Abstract
Starting from theophylline (1,3-dimethylxanthine) new thiazolidin-4-one derivatives 7a1–7, 7b1–7 have been synthesized as potential antidiabetic drugs. The structure of the new derivatives was confirmed using spectral methods (FT-IR, 1H-NMR, 13C-NMR). The in vitro antioxidant potential of the synthesized compounds was evaluated according to the ferric reducing power, the total antioxidant activity and the DPPH and ABTS radical scavenging assays. Reactive oxygen species (ROS) and free radicals are considered to be implicated in a variety of pathological events, such as diabetes mellitus and its micro- and macrovascular complications. The results of chemical modulation of the thiazolidin-4-one intermediaries 6a, 6b through condensation with several aromatic aldehydes is the improvement of the antioxidant effect. All benzylidenethiazolidin-4-one derivatives 7a1–7, 7b1–7 are more active than their parent thiazolidin-4-ones. The most active compounds are the ones obtained by reaction of condensation with 4-hydroxybenzaldehyde (compounds 7a5, 7a6), 4-dimethylaminobenzaldehyde (compounds 7a6, 7b6) and 2-nitrobenzaldehyde (compounds 7a7, 7b7).
Keywords: thiazolidin-4-one, xanthine, synthesis, spectral methods, antioxidant effect
1. Introduction
The thiazolidine ring system represents a very important structural unit in drug discovery [1]. A survey of the literature reveals that extensive studies have been carried out on the synthesis of thiazolidines. These compounds are known to exhibit interesting biological activity such as antimicrobial [2,3,4,5], antitubercular [6], antipsihotic [7], anticancer [8,9], antiviral [10], anti-HIV [11], anti-inflammatory [12,13] and antihyperglycemic [14,15,16,17] effects. As antidiabetic drugs, thiazolidinediones (pioglitazone—Actos® and rosiglitazone—Avandia®, Figure 1) have an important place in type 2 diabetes mellitus therapy [18].
Figure 1.
The structure of pioglitazone and rosiglitazone.
Diabetes mellitus, a chronic metabolic disorder, is a major health problem that is approaching pandemic proportions worldwide. It is characterized by absolute or relative deficiencies in insulin secretion and/or insulin action [19]. An inadequate glycaemic control can be the leading cause of cardiovascular disorders, blindness, renal failure and amputations [19,20]. Type 2 diabetes mellitus (T2DM) accounts for over 90% of the disease cases and it is characterized by high levels of glucose resulting from progressive pancreatic β-cell dysfunction caused by chronic insulin resistance [19]. As agonists of nuclear receptor peroxisome proliferator-activated receptor gamma (PPAR-γ), thiazolidinediones (TZD) reduce insulin resistance in the liver and peripheral tissues; increase the intake of insulin-dependent glucose and decrease withdrawal of glucose from the liver [14,18]. On the other hand, the thiazolidine derivatives have been reported to have antioxidant activities that could be effective in the T2DM therapy [21,22,23].
A relationship between reactive oxygen species and many diseases including diabetes, myocardial infarction, neurological disorders, asthma, cancer, rheumatoid arthritis has been observed [24], so in case of diabetic patients, elevated levels of blood glucose is the main cause which leads to an increased production of free radicals, especially reactive oxygen species resulted from glucose auto-oxidation, lipid peroxidation and protein glycosylation. Endothelial cells chronically exposed to oxidative stress lead to diabetes long term complications (micro- and macrovascular) [19].
The latest research regarding antidiabetic drugs has focused on dipeptidyl peptidase IV inhibitors that act by increasing the concentrations of the endogenous incretins GLP-1 and GI, and consequently reducing of fasting post-prandial hyperglycaemia [25]. The most recently marketed inhibitor is linagliptin (Ondero®, Trajenta®, Figure 2), a nonpetidommimetic drug with a xanthine structure, which received approval in 2011 [26]. At the same time xanthine derivatives are known to have antioxidant and radical scavenging activity that contributes to their pharmacological properties [27,28].
Figure 2.
The structure of linagliptin.
Based on the arguments presented above, we are reporting here the design, synthesis and antioxidant effects of new heterocyclic compounds which combine a thiazolidine structure with a xanthine one, as potential antidiabetic agents.
2. Results and Discussion
2.1. Chemistry
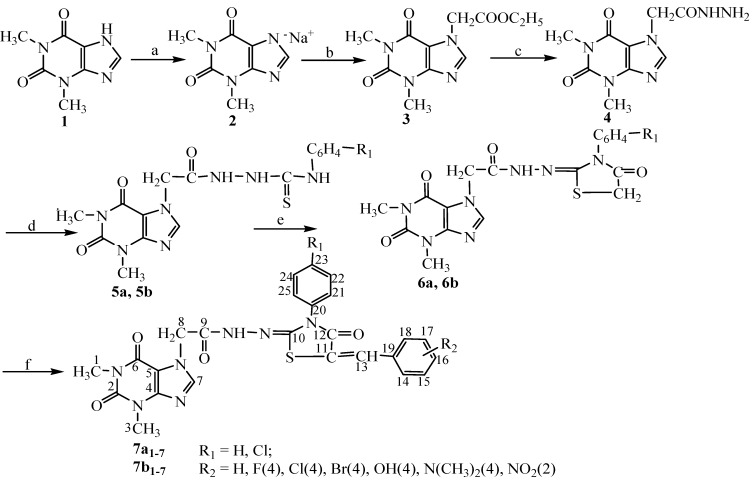
The thiazolidine derivatives 7a1–7; 7b1–7 were prepared using the method summarized in Scheme 1.
Scheme 1.
Synthetic procedure for compounds 7a1–7, 7b1–7.
Reagents and conditions: (a) sodium, absolute methanol; (b) ethyl chloroacetate, ethanol:DMFA, heating 7 h; (c) hydrazine hydrate, ethanol, heating 6 h; (d) phenyl isothiocyanate/4-chlorophenyl isothiocyanate, 1,4-dioxane:DMFA, heating 6 h; (e) chloroacetyl chloride, methanol:chloroform, heating 10 h; (f) aromatic aldehydes, 1,4-dioxane, piperidine, heating 6 h.
Through the esterification of theophylline (1,3-dimethylxanthine, 1) sodium salt 2 with ethylcloroacetate, (1,3-dimethylxanthin-7-yl)ethyl acetate (3) was obtained, which was turned into hydrazide derivative 4 [29]. During the next step this intermediate was reacted with phenylisothiocyanate and 4-chlorophenylisothiocyanate leading to the corresponding thiosemicarbazide derivatives 5a, 5b. By cyclization of 5a, 5b with chloracetyl chloride the corresponding thiazolidin-4-one derivatives 6a, 6b were obtained. The last step of the synthesis consisted in the condensation of the thiazolidin-4-one derivatives with several aromatic aldehydes—benzaldehyde, 4-bromo-/4-fluoro-/4-chloro-/4-hydroxy-/4-dimethylamino-/2-nitrobenzaldehyde.
The structure of the compounds was assigned on the basis of spectral data (IR, 1H-NMR, 13C-NMR). In the IR spectra of the thiosemicarbazide derivatives 5a, 5b the characteristic absorption band of the thione group (C=S) was observed at 1160 cm−1 (5a), and 1188 cm−1 (5b), respectively. The valence vibration of the bond C-Cl (5b) produced a narrow absorption band of medium intensity at 825 cm−1. In the 1H-NMR spectra of the compounds the aromatic protons resonated as multiplets in the 7.19–7.47 ppm region (5a), or the 7.31–7.51 ppm region (5b), respectively, confirming the success of the reaction between hydrazide derivative 4 and the phenyl isothiocyanates. The protons of the xanthine structure were also identified in the spectra of the thiosemicarbazides. The protons of the two methyl groups appeared as two singlets at 3.09 ppm and 3.45 ppm (5a), or at 3.22 ppm and 3.57 ppm (5b), respectively. The proton of the methine group resonated as a singlet at 8.09 ppm, while the protons of the methylene group also resonated as a singlet in the 5.08–5.09 ppm region.
In the IR spectra of thiazolidin-4-ones derivatives 6a, 6b the characteristic band of the thione group (C=S) disappeared and the appearance of the C-S bond characteristic of the thiazolidin-4-one it was observed in the range of 690–695 cm−1. The 1H-NMR spectra of the compounds showed the methylene protons of the thiazolidin-4-one ring at 3.67 ppm (6a) and at 3.78 ppm (6b) resonating as a singlet. That confirmed the formation of the thiazolidin-4-one ring.
The structure of the benzylidenethiazolidin-4-one derivatives 7a1–7, 7b1–7 was confirmed by the appearance in the 1H-NMR spectra of the proton signal characteristic for the methine group from the benzilidene thiazolidin-4-one ring as a sing let in the range of 7.28–7.38 ppm (7a1–7) and at 7.24–7.51 ppm (7b1–7). In the 13C-NMR spectra the characteristic signal for this methine group appeared in the 140.35–143.07 ppm range (7a1–7), or 138.51–142.84 (7b1–7), respectively.
