Abstract
A mild and efficient synthesis of pyrazolo[3,4-b]pyridine-6(7H)-one derivatives via a three-component reaction of an aldehyde, Meldrum’s acid and 3-methyl-1H-pyrazol-5-amine using recyclable polyethylene glycol (PEG)-400 as a reaction medium is described. This method has the advantages of accessible starting materials, good yields, mild reaction conditions and begin environmentally friendly.
Keywords: pyrazolo[3,4-b]pyridine-6(7H)-one; recyclable polyethylene glycol; aqueous media; synthesis
1. Introduction
Pyrazolo[3,4-b]pyridine-6-ones are a promising class of heterocyclic compounds that have been shown to have potential in the treatment of several diseases, including bipolar disorder, diabetes, dementia, Alzheimer’s disease, schizophrenia, depression and cancer [1,2]. Pyrazolo[3,4-b]pyridine-6-ones were first synthesized by condensing ethylidenemalonic acid diethyl ester and 1-ethyl-5-amino methylpyzole in refluxing dimethylformamide and water for 94 h and resulted in a 53% yield of the target product [3]. Quiroga et al. [4]. reported the synthesis of dihydropyrazolo[3,4-b]pyridine-6-ones by refluxing equimolar amounts of aminopyrazole and the appropriate Meldrum’s acid benzylidene derivatives in nitrobenzene for 30 min, resulting in yields ranging from 42% to 72%. Martinez-Teipel et al. [5]. developed a new synthetic route to this class of compound in 37%–92% yield involving an intermolecular cyclization of 2-methoxy-6-oxo-1,4,5,6-tetrahydropyridine-3-carbonitriles with hydrazines. Dress et al. [1]. also reported the synthesis of these compounds in 30%–75% yield by using condensation reactions that involved refluxing aminopyrazole with the appropriate aldehyde and dimedone derivatives in ethanol for 6–8 h. Recently, an efficient synthsis of pyrazolo[3,4-b]pyridine-6-one derivatives using intermolecular cyclization reactions under solvent-free microwave conditions and ultrasound irradiation conditions was reported, resulting in yields of 40%–60% and 60%–95%, respectively [6]. Shi et al. [7] reported the synthesis of 3-methyl-1,4-disubstituted-4,5-dihydro-1H-pyrazolo[3,4-b]pyridine-6(7H)-ones via the three-component L-proline-catalyzed reaction of an aldehyde, 3-methyl-1-phenyl-1H-pyrazol-5-amine, and Meldrum's acid. However, although these methods have successfully led to a large library synthesis of pyrazolo[3,4-b]pyridine-6-ones, many of them still suffer from drawbacks such as requiring the use of harmful organic reagents, unsatisfactory yields and long reaction times. Therefore, a method with higher yield and environmentally-friendly manipulation needs to be developed.
Multi-component reactions (MCRs), in which multiple reactions are combined into a synthetic operation have been used extensively in synthetic chemistry to form carbon-carbon bonds [8,9,10,11,12,13,14,15]. Such reactions offer a wide range of possibilities for the efficient construction of highly complex molecules in a single procedural step, thus avoiding the complicated purification operations and allowing savings of both solvents and reagents. In the past decade, there has been tremendous development in the area of three- and four-component reactions, and great efforts continue to be made to develop new MCRs [16,17,18,19,20,21,22,23,24]. Recently, Mamaghani et al. reported an one-pot three-component reaction for the synthesis of novel derivatives of pyrazolo[3,4-b]pyridine-6(7H)-ones in 3–4 min with excellent yields (87%–95%) from 5-amino-3-methyl-1H-pyrazole, Meldrum’s acid and aryl aldehydes under ultrasonic irradiation [25]. Nevertheless, this method needs specific experimental facilities, which limits its use in mass production.
