Abstract
Five new syringyl acylated flavonol glycosides, named leonurusoides A (1), B (2), C (3), D (4), and E (5), together with one known one 6 were obtained from the aerial parts of Leonurus japonicus. Their structures were elucidated by chemical and spectroscopic methods (UV, IR, HRESI-TOF-MS, 1D and 2D NMR). Compounds 1−6 showed triglyceride (TG) accumulation inhibitory effects in free fatty acid-induced HepG2 cells.
Keywords: Leonurus japonicus, syringyl acylated flavonol glycosides, TG accumulation inhibitory effects, HepG2 cells
1. Introduction
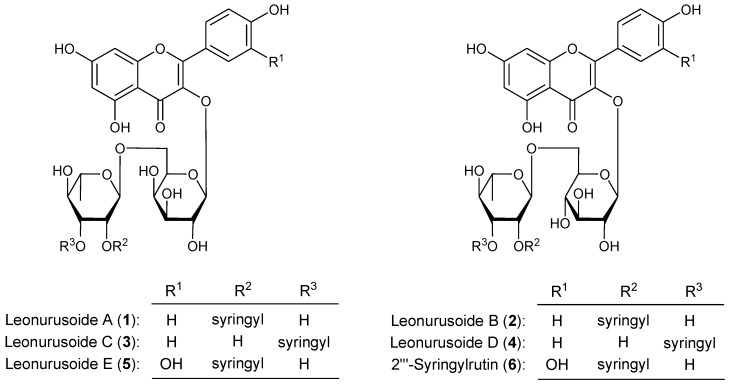
Leonurus japonicus Houtt is from the Lamiaceae family. As a Traditional Chinese Medicine (TCM), it has been commonly used to treat disorders of mammary gland [1]. Several metabolites such as alkaloids [2,3,4,5,6], flavonoids [6,7,8,9], and other compounds [10] have been isolated from this plant. During the course of our characterization studies on the bioactive constituents from the aerial parts of L. japonicus, five new syringyl acylated flavonol glycosides, named leonurusoides A (1), B (2), C (3), D (4), and E (5), together with the known one, 2'''-syringylrutin (6) (Figure 1) were obtained. This paper deals with the isolation and TG accumulation inhibitory effects in HepG2 cells elucidation of these compounds.
Figure 1.
Chemical structures of 1−6.
2. Results and Discussion
The aerial parts of L. japonicus were finely cut and refluxed with 50% ethanol-water. Evaporation of the solvent under reduced pressure provided a 50% ethanol-water extract, which was partitioned into a CHCl3-H2O (1:1, v/v) mixture to furnish a CHCl3-soluble fraction and an aqueous phase. The aqueous soluble fraction was subjected to column chromatography (CC) over macroporous resin D101, normal- and reversed-phase CC, and finally HPLC to give five new compounds, leonurusoides A (1), B (2), C (3), D (4), and E (5), together with the known one, 2'''-syringylrutin (6).
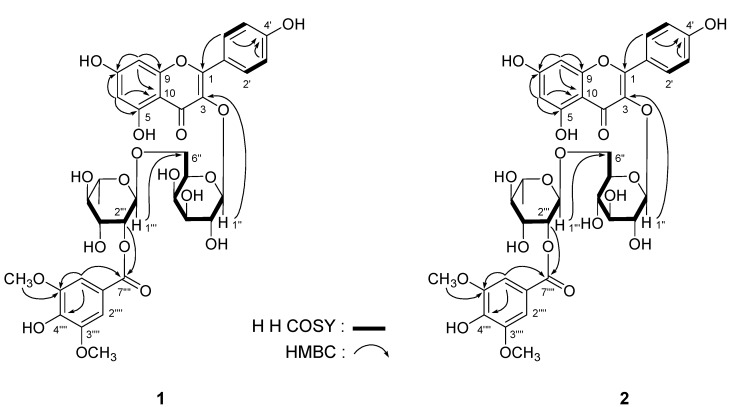
Leonurusoide A (1) was isolated as a yellow powder with negative optical rotation [[α]25D –13.0° (c = 0.54, MeOH)]. Its molecular formula was determined to be C36H38O19 by positive-ion HRESI-TOF-MS (m/z 775.2076 [M+H]+, calcd for C36H39O19 775.2080). The IR spectrum exhibited absorption bands typical of OH (3356 cm−1), ester carboxyl group (1699 cm–1), α,β-unsaturated ketone (1654 cm–1), aromatic ring (1608, 1516, 1457 cm–1), and an O-glycosidic linkage (1056 cm–1). The UV data of 1 showed the characteristic absorption maxima of a kampferol aglycon at 351 nm (4.00) and 268 nm (4.24). Acid hydrolysis of 1 with 1 M HCl afforded D-galactose and L-rhamnose, whose absolute configurations were determined by HPLC analysis using an optical rotation detector [11,12]. The 1H- (500 MHz) and 13C-NMR (125 MHz) spectra of 1 (DMSO-d6, Table 1), which were assigned by various NMR experiments including 1H-1H COSY, HSQC, and HMBC spectra, indicated there was a kampferol part [δ 6.14 (1H, br. s, H-6), 6.34 (1H, br. s, H-8), 6.81 (2H, d, J = 8.5 Hz, H-3',5'), 8.02 (2H, d, J = 8.5 Hz, H-2',6'), 12.53 (1H, br. s, 5-OH)], together with an α-L-rhamnopyranosyl and a β-D-galactopyranosyl moieties [δ 4.57 (1H, br. s, H-1'''), 5.30 (1H, d, J = 7.5 Hz, H-1'')] in the structure. On the other hand, the appearance of a methoxyl signal at δ 3.81 (6H, s, 3'''',5''''-OCH3) and a sharp aromatic singlet signal at δ 7.19 (2H, s, H-2'''',6'''') in the 1H-NMR spectrum, together with the signals at δ 55.9 (3'''',5''''-OCH3), 107.0 (C-2'''',6''''), 119.2 (C-1''''), 140.9 (C-4''''), 147.4 (C-3'''',5''''), 164.7 (C-7'''') in the 13C-NMR one, indicated the presence of a syringyl group. Furthermore, the assignment of glycoside protons was determined by proton-proton correlations observed from 1H-1H COSY and HSQC experiments. Finally, the linkages of α-L-rhamnopyranosyl, β-D-galactopyranosyl, and syringyl moieties were clarified on the basis of HMBC experiment, which showed long-range correlations between δH 5.30 (H-1'') and δC 133.2 (C-3); δH 4.57 (H-1''') and δC 65.3 (C-6''), and δH 4.92 (1H, br. d, ca. J = 3 Hz, H-2''') and δC 164.7 (C-7''''). On the basis of above mentioned, the structure of leonurusoide A was determined as kampferol-3-O-(2'''-syringyl)-α-L-rhamnopyranosyl(1→6)-β-D-galactopyranoside (1).
Table 1.
1H and 13C-NMR data for compounds 1 and 2 in DMSO-d6 (500 MHz for 1H and 125 MHz for 13C).
| No. | 1 | 2 | ||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 2 | 156.2 | — | 156.3 | — |
| 3 | 133.2 | — | 133.1 | — |
| 4 | 177.0 | — | 177.1 | — |
| 5 | 161.0 | — | 161.0 | — |
| 6 | 99.1 | 6.14 (1H, br. s) | 98.9 | 6.16 (1H, br. s) |
| 7 | 165.9 | — | 165.1 | — |
| 8 | 93.9 | 6.34 (1H, br. s) | 93.8 | 6.34 (1H, br. s) |
| 9 | 156.5 | — | 156.5 | — |
| 10 | 103.2 | — | 103.5 | — |
| 1' | 120.8 | — | 120.8 | — |
| 2',6' | 130.7 | 8.02 (2H, d, 8.5) | 130.7 | 7.96 (2H, d, 8.5) |
| 3',5' | 114.9 | 6.81 (2H, d, 8.5) | 114.9 | 6.83 (2H, d, 8.5) |
| 4' | 159.8 | — | 159.8 | — |
| 1'' | 102.2 | 5.30 (1H, d, 7.5) | 101.5 | 5.32 (1H, d, 7.5) |
| 2'' | 71.0 | 3.57 (1H, dd, 7.5, 9.0) | 74.1 | 3.18 (1H, dd, 7.5, 9.0) |
| 3'' | 72.9 | 3.43 (1H, dd, 3.0, 9.0) | 76.3 | 3.23 (1H, dd, 9.0, 9.0) |
| 4'' | 67.9 | 3.65 (1H, m, overlapped) | 69.7 | 3.08 (1H, dd, 9.0, 9.0) |
| 5'' | 73.2 | 3.63 (1H, m, overlapped) | 75.6 | 3.29 (1H, m) |
| 6'' | 65.3 | 3.32 (1H, dd, 5.0, 9.0) | 66.8 | 3.38 (1H, dd, 5.0, 9.0) |
| 3.66 (1H, m, overlapped) | 3.73 (1H, br. d, ca. 11) | |||
| 5-OH | 12.53 (1H, br. s) | 12.54 (1H, br. s) | ||
| 1''' | 96.9 | 4.57 (1H, br. s) | 97.6 | 4.53 (1H, br. s) |
| 2''' | 72.5 | 4.92 (1H, br. d, ca. 3) | 72.5 | 4.95 (1H, br. d, ca. 3) |
| 3''' | 68.4 | 3.63 (1H, m, overlapped) | 68.6 | 3.61 (1H, dd, 3.0, 9.0) |
| 4''' | 72.4 | 3.26 (1H, dd, 9.5, 9.5) | 72.4 | 3.24 (1H, dd, 9.0, 9.5) |
| 5''' | 68.6 | 3.52 (1H, m) | 68.4 | 3.41 (1H, m) |
| 6''' | 18.0 | 1.15 (3H, d, 6.0) | 17.8 | 1.07 (3H, d, 6.0) |
| 1'''' | 119.2 | — | 119.4 | — |
| 2'''',6'''' | 107.0 | 7.19 (2H, s) | 107.1 | 7.19 (2H, s) |
| 3'''',5'''' | 147.4 | — | 147.4 | — |
| 4'''' | 140.9 | — | 140.7 | — |
| 7'''' | 164.7 | — | 164.7 | — |