2.2. Biological Evaluation
2.2.1. Ferric Reducing Power
The measurement of the reducing power defines an important aspect of the compounds’ antioxidant activity. In the presence of the electron-donating compounds, the potassium ferric/ferricyanide complex is reduced to its ferrous form (Fe2+). The amount of Fe2+ is then quantitatively monitored by measuring the intensity of the Perls Prussian blue colour complex formed by reaction with ferric chloride, at 700 nm [30]. The reaction between the ferrous form and the ferric chloride is:
| 4FeCl3 + 3K4[Fe(CN)6] → Fe[Fe(CN)6]3 + 12KCl |
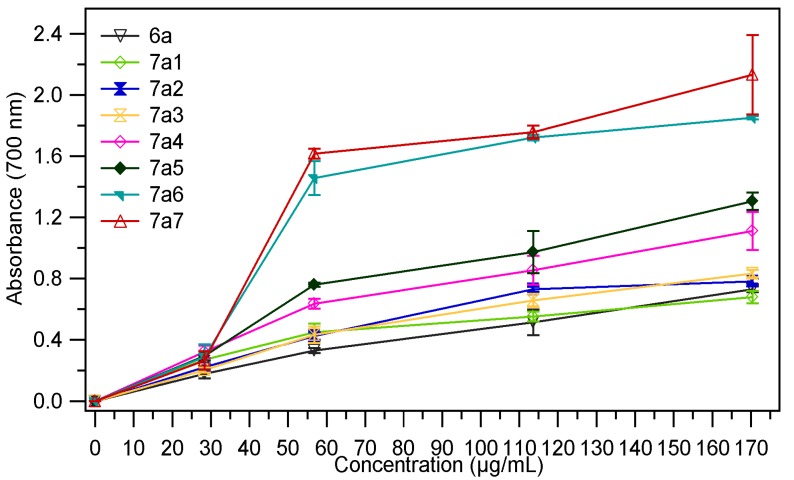
The absorbance value of the samples at different concentrations (28.4 µg/mL, 56.8 µg/mL, 113.63 µg/mL and 170.45 µg/mL) are presented in Figure 3, Figure 4. The results expressed as EC50 values (mg/mL) are shown in Table 1. Low values of EC50 demonstrate a higer ferric reducing power.
Figure 3.
The absorbance of 7a1–7 in ref. to 6a.
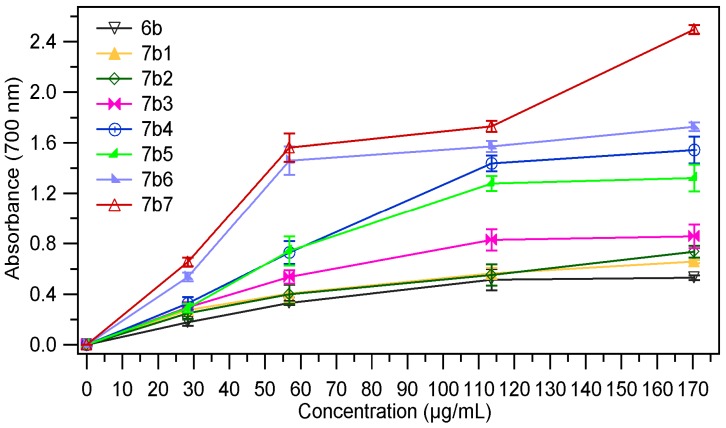
Figure 4.
The absorbance of 7b1–7 in ref. to 6b.
Table 1.
The ferric reducing power (EC50 mg/mL) of the derivatives6a, 6b, 7a1–7, 7b1–7.
| Sample | EC50 mg/mL | Sample | EC50 mg/mL |
|---|---|---|---|
| 6a | 0.137 ± 0.0036 | 6b | 0.155 ± 0.0038 |
| 7a1 | 0.109 ± 0.0014 | 7b1 | 0.109 ± 0.0045 |
| 7a2 | 0.097 ± 0.0018 | 7b2 | 0.102 ± 0.0024 |
| 7a3 | 0.055 ± 0.0036 | 7b3 | 0.046 ± 0.0015 |
| 7a4 | 0.096 ± 0.0023 | 7b4 | 0.066 ± 0.0017 |
| 7a5 | 0.033 ± 0.0019 | 7b5 | 0.027 ± 0.0013 |
| 7a6 | 0.033 ± 0.0012 | 7b6 | 0.023 ± 0.0011 |
| 7a7 | 0.058 ± 0.0041 | 7b7 | 0.041 ± 0.0010 |
| AA | 0.0075 ± 0.0002 |
Data are mean ± SD (n = 3, p < 0.005).
As we expected the absorbance of the sample increased with the concentration, thus proving the fact that reducing power of the tested compounds is concentration-dependent. The analysis of the data presented in the Table 1, revealed that the chemical modulation of the thiazolidin-4-one ring through condensation with several aromatic aldehydes had a good influence on the antioxidant potential; all tested compounds being more active than the corresponding thiazolidin-4-ones 6a, 6b. It was also observed that both series of compounds 7a1–7, 7b1–7 have a comparable reducing power. In the series of 3-phenylthiazolidin-4-one derivatives 7a1–7 the most active compounds were those which resulted from condensation with 4-hydroxybenzaldehyde (compound 7a5) and 4-dimethlaminobenzaldehyde (compound 7a6). For these compounds the EC50 values were 0.033 ± 0.0019 (for 7a5) and 0.033 ± 0.0012 (for 7a6), which means that they are about four times more active than the corresponding thiazolidin-4-one (6a, EC50 = 0.137 ± 0.0036). Regarding the compounds 7b1–7 obtained through condensation of 3-(4-chlorophenyl)-thiazolidin-4-one derivative 6b with several aromatic benzaldehydes, we could observe that the most active compounds were 7b5 and 7b6, the corresponding analogues of 7a5 and 7a6. These compounds were about 5.7 times (7b5, EC50 = 0.027 ± 0.0013) and 6.7 times (7b6, EC50 = 0.023 ± 0.0011) more active than the corresponding thiazolidin-4-one derivative (6b, EC50 = 0.155 ± 0.0038). The chemical modulation of the parent thiazolidin-4-one derivatives improved their ferric reducing power, all tested compounds being more active than 3-phenlythiazolidin-4-one, respectively the 3-(4-chlorophenyl)thiazolidin-4-one but they are less active than the ascorbic acid (AA) at the same concentration.
2.2.2. Total Antioxidant Activity
The total antioxidant activity was determined by the formation of phosphomolybdenum blue complex by the reduction of Mo(VI) to Mo(V) under the action of electron donating compounds. The maximum absorption of the complex was recorded at 695 nm [31]. The absorbance values of samples at different concentrations (23, 34, 45, 68, 91 and 136 µg/mL) are presented in Figure 5, Figure 6. The EC50 values (mg/mL) are shown in Table 2. Low values of EC50 indicate a higer effectiveness in antioxidant properties.
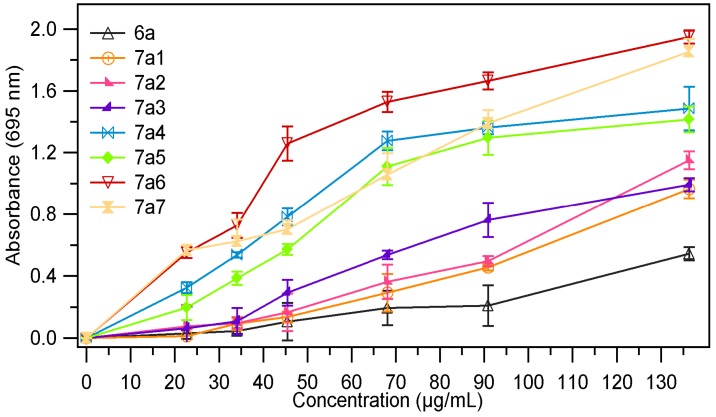
Figure 5.
The absorbance of 7a1–7 in ref. to 6a.
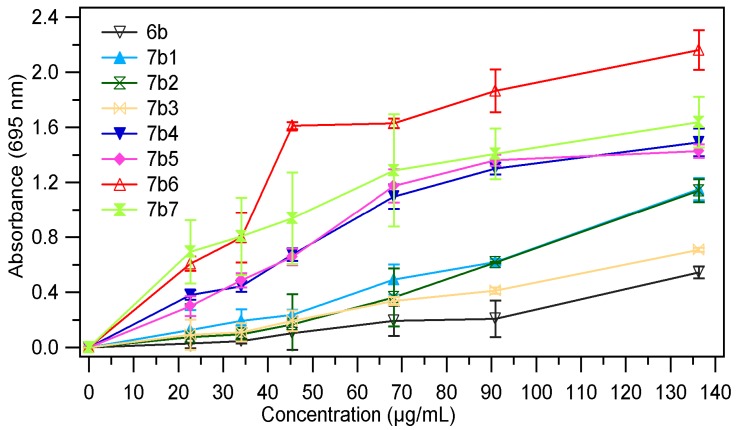
Figure 6.
The absorbance of 7b1–7 in ref. to 6b.
