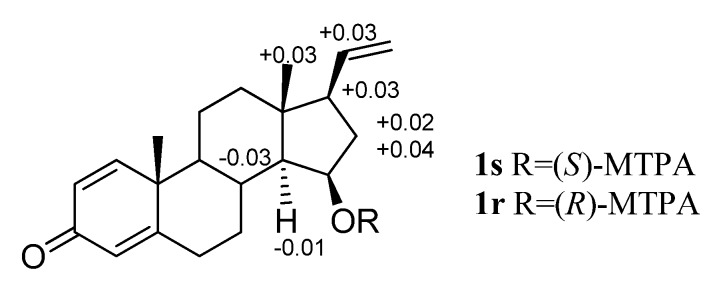
The absolute configuration of 1 was established by the modified Mosher method [16]. Treatment of 1 with (S)-(+)-α- and (R)-(–)-α-methoxy-α-(trifluoromethyl)phenylacetyl chloride (MTPA-Cl) gave the corresponding (R)- and (S)-MTPA esters 1r and 1s, respectively. The 1H-NMR signals of the two MTPA esters were assigned on the basis of their 1H–1H COSY spectra. The ΔδH(S–R) values were then calculated (Figure 4). Following the literature [16], the results indicated that the absolute configuration of C-15 was R. Therefore the absolute configurations at C-8, C-9, C-10, C-13, C-14 and C-17 in 1 were assigned as R, S, S, R, S and R, respectively. On the basis of the above evidence, the chemical conversion from 2 to 1 allowed the determination of the 8R,9S,10S,13R,14S,15R,17R configurations for 2 as the first report of its absolute configuration.
Figure 4.
Values of ΔδH(S–R) (measured in CDCl3) of the MTPA esters of compound 1.
Compound 2 differed from 1 only in the acetate group at C-15. Actually we used EtOAc for their extraction and isolation, but even though 1 was dissolved in EtOAc and stirred at 40 °C for one week, 2 was not detected in solution, therefore, it seems very unlikely that 2 is an artifact of the isolation procedure. Furthermore, 2 has also been isolated and confirmed as a new natural product from an Indopacific octocoral Carijoa sp. [13].
All the isolated compounds were evaluated for the cytotoxic activity against the human hepatoma Bel-7402 and human normal embryonic lung fibroblast MRC-5 cell lines. Compounds 1, 3 and 4 showed cytotoxicity to Bel-7402, with IC50 values of 9.33, 11.02 and 18.68 µM, respectively. Compound 2 exhibited moderate cytotoxicity against Bel-7402, with an IC50 = 73.47 µM. Additionally, compounds 1–4 showed weak cytotoxicity against MRC-5.
The antibacterial activity of compounds 1–4 was further evaluated in vitro against a panel of pathogenic bacteria [17], including Bacillus cereus, Tetragenococcus halophilus, Staphylococcus albus, Staphylococcus aureus, Escherichia coli, Pseudomonas putida, Nocardia brasiliensis, and Vibrio parahaemolyticus (Table 2). Compounds 1 and 2 showed a broad spectrum of antibacterial activity. Compound 1 showed significant antibacterial activity against S. aureus, S. albus, E. coli, V. parahaemolyticus and N. brasiliensis, with MIC values of 0.063, 1.00, 1.00, 4.00, and 0.500 μM, respectively, and exhibited promising inhibitory activity against P. putida, with an MIC value of 31 nM, which was approximately 5-fold more potent than that of ciprofloxacin (MIC = 156 nM). Compound 2 inhibited seven pathogenic bacteria, but not S. aureus, and was especially active against T. halophilus, with a MIC value of 312 nM. Additionally, Compound 3 showed antibacterial activity against B. cereus, S. aureus and T. halophilus with MIC values of 2.50, 0.156, and 1.25 μM, respectively.
Table 2.