| -OCH3 | 55.9 | 3.81 (6H, s) | 56.0 | 3.82 (6H, s) |
Leonurusoide B (2) was obtained as a yellow powder and exhibited a negative rotation [[α]25D –16.6° (c = 0.21, MeOH)]. The IR spectrum of 2 indicated the presences of carboxyl (1699 cm–1), γ-pyrone (1653 and 1569 cm–1) functions, and a glycoside structure (3218, 1055 cm–1). HRESI-TOF-MS analysis suggested that 2 had the same molecular formula as 1 [C36H39O19 (m/z 775.2070 [M+H]+, calcd for C36H39O19 775.2080). Comparison of the 1H- and 13C-NMR spectra of 2 with those of 1 indicated that the main difference lie in the region of β-D-galactopyranosy moiety of 1. The 1H- and 13C-NMR of 2 suggested a set of signals for the presence of β-D-glucopyranosyl group [δH 5.32 (1H, d, J = 7.5 Hz, H-1''); δC 66.8 (C-6''), 69.7 (C-4''), 74.1 (C-2''), 75.6 (C-5''), 76.3 (C-3''), and 101.5 (C-1'')]. In the HMBC spectrum, the long-range correlations between δH 5.32 (H-1'') and δC 133.2 (C-3); δH 4.53 (1H, br. s, H-1''') and δC 66.8 (C-6''), and δH 4.95 (1H, br. d, ca. J = 3 Hz, H-2''') and δC 164.7 (C-7'''') (Figure 2) were observed. Finally, the presences of L-rhamnopyranosyl and D-glucopyranosyl moieties in 2 were demonstrated by the acid hydrolysis like those of 1 [11,12]. Consequently, the structure of leonurusoide B was determined to be kampferol-3-O-(2'''-syringyl)-α-L-rhamnopyranosyl(1→6)-β-D-glucopyranoside (2).
Figure 2.
The main 1H-1H COSY and HMBC correlations of compounds 1 and 2.
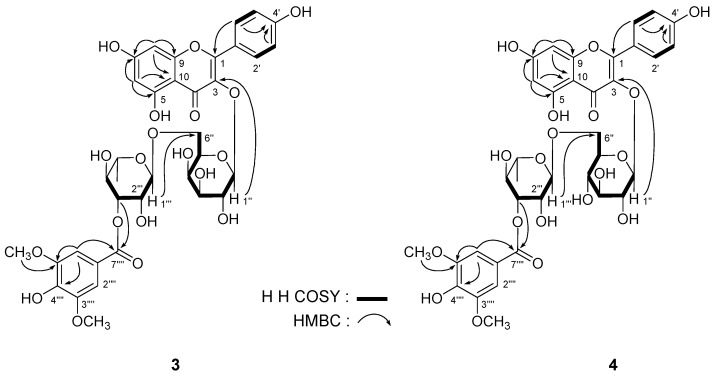
Leonurusoides C (3) and D (4) were both obtained with negative rotation [[α]25D: −27.2° (c = 0.38, MeOH) for 3; and −11.0° (c = 0.31, MeOH) for 4], too. The same molecular formula, C36H38O19, of them were determined from positive-ion HRESI-TOF-MS [m/z 797.1904 (3) and 797.1901 (4), both [M+Na]+, calcd for C36H38O19Na 797.1900]. Under acid hydrolysis with 1 M HCl [11,12], 3 gave L-rhamnose and D-galactose, but 4 afforded L-rhamnose and D-glucose. The 1H- and 13C-NMR (DMSO-d6, Table 2) spectra, which were assigned by various NMR experiments including 1H-1H COSY, HSQC, and HMBC spectra, indicated 3 and 4 had the same aglycon, kampferol [for 3: 6.18 (1H, br. s, H-6), 6.42 (1H, br. s, H-8), 6.87 (2H, d, J = 8.0 Hz, H-3',5'), 8.06 (2H, d, J = 8.0 Hz, H-2',6'), 12.57 (1H, br. s, 5-OH); for 4: 6.16 (1H, d, J = 1.5 Hz, H-6), 6.38 (1H, d, J = 1.5 Hz, H-8), 6.88 (2H, d, J = 8.5 Hz, H-3',5'), 7.98 (2H, d, J = 8.5 Hz, H-2',6'), 12.51 (1H, br. s, 5-OH)]. And both of them owned a syringyl moiety [for 3: 3.81 (6H, s, 3'''',5''''-OCH3), 7.27 (2H, s, H-2'''',6''''); for 4: 3.82 (6H, s, 3'''',5''''-OCH3), 7.28 (2H, s, H-2'''',6'''')]. Finally, the linkage position of α-L-rhamnopyranosyl, β-D-galacto-pyranosyl (in 3)/β-D-glucopyranosyl (in 4), and syringyl moieties were clarified on the basis of HMBC experiment (Figure 3). Consequently, the structure of leonurusoides C and D were determined to be kampferol-3-O-(3'''-syringyl)-α-L-rhamnopyranosyl(1→6)-β-D-galactopyranoside (3) and kampferol-3-O-(3'''-syringyl)-α-L-rhamnopyranosyl(1→6)-β-D-glucopyranoside (4), respectively.