Over the years, there has been a growing recognition that water has become an attractive medium for many organic reactions, such as Diels-Alder reactions [26], Claisen rearrangement reactions [27,28], Reformatsky reactions [29,30] and pinacol-coupling reactions [31], not only for the advantages concerning the avoidance of expensive drying reactions, catalysts and solvents, but also for some unique reactivity and selectivity [32,33,34,35]. On the other hand, organic reactions in water without using harmful organic solvents is one of the current focuses today, especially in our current environmentally conscious society, because water is abundant, nontoxic and environmentally-friendly when compared with the traditionally used organic solvents. As we all know, poly(ethylene glycol) (PEG) is a thermally stable, inexpensive, recoverable, and non-toxic hydrophilic polymer. Meanwhile, the high solubility of PEGs in water and several organic solvents including alcohol and acetone [36] instead of their insolubility in less polar solvents such as hexane makes them easy to recover and high performance solvents for organic reactions [37,38,39]. Therefore, the use of an obviously benign and inexpensive solvent like water and PEG could yield significant green chemistry benefits. Herein we envisaged a simple and efficient one-pot three-component protocol for the synthesis of pyrazolo[3,4-b]pyridine-6(7H)-ones in moderate to high yields, using environmentally-friendly polyethylene glycol (PEG) as a recyclable reaction medium.
2. Results and Discussion
When the three components, an aldehyde 1, Meldrum’s acid (2) and 3-methyl-1H-pyrazol-5-amine (3) were treated in water in the presence of PEG 400 at 90 °C for about 15 min, the desired products―4-substituted-3-methyl-4,5-dihydro-2H-pyrazolo[3,4-b]pyridin-6(7H)-ones 4 were obtained (Scheme 1).
Scheme 1.
The synthesis of pyrazolo[3,4-b]pyridine-6(7H)-one derivatives in aqueous media.
A range of novel valuable structures 4 were thus synthesized in good to excellent yields by this simple four-component reaction in aqueous media. The results are summarized in Table 1.
Table 1.
The synthesis of pyrazolo[3,4-b]pyridine-6(7H)-one derivatives in aqueous media.
| Entry | Product | R | Isolated Yield (%) |
|---|---|---|---|
| 1 | 4a | 4-CH3C6H4 | 88 |
| 2 | 4b | 3-CH3C6H4 | 93 |
| 3 | 4c | 4-CH3OC6H4 | 89 |
| 4 | 4d | 3-CH3OC6H4 | 84 |
| 5 | 4e | 4-BrC6H4 | 88 |
| 6 | 4f | 3-BrC6H4 | 92 |
| 7 | 4g | 2-CH3C6H4 | 89 |
| 8 | 4h | 3-ClC6H4 | 90 |
| 9 | 4i | n-propyl | 86 |
As shown in Table 1, we were pleased to find that the method was applicable to a broad substrate scope of substituted aldehydes. Aldehydes containing various electron-donating and electron-withdrawing substituents were reacted under the experimental conditions, and the corresponding products were obtained in good yields. Therefore, no remarkable electronic effects were observed in the reaction. Good yields was also obtained when the alkyl alhedyde butyraldehyde was reacted (Table 1, entry 9).
With regard to sustainable chemistry issues, reagent recyclability is an important question. The separation of the products and the reaction medium were explored for the synthesis of product 4a in PEG-400-H2O. We were pleased to find that the entire reaction medium could be successfully recycled for up to five runs with limited loss of activity (the yield decreased from 93% to 70% after 5 runs, Table 2).
Table 2.
