Abstract
A broad variety of oxygen-substituted diaryl ketones has been synthesized by solar energy-induced Friedel Crafts acylations of 1,4-benzo- and 1,4-naphthoquinones with benzaldehydes. The in vitro antiproliferative properties of the photoproducts were assessed on prostate (DU-145), bladder (T24) and breast (MCF7) human-derived tumor cell lines and compared to non-tumor mouse fibroblasts (Balb/3T3). Among the tested compounds, it was found that those containing a 3,4,5-trimethoxyphenyl A-ring, such as 12 and 22 are more active on DU-145, with EC50 values of 1.2 and 5.9 μM, respectively. By comparing their effects on the three cancer cell lines, the analogue 22 has the best mean selective index (2.4).
Keywords: photo-Friedel Crafts acylation, diaryl ketones, green chemistry, antiproliferative activity
1. Introduction
Acylhydroquinones are valuable building blocks of natural [1,2,3,4] and synthetic compounds endowed with a variety of biological properties [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20]. The classic procedures to construct the diaryl ketone framework of acylhydroquinones are based on the Friedel Crafts acylation and Fries rearrangement. Despite the efficiency of these synthetic methods, they have some disadvantages such as the lack of atom economy, the use of hazardous environmental Lewis acids, namely BF3, AlCl3, TiCl4, or ZnCl2 [21,22,23,24,25,26], and limitations regarding the use of precursors containing oxygen acid-labile functional groups. The photo-Friedel Crafts acylation of quinones with aliphatic and aromatic aldehydes to prepare acylhydroquinones is a green and efficient alternative method with respect to the classic aforementioned acylation methods. It is noteworthy that several examples of the preparation of acylhydroquinones by photoacylation of 1,4-quinone with aldehydes have been reported [27,28,29,30,31,32,33,34], however, the scope of this method to the synthesis of oxygen-substituted diaryl ketones had received relatively little attention.
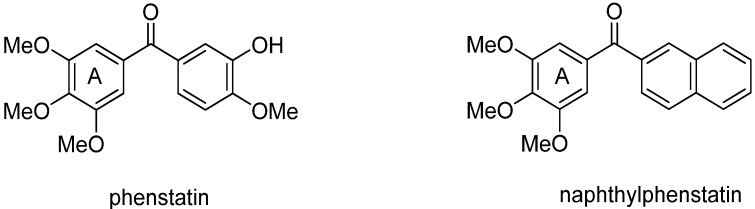
Among the broad variety of synthetic diaryl ketones the oxygen-substituted members, named phenstatin [35] and naphthylphenstatin [36] (Figure 1), stand out by their biological activity as microtubule-targeting agents. Based on these precedents we wanted to examine the synthetic flexibility of the eco-friendly solar photoacylation of 1,4-benzo- and 1,4-naphthoquinone with substituted benzaldehydes to the synthesis of diverse oxygen-substituted diaryl ketones. Taking advantage of this potentially simple access to oxygen-substituted diaryl ketones we were also interested in evaluating the series for in vitro antiproliferative activity on cancer cells. The aim of this study is mainly directed towards broadening the use of simple and eco-friendly methodologies in the synthesis of new oxygen-substituted diaryl ketones as well as to contribute to the search for new biological active members of this series.
Figure 1.
Structure of phenstatin and naphthylphenstatin.
2. Results and Discussion
2.1. Chemistry
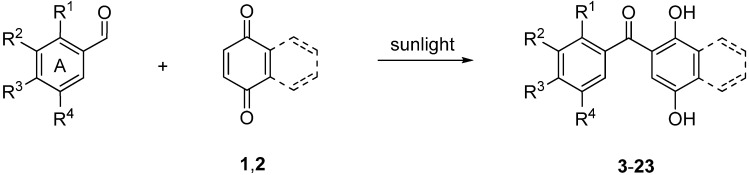
Based on our experience on the synthesis of heteroaroylhydroquinones by solar photoacylation of 1,4-benzo- and 1,4-naphthoquinone 1 and 2 with heteroarylcarbaldehydes in benzene [33], we initially examined the reaction of 1 and 2 with mono-substituted benzaldehydes. The reactions were carried out by using a 6.5 molar excess of the aldehyde with respect to the quinone. It is interesting to point out that molar excess of aldehyde is used to inhibit the dimerization of the quinone [30]. To avoid the use of hazardous benzene as the solvent, both the reaction of 1 and 2 with benzaldehyde and the liquid isomers of methyl- and methoxybenzaldehydes were accomplished using the appropriate aldehyde in excess as the solvent. In these experiments, performed by solar irradiation for 30 hours, the reaction mixtures were further submitted to column chromatography to give the respective photoproducts 3–8 and 14–18 (Scheme 1) in the 34%-77% yield range (Table 1). The formation yields of the products were determined on the basis of the initial and the amounts of the respective quinones recovered.
Scheme 1.
Photoacylation of quinones 1 and 2 with substituted benzaldehydes.
Table 1.
Oxygen-substituted diaryl ketones 3–23 prepared by solar photoacylation.
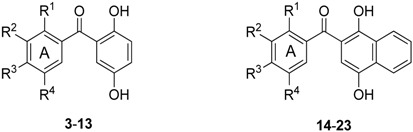
| Photoproduct | Substituents | Yield (%) a | ||||
|---|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | Method A b | Method B c | |
| 3 | H | H | H | H | 77 | 91 |
| 4 | Me | H | H | H | 52 | 80 |
| 5 | H | Me | H | H | 34 | 74 |
| 6 | H | H | Me | H | 58 | 82 |
| 7 | H | OMe | H | H | 38 | 74 |
| 8 | H | H | OMe | H | 59 | 79 |
| 9 | OMe | H | H | OMe | - | 70 |
| 10 | H | H | OMe | OMe | - | 70 |
| 11 | H | OMe | OH | H | - | 78 |
| 12 | H | OMe | OMe | OMe | - | 70 |
| 13 | H | OMe | OH | OMe | - | 70 |
| 14 | Me | H | H | H | 53 | 82 |
| 15 | H | Me | H | H | 41 | 69 |
| 16 | H | H | Me | H | 57 | 84 |
| 17 | H | OMe | H | H | 50 | 71 |
| 18 | H | H | OMe | H | 69 | 88 |
| 19 | OMe | H | H | OMe | - | 65 |
| 20 | H | H | OMe | OMe | - | 63 |
| 21 | H | H | OH | OMe | - | 73 |
| 22 | H | OMe | OMe | OMe | - | 60 |
| 23 | H | OMe | OH | OMe | - | 66 |
a Isolated by column chromatography; b Method A: the reaction was carried out using 1 or 2 (1 equiv.) and the aldehyde (7.5 equiv.) without benzene; c Method B: the reaction was carried out using 1 or 2 (1 equiv.), the aldehyde (7.5 equiv.) and benzene as the solvent.
In parallel experiments, the above reactions were run in benzene in order to compare the yields of the photo-acylation reactions with and without this solvent. The results of these assays are collected in Table 1. The data indicate that the photoacylation reactions of quinones 1 and 2 give higher yields in benzene solvent than in excess aldehyde.
The photoacylation of quinones 1 and 2 with the solid di- and trisubstituted benzaldehydes were carried out in benzene under the above mentioned solar irradiation conditions. The treatment provides access to the corresponding photoproducts 9–13 and 19–23 in good yields (Table 1). The new diaryl ketones 7, 9–13, 15, 19–21 and 23 were fully characterized by IR, 1H-, 13C-NMR and HRMS.
The synthesis of compounds 4 (65%), 6 (79%), 8 (77%), 14 (57%) and 16 (70%) have been previously reported by photoacylation of 1 and 2 with the respective aldehydes in the presence of catalytic amounts of benzophenone and using artificial light irradiation [29]. According to the data in Table 1 better yields on these compounds are achieved by using the solar chemical procedure (method B). Selected indoor photoacylation experiments performed in benzene by using irradiation with fluorescent lamps indicate that the photoproducts were generated in low yields.
