Abstract
Three flavone-C-glycosides—cassiaoccidentalin A (1), isovitexin (2) and isoorientin (3)—were isolated from the ethyl acetate (EtOAc) soluble fraction of the methanol crude extract of the African medicinal plant Biophytum umbraculum, This is the first report of these compounds in this plant. All compounds were identified by spectroscopic analysis and comparison with published data. Isoorientin (3) and the EtOAc extract showed the greatest antioxidant activity in the DPPH assay as well as the strongest inhibition of xanthine oxidase (XO) and 15-lipoxygenase (15-LO). From these results, the extract of B. umbraculum might be a valuable source of flavone C-glycosides.
Keywords: Biophytum umbraculum, flavone-C-glycosides, DPPH, xanthine oxidase, 15-lipoxygenase
1. Introduction
Biophytum umbraculum Welw. (common syn. Biophytum petersianum Klotzsch) (Oxalidaceae) is a slender annual herb distributed in tropical and subtropical Africa, and across to Asia and New Guinea. The aerial parts of the plant have several medicinal uses in Mali and other African countries [1]. Ethnopharmacological surveys on the use of B. umbraculum by practitioners in different districts in Mali (Bamako, Siby and Dioila) show that the plant is frequently used against cerebral malaria, but also against hemorrhoids, colonic ailments, wounds, stomach ache and fever [2,3,4]. In Nigeria the plant has been used against wounds, gonorrhea, urethral stones and stomach ache [1]. Other indications reported are constipation, hypertension, migraine, epilepsy, breathing difficulties and lack of fertility [1,5,6]. Various in vitro studies indicate that extracts of B. umbraculum may exert beneficial pharmaceutical effects on hypertension [5,7,8,9,10]. In addition, pectic polysaccharides isolated from B. umbraculum have demonstrated diverse effects on the immune system by virtue of complement fixation [3,4], activation of macrophages and dendritic cells [11], and modulation of intestinal Peyer’s patch cells [12]. Phytochemical investigations on B. umbraculum have so far been restricted to polysaccharides [3,4,11,12], although saponins of unknown structure have been stated to be present in aqueous extracts of the plant [13].
In the past decades, there has been a growing interest in antioxidants and free radical scavengers as they may have an important role in the prevention of pathologies in which reactive oxygen species (ROS) or free radicals are implicated, such as atherosclerosis, cardiovascular diseases (CVD), ischemia/reperfusion injury, neurodegenerative diseases and cancer [14,15]. As a part of an ongoing project on Malian medicinal plants [16,17,18], in the present study chemical characterization and investigation of antioxidant activity were performed on B. umbraculum.
2. Results and Discussion
2.1. Isolation and Structural Elucidation
Compounds 1, 2 and 3 were isolated from the EtOAc soluble fraction of the MeOH crude extract of Biophytum umbraculum by column chromatography (CC). Based on the weights of purified fractions, estimated concentrations of substances in the crude MeOH extract are 0.45% (1), 0.37% (2) and 0.17% (3). The compounds were identified as cassiaoccidentalin A (1), isovitexin (2) and isoorientin (3), respectively, by comparing their 1H-NMR and 13C-NMR spectroscopic data with those reported in the literature [19,20,21]. Besides, the COSY spectrum revealed 1H-1H couplings and helped assign proton resonances, especially those of the inner sugar of 1. The HSQC spectrum showed all 1J direct 1H-13C correlations and thus confirmed the assignments of all signals arising from the CH- and CH2- groups. Among all the 1H-13C long-range correlations observed in the HMBC spectrum, the most important were the correlations from the anomeric proton of the inner sugar (H-1′) to C-6 (2J), C-5 (3J) and C-7 (3J) of the aglycone, which confirmed the C-glycosylation at C-6.
