Abstract
Three new multiflorane-type triterpenes; 7α-methoxymultiflor-8-ene-3α,29-diol 3-acetate-29-benzoate (1), 7-oxomultiflor-8-ene-3α,29-diol 3-acetate-29-benzoate (2), and multiflora-7,9(11)-diene-3α,29-diol 3-p-hydroxybenzoate-29-benzoate (3), were isolated from seeds of Cucurbita maxima, along with three known compounds. Compound 3 and multiflora-7,9(11)-diene-3α-29-diol 3-benzoate (5) exhibited potent inhibitory effects on melanogenesis, with low cytotoxicities, and 2 exhibited single-digit micromolar cytotoxicity against HL-60 and P388 cells.
Keywords: Cucurbita maxima, multiflorane-type triterpenes, melanogenesis inhibitory activity, cytotoxic activity
1. Introduction
Pumpkins, including Cucurbita moschata, C. pepo, and C. maxima are gourd squashes of the genus Cucurbita and the family Cucurbitaceae. Cucurbita moschata seeds have been used as an anthelmintic [1], and Cucurbita pepo seeds as an anthelmintic and a diuretic [2].
Cucurbita maxima (English name: squash, pumpkin, Japanese name: kabocha) is indigenous to the plateaus of central and south America, but is cultivated throughout the World. Its fruits, flowers, and seeds have been eaten as vegetables containing vitamins A, C, and E. Several triterpenes such as cucurbita-5,24-dienol [3] and α− and β-amyrin [4] are present in the seeds of Cucurbita maxima. Additionally, it has been demonstrated that the seeds and flowers of C. maxima contain sterols [4,5,6]. Herein, we report the isolation and structural elucidation of three new multiflorane-type triterpenes along with three known compounds, multiflora-7,9(11)-diene-3α,29-diol 3,29-dibenzoate (4), multiflora-7,9(11)-diene-3α-29-diol 3-benzoate (5) and multiflora-5,7,9(11)-triene-3α,29-diol 3,29-dibenzoate (6), from seeds of C. maxima and describe their inhibitory effects on α-MSH-induced melanogenesis in B16 melanomas, and cytotoxic activities against the HL-60 and P388 leukemia cell lines.
2. Results and Discussion
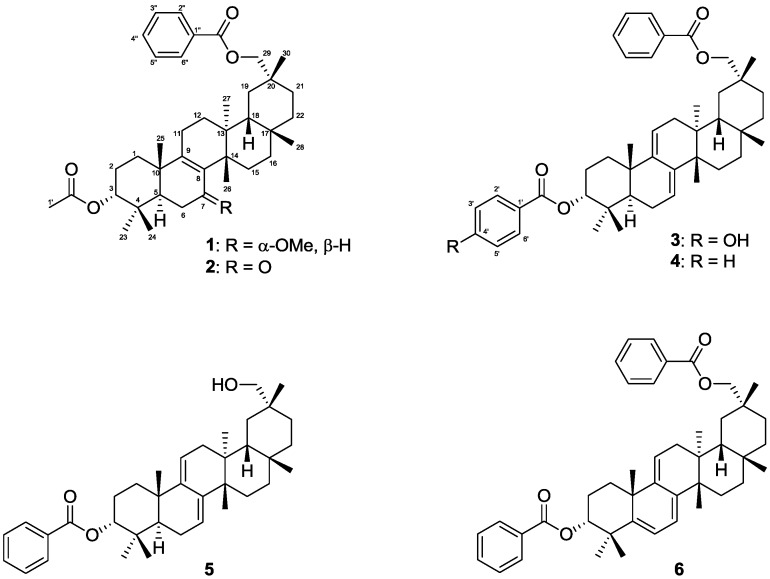
Three new multiflorane-type triterpenes 1–3 and three known multiflorane-type triterpenes 4–6 were isolated from the MeOH extract of C. maxima seeds (Figure 1).
Figure 1.
Chemical structures of isolated compounds 1–6.
