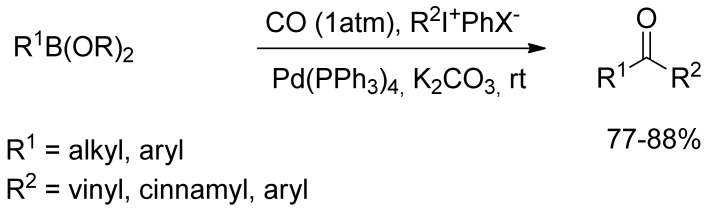Abstract
Since the first report and due to its handiness and wide scope, the Suzuki-Miyaura (SM) cross coupling reaction has become a routine methodology in many laboratories worldwide. With respect to other common transition metal catalyzed cross couplings, the SM reaction has been so far less exploited as a tool to introduce an acyl function into a specific substrate. In this review, the various approaches found in the literature will be considered, starting from the direct SM acylative coupling to the recent developments of cross coupling between boronates and acyl chlorides or anhydrides. Special attention will be dedicated to the use of masked acyl boronates, alkoxy styryl and alkoxy dienyl boronates as coupling partners. A final section will be then focused on the acyl SM reaction as key synthetic step in the framework of natural products synthesis.
Keywords: Suzuki-Miyaura cross-coupling, acylation, palladium, ketones
1. Introduction
The introduction of acyl functionalities represents a fruitful and widespread tool to construct building blocks for the synthesis of natural products and pharmaceutical compounds, and a number of approaches are well known among the synthetic organic chemistry community [1,2]. Traditionally, one potential approach is the Friedel-Crafts acylation of substituted aromatic rings [3,4,5]. However, this method entails some problems and limitations such as drastic reaction conditions, low regioselectivity, and a large amount of by-products [6]. Besides, the nucleophilic addition of organometallic compounds to carboxylic acid derivatives can be used to prepare ketones. Organotin [7], zinc [8], cadmium [9], Grignard or organolithium reagents have been successfully used as nucleophilic reagents [10], but low yields are often obtained because of tertiary alcohol formation [11]. The strategies using organoboron compound–mediated cross-coupling processes have been utilized most widely by synthetic chemists because of their non-toxicity and thermal, air, and moisture stability, and can be considered as a readily available method for new carbon–carbon bond formation in organic synthesis [12,13,14]. Suzuki-Miyaura cross-coupling has been successfully exploited to introduce an acyl function onto a specific substrate with high regioselectivity. To this purpose three different approaches can be envisaged. The first of them is the palladium-catalyzed cross-coupling reaction of arylboronic acids with carboxylic acid derivatives (see Section 2), and are based on the cleavage of the C–O bond of the carboxylic derivative in the presence of palladium catalyst. This approach is superior to the previous methods in terms of reaction conditions, efficiency, and functional group compatibility. The second one (Section 3) relies on the carbonylative version of the SM reaction. In most cases 1 atm of CO (g) and mild conditions are sufficient to promote the carbonylative coupling. The last approach (Section 4) deals with the use of masked acyl functionalities, namely masked acyl boronic esters (alkoxy dienylboronates, alkoxy styryl boronates), following a traditional cross coupling reaction with a coupling partner the original acyl function can be easily restored. The intent of this short review is to give a general overview of the most recent advances in the literature in this field.
2. Suzuki-Miyaura Coupling of Acyl Chlorides and Anhydrides
2.1. Coupling of Arylboronic Acid Derivatives with Acyl Chlorides
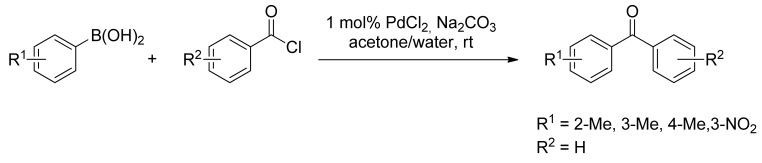
Many synthetic methods have been developed in the past for the synthesis of diaryl ketones, which are important building blocks in a large number of natural products and active pharmaceutical compounds [15,16,17]. Among them, the conversion of carboxylic acid derivatives into aryl ketones has proven to be a widely employed transformation in the natural product synthesis as well in drug discovery design processes. The most common synthetic approach to aromatic ketones is undoubtedly the Friedel-Crafts acylation of an arene [18]. However, due to its limited regioselectivity and to the incompatibility of certain functional groups, this approach often leads to hard to resolve isomeric mixtures. Moreover, the synthesis of ketones from organometallic reagents and acyl chlorides or esters often proceeds in low yield because of the addition of the organometallic reagent to the ketone to form tertiary alcohols [11,19]. Thus, the necessity of alternative approaches to the synthesis of diaryl ketones embraced the exponential growth of palladium catalyzed methods with the seminal report by Bumagin and coworkers in 1997 [20], where acyl chlorides were reacted for the first time with arylboronic acids in the presence of ligand-free palladium to form the corresponding aromatic ketones in high yields (Scheme 1). The reaction proceeded quantitatively with catalytic PdCl2 between arylboronic acids or Ph4BNa and acyl chlorides in the presence of Na2CO3 in aqueous acetone, while easily hydrolyzed acyl chlorides reacted only smoothly with Pd(OAc)2 in anhydrous acetone.
Scheme 1.
Bumagin’s first carbon monoxide free carbonylation of arylboronic acids.
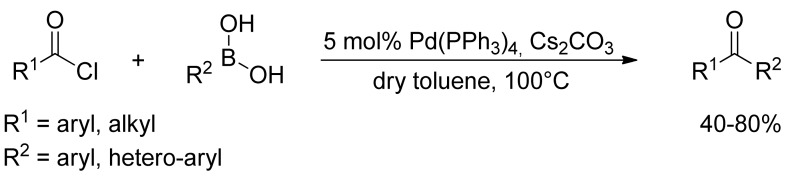
The classic Suzuki coupling reaction, which is generally carried out as a palladium-catalyzed reaction in organic solvent and water, has proven to be poorly suitable for the coupling of acyl chlorides with boronic acids. However, Haddach and coworkers [21] reported in 1999 as an alternative method the first palladium-catalyzed coupling of organoboronic acids with acyl chlorides under anhydrous reaction conditions (Scheme 2). By means of catalytic Pd(PPh3)4 and cesium carbonate in dry toluene, several symmetric and non-symmetric aromatic ketones have been synthesized in moderate to good yields.
Scheme 2.
Haddach’s carbonylation of arylboronic acids under anhydrous conditions.
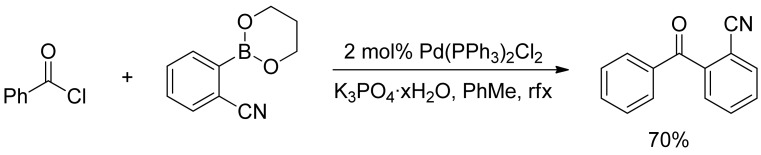
In a similar report, Bumagin and Korolev described the phosphine-free palladium-catalyzed acyldeboration of sodium tetraarylborates and arylboronic acids with acyl chlorides in dry acetone in the presence of Na2CO3 as a base and Pd(OAc)2 (1 mol%) as the catalyst precursor [22]. Few years later, Urawa and Ogura found that both Haddach’s [21] and Bumagin’s [22] anhydrous conditions were not suitable in the synthesis of ortho-cyanobenzophenone derivatives [23], where only low conversion were achieved. However, they reported in 2003 that the use of K3PO4 hydrate as a base in the reaction of boronic acids with acyl chlorides in toluene afforded the desired cyanobenzophenone in good yield, and the methodology was successfully applied to the synthesis of symmetric and asymmetric ketones in good to excellent yields (Scheme 3) [23].
Scheme 3.
Urawa’s synthesis of ortho-cyanobenzophenone derivatives.
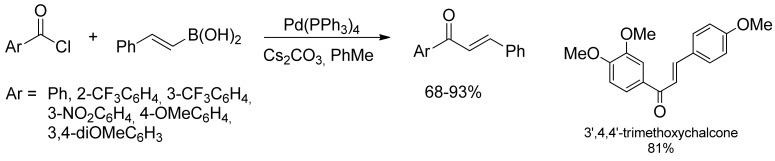
At the same time, Rolando and coworkers successfully applied Haddach’s conditions [21] in the coupling reaction between different aryl chlorides and substituted cinnamoyl chlorides leading to a variety of substituted chalcones in near quantitative yield [24]. This approach allowed the access to naturally occurring chalcones starting from suitably methoxylated substrates, as reported in Scheme 4.
Scheme 4.
Rolando’s approach to substituted chalcones derivatives by SM-coupling of cinnamyl boronic acids.
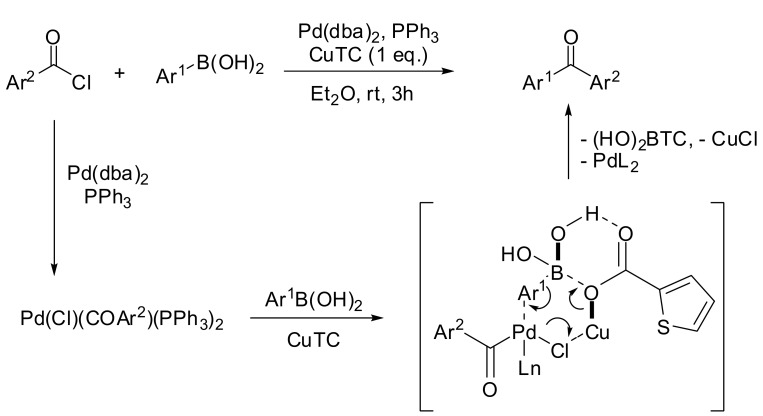
Several alternative procedures for the SM direct acylation of arylboronic acids have been investigated since the preliminary reports described above. For example, aromatic ketones were synthesized via a palladium catalyzed cross-coupling reaction of boronic acids with acyl chlorides in the presence of catalytic PdCl2 and dry Na2CO3 at room temperature under solvent-free conditions [25]. At the same time, Nishihara and colleagues reported the synthesis of unsymmetrical aromatic ketones by a Pd(dba)2 catalyzed reaction in diethyl ether in the presence of copper(I) thiophene-2-carboxylate (CuTC) as an activator [26]. These conditions were successfully applied to a wide range of substrates bearing an electron-donating or an electron-withdrawing substituent. The mechanism proposed by the authors involves a preliminary transmetalation of the acyl palladium(II) chloride by a six-membered transition state, where the soft Cu(I) ion accelerates the polarization of the Pd–Cl bond while the hard carboxylate counterion activates the boronic acid (see Scheme 5).
Scheme 5.
Copper (I)-Pd(II) promoted carbonylation of arylboronic acids.
Microwave-assisted methodologies have also been recently developed providing the ketones in high yields within short reaction times. Simple catalytic systems for cross-coupling reactions of acyl chlorides with arylboronic acids under microwave conditions (260 W, 98 °C, 10 min) were tested by Polàckovà and coworkers in 2006 [27], whereby Pd(PPh3)4 and Cs2CO3 were employed as catalytic system for the coupling in wet toluene under irradiation, affording ketones in good to high yields. In 2008 Wolf and coworkers described the SM cross-coupling of aromatic and aliphatic acyl chlorides with arylboronic acids in the presence of 2.5 mol% of (t-Bu2POH)2PdCl2 which was completed within 10 min under microwave irradiation (100 W, 80 °C), leading to benzophenone and acetophenone derivatives in good to high yields [28].