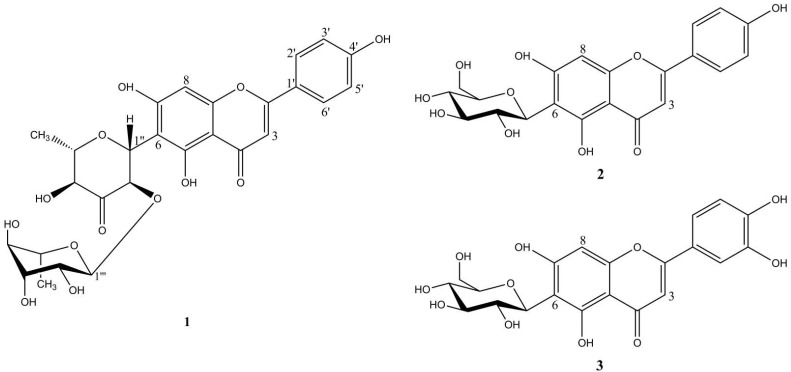
The chemical structures of the three flavone-C-glycosides are shown in Figure 1. To our knowledge, this is the first identification of flavone-C-glycosides in B. umbraculum. Compound 1 is a very rare flavone-C-glycoside which has only been identified once before, in Cassia occidentalis [19], whereas the more common flavone glucosides 2 and 3 have been identified in another Biophytum species, namely Biophytum sensitivum [22].
Figure 1.
Chemical structures of the three flavone-C-glycosides 1–3.
2.2. Antioxidant Activity
The dichloromethane (DCM) crude extract of B. umbraculum was virtually inactive as a DPPH radical scavenger, xanthine oxidase (XO) and 15-lipoxygenase (15-LO) inhibitor (IC50 > 167 µg/mL). The MeOH crude extract and the semi-polar extracts obtained by partitioning the MeOH extract showed fairly high radical scavenger activity and 15-LO inhibition (Table 1). The extracts showed moderate or low inhibitory activity towards XO compared to the positive control quercetin. The EtOAc extract exhibited the highest activity in all three assays.
Table 1.
DPPH radical scavenging, xanthine oxidase (XO) inhibition and 15-lipoxygenase (15-LO) inhibition of Biophytum umbraculum extracts. IC50 values ± SD (in µg/mL) are shown.
| Extract | DPPH | XO | 15-LO |
|---|---|---|---|
| DCM crude extract | >167 | >167 | >167 |
| MeOH crude extract | 13.4 ± 0.6 | 59.7 ± 6.1 | 68.9 ± 5.0 |
| EtOAc extract | 6.8 ± 0.6 | ca. 21 | 43.0 ± 3.6 |
| BuOH extract | 12.5 ± 1.9 | 102.6 ± 5.6 | 53.4 ± 2.1 |
| Aqueous residue | 29.8 ± 3.5 | >167 | >167 |
| Quercetin (positive control) | 4.4 ± 0.4 | 2.33 ± 0.09 | 33.4 ± 0.3 |
The activity of the isolated compounds as DPPH scavengers, XO- and 15-LO inhibitors is shown in Table 2. Flavone-C-glycosides were found to contribute both as DPPH scavengers and 15-LO inhibitors in the EtOAc extract. This is in good accordance with previous structure-activity studies indicating the importance of a 2,3 double bond in conjugation with a 4-oxo function in the C-ring and o-dihydroxy structure in the B-ring [23,24]. The strongest inhibitor of XO and 15-LO and the best DPPH scavenger was isoorientin. The strong DPPH radical scavenging activity of isoorientin and the much weaker activity of isovitexin are supported by previous results [25,26]. This antioxidant activity clearly shows that the 3′,4′-dihydroxy structural element of isoorientin is of importance for activity, isoorientin having an IC50 value similar to the positive control quercetin. This is in accordance with a previous report [27]. The EtOAc extract may contain additional unidentified XO inhibitors, since XO inhibition of the isolated compounds does not account for the activity observed in the EtOAc extract. It has previously been suggested that flavones without a glycosyl group were relatively strong inhibitors of XO, as the presence of a glycosyl group would decrease the inhibitory activity [28,29]. XO and 15-LO inhibitors may be beneficial for diseases and conditions such as ischemia/reperfusion, gout, renal stones, inflammation, arteriosclerosis, neurodegenerative diseases, cancer, aging, etc. [28,30,31,32]. Additionally, XO-inhibitors may have beneficial effects as adjuncts in the management of severe Plasmodium falciparum malaria [33].
Table 2.