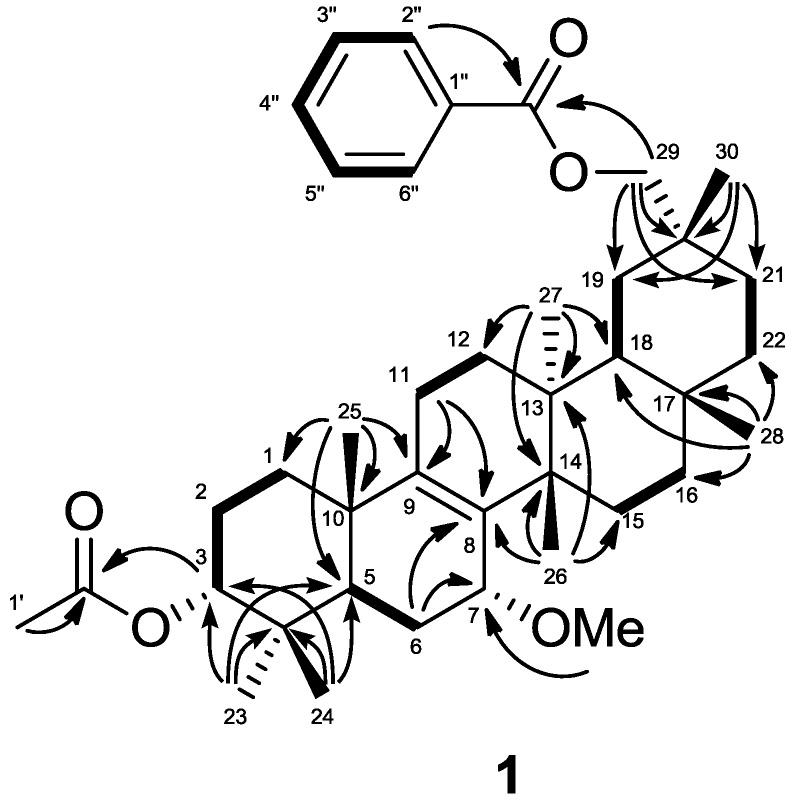
The compound 1 was obtained as an amorphous solid with a molecular ion at m/z 618.4282 [M]+ (calcd. for C40H58O5, 618.4285) in the HREIMS. The IR spectrum showed absorptions indicating two carbonyl groups [νmax 1743 (C=O), 1724 (C=O), 1267 (C–O), 1247 (C–O) cm−1]. The 1H- and 13C-NMR spectra (δH and δC in ppm, Table 1) displayed signals for seven tertiary methyl groups [δH 0.88, 0.93, 0.94, 1.04, 1.06, 1.08, 1.13 (each s)], an oxymethylene [δH 4.11, 4.15 (each d); δC 73.0 (t)], two oxymethines [δH 3.53 (brs), 4.68 (t); δC 74.0 (d), 77.2 (d)], a tetrasubstituted olefin [δC 135.2 (s), 140.0 (s)], an acetoxy group [2.06 (s); δC 21.5 (q), 171.1 (s)], a benzoyl group [δH 7.46 (2H, tt), 7.57 (1H, tt), 8.06 (2H, dd); δC 128.3 (d), 129.5 (d), 130.7 (s), 132.7 (d), 166.6 (s)], and a methoxyl group [δH 3.24 (s); 55.0 (q)]. In the HMBC experiment (Figure 2), the following correlations were observed: Me-23 [δH 0.88 (s)] to C-3 [δc 77.2 (d)], C-4, C-5, and C-24; Me-24 [δH 0.93 (s)] to C-3, C-4, C-5, and Me-23; Me-25 [δH 0.94 (s)] to C-1, C-5, C-9 [δC 140.0 (s)], and C-10; Me-26 [δH 1.04 (s)] to C-8 [δC 135.2 (s)], C-13, C-14, and C-15; Me-27 [δH 1.06 (s)] to C-12, C-13, C-14, and C-18; Me-28 [δH 1.13 (s)] to C-16, C-17, C-18, and C-22; H2-29 [δH 4.11, 4.15 (each d)] to C-19, C-20, C-21, C-30, and 29-OCO [δC 166.6 (s)]; Me-30 [δH 1.08 (s)] to C-19, C-20, C-21, and C-29 [δC 73.0 (t)]; H-3 [δH 4.68 (t)] to 3-OCO [δC 171.1 (s)]; H-5, H-6β, and 7-OMe [δH 3.24 (s)] to C-7 [δC 74.0 (d)]; H-6β, H-11, and Me-26 to C-8 [δC 135.2 (s)]; and H-11 and Me-25 to C-9 [δC 140.0 (s)] (Figure 2).
Table 1.
1H (500 MHz) and 13C (125 MHz) NMR spectroscopic data of compounds 1–3 (CDCl3) a.
| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δC (ppm), type | δH (ppm) (J in Hz) | δC (ppm), type | δH (ppm) (J in Hz) | δC (ppm), type | δH (ppm) (J in Hz) | |
| 1 | 29.7, t | α, 1.39, m | 29.5, t | 1.59, m | 31.8, t | α, 1.97, m |
| β, 1.45, m | β, 1.58 m | |||||
| 2 | 23.4, t | α, 1.64, m | 22.9, t | α, 1.75, m | 23.1, t | α, 1.87, m |
| β, 1.85, m | β, 1.95, m | β, 1.98, m | ||||
| 3 | 77.2, d | 4.68, t (2.8) | 76.9, d | 4.71, t (2.5) | 78.8, d | 4.82, brd (3.2) |
| 4 | 36.2, s | 36.5, s | 37.6, s | |||
| 5 | 39.7, d | 1.99, dd (12.5, 1.1) | 42.6, d | 2.07, dd (7.5, 3.9) | 43.9, d | 1.94, m |
| 6 | 22.4, t | α, 1.89, m | 36.2, t | 2.35, m | 23.7, t | α, 2.14, brt (5.0) |
| β, 1.30, m | β, 2.08, m | |||||
| 7 | 74.0, d | 3.53, brs | 198.3, s | 119.4, d | 5.60, brd (5.9) | |
| 8 | 135.2, s | 142.5, s | 142.3, s | |||
| 9 | 140.0, s | 163.3, s | 145.8, s | |||
| 10 | 38.5, s | 39.2, s | 36.4, s | |||
| 11 | 21.0, t | 1.95, 2H, m | 22.2, t | α, 2.30, m | 114.8, d | 5.29, brd (5.9) |
| β, 2.14, m | ||||||
| 12 | 31.2, t | α, 1.34, m | 29.8, t | α, 1.38, m | 39.1, t | α, 2.08, m |
| β, 1.60, m | β, 1.59, m | β, 1.79, m | ||||
| 13 | 37.0, s | 38.0, s | 37.5, s | |||
| 14 | 41.7, s | 39.1, s | 40.4, s | |||