Tests of MIC (μM) for compounds 1–4 against eight bacterial strains.
| Strains | Compounds | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Ciprofloxacin | |
| B. cereus | >64.0 | 5.00 | 2.50 | >50.0 | 0.078 |
| S. aureus | 0.063 | >50.0 | 0.156 | >50.0 | 0.019 |
| S. albus | 1.00 | 2.50 | >50.0 | >50.0 | 0.312 |
| T. halophilus | >64 | 0.312 | 1.25 | >50.0 | 0.019 |
| E. coli | 1.00 | 2.50 | >50.0 | >50.0 | 0.625 |
| P. putida | 0.031 | 1.25 | >50.0 | >50.0 | 0.156 |
| V. parahaemolyticus | 4.00 | 50.0 | >50.0 | >50.0 | 2.50 |
| N. brasiliensis | 0.500 | 1.25 | >50.0 | >50.0 | 0.078 |
3. Experimental
3.1. General Procedures
Optical rotations were measured on a JASCO P-2000 digital polarimeter at room temperature. IR spectra were recorded on a Nicolet 6700 spectrometer. UV spectra were measured on a Nicolet Evolution 300 spectrophotometer. ESI-MS and HR-ESI-MS were recorded on a Q-Tof Premier LC mass spectrometer. NMR spectra were recorded on an AVANCE III 400 (400 MHz for 1H-NMR and 100 MHz for 13C-NMR) spectrometer and a JEOL Eclips-600 spectrometer. Chemical shifts (δ) were reported in ppm relative to an internal TMS standard, and coupling constant (J) was reported in Hz. HPLC separation was performed in an Agilent 1200 semi-preparative HPLC system coupled with variable wavelength detector. A XDB-C18 preparative HPLC column (250 × 9.4 mm, 5 μm) was used. Analysis HPLC (Agilent 1200 HPLC system coupled with diode array detector) with XDB-C18 HPLC column (150 × 4.6 mm, 5 μm) was used. All solvents used were of analytical grade (Shanghai Chemical Plant, Shanghai, China). Silica gel (200–300 mesh; Qingdao Marine Chemical Group Co., Qingdao, China), octadecylsilyl (ODS) silica gel (45–60 mm; Merck KGaA, Darmstadt, Germany), and Sephadex LH-20 (Amersham Biosciences Inc., Piscataway, NJ, USA) were used for column chromatography. Precoated silica gel GF254 plates (Yantai Zifu Chemical Group Co., Yantai, China) were used for TLC analysis.
3.2. Animal Materials
Gorgonian Carijoa sp. (1.1 kg, wet weight) was collected off the coral reef of Weizhou Island in the South China Sea, China, in April 2011, and was identified by Dr. Xiu-Bao Li, South China Sea Institute of Oceanology, Chinese Academy of Science.
3.3. Extraction and Isolation
The frozen specimen was extracted with 95% EtOH (3 × 2000 mL) three times at room temperature for one week, and the solvent was evaporated in vacuo. The residue was partitioned in H2O (500 mL) and extracted with EtOAc three times (3 × 1,000 mL) at room temperature. The EtOAc extract was concentrated in vacuo to afford 8 g of EtOAc residue, which was subjected to column chromatography (CC) on silica gel, using petroleum ether (b.p. 60–90 °C)–EtOAc (from 20:1 to 0:10) as eluent. By combining the fractions according to TLC (GF254) monitoring, six fractions (Fr. 1−Fr. 6) were obtained. Fr. 2 (900 mg) was fractionated over silica gel CC eluted with petroleum ether−EtOAc gradients (from 25:1 to 3:1) to afford three sub-fractions (Fr.2.1−Fr.2.3). Repeated chromatography of Fr.2.3 using Sephadex LH-20 eluted with petroleum ether−CHCl3−MeOH (2:1:1) provided Fr.2.3.1−Fr.2.3.3, and then Fr.2.3.2 was purified by ODS CC eluted with MeOH to yield 2 (50 mg), and semi-preparative HPLC (MeOH:H2O = 80:20) to obtain 1 (4.3 mg), 3 (2.9 mg), and 4 (5.4 mg).
15β-Hydroxypregna-1,4,20-trien-3-one (1): pale yellow oil; [α]26D −4° (c 0.4, CHCl3); UV (MeOH) λmax (logε) 246 (4.00) nm, IR (KBr) νmax 3447, 2925, 2851, 1658, 1620, 1245, 910, 887 cm−1; 1H-NMR and 13C-NMR see Table 1; HRESIMS m/z: [M+H]+ 313.2168 (C21H29O2, calcd. 313.2168), [M+Na]+ 335.1987 (C21H28O2Na, calcd. 335.1987).
3.4. Hydrolysis of Compound 2
Compound 2 (20 mg) was dissolved in ethanol (2.0 mL), then NaOH (40 mg) was added, and the solution was allowed to stir at room temperature for 12 h. After that the mixture was neutralized with excess hydrochloric acid, water (10 mL) was added, and the solution was then extracted with EtOAc (10 mL × 3). The organic solvent was removed with a high-vacuum pump and the crude mixture was subjected to preparative HPLC to obtain 1 (15 mg).
