Abstract
A new phenolic glycoside, 3-methoxyphenol 1-O-α-L-rhamnopyranosyl-(1→6)-O-β-D-glucopyranoside (1), was isolated from the 90% acetone extract of the branches and leaves of Chamaecyparis obtusa var. breviramea f. crippsii along with another 10 known phenolics 2–11. Their structures were determined mainly by means of MS, 1D- and 2D-NMR data. Cytotoxicities of compounds 3 and 5–11 were tested on BGC-823, Hela and A549 cancer cell lines, the results showed that compound 8 was bioactive and its IC50 values were 6.9, 29.7 and 52.9 μM, respectively.
Keywords: Chamaecyparis obtusa var. breviramea f. crippsii, phenolic compounds, cytotoxicity
1. Introduction
There are six speciesin the genus Chamaecyparis, which are mainly distributed in North America, Japan, and Taiwan [1]. Chamaecyparis plants have been found to be rich sources of monoterpenes [2], sesquiterpenoids [3], diterpenes [2,4] and lignans [5,6,7], some of which have shown antitumor, antimalarial and antibacterial activities [8,9,10]. Chamaecyparis obtusa (Sieb. et Zucc.) Endl. var. breviramea f. crippsii is a cultivated variety of C. obtusa [11]. According to the literature, no chemical constituents of this plant has been reported until now. As part of serial investigations on the Cupressaceae and in order to seek more novel bioactive compounds, we carried out an extensive chemical study on C. obtusa var. breviramea f. crippsii. In this paper, we report the isolation and structure elucidation of a new phenolic glycoside 1, together with ten other known phenolics 2–11 from the branches and leaves of C. obtusa var. breviramea f. crippsii, in addition to a screening of their cytotoxicity.
2. Results and Discussion
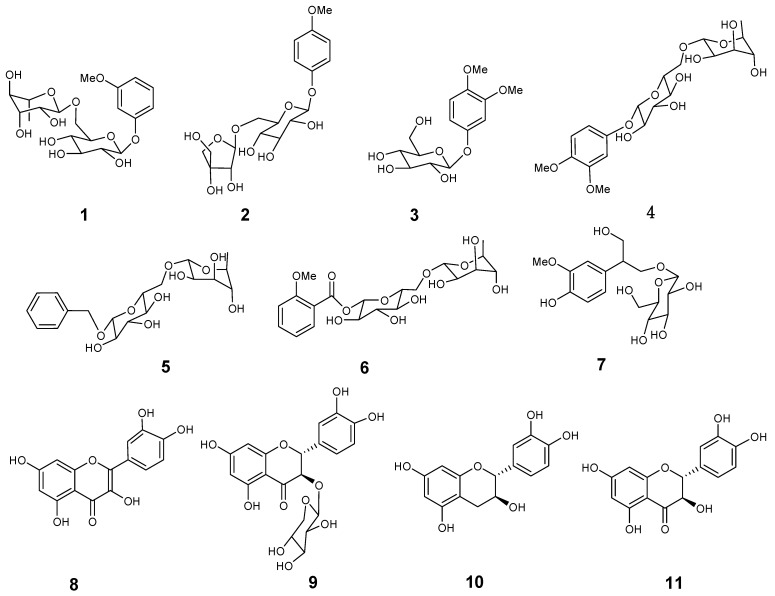
The n-BuOH fraction of the branches and leaves of C. obtusa var. breviramea f. crippsii was subjected to column chromatographies on silica gel, Sephadex LH-20 and MCI, and preparative HPLC, to afford a new phenolic glycoside 1, together with the 10 known phenolics 2–11, which were identified by comparison of spectra data with the reported literature values (Figure 1).
Figure 1.
Structures of compounds 1–11.