| 15 | 25.4, t | α, 2.18, m | 29.4, t | α, 2.43, m | 27.6, t | α, 1.63, m |
| β, 1.25, m | β, 1.59, m | β, 1.42, m | ||||
| 16 | 36.9, t | 1.56, 2H, m | 35.9, t | α, 1.39, m | 37.2, t | α, 1.76, m |
| β, 1.63, m | β, 1.49, m | |||||
| 17 | 31.1, s | 31.1, s | 31.9, s | |||
| 18 | 44.0, d | 1.59, m | 41.3, d | 1.66, m | 45.1, d | 1.68, m |
| 19 | 28.8, t | α, 1.84, m | 30.2, t | α, 1.63, m | 29.6, t | α, 1.76, m |
| β, 1.30, m | β, 1.29, dd (15.7, 3.9) | β, 1.30, m | ||||
| 20 | 31.9, s | 32.4, s | 29.9, s | |||
| 21 | 29.5, t | 1.51, 2H, m | 28.3, t | α, 1.56, m | 30.1, t | 1.63, 2H, m |
| β, 1.47, m | ||||||
| 22 | 35.7, t | α, 1.79, m | 38.5, t | α, 1.50, m | 33.0, t | α, 1.89, m |
| β, 0.97, m | β, 1.03, m | β, 0.95, m | ||||
| 23 | 27.2, q | 0.88, s | 26.7, q | 0.87, s | 28.0, q | 0.90, s |
| 24 | 22.3, q | 0.93, s | 21.4, q | 0.99, s | 21.6, q | 1.03, s |
| 25 | 18.2, q | 0.94, s | 18.0, q | 1.03, s | 21.1, q | 1.01, s |
| 26 | 26.1, q | 1.04, s | 26.7, q | 1.39, s | 21.2, q | 0.94, s |
| 27 | 18.9, q | 1.06, s | 18.3, q | 0.99, s | 19.9, q | 1.03, s |
| 28 | 31.2, q | 1.13, s | 30.6, q | 1.22, s | 31.4, q | 1.11, s |
| 29 | 73.0, t | a, 4.15, d (10.8) | 75.0, t | a, 4.05, d (10.5) | 74.2, t | a, 4.34, d (10.7) |
| b, 4.09, d (10.8) | b, 4.02, d (10.5) | b, 4.12, d (10.7) | ||||
| 30 | 29.7, q | 1.08, s | 26.5, q | 1.15, s | 31.3, q | 1.16, s |
| 3-OCO | 171.1, s | 170.6, s | 165.4, s | |||
| 1' | 21.5, q | 2.06, s | 21.3, q | 2.07, s | 123.1, s | |
| 2', 6' | 131.9, d | 7.85, 2H, dd (8.4, 2.8) | ||||
| 3', 5' | 115.3, d | 6.84, 2H, dd (8.4, 2.8) | ||||
| 4' | 160.8, s | |||||
| 29-OCO | 166.6, s | 166.8, s | 168.5, s | |||
| 1'' | 130.7, s | 130.6, s | 130.2, s | |||
| 2'', 6'' | 129.5, d | 8.06, 2H, dd (7.4, 1.3) | 129.5, d | 8.06, 2H, dd (8.0, 1.4) | 129.6, d | 8.04, 2H, dd (7.4, 1.4) |
| 3'', 5'' | 128.3, d | 7.46, 2H, tt (7.4, 1.3) | 132.8, d | 7.46, 2H, tt (8.0, 1.4) | 128.8, d | 7.46, 2H, tt (7.4, 1.4) |
| 4'' | 132.7, d | 7.57, tt (7.4, 1.3) | 128.4, d | 7.58, tt (8.0, 1.4) | 133.6, d | 7.56, tt (7.4, 1.4) |
| 7-OMe | 55.0, q | 3.24, s | ||||
| 4'-OH | 7.49, brs | |||||
a Assignments were based on 1H-1H COSY, HMQC, HMBC and NOESY spectroscopic data.
Figure 2.
Key HMBC ( ) and 1H-1H COSY (
) and 1H-1H COSY ( ) correlations of 1.
) correlations of 1.
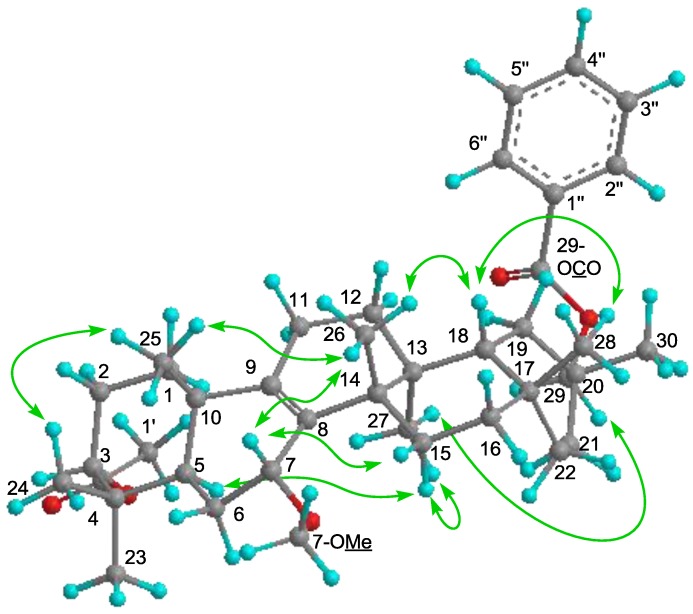
In the 1H-1H COSY experiment, H-7 [δH 3.53 (brs)] correlated with H2-6 [δH 1.30, 1.89]. The following significant NOE interactions were observed in 1: H-5/H-15α; H-15α/Me-27; Me-27/H2-29; Me-26/H-7, Me-25, and H-18; H-18/Me-28 (Figure 3). Therefore, the methoxy group at C-7 had the α (axial)-orientation. The configuration of the acetoxy group at C-3 was established as the α (axial)-orientation due to the NOE correlations between H-3 and Me-23 and Me-24, and the coupling constants of H-3 [δH 4.68 (t, J3β.2α;3β,2β = 2.8 Hz)]. Therefore, 1 was determined as 7α-methoxymultiflor-8-ene-3α,29-diol 3-acetate-29-benzoate.