Table 2.
The total antioxidant activity (EC50 mg/mL) of the derivatives 6a, 6b, 7a1–7, 7b1–7.
| Sample | EC50 mg/mL | Sample | EC50 mg/mL |
|---|---|---|---|
| 6a | 0.133 ± 0.0045 | 6b | 0.124 ± 0.0009 |
| 7a1 | 0.105 ± 0.0025 | 7b1 | 0.090 ± 0.0018 |
| 7a2 | 0.099 ± 0.0038 | 7b2 | 0.092 ± 0.0008 |
| 7a3 | 0.048 ± 0.0023 | 7b3 | 0.043 ± 0.0026 |
| 7a4 | 0.076 ± 0.0025 | 7b4 | 0.099 ± 0.0024 |
| 7a5 | 0.025 ± 0.0012 | 7b5 | 0.022 ± 0.0013 |
| 7a6 | 0.033 ± 0.0014 | 7b6 | 0.026 ± 0.0018 |
| 7a7 | 0.037 ± 0.0007 | 7b7 | 0.043 ± 0.0028 |
| AA | 0.0067 ± 0.0003 |
Data are mean ± SD (n = 3, p< 0.05).
The data of this study also support the conclusion that the antioxidant activity of the tested compounds increases with concentration and that all benzylidenethiazolidin-4-one derivatives 7a1–7, 7b1–7 are more active than parent thiazolidin-4-one derivatives (6a, 6b). It was observed that the presence of chloro radical on the phenyl ring at N3 of thiazolidin-4-one has a good influence on the antioxidant properties, the derivatives (7b1–7) being generally more active than their analogues from the 7a1–7 series. In both series the most active compounds were hydroxyl- (7a5, 7b5) and dimethylamino- (7a6, 7b6) analogues. The hydroxyl derivatives, 7a5 (EC50 = 0.025 ± 0.0012) and 7b5 (EC50 = 0.022 ± 0.0013) were 5.3 times and 5.6 times more active than corresponding thiazolidin-4-one, 6a (EC50 = 0.133 ± 0.0045), respectivelly 6b (EC50 = 0.124 ± 0.0009). The antioxidant effects of the dimethylamino analogues (7a6, 7b6) were similar with of hydroxyl derivatives, being 4 times (7a6, EC50 = 0.033 ± 0.0014), respectivelly 4.8 times (7b6, EC50 = 0.026 ± 0.0018), more active than parent compounds (6a, 6b). The tested compounds were less active than ascorbic acid (AA) at the same concentration.
2.2.3. DPPH Radical Scavenging Assay
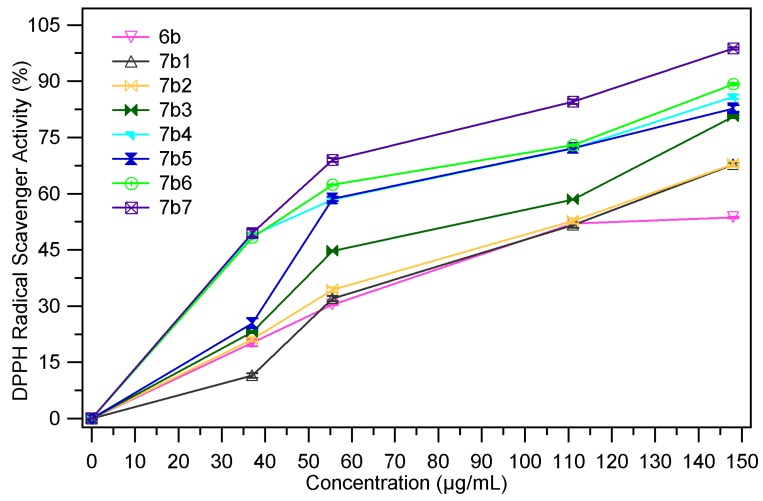
DPPH (1,1-diphenyl-2-picryl-hydrazyl) is a stable free radical with a violet colour that, under the action of proton donating compounds, is reduced to a light yellow colour that this change can be monitored at 517 nm [32]. The DPPH radical scavenging ability (%) of samples at different concentrations (37 µg/mL, 55.55 µg/mL, 111.11 µg/mL and 144.80 µg/mL) are presented in Figure 7, Figure 8. The EC50 values (mg/mL) are shown in Table 3. Low values of EC50 indicate a higer effectiveness in DPPH scavenging ability.
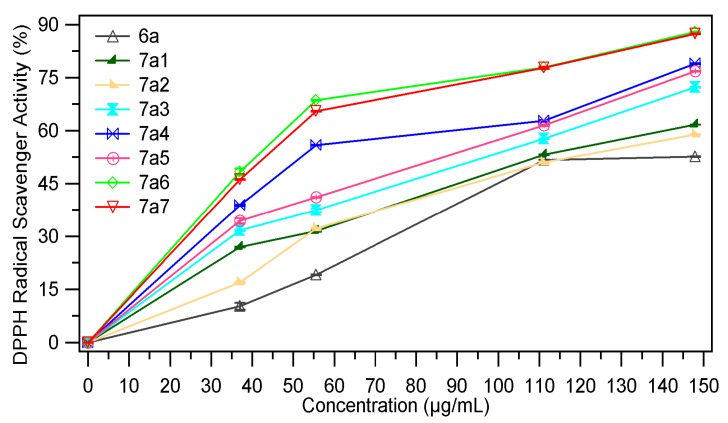
Figure 7.
The scavenging ability (%) of 7a1–7 in ref. to 6a.
Figure 8.
The scavenging ability (%) of 7b1–7 in ref. to 6b.
Table 3.
The DPPH scavenging ability (EC50 mg/mL) of the derivatives 6a, 6b, 7a1–7, 7b1–7.
| Sample | EC50 mg/mL | Sample | EC50 mg/mL |
|---|---|---|---|
| 6a | 0.136 ± 0.0006 | 6b | 0.127 ± 0.0013 |
| 7a1 | 0.094 ± 0.0013 | 7b1 | 0.106 ± 0.0011 |
| 7a2 | 0.107 ± 0.0018 | 7b2 | 0.101 ± 0.0044 |
| 7a3 | 0.066 ± 0.0011 | 7b3 | 0.049 ± 0.0006 |
| 7a4 | 0.077 ± 0.0005 | 7b4 | 0.085 ± 0.0017 |
| 7a5 | 0.038 ± 0.0044 | 7b5 | 0.038 ± 0.0024 |
| 7a6 | 0.039 ± 0.0008 | 7b6 | 0.037 ± 0.0037 |
| 7a7 | 0.048 ± 0.0001 | 7b7 | 0.038 ± 0.0014 |
| AA | 0.0065 ± 0.0031 |
Data are mean ± SD (n = 3, p< 0.05).
The chemical modulation of the thiazolidin-4-one derivatives 6a, 6b through condensation with several aromatic benzaldehydes determines the improvement of the DPPH radical scavenging ability, as both series of compounds 7a1–7 and 7b1–7 were more active than their parents. In reference to compound 6a, its benzylidene analogues 7a1–6 are 1.5–3.5 times more active. Under similar conditions the compounds 7b1–7 are slightly less active, being 1.2–3.4 times more active than the corresponding thiazolidin-4-one 6b. The most active compounds are hydroxyl- (compounds 7a5, 7b5), dimethylamino- (compounds 7a6, 7b6) and nitro- (compounds 7a7, 7b7) analogues.
2.2.4. ABTS Radical Scavenging Assay
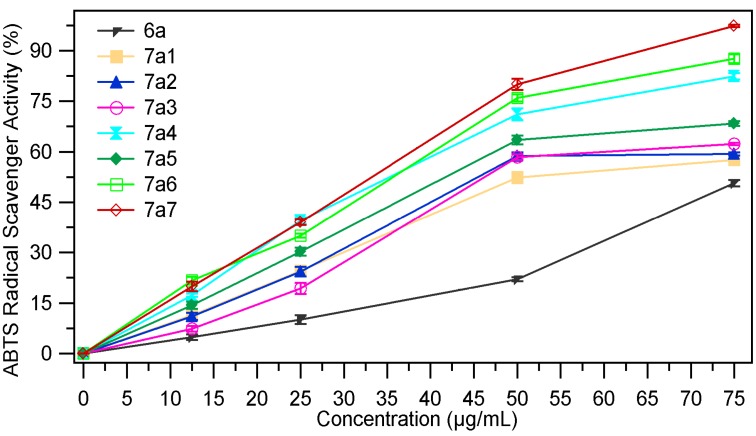
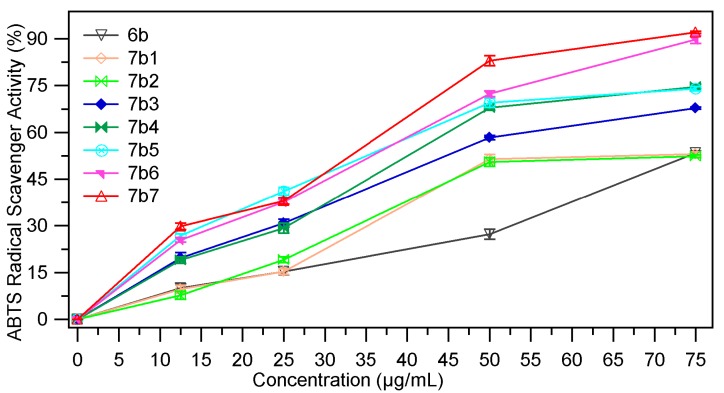
The ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) radical cation decolourisation assay is based on the ability of the hydrogen donating antioxidants to scavenge the long-life radical cation ABTS+. The ABTS+ which is a blue chromophore is generated by the reaction between 2.2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) and ammonium persulfate. The antioxidant compound produces a discoloration of the solution with a decrease in the absorbance measured at 734 nm [33]. The ABTS radical scavenging ability (%) of the samples at different concentrations (12.5 µg/mL, 25 µg/mL, 50 µg/mL and 75 µg/mL) are presented in Figure 9, Figure 10. The EC50 values (mg/mL) are presented in Table 4. Low values of EC50 indicate a higer effectiveness in ABTS scavenging ability.
