Abstract
A series of novel thiourea and urea derivatives containing 1,2,4-triazole moieties were synthesized and evaluated for their antifungal and larvicidal activity. Triazole derivatives 3a–e and 4a–e were synthesized by reacting thiocarbohydrazide with thiourea and urea compounds 1a–e and 2a–e, respectively, in a 130–140 °C oil bath. The proposed structures of all the synthesized compounds were confirmed using elemental analysis, UV, IR, 1H-NMR and mass spectroscopy. All compounds were evaluated for antifungal activity against plant pathogens, larvicidal and biting deterrent activity against the mosquito Aedes aegypti L. and in vitro cytotoxicity and anti-inflammatory activity against some human cell lines. Phomopis species were the most sensitive fungi to these compounds. Compounds 1b, 1c, 3a and 4e demonstrated selectively good activity against Phomopis obscurans and only 1b and 4e showed a similar level of activity against P. viticola. Compound 3d, with a LD50 value of 67.9 ppm, followed by 1c (LD50 = 118.8 ppm) and 3e (LD50 = 165.6 ppm), showed the highest toxicity against Aedes aegypti larvae. Four of these compounds showed biting deterrent activity greater than solvent control, with the highest activity being seen for 1c, with a proportion not biting (PNB) value of 0.75, followed by 1e, 2b and 1a. No cytotoxicity was observed against the tested human cancer cell lines. No anti-inflammatory activity was observed against NF-κB dependent transcription induced by phorbol myristate acetate (PMA) in human chondrosarcoma cells.
Keywords: thiourea; urea; 1,2,4-triazole; synthesis; fungicide; plant pathogens; larvicides; mosquitoes; anti-inflammatory; cytotoxicity
1. Introduction
Thioureas are important sulphur and nitrogen-containing compounds that have proved to be useful substances in drug research in recent years [1,2,3,4,5,6]. Some urea derivatives possess valuable antituberculosis, antibacterial and anticonvulsant properties [7,8,9,10]. Most of these compounds include heterocyclic rings such as oxadiazoles, thiadiazoles, triazoles, and pyrazoles. It is well known that the 1,2,4-triazole-derived N-bridged heterocycles find applications in the field of medicine, agriculture and industry [11,12,13,14]. The 1,2,4-triazole nucleus has also been incorporated into a wide variety of therapeutically important molecules to transform them into better drugs. Drugs such as fluconazole, itraconazole, and the new generation of triazoles posaconazole, voriconazole, and ravuconazole are the best examples of potent antifungal molecules possessing triazole nuclei [15,16,17].
Thioureas can be used in the control of plant pathogens like Penicillum expansum and Fusarium oxysporum [18]. 1,3-Dialkyl or diaryl thioureas exhibited significant antifungal activity against Pyricularia oryzae and Drechslera oryzae [19]. Cao et al. reported that γ-aryl-1H-1,2,4-triazole derivatives showed potent antifungal activity against Fusarium oxysporium, Rhizoctonia solani, Botrytis cinerea, Gibberella zeae, Dothiorella gregaria, and Colletotrichum gossypii species [20,21]. Crank et al. [22] reported for the first time the antifungal activity of 2-aminooxazole thiourea derivatives against Botrytis cinerea.
Data on the biological activity of new thiourea and urea derivatives bearing 1,2,4-triazole moieties is scanty. Considering the biological activities of these compounds, in this work a series of new thiourea and urea derivatives bearing 1,2,4-triazole moieties were prepared and evaluated for their antifungal activity against several filamentous fungal plant pathogens, mosquito (Aedes aegypti) larvicidal and biting deterrent activity and cytotoxicity and anti-inflammatory activity in some human cell lines.
2. Results and Discussion
2.1. Synthesis
A series of new thiourea and urea derivatives bearing 1,2,4-triazole rings were prepared according to Scheme 1. The thiourea derivatives 1a–e were previously synthesized and reported by Celen et al. [23]. Compounds 2a–e were prepared by refluxing equimolar amounts of 4-(aminophenyl)acetic acid and various isocyanates in acetone. The reactions of compounds 1a–e and 2a–e with thio-carbohydrazide in an oil bath afforded the corresponding 1,2,4-triazoles 3a–e and 4a–e. All compounds were isolated in satisfactory yields (45–83%) and readily purified by recrystallization from acetonitrile. The purity of the compounds was checked by TLC and elemental analyses. The chemical structures of all compounds were characterized by various spectroscopic methods, and both the analytical and spectral data of all the synthesized compounds were in full agreement with the proposed structures. Physical and chemical properties of all compounds are presented in Table 1.
Scheme 1.
General synthetic route for title compounds 1a–e, 2a–e, 3a–e and 4a–e.
Reagents and Conditions: (a) Ar-NCS, 100 °C; (b) Ar-NCO, 100 °C; (c) NH2NHCSNHNH2, 130–140 °C; (d) NH2NHCSNHNH2, 130–140 °C.
Table 1.
Structure and physical data of compounds 1a–e, 2a–e, 3a–e and 4a–e.
| Compd. | Ar | M.P. (°C) | M.F. | M.W. | Yield (%) |
|---|---|---|---|---|---|
| 1a | 2,4,6-Cl-C6H2 | 214–215 | C15H11Cl3N2O2S | 389.68 | 50.5 |
| 1b | 2,6-Cl2-C6H4 | 206–207 | C15H13ClN2O2S | 320.79 | 48.3 |
| 1c | 4-CH3S-C6H4 | 193–194 | C16H16N2O3S | 316.37 | 61.1 |
| 1d | 4-CF3-C6H5 | 240–241 | C15H13N3O4S | 331.34 | 45.5 |
| 1e | 4-NO2-C6H5 | 200–201 | C15H13FN2O2S | 304.34 | 67.2 |
| 2a | 2,4,6-Cl-C6H2 | 276–277 | C15H11Cl3N2O3 | 373.62 | 77.3 |
| 2b | 2,6-Cl2-C6H4 | 258–259 | C15H13ClN2O3 | 304.73 | 78.0 |
| 2c | 4-CH3S-C6H4 | 234–235 | C16H16N2O4 | 300.31 | 83.5 |
| 2d | 4-CF3-C6H5 | 250–251 | C15H13N3O5 | 315.28 | 67.4 |
| 2e | 4-NO2-C6H5 | 245–246 | C15H13FN2O3 | 288.27 | 80.9 |
| 3a | 2,4,6-Cl-C6H2 | 227–228 | C16H13Cl3N6S2 | 459.80 | 46.4 |
| 3b | 2,6-Cl2-C6H4 | 206–207 | C16H15ClN6S2 | 390.85 | 50.8 |
| 3c | 4-CH3S-C6H4 | 228–230 | C17H18N6OS2 | 386.56 | 59.3 |
| 3d | 4-CF3-C6H5 | 240–241 | C15H17N7O2S2 | 401.47 | 55.4 |
| 3e | 4-NO2-C6H5 | 234–236 | C16H15FN6S2 | 374.46 | 52.2 |
| 4a | 2,4,6-Cl-C6H2 | 237–238 | C16H13Cl3N6OS | 443.74 | 58.0 |
| 4b | 2,6-Cl2-C6H4 | 258–259 | C16H15ClN6OS | 374.85 | 55.9 |
| 4c | 4-CH3S-C6H4 | 180–182 | C17H19N6O2S | 371.43 | 51.8 |
| 4d | 4-CF3-C6H5 | 214–216 | C16H15N7O3S | 385.40 | 50.2 |
| 4e | 4-NO2-C6H5 | 240–241 | C16H15FN6OS | 358.39 | 48.8 |
In the IR spectra of compounds 3a–e, two C=S stretching bands due to the thiourea and thioxo groups were seen at 1320–1350 cm−1 and 1234–1294 cm−1. In the 1H-NMR spectrum, the carboxylic acid O-H peaks were observed as singlets at 11.98–12.58 ppm for compounds 1a–e [23], but were absent in the spectra of the 1,2,4-triazole derivatives 3a–e due to the ring closure. The N-H peaks of the triazole rings were seen at 11.70–12.30 ppm as singlets and the thiourea N-H peaks were observed at 7.83–9.79 ppm as two singlets. In the IR spectra of compounds 2a–e, two C=O stretching bands due to the carboxylic acid and urea groups were detected at 1629–1697 cm−1. Urea N-H peaks were seen at 8.22–9.33 ppm as two singlets. The carboxylic acid O-H peaks were observed at 12.13–12.24 ppm as singlets, but these peaks disappeared in the spectra of 1,2,4-triazole derivatives 4a–e due to the ring closure. The N-H peaks of the triazole rings were seen at 12.40-13.48 ppm as a singlet but some compounds (e.g., 4a, 4b) have no triazole ring-related N-H peaks due to deuterium exchange with the NMR solvent. The other aliphatic and aromatic protons were detected in the expected regions for all compounds. Mass spectra (MS-ES) of compounds showed [MH]+ peaks in agreement with their molecular formulae.