Figure 3.
Key NOE ( ) correlations of 1.
) correlations of 1.
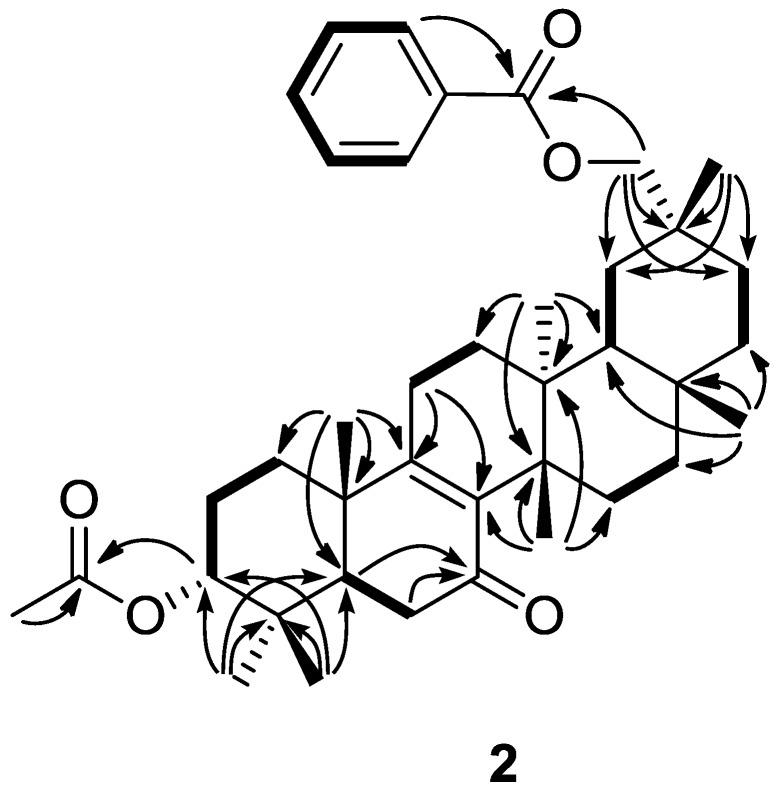
Compound 2 exhibited a [M]+ ion in the HREIMS data at m/z 602.3975 whose molecular formula was C39H54O5(calcd. 602.3972). The IR and UV spectra showed absorptions indicating two carbonyl groups [νmax 1739 (C=O), 1723 (C=O), 1270 (C−O), 1245 (C−O) cm−1] and an α,β-unsaturated six-membered ring ketone [νmax 1658 cm−1; λmax 233.0 nm (log ε 3.91)]. 2 is similar to 1 according to the 1H- and 13C-NMR spectra (δH and δC in ppm). In the HMBC experiment, cross-peaks were observed from H-5 and H-6 to C-7 [δC 198.3 (s)]; and from H2-11 to C-8 [δC 142.5 (s)] and C-9 [δC 163.3 (s)] (Figure 4). In the 1H-1H COSY experiment, H2-11 [δH 2.14, 2.30] correlated with H2-12 [δH 1.38, 1.59], but H2-6 [δH 2.35 (2H)] correlated with only H-5 [δH 2.07 (dd)] (Figure 4). NOESY experiments revealed that the relative of 2 to have the same conformation as 1. As a result, 2 was determined to be 7-oxomultiflor-8-ene-3α,29-diol 3-acetate-29-benzoate.
Figure 4.
Key HMBC ( ) and 1H-1H COSY (
) and 1H-1H COSY ( ) correlations of 2.
) correlations of 2.