Effects of isolated compounds from B. umbraculum on DPPH radical scavenging, xanthine oxidase (XO) inhibition and 15-lipoxygenase (15-LO) inhibition. IC50 values ± SD (in µM) are shown.
| Isolated compound | DPPH | XO | 15-LO |
|---|---|---|---|
| Cassiaoccidentalin A (1) | >167 | 149.5 ± 7.4 | 99.9 ± 2.5 |
| Isovitexin (2) | 96.0 ± 3.6 | >167 | 107.1 ± 2.1 |
| Isoorientin (3) | 18.1 ± 1.1 | 117.2 ± 13.5 | 86.4 ± 0.5 |
| Quercetin (positive control) | 13.7 ± 1.3 | 7.7 ± 0.3 | 110.6 ± 1.0 |
Since the solubility in water of the flavonoids reported here is unknown (although a calculation, as given on the SciFinder website, gives theoretical values of between 0.12 and 0.15 mg/mL), an analysis of aqueous extracts of the plant may be relevant for the evaluation of its ethnopharmacological use, and might thus represent a useful continuation of the present work.
These results imply that the extracts from B. umbraculum are a rich source of flavone-C-glycosides, and that herbal remedies obtained from this plant may have an effect against inflammations or other diseases related to oxidative stress. The results from our study would seem to be in accord with several of the reported ethnopharmacological usages of the plant.
3. Experimental
3.1. General
1D and 2D NMR spectra were recorded on Bruker DPX 300 or Bruker AVII 400 instruments with CD3OD as solvent and TMS as internal standard. CC was done over Sephadex LH-20 (Pharmacia) or reverse phase (RP) silica (VersaPak C18 cartridges; Supelco). Fractions from CC were combined as indicated by TLC. Foils coated with Si gel RP-18 F254S (Merck) were used for analytical and preparative TLC. In analytical TLC, spots were visualized by UV irradiation (254 and 366 nm) and by spraying with Ce(SO4)2 (1% in 10% aqueous H2SO4) followed by heating (100 °C, 10–15 min). For UV/VIS measurements, a Biochrom Libra S32 PC instrument was employed.
3.2. Plant Material
Flowering, whole aerial parts of Biophytum umbraculum Welw. (Oxalidaceae) were collected in Blendio, Mali. The plant was identified by one of the authors, Prof. Drissa Diallo. A voucher specimen (NO. 2653 DMT) was deposited in the herbarium at the Department of Traditional Medicine (DMT), Bamako, Mali.
3.3. Extraction and Isolation
The dried and powdered aerial parts of B. umbraculum (305 g) were extracted at RT with DCM (4 × 2.5 L), each time for 24 h, yielding 5.1 g of DCM extract (1.7% yield). The plant residue was then extracted similarly with MeOH (5 × 2.5 L) to yield 17.7 g (5.8%) of MeOH extract. The MeOH extract was suspended in 3 × 100 mL distilled water and successively partitioned with EtOAc (6 × 300 mL) and BuOH (5 × 200 mL). The solvents were removed under vacuum, affording an EtOAc extract (4.6 g), a BuOH extract (5.3 g) and an aqueous residue (5.0 g). The EtOAc (E) extract was chromatographed over Sephadex LH-20 (2.5 × 74 cm) with a gradient of H2O/MeOH (25%–100% MeOH) to yield 15 subfractions (E1-E15). E9 (334 mg), E10 (1,029 mg) and E11 (555 mg) were chosen for isolation of bioactive compounds based on fraction weights, pattern in NMR spectra and results from activity assays. E9 was flash chromatographed over RP C-18 Si gel (VersaPak, 2.3 × 11 cm) eluting with a H2O-MeOH gradient (40%–100% MeOH) to give 1 (57 mg). E10 was applied to a RP C-18 Si gel VersaPak column (4 × 15 cm) and eluted with a gradient of H2O-MeOH (20%–100% MeOH) to afford E10.1-E10.9. E10.6 (42 mg) and E10.7 (24 mg) were combined and rechromatographed over a smaller VersaPak column (2.3 × 11 cm) with a H2O-MeOH gradient (20%–100% MeOH) followed by preparative TLC to give 2 (8.8 mg). E11 was flash chromatographed with the same column as used for E10 and eluted with a gradient of H2O-MeOH (40%–100% MeOH) to yield E11.1-E11.13. E11.2 (50 mg) and E11.3 (21 mg) were combined and purified using the same system to give 3 (6.5 mg). A second batch of plant material (225 g) was subjected to Soxhlet extraction (2 L DCM for 48 h, followed by 3 L MeOH for 48 h). Yield of DCM extract was 7.5 g (3.3%), and of MeOH extract 17.2 g (7.6%). EtOAc extract (4.6 g), BuOH extract (3.3 g) and aqueous residue (5.1 g) were obtained similarly as above.