Compound 1 was obtained as a colorless amorphous solid. It possessed the molecular formula C19H28O11 according to HRTOFMS ([M+Cl]− peak at m/z 467.1313, calc. 467.1320), which was confirmed by the 13C-NMR spectrum. The IR spectrum of 1 showed absorption bands for OH (3423 cm−1) and aromatic (Ph) groups (1596 cm−1). Its UV spectrum revealed the presence of aromatic (Ph) groups (200, 220, 273 nm). The 1H and 13C spectra (see Table 1) showed the presence of a m-substituted aromatic group (δC 147.9 (C-1), 124.2 (C-2), 150.8 (C-3), 113.7 (C-4), 122.3 (C-5), 118.2 (C-6); δH7.03 (d, J = 2.9 Hz, H-2), 7.03 (m, H-4), 6.95 (m, H-5), 7.16 (d, J = 6.6 Hz, H-6)), a β-D-glucopyranose (δC 102.7 (C-1'), 74.9 (C-2'), 77.9 (C-3'), 71.6 (C-4'), 76.9 (C-5'), 67.7 (C-6'); δH4.86 (d, J = 6.3 Hz, H-1'), 3.50 (t, J = 7.6 Hz, H-2'), 3.48 (t, J = 7.6 Hz, H-3'), 3.40 (t, J = 7.6 Hz, H-4'), 3.54 (dt, J = 6.8, 1.5 Hz, H-5'), 4.02 (dd, J = 9.0, 1.5 Hz, H-6'a), 3.62 (dd, J = 9.0, 5.1 Hz, H-6'b)), an α-L-rhamnopyranose [δC 102.2 (C-1''), 72.2 (C-2''), 74.0 (C-3''), 72.4 (C-4''), 69.8 (C-5''), 18.0 (C-6''); δH4.72 (d, J = 1.2 Hz, H-1''), 3.83 (dd, J = 2.8, 1.4 Hz, H-2''), 3.70 (dd, J = 8.0, 2.8 Hz, H-3''), 3.37 (t, J = 8.0 Hz, H-4''), 3.66 (m, H-5''), 1.22 (d, J = 5.2 Hz, H-6'')], and a methoxy (δc 56.6), which suggested that compound 1 was a phenolic glycoside. The 1H- and 13C-NMR data of 1 (see Table 1) were a close match to those of itoside I [12].
Table 1.
13C- and 1H-NMR spectral data of compound 1 (δ in ppm, J in Hz).
| Position | δC | δH | δC | δH | |
|---|---|---|---|---|---|
| 1 | 147.9 | 4' | 71.6 | 3.40 (t, 7.6 Hz) | |
| 2 | 124.2 | 7.03 (d, 2.9 Hz) | 5' | 76.9 | 3.54 (dt, 6.8, 1.5 Hz) |
| 3 | 150.8 | 6' | 67.7 | 4.02 (dd, 9.0, 1.5 Hz) 3.62 (dd, 9.0, 5.1 Hz) | |
| 4 | 113.7 | 7.03 (m) | 1'' | 102.2 | 4.72 (d, 1.2 Hz) |
| 5 | 122.3 | 6.95 (m) | 2'' | 72.2 | 3.83 (dd, 2.8, 1.4 Hz) |
| 6 | 118.2 | 7.16 (d, 6.6 Hz) | 3'' | 74.0 | 3.70 (dd, 8.0, 2.8 Hz) |
| OMe | 56.6 | 3.88 (s) | 4'' | 72.4 | 3.37 (t, 8.0 Hz) |
| 1' | 102.7 | 4.86 (d, 6.3 Hz) | 5'' | 69.8 | 3.66 (m) |
| 2' | 74.9 | 3.50 (t, 7.6 Hz) | 6'' | 18.0 | 1.22 (d, 5.2 Hz) |
| 3' | 77.9 | 3.48 (t, 7.6 Hz) |
13C- (125 MHz) and 1H- (500 MHz) in CD3OD.
In the HMBC spectrum of 1 (Figure 2), the correlation from the anomeric proton 4.72 (d, J = 1.2 Hz, H-1'') to the methine at 67.7 (C-6') indicated that the linkage between the α-L-rhamnopyranose and the β-D-glucopyranose was C-1''→C-6'. The cross peak between δH4.86 (d, J = 6.3 Hz, H-1') and δC147.9 (C-1) suggested that the substituted site of the β-D-glucopyranose on the phenolic aglycone was C-1'→C-1. The correlation between δH3.88 (s, OMe) and δC150.8 (C-3) indicated that the OMe was located at C-3. Thus, the structure of 1 was identified as 3-methoxyphenol 1-O-α-L-rhamnopyranosyl-(1→6)-O-β-D-glucopyranoside.
Figure 2.
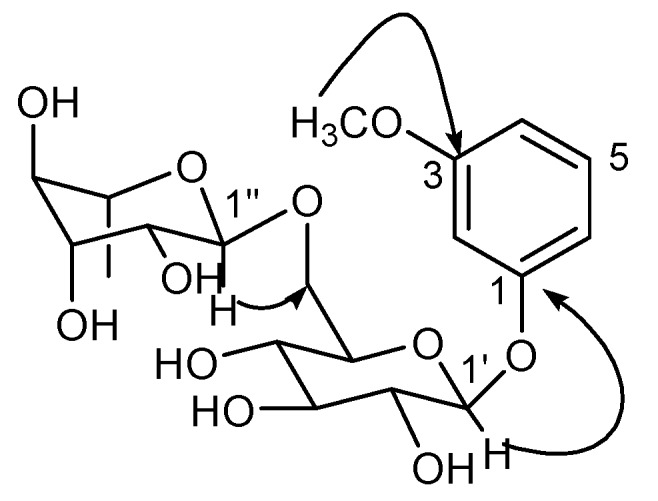
Key HMBC correlations of compound 1.