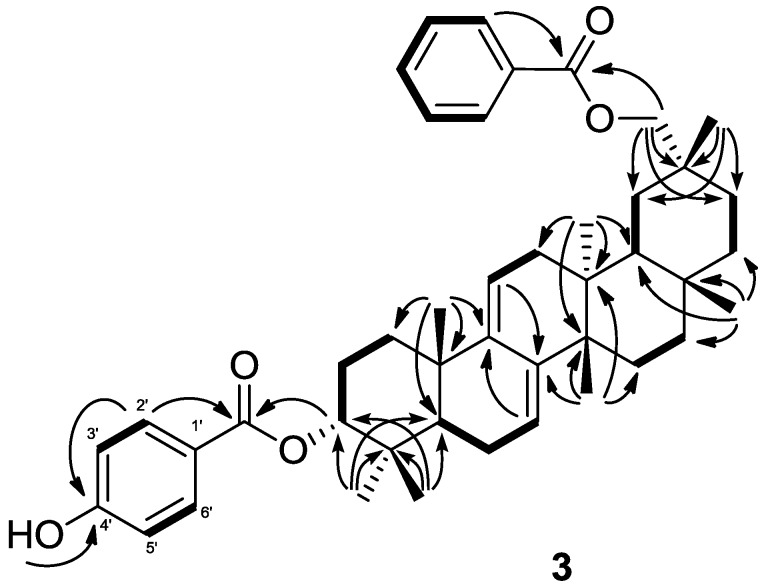
The molecular formula of 3 was determined as C44H56O5 based on the HREIMS (m/z 664.4127, calcd. 664.4127). In addition, m/z 526 [M−C7H6O3]+ indicated the presence of a hydroxybenzoyloxy group. The IR spectrum showed the existence of a hydroxy group (νmax3436 cm−1) and aryl esters (νmax 1716, 1683, 1509, 1456, 1274 cm−1). The 1H- and 13C NMR spectra of 3 displayed signals for seven tertiary methyl groups [δH 0.90, 0.94, 1.01, 1.03 (6H), 1.11, 1.16 (each s)], an oxymethylene [δH 4.12, 4.34 (each d); δC 74.2 (t)], an oxymethine [δH 4.82 (brd); δC 78.8 (d)], a heteroannular diene [δH 5.29, 5.60 (each brd); δC 114.8 (d), 119.4 (d), 142.3 (s), 145.8 (s)], two aryl ester groups [δH 6.84 (dd), 7.46 (tt), 7.56 (tt), 7.85 (dd), 8.04 (dd); δC 115.3 (d), 123.1 (s), 128.8 (d), 129.6 (d), 130.2 (s), 131.9 (d), 133.6 (d), 160.8 (s), 165.4 (s), 168.5 (s)], and a hydroxyl group [δH 7.49 (brs)]. The 1H and 13C-NMR spectra of 3 were similar to those of multiflora-7,9(11)-diene-3α-29-diol 3,29-dibenzoate (4) except for the signal of the C-4' [δC 160.8 (s) in 3, δC 133.6 (s) in 4]. In the HMBC experiment, the correlations were observed from 4'-OH [δH 7.49 (brs)] to C-4' (Figure 5). Therefore the structure of 3 was determined to be multiflora-7,9(11)-diene-3α,29-diol 3-p-hydroxybenzoate-29-benzoate.
Figure 5.
Key HMBC ( ) and 1H-1H COSY () correlations of 3.
) and 1H-1H COSY () correlations of 3.
The known compounds 4 [7,8] and 5 [9] were identified by comparing MS and 1H and 13C-NMR data with published data, and 6 [7] by MS and 1H NMR data.
The six multiflorane triterpenes 1−6 from C. maxima were evaluated for inhibitory activities against α-MSH-induced melanogenesis in B16 melanomas (Table 2). At a low concentration (10 μM), 5 inhibited melanogenesis (76.9% of melanin content) with low cytotoxicity (99.5% of cell viability). 5 also inhibited melanogenesis (70.9% of melanin content) with low cytotoxicity (97.7% cell viability) at 30 μM. At a high concentration (100 μM), 3 and 5 exhibited inhibitory activities (51.8 and 67.4% of melanin content, respectively) with low cytotoxicity (95.1 and 99.6% of cell viability, respectively). The activity levels of compounds 5 at 10 and 30 μM were comparable with or superior to those of the positive control, arbutin, which has been recognized as a useful depigmentation compound for skin whitening in the cosmetic industry [10]. It appears that two multiflorane-type triterpenes, 5 from C. maxima seeds, may be valuable as potential skin-whitening agents. The melanogenesis inhibitory activity of 2 (28.1% of melanin content at 100 μM) is thought to be due to their cytotoxic action (69.0% of cell viability at 100 μM).
Table 2.
Melanogenesis inhibitory activity and cytotoxicity in B16 mouse melanoma cells of multiflorane-type triterpenes isolated from Cucurbita maxima seeds a.
| Compound | mean ± S.D. (%) at 10 μM | mean ± S.D. (%) at 30 μM | mean ± S.D. (%) at 100 μM | |||
|---|---|---|---|---|---|---|
| Melanin content | Cell viability | Melanin content | Cell viability | Melanin content | Cell viability | |
| 1 | 94.8 ± 0.5 | 92.7 ± 2.2 | 77.1 ± 3.8 | 84.4 ± 4.0 | 73.7 ± 3.6 | 84.3 ± 5.2 |
| 2 | 106.8 ±9.3 | 106.3 ± 8.0 | 92.2 ± 5.4 | 107.1 ± 7.4 | 28.1 ± 2.3 | 69.0 ± 4.5 |
| 3 | 91.2 ± 2.2 | 107.2 ± 5.1 | 81.8 ± 4.0 | 105.6 ± 3.1 | 51.8 ± 8.0 | 95.1 ± 4.3 |
| 4 | 98.4 ± 3.2 | 110.8 ± 4.3 | 102.2 ± 11.7 | 103.0 ± 8.2 | 95.4 ± 8.4 | 101.1 ± 5.9 |
| 5 | 76.9 ± 4.0 | 99.5 ± 3.3 | 70.9 ± 0.1 | 97.7 ± 3.1 | 67.4 ± 3.6 | 99.6 ± 2.0 |
| 6 | 107.8 ± 2.6 | 91.0 ± 1.6 | 111.8 ± 7.1 | 81.8 ± 2.1 | 82.0 ± 5.1 | 74.4 ± 3.2 |
| arbutin b | 88.9 ± 2.3 | 100.0 ± 2.7 | 72.3 ± 3.1 | 94.4 ± 1.2 | 55.3 ± 1.0 | 89.9 ± 0.3 |
a Melanin content (%) and cell viability (%) were determined based on the absorbance at 450 nm, and 540 nm, respectively, by comparison with values for DMSO (100%). Each value represents the mean ± standard deviation (S.D.) of three determinations. The concentration of DMSO in the sample solution was 2 μL/mL. b Reference compound.