2.2. Biological Activity
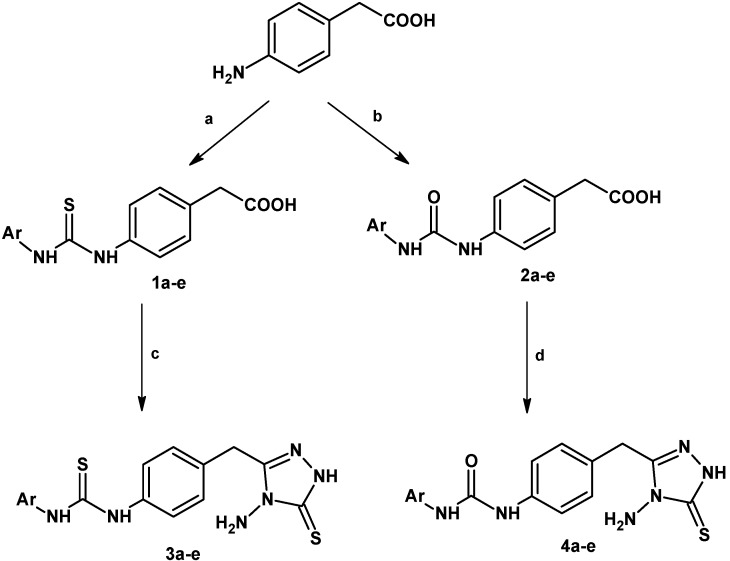
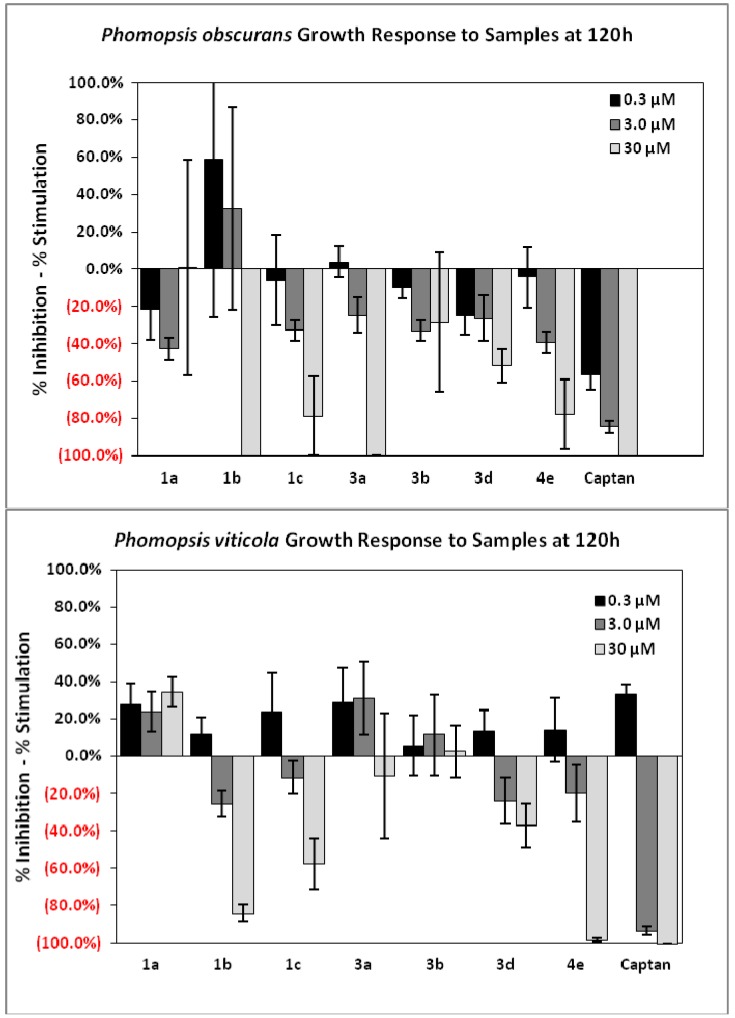
All compounds were evaluated for growth inhibition of filamentous plant pathogenic fungi belonging to genera Colletotrichum, Botrytis, Fusarium and Phomopsis using 96-well micro-dilution broth assays. Microbioassay results indicated that the thiourea derivatives 1b, 1c, 3a and 3d bearing 2,6-dichloro-, 2,4,6-trichloro-, 4-methysulfanyl- and 4-trifluoromethyl- groups on the phenylthiourea ringand the urea derivative 4e having a 4-nitro group were the most active against Phomopsis obscurans and P. viticola. Compounds 1b, 3a, and 1c and 4e at 30 μM inhibited the growth of P.obscurans by 100% and 80%, respectively, after 120 h exposure, whereas 1b, 3d and 4e showed 80%, 60% and 80% growth inhibition, respectively, at 30 μM, respectively (Figure 1). Compound 4e showed 100% growth inhibition of P.viticola at 30 μM, whereas 1b and 1c inhibited its growth by 80% and 60%, respectively (Figure 2). Compounds 1b, 3a and 4e having 2,6-dichloro-, 2,4,6-trichloro- and 4-nitro-groups on the phenylthiourea ring showed fungal growth inhibition that was similar to that of 30 μM of the positive control captan, a well known multisite inhibitor fungicide with no systemic activity, used as a commercial protectant fungicide to prevent anthracnose diseases in fruits and ornamentals [24]. Tested compounds showed a very weak activity against Colletotrichum, Botrytis and Fusarium species.