Compounds 2–11 were identified as 4-methoxyphenol 1-O-β-D-apiofuranosyl-(1→6)-O-β-D-glucopyranoside (2) [13], 3,4-dimethoxyphenyl-1-O-β-D-glucopyranoside (3) [14], 3,4-dimethoxyphenyl 1-O-α-L-rhamnopyranosyl-(1→6)-O-β-D-glucopyranoside (4) [15], 7-O-benzyl-α-L-rhamnopyranosyl-(1→6)-O-β-D-glucopyranoside (5) [16], 7-O-α-L-rhamnopyranosyl-(1→6)-O-β-D-glucopyranoside of methyl salicylate (6) [17], 1-O-β-D-glucopyranosyl-2-(3-methoxy-4-hydroxyphenyl)-propane-1,3-diol (7) [18], quercetin (8) [19], (+)-taxifolin-3-O-β-D-xylopyranoside (9) [20], (+)-catechin (10) [21] and (+)-taxifolin (11) [22] by comparison of their data ([α], MS and NMR) with those in the literature.
In the primary bioactivity test, the methanol extract of this plant showed cytotoxicities on the cancer cell lines A549, BGC-823, Du145 and MDA-MB-231 with IC50 values of 0.94, 1.07, 0.95 and 0.96 μg/mL, respectively. In order to find the cytotoxic constituents, among the 11 compounds obtained and identified from the n-BuOH fraction of this plant, compounds 3 and 5–11 were tested for cytotoxicity against the Hela, BGC-823 and A549 cancer cell lines, and only compound 8 showed cytotoxicity, with IC50 values of 6.9, 29.7 and 52.9 μM, respectively.
3. Experimental
3.1. General
Optical rotations were measured at 17 °C on a Horiba SEAP-300 polarimeter. IR spectra were obtained on a Bio-Red FTS-135 spectrophotometer. UV spectra were measured on a 2401PC spectrophotometer. NMR spectra were recorded on a Bruker AM-400 or DRX-500 spectrometer, using TMS as an internal standard. ESIMS spectra were obtained on a VG Autospec-3000 spectrometer. HPLC was performed using an Agilent 1100 autopurification system equipped with a DAD detector (190–950 nm). Precoated silica gel plates (Meijing, China) were used for TLC. Detection was done by spray plates with 8% anisaldehyde-sulfuric acid, followed by heating.
3.2. Plant Material
Branches and leaves of C. obtusa var. breviramea f. crippsii were collected from Kunming Botany Garden, Yunnan Province, People’s Republic of China, in August 2010. It was identified by Associated Prof. Zhong Shu Yue from Kunming Institute of Botany, Chinese Academy of Sciences.
3.3. Cytotoxicity Activity Assay
The human tumor cell lines Du145 (prostate carcinoma) and MDA-MB-231 (breast carcinoma), BGC-823 (gastric carcinoma), Hela (cervical carcinoma) and A549 (non-small cell lung carcinoma) were bought from the Chinese Academy of Medical Sciences. The cytotoxicity assays were performed according to a published procedure [23].
3.4. Extraction and Isolation
The powdered air-dried branches and leaves (12.5 kg) of C. obtusa var. breviramea f. crippsii were extracted with 90% acetone (3 × 20 L) at room temperature and then concentrated under reduced pressure. The concentrated acetone extract (860 g) was dissolved in hot water and extracted with petroleum ether, EtOAc and n-BuOH, respectively, to afford a 250 g petroleum ether fraction, a 110 g EtOAc fraction, a 210 g n-BuOH fraction and a 284 g water fraction. The n-BuOH fraction was purified by column chromatography (CC) on silica gel eluting with CH2Cl2/MeOH (9:1–0:1), to give sub-fractions 1–9. Sub-fraction 6 was respectively purified by CC and eluted with CH2Cl2/MeOH (8.5:1.5–7:3), MCI (MeOH/Water: 1:9–6:4), Sephadex LH-20 (MeOH/Water: 7:3), and then preparative HPLC using a Sunfire C-18 column (250 × 21.2 mm, 5 μm) with a mobile phase consisting of MeOH-Water (1.5:8.5–4:6) to afford 1 (11 mg), 2 (8 mg), 3 (26 mg), 4 (7 mg), 5 (21 mg), 6 (17 mg) and 7 (35 mg). Sub-fraction 2 was purified by CC and eluted with CH2Cl2/MeOH (9:1–8:2) to afford 8 (35 mg), 9 (24 mg), 10 (48 mg) and 11 (31 mg).