Figure 9.
The scavenging ability (%) of 7a1–7 in ref. with 6a.
Figure 10.
The scavenging ability (%) of 7b1–7 in ref. with 6b.
Table 4.
The ABTS scavenging ability (EC50 mg/mL) of the derivatives 6a, 6b, 7a1–7, 7b1–7.
| Sample | EC50 mg/mL | Sample | EC50 mg/mL |
|---|---|---|---|
| 6a | 0.074 ± 0.0039 | 6b | 0.069 ± 0.0031 |
| 7a1 | 0.047 ± 0.0032 | 7b1 | 0.048 ± 0.0014 |
| 7a2 | 0.049 ± 0.0026 | 7b2 | 0.041 ± 0.0041 |
| 7a3 | 0.037 ± 0.0015 | 7b3 | 0.031 ± 0.0035 |
| 7a4 | 0.043 ± 0.0028 | 7b4 | 0.040 ± 0.0029 |
| 7a5 | 0.027 ± 0.0017 | 7b5 | 0.024 ± 0.0047 |
| 7a6 | 0.025 ± 0.0012 | 7b6 | 0.021 ± 0.0041 |
| 7a7 | 0.036 ± 0.0098 | 7b7 | 0.030 ± 0.0017 |
| AA | 0.0055 ± 0.0024 |
Data are mean ± SD (n = 3, p< 0.05).
From the data presented in Table 4 it is obvious that the ABTS radical scavenging ability of the benzylidenethiazolidin-4-one derivatives 7a1–7, 7b1–7 is higher than that of the thiazolidin-4-ones 6a, 6b. As in the DPPH radical scavenging assay the most active compounds are the derivatives obtained by condensation of thiazolidin-4-ones with 4-hydroxy-, 4-dimethylamino- and 2-nitrobenzaldehydes. The hydroxy- (compounds 7a5, 7b5) and dimethylamino- (compounds 7a6, 7b6) analogues are about three times more active, while the nitro- (compounds 7a7, 7b7) are two times more active than the corresponding thiazolidin-4-ones 6a, 6b, but they are less active than ascorbic acid (AA) at the same concentration.
3. Experimental
3.1. General Procedures
The melting points were measured using a Buchi Melting Point B-540 apparatus and they are uncorrected. The FT-IR spectra were recorded on an ABB Bomen MB3000 spectrometer, over a 500–4,000 cm−1 range, after 32 scans at a resolution of 4 cm−1. The spectra processing was carried out with the Horizon MBTM FTIR Software. The 1H-NMR and 13C-NMR spectra were obtained on a Bruker Avance DRX-300 spectrometer using tetramethylsilane as internal standard and DMSO-d6 as solvent. The chemical shifts were shown in δ values (ppm). The progress of the reaction was monitored on TLC, using pre-coated Kieselgel 60 F254 plates (Merck) and the compounds were visualized by using UV light.
3.2. Synthetic Procedures
3.2.1. General Procedure for the Preparation of 1-[2-(1,3-Dimethylxanthin-7-yl)acetyl]-4-(4-R-phenyl)-thiosemicarbazides 5a, 5b
4-R-phenyl isothiocyanate (R = H, Cl; 23 mmol) was added to a solution of (1,3-dimethylxanthin-7-yl)acetyl hydrazine (7 g, 23 mmol) in mixture of 1,4-dioxane (250 mL) and DMFA (100 mL), and then heated under reflux for 6 hours. After cooling at room temperature the white solid was filtered off, dried and recrystallized from ethanol: 1,4-dioxane (3:1).
1-[2-(1,3-Dimethylxanthin-7-yl)acetyl]-4-(phenyl)thiosemicarbazides (5a). Yield 89%, m.p. 230–231 °C; FT-IR (KBr, cm−1): 3,238, 3,098 (-NH-), 3,012, 1,599, 1,520 (phenyl ring), 1,749 (-C=O amide), 1,680 (C=O), 1,645 (-C=N), 1,470 (-CH2-), 1,206 (-C-N), 1,160 (-C=S); 1H-NMR (DMSO-d6) δ in ppm: 8.09 (s, 1H, -CH=N); 8.03 (d, 1H, -NH-); 7.47, 7.35, 7.19 (dm, 5H, Ar-H); 5.09 (s, 2H, -CH2-); 3.45, 3.09 (s, 6H, 2CH3); 2.00 (s, 2H, -NH-C(=S)-NH-).
1-[2-(1,3-Dimethylxanthin-7-yl)acetyl]-4-(4-chlorophenyl)thiosemicarbazides (5b). Yield 84%, m.p. 240–242 °C; FT-IR (KBr, cm−1): 3,285, 3,115 (-NH-), 3,040, 3,013, 1,591, 1,531 (phenyl ring), 1,693 (C=O), 1,645 (-C=N), 1,474 (-CH2), 1,225 (-C-N), 1,188 (-C=S), 825 (C-Cl); 1H-NMR (DMSO-d6) δ in ppm: 8.09 (s, 1H, -CH=N); 7.99 (d, 1H, -NH-); 7.51, 7.31 (d, 4H, Ar-H), 5.08 (s, 2H, -CH2-); 3.57, 3.22 (s, 6H, 2CH3); 2.04 (s, 2H, -NH-C(=S)-NH-).
3.2.2. General Procedure for the Preparation of 2-{2-[2-(1,3-Dimethylxanthin-7-yl)acetyl]hydrazono}-3-(4-R-phenyl) Thiazolidin-4-ones (R = H, Cl) 6a, 6b
Chloroacetyl chloride (39 mmol) was added slowly at room temperature to a solution of 1-[2-(1,3-dimethylxanthin-7-yl)acetyl]-4-(4-R-phenyl)thiosemicarbazides 5a, 5b (13 mmol) in a mixture of methanol-chloroform (1:1, 250 mL). After 10 h of heating under reflux a yellow clear solution was obtained. The solvent was removed under reduced pressure and the residue was washed with cold water, (100 mL), filtered off, dried and recrystallized from ethanol 96%. The progress of the reaction was monitored by silica gel coated TLC plates.
2-{2-[2-(1,3-Dimethylxanthin-7-yl)acethyl]hydrazono}-3-(phenyl)thiazolidin-4-one (6a). Yield 52%, m.p. 239–241 °C; FT-IR (KBr, cm−1): 3,310, 3,201, 3,145 (-NH-), 3,048, 1,604, 1,553 (phenyl ring), 1,701 (-C=O), 1,642 (C=N), 1,471 (-CH2-), 1,234 (C-N), 690 (C-S); 1H-NMR (DMSO-d6) δ in ppm: 8.28 (s, 1H, -CH=N); 7.55, 7.33, 6.99 (dm, 5H, Ar-H); 7.01 (s, 1H, -NH-); 5.17 (s, 2H, -CH2-); 3.67 (s, 2H, S-CH2-); 3.44, 3.23 (s, 6H, 2CH3).
2-{2-[2-(1,3-Dimethylxanthin-7-yl)acethyl]hydrazono}-3-(4-chlorphenyl)thiazolidin-4-one (6b). Yield 50%, m.p. 248–249 °C; FT-IR (KBr, cm−1): 3,312, 3,223, 3,179 (-NH-), 3,051, 1,603, 1,547 (phenyl ring), 1,696 (-C=O), 1,644 (C=N), 1,466 (-CH2-), 1,216 (C-N), 812 (C-Cl), 695 (C-S); 1H-NMR 1H-NMR (DMSO-d6) δ in ppm: 7.99 (s, 1H, -CH=N ); 7.04 (s, 1H, -NH-); 7.52, 7.45 (d, 4H, Ar-H); 3.78 (s, 2H, S-CH2-); 5.08 (s, 2H, -CH2-); 3.41, 3.17 (s, 6H, 2CH3).