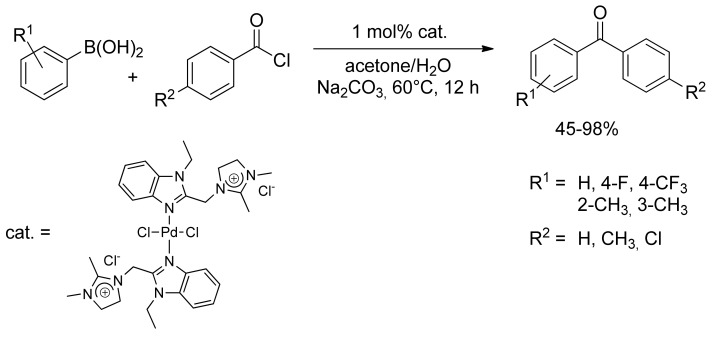
More recently, new palladium catalysts have been employed in the SM acylation of boronic acid derivatives, as reported by Li and collegues in 2011 [29]. An imidazolium chloride palladium(II) complex has proven to be active toward cross-coupling of arylboronic acid and benzoyl chloride in aqueous acetone solution, giving high yield of the desired aryl ketones (Scheme 6). Noteworthy, the phosphine-free (N–N)Pd(II) complex has been efficiently recycled more than four times almost without any loss of reactivity in the aqueous SM coupling reaction.
Scheme 6.
Li’s imidazolium catalyst applied to the synthesis of diaryl ketones.
2.2. Coupling of Arylboronic Derivatives with Anhydrides
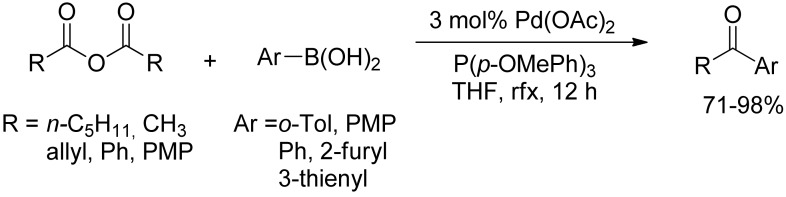
Despite the wide amount of diaryl ketones syntheses by SM-coupling reaction of arylboronic acids with acyl halides, the first development of a catalytic system capable of coupling anhydrides with boronic acids has been investigated only at the very beginning of the XXIst century with two independent reports by Yamamoto [30,31,32] and Gooßen [33]. The catalytic process described by the authors consists of the oxidative addition of the anhydride to an acyl palladium complex, exchange of the carboxylate for the aryl group by transmetalation of the boronic acid followed by reductive elimination of the ketone. The reactions were easily performed with many functionalized derivatives (Scheme 7), only required commercially available nontoxic chemicals, and produced a minimum amount of waste, thus making this approach superior to the previous methods in terms of mildness of reaction conditions, selectivity, and efficiency.
Scheme 7.
Gooßen’s SM-coupling of arylboronic acids with anhydrides.
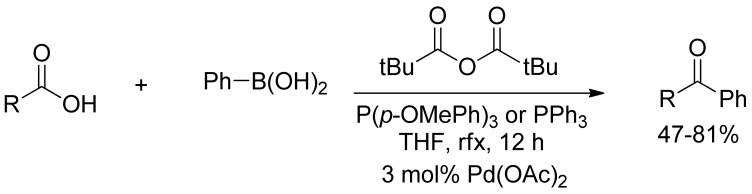
Moreover, Gooßen and coworkers reported an efficient method for the catalyzed SM-cross coupling of carboxylic acids to arylboronic acids in the presence of palladium(II) acetate (Scheme 8) [34]. This nicely described approach combined the activation and cross-coupling step of the carboxylic with boronic acids into a convenient one-pot procedure using pivalic anhydride as an activating agent, without any formation of tert-butylated ketones and formation of only pivalic acid and boric acid as side products.
Scheme 8.
Gooßen’s pivalic anhydride promoted coupling of arylboronic acids with carboxylic acids.
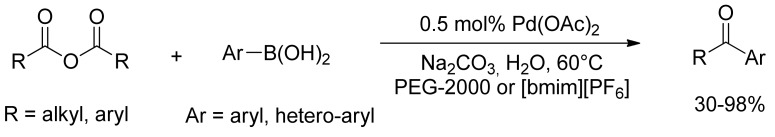
However, both Yamamoto’s and Gooßen’s studies showed that the phosphine ligands played a key role in the successful execution of the reactions, and no ketone product was obtained in the absence of phosphine ligands. Thus, the Pd(OAc)2 catalyzed coupling reaction of arylboronic acid with carboxylic anhydrides without phosphorous ligands was successfully investigated few years later. In 2006, Zhang and coworkers [35] turned out that this SM-cross coupling could be carried out smoothly in water in the presence of PEG or [bmim][PF6] to give high yields of ketones without the use of phosphine ligands with a good recycling of the catalytic system (Scheme 9). Moreover, the authors reported in 2007 that the presence of surfactants has a significant effect on the activity of the cross-coupling reaction in water in the absence of phosphine ligands [36]. Even the effect of acetone as a cosolvent in water was studied in 2008 by Xin [37], showing that a high efficiency without other additives and ligands can be achieved making the method useful and attractive for the synthesis of aryl ketones.
Scheme 9.
Zhang’s coupling of arylboronic acids with anhydrides in the presence of additives.
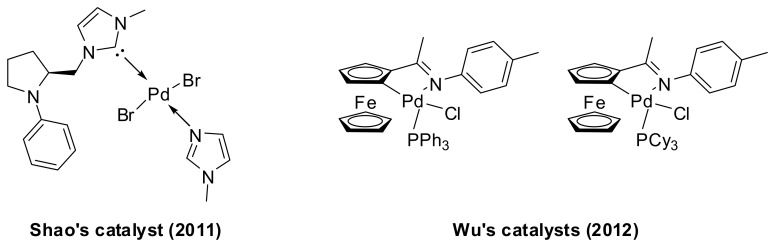
Traditional palladium catalysts for the coupling of arylboronic acids with anhydrides have been recently successfully replaced by new generation catalysts. For example, the use of coordinated monodentate NHC–Pd(II) complex derived from N-phenyl substituted proline has been reported by Shao in 2011 in the coupling reaction of arylboronic acids with benzoic anhydride in water [38], and cyclopalladated ferrocenylimines catalysts have been recently explored by Wu and coworkers [39] in the cross-coupling of arylboronic acids with carboxylic anhydrides (Figure 1).
Figure 1.
Shao’s (left) and Wu’s (right) catalysts applied in the synthesis of aromatic ketones by Pd-catalyzed coupling of arylboronic acids with anhydrides.
2.3. Coupling with Chloroformates and Carbamoyl Chlorides
Since aryl carboxylic esters and aryl carboxamides are useful building blocks both for laboratory synthesis as well as industrial processes [40], several synthetic methods for preparing these compounds have been developed in the past. Among them, metal-catalyzed carbonylation of aryl halides using carbon monoxide as the carbonyl source in the presence of amines or alcohols is the most popular way [41]. To avoid the use of toxic gaseous carbon monoxide, modified processes have been described by using transition metals complexes like nickel, molybdenum and iron as solid sources of monoxide [42,43,44]. Another attractive method for the synthesis of aryl carboxamides is the direct amidation of organometallic reagents with formamide derivatives, even if the organometallic reagents used for such an approach have been limited mainly to magnesium or lithium reagents [45,46,47,48].
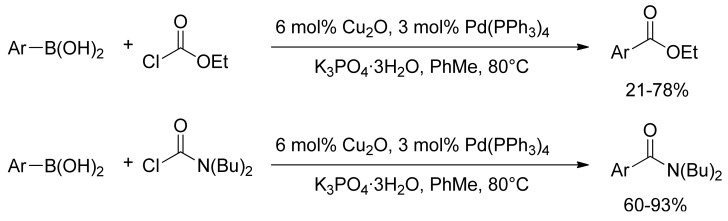
The first description of a palladium catalyzed reaction for the synthesis of this class of compounds has been reported by Deng and Duan in 2005 [49]. A convenient preparation of arylcarboxylic esters and arylcarboxamides has been described from arylboronic acids under mild conditions by means of a Suzuki-type cross-coupling reaction of arylboronic acids with ethyl chloroformate or N,N-dibutylcarbamoyl chloride in the presence of catalytic amounts of Cu2O and Pd(PPh3)4 (Scheme 10).
Scheme 10.
Pd/Cu catalyzed carboxylation and amidation of arylboronic acids derivatives.
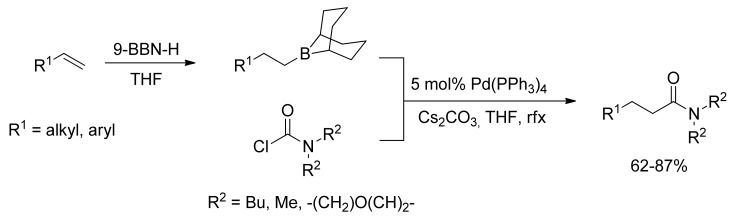
In 2007 Takemoto and coworkers reported a one-pot amidation of olefins through the first palladium-catalyzed coupling reaction of carbamoyl chlorides with alkylboranes [50]. Hydroboration of olefins and subsequent coupling of the resulting alkylboranes with carbamoyl chlorides have proven to be a promising synthetic route for the generation of amides starting from unactivated alkenes (Scheme 11). Moreover, carbamoyl chlorides have been successfully coupled with arylboronic acids in a copper-free Suzuki coupling reaction in the presence of catalytic Pd(PPh3)4.
Scheme 11.
Takemoto’s Pd-catalyzed amidation of olefins via 9-BBN intermediates.
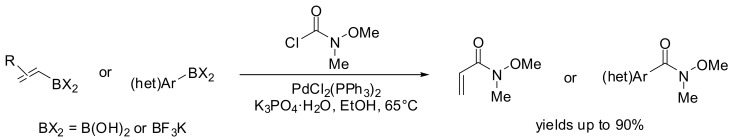
More recently, the Sukuzi-Miyaura cross-coupling of commercially available N-methoxy-N-methyl-carbamoyl chloride with both (hetero)aryl and alkenyl boronic acids or trifluoroborates has been investigated by Herr and coworkers (Scheme 12) [51]. This methodology has proven to be an improvement upon existing methods for the preparation of Weinreb amides, avoiding the use of toxic carbon monoxide in case of the metal catalyzed cross-coupling reactions approach.
Scheme 12.
Synthesis of Weinreb amides by SM-coupling of arylboronic acids/ organotrifluoro borates with N-methoxy-N-methyl-carbamoyl chloride.
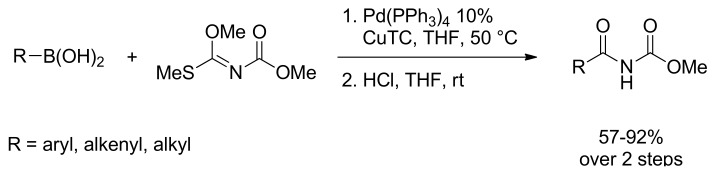
Very recently the preparation of imides via the palladium-catalyzed coupling reaction of aryl-, alkyl-, and alkenylboronic acids with N-[methoxy(methylthio)methylene]carbamate in the presence of Cu(I) thiophene-2-carboxylate (CuTC) has been described. The reaction afforded imino ethers that were easily converted to the corresponding imides by acidic hydrolysis in high yield. (Scheme 13) [52].