3-Methoxyphenol 1-O-α-L-rhamnopyranosyl-(1→6)-O-β-D-glucopyranoside (1): Colorless amorphous solid. m.p. 112–114 °C. [α]22D = −48.9 (c = 0.28, MeOH). IR νmax cm−1: 3423, 2928, 1676, 1596, 1504, 1257, 1068; UV λmax (logε): 200 (4.13), 220 (3.85), 273 (3.26). 1H- and 13C-NMR spectral data: see Table 1. ESIMS m/z: 467 [M+Cl]+. HRTOFMS m/z: 467.1313[M+Cl]+ C19H28O11Cl (calc. for 467.1320).
4. Conclusions
This work was part of a series of investigations on antitumor compounds from Cupressaceae plants. Compound 1 was found to be a new phenolic glycoside, and the other ten compounds were found for the first time in C. obtusa var. breviramea f. crippsii. The random cytotoxic screening results showed that the significant cytotoxicity of the methanol extract was not caused by these simple phenolics isolated from the n-BuOH fraction, so the sesquiterpenes, diterpenes and podophyllotoxin derivatives isolated from the petroleum ether and EtOAc fraction might be responsible for the strong cytotoxicity of the crude methanol extract, which will be extensively investigated and reported in future work.
Acknowledgments
This work was supported by the grants from the National Natural Science Foundation of China (20972168 and 30725048), the National New Drug Innovation Great Project of China (2011ZX09307-002-02), the Natural Science Foundation of Yunnan Province (2010CI048), National Basic Research Program of China (2009CB522300).
Footnotes
Sample Availability: Samples of the compounds are available from authors.
References
- 1.Editorial Committee of Flora Reipublicae Popularis Sinicae. Florae Reipublicae Popularis Sinicae. Science Press; Beijing, China: 1999. [Google Scholar]
- 2.Kuo Y.H., Chen C.H., Wein Y.S. New dimeric monoterpenes and dimeric diterpenes from the heartwood of Chamaecyparis obtusa var. formosana. Helv. Chim. Acta. 2002;85:2657–2663. doi: 10.1002/1522-2675(200209)85:9<2657::AID-HLCA2657>3.0.CO;2-B. [DOI] [Google Scholar]
- 3.Kuo Y.H., Chen C.H., Chien S.C., Lin Y.L. Five new cadinane-type sesquiterpenes from the heartwood of Chamaecyparis obtusa var formosana. J. Nat. Prod. 2002;65:25–28. doi: 10.1021/np0101402. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y.M., Tan N.H., Lu Y., Yang E.H., Jia R.R. Chamobtusin A, a novel skeleton diterpenoid alkaloid from Chamaecyparis obtusa cv. tetragon. Org. Lett. 2007;9:4579–4581. doi: 10.1021/ol702087h. [DOI] [PubMed] [Google Scholar]
- 5.Takaku N., Mikame K., Okunishi T., Suzuki S., Umezawa T., Shimada M. New lignan, isoactifolin, from Chamaecyparis obtusa cv. breviramea. J. Wood Sci. 2001;47:493–496. doi: 10.1007/BF00767904. [DOI] [Google Scholar]
- 6.Takaku N., Choi D.H., Mikame K., Okunishi T., Suzuki S., Ohashi H., Umezawa T., Shimada M. Lignans of Chamaecyparis obtusa. J. Wood Sci. 2001;47:476–482. doi: 10.1007/BF00767901. [DOI] [Google Scholar]
- 7.Chien S.C., Chang J.Y., Kuo C.C., Hsieh C.C., Yang N.S., Kuo Y.H. Cytotoxic and novel skeleton compounds from the heartwood of Chamaecyparis obtusa var. formosana. Tetrahedron Lett. 2007;48:1567–1569. [Google Scholar]