Figure 1.
Growth inhibition of Phomposis obscurans and P. viticola after 120 h using a 96 well microdilution broth assay in a dose response with the commercial fungicide standard captan as reference.
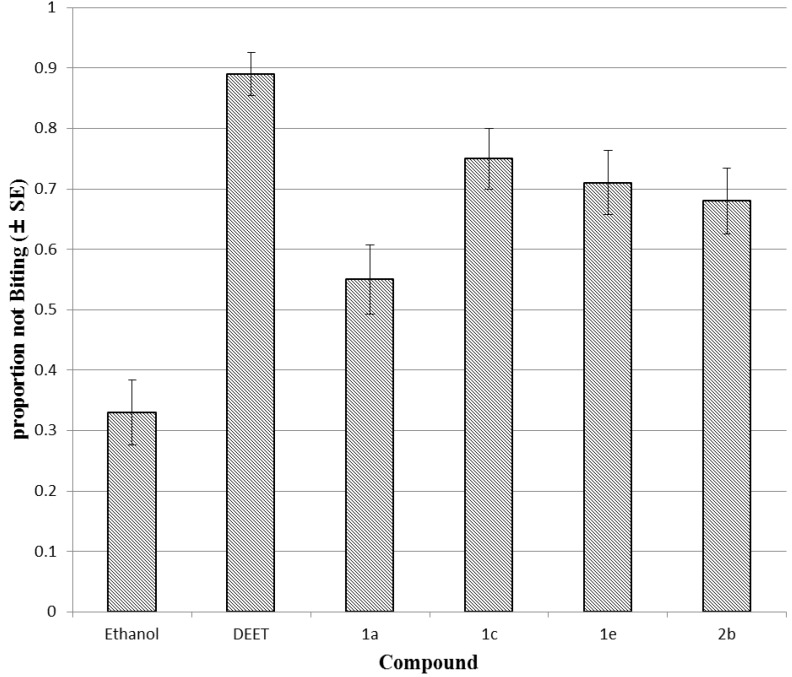
Figure 2.
Biting deterrent effects of DEET 1a, 1c and 1e and 2b at 25 nmol/cm2 against A. aegypti.
In biting deterrent bioassays with A. aegypti, compounds 1a, 2b, 1e and 1c showed significantly higher biting deterrent activity (Figure 2) than the control, while the activity in the other compounds was similar to that of the ethanol control. Compound 1c bearing a 2,4,6-trichloro group had the highest activity, with a portion not biting (PNB) value of 0.75, followed by 1e, 2b and 1a having 4-nitro-, 2,6-dichloro- and 2,4,6-trichloro-groups, with PNB values of 0.71, 0.68 and 0.55, respectively, at 25 nmol/cm2. However, biting deterrent activity in all these compounds was significantly lower than that of the reference compound DEET (PNB = 0.89).
In order to discover new mosquito toxicants, synthesized compounds were also screened against 1 d old larvae. The three compounds 1c, 3d and 3e which showed larvicidal activity in preliminary screening were selected for further study in dose response bioassays. Dose mortality data of these thiourea compounds are presented in Table 2.
Table 2.
Toxicity of substituted urea and thiourea 1,2,4-triazole derivatives 1c, 3d and 3e against 1-d old larvae of Aedes aegypti at 24-h post treatment.
| Compounds | LD50 (95% CI) * | LD90 (95% CI) * | Chi square | DF |
|---|---|---|---|---|
| 1c | 118.8 (105.3–135.2) | 216.4 (182.4–280.4) | 61.7 | 38 |
| 3d | 67.4 (59.0–77.0) | 139.4 (116.4–180.6) | 75.0 | 38 |
| 3e | 165.6 (141.7–205.2) | 370.95 (278.5–619.3) | 40.3 | 38 |
* LD values are in ppm; 95% CI = 95% confidential intervals.
Compound 3d carrying a 4-trifluorophenyl group (LD50 value of 67.9 ppm) showed the highest mortality, followed by 1c having a 4-methylsulfanylphenyl group on the thiourea moiety (LD50 = 118.8 ppm) and 3e with a 4-nitrophenyl moiety (LD50 = 165.6). The comparison of larvicidal activity of thiourea and urea derivatives showed that thiourea derivatives were much more potent than the corresponding urea derivatives. There was not not much increase in mortality at 48 h post treatment.
All synthesized compounds were tested for their cytotoxicity and anti-inflammatory activity in cellular assays. No anti-inflammatory activity was observed against NF-κB mediated transcription induced by phorbol myristate acetate (PMA) in human chondrosarcoma cells up to the highest tested concentration of 25 μg/mL and no cytotoxicity was observed against mammalian kidney cells (Vero and LLC-PK11) or human solid tumor cells (SK-MEL, SK-OV-3, BT-549 and KB) up to a highest concentration of 25 μg/mL, except for compound 3e having 4-nitro group, which showed mild cytotoxicity with IC50 values in the range of 11.0–25.0 μg/mL towards most of the tested cell lines.
3. Experimental
3.1. General
Reactions were monitored by thin layer chromatography (TLC) performed on 60 F-254 silica gel plates with visualization by UV-light using chloroform and methanol as solvent system and purity of the products was checked by High Performance Liquid Chromatography (HPLC, Agilent Technologies, Palo Alto, CA, USA). Melting points were determined on a SMP II apparatus (Schorpp Geaetetechnik Gehrden, Germany). The UV spectra were measured with a Shimadzu UV–2100 S (Shimadzu, Kyoto, Japan).The IR spectra were recorded on a Shimadzu FTIR 8400S spectrometer (Shimadzu, Kyoto, Japan). 1H-NMR spectra were recorded at 400 MHz on Bruker Avance-DPX-400 spectrometer (Bruker Spectrospin, Billerica, MA, USA) in DMSO-d6. Chemical shifts were recorded in parts per million (ppm) downfield from TMS. The splitting patterns of 1H-NMR were designated as follows: s: singlet, d: doublet, t: triplet, q: quartet, m: multiplet. Mass spectra were recorded on an Agilent 1100 LC-MS series system (Agilent Technologies, Palo Alto, CA, USA) in the electrospray mode. Elemental analysis was performed on a Leco CHNS-932 analyzer (Leco, Michigan, MI, USA). All chemicals and solvents were procured from Merck or Aldrich (Darmstad and Steinheim, Germany). The thiourea derivatives 1a–e were prepared by reacting 4-(aminophenyl)acetic acid with the corresponding isothiocyanate. Spectroscopic characterisation of 1a–e has been previously reported [23].
3.1.1. General Procedure for the Preparation of 2a–e
4-(Aminophenyl)acetic acid (0.500 g, 3.3 mmol) was dissolved in acetone (5 mL) at 100 °C. Then, a solution of the corresponding isocyanate (3.3 mmol) in acetone (5 mL) was added in three portions separated by 30 min. After 6–8 h, the reaction was finalized according to TLC monitoring. Solid material was filtered off and recrystallized from a suitable solvent.