2.2. In Vitro Antiproliferative Activity of Diaryl Ketones 3–23 again Select Cancer Cell Lines
The oxygen-substituted diaryl ketones 3–23 were evaluated for their in vitro antiproliferative activity on a panel of four cell lines, including non-tumor fibroblasts (Balb/3T3) and three human-derived tumor cell lines, namely DU-145 (prostate), T24 (bladder) and MCF7 (breast), using the conventional microculture tetrazolium reduction assay [37].
Table 2 summarizes the data from these evaluations: it shows the EC50 values (µM) of the respective benzo- and naphthohydroquinone derivatives. These values were calculated from their effects on MTT reduction in three cancer cell lines and Balb/3T3 non transformed mouse fibroblasts as a function of their concentration during 48 h of incubation. All three cancer cell lines were similarly sensitive to these compounds. With rather few exceptions, the members containing the dihydroxyphenyl fragment, such as 3–13, were in general less active than their corresponding naphthyl analogues 14–23 when tested against cancer cells, but just the opposite was observed in non-transformed fibroblasts. The data in Table 2 showed that compounds 12 and 22 appear as the most potent members of the series with lower EC50 values against T24 and DU-145 with respect to the reference drug mitomycin C. The biological activity differences of compounds 12 and 22 with respect to their analogues could be attributed to the 3,4,5-trimethoxy substitution on the A-phenyl ring. According to literature precedents, the 3,4,5-trimethoxyphenyl ring is considered essential for the antitubulin activity of a broad variety of antimitotic compounds [36,38,39,40,41,42,43,44,45]. Nevertheless, it should be noted that the EC50 values of compounds 12 and 22 are two orders of magnitude lower than to that reported to phenstatin when tested in the NCI screen [35] showing a mean panel GI50 (growth inhibitory) value of 6.01 × 10−8 M. In addition to the tendency showing that dihydroxynaphthyl analogues are more active than the dihydroxyphenyl derivatives, the vast majority of compounds did not have an adequate selectivity (Table 2), that is they affect both cancer and non-tumor cells in a similar way. In this context, only compound 22 have a good mean selectivity index (2.45).
Table 2.
In vitro inhibitory effect of compounds 3–23 on the proliferation of the human-derived tumor cell lines: T24 (bladder), DU-145 (prostate) and MCF7 (breast) and the non-tumor fibroblasts (Balb/3T3).

| EC50 ± SEM a (μM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| N° | R1 | R2 | R3 | R4 | T24 | DU-145 | MCF-7 | BALB/3T3 | MSI b |
| 3 | H | H | H | H | 186.4 ± 20.4 | 205.0 ± 21.5 | 189.8 ± 19.2 | 38.5 ± 5.5 | 0.20 |
| 4 | Me | H | H | H | 172.5 ± 13.2 | 172.5 ± 19.7 | 180.7 ± 15.8 | 183.9 ± 17.6 | 1.06 |
| 5 | H | Me | H | H | 202.3 ± 15.9 | 186.2 ± 17.8 | 225.7 ± 24.2 | 100.3 ± 9.5 | 0.49 |
| 6 | H | H | H | Me | 208.9 ± 19.5 | 100.8 ± 9.5 | 142.1 ± 15.3 | 78.3 ± 6.4 | 0.57 |
| 7 | H | OMe | H | H | 209.7 ± 20.5 | 166.1 ± 15.8 | 154.4 ± 12.3 | 117.6 ± 19.5 | 0.68 |
| 8 | H | H | OMe | H | 168.1 ± 12.2 | 170.2 ± 15.5 | 190.7 ± 17.5 | 85.2 ± 9.7 | 0.49 |
| 9 | OMe | H | H | OMe | 149.7 ± 18.4 | 130.3 ± 12.5 | 164.5 ± 13.8 | 43.7 ± 7.6 | 0.29 |
| 10 | H | H | OMe | OMe | 147.4 ± 19.2 | 140.0 ± 13.6 | 182.9 ± 17.7 | 35.4 ± 4.7 | 0.23 |
| 11 | H | OMe | OH | H | 154.5 ± 14.5 | 167.5 ± 15.9 | 176.7 ± 19.4 | 78.0 ± 9.3 | 0.47 |
| 12 | H | OMe | OMe | OMe | 3.6 ± 1.4 | 1.2 ± 0.6 | 53.6 ± 6.7 | 1.5 ± 0.4 | 0.57 |
| 13 | H | OMe | OH | OMe | >350 | 112.8 ± 10.7 | >350 | 19.1 ± 3.1 | - |
| 14 | Me | H | H | H | 143.3 ± 9.5 | 143.8 ± 17.7 | 152.3 ± 14.5 | 225.5 ± 25.4 | 1.54 |
| 15 | H | Me | H | H | 136.8 ± 11.7 | 147.4 ± 15.9 | 144.9 ± 12.7 | 142.4 ± 13.9 | 0.99 |
| 16 | H | H | Me | H | 134.6 ± 12.8 | 158.3 ± 13.7 | 133.8 ± 14.9 | 138.9 ± 15.2 | 0.98 |
| 17 | H | OMe | H | H | 133.3 ± 12.4 | 139.4 ± 11.3 | 127.9 ± 13.6 | 109.1 ± 11.1 | 0.82 |
| 18 | H | H | OMe | H | 126.8 ± 10.6 | 77.5 ± 6.5 | 124.9 ± 10.2 | 107.0 ± 12.3 | 1.03 |
| 19 | OMe | H | H | OMe | 129.8 ± 10.1 | 128.7 ± 17.2 | >310 | 100.3 ± 8.9 | 0.77 |
| 20 | H | H | OMe | OMe | 46.8 ± 5.1 | 61.8 ± 5.6 | 12.2 ± 3.8 | 2.8 ± 0.6 | 0.11 |
| 21 | H | H | OH | OMe | 86.6 ± 9.6 | 89.0 ± 7.3 | 15.7 ± 4.6 | 15.7 ± 3.9 | 0.45 |
| 22 | H | OMe | OMe | OMe | 13.5 ±1.9 | 5.9 ± 0.8 | 20.2 ± 4.5 | 25.1 ± 3.9 | 2.45 |
| 23 | H | OMe | OH | OMe | 112.0 ± 9.3 | 113.5 ± 9.5 | 130.2 ± 14.8 | 99.6 ± 7.5 | 0.84 |
| DOXc | - | - | - | - | 0.65 ± 0.07 | 0.42 ± 0.03 | 0.33 ± 0.05 | 0.19 ± 0.01 | 0.44 |
| MITd | - | - | - | - | 42.2 ± 5.8 | 14.3 ± 2.6 | 16.8 ± 2.9 | 27.3 ± 3.3 | 1.39 |
a Data represent EC50 mean values ± SEM of at least three different experiments; b MSI: Mean Selective Index = EC50 values fibroblasts/EC50 values tumor cells; c DOX: doxorubicin; d MIT: mitomycin C.
3. Experimental
3.1. General
All reagents were commercially available reagent grade and were used without further purification. Melting points were determined on a Stuart Scientific SMP3 apparatus and are uncorrected. The IR spectra were recorded on an FT Bruker spectrophotometer using KBr disks, and the wave numbers are given in cm−1. 1H-NMR spectra were run on Bruker AM-200 and AM-400 instruments in deuterochloroform (CDCl3) and dimethyl sulfoxide-d6 (DMSO-d6). Chemical shifts are expressed in ppm downfield relative to tetramethylsilane (TMS, δ scale), and the coupling constants (J) are reported in Hertz. 13C-NMR spectra were obtained in CDCl3 + DMSO-d6 at 50 and 100 MHz. Chemical shifts are reported in δ ppm downfield from TMS, and J-values are given in Hertz. HRMS data were obtained on Thermo Finnigan mass spectrometer, model MAT 95XP and LTQ-Orbitrap mass spectrometer (Thermo-Fisher Scientific) with the analysis performed using an APCI source operated in positive mode. Silica gel Merck 60 (70–230 mesh) was used for preparative column chromatography and TLC aluminum foil 60F254 for analytical TLC. The solar irradiation experiments were performed at the Canchones Experimental Center in Iquique/Chile (latitude 20°26′43.80"S, 990 m above sea level), located in the Atacama Desert.