Table 2.
1H and 13C-NMR data for compounds 3 and 4 in DMSO-d6 (500 MHz for 1H and 125 MHz for 13C).
| No. | 3 | 4 | ||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 2 | 156.4 | — | 156.7 | — |
| 3 | 133.2 | — | 133.2 | — |
| 4 | 177.3 | — | 177.2 | — |
| 5 | 161.1 | — | 161.1 | — |
| 6 | 98.7 | 6.18 (1H, br. s) | 98.7 | 6.16 (1H, d, 1.5) |
| 7 | 164.3 | — | 164.3 | — |
| 8 | 93.7 | 6.42 (1H, br. s) | 93.7 | 6.38 (1H, d, 1.5) |
| 9 | 156.3 | — | 156.4 | — |
| 10 | 103.7 | — | 103.8 | — |
| 1' | 120.7 | — | 120.8 | — |
| 2',6' | 130.9 | 8.06 (2H, d, 8.0) | 130.7 | 7.98 (2H, d, 8.5) |
| 3',5' | 115.0 | 6.87 (2H, d, 8.0) | 115.0 | 6.88 (2H, d, 8.5) |
| 4' | 159.9 | — | 159.8 | — |
| 1'' | 102.0 | 5.34 (1H, d, 8.0) | 101.5 | 5.32 (1H, d, 7.5) |
| 2'' | 71.0 | 3.57 (1H, dd, 8.0, 9.0) | 74.1 | 3.19 (1H, dd, 7.5, 8.5) |
| 3'' | 72.8 | 3.43 (1H, dd, 3.0, 9.0) | 76.3 | 3.24 (1H, dd, 8.5, 8.5) |
| 4'' | 67.7 | 3.67 (1H, m, overlapped) | 69.9 | 3.07 (1H, dd, 8.5, 9.0) |
| 5'' | 73.1 | 3.64 (1H, m, overlapped) | 75.6 | 3.33 (1H, m) |
| 6'' | 65.0 | 3.29 (1H, dd, 5.0, 9.0) | 67.3 | 3.37 (1H, dd, 5.5, 12.0) |
| 3.67 (1H, m, overlapped) | 3.75 (1H, dd, 2.0, 12.0) | |||
| 5-OH | 12.57 (1H, br. s) | 12.51 (1H, br. s) | ||
| 1''' | 99.9 | 4.51 (1H, br. s) | 100.8 | 4.46 (1H, d, 1.5) |
| 2''' | 68.0 | 3.75 (1H, br. d, ca. 3) | 67.8 | 3.77 (1H, dd, 1.5, 3.5) |
| 3''' | 74.7 | 4.84 (1H, dd, 3.0, 9.5) | 74.7 | 4.78 (1H, dd, 3.5, 9.5) |
| 4''' | 68.9 | 3.49 (1H, dd, 9.5, 9.5) | 68.8 | 3.49 (1H, dd, 8.5, 9.5) |
| 5''' | 68.3 | 3.58 (1H, m) | 68.3 | 3.47 (1H, m) |
| 6''' | 17.8 | 1.14 (3H, d, 6.0) | 17.6 | 1.11 (3H, d, 6.0) |
| 1'''' | 119.8 | — | 119.9 | — |
| 2'''',6'''' | 107.2 | 7.27 (2H, s) | 107.2 | 7.28 (2H, s) |
| 3'''',5'''' | 147.3 | — | 147.3 | — |
| 4'''' | 140.4 | — | 140.4 | — |
| 7'''' | 165.3 | — | 165.2 | — |
| -OCH3 | 56.0 | 3.81 (6H, s) | 56.0 | 3.82 (6H, s) |
Figure 3.
The main 1H-1H COSY and HMBC correlations of compounds 3 and 4.
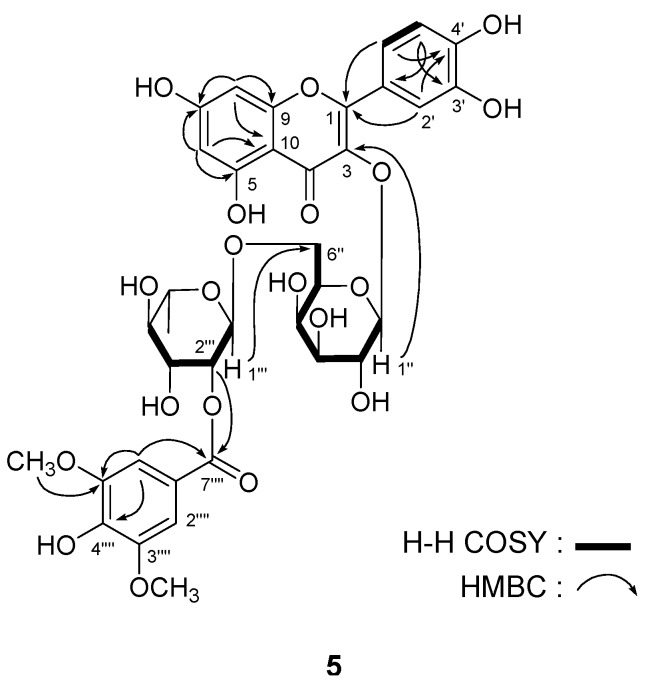
Leonurusoide E (5) was obtained as a yellow powder with negative rotation [[α]25D –18.2° (c = 1.09, MeOH)]. The molecular formula, C36H38O20, of 5 was determined by positive-ion HRESI-TOF-MS (m/z 813.1833 [M + Na]+, calcd for C36H38O20Na 813.1849). The 1H and 13C-NMR (DMSO-d6, Table 3), and various NMR experiments including 1H-1H COSY, HSQC, and HMBC spectra (Figure 4) of 5 revealed the presence of a syringyl group [δ 3.81 (6H, s, 3'''',5''''-OCH3), 7.20 (2H, s, H-2'''',6'''')], together with a hyperoside moiety {[δ 1.16 (3H, d, J = 6.0 Hz, 6'''-CH3), 4.62 (1H, d, J = 1.0 Hz, H-1'''], 5.35 (1H, d, J = 8.0 Hz, H-1''), 6.20 (1H, br. s, H-6), 6.39 (1H, br. s, H-8), 6.80 (1H, d, J = 8.5 Hz, H-5'), 7.54 (1H, d, J = 2.0 Hz, H-2'), 7.63 (1H, dd, J = 2.0, 8.5 Hz, H-6), 12.58 (1H, br. s, 5-OH)]}.