- 8.Barrero A.F., Quilez del Moral J.F., Mar Herrador M., Akssira M., Bennamara A., Akkad S., Aitigri M. Oxygenated diterpenes and other constituents from Moroccan Juniperus phoenicea and Juniperus thurifera var. africana. Phytochemistry. 2004;65:2507–2515. doi: 10.1016/j.phytochem.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 9.Okasaka M., Takaishi Y., Kashiwada Y., Kodzhimatov O., Ashurmetov O., Lin A.J., Consentino L.M., Lee K.H. Terpenoids from Juniperus polycarpus var. seravschanica. Phytochemistry. 2006;67:2635–2640. doi: 10.1016/j.phytochem.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 10.Smith E.C.J., Williamson E.M., Wareham N., Kaatz G.W., Gibbons S. Antibacterials and modulators of bacterial resistance from the immature cones of Chamaecyparis lawsoniana. Phytochemistry. 2007;68:210–217. doi: 10.1016/j.phytochem.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Kunming Botanical Garden; Kunming Institute of Botany; Chinese Academy of Sciences. An Enumeration of Plants Growing in Kunming Botanical Garden. Yunnan Science Press; Kunming, China: 2006. [Google Scholar]
- 12.Chai X.Y., Xu Z.R., Ren H.Y., Shi H.M., Lu Y.N., Li F.F., Tu P.F. Itosides A-I, new phenolic glycosides from Itoa orientalis. Helv. Chim. Acta. 2007;90:2176–2185. doi: 10.1002/hlca.200790225. [DOI] [Google Scholar]
- 13.Arevalo C., Ruiza I., Piccinellib A.L., Camponeb L., Rastrellib L. Phenolic derivatives from the leaves of Martinella obovata (Bignoniaceae) Nat. Prod. Commun. 2011;6:957–960. [PubMed] [Google Scholar]
- 14.Xuan W.D., Chen H.S., Bian J. A new indole alkaloid glycoside from stems of Nauclea officinalis. Acta Pharm. Sin. 2006;41:1064–1067. [PubMed] [Google Scholar]
- 15.Graikoua K., Aligiannisa N., Chinou I., Skaltsounisa A.L., Tillequin F., Litaudon M. Chemical constituents from Croton insularis. Helv. Chim. Acta. 2005;88:2654–2660. doi: 10.1002/hlca.200590206. [DOI] [Google Scholar]
- 16.Hamerski L., Bomm M.D., Silva D.H.S., Young M.C.M., Furlan M., Eberlin M.N., Gamboa I.C., Cavalheiro A.J., Bolzani V.S. Phenylpropanoid glucosides from leaves of Coussarea hydrangeifolia (Rubiaceae) Phytochemistry. 2005;66:1927–1932. doi: 10.1016/j.phytochem.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 17.Chassagne D., Crouzet J., Bayonove C.L., Baumes R.L. Glycosidically bound eugenol and methyl salicylate in the fruit of edible Passiflora species. J. Agric. Food Chem. 1997;45:2685–2689. doi: 10.1021/jf9608480. [DOI] [Google Scholar]
- 18.Kong C.S., Um Y.R., Lee J.I., Kim Y.A., Yea S.S., Seo Y. Constituents isolated from Glehnia littoralis suppress proliferations of human cancer cells and MMP expression in HT1080 cells. Food Chem. 2010;120:385–394. doi: 10.1016/j.foodchem.2009.09.096. [DOI] [Google Scholar]
- 19.Wagner H., Chari V.M., Sonnenbichler J. 13C-NMR-spektren natürlich vorkommender flavonoide. Tetrahedron Lett. 1976;17:1799–1802. doi: 10.1016/S0040-4039(00)93787-0. [DOI] [Google Scholar]
- 20.Liu J.F., Zhang X.M., Shi Y., Zhang Q., Ma Y.B., Chen J.J. Studies on chemical constituents of Illicium simonsii. China J. Chin. Mater. Med. 2011;36:1311–1315. [PubMed] [Google Scholar]
- 21.Zheng X.D., Hu H.B. Chemical constituents of Elsholtzia ciliate (Thunb) Hyland. Chem. Res. 2006;17:85–87. [Google Scholar]
- 22.Yang G.X., Song L., Li K.L., Hu C.Q. Studies on the chemical constituents of Polygonum orientale. Chin. Pharm. J. 2003;38:338–340. [Google Scholar]
- 23.Fan J.T., Su J., Peng Y.M., Li Y., Li J., Zhou Y.B., Zeng G.Z., Yan H., Tan N.H. Rubiyunnanins C-H, cytotoxic cyclic hexapeptides from Rubia yunnanensis inhibiting nitric oxide production and NF-κB activation. Bioorg. Med. Chem. 2010;18:8226–8234. doi: 10.1016/j.bmc.2010.10.019. [DOI] [PubMed] [Google Scholar]