3.2.3. General Procedure for the Preparation of 2-{2-[2-(1,3-Dimethylxanthin-7-yl)acetyl]hydrazono}-3-(4-R1-phenyl-5-(R2-benzyliden)thiazolidin-4-ones 7a1-7, 7b1-7
The aromatic aldehydes (79 mmol) and piperidine (79 mmol) as catalyst were added slowly to a solution of 2-{2-[2-(1,3-dimethylxanthin-7-yl)acethyl]hydrazono}-3-(4-R1-phenyl)thiazolidine-4-one 6a, 6b (79 mmol) in 1,4-dioxane (100 mL). The mixture of reaction was heated under reflux for 6 h and then the solvent was removed under reduced pressure. The crude product was precipitated in cold water, filtered off, washed with cold water and recrystallised from 1,4- dioxane.
2-{2-[2-(1,3-Dimethylxanthin-7-yl)acethyl]hydrazono}-3-phenyl-5-(benzyliden)thiazolidin-4-one (7a1). Yield 72%, m.p. 260–262 °C; FT-IR (KBr, cm−1): 3,255, 3,191 (-NH-), 3,082, 1,598, 1,547 (phenyl ring), 1,696 (-C=O), 1,641 (C=N), 1,376 (-CH2-), 1,229 (C-N), 680 (C-S-C); 1H-NMR (DMSO-d6) δ in ppm: 8.27 (s, 1H, -CH=N-); 7.64–6.99 (dm, 10H, Ar-H); 7.35 (s, 1H, C=CH-); 6.92 (s, 1H, -NH-); 5.79 (s, 2H, -CH2-); 3.44, 3.23 (s, 6H, 2CH3); 13C-NMR (DMSO-d6) δ in ppm: 173.70 (C9), 165.63 (C12), 154.44, 150.95 (C6, C2), 154.05 (C10), 148.41 (C4), 146.60 (C7), 142.94 (C13), 135.27 (C19), 132.19 (C20), 129.08 (C22, C24), 128.68 (C15, C17), 127.99 (C16), 127.52 (C14, C18), 124.77 (C23), 121.97 (C21, C25), 117.37 (C11), 105.77 (C5), 30.65 (C8), 29.47, 27.5 (C1, C3).
2-{2-[2-(1,3-Dimethylxanthin-7-yl)acethyl]hydrazono}-3-phenyl-5-(4-fluorobenzyliden)thiazolidin-4-one (7a2). Yield 70%, m.p. 263–265 °C; FT-IR (KBr, cm−1): 3,254, 3,138 (-NH-), 3,082, 1,599, 1,547 (phenyl ring), 1,696 (-C=O), 1,642 (C=N), 1,352 (-CH2-), 1,229 (C-N), 1,153 (C-F), 680 (C-S-C); 1H-NMR (DMSO-d6) δ in ppm: 8.18 (s, 1H, -CH=N-); 7.50–6.96 (dm, 9H, Ar-H); 7.28 (s, 1H, C=CH-); 6.89 (s, 1H, -NH-); 5.73 (s, 2H, -CH2-); 3.40, 3.18 (s, 6H, 2CH3); 13C-NMR (DMSO-d6) δ in ppm: 172.29 (C9), 166.36 (C12), 163.12 (C16), 155.11, 154.96 (C6, C2), 151.63 (C10), 148.75 (C4), 146.67 (C7), 143.07 (C13), 133.24 (C19), 129.65 (C20), 129.26 (C22, C24), 128.83 (C14, C18), 124.19 (C23), 122.71 (C21, C25), 118.05 (C11), 117.85 (C15, C17), 106.36 (C5), 31.06 (C8), 30.04, 28.09 (C1, C3).
2-{2-[2-(1,3-Dimethylxanthin-7-yl)acethyl]hydrazono}-3-phenyl-5-(4-chlorobenzyliden)thiazolidin-4-one (7a3). Yield 74%, m.p. 235–237 °C; FT-IR (KBr, cm−1): 3,254, 3,140 (-NH-), 3,082, 1,598, 1,547 (phenyl ring), 1,696 (-C=O), 1,641 (C=N), 1,350 (-CH2-), 1,229 (C-N), 804 (C-Cl), 680 (C-S-C); 1H-NMR (DMSO-d6) δ in ppm: 8.24 (s, 1H, -CH=N-); 7.55-6.99 (dm, 9H, Ar-H); 7.34 (s, 1H, C=CH-); 6.94 (s, 1H, -NH-); 5.77 (s, 2H, -CH2-); 3.42, 3.21 (s, 6H, 2CH3); 13C-NMR (DMSO-d6) δ in ppm: 174.41 (C9), 166.53 (C12), 154.95, 154.82 (C6, C2), 151.52 (C10), 148.86 (C4), 145.41 (C7), 140.76 (C13), 133.87 (C19), 134.02 (C16), 133.35 (C20), 129.61 (C22, C24), 129.26 (C15, C17), 127.88 (C14, C18), 125.47 (C23), 122.66 (C21, C25), 117.93 (C11), 106.24 (C5), 30.01 (C8), 29.03, 28.04 (C1, C3).
2-{2-[2-(1,3-Dimethylxanthin-7-yl)acethyl]hydrazono}-3-phenyl-5-(4-bromobenzyliden)thiazolidin-4-one (7a4). Yield 62%, m.p. 258–260 °C; FT-IR (KBr, cm−1): 3,254, 3,139 (-NH-), 3,081, 1,599, 1,547 (phenyl ring), 1,696 (-C=O), 1,642 (C=N), 1,352 (-CH2-), 1,229 (C-N), 680 (C-S-C), 660 (C-Br); 1H-NMR (DMSO-d6) δ in ppm: 8.27 (s, 1H, -CH=N-); 7.55–6.99 (dm, 9H, Ar-H); 7.38 (s, 1H, C=CH-); 6.92 (s, 1H, -NH-); 5.84 (s, 2H, -CH2-); 3.48, 3.26 (s, 6H, 2CH3); 13C-NMR (DMSO-d6) δ in ppm: 172.81 (C9), 165.63 (C12), 155.29, 154.44 (C6, C2), 151.22 (C10), 148.41 (C4), 146.87 (C7), 140.35 (C13), 134.63 (C19), 133.45 (C20), 131.57 (C15, C17), 129.06 (C22, C24), 128.44 (C14, C18), 125.87 (C23), 123.54 (C21, C25), 121.83 (C16), 117.38 (C11), 106.88 (C5), 31.07 (C8), 29.48, 27.51 (C1, C3).
2-{2-[2-(1,3-Dimethylxanthin-7-yl)acethyl]hydrazono}-3-phenyl-5-(4-hydroxybenzyliden)thiazolidin-4-one (7a5). Yield 78%, m.p. 243–245 °C; FT-IR (KBr, cm−1): 3,267 (OH), 3,253, 3,138 (-NH-), 3,081, 1,598, 1,547 (phenyl ring), 1,695 (-C=O), 1,641 (C=N), 1,350 (-CH2-), 1229 (C-N), 1152 (C-O), 680 (C-S-C); 1H-NMR (DMSO-d6) δ in ppm: 8.28 (s, 1H, -CH=N-); 7.75–6.99 (dm, 9H, Ar-H), 7.35 (s, 1H, C=CH-); 6.93 (s, 1H, -NH-); 5.79 (s, 2H, -CH2-); 3.32, 3.23 (s, 6H, 2CH3); 13C-NMR (DMSO-d6) δ in ppm: 173.33 (C9), 165.64 (C12), 158.33 (C16), 154.44, 154.21 (C6, C2), 150.95 (C10), 148.41 (C4), 146.47 (C7), 140.36 (C13), 132.06 (C20), 129.05 (C22, C24), 127.99 (C14, C18, C19), 125.27 (C23), 123.37 (C21, C25), 117.37 (C11), 115.79 (C15, C17), 105.77 (C5), 31.44 (C8), 29.47, 27.51 (C1, C3).
2-{2-[2-(1,3-Dimethylxanthin-7-yl)acethyl]hydrazono}-3-phenyl-5-(4-N-dimethylaminobenzyliden) thiazolidin-4-one (7a6). Yield 88%, m.p. 255–257 °C; FT-IR (KBr, cm−1): 3,256, 3,139 (-NH-), 1,599, 1,549 (phenyl ring), 1,699 (-C=O), 1,643 (C=N), 1,353 (-CH2-), 1,230 (C-N), 681 (C-S-C); 1H-NMR (DMSO-d6) δ in ppm: 8.19 (s, 1H, -CH=N-); 7.52-6.96 (dm, 9H, Ar-H); 7.35 (s, 1H, C=CH-); 6.92 (s, 1H, -NH-); 5.74 (s, 2H, -CH2-); 3.41, 3.19 (s, 6H, 2CH3); 2.52, 2.49 (s, 6H, 2CH3, N(CH3)2); 13C-NMR (DMSO-d6) δ in ppm: 173.23 (C9), 166.29 (C12), 154.95, 154.65 (C6, C2), 151.59 (C10), 148.79 (C4), 148.46 (C16), 147.26 (C7), 140.63 (C13), 132.07 (C20), 129.63 (C14, C18), 129.05 (C22, C24), 127.26 (C23), 124.64 (C19), 122.85 (C21, C25), 117.98 (C11), 115.62 (C15, C17), 106.33 (C5), 39.87 (2C, N(CH3)2), 33.45 (C8), 30.02, 28.07 (C1, C3).