Recycling and reuse of PEG-400-H2O.
| Run | Yield (%) |
|---|---|
| 1 | 93 |
| 2 | 89 |
| 3 | 82 |
| 4 | 76 |
| 5 | 70 |
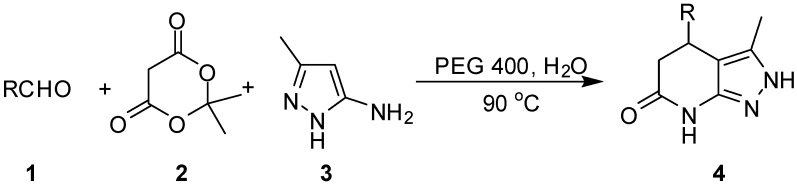
All the products were characterized by 1H-NMR, IR and HRMS spectra. The structure of compound 4c was further confirmed by X-ray diffraction analysis [40]. The molecular structure of 4c is shown in Figure 1.
Figure 1.
Molecular structure of 4c.
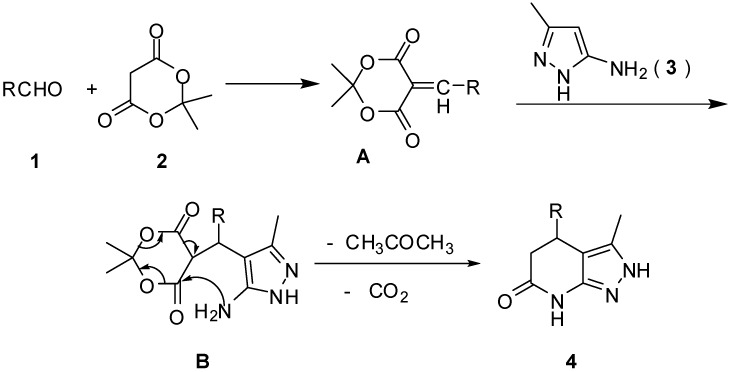
Although the mechanism of the reaction has not yet been established, a possible explanation is proposed in Scheme 2. The reaction might thus proceed via sequential condensation, addition, cyclization, and elimination. First, a Knoevenagel condensation between aldehydes 1 with Meldrum’s acid (2) affords intermediate A. The Michael addition of A with 3-methyl-1H-pyrazol-5-amine (3) would then furnish the intermediate product B, which subsequently undergoes an intramolecular cyclization and then releases acetone and carbon dioxide to give product 4.
Scheme 2.
Possible mechanism for the formation of product 4.
3. Experimental
3.1. General Information
Commercial solvents and reagents were used as received. IR spectra were obtained on a Nicolet 6700 spectrophotometer. 1H-NMR spectra were recorded using a Bruker DPX-400 MHz instrument, at 293 K unless otherwise noted, with the residual peaks of the solvent DMSO-d6 (δ = 2.50) used for reference. HRMS were obtained on a micromass GCT-TOF instrument. X-Ray crystallographic analysis was performed with a Rigaku Mercury diffractometer.
3.2. General Procedure for the Synthesis of Pyrazolo[3,4-b]pyridine-6(7H)-ones 4 in Aqueous Media
To a stirred solution of polyethylene glycol (PEG)-400 (5 mL) in water (10 mL), aldehyde 1 (2 mmol), Meldrum’s acid (2, 2 mmol) and 3-methyl-1H-pyrazol-5-amine (3, 2 mmol) were added and the mixture stirred at 90 °C, until the reaction was complete as indicated by TLC (about 15 min). After completion of the reaction, the crystalline powder formed was collected by filtration, washed with water and recrystallized from ethanol to give pure 4. The recovered PEG with water was reused for further cycles.
3-Methyl-4-p-tolyl-4,5-dihydro-2H-pyrazolo[3,4-[3,4-b]pyridin-6(7H)-one (4a). M.p. >300 °C; IR (KBr): 3203, 3160 cm−1 (NH), 1650 cm−1 (C=O). 1H-NMR (DMSO-d6) δ: 1.87 (s, 3H, CH3), 2.26 (s, 3H, CH3), 2.54 (d, J = 6.0 Hz, 1H, CH), 2.74–2.78 (m, 1H, CH), 4.09 (t, J = 6.4 Hz, 1H, CH), 7.04–7.12 (m, 4H, ArH), 10.27 (s, 1H, NH), 11.79 (s, 1H, NH); HRMS: m/z calcd. for C14H16N3O: 242.12879 (M+H); found 242.12880.