(4-{[(2,4,6-Trichlorophenyl)carbamoyl]amino}phenyl)acetic acid (2a). UV λmax. (EtOH) nm (log ε): 251 (4.45). IR (νmax, cm−1): 3286 (N-H, O-H), 3286 (C-H), 1687, 1651 (C=O), 1543, 1510 (N-H). 1H-NMR δ: 3.48 (2H, s, -CH2), 6.99–7.83 (8H, m, Ar-H), 8.22 (1H, s, -NH-), 8.94 (1H, s, -NH-), 12.24 (1H, s, OH). Anal. Calcd. for C15H11Cl3N2O3; C: % 48.22; H: % 2.97; N: % 7.50 Found: C: % 47.95; H: % 3.00; N: % 7.30. MS-ES (m/z): 374.62 (MH+).
(4-{[(2,6-Dichlorophenyl)carbamoyl]amino}phenyl)acetic acid (2b). UV λmax. (EtOH) nm (log ε): 251 (4.41). IR (υmax, cm−1): 3252 (N-H, O-H), 3016 (C-H), 1697, 1651 (C=O), 1573, 1545 (N-H). 1H-NMR δ: 3.49 (2H, s, -CH2), 7.09–7.60 (7H, m, Ar-H), 8.18 (1H, s, -NH-), 8.91 (1H, s, -NH-), 12.24 (1H, s, OH). Anal. Calcd. for C15H12Cl2N2O3; C: % 53.12; H: % 3.57; N: % 8.26 Found: C: % 52.84; H: % 3.55; N: % 8.15. MS-ES (m/z): 340.18 (MH+).
4-{[(4-(Methylsulfanyl)phenyl)carbamoyl]amino}phenyl)acetic acid (2c). UV λmax. (EtOH) nm (log ε): 279 (3.12). IR (νmax, cm−1): 3290 (N-H, O-H), 3007 (C-H), 1695,1629 (C=O), 1550 (N-H). 1H-NMR δ: 3.35 (3H, s, CH3), 3.54 (2H, s, -CH2), 7.02–7.62 (8H, m, Ar-H), 8.63 (1H, s, -NH-), 8.70 (1H, s, -NH-), 12.24 (1H, s, OH). Anal. Calcd. for C16H16N2O3S; C: % 60.74; H: % 5.10; N: % 8.85, S: 10.14 Found: C: % 60.54; H: % 5.09; N: % 8.73, S: 10.00. MS-ES (m/z): 317.37 (MH+).
(4-{[(4-Trifluoromethylphenyl)carbamoyl]amino}phenyl)acetic acid (2d). UV λmax. (EtOH) nm (log ε): 261 (4.06). IR (νmax, cm−1): 3304 (N-H, O-H), 3304 (C-H), 1697,1630 (C=O), 1552, 1512 (N-H). 1H-NMR δ: 3.48 (2H, s, -CH2), 6.81–7.42 (8H, m, Ar-H), 8.43 (1H, s, -NH-), 8.53 (1H, s, -NH-), 12.21 (1H, s, OH). Anal. Calcd. for C16H13F3N2O3; C: % 56.81; H: % 3.87; N: % 8.28 Found: C: % 56.60; H: % 3.72; N: % 8.16. MS-ES (m/z): 339.28 (MH+).
(4-{[(4-Nitrophenyl)carbamoyl]amino}phenyl)acetic acid (2e). Compound 2e was previously synthesized by Deny et al. [25]. UV λmax. (EtOH) nm (log ε): 249 (4.08). IR (νmax, cm−1): 3367 (N-H, O-H), 3308 (C-H), 1672, 1640 (C=O), 1556,1496 (N-H). 1H-NMR δ: 3.25 (2H, s, -CH2), 7.11–8.30 (8H, m, Ar-H), 8.70 (1H, s, -NH-), 9.33 (1H, s, -NH-), 12.13 (1H, s, OH). Anal. Calcd. for C15H13N3O5: C: % 57.14; H: % 4.16; N: % 13.33 Found: C: % 57.27; H: % 4.10; N: % 13.38. MS-ES (m/z): 316.28 (MH+).
3.1.2. General Procedure for the Preparation of 3a–e and 4a–e
Equimolar mixtures of thiocarbohydrazide (0.1 mol) and thiourea or urea derivatives 1a–e, 2a–e were heated in an oil-bath at 130–140 °C for 2–3 h. The fused mass obtained was dispersed with hot water to obtain the new thiourea and urea derivatives 3a–e, 4a–e containing 1,2,4-triazole moieties. The products were recrystallized from methanol.
1-{4-[(4-Amino-5-thioxo-4,5-dihydro-1H-1,2,4-triazole-3-yl)methyl]phenyl}-3-(2,4,6-trichlorophenyl)-thiourea (3a). UV λmax. (EtOH) nm (log ε): 258 (3.97). IR (νmax, cm−1): 3252 (N-H), 3020 (C-H) 1651 (C=N), 1600, 1510 (N-H), 1345, 1246 (C=S), 1182 (C-N). 1H-NMR δ: 3.20 (2H, s, -CH2), 5.48 (2H, s, NH2), 7.20–7.46 (6H, m, Ar-H), 7.83 (1H, s, -NH-), 8.55 (1H, s, -NH-), 11.70 (1H, s, triazole NH). Anal. Calcd. for C16H13Cl3N6S2; C: % 41.79; H: % 2.85; N: % 18.28; S: % 13.95 Found: C: % 41.64; H: % 3.09; N: % 18.25; S: % 13.72. MS-ES (m/z): 460.80 (MH+).
1-{4-[(4-Amino-5-thioxo-4,5-dihydro-1H-1,2,4-triazole-3-yl)methyl]phenyl}-3-(2,6-dichlorophenyl)-thiourea (3b). UV λmax. (EtOH) nm (log ε): 278 (3.51). IR (νmax, cm−1): 3194 (N-H), 3012 (C-H), 1650 (C=N), 1541 (N-H), 1350, 1240 (C=S), 1180 (C-N). 1H-NMR δ: 3.47 (2H, s, -CH2), 5.50 (2H, s, NH2), 7.15–7.59 (7H, m, Ar-H), 9.68 (1H, s, -NH-), 9.79 (1H, s, -NH-), 11.80 (1H, s, triazole NH). Anal. Calcd. for C16H14Cl2N6S2; C: % 45.18; H: % 3.32; N: % 19.76; S: % 15.08. Found: C: % 45.10; H: % 3.30; N: % 19.66; S: % 15.00. MS-ES (m/z): 426.36 (MH+).
1-{4-[(4-Amino-5-thioxo-4,5-dihydro-1H-1,2,4-triazole-3-yl)methyl]phenyl}-3-(4-(methylsulfanyl)-phenyl)thiourea (3c). UV λmax. (EtOH) nm (log ε): 282 (3,30). IR (νmax, cm−1): 3220 (N-H), 3026 (C-H), 1537 (N-H), 1345, 1234 (C=S), 1178 (C-N). 1H-NMR δ: 3.30 (3H, s, CH3), 3.49 (2H, s, -CH2), 5.10 (2H, s, NH2), 6.90–7.40 (8H, m, Ar-H), 9.40 (1H, s, -NH-), 9.46 (1H, s, -NH-), 12.00 (1H, s, triazole NH). Anal. Calcd. for C17H18N6OS2; C: % 50.72; H: % 4.51; N: % 20.88; S: % 23.90. Found: C: % 50.27; H: % 4.70; N: % 20.69; S: % 23.92. MS-ES (m/z): 403.56 (MH+).