Six triterpenes and a reference compound, 5-fluorouracil (5-FU), were also evaluated for cytotoxic activities against human leukemia (HL-60) and murine leukemia (P388) cell lines by means of the MTT assay (Table 3). Compound 2 exhibited single-digit micromolar cytotoxicity with IC50 values of 7.0 and 9.5 μM against HL-60 and P388 cells, respectively. It was slightly less cytotoxic than 5-FU [IC50 2.3 (HL-60); 1.9 (P388) μM] (Table 3).
Table 3.
Cytotoxic activities of multiflorane-type triterpenes from Cucurbita maxima seeds.
| Compound | IC50 (μM) a | |
|---|---|---|
| HL-60 | P388 | |
| (human leukemia) | (murine leukemia) | |
| 1 | >100 | >100 |
| 2 | 7.0 ± 1.1 | 9.5 ± 1.1 |
| 3 | 55.9 ± 1.1 | 92.6 ± 1.3 |
| 4 | >100 | >100 |
| 5 | >100 | >100 |
| 6 | 54.1 ± 1.3 | 46.7 ± 1.2 |
| 5-fluorouracil b | 2.3 ± 0.2 | 1.9 ± 0.2 |
a HL-60 and P388 cell lines (each 1 × 104 cells in 100 μL) were treated with test compounds for 72 h, and MTT solution was added to the wells. The grown cells were labeled with 5 mg/mL MTT in phosphate-buffered saline (PBS), and the absorbance of formazan dissolved with 20% sodium dodecyl sulfate (SDS) in 0.1 N HCl was measured at 550 nm using a microplate reader. Data are expressed as mean ± S.D. (n = 3); b Reference compound.
3. Experimental
3.1. General Procedures
Chemicals and reagents were purchased as follows: fetal bovine serum (FBS) from Invitrogen Co. (Carlsbad, CA, USA), 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) from Sigma-Aldrich Japan Co. (Tokyo, Japan), and 5-fluorouracil (5-FU) (purity ≥ 98.5%), arbutin (purity ≥ 95.0%), Roswell Park Memorial Institute (RPMI) 1640 medium, Dulbecco’s modified Eagle’s medium (D-MEM), and antibiotics from Nacalai Tesque, Inc. (Kyoto, Japan). All other chemicals and reagents were of analytical grade. Melting points were determined on a Yanagimoto micro-melting point apparatus and are uncorrected. Optical rotations were measured with a JASCO DIP-1000 digital polarimeter. IR spectra were recorded on a Perkin-Elmer 1720X FTIR spectrophotometer. The 1H (500 MHz) and 13C (125 MHz) NMR spectra were recorded on a Varian INOVA 500 spectrometer in CDCl3 with tetramethylsilane as the internal standard. The EIMS was recorded on a Hitachi 4000H double-focusing mass spectrometer (70 eV). Silica gel (70–230 mesh, Merck) and silica gel 60 (230–400 mesh, Nacalai Tesque, Inc., Kyoto, Japan) were used for column chromatography and medium-pressure liquid chromatography, respectively. The 20% AgNO3/SiO2 (w/w) used for chromatography was prepared from silica gel 60 and AgNO3 (Nacalai Tesque, Inc., Kyoto, Japan). HPLC was carried out on an SiO2 column (Cosmosil 5SL-II column, 25 cm × 20 mm i.d., Nacalai Tesque, Inc., Kyoto, Japan) at 25 °C with n-hexane/EtOAc [10:1 (HPLC system I) and 5:1 (HPLC system II), flow rate 8.0 mL/min].
3.2. Plant Material
The seeds of Cucurbita maxima, produced in Japan (Nara prefecture), were purchased from JA (Japan Agricultural Co-operation)-Takatsuki in 2011. A voucher specimen was deposited in the Herbarium of the Laboratory of Medicinal Chemistry, Osaka University of Pharmaceutical Sciences.