3.4. DPPH Radical Scavenging
Pure substances and crude extracts (Soxhlet) were dissolved in DMSO, and the assay was carried out as reported previously [34]. Quercetin (Sigma-Aldrich) was used as positive control.
3.5. Inhibition of Xanthine Oxidase (XO)
Pure substances and crude extracts (Soxhlet) were dissolved in DMSO, and the assay was carried out as reported previously [35]. Quercetin (Sigma-Aldrich) was used as positive control.
3.6. Inhibition of 15-Lipoxygenase (15-LO)
Pure substances and crude extracts (Soxhlet) were dissolved in DMSO, and the assay was carried out as reported previously [34]. Quercetin (Sigma-Aldrich) was used as positive control.
3.7. Statistical Analysis
All samples were analyzed in triplicates and the results are shown as mean ± standard deviation (SD).
4. Conclusions
Our phytochemical study led to the isolation and characterization of three flavone-C-glycosides from the aerial parts of B. umbraculum. This is the first report on this type of compound from this plant. Compound 3 and the EtOAc extract of the plant revealed strong antioxidant activity towards DPPH radical and 15-LO, and moderate activity towards XO. Further studies on these extracts with respect to antioxidant properties in vivo are needed.
Acknowledgments
The NMR laboratory at the Chemistry Department, University of Oslo, Norway is acknowledged for the spectrometer facilities.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 1–3 are available from the authors.
References
- 1.Burkill H.M. The Useful Plants of West Tropical Africa. 2nd. Volume 4. Royal Botanic Gardens, Kew; Surrey, UK: 1997. pp. 336–338. [Google Scholar]
- 2.Grønhaug T.E., Glæserud S., Skogsrud M., Ballo N., Bah S., Diallo D., Paulsen B.S. Ethnopharmacological survey of six medicinal plants from Mali, West-Africa. J. Ethnobiol. Ethnomed. 2008;4:26. doi: 10.1186/1746-4269-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inngjerdingen K.T., Coulibaly A., Diallo D., Michaelsen T.E., Smestad P.B. A complement fixing polysaccharide from Biophytum petersianum Klotzsch, a medicinal plant from Mali, West Africa. Biomacromolecules. 2006;7:48–53. doi: 10.1021/bm050330h. [DOI] [PubMed] [Google Scholar]
- 4.Diallo D., Sogn C., Samaké F.B., Paulsen B.S., Michaelsen T.E., Keita A. Wound healing plants in Mali, the Bamako region. An ethnobotanical survey and complement fixation of water extracts from selected plants. Pharm. Biol. 2002;40:117–128. doi: 10.1076/phbi.40.2.117.5846. [DOI] [Google Scholar]
- 5.Mouzou A.P., Titrikou S., Constantin B., Sebille S., Cognard C., Gbeassor M., Raymond G. Effets du décocté de Biophytum petersianum (Oxalidaceae) sur la libération du calcium du réticulum sarcoplasmique des cellules musculaires squelettiques (in French) Phytothérapie. 2010;8:231–235. doi: 10.1007/s10298-010-0563-8. [DOI] [Google Scholar]
- 6.Focho D.A., Ndam W.T., Fonge B.A. Medicinal plants of Aguambu—Bamumbu in the lebialem highlands, southwest province of Cameroon. Afr. J. Pharm. Pharmacol. 2009;3:1–13. [Google Scholar]
- 7.Kodjo K.M., Contesse V., Do R.J.L., Aklikokou K., Titrikou S., Gbeassor M., Vaudry H. In vitro effects of crude extracts of Parkia biglobosa (Mimosaceae), Stereospermum kunthianum (Bignoniaceae), and Biophytum petersianum (Oxalidaceae) on corticosteroid secretion in rat. J. Steroid. Biochem. Mol. Biol. 2006;100:202–208. doi: 10.1016/j.jsbmb.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 8.Titrikou S., Eklu-Gadegbeku K., Mouzou A., Aklikokou K., Gbeassor M. Calcium antagonistic activity of Biophytum petersianum on vascular smooth muscles of wistar rat. Iran. J. Pharmacol. Ther. 2007;6:185–189. [Google Scholar]