1-{4-[(4-amino-5-thioxo-4,5-dihydro-1H-1,2,4-triazole-3-yl)methyl]phenyl}-3-(4-(trifluoromethyl)-phenyl)thiourea (3d). UV λmax. (EtOH) nm (log ε): 257 (4.62). IR (νmax, cm−1): 3288 (N-H, C-H), 1697 (C=N), 1627, 1552 (N-H), 1346, 1294 (C=S), 1188 (C-N). 1H-NMR δ: 3.41 (4H, d, -NH2, -CH2), 7.05–7.52 (8H, m, Ar-H), 8.45 (1H, s, -NH-), 8.69 (1H, s, -NH-), 12.30 (1H, s, triazole NH). Anal. Calcd. for C17H15F3 N6S2; C: % 48.10; H: % 3.56; N: % 19.80; S: % 15.11 Found: C: % 50.29; H: % 4.12; N: % 22.50; S: % 17.17. MS-ES (m/z): 425.47 (MH+).
1-{4-[(4-Amino-5-thioxo-4,5-dihydro-1H-1,2,4-triazole-3-yl)methyl]phenyl}-3-(4-nitrophenyl)thiourea (3e). UV λmax. (EtOH) nm (log ε): 246 (4.06). IR (νmax, cm−1): 3280 (N-H), 3023 (C-H), 1593 (C-NO2 assymetrical stretching), 1494 (N-H), 1320, 1298 (C=S), 1170 (C-N). 1H-NMR δ:3.55 (2H, s, -CH2), 5.25 (2H, s, NH2), 7.11–8.36 (8H, m, Ar-H), 9.20 (1H, s, -NH-), 9.40 (1H, s, -NH-), 12.20 (1H, s, triazole NH). Anal. Calcd. for C16H15N7O2S2; C: % 47.87; H: % 3.77; N: % 24.42; S: % 15.97. Found: C: % 47.80; H: % 3.74; N: % 24.40; S: % 15.96. MS-ES (m/z): 402.47 (MH+).
1-{4-[(4-Amino-5-thioxo-4,5-dihydro-1H-1,2,4-triazole-3-yl)methyl]phenyl}-3-(2,4,6-trichlorophenyl)-urea (4a). UV λmax. (EtOH) nm (log ε): 253 (3.24). IR (νmax, cm−1): 3271 (N-H, C-H), 1651 (C=O), 1595 (C=N), 1539, 1508 (N-H), 1284–1253 (C=S), 1182 (C-N). 1H-NMR δ: 4.96 (4H, s, -NH2, -CH2), 7.15–7.79 (6H, m, Ar-H), 9.12 (1H, s, -NH-), 9.63 (1H, s, -NH-). Anal. Calcd. for C16H13Cl3N6OS; C: % 43.31; H: % 2.95; N: % 18.94; S: % 7.23 Found: C: % 42.95; H: % 2.91; N: % 19.03; S: % 7.24. MS-ES (m/z): 444.74 (MH+).
1-{4-[(4-Amino-5-thioxo-4,5-dihydro-1H-1,2,4-triazole-3-yl)methyl]phenyl}-3-(2,6-dichlorophenyl)-urea (4b). UV λmax. (EtOH) nm (log ε): 263 (4.60). IR (νmax, cm−1): 3300 (N-H, C-H), 1699 (C=O), 1633 (C=N), 1591, 1541 (N-H), 1228 (C=S), 1089 (C-N). 1H-NMR δ: 3.47 (4H, s, -NH2, -CH2), 7.10–7.58 (7H, m, Ar-H), 9.29 (1H, s, -NH-), 9.48 (1H, s, -NH-). Anal. Calcd. for C16H14Cl2N6OS2; C: % 45.18; H: % 3.45; N: % 20.53; S: % 7.83 Found: C: % 47.00; H: % 3.42; N: % 20.50; S: % 7.92. MS-ES (m/z): 410.29 (MH+).
1-{4-[(4-Amino-5-thioxo-4,5-dihydro-1H-1,2,4-triazole-3-yl)methyl]phenyl}-3-(4-methylsulfanyl-phenyl)urea (4c). UV λmax. (EtOH) nm (log ε): 261 (4.06). IR (νmax, cm−1): 3304 (N-H, O-H), 3304 (C-H), 1697, 1633 (C=O), 1552,1512 (N-H). 1H-NMR δ: 3.32 (3H, s, CH3), 3.48 (2H, s, -CH2), 5.20 (2H, s, -NH2), 6.81–7.42 (8H, m, Ar-H), 8.43 (1H, s, -NH-), 8.53 (1H, s, -NH-), 12.40 (1H, s, triazole NH). Anal. Calcd. for C17H18N6OS; C: % 52.83; H: % 4.69; N: % 21.70; S: 16.59. Found: C: % 52.70; H: % 4.49; N: % 21.67; S: 16.50. MS-ES (m/z): 387.49 (MH+).
1-{4-[(4-Amino-5-thioxo-4,5-dihydro-1H-1,2,4-triazole-3-yl)methyl]phenyl}-3-(4-trifluoromethyl-phenyl)urea (4d). UV λmax. (EtOH) nm (log ε): 258 (4.33). IR (νmax, cm−1): 3290 (N-H), 1699 (C=O), 1556 (C=N), 1510, 1494 (N-H), 1211-1186 (C=S), 1151, 1095 (C-N). 1H-NMR δ: 3.42 (4H, d, -NH2, -CH2), 7.02–7.66 (8H, m, Ar-H), 8.65 (2H, d, -NH-, -NH-), 12.50 (1H, s, triazole NH). Anal. Calcd. for C17H15F3 N6OS; C: % 50.00; H: % 3.70; N: % 20.58; S: % 7.85 Found: C: % 50.10; H: % 3.67; N: % 20.50; S: % 7.89. MS-ES (m/z): 409.40 (MH+).
1-{4-[(4-Amino-5-thioxo-4,5-dihydro-1H-1,2,4-triazole-3-yl)methyl]phenyl}-3-(4-nitrophenyl)urea (4e). UV λmax. (EtOH) nm (log ε): 253 (3,36). IR (νmax, cm−1): 3290 (N-H, C-H), 1695 (C=O), 1629 (C=N), 1589, 1545 (N-H), 1296 (C=S), 1111 (C-N). 1H-NMR δ: 3.48 (2H, s, -CH2), 5.40 (2H, s, -NH2), 7.01–7.62 (8H, m, Ar-H), 8.52 (1H, s, -NH-), 9.93 (1H, s, -NH-), 13.48 (1H, s, triazole NH). Anal. Calcd. for C16H15FN7O3S; C: % 49.86; H: % 3.92; N: % 25.44; S: % 8.32 Found: C: % 50.08; H: % 4.08; N: % 25.37; S: % 8.26. MS-ES (m/z): 386.40 (MH+).