3.5. Preparation of the (S)- and (R)-MTPA Ester Derivatives of Compound 1
4-(Dimethylamino)pyridine (2 mg) and (R)-(–)-α-methoxy-α-(trifluoromethyl)phenylacetyl chloride (MTPA-Cl, 10 μL) were added in a solution of 1 (1.5 mg) in pyridine (500 μL). The mixture was stirred at room temperature for 12 h. The reaction mixture was then passed through a disposable pipet packed with silica gel and eluted with petroleum ether–EtOAc (5:1) to give the (S)-Mosher ester 1s. Treatment of 1 (1.5 mg) with (S)-MTPA-Cl (10 μL) as described above yielded the corresponding (R)-Mosher ester 1r. Selected 1H-NMR (CDCl3, 600 MHz) of (S)-MTPA ester (1s): δH 7.42–7.56 (5H, Ph), 7.01 (1H, d, J = 10.1 Hz, H-1), 6.21 (1H, dd, J = 10.1, 1.9 Hz, H-2), 6.03 (1H, br t, H-4), 5.71(1H, ddd, J = 17.1, 10.3, 7.3 Hz, H-20), 5.34 (1H, m, H-15), 5.05 (1H, dd, J = 10.3, 1.5 Hz, H-21a), 4.99 (1H, dd, J = 17.1, 1.5 Hz, H-21b), 2.55 (1H, m, H-16a), 1.99 (1H, m, H-17), 1.68 (1H, m, H-8), 1.64 (1H, m, H-16b), 1.14 (3H, s, H3-19), 1.06 (1H, m, H-14), 0.72 (3H, s, H3-18); selected 1H-NMR (CDCl3, 600 MHz) of (R)-MTPA ester (1r): δH 7.43–7.55 (5H, Ph), 7.02 (1H, d, J = 10.1 Hz, H-1), 6.22 (1H, dd, J = 10.1, 1.9 Hz, H-2), 6.05 (1H, br t, H-4), 5.68 (1H, ddd, J = 17.1, 10.3, 7.3 Hz, H-20), 5.31 (1H, m, H-15), 5.03 (1H, dd, J = 10.3, 1.5 Hz, H-21a), 4.97 (1H, dd, J = 17.1, 1.5 Hz, H-21b), 2.53 (1H, m, H-16a), 1.96 (1H, m, H-17), 1.71 (1H, m, H-8), 1.60 (1H, m, H-16b), 1.17 (3H, s, H3-19), 1.07 (1H, m, H-14), 0.69 (3H, s, H3-18).
3.6. Brine Shrimp Lethality Assay
The growth inhibitory activity of the EtOAc extract was evaluated against the brine shrimp Artemia salina [18]. After incubation for 48 h, the brine shrimp lethality assay was performed on larvae of A. salina according to the published protocols [19]. Dimethyl sulfoxide (DMSO) was used as a negative control.
3.7. Cytotoxicity Test
The isolated compounds were screened for cytotoxic activity in vitro against the human hepatoma cell line Bel-7402 and the normal human embryonic lung fibroblast cell line MRC-5 using the tetrazolium (MTT) microculture method [20]. Well-growing carcinoma cells were collected and seeded in 96-well plates at 1 × 105/mL density. When the cells anchored to the plates, the culture medium was replaced with fresh medium containing various concentrations of the compounds. Three duplicate wells were used for each sample. After incubation at 37 °C, 5% CO2 for 48 h, 50 μL MTT was added to each well for another 4 h incubation. Then, the MTT medium was discarded and warm dimethylsulfoxide (DMSO, 150 μL) was added. Absorbance was measured at 550 nm. Cisplatin was used as a positive control, and phosphate buffered saline was used as a blank control.
3.8. Antibacterial Activity Assay
Antibacterial activity was evaluated by the conventional broth dilution assay [17]. Eight bacterial strains: B. cereus (ACCC 11077), S. albus (ATCC 8799), S. aureus (ATCC 27154), T. halophilus (ATCC 13623), E. coli (ATCC 25922), P. putida (ATCC 17485), N. brasiliensis (ATCC 19019) and V. parahaemolyticus (ATCC 17802) were used, and ciprofloxacin was used as a positive control.