2-{2-[2-(1,3-Dimethylxanthin-7-yl)acethyl]hydrazono}-3-phenyl-5-(2-nitrobenzyliden)thiazolidin-4-one (7a7). Yield 91%, m.p. 247–250 °C; FT-IR (KBr, cm−1): 3,255, 3,139 (-NH-), 3,082, 1,598, 1,547 (phenyl ring), 1,696 (-C=O), 1,641 (C=N), 1,499 (NO2), 1,350 (-CH2-), 1,229 (C-N), 680 (C-S-C); 1H-NMR (DMSO-d6) δ in ppm: 8.22 (s, 1H, -CH=N-); 8.21–6.99 (dm, 9H, Ar-H), 7.34 (s, 1H, C=CH-); 6.91 (s, 1H, -NH-); 5.76 (s, 2H, -CH2-); 3.42, 3.21 (s, 6H, 2CH3); 13C-NMR (DMSO-d6) δ in ppm: 170.61 (C9), 166.22 (C12), 155.08, 154.85 (C6, C2), 151.54 (C10), 148.84 (C4), 145.40 (C18), 144.45 (C7), 140.99 (C13), 133.91 (C20), 131.53 (C15), 130.12 (C19), 129.61 (C22, C24), 128.41 (C16), 127.63 (C14), 126.37 (C23), 122.72 (C21, C25), 121.11 (C17), 117.94 (C11), 106.31 (C5), 31.11 (C8), 30.01, 28.05 (C1, C3).
2-{2-[2-(1,3-Dimethylxanthin-7-yl)acethyl]hydrazono}-3-(4-chlorophenyl)-5-(benzyliden)thiazolidin-4-one (7b1). Yield 68%, m.p. 265–267 °C; FT-IR (KBr, cm−1): 3,297, 3,118 (-NH-), 2,994, 1,591, 1,545 (phenyl ring), 1,699 (-C=O), 1,639 (C=N), 1,348 (-CH2-), 1,214 (C-N), 777 (C-Cl), 710 (C-S-C); 1H-NMR (DMSO-d6) δ in ppm: 8.06 (s, 1H, -CH=N-); 7.51-7.31 (dm, 9H, Ar-H); 7.24 (s, 1H, C=CH-); 7.01 (s, 1H, -NH-); 5.17 (s, 2H, -CH2-); 3.32, 3.20 (s, 6H, 2CH3); 13C-NMR (DMSO-d6) δ in ppm: 173.73 (C9), 167.63 (C12), 156.18 (C10), 154.39, 150.97 (C6, C2), 148.24 (C4), 146.52 (C7), 142.81 (C13), 135.18 (C19), 132.41 (C20), 128.57 (C22, C24), 128.02 (C15, C17), 127.03 (C16), 126.87 (C14, C18), 120.84 (C23), 119.76 (C21, C25), 117.08 (C11), 106.29 (C5), 29.45 (C8), 29.01, 27.42, (C1, C3).
2-{2-[2-(1,3-Dimethylxanthin-7-yl)acethyl]hydrazono}-3-(4-chlorophenyl)-5-(4-fluorobenzyliden) thiazolidin-4-one (7b2). Yield 70%, m.p. 255–257 °C; FT-IR (KBr, cm−1): 3,229, 3,186 (-NH-), 3,000, 1,605, 1,545 (phenyl ring), 1,694 (-C=O), 1,647 (C=N), 1,344 (-CH2-), 1,225 (C-N), 1,149 (C-F), 759 (C-Cl), 747 (C-S-C); 1H-NMR (DMSO-d6) δ in ppm: 8.28 (s, 1H, -CH=N-); 7.62-7.36 (d, 8H, Ar-H); 7.36 (s, 1H, C=CH-); 7.02 (s, 1H, -NH-); 5.80 (s, 2H, -CH2-); 3.44, 3.23 (s, 6H, 2CH3); 13C-NMR (DMSO-d6) δ in ppm: 173.38 (C9), 165.31 (C12), 163.83 (C16), 155.93 (C10), 154.44, 150.95 (C6, C2), 148.42 (C4), 145.43 (C7), 142.84 (C13), 132.68 (C20), 130.64 (C19), 128.87 (C22, C24), 125.35 (C14, C18), 121.32 (C21, C25), 120.41 (C23), 118.88 (C11), 116.16 (C15, C17), 105.76 (C5), 30.43 (C8), 29.47, 27.51 (C1, C3).
2-{2-[2-(1,3-Dimethylxanthin-7-yl)acethyl]hydrazono}-3-(4-chlorophenyl)-5-(4-chlorobenzyliden) thiazolidin-4-one (7b3). Yield 59%, m.p. 250–252 °C; FT-IR (KBr, cm−1): 3,296, 3,118 (-NH-), 2,994, 1,591, 1,547 (phenyl ring), 1,699 (-C=O), 1,641 (C=N), 1,348 (-CH2-), 1,214 (C-N), 777 (C-Cl), 712 (C-S-C); 1H-NMR (DMSO-d6) δ in ppm: 8.28 (s, 1H, -CH=N-); 7.63-7.36 (d, 8H, Ar-H); 7.35 (s, 1H, C=CH-); 7.01 (s, 1H, -NH-); 5.84 (s, 2H, -CH2-); 3.48, 3.29 (s, 6H, 2CH3); 13C-NMR (DMSO-d6) δ in ppm: 173.62 (C9), 165.31 (C12), 154.66 (C10), 154.32, 150.87 (C6, C2), 151.78 (C4), 148.41 (C7), 142.07 (C13), 139.23 (C19), 133.35 (C16), 131.36 (C20), 129.19 (C15, C17), 128.84 (C14, C18), 125.36 (C22, C24), 123.99 (C21, C25), 121.25 (C23), 118.87 (C11), 105.76 (C5), 30.32 (C8), 29.44, 27.48 (C1, C3).
2-{2-[2-(1,3-Dimethylxanthin-7-yl)acethyl]hydrazono}-3-(4-chlorophenyl)-5-(4-bromobenzyliden) thiazolidin-4-one (7b4). Yield 69%, m.p. 245–247 °C; FT-IR (KBr, cm−1): 3,297, 3,117 (-NH-), 2,994, 1,589, 1,545 (phenyl ring), 1,699 (-C=O), 1,635 (C=N), 1,348 (-CH2-), 1,213 (C-N), 775 (C-Cl), 710 (C-S-C), 635 (C-Br); 1H-NMR (DMSO-d6) δ in ppm: 8.04 (s, 1H, -CH=N-); 7.49–7.33 (d, 8H, Ar-H), 7.24 (s, 1H, C=CH-); 7.03 (s, 1H, -NH-); 5.17 (s, 2H, -CH2-); 3.30, 3.20 (s, 6H, 2CH3); 13C-NMR (DMSO-d6) δ in ppm: 173.32 (C9), 167.61 (C12), 163.57 (C16), 154.58 (C10), 154.39, 154.39 (C6, C2), 150.97 (C4), 147.95 (C7), 142.04 (C13), 131.89 (C20), 130.53 (C19), 128.58 (C14, C18), 128.02 (C22, C24), 122.87 (C21, C25), 120.84 (C23), 119.77 (C11), 117.32 (C15, C17), 106.29 (C5), 33.78 (C8), 29.45, 27.42 (C1, C3).
2-{2-[2-(1,3-Dimethylxanthin-7-yl)acethyl]hydrazono}-3-(4-chlorophenyl)-5-(4-hydroxybenzyliden) thiazolidin-4-one (7b5). Yield 58%, m.p. 248–250 °C; FT-IR (KBr, cm−1): 3,318 (OH), 3,229, 3,187 (-NH-), 3,000, 1,607, 1,547 (phenyl ring), 1,695 (-C=O), 1,650 (C=N), 1,344 (-CH2-), 1,227 (C-N), 1,150 (C-O), 799 (C-Cl), 748 (C-S-C); 1H-NMR (DMSO-d6) δ in ppm: 8.28 (s, 1H,-CH=N-); 7.60-7.34 (d, 8H, Ar-H); 7.28 (s, 1H, C=CH-); 7.01 (s, 1H, -NH-); 5.77 (s, 2H, -CH2-); 3.42, 3.21 (s, 6H, 2CH3); 13C-NMR (DMSO-d6) δ in ppm: 173.33 (C9), 165.33 (C12), 157.43 (C16), 154.80 (C10), 151.03 (C4), 154.43, 150.26, (C6, C2), 148.36 (C7), 142.72 (C13), 131.82 (C20), 128.87 (C14, C18), 127.03 (C19), 125.50 (C22, C24), 124.76 (C21, C25), 123.53 (C23), 118.67 (C11), 117.08 (C15, C17), 105.79 (C5), 30.61 (C8), 29.49, 27.52 (C1, C3).