3.2. Biological Activity
3.2.1. Antifungal Activity
A standardized 96-well micro-dilution broth assay as developed by Wedge and Kuhajek [26] was used to evaluate the antifungal activity of the compounds towards Botrytis cinerea, Colletotrichum acutatum, C. fragariae, C. gloeosporioides, P. viticola, P. obscurans and Fusarium oxysporum. The fungicide captan was used as an internal fungicide standard in all assays. Each fungus was challenged in duplicate in a dose-response format using test compounds where the final treatment concentrations were 0.3, 3.0 and 30.0 μM. Microtiter plates (Nunc MicroWell, untreated, Roskilde, Denmark) were covered with a plastic lid and incubated in a growth chamber as described previously [27]. Fungal growth was then evaluated by measuring absorbance of each well at 620 nm using a microplate photometer (Packard Spectra Count, Packard Instrument Co., Downers Grove, IL, USA). Sixteen wells containing broth and inoculum served as positive growth controls, eight wells containing solvent at the appropriate concentration and broth without inoculum were used as negative growth controls. The experiments were repeated at three times over a period of time. Mean absorbance values and standard errors were used to evaluate fungal growth at 48 h and 72 h except for P. obscurans and P. viticola where the data were recorded at 144 h. Means for percent inhibition of each fungus at each dose of test compound relative to the untreated positive growth controls were used to evaluate fungal growth. The SAS Proc ANOVA module [28] was used to identify significant factors, and Fisher’s protected LSD was used to separate means [29].
3.2.2. Mosquito Larval Bioassay
Aedes aegypti (L.) used in these studies were from a laboratory colony maintained since 1952 at the Mosquito and Fly Research Unit at Center for Medical, Agricultural and Veterinary Entomology, USDA-ARS, Gainesville, FL, USA. This colony is maintained using standard procedures [30]. Bioassays to determine the larvicidal activity of urea and thiourea derivatives against A. aegypti were conducted by using the system described by Pridgeon et al. [31]. In brief, the eggs were hatched under vacuum (about 1 h) by placing a piece of a paper towel with eggs in a cup filled with 100 mL of deionized water containing small quantity of larval diet. Larvae were removed from vacuum and held overnight in the cup in a temperature-controlled room maintained at a temperature of 27 ± 2 °C and 60 ± 10% RH at a photoperiod regimen of 12:12 (L:D) h. Five 1-d-old first instar A. aegypti were added to each well of 24-well plates in a droplet of water using a disposable 22.5-cm Pasteur pipette by placing on an illuminated light box. One mL deionized water was added to each well using a Finnpipette stepper (Thermo Fisher, Vantaa, Finland). Fifty µL of larval diet (2% slurry of 3:2 beef liver powder (Now Foods, Bloomingdale, IL, USA) and brewer’s yeast (Lewis Laboratories Ltd., Westport, CT, USA) were then added to each well. All chemicals to be tested were diluted in dimethyl sulfoxide (DMSO). Eleven microliters of the test chemical was added to the labeled wells, and in control treatments 11 µL of DMSO alone was added. After the treatment, the plates were swirled in clockwise and counter clockwise motions and front and back and side to side five times to ensure even mixing of the chemicals. Larval mortality was recorded 24- and 48-h after treatment. Larvae that showed no movement in the well after manual disturbance of water by a pipette tip were recorded as dead. A series of concentrations were used in each treatment to get a range of mortality. Treatments were replicated 15 times for each compound. LD50 values for larvicidal data were calculated by using the SAS Proc Probit module [28]. Control mortality was corrected by using Abbott’s formula.
3.2.3. Mosquito Biting Bioassays
Experiments were conducted using a six-celled in vitro Klun & Debboun (K & D) module bioassay system as developed by Klun et al. [32] for quantitative evaluation of bite deterrent properties of candidate compounds. The term deterrent refers to a chemical that inhibits feeding when present in a place where the insects feed in its absence and the repellent is a chemical that causes insects to make oriented movement away from its source (Dethier [33]). Briefly, the assay system consists of a six well reservoir with each of the 3 cm × 4 cm wells containing 6 mL of blood. As described by Ali et al. [34], a feeding solution consisting of CPDA-1 and ATP was used instead of blood. N,N-diethyl-meta-toluamide (DEET) was obtained from Sigma Aldrich (St. Louis, MO, USA) and used as a positive control. Molecular biology grade ethanol was obtained from Fisher Scientific Chemical Co. (Fairlawn, NJ, USA). All synthesized compounds (Table 1) were screened in this study and DEET at 25 nmol/cm2 was used as positive control. All the treatments were freshly prepared in ethanol at the time of bioassay.
The temperature of the solution in the reservoirs was maintained at 37.5 °C by continuously passing the warm water through the reservoir using a circulatory bath. The reservoirs were covered with a layer of collagen membrane. The test compounds were randomly applied to six 4 cm × 5 cm areas of organdy cloth and positioned over the membrane-covered CPDA-1+ATP solution with a separator placed between the treated cloth and the six-celled module. A six celled K & D module containing five females per cell was positioned over cloth treatments covering the six CPDA-1+ATP solution membrane wells, and trap doors were opened to expose the treatments to these females. The number of mosquitoes biting through cloth treatments in each cell was recorded after a 3 min exposure and mosquitoes were prodded back into the cells. These mosquitoes were then squashed to determine the number which has actually engorged the solution. A replicate consisted of six treatments: four test compounds, DEET (a standard biting deterrent) and ethanol treated cloth as solvent control. A set of five replications was conducted on different days using a new batch of females in each replication. Treatments were replicated 15 times.
3.2.4. Cytotoxicity Activity
In vitro cytotoxicity was determined against a panel of human cancer cell lines (SK-MEL, KB, BT-549, SK-OV-3) as well as noncancerous pig kidney epithelial (LLC-PK11) and monkey kidney fibroblast (VERO) cells. The assay was performed in 96-well tissue culture-treated microplates. Cells were seeded to the wells of the plate (25,000 cells/well) and incubated for 24 hours. Samples were added and again incubated for 48 h. The number of viable cells was determined using the supravital dye Neutral Red according to the procedure described earlier [35]. Doxorubicin was used as control drug for cytotoxicity.
3.2.5. Anti-Inflammatory Activity
Inhibition of NF-κB mediated transcription was determined in human chondrosarcoma (SW1353) cells by a reporter gene assay as described earlier [36,37]. In brief, at about 75% confluency, cells were harvested and transfected with NF-κB reporter luciferase plasmid construct at 160 V and one 70-ms pulse in a BTX Electro Square Porator T 820. Transfected cells were plated in 96-well plates (1 × 105 cells/well) and incubated for 24 h. After 24 h, cells were treated with test samples and after 30 min treated with PMA (70 ng/mL) for the activation of NF-κB and incubated for 8 h. After removing medium, cells were lysed by adding 40 µL of a 1:1 mixture of LucLite reagent and PBS containing 1 mM calcium and magnesium. Luciferase activity was measured as light output on a SpectraMax plate reader. Sp-1 was used as a control transcription factor to evaluate the toxicity of tested compounds in the same assay and parthenolide was used as a positive control as described earlier [37].