Table 3.
1H and 13C-NMR data for compound 5 in DMSO-d6 (500 MHz for 1H and 125 MHz for 13C).
| δC | δH (J in Hz) | δC | δH (J in Hz) | ||
|---|---|---|---|---|---|
| 2 | 156.2 | — | 3'' | 72.9 | 3.43 (1H, dd, 3.0, 9.5) |
| 3 | 133.4 | — | 4'' | 67.8 | 3.68 (1H, m, overlapped) |
| 4 | 177.2 | — | 5'' | 73.2 | 3.64 (1H, m, overlapped) |
| 5 | 161.1 | — | 6'' | 65.1 | 3.33 (1H, dd, 5.5, 9.5) |
| 6 | 98.6 | 6.20 (1H, br. s) | 3.67 (1H, m, overlapped) | ||
| 7 | 164.3 | — | 1''' | 96.8 | 4.62 (1H, d, 1.0) |
| 8 | 93.5 | 6.39 (1H, br. s) | 2''' | 72.6 | 4.93 (1H, dd, 1.0, 3.0) |
| 9 | 156.2 | — | 3''' | 68.6 | 3.65 (1H, m, overlapped) |
| 10 | 103.7 | — | 4''' | 72.4 | 3.28 (1H, dd, 9.0, 9.5) |
| 1' | 121.0 | — | 5''' | 68.4 | 3.54 (1H, m) |
| 2' | 115.9 | 7.54 (1H, d, 2.0) | 6''' | 18.0 | 1.16 (3H, d, 6.0) |
| 3' | 144.7 | — | 1'''' | 119.2 | — |
| 4' | 148.4 | — | 2'''',6'''' | 107.0 | 7.20 (2H, s) |
| 5' | 115.0 | 6.80 (1H, d, 8.5) | 3'''', 5'''' | 147.4 | — |
| 6' | 121.7 | 7.63 (1H, d, 2.0, 8.5) | 4'''' | 140.7 | — |
| 5-OH | 12.58 (1H, br. s) | 7'''' | 164.7 | ||
| 1'' | 101.9 | 5.35 (1H, d, 8.0) | -OCH3 | 55.9 | 3.81 (6H, s) |
| 2'' | 71.0 | 3.60 (1H, dd, 8.0, 9.0) |
Figure 4.
The main 1H-1H COSY and HMBC correlations of compound 5.
The position of syringyl group was determined by the HMBC experiment, which showed long-range correlations between the protons of position-2'" [δH 4.93 (1H, dd, J = 1.0, 3.0 Hz)] and ester carboxyl carbon [δC 164.7 (C-7"'')]. Finally, acid hydrolysis of 5 with 1 M HCl afforded L-rhamnose and D-galactose determined by HPLC analysis [11,12]. Consequently, the structure of leonurusoide E was determined to be quercetin-3-O-(2'''-syringyl)-α-L-rhamnopyranosyl(1→6)-β-D-galactopyranoside (5). The known acylated flavonoid glycoside compound 6 was identified as 2'''-syringylrutin by comparing its physical properties and spectral data with that reported in the literature [6].
Intracellular excess lipid accumulation (especially in liver and muscle) is a mediator of metabolic syndrome, which comprises a cluster of risk factors such as diabetes, hyperlipidemia, and hypertension. Free fatty acid induced TG accumulation HepG2 cells is commonly used for research on lipid metabolism regulation effects. The TG accumulation inhibitory effects of the isolates 1–6 were tested. All compounds showed inhibitory effects on TG accumulation in free fatty acid induced HepG2 cells (Table 4).
Table 4.