- 9.Titrikou S., Aklikokou A.K., Gbeassor M. Effets de l'extrait de Biophytum petersianum (Oxalidaceae), Klotzsh, sur le systeme cardiovasculaire de cobaye (in French) Pharmacopée et Médecine Traditionelle Africaines. 1998;10:32–41. [Google Scholar]
- 10.Titrikou S., Eklu-Gadegbeku K., Aklikokou K.A., Gbeassor M. Effets de Biophytum petersianum (Oxalidaceae) sur la pression artérielle chez le rat Wistar (in French) Phytothérapie. 2008;6:215–218. doi: 10.1007/s10298-008-0321-3. [DOI] [Google Scholar]
- 11.Inngjerdingen M., Inngjerdingen K.T., Patel T.R., Allen S., Chen X., Rolstad B., Morris G.A., Harding S.E., Michaelsen T.E., Diallo D., et al. Pectic polysaccharides from Biophytum petersianum Klotzsch, and their activation of macrophages and dendritic cells. Glycobiology. 2008;18:1074–1084. doi: 10.1093/glycob/cwn090. [DOI] [PubMed] [Google Scholar]
- 12.Grønhaug T.E., Kiyohara H., Sveaass A., Diallo D., Yamada H., Paulsen B.S. Beta-D-(1→4)-galactan-containing side chains in RG-I regions of pectic polysaccharides from Biophytum petersianum Klotzsch contribute to expression of immunomodulating activity against intestinal Peyer’s patch cells and macrophages. Phytochemistry. 2011;72:2139–2147. doi: 10.1016/j.phytochem.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Santoso B., Kilmaskossu A., Sambodo P. Effects of saponin from Biophytum petersianum Klotzsch on ruminal fermentation, microbial protein synthesis and nitrogen utilization in goats. Anim. Feed Sci. Tech. 2007;137:58–68. doi: 10.1016/j.anifeedsci.2006.10.005. [DOI] [Google Scholar]
- 14.Valko M., Leibfritz D., Moncol J., Cronin M.T.D., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Ghasemzadeh A., Ghasemzadeh N. Flavonoids and phenolic acids: Role and biochemical activity in plants and human. J. Med. Plants Res. 2011;5:6697–6703. [Google Scholar]
- 16.Le N.H.T., Malterud K.E., Diallo D., Paulsen B.S., Nergard C.S., Wangensteen H. Bioactive polyphenols in Ximenia americana and the traditional use among Malian healers. J. Ethnopharmacol. 2012;139:858–862. doi: 10.1016/j.jep.2011.12.031. [DOI] [PubMed] [Google Scholar]
- 17.Austarheim I., Mahamane H., Sanogo R., Togola A., Khaledabadi M., Vestrheim A.C., Inngjerdingen K.T., Michaelsen T.E., Diallo D., Paulsen B.S. Anti-ulcer polysaccharides from Cola cordifolia bark and leaves. J. Ethnopharmacol. 2012;143:221–227. doi: 10.1016/j.jep.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 18.Pham A.T., Dvergsnes C., Togola A., Wangensteen H., Diallo D., Paulsen B.S., Malterud K.E. Terminalia macroptera, its current medicinal use and future perspectives. J. Ethnopharmacol. 2011;137:1486–1497. doi: 10.1016/j.jep.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 19.Hatano T., Mizuta S., Ito H., Yoshida T. C-glycosidic flavonoids from Cassia occidentalis. Phytochemistry. 1999;52:1379–1383. doi: 10.1016/S0031-9422(99)00437-9. [DOI] [Google Scholar]
- 20.Ramarathnam N., Osawa T., Namiki M., Kawakishi S. Chemical studies on novel rice hull antioxidants. 2. Identification of isovitexin, a C-glycosyl flavonoid. J. Agric. Food Chem. 1989;37:316–319. doi: 10.1021/jf00086a009. [DOI] [Google Scholar]
- 21.Kumarasamy Y., Byres M., Cox P.J., Delazar A., Jaspars M., Nahar L., Shoeb M., Sarker S.D. Isolation, structure elucidation, and biological activity of flavone 6-C-glycosides from Allaria petiolata. Chem. Nat. Compd. 2004;40:122–128. doi: 10.1023/B:CONC.0000033926.72396.41. [DOI] [Google Scholar]