3.3. Extraction and Isolation
The seeds of Cucurbita maxima (3 kg) were subjected to extraction with MeOH (10 L) under reflux (1 week, 4 times). After concentration the MeOH extract (102.2 g) was then partitioned between Et2O and H2O. The Et2O-soluble fraction (62.2 g) was subjected to SiO2 column chromatography (CC) [SiO2 (1.5 kg); CHCl3/EtOAc 1:0, 5:1, 2:1, 0:1 and MeOH, in increasing order of polarity] resulting in seven fractions (Fr. A–G). Fr. B, eluted with CHCl3, was subjected to SiO2 CC to yield 10 fractions, B1–B10. Among them, Fr. B3, eluted with hexane/EtOAc (5:1), was subjected to SiO2 CC to yield 11 fractions; B3-1–B3-11. Preparative HPLC of B3-4 (123.0 mg), eluted with hexane/EtOAc (5:1), gave 4 (15.5 mg; tR 11.2 min) (HPLC system I). Fr. C, eluted with CHCl3, was subjected to SiO2 CC to yield 22 fractions, C1–C22. Preparative HPLC of C3 (14.8 mg), eluted with hexane/EtOAc (10:1), gave 4 (5.1 mg) and 6 (3.1 mg; tR 12.0 min), respectively (HPLC system I). Fr. C11 (1.3 g), eluted with hexane/EtOAc (10:1), was subjected to CC with 20% AgNO3/SiO2 to give C11-1–C11-11, followed by CC of C11-4 (795.6 mg), eluted with hexane/CHCl3 (20:1), with 20% AgNO3/SiO2 to yield C11-4-1–C11-4-9. Preparative HPLC of C11-4-3 (15.0 mg), eluted with hexane/EtOAc (2:1), gave 2 (2.0 mg; tR 18.9 min) (HPLC system II). Fr. D, eluted with CHCl3, was fractionated with SiO2 CC to D1–D16. Fr. D4 (1369.0 mg), eluted with hexane/EtOAc (5:1) was subjected to SiO2 CC to yield D4-1–D4-12. Preparative HPLC of D4-5 (10.0 mg), eluted with hexane/EtOAc (5:1), gave 1 (1.6 mg; tR 15.3 min) (HPLC system II). Fr. D4-7 (194.3 mg), eluted with hexane/EtOAc (5:1), was subjected to SiO2 CC to yield D4-7-1–D4-7-7, followed by preparative HPLC of D4-7-2 (99.9 mg), eluted with hexane/EtOAc (5:1), for the isolation of 2 (3.0 mg; tR 22.2 min) and 1 (1.4 mg) (HPLC system II). Fr. D6 (265.4 mg), eluted with hexane/EtOAc (5:1) was subjected to SiO2 CC to yield D6-1–D6-11, followed by preparative HPLC of D6-2 (6.7 mg), eluted with hexane/EtOAc (10:1), for the isolation of 5 (3.6 mg; tR 35.6 min) (HPLC system I). Fr. D7 (176.3 mg), eluted with hexane/EtOAc (10:1), was subjected to SiO2 CC with hexane/EtOAc (10:1) for fractionation to D7-1–D7-12. SiO2 CC of D7-8 gave 3 (1.7 mg).
7α-Methoxymultiflor-8-ene-3α,29-diol 3-acetate-29-benzoate (1). Amorphous solid; [α]22 D −74.8 (c 0.1, CHCl3); UV (EtOH) λmax (logε) 206.0 (3.58), 228.5 (3.68), 271 (2.81) nm; IR (KBr) νmax: 2,924, 2,855, 1,743, 1,724, 1,467, 1,452, 1,374, 1,267, 1,247, 1,110, 1,072 cm−1; 1H- and 13C-NMR spectroscopic data (in ppm), see Table 1; EIMS m/z 618 [M]+ (10), 586 (26), 526 (30), 511 (100), 483 (9), 389 (13), 105 (66); HREIMS m/z 618.4282 (calcd for C40H58O5, 618.4285).
7-Oxomultiflor-8-ene-3α,29-diol 3-acetate-29-benzoate (2). Amorphous solid; [α]22 D −67.4 (c 0.1, CHCl3); UV (EtOH) λmax (logε) 233.0 (3.91), 250.5 (3.81) nm; IR (KBr) νmax: 2,937, 2,883, 1,739, 1,723, 1,658, 1,270, 1,245 cm−1; 1H- and 13C-NMR spectroscopic data (in ppm), see Table 1; EIMS m/z 602 [M]+ (100), 587 (11), 542 (8), 480 (14), 465 (16), 420 (7), 371 (4), 325 (10), 303 (11), 278 (47), 243 (33), 203 (34), 105 (39); HREIMS m/z 602.3975 (calcd for C39H54O5, 602.3972).
Multiflora-7,9(11)-diene-3α,29-diol 3-p-hydroxybenzoate-29-benzoate (3). Amorphous solid; [α]22 D −3.9 (c 0.09, CHCl3); UV (EtOH) λmax (logε) 231.0 (4.25), 246.5 (4.12), 266.5 (3.95) nm; IR (KBr) νmax: 3,436, 2,941, 2,863, 1,716, 1,683, 1,636, 1,509, 1,456, 1,384, 1,274, 1,166, 1,111 cm−1; 1H- and 13C-NMR spectroscopic data (in ppm), see Table 1; EIMS m/z 664 (16) [M]+, 526 (90) [M−C7H6O3]+, 511 (32), 389 (26), 253 (41), 121 (100); HREIMS m/z 664.4127 (calcd for C44H56O5, 664.4127).
3.4. Cell Cultures
The cell lines HL-60 (human leukemia) and P388 (murine leukemia) were grown in RPMI 1640 medium, while B16 4A5 cells were grown in D-MEM. The medium was supplemented with 10% FBS and antibiotics (100 units/mL penicillin and 100 μg/mL streptomycin). The cells were incubated at 37 °C in a 5% CO2 humidified incubator.
3.5. Determination of B16 4A5 Cells Proliferation
B16 4A5 cell proliferation was examined according to a method reported previously [11] with slight modifications. Briefly, B16 4A5 cells (obtained from the Riken Cell Bank, Tsukuba, Ibaraki, Japan) (3 × 104 cells in 500 μL), preincubated for 24 h were treated for 48h with test samples dissolved in dimethyl sulfoxide (DMSO) at a final concentration of 100, 30, or 10 μM, and MTT solution was added. After 3 h of incubation, 2-propanol containing 0.08 M HCl was added to dissolve the formazan produced in the cells. The absorbance of each well was read at 550 nm using a microplate reader.
3.6. Assay of Melanin Content
The assay of melanin content was performed as described previously [11] with small modifications. B16 4A5 cells (3 × 104 cells in 500 μL) were pre-incubated as above in α-MSH (100 nM)-containing medium. Test samples dissolved in DMSO were added to the medium and the cells were cultured for 48 h. The medium was removed and the cells were dissolved in 2 M NaOH containing 10% DMSO. The amount of melanin was determined spectrophotometrically by measuring absorbance at 450 nm using a microplate reader. The optical density of control cells was assumed to be 100%.