3-Methyl-4-m-tolyl-4,5-dihydro-2H-pyrazolo[3,4-b]pyridin-6(7H)-one (4b). M.p. >300 °C; IR (KBr): 3200, 3150 cm−1 (NH), 1640 cm−1 (C=O). 1H-NMR (DMSO-d6) δ: 1.83 (s, 3H, CH3), 2.26 (s, 3H, CH3), 2.54 (d, J = 6.0 Hz, 1H, CH), 2.74–2.79 (m, 1H, CH), 4.09 (t, J = 6.4 Hz, 1H, CH), 7.04–7.12 (m, 4H, ArH), 10.27 (s, 1H, NH), 11.79 (s, 1H, NH); HRMS: m/z calcd. for C14H16N3O: 242.12879 (M+H); found 242.12878.
4-(4-Methoxyphenyl)-3-methyl-4,5-dihydro-2H-pyrazolo[3,4-b]pyridin-6(7H)-one (4c). M.p. >300 °C; IR (KBr): 3180, 3155 cm−1 (NH), 1640 cm−1 (C=O). 1H-NMR (DMSO-d6) δ: 1.83 (s, 3H, CH3), 2.72–2.78 (m, 2H, CH2), 3.73 (s, 3H, OCH3), 4.09 (t, J = 6.4 Hz, 1H, CH), 6.86–6.88 (m, 2H, ArH), 7.07–7.09 (m, 2H, ArH),10.25 (s, 1H, NH), 11.78 (s, 1H, NH); HRMS: m/z calcd. for C14H16N3O2: 258.12370 (M+H); found 258.12369.
4-(3-Methoxyphenyl)-3-methyl-4,5-dihydro-2H-pyrazolo[3,4-b]pyridin-6(7H)-one (4d). M.p. >300 °C; IR (KBr): 3180, 3150 cm−1 (NH), 1640 cm−1 (C=O). 1H-NMR (DMSO-d6) δ: 1.85 (s, 3H, CH3), 2.56 (d, J = 5.6 Hz, 1H, CH), 2.78–2.84 (m, 1H, CH), 3.72 (s, 3H, OCH3), 4.17 (t, J = 6.4 Hz, 1H, CH), 6.73–6.81 (m, 3H, ArH), 7.23 (t, J = 8.0 Hz, 1H, ArH), 10.28 (s, 1H, NH), 11.81 (s, 1H, NH); HRMS: m/z calcd. for C14H16N3O2: 258.12370 (M+H); found 258.12372.
4-(4-Bromophenyl)-3-methyl-4,5-dihydro-2H-pyrazolo[3,4-b]pyridin-6(7H)-one (4e). M.p. >300 °C; IR (KBr): 3190, 3160 cm−1 (NH), 1643 cm−1 (C=O). 1H-NMR (DMSO-d6) δ: 1.85 (s, 3H, CH3), 2.56 (d, J = 5.6 Hz, 1H, CH), 2.78–2.84 (m, 1H, CH), 4.17 (t, J = 6.4 Hz, 1H, CH), 7.13–7.15 (m, 2H, ArH), 7.50–7.52 (m, 2H, ArH), 10.32 (s, 1H, NH), 11.85 (s, 1H, NH); HRMS: m/z calcd. for C13H13BrN3O: 306.02365 (M+H); found 306.02362.
4-(3-Bromophenyl)-3-methyl-4,5-dihydro-2H-pyrazolo[3,4-b]pyridin-6(7H)-one (4f). M.p. >300 °C; IR (KBr): 3190, 3160 cm−1 (NH), 1640 cm−1 (C=O). 1H-NMR (DMSO-d6) δ: 1.86 (s, 3H, CH3), 2.50–2.58 (m, 1H, CH), 2.79–2.83 (m, 1H, CH), 4.17 (t, J = 6.0 Hz, 1H, CH), 7.16–7.41 (m, 4H, ArH), 10.33 (s, 1H, NH), 11.86 (s, 1H, NH); HRMS: m/z calcd. for C13H13BrN3O: 306.02365 (M+H); found 306.02371.
