Abstract
A new indole alkaloid, 12-hydroxy-N-acetyl-21(N)-dehydroplumeran-18-oic acid (13), and 11 known indole alkaloids: 3,4,5,6-tetradehydro-β-yohimbine (3), 19(E)-hunteracine (4), β-yohimbine (5), yohimbine (6), 19,20-dehydro-17-α-yohimbine (7), uleine (10), 20-epi-dasycarpidone (11), olivacine (8), 20-epi-N-nor-dasycarpidone (14), N-demethyluleine (15) and 20(E)-nor-subincanadine E (12) and a boonein δ-lactone 9, ursolic acid (1) and 1D,1O-methyl-chiro-inositol (2) were isolated from the EtOH extracts of different parts of Aspidosperma ulei Markgr. (Apocynaceae). Identification and structural elucidation were based on IR, MS, 1H- and 13C-NMR spectral data and comparison to literature data. The antiplasmodial and antimalarial activity of 1, 5, 6, 8, 10 and 15 has been previously evaluated and 1 and 10 have important in vitro and in vivo antimalarial properties according to patent and/or scientific literature. With the aim of discovering new antiplasmodial indole alkaloids, 3, 4, 11, 12 and 13 were evaluated for in vitro inhibition against the multi-drug resistant K1 strain of the human malaria parasite Plasmodium falciparum. IC50 values of 14.0 (39.9), 4.5 (16.7) and 14.5 (54.3) μg/mL (μM) were determined for 3, 11 and 12, respectively. Inhibitory activity of 3, 4, 11, 12 and 13 was evaluated against NIH3T3 murine fibroblasts. None of these compounds exhibited toxicity to fibroblasts (IC50 > 50 μg/mL). Of the five compounds screened for in vitro antiplasmodial activity, only 11 was active.
Keywords: Apocynaceae, indole alkaloids, antiplasmodial, Plasmodium falciparum K1, murine fibroblasts, cytotoxic evaluation, NMR, Aspidosperma ulei, dasycarpane
1. Introduction
Malaria continues to be a disease that afflicts the whole World, especially the African continent. However, data from 99 countries reveals that based on the overall number of deaths malaria is in decline [1]. The main antimalarials available today are the quinolines that are structural mimics of the plant-derived natural product quinine and the semi-synthetic derivatives of another plant-derived natural product, artemisinin. Resistance of the malaria parasites to these drugs is an issue of concern and it is important to discover new compounds that may be developed into the next generation of antimalarial drugs [2].
The Aspidosperma spp. (Apocynaceae) comprise trees distributed in Central and South America. Aspidosperma spp. extracts exhibit antimalarial activity and remedies prepared from the bark are used in traditional medicine for the treatment of malaria [3]. Screening of bark extracts representing six Aspidosperma spp. for in vitro inhibition against chloroquine-resistant W2 and chloroquine-sensitive 3D7 strains of the human malaria parasite Plasmodium falciparum revealed good activity (IC50 = 5.0–65.0 μg/mL). Thus, A. ulei (syn. A. parvifolium) trunk bark EtOH extracts were found to be active, as were the extracts of two other Aspidosperma spp. [4].
Approximately 250 indole alkaloids have been isolated from Aspidosperma spp. [5,6,7]. Uleine, 2-methyltetrahydroellipticine, dihydroolivacine and 2-methyltetrahydroolivacine have been previously isolated from A. ulei [8,9]. Aspidospermine-type alkaloids inhibit P. falciparum in vitro (IC50 = 3.2–8.7 μM) [10]. Uleine and uleine-containing extracts have received attention as antiparasitic agents. A plant extract containing uleine as a preventive medication for the treatment of infectious diseases, especially malaria has been patented [11]. Also, standardized extracts of A. ulei (cited A. parvifolium) containing uleine that exhibit potent antiplasmodial effects against P. falciparum [12] have also been patented. There is also experimental evidence that uleine´s pharmacological effects are due to action in the P. falciparum digestive vacuole [13]. Other indole alkaloids, aspidocarpine, ellipticine and olivacine isolated from the bark of A. desmanthum, A. vargasii and A. olivaceum, respectively, exhibited significant in vitro antiplasmodial activity against the K1 strain of P. falciparum [14,15]. Furthermore, ellipticine and olivacine exhibited low cytotoxicity and high in vivo antimalarial activity in Plasmodium berghei-infected mice at daily doses of 50-100 mg/kg/day in the 4-day suppressive test [15].