3.2. Chemistry
General Procedure for Photoacylation of 1 and 2 with Substituted Benzaldehydes in the Absence of Benzene (Method A)
Quinone 1 or 2 (1 mmol) and the liquid aldehyde (7.5 mmol), were placed into a test tube, nitrogen was bubbled through the solution for 2 min and then the tube was sealed with a septum. The mixture was irradiated for six days (total illumination time of 30 h), under solar radiation conditions in the range 800–1100 Watts/m2 (November–March). The mixture reaction was chromatographed on silica gel (3:1 petroleum ether/ethyl acetate) to give pure samples of the corresponding diaryl ketones. The remaining precursors were recovered to be used in further preparations.
General Procedure for Photoacylation of 1 and 2 with Substituted Benzaldehydes in Benzene (Method B).
A 100 mL benzene solution of the required quinone 1 or 2 (1 mmol) and the substituted benzaldehyde (7.5 mmol), was placed into the outer jacket of a Liebig condenser type. The solution was bubbled with nitrogen (2 min), the flask was sealed with a septum and then irradiated for six days (total illumination time of 30 h), under solar radiation conditions in the range 800–1100 Watts/m2 (November–March). The solvent was evaporated under reduced pressure and the residue was chromatographed on silica gel (3:1 petroleum ether/ethyl acetate). The starting aldehyde and the solvent were recovered and employed in the next batches.
(2,5-Dihydroxyphenyl)(phenyl)methanone (3). This compound was prepared from quinone 1 and benzaldehyde and was isolated in 77 and 91% yield by following methods A and B, respectively; orange solid, mp 121–122 °C (lit. [46]: 125–126 °C). IR (KBr) νmáx cm–1: 3456 (O-H), 3358 (O-H), 1637 (C=O); 1H-NMR (CDCl3): δ 6.98 (m, 2H, 4'-H + 5-OH), 7.03 (s, 1H, 6-H), 7.11 (d, 2H, J = 7.2 Hz, 2'-H + 6'-H), 7.19 (m, 2H, 3'-H + 5'-H), 8.10 (d, 1H, J = 7.8 Hz, 3-H or 4-H), 8.21 (d, 1H, J = 7.8 Hz, 4-H or 3-H), 11.42 (bs 1H, 2-OH); 13C-NMR (CDCl3): δ 119.6, 119.9, 123.4, 125.5, 129.1, 129.5, 129.7, 130.4, 130.6, 132.7, 138.9, 149.9, 206.1; HRMS (M+): m/z calcd for C13H10O3: 214.06299; found: 214.06189.
(2,5-Dihydroxyphenyl)(2'-methylphenyl)methanone (4). This compound was prepared from quinone 1 and 2-methylbenzaldehyde in 52 and 80% yield following methods A and B, respectively; yellow solid, mp 104–105 °C (lit. [47]: 106–108 °C). IR (KBr) νmáx cm–1: 3287 (O-H), 1638 (C=O); 1H-NMR (CDCl3): δ 2.29 (s, 3H, Me), 4.76 (s, 1H, 5-OH), 6.71 (d, 1H, J = 3.0 Hz, 6-H), 6.95 (d, 1H, J = 8.9 Hz, 3-H), 7.04 (dd, 1H, J = 8.9, 3.0 Hz, 4-H), 7.27 (m, 3H, 3'-H + 4'-H + 6'-H), 7.39 (m, 1H, 5’-H), 11.81 (s, 1H, 2-OH); 13C-NMR (CDCl3): δ 19.6, 118.1, 119.3, 119.5, 125.4, 125.5, 127.3, 130.2, 130.9, 135.4, 137.7, 147.4, 157.5, 203.9; HRMS (M+): m/z calcd for C14H12O3: 228.07864; found: 228.07767.
(2,5-Dihydroxyphenyl)(3'-methylphenyl)methanone (5). This compound was prepared from 1 and 3-methylbenzaldehyde in 34 and 74% yield following methods A and B, respectively; yellow solid, mp 119–120 °C (lit. [47]: 114–116 °C). IR (KBr) νmáx cm–1: 3285 (O-H), 1637 (C=O); 1H-NMR (CDCl3): δ 2.36 (s, 3H, Me), 5.82 (s, 1H, 5-OH), 6.91 (m, 1H, 3-H or 4-H), 7.01 (m, 2H, 4-H or 3-H + 6-H), 7.31 (m, 2H, 4'-H + 5'-H or 5'-H + 6'-H), 7.38 (d, 1H, J = 7.2 Hz, 4'-H or 6'-H), 7.41 (s, 1H, 2'-H), 11.63 (s. 1H, 2-OH); 13C-NMR (CDCl3): δ 21.4, 118.6, 118.9, 119.1, 124.9, 126.2, 128.2, 129.4, 132.9, 137.6, 138.4, 147.4, 157.0, 201.7; HRMS (M+): m/z calcd for C14H12O3: 228.07864; found: 228.07809.
(2,5-Dihydroxyphenyl)(4'-methylphenyl)methanone (6). This compound was prepared from 1 and 4-methylbenzaldehyde in 58 and 82% yield following methods A and B, respectively; yellow solid, mp 135–136 °C. IR (KBr) νmáx cm−1: 3442 (O-H), 1629 (C=O); 1H-NMR (CDCl3): δ 2.40 (s, 3H, Me), 5.44 (s, 1H, 5-OH), 6.93 (d, 1H, J = 7.6 Hz, 3-H), 7.02 (m, 2H, 4-H + 6H), 7.24 (d, 2H, J = 8.1 Hz, 2'-H + 3'-H or 5'-H + 6'-H), 7.54 (d, 2H, J = 8.1 Hz, 3'-H + 2'-H or 6'H + 5'-H), 11.58 (s, 1H, 2-OH); 13C-NMR (CDCl3): δ 21.6, 118.4, 119.0, 119.2, 124.7, 129.1 (2 × C), 129.4 (2 × C), 134.9, 142.9, 147.3, 157.0, 201.7; HRMS (M+): m/z calcd for C14H12O3: 228.07864; found: 228.07831.
(2,5-Dihydroxyphenyl)(3'-methoxyphenyl)methanone (7). This compound was prepared from 1 and 3-methoxybenzaldehyde in 38 and 54% yield following methods A and B, respectively; yellow solid, mp 97–98 °C. IR (KBr) νmáx cm–1: 3344 (O-H), 1635 (C=O); 1H-NMR (CDCl3): δ 3.80 (s, 3H, OMe), 5.80 (s, 1H, 5-OH), 6.91 (d, 1H, J = 9.8 Hz, 3-H or 4-H) 7.02 (m, 2H, 4-H or 3-H + 6-H), 7.06 (dd, 1H, J = 8.2, 2.2, Hz 4'-H or 6'-H), 7.14 (d, 1H, J = 2.2 Hz 2'-H), 7.16 (d, 1H, J = 7.9 Hz, 6'-H or 4'-H), 7.32 (t, 1H, J = 7.9 Hz, 5'-H), 11.56 (s, 1H-2-OH); 13C-NMR (CDCl3): δ 55.5, 113.9, 118.1, 118.4, 118.8, 119.2, 121.6, 125.1, 129.4, 138.8, 147.5, 157.0, 159.4, 201.2; HRMS (M+): m/z calcd for C14H12O4: 244.07356; found: 244.07361.