3-Methyl-4-o-tolyl-4,5-dihydro-2H-pyrazolo[3,4-b]pyridin-6(7H)-one (4g). M.p. >300 °C; IR (KBr): 3180, 3150 cm−1 (NH), 1645 cm−1 (C=O). 1H-NMR (DMSO-d6) δ: 1.73 (s, 3H, CH3), 2.37 (s, 3H, CH3), 2.39–2.47 (m, 1H, CH), 2.73–2.78 (m, 1H, CH), 4.34 (t, J = 6.4 Hz, 1H, CH), 6.88–7.20 (m, 4H, ArH), 10.30 (s, 1H, NH), 11.81 (s, 1H, NH); HRMS: m/z calcd. for C14H16N3O: 242.12879 (M+H); found 242.12871.
4-(3-Chlorophenyl)-3-methyl-4,5-dihydro-2H-pyrazolo[3,4-b]pyridin-6(7H)-one (4h) M.p. >300 °C; IR (KBr): 3190, 3155 cm−1 (NH), 1645 cm−1 (C=O). 1H-NMR (DMSO-d6) δ: 1.86 (s, 3H, CH3), 2.77 (dd, J1 = 5.6 Hz, J2 = 16 Hz, 1H, CH), 2.79–2.84 (m, 1H, CH), 4.20 (t, J = 6.0 Hz, 1H, CH), 7.14–7.35 (m, 4H, ArH), 10.34 (s, 1H, NH), 11.87 (s, 1H, NH); HRMS: m/z calcd. for C13H13ClN3O: 262.07417 (M+H); found 262.07416.
3-methyl-4-propyl-4,5-dihydro-2H-pyrazolo[3,4-b]pyridin-6(7H)-one (4i) M.p. 259–260 °C; IR (KBr): 3175, 3140 cm−1 (NH), 1645 cm−1 (C=O). 1H-NMR (DMSO-d6) δ: 0.85–0.86 (m, 3H, CH3), 1.17–1.93 (m, 4H, 2CH2), 2.18 (s, 3H, CH3), 2.24–2.26 (m, 1H, CH), 2.77–2.78 (m, 1H, CH), 3.51 (t, J = 7.2 Hz, 1H, CH), 10.13 (s, 1H, NH), 11.66 (s, 1H, NH); HRMS: m/z calcd. for C10H16N3O: 194.12879 (M+H); found 194.12874.
4. Conclusions
In summary, we have demonstrated a mild and highly efficient protocol for the synthesis of 4-substituted-3-methyl-4,5-dihydro-2H-pyrazolo[3,4-b]pyridin-6(7H)-ones in excellent yields by using recyclable polyethylene glycol (PEG)-400 as a reaction medium. Environmental acceptablility, high yields, easy work-up, cleaner reaction profiles, environmentally friendly solvent, and recyclability of PEG are the notable features of this protocol.
Acknowledgments
We are grateful to The Joint Funds of the National Natural Science Foundation of China and Shenhua Group Corporation Limited (No. 51134020), the National Natural Science Foundation of China Youth Science Foundation (Grant No. 51204171 and 51004105), and A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 4 are available from the authors.