TG accumulation inhibitory effects of 1–6 in HepG2 cells.
| Concentration ( μM) | Inhibitory rate (%) | |
|---|---|---|
| Control | 0 | 0.0 ± 1.2 |
| Orlistat | 1 | 43.3 ± 4.1 * |
| 1 | 10 | 61.4 ± 10.0 ** |
| 2 | 10 | 52.8 ± 4.2 * |
| 3 | 10 | 56.9 ± 9.6 ** |
| 4 | 10 | 51.7 ± 6.9 * |
| 5 | 10 | 49.1 ± 4.9 * |
| 6 | 10 | 44.3 ± 4.3 * |
Intracellular TG accumulation inhibitory rate of control group was set as 0%. Values represent the mean ± SD of six determinations. * p < 0.05; ** p < 0.01 vs. control group.
3. Experimental
3.1. General
Optical rotations were measured on a Rudolph Autopol® IV automatic polarimeter. IR spectra were recorded on a Varian 640-IR FT-IR spectrophotometer. UV spectra were obtained on a Varian Cary 50 UV-Vis spectrophotometer. NMR spectra were determined on a Bruker 500 MHz NMR spectrometer at 500 MHz for 1H and 125 MHz for 13C-NMR, with TMS as an internal standard. Positive- and Negative-ion HRESI-TOF-MS were recorded on an Agilent Technologies 6520 Accurate-Mass Q-Tof LC/MS spectrometer. Column chromatographies (CCs) were performed on macroporous resin D101 (Haiguang Chemical Co., Ltd., Tianjin, China), silica gel (48–75 μm, Qingdao Haiyang Chemical Co., Ltd., Qingdao, China), Sephadex LH-20 (Ge Healthcare Bio-Sciences, Uppsala, Sweden), and ODS (40-63 μm, YMC Co., Ltd., Tokyo, Japan). A Cosmosil 5C18-MS-II (20 mm i.d. × 250 mm, Nakalai Tesque, Inc., Tokyo, Japan) preparative HPLC column was used to purify the constituents. Pre-coated TLC plates with Silica gel GF254 (Tianjin Silida Technology Co., Ltd., Tianjin, China) were used to detect the purity of isolate; visualization was achieved by spraying with 10% aqueous H2SO4-EtOH, following by heating.
3.2. Plant Material
The aerial parts of L. japonicus Houtt were purchased from Anguo Medicinal Market, Hebei Province, China and identified by Dr. Li Tianxiang. The voucher specimen was deposited at the Academy of Traditional Chinese Medicine of Tianjin University of TCM (No. 20110909).
3.3. Extraction and Isolation
The aerial parts of L. japonicus (6.0 kg) were finely cut and refluxed with 50% ethanol-water (48 L, 2 h) three times. Evaporation of the solvent under reduced pressure provided a 50% ethanol-water extract (600.0 g). The 50% ethanol-water extract (480.1 g) was partitioned into a CHCl3-H2O (1:1, v/v) mixture to furnish a CHCl3-soluble fraction (46.1 g) and an aqueous phase (430.2 g). The aqueous soluble fraction (362.0 g) was subjected to D101 resin CC, and eluted with H2O, 70% and 95% EtOH, successively, and the H2O (255.1 g), 70% EtOH (78.3 g) and 95% EtOH (32.2 g) eluted fractions were obtained, respectively. The 70% EtOH eluted fraction (75.0 g) was separated by silica gel CC [CHCl3 → CHCl3-MeOH (100:5, v/v) → CHCl3-MeOH-H2O (10:3:1 → 7:3:1 → 6:4:1, v/v/v, the lower layer) → MeOH] to afford nine fractions (Fr. 1−9). Fraction 2 (15.0 g) was separated by ODS CC [MeOH-H2O (10% → 20% → 30% → 40% → 50% → 60% → 100%, v/v)], and 12 fractions (Fr. 2-1−2-12) were obtained. Fraction 2-9 (0.97 g) was separated by preparative HPLC (PHPLC) [CH3CN-H2O (22:78, v/v)] to afford eight fractions (Fr. 2-9-1−2-9-8). Fractions 2-9-7 (85.3 mg) and 2-9-8 (90.1 mg) were further purified by PHPLC [MeOH-H2O (54:46, v/v)] to give leonurusoides E (5, 24.2 mg) and C (3, 9.7 mg), respectively. Fraction 2-10 (1.0 g) was separated by PHPLC [CH3CN-H2O (23:77, v/v)] to yield 10 fractions [Fr. 2-10.1−2-10.10]. Fraction 2-10-4 (440.1 mg) was purified by PHPLC [MeOH-H2O (46:54, v/v)] to give leonurusoide E (5, 34.1 mg). Fraction 2-10-10 (61.8 mg) was further separated by PHPLC [MeOH-H2O (47:53, v/v) to afford leonurusoide D (4, 6.7 mg). Fraction 2-11 (1.13 g) was separated by PHPLC [CH3CN-H2O (23:77, v/v)] to give six fractions (Fr. 2-11-1−2-11-6). Fractions 2-11-1 (381.8 mg) and 2-11-3 (56.2 mg) were purified by PHPLC [MeOH-H2O (52:48, v/v)] to give 2'''-syringylrutin (6, 5.8 mg) and leonurusoide A (1, 13.4 mg). Fraction 2-11-4 (188.9 mg) was further subjected to PHPLC [MeOH-H2O (53:47, v/v)] to afford leonurusoide B (2, 3.8 mg).