Scheme 13.
SM cross coupling of Methyl N-[Methoxy(methylthio)methylene]carbamate.
3. Carbonylative SM Reactions
Despite the direct acylation of organoboron derivatives has proved to be an attractive approach to the formation of symmetric and unsymmetrically substituted ketones, the carbonylative Pd-catalyzed coupling reaction has been widely employed for the synthesis of aromatic ketones [12]. Depending on the substrates used, catalytic carbonylation leads to a variety of carbonyl-containing compounds and dicarbonyl compounds. As mentioned above, diaryl ketones are target molecules of importance for organic synthesis since the diarylketo moiety occurs in many natural and synthetic biologically active molecules, as well as in non-steroid anti-inflamatory drugs [53]. Aryl halides with various coupling partners have found success including stannanes [54,55,56], magnesium [57], aluminum [58], and silane derivatives [59], as well as boronic acids [60,61].
However, the application of these methods is limited due to the side reaction which prevent the insertion of carbon monoxide into the ArPdX intermediates and promotes the formation of the Ar-Pd-Ar species to give biaryls. The use of pressured carbon monoxide is a general method for suppressing such a side reaction, which was applied in the reaction of aryltin derivatives with aryltriflates. Relatively high temperature conditions must be used to induce this kind of carbonylative coupling, and the poor reactivity of electron-deficient aryl halides makes this type of reaction less accessible. Moreover, the form of the palladium catalyst, ligands, base, additives, solvent, and temperature have been shown to have an effect on the ketone:biaryl ratio.
α,β-Unsaturated ketones are important key reagents in organic synthesis, as they are widely used as Michael acceptors in conjugated reactions [62,63,64], as activated olefins in Heck-type couplings [65] or in epoxidation reactions [66]. The most common synthetic approach proceeds through an aldol condensation reaction between one equivalent of ketone and one equivalent of an aldehyde derivative followed by a dehydration step. The reaction is extremely efficient especially for the synthesis of chalcone derivatives. Other alternative strategies have been developed to access aryl α,β-unsaturated ketones and among them, the most efficient ones has proven to be the selective oxidation of an allylic alcohol [67] and Friedel-Crafts acylation reaction of arenes using α,β-unsaturated acyl halides [68,69].
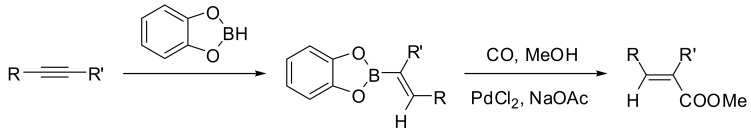
While the synthesis of diaryl ketones has been widely described [12], only a limited number of reactions involve a Pd-catalyzed carbonylation step have been reported for the synthesis of vinyl ketones. Among them, the first synthetic application of the carbonylative Pd-catalyzed cross coupling reaction for the synthesis of α,β-unsaturated systems has been described in the seminal report by Suzuki and Miyaura in 1981 [70]. Alkenylboranes were reacted with carbon monoxide in the presence of palladium chloride and sodium acetate in methanol to give the corresponding α,β-unsaturated carboxylic esters with retention of configuration with respect to alkenylboranes in good yields (Scheme 14).
Scheme 14.
Suzuki-Miyaura carbomethoxylation of alkenylborane derivatives.
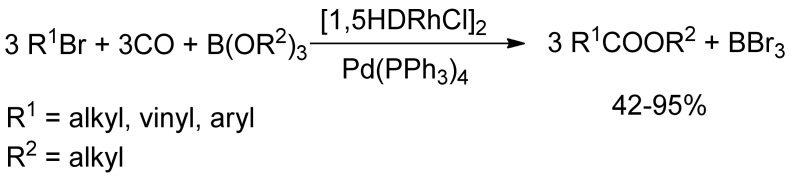
Few years later, Alper and coworkers [71] reported in a similar way the synthesis of α,β-unsaturated esters by reaction of alkyl, vinyl and aromatic bromides with organoborates and carbon monoxide, in the presence of catalytic bimetallic system of 1,5-hexadienerhodium(I) chloride dimer and tetrakis(triphenylphosphine) palladium (Scheme 15).
Scheme 15.
Alper’s Rh/Pd catalyzed carbomethoxylation of alkyl and vinyl bromides.
In 1998 Kang and coworkers [72] described the Pd-catalyzed carbonylative cross-coupling of organoboranes with hypervalent iodonium salts in the presence of Pd(PPh3)4 and potassium carbonate under an atmospheric pressure of carbon monoxide at room temperature (Scheme 16). The reaction was successfully applied with aryl-, alkenyl-, and alkynyliodonium salts at room temperature affording unsymmetric aromatic ketones and chalcones in moderate yields.
Scheme 16.
Hypervalent iodonium salts promoted synthesis of chalcone derivatives.
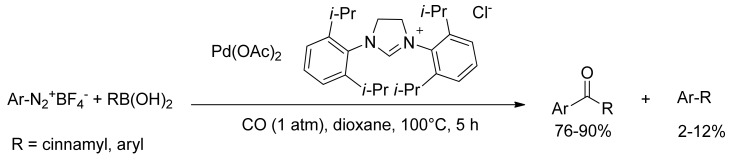
Andrus and coworkers reported the first example of carbonylative coupling of aryl diazonium tetrafluoroborate salts and aryl boronic acids to form aryl ketones and chalcones [73]. Palladium(II) acetate and N,N-bis-(2,6-diisopropylphenyl)dihydroimidazolium chloride (2 mol%) were used as catalytic system in the presence of an atmospheric pressure of carbon monoxide in 1,4-dioxane at 100 °C for 5 h (Scheme 17). Yields ranged from 76% to 90% with only minor amounts of biaryl coupling product observed (2%–12%).
Scheme 17.
Andrus’s carbonylative coupling of aryl diazonium salts.
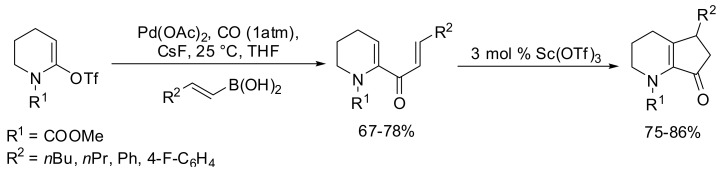
The carbonylative Suzuki-Miyaura coupling of vinyl triflates with alkenylboronic acids has subsequently been reported for the first time by Occhiato and coworkers in 2006 [74]. A short series of alkenylboronic acids were tested as substrates for the SM carbonylative coupling with lactam derived triflate in the presence of 5% Pd(OAc)2 and 10% Ph3P as the catalyst, and CsF (3 equiv) in THF at 100 °C (Scheme 18). The best results were achieved by carrying out the reaction at room temperature, which avoided some degradation of the starting material. This efficient carbonylative Suzuki-Miyaura coupling reaction using alkenylboronic acids as the nucleophiles, provided 2-(N-methoxycarbonylamino)-1,4-pentadien-3-ones which have been employed as substrates for the Lewis acid-catalyzed Nazarov reaction.
Scheme 18.
Pd-catalyzed carbovinylation of lactam-derived triflates.
This methodology for the preparation of unsymmetrical divinyl ketones was subsequently extended by the same research group to five- and six-membered heterocyclic systems namely lactams, lactones, and thiolactones [75,76,77]. Good to excellent yields (60%–86%) were obtained in particular with N-heterocyclic vinyl triflates, while good overall yields over two steps (50%–57%) were also obtained with lactone- and thiolactone-derived enol triflates.
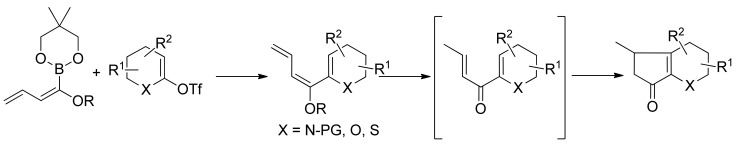
4. SM with Masked Acyl Boronates
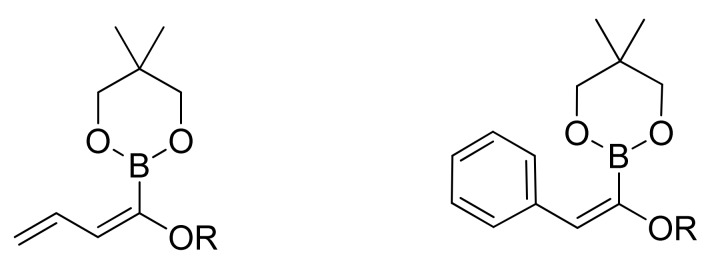
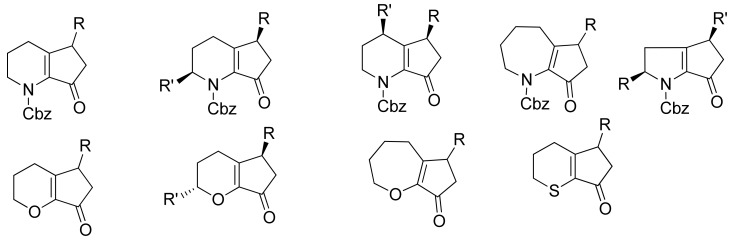
The introduction of a masked acyl function onto a substrate that can be successively unmasked is a general and most widely applied method for acylation reaction. The use of masked acyl boronates offers a complementary alternative to carbonylative approaches. To this purpose alkoxy dienyl and alkoxy styryl boronates (Figure 2) have been synthesized and used as coupling partners in SM reactions.
Figure 2.
Alkoxydienyl and alkoxystyryl boronates.
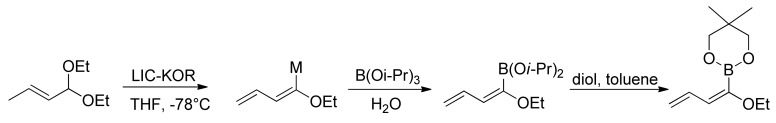
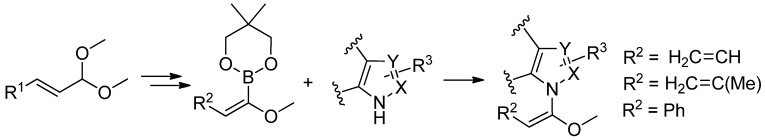
In our studies, we have developed a new synthesis of butadienylboronic esters, starting from α,β-unsaturated acetals, in the presence of LIC–KOR superbase (Scheme 19) [78].
Scheme 19.
Synthesis of butadienyl boronic esters.
The coupling products thus obtained can be used directly or after mild unmasking procedure as substrates for Nazarov electrocyclization (Scheme 20).
Scheme 20.
SM coupling between alkoxy dienyl boronates and heterocycle derived triflates.