References
- 1.Dress B.E., Chakravarty L., Prestwich G.D., Dorman G., Kavecz M., Lukacs A., Urge L., Darvas F., Rzepecki P.W., Ferguson C.G. Compounds having inhibitive activity of phosphatidylinositol 3-kinase and methods of use thereof. W.O. Patent 016,245, 2005. Chem. Abstr. 2005;142:261534. [Google Scholar]
- 2.Kung D.W. S., Fuller G. Pyrazolo[3,4-b]pyridin-6-ones as GSK-3 inhibitors. U.S. Patent 266,815, 2004. Chem. Abstr. 2005;142:266815. [Google Scholar]
- 3.Hoehn H. 1,4,5,7-Tetrahydropyrazolo[3,4-b]pyridin-6-ones. U.S. Patent 3,935,222, 1976. Chem. Abstr. 1976;85:21351. [Google Scholar]
- 4.Quiroga J., Hormanza A., Insuasty B. Reaction of 5-amino-1-aryl-3-methylpyrazoles with benzylidene derivatives of meldrum’s acid: Synthesis and characterization of pyrazolo[3,4-b]pyridinones. J. Heterocycl. Chem. 1998;35:409–412. doi: 10.1002/jhet.5570350225. [DOI] [Google Scholar]
- 5.Martinez-Teipel B., Teixido J., Pascual R., Mora M., Pujola J., Fujimoto T., Borrell I.I., Michelotti E.L. 2-Methoxy-6-oxo-1,4,5,6-tetrahydropyridine-3-carbonitriles: Versatile starting materials for the synthesis of libraries with diverse heterocyclic scaffolds. J. Comb. Chem. 2005;7:436–448. doi: 10.1021/cc049828y. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigues-Santos C.E., Echevarria A. Convenient syntheses of pyrazolo[3,4-b]pyridin-6-ones using either microwave or ultrasound irradiation. Tetrahedron Lett. 2011;52:336–340. doi: 10.1016/j.tetlet.2010.11.054. [DOI] [Google Scholar]
- 7.Shi C.L., Chen H., Shi D.Q. An efficient one-pot synthesis of pyrazolo[3,4-b]pyridinone derivatives catalyzed by L-proline. J. Heterocycl. Chem. 2011;48:351–354. doi: 10.1002/jhet.573. [DOI] [Google Scholar]
- 8.Bienayme H., Hulme C., Oddon G., Schmitt P. Maximizing synthetic efficiency: Multi-component transformations lead the way. Chem-Eur. J. 2000;6:3321–3329. doi: 10.1002/1521-3765(20000915)6:18<3321::AID-CHEM3321>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 9.Tietze L.F., Modi A. Multicomponent domino reactions for the synthesis of biologically active natural products and drugs. Med. Res. Rev. 2000;20:304–322. doi: 10.1002/1098-1128(200007)20:4<304::AID-MED3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Dömling A., Ugi I. Multicomponent reactions with isocyanides. Angew. Chem. Int. Ed. 2000;39:3168–3210. doi: 10.1002/1521-3773(20000915)39:18<3168::AID-ANIE3168>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 11.Zhu J. Recent developments in the isonitrile-based multicomponent synthesis of heterocycles. Eur. J. Org. Chem. 2003:1133–1144. doi: 10.1002/ejoc.200390167. [DOI] [Google Scholar]
- 12.Orru R.V.A., de Greef M. Recent advances in solution-phase multicomponent methodology for the synthesis of heterocyclic compounds. Synthesis. 2003;10:1471–1499. doi: 10.1055/s-2003-40507. [DOI] [Google Scholar]
- 13.Nair V., Rajesh C., Vinod A.V., Bindu S., Sreekanth A.R., Mathen J.S., Balagopal L. Strategies for heterocyclic construction via novel multicomponent reactions based on isocyanides and nucleophilic carbenes. Account. Chem. Res. 2003;36:899–907. doi: 10.1021/ar020258p. [DOI] [PubMed] [Google Scholar]