2-{2-[2-(1,3-Dimethylxanthin-7-yl)acethyl]hydrazono}-3-(4-chlorophenyl)-5-(4-N-dimethylamino-benzyliden)thiazolidin-4-one (7b6). Yield 65%, m.p. 258–260 °C; FT-IR (KBr, cm−1): 3,294, 3,118 (-NH-), 2,994, 1,590, 1,548 (phenyl ring), 1,699 (-C=O), 1,638 (C=N), 1,348 (-CH2-), 1,214 (C-N), 776 (C-Cl), 711 (C-S-C); 1H-NMR (DMSO-d6) δ in ppm: 8.06 (s, 1H, -CH=N-); 7.70-7.31 (d, 8H, Ar-H), 7.16 (s, 1H, C=CH-); 7.02 (s, 1H, -NH-); 5.17 (s, 2H, -CH2-); 3.32, 3.20 (s, 6H, 2CH3); 3.05, 2.97 (s, 6H, 2CH3, N(CH3)2); 13C-NMR (DMSO-d6) δ in ppm: 173.54 (C9), 167.60 (C12), 154.39 (C10), 150.97 (C4), 155.28, 150.33, (C6, C2), 150.04 (C16), 147.95 (C7), 138.51 (C13), 131.41 (C20), 128.71 (C14, C18), 125.45 (C22, C24), 124.92 (C19), 124.12 (C21, C25), 123.11 (C23), 119.78 (C11), 113.88 (C15, C17), 105.94 (C5), 40.32 (2C, N(CH3)2), 30.56 (C8), 29.45, 27.43 (C1, C3).
2-{2-[2-(1,3-Dimethylxanthin-7-yl)acethyl]hydrazono}-3-(4-chlorophenyl)-5-(2-nitrobenzyliden) thiazolidin-4-one (7b7). Yield 75%, m.p. 235–237 °C; FT-IR (KBr, cm−1): 3,296, 3,118 (-NH-), 2,994, 1,591, 1,547 (phenyl ring), 1,699 (-C=O), 1,641 (C=N), 1,499 (NO2), 1,348 (-CH2-), 1,214 (C-N), 777 (C-Cl), 712 (C-S-C); 1H-NMR (DMSO-d6) δ in ppm: 8.28 (s, 1H, -CH=N-); 8.06-7.38 (d, 8H, Ar-H); 7.18 (s, 1H, C=CH-); 7.02 (s, 1H, -NH-); 5.80 (s, 2H, -CH2-); 3.44, 3.23 (s, 6H, 2CH3); 13C-NMR (DMSO-d6) δ in ppm: 173.96 (C9), 165.27 (C12), 154.72 (C10), 154.44, 148.42 (C6, C2), 150.95 (C4), 147.82 (C7), 147.42 (C18), 139.63 (C13), 137.86 (C15), 131.74 (C20), 129.64 (C19), 129.38 (C16), 128.82 (C22, C24), 127.51 (C14), 122.97 (C21, C25), 120.83 (C23), 120.6 (C17), 119.03 (C11), 105.76 (C5), 33.64 (C8), 29.46, 27.49 (C1, C3).
3.3. Biological Evaluation
3.3.1. Antioxidant Assays
The antioxidant activity was estimated using in vitro tests: ferric reducing power, total antioxidant capacity, DPPH radical scavenging assay and ABTS radical scavenging ability.
3.3.2. Ferric Reducing Power
The ferric reducing power of the compounds was quantified by the method described by [30] with slight modifications. phosphate buffer (1 mL, 0.2 M, pH 6.6) and potassium ferricyanide (1 mL, 1% w/v) were added in a test tube to the sample (50 µL, 100 µL, 200 µL, 300 µL) obtained from a stock solution (5 mg/mL in DMSO). The mixture was incubated at 50 °C for 20 min in a water bath and then the reaction was stopped by adding trichloroacetic acid (1 mL, 10% w/v). After centrifugation at 4,500 rpm for 15 min, the upper layer (1 mL) was collected and diluted with deionised water (1 mL) and then ferric chloride (0.2 mL, 0.1% w/v) was added. The mixture was left at room temperature for 10 min and then the absorbance was measured at 700 nm against a blank solution (DMSO mixed with the reagents). All the tests were performed in triplicate. For each sample the effective concentration (EC50) was calculated and the ascorbic acid (AA) was used as positive control.
3.3.3. Total Antioxidant Activity
The total antioxidant activity of tested compounds was evaluated using the phosphomolybdenum method according to the procedure of [31] with minor modifications. The method is based on the reduction of Mo (VI) to Mo (V) by the tested compounds followed by the formation of a green phosphate/Mo(V) complex at acid pH. There were used different sample volumes (10, 15, 20, 30, 40, 60 µL) obtained from a stock solution (5 mg/mL in DMSO). The samples were mixed with the reagent solution (2 mL, 28 mM sodium phosphate; 4 mM ammonium molybdate; 0.6 M sulphuric acid), incubated at 95 °C for 90 min, cooled at room temperature and then centrifuged at 4,500 rpm for 15 min. The upper layer was collected and the absorbance was measured at 695 nm against a blank solution (DMSO mixed with the reagents). For each sample the effective concentration (EC50) was calculated and the ascorbic acid (AA) was used as positive control. All tests were performed in triplicate.
3.3.4. DPPH Radical Scavenging Assay
The radical scavenging activity of the tested compounds towards the radical 1,1-diphenyl-2-picrylhydrazyl (DPPH) was measured as described by [32] with slight modifications. Various concentrations of the tested compounds (50 µL, 75 µL, 150 µL, 200 µL) from a stock solution (5 mg/mL in DMSO) were mixed with DMSO to obtain 200 μL sample, and then DPPH in methanol (2,500 μL, 0.1 mM) was added. The mixture was left for 1 hour at room temperature, in the dark, and after that the absorbance was measured at 517 nm against a blank solution. The radical scavenging capacity was calculated according to the following equation: Scavenging activity % = [(Ao − At)/A0] × 100. A0 is the absorbance of DPPH methanol solution. At is the absorbance of the sample after 1 h. For each sample the effective concentration (EC50) was calculated and the ascorbic acid (AA) was used as positive control. All tests were performed in triplicate.
3.3.5. ABTS Radical Scavenging Assay
The ABTS+ radicals were activated by reacting of ABTS (7 mM) with ammonium persulphate (2.45 mM). The mixture was left at room temperature for 16 hours in the dark as described by [33]. The intensely coloured ABTS+ radical cation solution was diluted with ethanol to obtain an absorbance value of 0.7 ± 0.02 at 734 nm. Different sample volumes (5 µL, 10 µL, 20 µL, 25 µL) from a stock solution (5 mg/mL in DMSO) were mixed with DMSO to 25 µL and then ABTS solution (1975 µL) was added. After 6 min the absorbance was measured and the radical scavenging capacity was calculated according to the following equation: Scavenging activity % = [(Ao − At)/A0] × 100. A0 is the absorbance before adding the sample. At is the absorbance after 6 min of reaction. For each sample the effective concentration (EC50) was calculated and the ascorbic acid (AA) was used as positive control. All tests were performed in triplicate.
3.3.6. Statistical Analysis
All antioxidant assays were carried out in triplicate. Data were analyzed by an analysis of variance (ANOVA) (p < 0.05) and were expressed as means ± SD. The EC50 values were calculated by linear interpolation between the values registered above and below 50% activity.
4. Conclusions
In this study new heterocyclic compounds that combine a xanthine structure with a thiazolidine one have been synthesized. The structures of all new compounds were proven using spectral methods. The compounds were evaluated for their antioxidant activity using in vitro assays: ferric reducing power, total antioxidant activity, ABTS and DPPH radical scavenging ability. The all benzylidenethiazolidin-4-one derivatives 7a1-7, 7b1-7 showed improved antioxidant effects in reference to the corresponding thiazolidin-4-ones 6a, 6b. The encouraging preliminary results support the antioxidant potential of the synthesized compounds and their possible applications in several diseases mediated by reactive oxygen species (ROS), including diabetes mellitus, and motivate our next research focused on their potential antidiabetic effects.
Acknowledgments
This work was supported by the project “Doctoral Scholarships for increasing competitiveness in the medical and pharmaceutical field” (POSDRU/88/1.5/S/58965). The authors thank Polymer Research Group, Department of Organic Chemistry, University of Ghent, Belgium for their help to perform IR and NMR analyses.
Conflicts of Interest
The authors declared no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 7a1–7, 7b1–7 are available from the authors.