4. Conclusions
The molecules described in this study demonstrated no cytotoxicity and no anti-inflammatory activity, but some of them showed good antifungal activity against Phomopsis species. Based on the biological activity evaluation these compounds may not be useful as therapeutic or pharmaceutical agents. However, selective compounds exhibiting antifungal activity could have a potential to be developed as agrochemicals, and further work should be performed to modify their structure with an aim to increase their activity. Phomopsis appeared to be the most sensitive fungal species to the tested compounds. Phomopsis cane and leaf spot (P. viticola) causes serious economic losses to the vine grape production in the United States of America and Europe, while P. obscurans causes Phomopsis leaf blight and fruit rot of strawberry. Compound 3d was the most active derivative among the tested compounds in a larvicidal assay against A. aegypti. The comparison of antifungal and insecticidal activities of thiourea and urea derivatives showed that thiourea derivatives bearing 2,6-dichlorophenyl, 2,4,6-trichlorophenyl, 4-nitophenyl, 4-trifluoromethylphenyl substituents were the active compounds. Thiourea compounds, 1b, 1c, 3a, 3d, 3e, and urea compound 4e show sufficient potential to warrant further modification to discover new antifungal and insecticidal molecules.
Acknowledgments
The authors wish to thank Marmara University Scientific Research Projects Commission (BAPKO, SAG-C-YLP-270109-0013, 2009 and SAG-D-200611-0205 2011) for financial support. This study was supported in part by USDA/ARS grant No. 56-6402-1-612 and Deployed War-Fighter Protection Research Program Grant funded by the U.S. Department of Defense through the Armed Forces Pest Management Board. We thank Jessie Linda Robertson, Ramona Pace, John Trott, and Katherine Martin for the bioassays. We thank James J. Becnel, Mosquito and Fly Research Unit, Center for Medical, Agricultural and Veterinary Entomology (USDA-ARS, Gainesville, FL, USA) for supplying A. aegypti eggs.
Footnotes
Sample Availability: Contact the authors.
References
- 1.Vega-Pérez J.M., Periñán I., Argandoña M., Vega-Holm M., Palo-Nieto C., Burgos-Morón E., López-Lázaro M., Vargas C., Nieto J.J., Iglesias-Guerra F. Isoprenyl-thiourea and urea derivatives as new farnesyl diphosphate analogues: Synthesis and in vitro antimicrobial and cytotoxic activities. Eur. J. Med. Chem. 2012;58:591–612. doi: 10.1016/j.ejmech.2012.10.042. [DOI] [PubMed] [Google Scholar]
- 2.Yao J., Chen J., He Z., Sun W., Xu W. Design, synthesis and biological activities of thiourea containing sorafenib analogs as antitumor agents. Bioorg. Med. Chem. 2012;20:2923–2929. doi: 10.1016/j.bmc.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 3.Shantharam C.S., Suyoga V.D.M., Suhas R., Sridhara M.B., Channe G.D. Inhibition of protein glycation by urea and thiourea derivatives of glycine/proline conjugated benzisoxazole analogue—Synthesis and structure-activity studies. Eur. J. Med. Chem. 2013;60:325–332. doi: 10.1016/j.ejmech.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 4.Yang W., Hu Y., Yang Y.S., Zhang F., Zhang Y.B., Wang X.L., Tang J.F., Zhong W.Q., Zhu H.L. Design, modification and 3D QSAR studies of novel naphthalin-containing pyrazoline derivatives with/without thiourea skeleton as anticancer agents. Bioorg. Med. Chem. 2013;21:1050–1063. doi: 10.1016/j.bmc.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Keche A.P., Hatnapure G.D., Tale R.H., Rodge A.H., Kamble V.M. Synthesis, anti-inflammatory and antimicrobial evaluation of novel 1-acetyl-3,5-diaryl-4,5-dihydro (1H) pyrazole derivatives bearing urea, thiourea and sulfonamide moieties. Bioorg. Med. Chem. 2012;22:6611–6615. doi: 10.1016/j.bmcl.2012.08.118. [DOI] [PubMed] [Google Scholar]
- 6.Burgeson J.R., Moore A.L., Boutilier J.K., Cerruti N.R., Gharaibeh D.N., Lovejoy C.E., Amberg S.M., Hruby D.E., Tyavanagimatt S.R., Allen R.D., et al. Analysis of a series of acylthiourea derivatives possessing broad-spectrum antiviral. Bioorg. Med. Chem Lett. 2012;22:4263–4272. doi: 10.1016/j.bmcl.2012.05.035. [DOI] [PubMed] [Google Scholar]
- 7.Upadhayaya R.S., Kulkarni G.M., Vasireddy N.R., Vandavasi J.K., Dixit S.S., Sharma V. Chattapadhayaya, Design, synthesis and biological evaluation of novel triazole, urea and thiourea derivatives of quinoline against Mycobacterium tuberculosis. Bioorg. Med. Chem. 2009;17:4681–4692. doi: 10.1016/j.bmc.2009.04.069. [DOI] [PubMed] [Google Scholar]
- 8.Khan S.A., Singh N., Saleem K. Synthesis, characterization and in vitro antibacterial activity of thiourea and urea derivatives of stereoids. Eur. J. Med. Chem. 2008;43:2272–2277. doi: 10.1016/j.ejmech.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Sett P.P., Ranken R., Robinson D.E., Osgood S.A., Risen L.M., Rodgers E.L., Migawa M.T., Jefferson E.A., Swayze E.E. Aryl urea analogs with broad-spectrum antibacterial activity. Bioorg. Med. Chem. Lett. 2004;14:5569–5572. doi: 10.1016/j.bmcl.2004.08.059. [DOI] [PubMed] [Google Scholar]
- 10.Koçyiğit-Kaymakçıoğlu B., Rollas S., Körceğez E., Arıcıoğlu F. Synthesis and bological evaluation of new N-substituted-N′-(3,5-di/1,3,5-trimethylpyrazole-4-yl)thiourea/urea derivatives. Eur. J. Pharm. Sci. 2005;26:97–103. doi: 10.1016/j.ejps.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Holla B.S., Veerenda B., Shivananda K., Boja P. Synthesis characterization and anticancer activity studies on some Mannich bases derived from 1,2,4-triazoles. Eur. J.Med. Chem. 2003;38:759–767. doi: 10.1016/S0223-5234(03)00128-4. [DOI] [PubMed] [Google Scholar]
- 12.Abdel-Megeed A.M., Abdel-Rahman H.M., Alkaramany G.E., El-Gendy M.A. Design, synthesis and molecular modeling study of acylated 1,2,4-triazole-3-acetates with potential anti-inflammatory activity. Eur. J. Med. Chem. 2009;44:117–123. doi: 10.1016/j.ejmech.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Kaplancikli Z.A., Turan-Zitouni G., Ozdemir A., Revial G. New triazole and triazolothiadiazine derivatives as possible antimicrobial agents. Eur. J. Med. Chem. 2008;43:155–159. doi: 10.1016/j.ejmech.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 14.Koçyiğit-Kaymakçıoğlu B., Çalışır M.M., Özbek B., Ötük G. Synthesis and antimicrobial activities of schiff bases derived from 4-amino-5-(1-phenylethyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione. E. J. Chem. 2010;7:458–464. doi: 10.1155/2010/195725. [DOI] [Google Scholar]