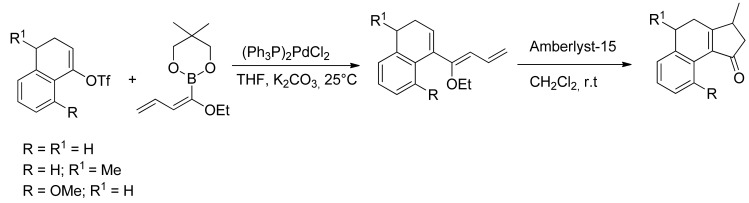
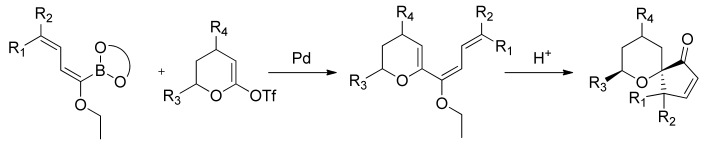
The ethoxydienylboronates thus formed can be used in an array of SM reactions with triflates or halides as coupling partners. In particular the reaction with α-tetralones-derived triflate, according to Suzuki-Miyaura conditions affords conjugated ethoxytrienes, which undergo cyclization in the presence of Amberlyst-15®, giving tricarbocyclic derivatives (Scheme 21) [78].
Scheme 21.
Synthesis of tricarbocyclic derivatives.
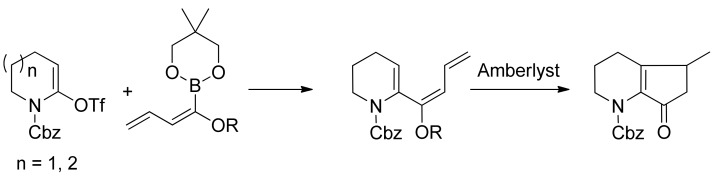
Alkoxydienyl- and alkoxystyrylboronates were used for Pd-catalyzed cross-coupling reactions with lactam-derived vinyl triflates (Scheme 22). The hydrolysis of the coupling products with alkoxystyrylboronates provided the corresponding N-acyl-substituted 3,4-dihydro-(2H)-pyridines and 2,3,4,5-tetrahydroazepines in good to high yields. The hydrolysis of the coupling products with alkoxydienylboronates, performed in the presence of Amberlyst-15®, resulted in a Nazarov-type cyclization that afforded hexahydro[1]pyrindin-7-ones and 3,4,5,6,7,8-hexahydro-(2H)-cyclopenta[b]azepin-8-ones. This methodology represents a novel and efficient procedure for the preparation of these classes of azacyclic compounds [79].
Scheme 22.
Synthesis of azepines.
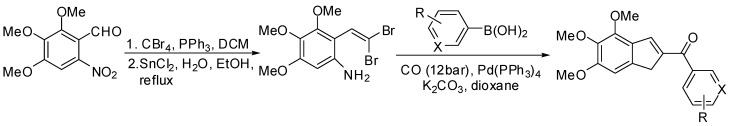
The whole process has been extended to lactone and thiolactone-derived vinyl triflates and phosphates. As usual, if subjected to mild acidic hydrolysis, these compounds undergo a 4π-electrocyclization process which furnishes cyclopenta-fused O- and S-heterocycles in good yields. The scope of the work has been that of closely examining the role and effect of both the heteroatom and the heterocycle ring size on the outcome of the electrocyclization, as well as the torquoselectivity of this process. The presence of the heteroatom was essential in stabilizing the oxyallyl cation intermediate, thus allowing the reaction to occur [80]. The ring size was also a basic parameter in the cyclization step: five-membered azacycles required more drastic conditions to give 5–5 fused systems and did so only after an initial hydrolysis to the corresponding divinyl ketones. As for the torquoselectivity, with both 2-methyl and 4-methyl substituted lactam derivatives steric interactions seem to have a role in forcing the conrotatory process to take place in one sense only: allowing the synthesis of diastereomerically pure compounds to be realized [81,82]. Because different patterns of substitution on the heterocycle are compatible with the reaction conditions, the methodology developed could be very useful for the synthesis of natural products and biologically active compounds containing cyclopenta-fused O- and N-heterocycle moieties (Figure 3).
Figure 3.
Representation of some of the scaffolds that can be synthesized through SM coupling between heterocyles derived triflates or phosphates and alkoxydienyl boronates.
The methodology has been then applied to the synthesis of spirocyclic ketones. As a matter of fact, the Suzuki-Miyaura cross-coupling reaction between α-ethoxydienyl boronates and lactone-derived vinyl triflates affords functionalized 6-(1-ethoxy-1,3-butadienyl)dihydropyran derivatives that give functionalized spirocyclic ketones after hydrolysis under mild acidic conditions. The product distribution and the stereoselectivity of the process are strongly dependent on the substitution of both the ethoxydiene and dihydropyran moieties. High stereoselectivity is observed in the presence of a C2-substituent on the dihydropyran moiety (Scheme 23) [83], which has been explained in terms of transition state geometries.
Scheme 23.
Synthesis of spyrocyclic ketones.
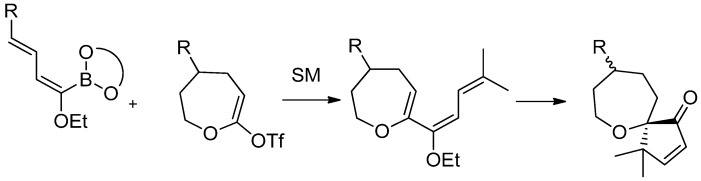
The same synthetic approach was finally applied to seven-membered oxacycles, in order to obtain spirooxepanes. Coupling products were obtained by the usual Suzuki-Miyaura cross-coupling reaction and treated with Amberlyst-15® affording the spiro products. As a consequence of the conformational flexibility of the seven-membered rings, a lack in diastereoselection has been observed in the cyclization process (Scheme 24).
Scheme 24.
Synthesis of seven membered oxacycles.
Furthermore, alkoxydienylboronates and alkoxystyrylboronates have been used in various copper mediated cross-coupling reactions with azoles. A variety of N-alkoxydienyl- and N-alkoxystyrylazoles have been synthesized under mild conditions. The process utilizes Cu(OAc)2 in CH2Cl2 at room temperature in the presence of CsF (Scheme 25) [84].
Scheme 25.
Coupling reactions between alkoxydienyl and alkoxystyrylboronates and azoles.
5. Acylation of Heterocycles by SM in the Synthesis of Natural Products
Nowadays, there are numerous applications of palladium catalysts in the preparation of pharmaceuticals, agrochemicals, and also advanced materials both on laboratory and industrial scale [85]. Among the wide number of Pd-catalyzed coupling reactions, the direct acylation of heterocycles and the carbonylation reactions also have experienced impressive improvements for the construction of diaryl ketones (see above) or substituted heteroaromatic moieties which constitute important building blocks of polymers [86], ligands [87], natural products as well as some biologically active pharmaceuticals [88]. In this last section we provide herein an overview of the direct acylation of heterocycles applied as key synthetic step in the preparation of natural products.
5.1. SM Acylation Reaction for the Synthesis of Strigolactones (SLs)
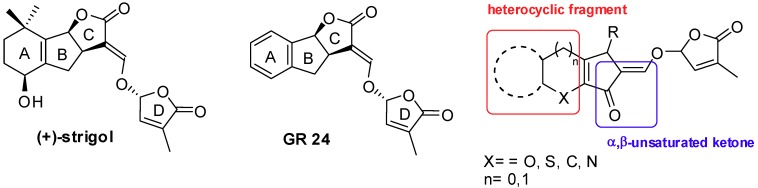
Since 2008 our research has been focused on the total synthesis of a family of plant hormones called strigolactones (SLs). This class of molecules proved to play multiple roles in regulating the rhizosphere: they are involved in the signaling between plants and symbiotic fungi and in the signaling of other different biological systems such as the germination of parasitic plants seeds, fungi hyphal branching, bacterial mitosis and plant shoot branching [89,90]. The SLs chemical structure is generally based on an ABC tricycle core linked to a D cycle by an enol ether bridge. Our work in this field has been mainly focused on the development of a innovative synthetic procedure aimed at the extension of the conjugated system to the ABC framework across an heterocyclic B ring and the replacement of the carboxylic moiety (present on the C ring) with a more reactive CO moiety (Figure 4) [91,92].
Figure 4.
Naturally occurring strigol with reference compound GR 24 (left) and our target analogue (right).
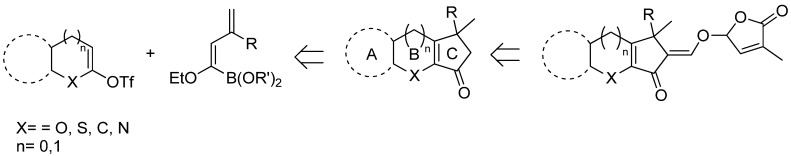
To this purpose, a retrosynthetic approach to the tricyclic framework ABC where a SM-coupling of a masked acyl anion with a heterocyclic triflate has been designed and exploited as the key step for the construction of the desired scaffold, as shown in Scheme 26.
Scheme 26.
Retrosynthetic approach to SL’s ABC core through SM-coupling of dienyl boronate derivatives.
Thus, a simple Suzuki coupling between indolyl triflate and alkoxy dienyl boronates in the presence of palladium(II) catalyst and aqueous potassium carbonate in THF afforded indolyl functionalized dienes in good yields at room temperature (Scheme 27) [89,90]. Upon treatment under mild acidic conditions, the alkoxy vinyl ethers unmasked the corresponding vinyl ketones that underwent Nazarov cyclization leading to the desired tricyclic core.
Scheme 27.
SM coupling of dienyl boronates for the synthesis of strigolactones ABC core.
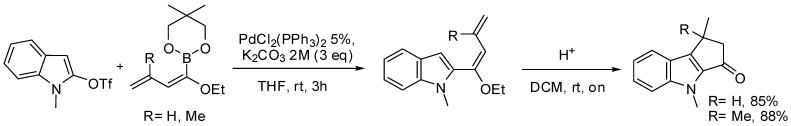
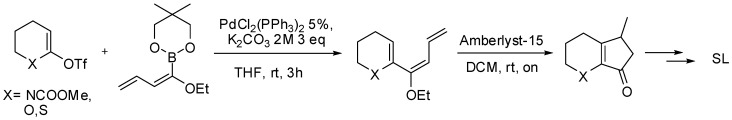
The same procedure was then extended to the synthesis of new SL analogues missing the A ring [89]. To this purpose N-protected δ-valerolactam, δ-valerolactone or thiolactone were successfully coupled with the dienyl boronate under the same reaction conditions. The coupling products were then subjected to a Nazarov cyclization with Amberlyst-15 to afford the bicyclic systems (Scheme 28) that were subsequently converted to the corresponding strigolactone derivatives.
Scheme 28.
Pd-catalyzed alkenylation of lactones and lactames applied to the synthesis of bicyclic SL’s analogues.
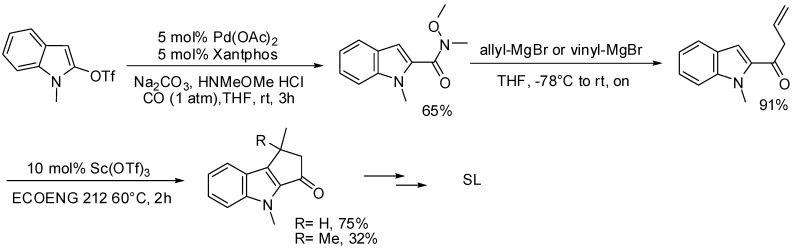
As an alternative procedure to the synthesis of the SL’s bi- or tricyclic core, we have also exploited a classic carbonylative approach for the preparation of heterocyclic Weinreb amides by means of a palladium catalyzed coupling of indolyl triflate with N-methoxy-N-methylamine hydrochloride [89]. The resulting amide was successfully coupled with allylmagnesium bromide and the resulting dienone, which possesses the suitable electronic arrangement to undergo an acid catalyzed Nazarov reaction, was subsequently subjected to ring closure under mild acidic conditions (Scheme 29).