(2,5-Dihydroxyphenyl)(4'-methoxyphenyl)methanone (8). This compound was prepared from 1 and 4-methoxybenzaldehyde, in 58 and 79% yield according methods A and B, respectively; yellow solid, mp 144–145 °C. IR (KBr) νmáx cm−1: 3343 (O-H), 1631 (C=O); 1H-NMR (CDCl3): δ 3.87 (s, 3H, OMe), 6.90 (d, 1H, J = 8.8 Hz, 3-H or 4-H), 6.97 (d, 2H, J = 8.8 Hz, 2'-H + 3'-H or 5'-H + 6'-H), 7.08 (m, 2H, 4-H or 3-H + 6-H), 7.72 (d, 2H, J = 8.8 Hz, 6'-H + 5'-H or 3'-H + 2'-H), 8.32(s, 1H, 5-OH), 11.38 (s, 1H, 2-OH); 13C-NMR (CDCl3): δ 55.5, 113.6, 115.9, 118.1, 118.7, 119.2, 122.4, 124.4, 130.4, 131.8, 148.7, 155.9, 162.8, 199.7; HRMS (M+): m/z calcd for C14H12O4: 244.07356; found: 244.07360.
(2,5-Dihydroxyphenyl)(2',5'-dimethoxyphenyl)methanone (9). This compound was prepared from 1 and 2,5-dimethoxybenzaldehyde in 70% yield (method B); yellow solid, mp 135–136 °C. IR (KBr) νmáx cm−1: 3299 (O-H), 1637 (C=O); 1H-NMR (CDCl3 + DMSO-d6): δ 3.74 (s, 3H, OMe), 3.78 (s, 3H, OMe), 6.82 (s, 2H, 6-H + 6'-H), 6.87 (d, 1H, J = 8.8 Hz, 3-H), 6.96 (m, 2H, 3'-H + 4'-H), 7.06 (d, 1H, J = 8.8 Hz, 4-H), 8.39 (s, 1H, 5-OH), 11.60 (s, 1H, 2-OH); 13C-NMR (CDCl3 + DMSO-d6): δ 55.9, 56.4, 113.1, 113.8, 116.0, 116.8, 118.2, 118.4, 119.8, 125.6, 149.1, 150.6, 153.4, 156.3, 201.3; HRMS (M+): m/z calcd for C15H14O5: 274.08412; found: 274.08316.
(2,5-Dihydroxyphenyl)(3,4-dimethoxyphenyl)methanone (10). This compound was prepared from 1 and 3,4-dimethoxybenzaldehyde in 70% yield (method B); brown solid, mp 79–81 °C. IR (KBr) νmáx cm–1: 3354 (O-H), 1630 (C=O); 1H-NMR (CDCl3 + DMSO-d6): δ 3.86 (s, 6H, 2 × OMe), 6.83 (s, 1H, 6'-H), 6.82 (s, 1H, 5'-H), 6.89 (d, 1H, J = 9.2 Hz, 3-H), 6.94 (s, 1H, 2'-H), 6.97 (d, 1H, J = 8.8 Hz, 4-H), 7.18 (s, 1H, 6-H), 8.69 (s, 1H, 5-OH), 11.34 (s, 1H, 2-OH); 13C-NMR (CDCl3 + DMSO-d6): δ 55.9, 60.5, 104.5, 111.2, 117.9, 118.4, 118.8, 122.5, 124.6, 133.0, 149.7, 149.8, 152.8, 155.7, 200.0; HRMS (APCI): [M+H]+ calcd for C15H14O5: 275.08412; found: 275.09072.
(2,5-Dihydroxyphenyl)(4'-hydroxy-3'-methoxyphenyl)methanone (11). This compound was prepared in 78% yield (method B) from 1 and 4-hydroxy-3-methoxybenzaldehyde; yellow solid, mp 221–222 °C. IR (KBr) νmáx cm–1: 3329 (O-H), 1639 (C=O); 1H-NMR (CDCl3 + DMSO-d6): δ 3.77 (s, 3H, OMe), 6.86 (d, 1H, J = 8.0 Hz, 4-H or 3-H), 6.95 (d, 1H, J = 8.0 Hz, 3-H or 4-H), 7.03 (d, 1H, J = 8.8 Hz, 6'-H), 7.11 (s, 1H, 2'-H), 7.26 (d, 1H, J = 8.4 Hz, 5'-H), 7.31 (s, 1H, 6-H), 7.63 (s, 1H, 4'-OH), 8.89 (s, 1H, 5-OH), 11.10 (s, 1H, 2-OH); 13C-NMR (CDCl3 + DMSO-d6): δ 55.5, 107.1, 111.5, 112.2, 114.1, 121.7, 123.7, 125.4, 128.5, 143.7, 146.7, 149.7, 156.1, 198.9; HRMS (M+): m/z calcd for C14H12O5: 260.06847; found: 260.06764.
(2,5-Dihydroxyphenyl)(3',4',5'-trimethoxyphenyl)methanone (12). This compound was prepared in 70% yield (method B) from 1 and 3,4,5-trimethoxybenzaldehyde; yellow solid, mp 68–70 °C. IR (KBr) νmáx cm–1:3445 (OH), 3200 (O-H), 1639 (C=O); 1H-NMR (CDCl3 + DMSO-d6): δ 3.81 (s, 9H, 3 × OMe), 6.81 (d, 1H, J = 8.8 Hz, 3-H), 6.87 (s, 2H, 2'-H + 6'-H), 7.0 (d, 1H, J = 8.8 Hz, 4-H), 7.07 (s, 1H, 6-H), 8.58 (s, 1H, 5-OH), 11.23 (s, 1H, 2-OH); 13C-NMR (CDCl3 + DMSO-d6): δ 56.3 (2 × C), 60.8, 107.0, 117.9, 118.7, 118.8, 124.9, 133.0, 141.3, 149.0 (2 × C), 149.9, 152.8, 156.0, 199.9; HRMS (M+): m/z calcd for C16H16O6: 304.09469; found: 304.09378.
(2,5-Dihydroxyphenyl)(4'-hydroxy-3',5'-dimethoxyphenyl)methanone (13). This compound was prepared in 70% (method B) from 1 and 4-hydroxy-3,5-dimethoxybenzaldehyde; yellow solid, mp 200–201 °C. IR (KBr) νmáx cm–1: 3331(O-H), 1635 (C=O); 1H-NMR (CDCl3 + DMSO-d6): δ 3.92 (s, 6H, 2 × OMe), 6.87 (d, 1H, J = 9.0 Hz, 3-H), 7.03 (s, 3H, 6-H + 2'-H + 6'-H), 7,16 (d, 1H, J = 9.0 Hz, 4-H), 8.47 (s, 1H, 4-OH), 8.78 (s, 1H, 5-OH), 11.15 (s, 1H, 2-OH); 13C-NMR (CDCl3 + DMSO-d6): δ 56.0 (2 × C), 107.2 (2 × C), 117.3, 118.0, 118.9, 123.5, 127.5, 139.6, 146.8 (2 × C), 148.6, 154.7, 198.6; HRMS (M+): m/z calcd for C15H14O6: 290.07904; found: 290.07830.