- 15.Ashok M., Holla B.S., Boja P. Convenient one pot synthesis and antimicrobial evaluation of some new Mannich bases carrying 4-methylthiobenzyl moiety. Eur. J. Med. Chem. 2007;42:1095–1101. doi: 10.1016/j.ejmech.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Sabatelli F., Patel R., Mann P.A., Mendrick C.A., Norris C.C., Hare R., Loebenberg D., Black T.A., McNicholas P.M. In vitro activities of posaconazole, fluconazole, itraconazole, voriconazole, and amphotericin B against a large collection of clinically important molds and yeats. Antimicrob. Agents Chemother. 2006;50:2009–2015. doi: 10.1128/AAC.00163-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson E., Espinel-Ingroff A., Szekely A., Hockey H., Troke P. Activity of voriconazole, itraconazole, fluconazole and amphotericin B in vitro against 1763 yeasts from 472 patients in the voriconazole phase III clinical studies. Int. J. Antimic. Agents. 2008;32:511–514. doi: 10.1016/j.ijantimicag.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Fernandez E., Manzano J.L., Benito J.J., Hermosa R., Monte E., Criado J.J. Thiourea, trizole and thiadiazine compounds and their metal complexes as antifungal agents. J. Inorg. Biochem. 2005;99:1558–1572. doi: 10.1016/j.jinorgbio.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Rauf M.K., Ebihara M., Imtiaz-ud-Din B.A. 1-Benzoyl-3-(2,4,5-trichloro-phenyl)thiourea. Acta Cryst. 2012 doi: 10.1107/S1600536811052780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao X., Li F., Hu M., Li M., Yu G.A., Liu S.H. Chiral γ-Aryl-1H-1,2,4-triazole derivatives as highly potential antifungal agents: Design, synthesis, structure, and in vitro fungicidal activities. J. Agric. Food Chem. 2008;56:11367–11375. doi: 10.1021/jf8026843. [DOI] [PubMed] [Google Scholar]
- 21.Cao X., Li F., Hu M., Zhang J., Li F., Yang Y., Liu D., Liu S.H. Determination of stereoselective ınteraction between enantiomers of chiral γ-Aryl-1H-1,2,4-triazole derivatives and Penicillium digitatum. J. Agric. Food Chem. 2009;57:6914–6919. doi: 10.1021/jf901554x. [DOI] [PubMed] [Google Scholar]
- 22.Crank G., Neville M., Ryden R. Thiourea derivatives of 2-aminooxazoles showing antibacterial and antifungal activity. J. Med. Chem. 1973;16:1402–1405. doi: 10.1021/jm00270a020. [DOI] [PubMed] [Google Scholar]
- 23.Celen A.Ö., Koçyiğit-Kaymakçıoğlu B., Gümrü S., Toklu H.Z., Arıcıoğlu F. Synthesis and anticonvulsant activity of substituted thiourea derivatives. Marmara Pharm. J. 2011;15:43–47. [Google Scholar]
- 24.Wedge D.E., Smith B.J., Quebedeaux J.P., Constantin R.J. Fungicide management strategies for control of strawberry fruit rot diseases in Louisiana and Mississippi. Crop Protect. 2007;26:1449–1458. doi: 10.1016/j.cropro.2006.12.007. [DOI] [Google Scholar]
- 25.Denny W.A., Cain B.F., Atwell G.J., Hansch C., Augustine Leo P.A. Potential antitumor agents. 36. Quantitative relationships between experimental antitumor activity, toxicity, and structure for the general class of 9-anilinoacridine antitumor agents. J. Med. Chem. 1982;25:276–315. doi: 10.1021/jm00345a015. [DOI] [PubMed] [Google Scholar]
- 26.Wedge D.E., Kuhajek J. Microbioassay for fungicide discovery. SAAS Bull. Biochem. Biotechnol. 1998;11:1–7. [Google Scholar]
- 27.Sobolev V.S., Khan S.I., Tabanca N., Wedge D.E., Mainly S.P., Cutler S.J., Coy M.R., Neff S.A., Gloer J.B. Biological activity of peanut (Arachis hypogaea) phytoalexins and selected natural and synthetic stilbenoids. J. Agric. Food Chem. 2011;59:1673–1682. doi: 10.1021/jf104742n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.SAS Online Document Version 9.2. SAS Institute; Cary, NC, USA: 2007. [Google Scholar]
- 29.Steel R.G.D., Torrie J.H. Principles and Procedures of Statistics: A Biometrical Approach. 2nd ed. McGraw Hill Book Company; New York, NY, USA: 1980. [Google Scholar]
- 30.Pridgeon J.W., Meepagala K.M., Becnel J.J., Clark G.G., Pereira R.M., Linthicum K.J. Structure-activity relationships of 33 piperidines as toxicants against female adults of Aedes aegypti (Diptera: Culicidae) J. Med. Entomol. 2007;44:263–269. doi: 10.1603/0022-2585(2007)44[263:SROPAT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 31.Pridgeon J.W., Becnel J.J., Clark G.G., Linthicum K.J. A high-throughput screening method to identify potential pesticides for mosquito control. J. Med. Entomol. 2009;46:335–341. doi: 10.1603/033.046.0219. [DOI] [PubMed] [Google Scholar]
- 32.Klun J.A., Kramer M., Debboun M. A new in vitro bioassay system for discovery of novel human-use mosquito repellents. J. Am. Mosq. Control Assoc. 2005;21:64–70. doi: 10.2987/8756-971X(2005)21[64:ANIVBS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 33.Dethier V.G., Browne B.L., Smith C.N. The Designation of chemicals in terms of the responses they elicit from insects. J. Econ. Entomol. 1960;53:134–136. doi: 10.1603/029.102.0606. [DOI] [PubMed] [Google Scholar]
- 34.Ali A., Cantrell C.L., Bernier U.R., Duke S.O., Schneider J.C., Khan S.I. Aedes aegypti (Diptera: Culicidae) Biting deterrence: Structure-activity relationship of saturated and unsaturated fatty acids. J. Med. Entomol. 2012 doi: 10.1603/me12026. accepted. [DOI] [PubMed] [Google Scholar]
- 35.Tabanca N., Bedir E., Kırımer N., Başer K.H.C., Khan S.I., Jacob M.R., Khan I.A. Antimicrobial compounds from Pimpinella species growing in Turkey. Planta Med. 2003;69:933–938. doi: 10.1055/s-2003-45103. [DOI] [PubMed] [Google Scholar]
- 36.Tabanca N., Ma G., Pasco D.S., Bedir E., Kırımer N., Başer K.H.C., Khan I.A., Khan S.I. Effect of essential oils and ısolated compounds from Pimpinella species on NF-kB: A target for anti-inflammatory action. Phytotherap. Res. 2007;21:741–745. doi: 10.1002/ptr.2154. [DOI] [PubMed] [Google Scholar]
- 37.Koçyiğit-Kaymakçıoğlu B., Oruç-Emre E.E., Ünsalan S., Tabanca N., Wedge D.E., Khan S.I., Demirci F., Rollas S. Synthesis and biological activities of hydrazide hydrazones and their corresponding 3-acetyl-2,5-disubstituted-1,3,4-oxadiazolines. Med. Chem. Res. 2012 doi: 10.1007/s00044-011-9882-z. [DOI] [Google Scholar]