4. Conclusions
In this study, four pregnane steroids 1–4 were obtained from a gorgonian Carijoa sp., of which compound 1 is a new pregnane. The absolute configuration of 1 was determined by application of the modified Mosher method. In addition, the absolute configuration for 2 was also reported for the first time. Among steroids from corals, C21 pregnane and their glycosides characterized by the unusual vinyl side chain represent a minor group of metabolites, and most of them are substituted by glycosides and present hydroxy or acetyl groups at C-3 instead of uncommon 3-one dienone [9,21,22,23]. In this work, we found pregnanes all with rare 3-one dienones, suggesting some specific metabolic pathway in this species.
Acknowledgments
We thank Xiu-Bao Li from the South China Sea Institute of Oceanology, Chinese Academy of Science for identification of gorgonian. This work was supported by the Key Program of National Natural Science Foundation of China (No. 41130858), the National Natural Science Foundation of China (Nos. 30901879; 81172977; 41176121), the Natural Science Foundation of Shandong Province (ZR2011DQ019), and the Program for New Century Excellent Talents in University, Ministry of Education of China (No. NCET-11-0472).
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/18/3/3458/s1.
Footnotes
Sample Availability: Samples of the compounds 2, 3, 4 are available from the authors.
References
- 1.Poza J.J., Fernández R., Reyes F., Rodríguez J., Jiménez C. Isolation, biological significance, synthesis, and cytotoxic evaluation of new natural parathiosteroids A–C and analogues from the soft coral Paragorgia sp. J. Org. Chem. 2008;73:7978–7984. doi: 10.1021/jo801198u. [DOI] [PubMed] [Google Scholar]
- 2.Díaz-Marrero A.R., Porras G., Aragón Z., de la Rosa J.M., Dorta E., Cueto M., D’Croz L., Maté J., Darias J. Carijodienone from the octocoral Carijoa multiflora. a spiropregnane-based steroid. J. Nat. Prod. 2011;74:292–295. doi: 10.1021/np1007608. [DOI] [PubMed] [Google Scholar]
- 3.Wu S.L., Wang G.H., Dai C.F., Sheu J.H. Pregnane-based steroids from a Formosan gorgonian Subergorgia mollis. J. Chin. Chem. Soc. 2004;51:205–208. [Google Scholar]
- 4.Reimão J.Q., Migotto A.E., Kossuga M.H., Berlinck R.G.S., Tempone A.G. Antiprotozoan activity of Brazilian marine cnidarian extracts and of a modified steroid from the octocoral Carijoa riisei. Parasitol Res. 2008;103:1445–1450. doi: 10.1007/s00436-008-1154-6. [DOI] [PubMed] [Google Scholar]
- 5.Regalado E.L., Tasdemir D., Kaiser M., Cachet N., Amade P., Thomas O.P. Antiprotozoal steroidal saponins from the marine sponge Pandaros acanthifolium. J. Nat. Prod. 2010;73:1404–1410. doi: 10.1021/np100348x. [DOI] [PubMed] [Google Scholar]
- 6.Chao C.H., Wen Z.H., Su J.H., Chen I.M., Huang H.C., Dai C.F., Sheu J.H. Further study on anti-inflammatory oxygenated steroids from the octocoral Dendronephthya griffin. Steroids. 2008;73:1353–1358. doi: 10.1016/j.steroids.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Fang H.Y., Liaw C.C., Chao C.H., Wen Z.H., Wu Y.C., Hsu C.H., Dai C.F., Sheu J.H. Bioactive pregnane-type steroids from the soft coral Scleronephthya gracillimum. Tetrahedron. 2012;68:9694–9700. doi: 10.1016/j.tet.2012.09.060. [DOI] [Google Scholar]
- 8.Wang S.K., Dai C.F., Duh C.Y. Cytotoxic pregnane steroids from the Formosan soft coral Stereonephthya crystalliana. J. Nat. Prod. 2006;69:103–106. doi: 10.1021/np050384c. [DOI] [PubMed] [Google Scholar]