(1,4-Dihydroxynaphthalen-2-yl)(2'-methylphenyl)methanone (14). This compound was prepared from quinone 2 and 2-methylbenzaldehyde in 53 and 82% yield following methods A and B, respectively; orange solid, mp 132–133 °C. IR (KBr) νmáx cm−1: 3357 (O-H), 1638 (C=O); 1H-NMR (CDCl3): δ 2.23 (s, 3H, Me), 5.53 (s, 1H, 4-OH), 6.46 (s, 1H, 3-H), 7.17 (m, 3H, 3'-H + 4'-H + 6'-H), 7.26 (m, 1H, 5'-H), 7.58 (t, 1H, J = 7.6 Hz, 6-H or 7-H), 7.66 (t, 1H, J =7.6 Hz, 7-H or 6-H), 8.07 (d, 1H, J = 8.3 Hz, 5-H or 8-H), 8.50 (d, 1H, J = 8.3 Hz, 8-H or 5-H), 13.63 (s, 1H, 1-OH); 13C-NMR (CDCl3): δ 19.7, 107.5, 112.4, 121.9, 124.0, 125.4, 125.9, 126.7, 127.0, 129.8, 129.9, 130.3, 130.8, 135.2, 138.0, 143.1, 158.8, 203.7; HRMS (APCI): [M+H]+ calcd for C18H14O3: 279.09429; found: 279.10136.
(1,4-Dihydroxynaphthalen-2-yl)(3'-methylphenyl)methanone (15). This compound was prepared from quinone 2 and 3-methylbenzaldehyde in 41 and 69% yield according methods A and B, respectively; orange solid, mp 154–155 °C. IR (KBr) νmáx cm–1: 3313 (O-H), 1633 (C=O); 1H-NMR (CDCl3): δ 2.44 (s, 3H, Me), 6.98 (s, 1H, 3-H), 7.39 (m, 2H, 4'-H or 6'-H + 2'-H), 7.51 (m, 2H, 6'-H or 4'-H + 5'-H), 7.56 (m, 1H, 6-H or 7-H), 7.66 (t, 1H, J = 7.6 Hz, 7-H or 6-H), 8.20 (d, 1H, J = 8.3 Hz, 5-H or 8-H), 8.46 (d, 1H, J = 8.3 Hz, 8-H or 5-H), 8.87 (s, 1H, 4-OH), 13.50 (s, 1H, 1-OH); 13C-NMR (CDCl3): δ 21.4, 107.4, 111.9, 122.3, 124.2, 125.8 (2 × C), 126.1, 127.9, 129.3, 129.5, 130.1, 132.0, 138.1, 138.5, 144.3, 157.4, 201.2; HRMS (APCI): [M+H]+ calcd for C18H14O3: 279.09429; found: 279.10124.
(1,4-Dihydroxynaphthalen-2-yl)(4'-methylphenyl)methanone (16). This compound was prepared from 2 and 4-methylbenzaldehyde in 57 and 84% yield according methods A and B, respectively; orange solid, mp 150–151 °C. IR (KBr) νmáx cm−1: 3422 (O-H), 1635 (C=O); 1H-NMR (CDCl3): δ 2.43 (s, 3H, Me), 7.0 (s, 1H, 3-H), 7.30 (d, 2H, J = 7.8 Hz, 2'-H + 3'-H or 5'-H + 6'-H), 7.56 (t, 1H, J = 7.5 Hz, 6-H or 7-H), 7.64 (d, 2H, J = 7.8 Hz, 3'-H + 2'-H or 6'-H + 5'-H), 7.65 (m, 1H, 7-H or 6-H), 8.20 (d, 1H, J = 8.3 Hz, 5-H or 8-H), 8.47 (d, 1H, J = 8.3 Hz, 8-H or 5-H), 8.71 (s, 1H, 4-OH), 13.48 (s, 1H, 1-OH); 13C-NMR (CDCl3): δ 21.6, 107.6, 111.9, 122.3, 124.2, 125.9, 126.1, 128.9 (2 × C), 129.3 (2 × C), 129.4, 129.9, 135.8, 141.9, 144.2, 157.3, 200.8; HRMS (APCI): [M+H]+ calcd for C18H14O3: 279.09429; found: 279.10122.
(1,4-Dihydroxynaphthalen-2-yl)(3'-methoxyphenyl)methanone (17). This compound was prepared from 2 and 3-methoxybenzaldehyde in 50 and 71% yield according methods A and B, respectively; orange solid, mp 149–150 °C (lit. [34]: 142-144 °C). IR (KBr) νmáx cm–1: 3344 (O-H), 1633 (C=O); 1H-NMR (CDCl3): δ 3.85 (s, 3H, OMe), 6.99 (s, 1H, 3-H), 7.08 (dd, 1H, J = 8.1, 2.0 Hz, 4'-H or 6'-H), 7.23 (s, 1H, 2'-H), 7.28 (m, 1H, 6'-H or 4'-H), 7.39 (t, 1H, J = 7.9 Hz, 5'-H), 7.56 (t, 1H, J = 7.6 Hz, 6-H or 7-H), 7.66 (t, 1H, J = 7.6 Hz 7-H or 6-H), 8.20 (d, 1H, J = 8.3 Hz, 5-H or 8-H), 8.47 (d, 1H, J = 8.3 Hz 8-H or 5-H), 8.65 (s, 1H, 4-OH), 13.46 (bs, 1H, 1-OH); 13C-NMR (CDCl3): δ 55.5, 107.3, 111.9, 113.7, 117.6, 121.4, 122.3, 124.3, 125.9, 126.2, 129.3, 129.6, 130.1, 139.8, 144.3, 157.7, 159.3, 200.7; HRMS (M+): m/z calcd for C18H14O4: 294.08921; found: 294.08854.
(1,4-Dihydroxynaphthalen-2-yl)(4'-methoxyphenyl)methanone (18). This compound was prepared from 2 and 4-methoxybenzaldehyde in 69 and 88% yield according methods A and B, respectively; yellow solid, mp 150-151°C (lit. [34]: 130–132 °C). IR (KBr) νmáx cm–1: 3470 (O-H), 1631 (C=O); 1H-NMR (CDCl3): δ 3.86 (s, 3H, OMe), 6.97 (d, 2H, J = 8.7 Hz, 2'-H + 3'-H or 5'-H + 6'-H), 7.02 (s, 1H, 3-H), 7.56 (t, 1H, J = 7.4 Hz, 6-H or 7-H), 7.65 (t, 1H, J = 7.4 Hz, 7-H or 6-H), 7.75 (d, 2H, J = 8.7 Hz, 6'-H + 5'-H or 3'-H + 2'-H), 8.20 (d, 1H, J = 8.3 Hz, 5-H or 8-H), 8.47 (m, 2H, 8-H or 5-H + 4-OH), 13.43 (s, 1H, 1-OH); 13C-NMR (CDCl3): δ 55.5, 107.8, 111.9, 113.5, 122.3, 124.2, 125.9, 126.1, 129.3 (2 × C), 129.8, 130.9, 131.6 (2 × C), 144.1, 157.2, 162.4, 199.6; HRMS (APCI): [M+H]+ calcd for C18H14O4: 295.08921; found: 295.08059.
(1,4-Dihydroxynaphthalen-2-yl)(2',5'-dimethoxyphenyl)methanone (19). This compound was prepared from 2 and 2,5-dimethoxybenzaldehyde in 65% yield (method B); yellow solid, mp 160–161 °C. IR (KBr) νmáx cm–1: 3389 (O-H), 1630 (C=O); 1H-NMR (CDCl3 + DMSO-d6): δ 3.75 (s, 3H, OMe), 3.79 (s, 3H, OMe), 6.70 (s, 1H, 6'-H), 6.90 (s, 1H, 3-H), 6.97 (m, 2H, 3'-H + 4'-H), 7.56 (t, 1H, J = 8.8 Hz, 6-H or 7-H), 7.66 (t, 1H, J = 8.8 Hz, 7-H or 6-H), 8.18 (d, 1H, J = 8.4 Hz, 8-H), 8.47 (s, 1H, 4-OH), 8.48 (d, 1H, J = 8.4 Hz, 5-H), 13.48 (s, 1H, 1-OH); 13C-NMR (CDCl3 + DMSO-d6): δ 56.0, 56.6, 107.4, 113.1, 113.2, 113.9, 117.0, 122.4, 124.5, 125.9, 126.2, 129.1, 129.7, 130.5, 144.4, 150.7, 153.5, 157.4, 200.8; HRMS (M+): m/z calcd for C19H16O5: 324.09978; found: 324.09914.