Scheme 29.
Acylation of indolyl triflates applied to the synthesis of SL’s tricyclic core.
5.2. SM Coupling Applied to the Synthesis of Other Natural Products
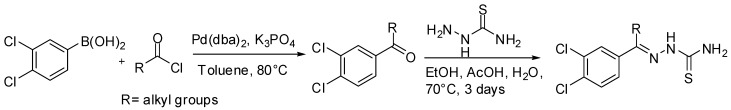
Despite the direct acylation of heterocycles has not been widely described, few examples of natural products syntheses involving SM-couplings acylation steps have been reported in the literature so far. The first example, reported by Guy and coworkers [93,94], is based on the discovery of potent thiosemicarbazone inhibitors of rhodesain [95] and cruzain [96] for the treatment of Chagas’ disease and sleeping sickness. Thiosemicarbazones have potent activity against rhodesain [97], protease of the papain-like family expressed by the protozoan parasite Trypanosoma brucei which causes Human African trypanosomiasis (HAT), a major health concern in sub-Saharan Africa with an estimated 50,000 cases and 60 million at risk of infection. In the synthetic sequence to rhodesain and cruzain inhibitors described by Guy, the key ketone intermediate was prepared by a SM coupling between an appropriate acyl chloride and a boronic acid by means of Pd(dba)2 in toluene. Finally, an acid catalyzed reaction with thiosemicarbazide afforded the target thiosemicarbazones in moderate to good yields (Scheme 30).
Scheme 30.
Guy’s synthesis of thiosemicarbazone inhibitors.
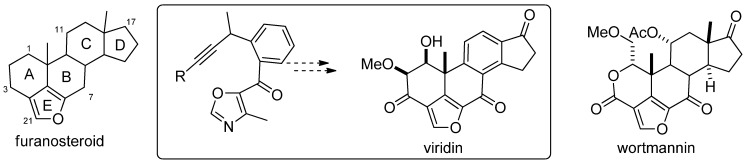
The second example we are presenting herein is focused on the synthesis of furanosteroids, performed by Jacobi and Sessions in 2006 [98]. The furanosteroids are a class of novel pentacyclic fungal metabolites that share in common a furan ring, bridging positions 4 and 6 of the steroid skeleton (Figure 4) [98]. Members of this class have attracted attention for many years because of their potent antiinflammatory and antibiotic properties [99] and more recently because of their ability to selectively block certain intracellular signaling pathways associated with cell growth and development [100,101]. Prominent members include those of the viridin and wortmannin families (Figure 5). In his report, Jacobi described the synthesis of viridin via an acyl oxazole intermediate, which its synthetic key step required a SM acylation.
Figure 5.
Furanosteroid class of PI3-kinase inhibitors.
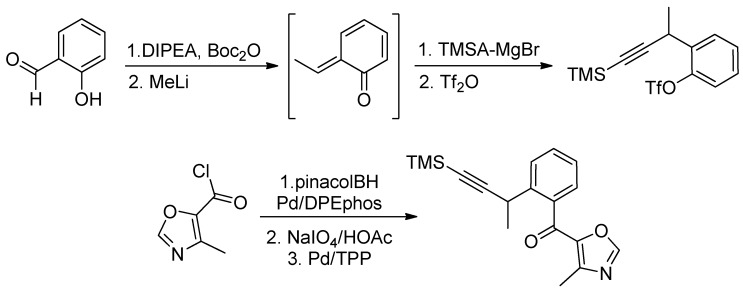
The salicylaldehyde was converted to the Boc derivative and next, the treatment with MeLi generated the reactive o-quinone methide, by a pathway involving nucleophilic addition to the aldehyde, followed by intramolecular transfer of the Boc group and conjugated elimination. Quenching with the Grignard reagent derived from trimethylsilylacetylene (TMSA) followed by triflation gave the corresponding alkyne in 74% yield. Finally, the TMS-alkynyl acyl oxazole was obtained in a three-step sequence starting from triflate via an elaboration of the corresponding boronic acid and a Suzuki coupling with the readily available acyl chloride (Scheme 31).
Scheme 31.
Jacobi’s synthesis of furanosteroid viridin’s core.
In the world of natural products diaryl ketones are important structural motifs which are present in a large number of biological and medicinal interest compounds (e.g., cotoin, papaveraldine, suprofen, ketoprofen), and the synthesis by direct acylation in SM-couplig reactions has already been described in the previous sections of this survey.
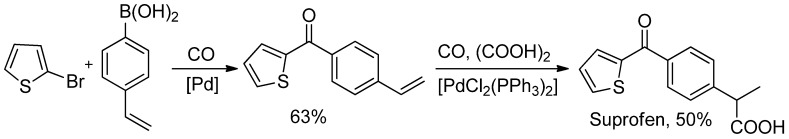
Differently from direct acylation of heterocycles, more examples about carbonylative SM reaction applied to the synthesis of natural products are available. For example, in 2008 Beller and co-workers [102] synthesized suprofen, a commercial nonsteroidal anti-inflammatory drug, in only two-steps (Scheme 32).
Scheme 32.
Beller’s two-steps synthesis of Suprofen.
Thus, 2-bromothiophene was reacted with 4-vinylphenylboronic acid in the presence of CO to give exclusively the desired diarylketone in 63% yields. Hence, 4-vinylphenyl 2-thienyl ketone was easily hydroxycarbonylated [103] in one step to suprofen.
Recently, Florent et al. [104] synthesized novel heterocyclic combretastatin A4 analogues. Combretastatin A4 (CA4) is a natural product isolated by Pettit et al. from the South African willow tree Combretum caffrum [105]. It strongly inhibits tubulin polymerization and the proliferation of murine and human cancer cell lines (Scheme 33) [106].
Scheme 33.
Synthesis of Combrestatin A4 analogues.
The required trimethoxyanilines were easily prepared in a two-step sequence (Ramirez–Corey–Fuchs olefination [107] followed by reduction of the nitro group) from the corresponding o-nitrobenzaldehyde derivatives [108]. The domino reaction was then performed heating the dibromoolefine and phenylboronic acid at 85 °C for 24 h, and the desired 2-aroylindole was isolated in satisfactory yield (65%), opening the possibility to get a novel series of CA4 analogues.
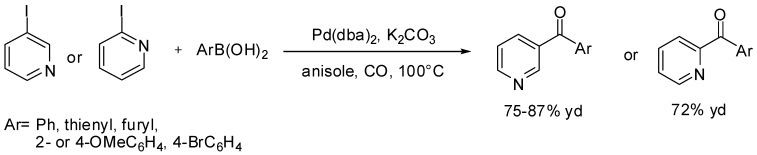
Finally, Yang and Xue [109] and Bhanage [110] synthesized heteroaryl-substituted pyridine derivatives by means of Suzuki carbonylative reactions (Scheme 34). These molecules are a very important family of compounds with several applications in diverse areas of chemistry such as metal-coordination complexes [111,112], pharmaceutical agents [113,114], and molecular electronic device materials [115,116,117].
Scheme 34.
Yang and Xue’s synthesis of heteroaryl-substituted pyridine derivatives.
6. Conclusions
The Suzuki palladium-catalyzed cross-coupling reaction of organoboron reagents with organic halides or pseudohalides to form biaryl derivatives has emerged over the past two decades as an extremely powerful tool in organic synthesis. More recently, the SM coupling has been exploited as an efficient method to introduce acyl functionalities on a target substrate. The intent of this short review is to give an overview of the most recent approaches found in the literature to reach this goal. Three different strategies can be pinpointed. The first of all relies on the coupling between organoboron reagents and acyl derivatives (acyl chloride, anhydrides, chloroformates and carbamoyl chlorides). The palladium-catalyzed three-component cross-coupling of aryl halides, carbon monoxide, and boronic acids, which is closely related to the Suzuki reaction, also provides convenient access to asymmetric ketones. However, the main drawback of the carbonylative Suzuki reaction often lies in the formation of significant amounts of the direct coupling product without carbon monoxide insertion, particularly with electron-deficient aryl halides. Finally, boronic derivatives with masked acyl functions (alkoxy dienyl or styryl boronates) can be used as a tool to introduce a masked acyl function by means of a traditional SM coupling, followed by a mild unmasking step. A selection of natural product syntheses in which SM acylation represents a key step is presented in the last section.
Acknowledgments
The authors gratefully thank MIUR and Regione Piemonte for financial support (Cipe 2007, Converging Technologies).