- 22.Bucar F., Jachak S.M., Karting T., Schubert-Zsilavecz M. Phenolic compounds from Biophytum sensitivum. Pharmazie. 1998;53:651–653. [Google Scholar]
- 23.Rice-Evans C.A., Miller N.J., Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biol. Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 24.Waki T., Nakanishi I., Matsumoto K.-i., Kitajima J., Chikuma T., Kobayashi S. Key role of chemical hardness to compare 2,2-diphenyl-1-picrylhydrazyl radical scavenging power of flavone and flavonol O-glycoside and C-glycoside derivatives. Chem. Pharm. Bull. 2012;60:37–44. doi: 10.1248/cpb.60.37. [DOI] [PubMed] [Google Scholar]
- 25.Yao H., Chen Y., Shi P.Y., Hu J., Li S.G., Huang L.Y., Lin J.H., Lin X.H. Screening and quantitative analysis of antioxidants in the fruits of Livistona chinensis R. Br using HPLC-DAD-ESI/MS coupled with pre-column DPPH assay. Food Chem. 2012;135:2802–2807. doi: 10.1016/j.foodchem.2012.07.076. [DOI] [PubMed] [Google Scholar]
- 26.Du Q.Z., Li B. Identification of antioxidant compounds of Mucuna sempervirens by high-speed counter-current chromatographic separation-DPPH radical scavenging detection and their oestrogenic activity. Food Chem. 2012;131:1181–1186. doi: 10.1016/j.foodchem.2011.09.095. [DOI] [Google Scholar]
- 27.Zielinska D., Zielinski H. Antioxidant activity of flavone C-glucosides determined by updated analytical strategies. Food Chem. 2011;124:672–678. doi: 10.1016/j.foodchem.2010.06.051. [DOI] [Google Scholar]
- 28.Hayashi T., Sawa K., Kawasaki M., Arisawa M., Shimizu M., Morita M. Inhibition of cow’s milk xanthine oxidase by flavonoids. J. Nat. Prod. 1988;51:345–348. doi: 10.1021/np50056a030. [DOI] [PubMed] [Google Scholar]
- 29.Lin C.M., Chen C.S., Chen C.T., Liang Y.C., Lin J.K. Molecular modeling of flavonoids that inhibits xanthine oxidase. Biochem. Biophys. Res. Commun. 2002;294:167–172. doi: 10.1016/S0006-291X(02)00442-4. [DOI] [PubMed] [Google Scholar]
- 30.Da Silva S.L., da Silva A., Honorio K.M., Marangoni S., Toyama M.H., da Silva A.B.F. The influence of electronic, steric and hydrophobic properties of flavonoid compounds in the inhibition of the xanthine oxidase. J. Mol. Struct. 2004;684:1–7. doi: 10.1016/j.theochem.2004.04.003. [DOI] [Google Scholar]
- 31.Yigitkanli K., Pekcec A., Karatas H., Pallast S., Mandeville E., Joshi N., Smirnova N., Gazaryan I., Ratan R.R., Witztum J.L., et al. Inhibition of 12/15-lipoxygenase as therapeutic strategy to treat stroke. Ann. Neurol. 2013;73:129–135. doi: 10.1002/ana.23734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Del Rio D., Rodriguez-Mateos A., Spencer J.P.E., Tognolini M., Borges G., Crozier A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Sign. 2013;18:1818–1892. doi: 10.1089/ars.2012.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwalokun B.A., Bamiro S.B., Ogunledun A. Levels and interactions of plasma xanthine oxidase, catalase and liver function parameters in Nigerian children with Plasmodium falciparum infection. APMIS. 2006;114:842–850. doi: 10.1111/j.1600-0463.2006.apm_457.x. [DOI] [PubMed] [Google Scholar]
- 34.Wangensteen H., Samuelsen A.B., Malterud K.E. Antioxidant activity in extracts from coriander. Food Chem. 2004;88:293–297. doi: 10.1016/j.foodchem.2004.01.047. [DOI] [Google Scholar]
- 35.Pham A.T., Malterud K.E., Paulsen B.S., Diallo D., Wangensteen H. DPPH radical scavenging and xanthine oxidase inhibitory activity of Terminalia macroptera leaves. Nat. Prod. Commun. 2011;6:1125–1128. [PubMed] [Google Scholar]