(1,4-Dihydroxynaphthalen-2-yl)(3',4'-dimethoxyphenyl)methanone (20). This compound was prepared from 2 and 3,4-dimethoxybenzaldehyde in 63% yield (method B); orange solid, mp 212–213 °C. IR (KBr) νmáx cm–1: 3462 (O-H), 1638 (C=O); 1H-NMR (CDCl3 + DMSO-d6): δ 3.65 (s, 6H, 2 × OMe), 6.82 (s, 1H, 5'-H), 6.88 (s, 1H, 6'-H or 2'-H), 7.00 (s, 1H, 2'-H or 6'-H), 7.12 (s, 1H, 3-H), 7.56 (d, 1H, J = 6.8 Hz, 8-H), 8.19 (d, 1H, J = 6.8 Hz, 5-H), 8.46 (t, 2H, J = 8.4 Hz, 6-H + 7-H), 9.05 (s, 1H, 4-OH), 13.42 (s, 1H, 1-OH); 13C-NMR (CDCl3 + DMSO-d6): δ 56.0, 60.7, 104.7, 107.3, 111.0, 111.8, 122.3, 124.1, 126.0, 126.7, 128.7, 129.3, 129.9, 130.9, 144.3, 149.8, 152.9, 157.1, 199.9; HRMS (APCI): [M+H]+ calcd for C19H16O5: 325.09977; found: 325.09146.
(1,4-Dihydroxynaphthalen-2-yl)(4'-hydroxy-3'-methoxyphenyl)methanone (21). This compound was prepared from 2 and 4-hydroxy-3-methoxybenzaldehyde in 73% yield (method B); orange solid, mp 221–222 °C. IR (KBr) νmáx cm–1: 3469 (O-H), 1635 (C=O); 1H-NMR (CDCl3 + DMSO-d6): δ 3.96 (s, 3H, OMe), 7.00 (d, 1H, J = 7.8 Hz, 8-H), 7.13 (s, 1H, 2'-H), 7.33 (d, 1H, J = 7.6 Hz, 5-H), 7.43 (s, 1H, 3-H), 7.56 (t, 1H, J = 7.8 Hz, 6-H or 7-H), 7.65 (t, 1H, J = 7.8 Hz, 7-H or 6-H), 8.20 (d, 1H, J = 8.0 Hz, 6'-H or 5'-H), 8.44 (d, 1H, J = 8.0 Hz, 5'-H o 6'-H), 8.81 (s, 1H, 4'-OH), 9.04 (s, 1H, 4-OH), 13.38 (s, 1H, 1-OH); 13C-NMR (CDCl3 + DMSO-d6): δ 55.9, 107.5, 112.0, 112.6, 114.5, 122.1, 123.9, 124.1, 125.7, 125.8, 128.9, 129.5, 129.7, 144.1, 147.1, 150.1, 156.5, 199.3; HRMS (M+): m/z calcd for C18H14O5: 310.08413; found: 310.08401.
(1,4-Dihydroxynaphthalen-2-yl)(3',4',5'-trimethoxyphenyl)methanone (22). This compound was prepared from 2 and 3,4,5-trimethoxybenzaldehyde in 60% yield (method B); brown solid, mp 189–191 °C. Mp and the spectral properties of 22 were in agree to those reported in literature [19].
(1,4-Dihydroxynaphthalen-2-yl)(4'-hydroxy-3',5'-dimethoxyphenyl)methanone (23). This compound was prepared from 2 and 4-hydroxy-3,5-dimethoxybenzaldehyde in 66% yield (method B); yellow solid, mp 173–174 °C. IR (KBr) νmáx cm–1: 3346 (O-H), 1630 (C=O); 1H-NMR (CDCl3 + DMSO-d6): δ 3.95 (s, 6H, 2 × OMe), 7.08 (s, 2H, 2'-H + 6'-H), 7.16 (s, 1H, 3-H), 7.56 (t, 1H, J = 7.2 Hz, 6-H or 7-H), 7.66 (t, 1H, J = 7.2 Hz, 7-H or 6-H), 8.20 (d, 1H, J = 8.4 Hz, 8-H), 8.45 (d, 1H, J = 8.4 Hz, 5-H), 9.11 (s, 2H, 4-OH), 13.34 (s, 1H, 1-OH); 13C-NMR (CDCl3 + DMSO-d6): δ 56.3 (2 × C), 107.2 (2 × C), 111.8, 122.1, 122.5, 123.9, 125.7, 125.9, 128.6, 129.0, 129.5, 139.0, 144.2, 146.9, (2 × C), 149.3, 199.2; HRMS (M+): m/z calcd for C19H16O6: 340.09469; found: 340.09380.
3.3. Antiproliferative Assay
3.3.1. Cell Lines and Culture Conditions
Human cancer cell lines (T24, DU-145, MCF7) were cultured in high-glucose Dulbecco's modified Eagle medium (Gibco, Grand Island, NY, USA) supplemented with 10% foetal calf serum, penicillin (100 U/mL), and streptomycin (100 μg/mL). Balb/3T3 cells (normal mouse fibroblasts) were cultured in the same medium, except that the foetal calf serum was replaced by 10% newborn calf serum. All cultures were kept at 37 °C in 95% air/5% CO2 at 100% humidity. Phosphate-buffered saline (PBS) was purchased from Gibco. Cells were incubated at the indicated times at 37 °C with or without hydroquinones at various concentrations.
3.3.2. Cellular Assays
The cytotoxicity of the bis aryl ketones was assessed by following the reduction of MTT (3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide) to formazan blue [37]. Briefly, cells were seeded into 96-well plates at a density of 10,000 cells/well for 24 h and then incubated for 48 h with or without the compounds. Cells were then washed twice with warm PBS and incubated with MTT (0.5 mg/mL) for 2 h at 37 °C. Incubation medium was thereafter discarded and the blue formazan crystals were solubilized by adding 100 μL DMSO/well. The colour solution was subsequently read at 550 nm. Results are expressed as % of MTT reduction compared to untreated control conditions. The calculation of EC50 values was performed by using GraphPad Prism software (San Diego, CA, USA).
4. Conclusions
We have extended the photo-Friedel Crafts acylation of 1,4-benzo- and 1,4-naphthoquinone with aldehydes to the synthesis of a significant number of oxygen-substituted diaryl ketones. The main advantages of this general procedure respect to other methods to construct oxygen-substituted diaryl ketone framework are the atom economy, simplicity, cheap and the chemical stability of the oxygen substituent of precursors and/or products. From the antiproliferative screening of the oxygen-substituted diaryl ketones, compounds 12 and 22 stand out by their biological activity levels on prostate DU-145 (EC50: 1.2 and 5.9 μM) and bladder T24 (EC50: 3.6 and 13.5 μM) cell lines, compared to those of the anticancer drug mitomycin C (EC50: 14.3 and 42.2 μM). Even though compound 22 displayed less potency than the analog 12, it exhibited a better mean selective index. Although compounds 12 and 22 have EC50 values lower than to GI50 values reported for phenstatin, due to their structural similarity it may be hypothesized that inhibition of microtubule assembly is involved in the antiproliferative mechanism of these two compounds. Chemical modifications of compounds 12 and 22 directed to access to the scaffold of future active tubulin polymerization inhibitors are in progress.