References
- 1.Lesyk R., Zimenkovsky B. 4-Thiazolidones: Current status and perspectives for modern organic and medicinal chemistry. Curr. Org. Chem. 2004;8:1547–1578. doi: 10.2174/1385272043369773. [DOI] [Google Scholar]
- 2.Vicini P., Geronikaki A., Anastasia K., Incerti M., Zani F. Synthesis and antimicrobial activity of novel 2-thiazolimino-5-arylidene-4-thiazolidinones. Bioor. Med. Chem. 2006;14:3859–3864. doi: 10.1016/j.bmc.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 3.Alagawadi K.R., Alegaon S.G. Synthesis, characterization and antimicrobial activity evaluation of new 2,4-thiazolidinediones bearing imidazo [2,1-b][1,3,4]thiadiazole moiety. Arab. J. Chem. 2011;4:465–472. doi: 10.1016/j.arabjc.2010.07.012. [DOI] [Google Scholar]
- 4.Prasad D., Kumar A., Kumar S.P., Nath M. Design, synthesis and antimicrobial evaluation of novel 2-aryl-thiazolidin-4-one derivatives. Org. Med. Chem. Lett. 2011;14:2–7. doi: 10.1186/2191-2858-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhagat T.M., Swamy D.K., Badne S.G., Kuberkar S.V. Synthesis and antibacterial activity of 4-thiazolidinone containing benzothiazolyl moiety. Rasayan J. Chem. 2011;4:24–28. [Google Scholar]
- 6.Sharma R., Samadhiya P., Srivastava S., Srivastava S. Synthesis of some new thiazolidine derivatives and their biological significance. Latv. J. Chem. 2012;50:296–307. [Google Scholar]
- 7.Amin K.M., Rahman D.E., Al-Eryani Y.A. Synthesis and preliminary evaluation of some substituted coumarins as anticonvulsant agents. Bioorg. Med. Chem. 2008;16:5377–5388. doi: 10.1016/j.bmc.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 8.Parameswaran M., Thengungal K.R., Subbuchettiar G. Synthesis of coumarin heterocyclic derivatives with antioxidant activity and in vitro cytotoxic activity against tumour cells. Acta Pharm. 2009;59:159–170. doi: 10.1111/j.1600-0773.1986.tb02732.x. [DOI] [PubMed] [Google Scholar]
- 9.Isloor A.M., Sunil D., Shetty P., Malladi S., Pai K.S.R., Maliyakki N. Synthesis, characterization, anticancer, and antioxidant activity of some new thiazolidin-4-ones in MCF-7 cells. Med. Chem. Res. 2013;22:758–767. doi: 10.1007/s00044-012-0071-5. [DOI] [Google Scholar]
- 10.Liu Y., Jing F., Xu Y., Xie Y., Shi F., Fangm H., Lim M., Xu W. Design, synthesis and biological activity of thiazolidine-4-carboxylic acid derivatives as novel influenza neuraminidase inhibitors. Bioorg. Med. Chem. 2011;19:2342–2348. doi: 10.1016/j.bmc.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 11.Balzarini J., Orzeszko B., Maurin J.K., Orzeszko A. Synthesis and anti-HIV studies of 2-adamantyl-substituted thiazolidin-4-ones. Eur. J. Med. Chem. 2007;42:993–1003. doi: 10.1016/j.ejmech.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Barros C.D., Amato A.A., de Oliveira T.B., Iannini K.B., Silva A.L., Silva T.G., Leitem, Hernandes M.Z., de Lima M.C.A., Galdino S.L., et al. Synthesis and anti-inflammatory activity of new arylidene-thiazolidine-2,4-diones as PPARgamma ligands. Bioorg. Med. Chem. 2010;18:3805–3811. doi: 10.1016/j.bmc.2010.04.045. [DOI] [PubMed] [Google Scholar]
- 13.Vazzana I., Terranova E., Mattioli F., Sparatore F. Aromatic schiff bases and 2,3-disubstituted-1,3-thiazolidin-4-one derivatives as antiinflammatory agents. ARKIVOC. 2004;v:364–374. [Google Scholar]
- 14.Kim H., Haluzik M., Gavrilova O., Yakar S., Portas J., Sun H., Pajvani U.B., Scherer P.E., LeRoith D. Thiazolidinediones improve insulin sensitivity in adipose tissue and reduce the hyperlipidemia without affecting the hyperglycaemia in a transgenic model of type 2 diabetes. Diabetologia. 2004;47:2215–2225. doi: 10.1007/s00125-004-1581-6. [DOI] [PubMed] [Google Scholar]
- 15.Iqbal A.K.M., Khan A.Y., Kalashetti M.B., Belavagi N.S., Gong Y.D., Khazi I.A. Synthesis, hypoglycemic and hypolipidemic activities of novel thiazolidinedione derivatives containing thiazole/triazole/oxadiazole ring. Eur. J. Med. Chem. 2012;53:308–315. doi: 10.1016/j.ejmech.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 16.Da Costa Leite L.F., Veras Mourão R.H., de Lima Mdo C., Galdino S.L., Hernandes M.Z., de Assis Rocha Neves F., Vidal S., Barbe J., da Rocha Pitta I. Synthesis, biological evaluation and molecular modeling studies of arylidene-thiazolidinediones with potential hypoglycemic and hypolipidemic activities. Eur. J. Med. Chem. 2007;42:1263–1271. doi: 10.1016/j.ejmech.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 17.Kishore A., Nampurath G.K., Mathew S.P., Zachariah R.T., Potu B.K., Rao M.S., Valiathan M., Chamallamudi M.R. Antidiabetic effect through islet cell protection in streptozotocin diabetes: A preliminary assessment of two thiazolidin-4-ones in Swiss albino mice. Chem. Biol. Interact. 2009;177:242–266. doi: 10.1016/j.cbi.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 18.Derosa G., Maffioli P. Effects of thiazolidinediones and sulfonylureas in patients with diabetes. Diabetes Technol. Ther. 2010;12:491–501. doi: 10.1089/dia.2009.0172. [DOI] [PubMed] [Google Scholar]
- 19.Alberti K.G., Zimmet P., Shaw J. Metabolic syndrome—A new world-wide definition. A consensus statement from the international diabetes federation. Diabet. Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 20.Hughes T.E. Emerging therapies for metabolic diseases—The focus is on diabetes and obesity. Curr. Opin. Chem. Biol. 2009;13:1–6. doi: 10.1016/j.cbpa.2009.04.622. [DOI] [PubMed] [Google Scholar]
- 21.Kushwaha S.P., Rawat S.K., Abhishekm P.K., Tripathim K. Coupling antioxidant and antidiabetic assests of 2,4-thiazolidinedione derivatives. Asian J. Pharm. 2011;1:71–73. [Google Scholar]
- 22.El Nezhawy A.O.H., Ramla M.M., Khalifa N.M., Abdulla M.M. Synthesis and antioxidant activity of some thiazolidin-4-one derivatives. Monatsh. Chem. 2009;140:531–539. doi: 10.1007/s00706-008-0085-3. [DOI] [Google Scholar]
- 23.Lupaşcu F., Dragostin O.M., Apotrosoaei M., Pânzariu A., Lupaşcu D., Vasile C., Profire L. Synthesis and evaluation of antioxidant activity of some new benzylidene-thiazolidine-xanthine derivatives. Rev. Med. Chir. 2013;117:244–249. [PubMed] [Google Scholar]
- 24.Kusano C., Ferrari B. Total Antioxidant Capacity: A biomarker in biomedical and nutritional studies. J. Cell Mol. Biol. 2008;7:1–15. [Google Scholar]
- 25.Schwartz S.L. Treatment of Elderly Patients With Type 2 Diabetes mellitus: A systematic review of the benefits and risks of dipeptidyl peptidase-4 inhibitors. Am. J. Geriatr. Pharmacother. 2010;5:405–418. doi: 10.1016/j.amjopharm.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Baetta R., Corsini A. Pharmacology of dipeptidyl peptidase-4 inhibitors: Similarities and differences. Drugs. 2011;71:1441–1467. doi: 10.2165/11591400-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 27.Bhat V.B., Madyastha K.M. Antioxidant and radical scavenging properties of 8-oxo derivatives of xanthine drugs pentoxifylline and lisofylline. Biochem. Biophys. Res. Commun. 2001;288:1212–1217. doi: 10.1006/bbrc.2001.5922. [DOI] [PubMed] [Google Scholar]
- 28.Kaptanoglu E., Solaroglu I., Akbiyik F., Demirpence E., Ergungor M.E. The antioxidant effect of aminophylline in rat brain and spinal cord homogenates. Turk. Neurosurg. 2003;13:9–13. [Google Scholar]
- 29.Lupascu F., Dash M., Samal S.K, Lupusoru C.E., Lupusoru P., Profire L., Dubruel P. Delivery of hydrophobic antioxidant drugs via chitosan: An approach to enhance their oral bioavailability. J. Mater. Sci. Mater. Med. 2013 submitted. [Google Scholar]
- 30.Lungu Apetrei C., Tuchilus C., Aprotosoaie A.C. Chemical, antioxidant and antimicrobial investigations of pinus cembra L. bark and needles. Molecules. 2011;16:7773–7788. doi: 10.3390/molecules16097773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cacic M., Molnar M., Sarkanj B., Has-Schon E., Rajkovic V. Synthesis and antioxidant activity of some new coumarinyl-1,3-Thiazolidine-4-ones. Molecules. 2010;15:6795–6809. doi: 10.3390/molecules15106795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shih M-H., Ke F.-Y. Syntheses and evaluation of antioxidant activity of sydnonyl substituted thiazolidinone and thiazoline derivatives. Bioorg. Med. Chem. 2004;12:4633–4643. doi: 10.1016/j.bmc.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 33.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]