References
- 1.Dieter R.K. Reaction of acyl chlorides with organometallic reagents: A banquet table of metals for ketone synthesis. Tetrahedron. 1999;55:4177–4236. doi: 10.1016/S0040-4020(99)00184-2. [DOI] [Google Scholar]
- 2.Zhang Y.D., Rovis T. A unique catalyst effects the rapid room-temperature cross-coupling of organozinc reagents with carboxylic acid fluorides, chlorides, anhydrides, and thioesters. J. Am. Chem. Soc. 2004;126:15964–15965. doi: 10.1021/ja044113k. [DOI] [PubMed] [Google Scholar]
- 3.Furstner A., Voigtlander D., Schrader W., Giebel D., Reetz M.T. A “hard/soft” mismatch enables catalytic Friedel-Crafts acylations. Org. Lett. 2001;3:417–420. doi: 10.1021/ol0069251. [DOI] [PubMed] [Google Scholar]
- 4.Gmouth S., Yang H.L., Vaultier M. Activation of bismuth(III) derivatives in ionic liquids: Novel and recyclable catalytic systems for Friedel-Crafts acylation of aromatic compounds. Org. Lett. 2003;5:2219–2222. doi: 10.1021/ol034529n. [DOI] [PubMed] [Google Scholar]
- 5.Fillion E., Fishlock D., Wilsily A., Goll J.M. Meldrum’s acids as acylating agents in the catalytic intramolecular Friedel-Crafts reaction. J. Org. Chem. 2005;70:1316–1327. doi: 10.1021/jo0483724. [DOI] [PubMed] [Google Scholar]
- 6.Ross J., Xiao J.L. Friedel-Crafts acylation reactions using metal triflates in ionic liquid. Green Chem. 2002;4:129–133. doi: 10.1039/b109847k. [DOI] [Google Scholar]
- 7.Labadie J.W., Stille J.K. Stereochemistry of transmetalation in the palladium-catalyzed coupling of acyl chloride and organostannanes. J. Am. Chem. Soc. 1983;105:669–670. doi: 10.1021/ja00341a083. [DOI] [Google Scholar]
- 8.Reddy C.K., Knochel P. New cobalt- and iron-catalyzed reactions of organozinc compounds. Angew. Chem. Int. Ed. Engl. 1996;35:1700–1701. doi: 10.1002/anie.199617001. [DOI] [Google Scholar]
- 9.Wu T.C., Xiong H.P., Rieke R.D. Organocalcium Chemistry: Preparation and Reactions of Highly Reactive Calcium. J. Org. Chem. 1990;55:5045–5051. doi: 10.1021/jo00304a016. [DOI] [Google Scholar]
- 10.Tatamidani H., Kakiuchi F., Chatani N. A new ketone synthesis by palladium-catalyzed cross-coupling reactions of esters with organoboron compounds. Org. Lett. 2004;6:3597–3599. doi: 10.1021/ol048513o. [DOI] [PubMed] [Google Scholar]
- 11.Eberle M.K., Kahle G.G. Preparation of functionalized ketones via low temperature grignard reaction. Tetrahedron Lett. 1980;21:2303–2304. doi: 10.1016/S0040-4039(00)92590-5. [DOI] [Google Scholar]
- 12.Miyaura N., Suzuki A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995;95:2457–2483. doi: 10.1021/cr00039a007. [DOI] [Google Scholar]
- 13.Littke A.F., Fu G.C. Palladium-catalyzed coupling reactions of aryl chlorides. Angew. Chem. Int. Ed. 2002;41:4176–4211. doi: 10.1002/1521-3773(20021115)41:22<4176::AID-ANIE4176>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 14.Hassan J., Sevignon M., Gozzi C., Schulz E., Lemaire M. Aryl-aryl bond formation one century after the discovery of the Ullmann reaction. Chem. Rev. 2002;102:1359–1469. doi: 10.1021/cr000664r. [DOI] [PubMed] [Google Scholar]
- 15.March J. Advanced Organic Chemistry. 3rd ed. Wiley Interscience; New York, NY, USA: 1985. pp. 433–435, 824–827. [Google Scholar]
- 16.Larock R.C. Comprehensive Organic Transformations: A Guide to Functional Group Preparation. VCH; New York, NY, USA: 1989. pp. 685–702. [Google Scholar]
- 17.O'Nell B.T. Comprehensive Organic Synthesis. Pergamon; Oxford, UK: 1991. pp. 397–458. [Google Scholar]
- 18.Olah G.A. Friedel.–Crafts. and Related Reactions. Volume 1 Interscience; New York, NY, USA: 1963. [Google Scholar]
- 19.Sato F., Inoue M., Oguro K., Sato M. Preparation of ketones by direct reaction of grignard reagents with acyl chlorides in tetrahydrofuran. Tetrahedron Lett. 1979;20:4303–4306. doi: 10.1016/S0040-4039(01)86573-4. [DOI] [Google Scholar]
- 20.Bykov V.V., Korolev D.N., Bumagin N.A. Palladium-catalyzed reactions of organoboron compounds with acyl chlorides. Russ. Chem. Bull. 1997;46:1631–1632. doi: 10.1007/BF02502959. [DOI] [Google Scholar]
- 21.Haddach M., McCarthy J.R. A new method for the synthesis of ketones: The palladium-catalyzed cross-coupling of acyl chlorides with arylboronic acids. Tetrahedron Lett. 1999;40:3109–3112. doi: 10.1016/S0040-4039(99)00476-1. [DOI] [Google Scholar]
- 22.Bumagin N.A., Korolev D.N. Synthesis of unsymmetric ketones via ligandless Pd-catalyzed reaction of acyl chlorides with organoboranes. Tetrahedron Lett. 1999;40:3057–3060. doi: 10.1016/S0040-4039(99)00364-0. [DOI] [Google Scholar]
- 23.Urawa Y., Ogura K. A convenient method for preparing aromatic ketones from acyl chlorides and arylboronic acids via Suzuki-Miyaura type coupling reaction. Tetrahedron Lett. 2003;44:271–273. doi: 10.1016/S0040-4039(02)02501-7. [DOI] [Google Scholar]
- 24.Eddarir S., Cotelle N., Bakkour Y., Rolando C. An efficient synthesis of chalcones based on the Suzuki reaction. Tetrahedron Lett. 2003;44:5359–5363. doi: 10.1016/S0040-4039(03)01140-7. [DOI] [Google Scholar]
- 25.Bandgar B.P., Patil A.V. A rapid, solvent-free, ligandless and mild method for preparing aromatic ketones from acyl chlorides and arylboronic acids via a Suzuki-Miyaura type of coupling reaction. Tetrahedron Lett. 2005;46:7627–7630. doi: 10.1016/j.tetlet.2005.08.111. [DOI] [Google Scholar]
- 26.Nishihara Y., Inoue Y., Fujisawa M., Takagi K. Room-Temperature Palladium-Catalyzed and Copper(I)-Mediated Coupling Reactions of Acyl Chlorides with Boronic Acids under Neutral Conditions. Synlett. 2005:2309–2312. doi: 10.1055/s-2005-872661. [DOI] [Google Scholar]
- 27.Polàckovà V., Toma S., Augustìnovà I. Microwave-promoted cross-coupling of acyl chlorides with arylboronic acids: A convenient method for preparing aromatic ketones. Tetrahedron. 2006;62:11675–11678. doi: 10.1016/j.tet.2006.09.055. [DOI] [Google Scholar]
- 28.Ekoue-Kovi K., Xu H., Wolf C. Palladium-phosphinous acid-catalyzed cross-coupling of aliphatic and aromatic acyl chlorides with boronic acids. Tetrahedron Lett. 2008;49:5773–5776. doi: 10.1016/j.tetlet.2008.07.115. [DOI] [Google Scholar]
- 29.Zhang L., Wu J., Shi L., Xia C., Li F. Ionically tagged benzimidazole palladium(II) complex: Preparation and catalytic application in cross-coupling reactions. Tetrahedron Lett. 2011;52:3897–3901. doi: 10.1016/j.tetlet.2011.05.079. [DOI] [Google Scholar]
- 30.Kakino R., Shimizu I., Yamamoto A. Synthesis of Trifluoromethyl Ketones by Palladium-Catalyzed Cross-Coupling Reaction of Phenyl Trifluoroacetate with Organoboron Compounds. Bull. Chem. Soc. Jpn. 2001;74:371–376. doi: 10.1246/bcsj.74.371. [DOI] [Google Scholar]
- 31.Kakino R., Yasumi S., Shimizu I., Yamamoto A. Synthesis of Unsymmetrical Ketones by Palladium-Catalyzed Cross-Coupling Reaction of Carboxylic Anhydrides with Organoboron Compounds. Bull. Chem. Soc. Jpn. 2002;75:137–148. doi: 10.1246/bcsj.75.137. [DOI] [Google Scholar]
- 32.Kakino R., Narahashi H., Shimizu I., Yamamoto A. Palladium-Catalyzed Direct Conversion of Carboxylic Acids into Ketones with Organoboronic Acids Promoted by Anhydride Activators. Bull. Chem. Soc. Jpn. 2002;75:1333–1345. doi: 10.1246/bcsj.75.1333. [DOI] [Google Scholar]
- 33.Gooßen L.J., Ghosh K. Palladium-Catalyzed Synthesis of Aryl Ketones from Boronic Acids and Carboxylic Acids or Anhydrides. Angew. Chem. Int. Ed. 2001;40:3458–3460. doi: 10.1002/1521-3773(20010917)40:18<3458::AID-ANIE3458>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 34.Gooßen L.J., Ghosh K. Palladium-Catalyzed Synthesis of Aryl Ketones from Boronic Acids and Carboxylic Acids Activated in situ by Pivalic Anhydride. Eur. J. Org. Chem. 2002:3254–3267. [Google Scholar]
- 35.Xin B., Zhang Y., Cheng K. Phosphine-Free Cross-Coupling Reaction of Arylboronic Acids with Carboxylic Anhydrides or Acyl Chlorides in Aqueous Media. J. Org. Chem. 2006;71:5725–5731. doi: 10.1021/jo060749d. [DOI] [PubMed] [Google Scholar]
- 36.Xin B., Zhang Y., Cheng K. The Surfactant-Promoted Cross-Coupling Reactions of Arylboronic Acids with Carboxylic Anhydrides or Acyl Chlorides in Water. Synthesis. 2007:1970–1978. doi: 10.1002/chin.200747092. [DOI] [PubMed] [Google Scholar]
- 37.Xin B.-W. Synthesis of Aryl Ketones by Cross-Coupling Reaction of Arylboronic Acids with Carboxylic Anhydrides in Aqueous Phase. Synth. Commun. 2008;38:2826–2837. doi: 10.1080/00397910801979346. [DOI] [Google Scholar]
- 38.Shen X.-B., Gao T.-T., Lu J.-M., Shao L.-X. Imidazole-coordinated monodentate NHC–Pd(II) complex derived from proline and its application to the coupling reaction of arylboronic acids with carboxylic acid anhydrides in water at room temperature. Appl. Organomet. Chem. 2011;25:497–501. doi: 10.1002/aoc.1792. [DOI] [Google Scholar]
- 39.Yu A., Shen L., Cui X., Peng D., Wu Y. Palladacycle-catalyzed cross-coupling reactions of arylboronic acids with carboxylic anhydrides or acyl chlorides. Tetrahedron. 2012;68:2283–2288. doi: 10.1016/j.tet.2012.01.053. [DOI] [Google Scholar]
- 40.Thompson D.J. Comprehensive Organic Synthesis. Volume 4. Pergamon; Oxford, UK: 1991. pp. 1028–1035. [Google Scholar]
- 41.Schoenberg A., Heck R.F. Palladium-catalyzed amidation of aryl, heterocyclic, and vinylic halides. J. Org. Chem. 1974;39:3327–3331. doi: 10.1021/jo00937a004. [DOI] [Google Scholar]
- 42.Corey E.J., Hegedus L.S. Base-catalyzed carboxylation of organic halides by nickel carbonyl in protic media. J. Am. Chem. Soc. 1969;91:1233–1234. doi: 10.1021/ja01033a044. [DOI] [Google Scholar]