- 14.Simon C., Constantieux T., Rodriguez J. Utilisation of 1,3-dicarbonyl derivatives in multicomponent reactions. Eur. J. Org. Chem. 2004:4957–4980. [Google Scholar]
- 15.Ramon D.J., Yus M. Asymmetric multicomponent reactions (AMCRs): The new frontier. Angew. Chem. Int. Ed. 2005;44:1602–1634. doi: 10.1002/anie.200460548. [DOI] [PubMed] [Google Scholar]
- 16.Nair V., Vinod A.U., Rajesh C. A novel synthesis of 2-aminopyrroles using a three-component reaction. J. Org. Chem. 2001;66:4427–4429. doi: 10.1021/jo001714v. [DOI] [PubMed] [Google Scholar]
- 17.List B., Castello C. A novel proline-catalyzed three-component reaction of ketones, aldehydes, and Meldrum’s acid. Synlett. 2001;11:1687–1689. doi: 10.1055/s-2001-18095. [DOI] [Google Scholar]
- 18.Shestopalov A.M., Emeliyanova Y.M., Shestiopolov A.A., Rodinovskaya L.A., Niazimbe tova Z.I., Evans D.H. One-step synthesis of substituted 6-amino-5-cyanospiro-4-(piperidine-4')-2H,4H-dihydropyrazolo[3,4-b]pyrans. Org. Lett. 2002;4:423–425. doi: 10.1021/ol0102747. [DOI] [PubMed] [Google Scholar]
- 19.Bertozzi F., Gustafsson M., Olsson R. A novel metal iodide promoted three-component synthesis of substituted pyrrolidines. Org. Lett. 2002;4:3147–3150. doi: 10.1021/ol0264814. [DOI] [PubMed] [Google Scholar]
- 20.Yuan Y., Li X., Ding K. Acid-free aza Diels-Alder reaction of Danishefsky’s diene with imines. Org. Lett. 2002;4:3309–3311. doi: 10.1021/ol0265822. [DOI] [PubMed] [Google Scholar]
- 21.Cheng J.F., Chen M., Arthenius T., Nadzen A. A convenient solution and solid-phase synthesis of Δ5–2-oxopiperazines via N-acyliminium ions cyclization. Tetrahedron Lett. 2002;43:6293–6295. [Google Scholar]
- 22.Huma H.Z.S., Halder R., Kalra S.S., Das J., Iqbal J. Cu(I)-catalyzed three component coupling protocol for the synthesis of quinoline derivatives. Tetrahedron Lett. 2002;43:6485–6488. [Google Scholar]
- 23.Bora U., Saikia A., Boruah R.C. A novel microwave-mediated one-pot synthesis of indolizines via a three-component reactio. Org. Lett. 2003;5:435–438. doi: 10.1021/ol020238n. [DOI] [PubMed] [Google Scholar]
- 24.Dallinger D., Gorobets N.Y., Kappe C.O. High-throughput synthesis of N3-acylated dihydropyrimidines combining microwave-assisted synthesis and scavenging techniques. Org. Lett. 2003;5:1205–1208. doi: 10.1021/ol034085v. [DOI] [PubMed] [Google Scholar]
- 25.Roshan A.A., Mamaghani M., Mahmoodi N.O., Shirini F. An efficient regioselective sonochemical synthesis of novel 4-aryl-3-methyl-4,5-dihydro-1H-pyrazolo[3,4-b]pyridin-6(7H)-ones. Chin. Chem. Lett. 2012;23:399–402. doi: 10.1016/j.cclet.2011.12.009. [DOI] [Google Scholar]
- 26.Breslow R., Maitra U. On the origin of product selectivity in aqueous diels-alder reactions. Tetrahedron Lett. 1984;25:1239–1240. doi: 10.1016/S0040-4039(01)80122-2. [DOI] [Google Scholar]
- 27.Ponaras A.A. A new variant of the Claisen rearrangement capable of creating the bond between two quaternary centers. J. Org. Chem. 1983;48:3866–3868. doi: 10.1021/jo00169a071. [DOI] [Google Scholar]