- 9.Han L., Wang C.Y., Huang H., Shao C.L., Liu Q.A., Qi J., Sun X.P., Zhai P., Gu Y.C. A new pregnane analogue from Hainan soft coral Scleronephthya gracillimum Kuekenthal. Biochem. Sys. Ecol. 2010;38:243–246. doi: 10.1016/j.bse.2009.12.030. [DOI] [Google Scholar]
- 10.Wang C.Y., Zhao J., Liu H.Y., Shao C.L., Liu Q.A., Liu Y., Gu Y.C. Two new eicosanoids with a unique isovalerianic acid ester moiety from the South China Sea gorgonian Dichotella gemmacea. Lipids. 2011;46:81–85. doi: 10.1007/s11745-010-3489-x. [DOI] [PubMed] [Google Scholar]
- 11.Li R., Shao C.L., Qi X., Li X.B., Li J., Sun L.L., Wang C.Y. Polyoxygenated sterols from the South China Sea soft coral Sinularia sp. Mar. Drugs. 2012;10:1422–1432. doi: 10.3390/md10071422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L., Sheng L., Wang C.Y., Zhou Y.B., Huang H., Li X.B., Li J., Mollo E., Gavagnin M., Guo Y.W. Diterpenes from the Hainan soft coral Lobophytum cristatum Tixier-Durivault. J. Nat. Prod. 2011;74:2089–2094. doi: 10.1021/np2003325. [DOI] [PubMed] [Google Scholar]
- 13.Ciavatta M.L., Gresa M.P.L., Manzo E., Gavagnin M., Wahidulla S., Cimino G. New C21Δ20 pregnanes, inhibitors of mitochondrial respiratory chain, from Indopacific octocoral Carijoa sp. Tetrahedron Lett. 2004;45:7745–7748. [Google Scholar]
- 14.Kingston J.F., Gregory B., Fallis A.G. Pregna-1,4,20-triene-3-one, a novel marine steroid from the sea raspberry Gersemia rubiformis. Tetrahedron Lett. 1977;49:4261–4264. doi: 10.1016/S0040-4039(01)83480-8. [DOI] [Google Scholar]
- 15.Higgs M.D., Faulkner D.J. 5α-pregna-1,20-dien-3-one and related compounds from a soft coral. Steroids. 1977;30:379–388. doi: 10.1016/0039-128X(77)90028-9. [DOI] [PubMed] [Google Scholar]
- 16.Ohtani I., Kusuni T., Kashman Y., Kakisawa H. High-field FT NMR application of Mosher’s method. The absolute configurations of marine terpenoids. J. Am. Chem. Soc. 1991;113:4092–4096. doi: 10.1021/ja00011a006. [DOI] [Google Scholar]
- 17.Appendio G., Gibbons S., Giana A., Pagani A., Grassi G., Stavri M., Smith E., Rahman M.M. Antibacterial cannabinoids from Cannabis satiνa: A structure-activity study. J. Nat. Prod. 2008;71:1427–1430. doi: 10.1021/np8002673. [DOI] [PubMed] [Google Scholar]
- 18.Evidente A., Andolfi A., Vurro M., Zonno M.C., Motta A. Cytochalasins Z4, Z5, and Z6, three new 24-oxa[14] cytochalasans produced by Phoma exigua var. heteromorpha. J. Nat. Prod. 2003;66:1540–1544. doi: 10.1021/np030252o. [DOI] [PubMed] [Google Scholar]
- 19.Meyer B.N., Ferrigni N.R., Putnam J.E., Jacobson L.B., Nicols D.E., Mclaughlin J.L. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982;45:31–34. doi: 10.1055/s-2007-971236. [DOI] [PubMed] [Google Scholar]
- 20.Mosmann T. Rapid Colorimetric Assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 21.Gutiérrez M., Capson T., Guzmán H.M., Quiñoá E., Riguera R. L-Galactose as a natural product: Isolation from a marine octocoral of the first α-L-galactosyl saponin. Tetrahedron. Lett. 2004;45:7833–7836. doi: 10.1016/j.tetlet.2004.08.170. [DOI] [Google Scholar]
- 22.Gutiérrez M., Capson T.L., Guzmán H.M., González J., Ortega-Barría E., Quiñoá E., Riguera R. Antiplasmodial metabolites isolated from the marine octocoral Muricea austere. J. Nat. Prod. 2006;69:1379–1383. doi: 10.1021/np060007f. [DOI] [PubMed] [Google Scholar]
- 23.Ioannou E., Abdel-Razik A.F., Alexi X., Vagias C., Alexis M.N., Roussis V. Pregnanes with antiproliferative activity from the gorgonian Eunicella cavolini. Tetrahedron. 2008;64:11797–11801. doi: 10.1016/j.tet.2008.09.078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.