- 43.Kaiser N.-F.K., Hallberg A., Larhed M. In Situ Generation of Carbon Monoxide from Solid Molybdenum Hexacarbonyl. A Convenient and Fast Route to Palladium-Catalyzed Carbonylation Reactions. J. Comb. Chem. 2002;4:109–111. doi: 10.1021/cc010085f. [DOI] [PubMed] [Google Scholar]
- 44.Collman J.P., Winter S.R., Komoto R.G. Selective syntheses of aliphatic carboxylic acids, esters, and amides using sodium tetracarbonylferrate(-II) J. Am. Chem. Soc. 1973;95:249–250. doi: 10.1021/ja00782a050. [DOI] [Google Scholar]
- 45.Blicke F.F., Zinnes H. The Reaction of the Chloromagnesium Derivative of Chloromagnesium Phenylacetate with Isocyanates, Carbamyl Chlorides and Isothiocyanates. J. Am. Chem. Soc. 1955;77:4849–4851. doi: 10.1021/ja01623a049. [DOI] [Google Scholar]
- 46.Mills R.J., Taylor N.J., Snieckus V. Directed ortho metalation of N,N-diethylbenzamides. Silicon protection of ortho sites and the o-methyl group. J. Org. Chem. 1989;54:4372–4385. doi: 10.1021/jo00279a028. [DOI] [Google Scholar]
- 47.Nagao Y., Miyamoto S., Miyamoto M., Takeshige H., Hayashi K., Sano S., Shiro M., Yamaguchi K., Sei Y. Highly Stereoselective Asymmetric Pummerer Reactions That Incorporate Intermolecular and Intramolecular Nonbonded S···O Interactions. J. Am. Chem. Soc. 2006;128:9722–9729. doi: 10.1021/ja056649r. [DOI] [PubMed] [Google Scholar]
- 48.Lemoucheux L., Seitz T., Rouden J., Lasne M.-C. Preparation of Tertiary Amides from Carbamoyl Chlorides and Organocuprates. Org. Lett. 2004;6:3703–3706. doi: 10.1021/ol0487130. [DOI] [PubMed] [Google Scholar]
- 49.Duan Y.-Z., Deng M.-Z. Palladium-Catalyzed Cross-Coupling Reaction of Arylboronic Acids with Chloroformate or Carbamoyl Chloride. Synlett. 2005:355–357. doi: 10.1002/chin.200528094. [DOI] [Google Scholar]
- 50.Yasui Y., Tsuchida S., Miyabe H., Takemoto Y. One-Pot Amidation of Olefins through Pd-Catalyzed Coupling of Alkylboranes and Carbamoyl Chlorides. J. Org. Chem. 2007;72:5898–5900. doi: 10.1021/jo070724u. [DOI] [PubMed] [Google Scholar]
- 51.Krishnamoorthy R., Lam S.Q., Manley C.M., Herr R.J. Palladium-Catalyzed Preparation of Weinreb Amides from Boronic Acids and N-Methyl-N-methoxycarbamoyl Chloride. J. Org. Chem. 2010;75:1251–1258. doi: 10.1021/jo902647h. [DOI] [PubMed] [Google Scholar]
- 52.Tomizawa T., Orimoto K., Niwa T., Nakada M. Preparation of Imides via the Palladium-Catalyzed Coupling Reaction of Organoborons with Methyl N-[Methoxy(methylthio)methylene]carbamate as a One-Carbon Elongation Reaction. Org. Lett. 2012 doi: 10.1021/ol303062a. [DOI] [PubMed] [Google Scholar]
- 53.Brunet J.J., Chauvin R. Synthesis of Diarylketones Through Carbonylative Coupling. Chem. Soc. Rev. 1995;24:89–95. doi: 10.1039/cs9952400089. [DOI] [Google Scholar]
- 54.Echavarren A.M., Stille J.K. Palladium-catalyzed carbonylative coupling of aryl triflates with organostannanes. J. Am. Chem. Soc. 1988;110:1557–1565. doi: 10.1021/ja00213a032. [DOI] [Google Scholar]
- 55.Kang S.-K., Yamaguchi T., Kim T.-H., Ho P.-S. Copper-Catalyzed Cross-Coupling and Carbonylative Cross-Coupling of Organostannanes and Organoboranes with Hypervalent Iodine Compounds. J. Org. Chem. 1996;61:9082–9083. doi: 10.1021/jo962033w. [DOI] [Google Scholar]
- 56.Ceccarelli S., Piarulli U., Gennari C. Effect of Ligands and Additives on the Palladium-Promoted Carbonylative Coupling of Vinyl Stannanes and Electron-Poor Enol Triflates. J. Org. Chem. 2000;65:6254–6256. doi: 10.1021/jo0004310. [DOI] [PubMed] [Google Scholar]
- 57.Yamamoto T., Kohara T., Yamamoto A. Selective Formation of Ketone, Diketone and Aldehyde by the CO Insertion into Nickel-Alkyl Bond of Dialkylnickel Complexes. A Novel Nickel-Catalyzed Syntheses of Ketones and Tertiary Alcohols from Grignard Reagents, Aryl Halides, and Carbon Monoxide. Chem. Lett. 1976;5:1217–1220. doi: 10.1246/cl.1976.1217. [DOI] [Google Scholar]
- 58.Bumagin N.A., Ponomaryov A.B., Beletskaya I.P. Ketone synthesis via palladium-catalyzed carbonylation of organoaluminium compounds. Tetrahedron Lett. 1985;26:4819–4822. doi: 10.1016/S0040-4039(00)94960-8. [DOI] [Google Scholar]
- 59.Hatanaka Y., Fukushima S., Hiyama T. Carbonylative coupling reaction of organofluorosilanes with organic halides promoted by fluoride ion and palldium catalyst. Tetrahedron. 1992;48:2113–2126. doi: 10.1016/S0040-4020(01)88878-5. [DOI] [Google Scholar]
- 60.Ishiyama T., Kizaki H., Miyaura N., Suzuki A. Synthesis of unsymmetrical biaryl ketones via palladium-catalyzed carbonylative cross-coupling reaction of arylboronic acids with iodoarenes. Tetrahedron Lett. 1993;34:7595–7598. [Google Scholar]
- 61.Skoda-Foldes R., Székvolgi Z., Kollàr L., Berente Z., Horvàth J., Tuba Z. Facile Synthesis of Steroidal Phenyl Ketones via Homogeneous Catalytic Carbonylation. Tetrahedron. 2000;56:3415–3418. doi: 10.1016/S0040-4020(00)00241-6. [DOI] [Google Scholar]
- 62.Knopff O., Alexakis A. Tandem Asymmetric Conjugate Additional Silylation of Enantiomerically Enriched Zinc Enolates. Synthetic Importance and Mechanistic Implications. Org. Lett. 2002;4:3835–3837. doi: 10.1021/ol026644o. [DOI] [PubMed] [Google Scholar]
- 63.Harutyunyan S.R., López F., Browne W.R., Correa A., Peña D., Badorrey R., Meetsma A., Minnaard A.J., Feringa B.L. On the Mechanism of the Copper-Catalyzed Enantioselective 1,4-Addition of Grignard Reagents to alfa-beta-Unsaturated Carbonyl Compounds. J. Am. Chem. Soc. 2006;128:9103–9118. doi: 10.1021/ja0585634. [DOI] [PubMed] [Google Scholar]
- 64.Martin D., Kehrli S., d’Augustin M., Clavier H., Mauduit M., Alexakis A. Copper-Catalyzed Asymmetric Conjugate Addition of Grignard Reagents to Trisubstituted Enones. Construction of All-Carbon Quaternary Chiral Centers. J. Am. Chem. Soc. 2006;128:8416–8417. doi: 10.1021/ja0629920. [DOI] [PubMed] [Google Scholar]
- 65.Bianco A., Cavarischia C., Guiso M. The Heck Coupling Reaction Using Aryl Vinyl Ketones: Synthesis of Flavonoids. Eur. J. Org. Chem. 2004:2894–2898. doi: 10.1002/ejoc.200400032. [DOI] [Google Scholar]
- 66.Hinch M., Jacques O., Drago C., Caggiano L., Jackson R.F.W., Dexter C., Anson M.S., Macdonald S.J.F. Effective asymmetric oxidation of enones and alkyl aryl sulfides. J. Mol. Catal. A: Chem. 2006;251:123–128. doi: 10.1016/j.molcata.2006.02.006. [DOI] [Google Scholar]
- 67.Shibuya M., Ito S., Takahashi M., Iwabuchi Y. Oxidative Rearrangement of Cyclic Tertiary Allylic Alcohols with IBX in DMSO. Org. Lett. 2004;6:4303–4306. doi: 10.1021/ol048210u. [DOI] [PubMed] [Google Scholar]
- 68.Yin W., Ma Y., Xu J., Zhao Y. Microwave-Assisted One-Pot Synthesis of 1-Indanones from Arenes and α,β-Unsaturated Acyl Chlorides. J. Org. Chem. 2006;71:4312–4315. doi: 10.1021/jo060022p. [DOI] [PubMed] [Google Scholar]
- 69.Taylor M.S., Zalatan D.N., Lerchner A.M., Jacobsen E.N. Highly Enantioselective Conjugate Additions to alfa,beta-Unsaturated Ketones Catalyzed by a (Salen)Al Complex. J. Am. Chem. Soc. 2005;127:1313–1317. doi: 10.1021/ja044999s. [DOI] [PubMed] [Google Scholar]
- 70.Miyaura N., Suzuki A. A Convenient Stereospecific Synthesis of Alpha,Beta-Unsaturated Carboxylic Esters via the Palladium-Catalyzed Carbonylation of 1-Alkenylboranes. Chem. Lett. 1981;7:879–882. doi: 10.1246/cl.1981.879. [DOI] [Google Scholar]
- 71.Hashem K.E., Woell J.B., Alper H. Palladium(0) and rhodium(I) catalysis of the carbonylation of unactivated bromides. Tetrahedron Lett. 1984;25:4879–4880. doi: 10.1016/S0040-4039(01)91248-1. [DOI] [Google Scholar]
- 72.Kang S.K., Lim K.H., Ho P.S., Yoon S.K., Son H.J. Palladium-catalyzed carbonylative cross-coupling of organoboranes with hypervalent iodonium salts: Synthesis of aromatic ketones. Synth. Commun. 1998;28:1481–1489. doi: 10.1080/00397919808006847. [DOI] [Google Scholar]
- 73.Andrus M.B., Ma Y.D., Zang Y.F., Song C. Palladium-imidazolium-catalyzed carbonylative coupling of aryl diazonium ions and aryl boronic acids. Tetrahedron Lett. 2002;43:9137–9140. doi: 10.1016/S0040-4039(02)02186-X. [DOI] [Google Scholar]
- 74.Larini P., Guarna A., Occhiato E.G. The Lewis acid-catalyzed Nazarov reaction of 2-(N-methoxycarbonylamino)-1,4-pentadien-3-ones. Org. Lett. 2006;8:781–784. doi: 10.1021/ol053071h. [DOI] [PubMed] [Google Scholar]
- 75.Bartali L., Larini P., Guarna A., Occhiato E.G. Preparation of cyclopenta-fused N-, O-, and S-heterocycles by Lewis acid catalyzed Nazarov reaction. Synthesis. 2007:1733–1737. doi: 10.1002/chin.200741034. [DOI] [Google Scholar]
- 76.Bartali L., Guarna A., Larini P., Occhiato E.G. Carbonylative Suzuki-Miyaura coupling reaction of lactam-, lactone-, and thiolactone-derived enol triflates for the synthesis of unsymmetrical dienones. Eur. J. Org. Chem. 2007:2152–2163. doi: 10.1002/ejoc.200601089. [DOI] [Google Scholar]
- 77.Bartali L., Scarpi D., Guarna A., Prandi C., Occhiato E.G. Chemistry of Lactam-Derived Vinyl Phosphates: Stereoselective Synthesis of (+)-Fagomine. Synlett. 2009;2009:913–916. [Google Scholar]
- 78.Deagostino A., Prandi C., Venturello P. alpha,beta-unsaturated acetals in synthesis. Curr. Org. Chem. 2003;7:821–839. doi: 10.2174/1385272033486666. [DOI] [Google Scholar]
- 79.Occhiato E.G., Prandi C., Ferrali A., Guarna A., Deagostino A., Venturello P. Synthesis of alpha-acyl-functionalized azacycles by Pd-catalyzed cross-coupling reactions of alpha-alkoxyboronates with lactam-derived vinyl triflates. J. Org. Chem. 2002;67:7144–7146. doi: 10.1021/jo025930a. [DOI] [PubMed] [Google Scholar]
- 80.Occhiato E.G., Prandi C., Ferrali A., Guarna A., Venturello P. New synthetic approach to cyclopenta-fused heterocycles based upon a mild Nazarov reaction. J. Org. Chem. 2003;68:9728–9741. doi: 10.1021/jo034939p. [DOI] [PubMed] [Google Scholar]