Acknowledgments
We thank the Fondo Nacional de Ciencia y Tecnología, Chile (Grant NO. 1100376), the Fonds Spéciaux de Recherche (FSR), Université catholique de Louvain, Belgium, and the FNRS (FRFC Grant 2.4555.08) for financial support to this study. The authors are grateful to Véronique Allaeys and Isabelle Blave for their excellent technical assistance.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/18/8/9818/s1.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Thompson R.H. Naturally Occurring Quinones III, Recent Advances. Chapman and Hall; London, UK: 1987. [Google Scholar]
- 2.Maruyama K., Naruta Y. Syntheses of alpha- and beta-lapachones and their homologues by way of photochemical side chain introduction to quinone. Chem. Lett. 1977;8:847–850. doi: 10.1246/cl.1977.847. [DOI] [Google Scholar]
- 3.Uno H. Allylation of 2-alkanoyl 1,4-quinones with allylsilanes and allylstannanes. Efficient synthesis of pyranonaphthoquinone antibiotics. J. Org. Chem. 1986;51:350–358. doi: 10.1021/jo00353a015. [DOI] [Google Scholar]
- 4.Brimble M.A., Lynds S.M. A short synthesis of deoxyfrenolicin. J. Chem. Soc. Perkin. Trans. 1. 1994;1:493–496. doi: 10.1039/p19940000493. [DOI] [Google Scholar]
- 5.Kraus G.A., Maeda H. A direct preparation of 1,4-benzodiazepines. The synthesis of medazepam and related compounds via a common intermediate. Tetrahedron Lett. 1994;35:9189–9190. doi: 10.1016/0040-4039(94)88461-7. [DOI] [Google Scholar]
- 6.Waske P.A., Mattay J., Oelgemöller M. Photoacylations of 2-substituted 1,4-naphthoquinones: A concise access to biologically active quinonoid compounds. Tetrahedron Lett. 2006;47:1329–1332. doi: 10.1016/j.tetlet.2005.12.060. [DOI] [Google Scholar]
- 7.Valderrama J.A., Pessoa-Mahana D., Tapia R.A., Rojas de Arias A., Nakayama H., Torres S., Miret J., Ferreira M.E. Studies on quinones. Part 34: The reaction of styrene with activated 1,4-benzoquinones: Access to potential antiprotozoal pyranobenzoquinones. Tetrahedron. 2001;57:8653–8658. doi: 10.1016/S0040-4020(01)00860-2. [DOI] [Google Scholar]
- 8.Valderrama J.A., Benites J., Cortés M., Pessoa-Mahana D., Prina E., Fournet A. Studies on quinones. Part 35: Access to antiprotozoal active euryfurylquinones and hydroquinones. Tetrahedron. 2002;58:881–886. doi: 10.1016/S0040-4020(01)01186-3. [DOI] [Google Scholar]
- 9.Valderrama J.A., Zamorano C., González M.F., Prina E., Fournet A. Studies on quinones. Part 39: Synthesis and leishmanicidal activity of acylchloroquinones and hydroquinones. Bioorg. Med. Chem. 2005;13:4153–4159. doi: 10.1016/j.bmc.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 10.Valderrama J.A., González M.F., Pessoa-Mahana D., Tapia R.A., Fillion H., Pautet F., Rodríguez J.A., Theoduloz C., Schmeda-Hishmann G. Studies on quinones. Part 41: Synthesis and cytotoxicity of isoquinoline-containing polycyclic quinones. Bioorg. Med. Chem. 2006;14:5003–5011. doi: 10.1016/j.bmc.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Valderrama J.A., González M.F., Colonelli P., Vásquez D. Design and synthesis of angucyclinone 5-aza analogues. Synlett. 2006;17:2777–2780. [Google Scholar]
- 12.Valderrama J.A., Vásquez D. Design and synthesis of angucyclinone AB-pyrido[2,3-d]pyrimidine analogues. Tetrahedron Lett. 2008;49:703–706. doi: 10.1016/j.tetlet.2007.11.133. [DOI] [Google Scholar]
- 13.Vásquez D., Rodríguez J.A., Theoduloz C., Buc Calderon P., Valderrama J.A. Studies on quinones. Part 46. Synthesis and in vitro antitumor evaluation of aminopyrimidoisoquinolinequinones. Eur. J. Med. Chem. 2010;45:5234–5242. doi: 10.1016/j.ejmech.2010.08.040. [DOI] [PubMed] [Google Scholar]
- 14.Valderrama J.A., Ibacache A., Rodriguez J.A., Theoduloz C., Benites J. Studies on Quinones. Part 47. Synthesis of novel phenylaminophenanthridinequinones as potential antitumor agents. Eur. J. Med. Chem. 2011;46:3398–3409. doi: 10.1016/j.ejmech.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Vásquez R., Verrax J., Valderrama J.A., Buc Calderon P. Aminopyrimidoisoquinolinequinone (APIQ) redox cycling is potentiated by ascorbate and induces oxidative stress leading to necrotic-like cancer cell death. Invest. New Drug. 2012;30:1003–1011. doi: 10.1007/s10637-011-9661-1. [DOI] [PubMed] [Google Scholar]
- 16.Monsalve F.A., Valderrama J.A., Vásquez D., Ibacache A., Rodríguez J.A., González D.R., González E. Inhibition of human topoisomerase I and activation of caspase-3 by aza-angucyclinones and arylaminopyrimido[4,5-c]isoquinoline-7,10-quinones. Int. J. Mol. Med. 2012;30:151–156. doi: 10.3892/ijmm.2012.961. [DOI] [PubMed] [Google Scholar]
- 17.Kviecinski M.R., Pedrosa R.C., Felipe K.B., Farias M.S., Glorieux C., Valenzuela M., Sid B., Benites J., Valderrama J.A., Verrax J., et al. Inhibition of cell proliferation and migration by oxidative stress from ascorbate-driven juglone redox cycling in human bladder-derived T24 cells. Biochem. Biophys. Res. Commun. 2012;421:268–273. doi: 10.1016/j.bbrc.2012.03.150. [DOI] [PubMed] [Google Scholar]
- 18.Delgado V., Ibacache A., Theoduloz C., Valderrama J.A. Synthesis and in vitro cytotoxic evaluation of aminoquinones structurally related to marine isoquinolinequinones. Molecules. 2012;17:7042–7056. doi: 10.3390/molecules17067042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vásquez D., Theoduloz C., Benites J., Ríos D., Valderrama J.A. Synthesis and antitumor evaluation of 6-arylsubstituted benzo[j]phenanthridine- and benzo[g]pyrimido[4,5-c]isoquinolinequinones. Molecules. 2012;17:11616–11629. doi: 10.3390/molecules171011616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ríos D., Benites J., Valderrama J.A., Farias M., Pedrosa R.C., Buc Calderon P., Verrax J. Biological evaluation of 3-acyl-2-arylamino-1,4-naphthoquinones as inhibitors of Hsp90 chaperoning function. Curr. Top. Med. Chem. 2012;12:2094–2102. doi: 10.2174/156802612804910188. [DOI] [PubMed] [Google Scholar]
- 21.Naeimi H., Moradi L. Microwave assisted direct ortho-acylation of phenol and naphthol derivatives by BF3.(C2H5)2O. Bull. Chem. Soc. Jpn. 2005;78:284–287. doi: 10.1246/bcsj.78.284. [DOI] [Google Scholar]