- 28.Coates R.M., Rogers B.D., Hobbs S.J., Peck D.R., Curran D.P. Synthesis and Claisen rearrangement of alkoxyallyl enol ethers. Evidence for a dipolar transition state. J. Am. Chem. Soc. 1987;109:1160–1170. doi: 10.1021/ja00238a028. [DOI] [Google Scholar]
- 29.Mattes H., Benezra C. Reformatsky-type reactions in aqueous media. Use ofbronometryl-acrylic acid for the synthesis of α-methylene-γ-butyrolactones. Tetrahedron Lett. 1985;26:5697–5698. doi: 10.1016/S0040-4039(01)80923-0. [DOI] [Google Scholar]
- 30.Zhou J.Y., Lu G.D., Wu S.H. A new approach for the synthesis of alpha-methylene-gamma-butyrolactones from alpha-bromomethylacrylicacids (or esters) Synth. Commun. 1992;22:481–487. doi: 10.1080/00397919208019246. [DOI] [Google Scholar]
- 31.Delair P., Luche J.L. A new sonochemical carbonyl cross-coupling reaction. J. Chem. Soc. Chem. Commun. 1989:398–399. doi: 10.1039/c39890000398. [DOI] [Google Scholar]
- 32.Brelow R., Maitra U., Rideout D.C. Selective diels-alder reactions in aqueous solutions and suspensions. Tetrahedron Lett. 1983;24:1901–1904. [Google Scholar]
- 33.Tan X.H., Hou Y.Q., Huang C., Liu L., Guo Q.X. SnCl2-mediated carbonyl allylation in fully aqueous media. Tetrahedron. 2004;60:6129–6136. [Google Scholar]
- 34.Copley S.D., Khowles J.R. The conformational equilibrium of chorismate in solution: Implications for the mechanism of the non-enzymic and the enzyme-catalyzed rearrangement of chorismate to prephenate. J. Am. Chem. Soc. 1987;109:5008–5013. doi: 10.1021/ja00250a040. [DOI] [Google Scholar]
- 35.Khosropour A.R., Khodaei M.M., Kookhazadeh M. A mild, efficient and environmentally friendly method for the regio- and chemoselective synthesis of enaminones using Bi(TFA)3 as a reusable catalyst in aqueous media. Tetrahedron Lett. 2004;45:1725–1728. doi: 10.1016/j.tetlet.2003.12.093. [DOI] [Google Scholar]
- 36.Zhang Z.H., Yin L., Wang Y.M., Liu J.Y., Li Y. Indium tribromide in poly(ethylene glycol) (PEG): a novel and efficient recycle system for chemoselective deprotection of 1,1-diacetates. Green Chem. 2004;6:563–565. [Google Scholar]
- 37.Fulwa V.K., Manivannan V. Synthesis of 3-substituted imidazo[1,5-a]pyridines having 1-(N-picolinamidin-2-yl) group. Tetrahedron. 2012;68:3927–3931. doi: 10.1016/j.tet.2012.03.095. [DOI] [Google Scholar]
- 38.Nagarapu L., Mallepalli R., Kumar U.N., Venkateswarlu P., Bantu R., Yeramanchi L. Synthesis of α1-oxindole-α-hydroxyphosphonates under catalyst-free conditions using polyethylene glycol (PEG-400) as an efficient and recyclable reaction medium. Tetrahedron Lett. 2012;53:1699–1700. [Google Scholar]
- 39.Reddy B.S., Naidu A., Dubey P.K. PEG-600-mediated, green and efficient, tandem syntheses of N-subtituted-2- styrylquinazolin-4-ones. Green Chem. Lett. Rev. 2013;6:254–261. doi: 10.1080/17518253.2012.742142. [DOI] [Google Scholar]
- 40.Crystallographic data for the structure of 4c (CCDC-967181) have been deposited at the Cambridge Crystallographic Data Centre. Copies of available material can be obtained free of charge on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK [fax: +44 (1223)336033; email: deposit@ccdc.cam.ac.uk].