Leonurusoide A (1). Yellow powder. [α]25D –13.0° (c = 0.54, MeOH); IR νmax (KBr) cm–1: 3356, 2922, 2840, 1699, 1654, 1608, 1516, 1457, 1361, 1281, 1210, 1180, 1116, 1056, 841, 762; UV λmax (MeOH) nm (log ε): 351 (4.00), 268 (4.24). 1H-NMR (DMSO-d6) and 13C-NMR (DMSO-d6) spectroscopic data, see Table 1. HRESI-TOF-MS: Positive-ion mode m/z 775.2076 [M+H]+ (calcd for C36H39O19 775.2080).
Leonurusoide B (2). Yellow powder. [α]25D –16.6° (c = 0.21, MeOH); IR νmax (KBr) cm–1: 3218, 2948, 2841, 1699, 1653, 1608, 1569, 1517, 1458, 1361, 1281, 1210, 1180, 1113, 1055, 841, 762; UV λmax (MeOH) nm (log ε): 349 (3.88), 267 (4.13). 1H-NMR (DMSO-d6) and 13C-NMR (DMSO-d6) spectroscopic data, see Table 1. HRESI-TOF-MS: Positive-ion mode m/z 775.2070 [M+H]+ (calcd for C36H39O19 775.2080).
Leonurusoide C (3). Yellow powder. [α]D25 –27.2° (c = 0.38, MeOH); IR νmax (KBr) cm–1: 3231, 2972, 2860, 1699, 1653, 1608, 1588, 1516, 1457, 1362, 1281, 1210, 1181, 1116, 1056, 765, 680; UV λmax (MeOH) nm (log ε): 348 (4.16), 268 (4.41). 1H-NMR (DMSO-d6) and 13C-NMR (DMSO-d6) spectroscopic data, see Table 2. HRESI-TOF-MS: Positive-ion mode m/z 797.1904 [M+Na]+ (calcd for C36H38O19Na 797.1900).
Leonurusoide D (4). Yellow powder. [α]25D –11.0° (c = 0.31, MeOH); IR νmax (KBr) cm–1: 3322, 2921, 2843, 1699, 1653, 1589, 1518, 1458, 1357, 1290, 1213, 1181, 1118, 1055, 772, 677; UV λmax (MeOH) nm (log ε): 346 (3.86), 267 (4.13). 1H-NMR (DMSO-d6) and 13C-NMR (DMSO-d6) spectroscopic data, see Table 2. HRESI-TOF-MS: Positive-ion mode m/z 797.1901 [M+Na]+ (calcd for C36H38O19Na 797.1900).
Leonurusoide E (5). Yellow powder. [α]25D –18.2° (c = 1.09, MeOH); IR νmax (KBr) cm–1: 3323, 2960, 1697, 1652, 1608, 1516, 1457, 1424, 1340, 1302, 1206, 1117, 1070, 815, 764, 675; UV λmax (MeOH) nm (log ε): 361 (4.24), 265 (4.43). 1H-NMR (DMSO-d6) and 13C-NMR (DMSO-d6) spectroscopic data, see Table 3. HRESI-TOF-MS: Positive-ion mode m/z 813.1833 [M+Na]+ (calcd for C36H38O20Na 813.1849).
Acid Hydrolysis of 1–5
A solution of leonurusoides A–E (1–5, each 1.5 mg) in 1 M HCl (1 mL) was heated under reflux for 3 h, respectively. The reaction mixture was neutralized with Amberlite IRA-400 (OH– form) and removed by filtration. The aqueous layer was subjected to the HPLC analysis under the following condition, respectively: HPLC column, Kaseisorb LC NH2-60-5 (4.6 mm i.d. × 250 mm, Tokyo Kasei Co. Ltd., Tokyo, Japan); detection, optical rotation [Chiralyser (IBZ Messtechnik GMBH, Mozartstrasse 14-16 D-30173 Hannover, Germany)]; mobile phase, CH3CN-H2O (75:25, v/v); flow rate 1.0 mL/min. Identification of L-rhamnose (i) and D-galactose (iii) from 1, 3 and 5; L-rhamnose (i) and D-glusose (ii) from 2 and 4 presented in the aqueous was carried out by comparison of its retention time and optical rotation with that of authentic samples, tR: (i) 7.5 min (negative, L-rhamnose), (ii) 13.9 min (positive, D-glusose), and (iii) 14.5 min (positive, D-galactose).
3.4. TG Accumulation Inhibitory Effects Assay
The hepatic cell line HepG2 (IBMS, CAMS/PUMC, Beijing, China) were maintained in high glucose Minimum Essential Medium (MEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin under a humidified atmosphere of 5% CO2 in air. After growth to 80% confluence, cells were seeded at 4 × 104 cells/mL on 48-well dish. After 24 h incubation, the medium was switched to high glucose MEM and supplemented with 10% FBS and 0.2 mM oleic acid sodium salt, together with sample DMSO solution (final concentration of DMSO was less than 0.1%). After 48 h incubation, the amount of intracellular triglycerides was determined with a Triglycerides kit (BioSino Bio-technology and Science Inc., Beijing, China) after cell lysis.