- 22.Trost B.M., Saulnier M.G. Regioselectivity in lithiation of t-butyldimethylsiloxy-3,5-dimethoxybenzene. A synthesis of the trimethyl ether of sophoraflavanone A. Tetrahedron Lett. 1985;26:123–126. doi: 10.1016/S0040-4039(00)61859-2. [DOI] [Google Scholar]
- 23.Crombie L., Jones R.C.F., Palmer C.J. Synthesis of the insecticidal 1'-acetoxy-mammeins and surangin B. Tetrahedron Lett. 1985;26:2933–2936. doi: 10.1016/S0040-4039(00)98875-0. [DOI] [Google Scholar]
- 24.Duplais C., Bures F., Sapountzis I., Korn T.J., Cahiez G., Knochel P. An efficient synthesis of diaryl ketones by iron-catalyzed arylation of aroyl cyanides. Angew. Chem. Int. Ed. Engl. 2004;43:2968–2970. doi: 10.1002/anie.200453696. [DOI] [PubMed] [Google Scholar]
- 25.Satori G., Casnati G., Bigi F., Predieri G. Ortho-coordinated acylation of phenol systems. J. Org. Chem. 1990;55:4371–4377. doi: 10.1021/jo00301a031. [DOI] [Google Scholar]
- 26.Boyer J.L., Krum J.E., Myers M.C., Fazal A.N., Wigal C.T. Synthetic utility and mechanistic implications of the Fries rearrangement of hydroquinone diesters in boron trifluoride complexes. J. Org. Chem. 2000;65:4712–4714. doi: 10.1021/jo000412q. [DOI] [PubMed] [Google Scholar]
- 27.Klinger H., Kolvenbach W. Die bildung von acetohydrochinon aus acetaldehyd und benzochinon im sonnenlicht. Ber. Dtsch. Chem. Ges. 1898;31:1214–1216. doi: 10.1002/cber.189803101220. [DOI] [Google Scholar]
- 28.Patai S. The Chemistry of the Quinoid Compounds, Part 1. Vol. 1. John Wiley and Sons; New York, NY, USA: 1974. p. 503. [Google Scholar]
- 29.Kraus G.A., Liu P. Benzophenone-mediated conjugate additions of aromatic aldehydes to quinones. Tetrahedron Lett. 1994;35:7723–7726. [Google Scholar]
- 30.Oelgemöller M., Schiel C., Fröhlich R., Mattay J. The photo-Friedel-Crafts acylation of 1,4-naphthoquinones. Eur. J. Org. Chem. 2002;15:2465–2474. [Google Scholar]
- 31.Oelgemöller M., Jung C., Ortner J., Mattay J., Schiel C., Zimmermann E. “Green photochemistry” with moderately concentrated sunlight. Spectrum. 2005;18:28–33. [Google Scholar]
- 32.Murphy B., Goodrich P., Hardacre C., Oelgemöller M. Green photochemistry: Photo-Friedel–Crafts acylations of 1,4-naphthoquinone in room temperature ionic liquids. Green Chem. 2009;11:1867–1870. doi: 10.1039/b913252j. [DOI] [Google Scholar]
- 33.Benites J., Ríos D., Díaz P., Valderrama J.A. The solar-chemical photo-Friedel–Crafts heteroacylation of 1,4-quinones. Tetrahedron Lett. 2011;52:609–611. doi: 10.1016/j.tetlet.2010.11.149. [DOI] [Google Scholar]
- 34.De Leon F., Kalagara S., Navarro A.A., Mito S. Synthesis of 6-acyl-5,8-quinolinediols by Photo-Friedel–Crafts acylation using sunlight. Tetrahedron Lett. 2013;54:3147–3149. doi: 10.1016/j.tetlet.2013.04.021. [DOI] [Google Scholar]
- 35.Pettit G.R., Toki B., Herald D.L., Verdier-Pinard P., Boyd M.R., Hamel E., Pettit R.K. Antineoplastic agents. 379. Synthesis of phenstatin phosphate. J. Med. Chem. 1998;41:1688–1698. doi: 10.1021/jm970644q. [DOI] [PubMed] [Google Scholar]
- 36.Alvarez C., Alvarez R., Corchete P., Pérez-Melero C., Peláez R., Medarde M. Synthesis and biological activity of naphthalene analogues of phenstatins: Naphthylphenstatins. Bioorg. Med. Chem. Lett. 2007;17:3417–3420. doi: 10.1016/j.bmcl.2007.03.082. [DOI] [PubMed] [Google Scholar]
- 37.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 38.Cushman M., Nagarathnam D., Gopal D., Chakraborti A.K., Lin C.M., Hamel E. Synthesis and evaluation of stilbene and dihydrostilbene derivatives as potential anticancer agents that inhibit tubulin polymerization. J. Med. Chem. 1991;34:2579–2588. doi: 10.1021/jm00112a036. [DOI] [PubMed] [Google Scholar]
- 39.Pettit G.R., Grealish M.P., Jung M.K., Hamel E., Pettit R.K., Chapuis J.C., Schmidt J.M. Antineoplastic agents. 465. Structural modification of resveratrol: sodium. Resverastatin phosphate. J. Med. Chem. 2002;45:2534–2542. doi: 10.1021/jm010119y. [DOI] [PubMed] [Google Scholar]
- 40.Liou J.P., Chang Y.L., Kuo F.M., Chang C.W., Tseng H.Y., Wang C.C., Yang Y.N., Chang J.Y., Lee S.J., Hsieh H.P. Concise synthesis and structure-activity relationships of combretastatin A-4 analogues, 1-aroylindoles and 3-aroylindoles, as novel classes of potent antitubulin agents. J. Med. Chem. 2004;47:4247–4257. doi: 10.1021/jm049802l. [DOI] [PubMed] [Google Scholar]
- 41.Tron G.C., Pirali T., Sorba G., Pagliai F., Busacca S., Genazzani A.A. Medicinal chemistry of combretastatin A4: Present and future directions. J. Med. Chem. 2006;49:3033–3044. doi: 10.1021/jm0512903. [DOI] [PubMed] [Google Scholar]
- 42.Romagnoli R., Baraldi P.G., Carrion M.D., Lopez Cara C., Preti D., Fruttarolo F., Pavani M. G., Tabrizi M.A., Tolomeo M., Grimaudo S., et al. Synthesis and biological evaluation of 2- and 3-aminobenzo[b]thiophene derivatives as antimitotic agents and inhibitors of tubulin polymerization. J. Med. Chem. 2007;50:2273–2277. doi: 10.1021/jm070050f. [DOI] [PubMed] [Google Scholar]
- 43.Hu L., Jiang J.D., Qu J., Li Y., Jin J., Li Z.R., Boykin D.W. Novel potent antimitotic heterocyclic ketones: Synthesis, Antiproliferative activity, and structure–activity relationships. Bioorg. Med. Chem. Lett. 2007;17:3613–3617. doi: 10.1016/j.bmcl.2007.04.048. [DOI] [PubMed] [Google Scholar]
- 44.Ty N., Kaffy J., Arrault A., Thoret S., Pontikis R., Dubois J., Morin-Allory L., Florent J.C. Synthesis and biological evaluation of cis-locked vinylogous combretastatin-A4 analogues: Derivatives with a cyclopropyl-vinyl or a cyclopropyl-amide bridge. Bioorg. Med. Chem. Lett. 2009;19:1318–1322. doi: 10.1016/j.bmcl.2009.01.062. [DOI] [PubMed] [Google Scholar]
- 45.Ghinet A., Rigo B., Hénichart J.P., Le Broc-Ryckewaert D., Pommery J., Pommery N., Thuru X., Quesnel B., Gautret P. Synthesis and biological evaluation of phenstatin metabolites. Bioorgn. Med. Chem. 2011;19:6042–6054. doi: 10.1016/j.bmc.2011.08.047. [DOI] [PubMed] [Google Scholar]
- 46.Bogert M.T., Howells H.P. The chemistry of the acyl para-quinones. A contribution to the solution of the “Pechmann dyes” problem. J. Am. Chem. Soc. 1930;52:837–850. doi: 10.1021/ja01365a061. [DOI] [Google Scholar]
- 47.Abdulla K.A., Abdul-Rahman A.L., Al-Hamdany R., Al-Saigh Z. Preparation and light induced reactions of substituted 1,4-benzoquinones. J. Prakt. Chem. 1982;34:498–504. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.