3.7. Cytotoxicity Assay against Cancer Cell Lines
The cytotoxicity assay against HL-60 and P388 cells was determined as described previously [12].
4. Conclusions
Six multiflorane-type triterpenes, including the three new compounds 7α-methoxymultiflor-8-ene-3α,29-diol 3-acetate-29-benzoate (1), 7-oxomultiflor-8-ene-3α,29-diol 3-acetate-29-benzoate (2) and multiflora-7,9(11)-diene-3α,29-diol 3-p-hydroxybenzoate-29-benzoate (3) were isolated from the MeOH extract of Cucurbita maxima seeds. The seeds included more 4 than other multiflorane-type triterpenes. It was suggested that multiflorane-type triterpenes in C. maxima were biosynthesized from 4, or consumed to biosynthesize 4. The melanogenesis inhibitory activity of 5 suggests they may be potential skin whitening agents. On the other hand, the results of cytotoxicity assays suggest that 2 may be valuable as an anticancer lead compound.
Acknowledgments
We thank Katsuhiko Minoura and Mihoyo Fujitake (this university) for NMR and MS measurements.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/18/5/5568/s1.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Not available.
References
- 1.Okada M., editor. Newly Revised Illustrated Medicinal Plants of World. Hokuryukan Publishing Co.,Ltd.; Tokyo, Japan: 2002. p. 514. [Google Scholar]
- 2.Chevallier Andrew. The Encyclopedia of Medicinal Plants. Seibundo Shinkosha Publishing Co., Ltd.; Tokyo, Japan: 2000. p. 194. [Google Scholar]
- 3.Akihisa T., Ghosh P., Thakur S., Rosenstein F.U., Tamura T., Matsumoto T. Widespread occurrence of cucurbita-5,24-dienol in Cucurbitaceae. Yukagaku. 1986;35:1036–1040. [Google Scholar]
- 4.Cattel L., Balliano G., Caputo O. Sterols and triterpenes from Cucurbita maxima. Planta Med. 1979;37:264–267. doi: 10.1055/s-0028-1097338. [DOI] [Google Scholar]
- 5.Fenner G.P., Patterson G.W., Koines P.M. Sterol composition during the life cycle of the soybean and the squash. Lipids. 1986;21:48–51. doi: 10.1007/BF02534302. [DOI] [PubMed] [Google Scholar]
- 6.Fenner G.P., Patterson G.W., Lusby W.R. Developmental regulation of sterol biosynthesis in Cucurbita maxima L. Lipids. 1989;24:271–277. doi: 10.1007/BF02535162. [DOI] [Google Scholar]
- 7.Ukiya M., Akihisa T., Tokuda H., Toriumi M., Mukainaka T., Banno N., Kimura Y., Hasegawa J., Nishino H. Inhibitory Effects of Cucurbitane Glycosides and Other Triterpenoids from the Fruit of Momordica grosvenori on Epstein-Barr Virus Early Antigen Induced by Tumor Promoter 12-O-Tetradecanoylphorbol-13-acetate. J. Agric. Food Chem. 2002;50:6710–6715. doi: 10.1021/jf0206320. [DOI] [PubMed] [Google Scholar]
- 8.Sekine T., Kurihara H., Waku M., Ikegami F., Ruangrungsi N. A New Pentacyclic Cucurbitane Glucoside and a New Triterpene from the Fruits of Gymnopetalum integrifolium. Chem. Pharm. Bull. 2002;50:645–648. doi: 10.1248/cpb.50.645. [DOI] [PubMed] [Google Scholar]
- 9.Akihisa T., Tamura T., Matsumoto T., Eggleston D.S., Kokke W.C.M.C. Karounidiol [D:C-friedo-oleana-7,9(11)-diene-3α,29-diol] and its 3-O-benzoate: novel pentacyclic triterpenes from Trichosanthes kirilowii. X-ray molecular structure of karounidiol diacetate. J. Chem. Soc. Perkin Trans. I. 1988;3:439–443. [Google Scholar]
- 10.Lim Y.-J., Lee E.H., Kang T.H., Ha S.K., Oh M.S., Kim S.M., Yoon T.-J., Kang C., Park J.-H., Kim S.Y. Inhibitory effects of arbutin on melanin biosynthesis of α-melanocyte stimulating hormone-induced hyperpigmentation in cultured Brownish guinea pig skin tissues. Arch. Pharm. Res. 2009;32:367–373. doi: 10.1007/s12272-009-1309-8. [DOI] [PubMed] [Google Scholar]
- 11.Akihisa T., Seino K., Kaneko E., Watanabe K., Tochizawa S., Fukatsu M., Banno N., Metori K., Kimura Y. Melanogenesis inhibitory activities of iridoid-, hemiterpene-, and fatty acid-glycosides from the fruits of Morinda citrifolia (Noni) J. Oleo Sci. 2010;59:49–57. doi: 10.5650/jos.59.49. [DOI] [PubMed] [Google Scholar]
- 12.Yamada T., Muroga Y., Jinno M., Kajimoto T., Usami Y., Numata A., Tanaka R. New class azaphilone produced by a marine fish-derived Chaetomium globosum. The stereochemistry and biological activities. Bioorg. Med. Chem. 2011;19:4106–4113. doi: 10.1016/j.bmc.2011.05.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.