Statistical Analysis
Values are expressed as mean ± S.D. All the grouped data were statistically performed with SPSS 11.0. Significant differences between means were evaluated by one-way analysis of variance (ANOVA) and Tukey’s Studentized range test was used for post hoc evaluations. p < 0.05 was considered to indicate statistical significance.
4. Conclusions
In summary, five new syringyl acylated flavonoid glycosides 1−5, together with the known compound, 2'''-syringylrutin (6) were obtained from the aerial parts of L. japonicas. Although various acylated flavonol glycosides distribute widely in the plant kingdom [13], syringates such as the isolates 1−6 are quite rare. Compound 6 and quercetin 3-(3'''-syringylrobinobioside), with a syringyl acylated at positions 2 and 3 of rhamnose, respectively, are the first examples of natural flavonol glycosides from the same genus, Leonurus [6,7], and are only found in the genus. All of the syringyl acylated flavonoid glycosides 1−6 showed TG accumulation inhibitory effects in free fatty acid-induced HepG2 cells.
Acknowledgments
This research was supported by Program for New Century Excellent Talents in University (NCET-10-0958), Applied Basic and Advanced Research Program of Tianjin (10SYSYJC28900, 10ZCKFSY09300), and Important Drug Development Fund, Ministry of Science and Technology of China (2011ZX09307-002-01).
Footnotes
Sample Availability: Samples of the compounds 1 and 5 are available from the authors.
References
- 1.National Pharmacopoeia Committee. The People’s Republic of China Pharmacopoeia. Chinese medicine science and Technology Press; Beijing, China: 2010. p. 273. [Google Scholar]
- 2.Giang P.M., Son P.T., Matsunami K., Otsuka H. New labdane-type diterpenoids from Leonurus heterophyllus SW. Chem. Pharm. Bull. 2005;53:938–941. doi: 10.1248/cpb.53.938. [DOI] [PubMed] [Google Scholar]
- 3.Giang P.M., Son P.T., Matsunami K., Otsuka H. New bis-spirolabdane-type diterpenoids from Leonurus heterophyllus SW. Chem. Pharm. Bull. 2005;53:1475–1479. doi: 10.1248/cpb.53.1475. [DOI] [PubMed] [Google Scholar]
- 4.Cai X.H., Che C.T., Lam C.K., Mak T.C., Wu L.J. A new labdane diterpene from Leonurus heterophyllus. J. Asian Nat. Prod. Res. 2006;8:599–603. doi: 10.1080/10286020500208394. [DOI] [PubMed] [Google Scholar]
- 5.Romero-González R.R., Avila-Núñez J.L., Aubert L., Alonso-Amelot M.E. Labdane diterpenes from Leonurus japonicus leaves. Phytochemistry. 2006;67:965–970. doi: 10.1016/j.phytochem.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Seo H.K., Kim J.S., Kang S.S. Labdane diterpenes and flavonoids from Leonurus japonicus. Helvetica Chimica Acta. 2010;93:2045–2051. doi: 10.1002/hlca.201000043. [DOI] [Google Scholar]
- 7.Cong Y., Wang J.H., Li X. A new flavonoside from Leonurus heterophyllus. J. Asian Nat. Prod. Res. 2005;7:273–277. doi: 10.1080/10286020410001690109. [DOI] [PubMed] [Google Scholar]
- 8.Cai X., Che Z., Wu B., Gao H., Wu L. Studies on chemical constituents of Leonurus japonicus. Shenyang Yaoke Daxue Xuebao. 2006;23:13–21. [Google Scholar]
- 9.Cong Y., Guo J., Wang X., Li M., Li K., Wang J., Li Q. Chemical constituents and antitumor activity on leukem ia K562 cell of Leonurus heterophyllus. Zhongguo Zhong Yao Za Zhi. 2009;34:1816–1818. [PubMed] [Google Scholar]
- 10.Li Y., Chen Z., Feng Z., Yang Y., Jiang J., Zhang P. Hepatoprotective glycosides from Leonurus japonicus Houtt. Carbohydr. Res. 2012;348:42–46. doi: 10.1016/j.carres.2011.10.034. [DOI] [PubMed] [Google Scholar]
- 11.Morikawa T., Zhang Y., Nakamura S., Matsuda H., Muraoka O., Yoshikawa M. Bioactive constituents from Chinese natural medicines. XXII. Absolute structures of new megastigmane glycosides, sedumosides E1, E2, E3, F1, F2, and G, from Sedum sarmentosum (Crassulaceae) Chem. Pharm. Bull. 2007;55:435–441. doi: 10.1248/cpb.55.435. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y., Morikawa T., Nakamura S., Ninomiya K., Matsuda H., Muraoka O., Yoshikawa M. Bioactive constituents from Chinese Natural Medicines. XXV. New flavonol bisdesmosides, sarmenosides I, II, III, and IV, with hepatoprotective activity from Sedum sarmentosum (Crassulaceae) Heterocycles. 2007;71:1565–1576. doi: 10.3987/COM-07-11050. [DOI] [Google Scholar]
- 13.Williams C.A. Flavonoids. In: Andersen Ø.M., Markham K.R., editors. Chemistry, Biochemistry and Applications. CRC Press; Boca Raton, FL, USA: 2006. p. 749. [Google Scholar]