- 81.Cavalli A., Masetti M., Recanatini M., Prandi C., Guarna A., Occhiato E.G. Density functional studies on the Nazarov reaction involving cyclic systems. Chem. Eur. J. 2006;12:2836–2845. doi: 10.1002/chem.200501391. [DOI] [PubMed] [Google Scholar]
- 82.Cavalli A., Pacetti A., Recanatini M., Prandi C., Scarpi D., Occhiato E.G. Predicting Reactivity and Stereoselectivity in the Nazarov Reaction: A Combined Computational and Experimental Study. Chem. Eur. J. 2008;14:9292–9304. doi: 10.1002/chem.200801030. [DOI] [PubMed] [Google Scholar]
- 83.Prandi C., Deagostino A., Venturello P., Occhiato E.G. Stereoselective synthesis of spirocyclic ketones by Nazarov reaction. Org. Lett. 2005;7:4345–4348. doi: 10.1021/ol051464a. [DOI] [PubMed] [Google Scholar]
- 84.Deagostino A., Prandi C., Zavattaro C., Venturello P. N-Functionalization of azoles through coupling reactions with alkoxydienyl and alkoxystyryl boronic esters. Eur. J. Org. Chem. 2007:1318–1323. doi: 10.1002/ejoc.200600872. [DOI] [Google Scholar]
- 85.Wu X.-F., Neumann H., Beller M. Synthesis of Heterocycles via Palladium-Catalyzed Carbonylations. Chem. Rev. 2012 doi: 10.1021/cr300100s. [DOI] [PubMed] [Google Scholar]
- 86.Kertesz M., Choi C.H., Yang S. Conjugated Polymers and Aromaticity. Chem. Rev. 2005;105:3448–3481. doi: 10.1021/cr990357p. [DOI] [PubMed] [Google Scholar]
- 87.Kaye S., Fox J.M., Hicks F.A., Buchwald S.L. The Use of Catalytic Amounts of CuCl and Other Improvements in the Benzyne Route to Biphenyl-Based Phosphine Ligands. Adv. Synth. Catal. 2001;343:789–794. doi: 10.1002/1615-4169(20011231)343:8<789::AID-ADSC789>3.0.CO;2-A. [DOI] [Google Scholar]
- 88.Kotha S., Lahiri K., Kashinath D. Recent applications of the Suzuki-Miyaura cross-coupling reaction in organic synthesis. Tetrahedron. 2002;58:9633–9695. doi: 10.1016/S0040-4020(02)01188-2. [DOI] [Google Scholar]
- 89.Bhattacharya C., Bonfante P., Deagostino A., Kapulnik Y., Larini P., Occhiato E.G., Prandi C., Venturello P. A new class of conjugated strigolactone analogues with fluorescent properties: Synthesis and biological activity. Org. Biomol. Chem. 2009;7:3413–3420. doi: 10.1039/b907026e. [DOI] [PubMed] [Google Scholar]
- 90.Prandi C., Occhiato E.G., Tabasso S., Bonfante P., Novero M., Scarpi D., Bova M.E., Miletto I. New Potent Fluorescent Analogues of Strigolactones: Synthesis and Biological Activity in Parasitic Weed Germination and Fungal Branching. Eur. J. Org. Chem. 2011:3781–3793. doi: 10.1002/ejoc.201100616. [DOI] [Google Scholar]
- 91.Cohen M., Prandi C., Occhiato E.G., Tabasso S., Wininger S., Resnick N., Steineberger Y., Koltai H., Kapulnik Y. Structure-Function Relations of Strigolactone Analogues: Activity as Plant Hormones and Plant Interactions. Mol. Plant. 2012 doi: 10.1093/mp/sss1134. [DOI] [PubMed] [Google Scholar]
- 92.Prandi C., Rosso H.N., Lace B., Occhiato E.G., Oppedisano A., Tabasso S., Alberto G., Blangetti M. Strigolactone analogues as molecular probes in chasing the (SLs) receptor/s: Design and synthesis of labeled molecules. Mol. Plant. 2012 doi: 10.1093/mp/sss1133. [DOI] [PubMed] [Google Scholar]
- 93.Fujii N., Mallari J.P., Hansell E.J., Mackey Z., Doyle P., Zhou Y.M., Gut J., Rosenthal P.J., McKerrow J.H., Guy R.K. Discovery of potent thiosemicarbazone inhibitors of rhodesain and cruzain. Bioorg. Med. Chem. Lett. 2005;15:121–123. doi: 10.1016/j.bmcl.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 94.Mallari J.P., Shelat A., Kosinski A., Caffrey C.R., Connelly M., Zhu F., McKerrow J.H., Guy R.K. Discovery of trypanocidal thiosemicarbazone inhibitors of rhodesain and TbcatB. Bioorg. Med. Chem. Lett. 2008;18:2883–2885. doi: 10.1016/j.bmcl.2008.03.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kerr I.D., Wu P., Marion-Tsukamaki R., Mackey Z.B., Brinen L.S. Crystal Structures of TbCatB and Rhodesain, Potential Chemotherapeutic Targets and Major Cysteine Proteases of Trypanosoma brucei. PLoS Negl. Trop. Dis. 2010;4:e701. doi: 10.1371/journal.pntd.0000701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McGrath M.E., Eakin A.E., Engel J.C., McKerrow J.H., Craik C.S., Fletterick R.J. The Crystal Structure of Cruzain: A Therapeutic Target for Chagas’ Disease. J. Mol. Biol. 1995;247:251–259. doi: 10.1006/jmbi.1994.0137. [DOI] [PubMed] [Google Scholar]
- 97.Greenbaum D.C., Mackey Z., Hansell E., Doyle P., Gut J., Caffrey C.R., Lehrman J., Rosenthal P.J., McKerrow J.H., Chibale K. Synthesis and Structure-Activity Relationships of Parasiticidal Thiosemicarbazone Cysteine Protease Inhibitors against Plasmodium falciparum, Trypanosoma brucei, and Trypanosoma cruzi. J. Med. Chem. 2004;47:3212–3219. doi: 10.1021/jm030549j. [DOI] [PubMed] [Google Scholar]
- 98.Sessions E.H., Jacobi P.A. Studies on the Synthesis of Furanosteroids. I. Viridin Models. Org. Lett. 2006;8:4125–4128. doi: 10.1021/ol061697h. [DOI] [PubMed] [Google Scholar]
- 99.Brian P.W., MCGOWAN J.G. Viridin: A highly fungistatic substance produced by Trichoderma viride. Nature. 1945;156:144–145. doi: 10.1038/156144a0. [DOI] [Google Scholar]
- 100.Powis G., Bonjouklian R., Berggren M.M., Gallegos A., Abraham R., Ashendel C., Zalkow L., Matter W.F., Dodge J., Grindey G., et al. Wortmannin, a Potent and Selective Inhibitor of Phosphatidylinositol-3-kinase. Cancer Res. 1994;54:2419–2423. [PubMed] [Google Scholar]
- 101.Ward S., Sotsios Y., Dowden J., Bruce I., Finan P. Therapeutic Potential of Phosphoinositide 3-Kinase Inhibitors. Chem. Biol. 2003;10:207–213. doi: 10.1016/S1074-5521(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 102.Neumann H., Brennführer A., Beller M. A General Synthesis of Diarylketones by Means of a Three-Component Cross-Coupling of Aryl and Heteroaryl Bromides, Carbon Monoxide, and Boronic acids. Chem. Eur. J. 2008;14:3645–3652. doi: 10.1002/chem.200800001. [DOI] [PubMed] [Google Scholar]
- 103.Del Rio I., Ruiz N., Claver C., van der Veen L.A., van Leeuwen P. Hydroxycarbonylation of styrene with palladium catalysts The influence of the mono- and bidentate phosphorus ligand. J. Mol. Catal. A.-Chem. 2000;161:39–48. doi: 10.1016/S1381-1169(00)00359-9. [DOI] [Google Scholar]
- 104.Arthuis M., Pontikis R., Chabot G.G., Quentin L., Scherman D., Florent J.-C. Domino approach to 2-aroyltrimethoxyindoles as novel heterocyclic combretastatin A4 analogues. Eur. J. Med. Chem. 2011;46:95–100. doi: 10.1016/j.ejmech.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 105.West C.M.L., Price P. Combretastatin A4 phosphate. Anti-Cancer Drugs. 2004;15:179–187. doi: 10.1097/00001813-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 106.Siemann D.W., Chaplin D.J., Walicke P.A. A review and update of the current status of the vasculature-disabling agent combretastatin-A4 phosphate (CA4P) Expert Opin. Investig. Drugs. 2009;18:189–197. doi: 10.1517/13543780802691068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Corey E.J., Fuchs P.L. A synthetic method for formyl→ethynyl conversion (RCHO→’RC≡CH or RC≡CR') Tetrahedron Lett. 1972;13:3769–3772. doi: 10.1016/S0040-4039(01)94157-7. [DOI] [Google Scholar]
- 108.Fang Y.-Q., Lautens M. A Highly Selective Tandem Cross-Coupling of gem-Dihaloolefins for a Modular, Efficient Synthesis of Highly Functionalized Indoles. J. Org. Chem. 2007;73:538–549. doi: 10.1021/jo701987r. [DOI] [PubMed] [Google Scholar]
- 109.Li H., Yang M., Qi Y., Xue J. Ligand-Free Pd-Catalyzed Carbonylative Cross-Coupling Reactions under Atmospheric Pressure of Carbon Monoxide: Synthesis of Aryl Ketones and Heteroaromatic Ketones. Eur. J. Org. Chem. 2011:2662–2667. doi: 10.1002/ejoc.201001685. [DOI] [Google Scholar]
- 110.Qureshi Z.S., Deshmukh K.M., Tambade P.J., Bhanage B.M. A Simple, Efficient, and Recyclable Phosphine-Free Catalytic System for Carbonylative Suzuki Coupling Reaction of Aryl and Heteroaryl Iodides. Synthesis. 2011:243–250. doi: 10.1002/chin.201121089. [DOI] [Google Scholar]
- 111.Pickup P.G. Conjugated metallopolymers. Redox polymers with interacting metal based redox sites. J. Mater. Chem. 1999;9:1641–1653. doi: 10.1039/a902244i. [DOI] [Google Scholar]
- 112.Rice C.R., Worl S., Jeffery J.C., Paul R.L., Ward M.D. New multidentate ligands for supramolecular coordination chemistry: Double and triple helical complexes of ligands containing pyridyl and thiazolyl donor units. J. Chem. Soc. Dalton Trans. 2001:550–559. doi: 10.1039/b007922g. [DOI] [Google Scholar]
- 113.Ishikura M., Kamada M., Terashima M. An Efficient Synthesis of 3-Heteroarylpyridines via Diethyl-(3-pyridyl)-borane. Synthesis. 1984:936–938. doi: 10.1055/s-1984-31026. [DOI] [Google Scholar]
- 114.Thompson W.J., Jones J.H., Lyle P.A., Thies J.E. An efficient synthesis of arylpyrazines and bipyridines. J. Org. Chem. 1988;53:2052–2055. doi: 10.1021/jo00244a037. [DOI] [Google Scholar]
- 115.Mitschke U., Bauerle P. The electroluminescence of organic materials. J. Mater. Chem. 2000;10:1471–1507. doi: 10.1039/a908713c. [DOI] [Google Scholar]
- 116.Wang C., Jung G.-Y., Hua Y., Pearson C., Bryce M.R., Petty M.C., Batsanov A.S., Goeta A.E., Howard J.A.K. An Efficient Pyridine- and Oxadiazole-Containing Hole-Blocking Material for Organic Light-Emitting Diodes: Synthesis, Crystal Structure, and Device Performance. Chem. Mater. 2001;13:1167–1173. doi: 10.1021/cm0010250. [DOI] [Google Scholar]
- 117.Chapman G.M., Stanforth S.P., Tarbit B., Watson M.D. Arylated pyridines: Suzuki reactions of O-substituted 2,6-dihalogenated-3-hydroxypyridines. J. Chem. Soc. Perkin Trans. 1. 2002:581–582. doi: 10.1039/b111620g. [DOI] [Google Scholar]