The aim of the present work was to perform a compositional study on the extracts of A. ulei and isolate indole alkaloids from this traditionally used antimalarial plant. Several isolated indole alkaloids were evaluated for in vitro antiplasmodial activity and cytotoxicity against fibroblasts as a means to discover new antiplasmodial compounds from this species.
2. Results and Discussion
2.1. Isolated Substances from A. ulei
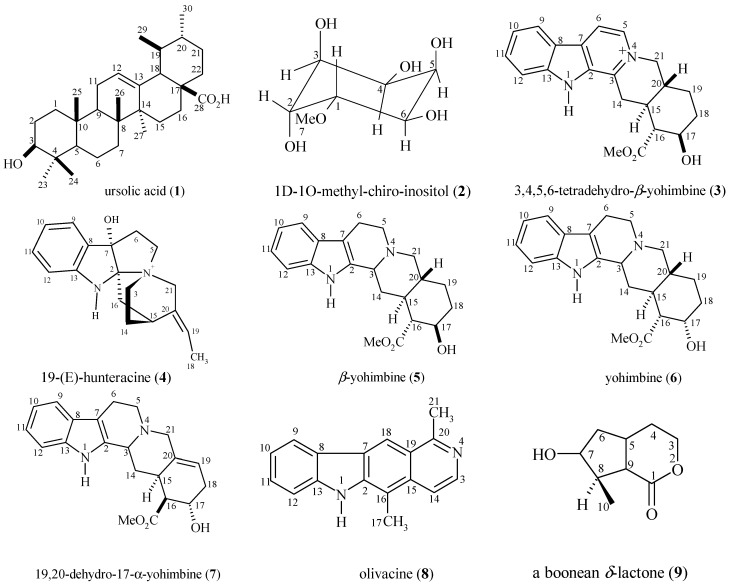
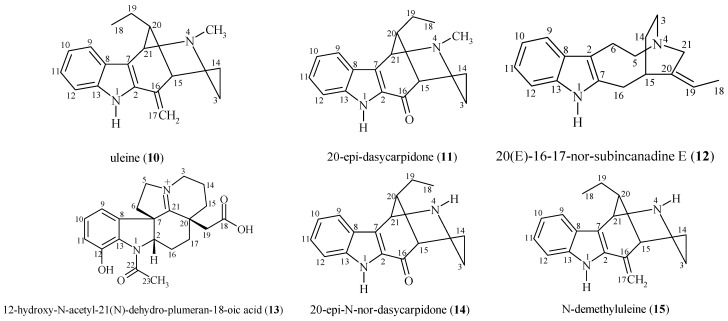
Phytochemical investigation of the leaf, bark, trunk wood, root wood and root bark EtOH extracts of Aspidosperma ulei led to the isolation and structural elucidation of several classes of compounds (Figure 1). The indole alkaloids β-yohimbine (5) [16], uleine (10) [8,9,16], olivacine (8) [17], N-demethyluleine (15) [16] and 20(E)-nor-subincanadine E (12) [16] were isolated in the present work and have been isolated previously from A. ulei.
Figure 1.
Structures of substances isolated from Aspidosperma ulei.
The following known compounds were isolated from A. ulei for the first time in the present study: indole alkaloids 3,4,5,6-tetradehydro-β-yohimbine (3), yohimbine (6), 19,20-dehydro-17α-yohimbine (7) and 20-epi-dasycarpidone (11), a triterpene, ursolic acid (1), an inositol derivative, methyl-chiro-inositol (2) and a boonein δ-lactone (6S-hydroxy-7R-methyl-(4aS,7aS)-hexahydrocyclopenta[c]pyran-1(3H)-one, 9). The alkaloids 19E-hunteracine (4) and 20-epi-N-nor-dasycarpidone (14) were isolated and have not been previously reported in a species of Aspidosperma. A new indole alkaloid, 12-hydroxy-N-acetyl-21(N)-dehydro-plumeran-18-oic acid (13), was isolated from the root wood of A. ulei (Figure 1).
2.2. Analysis of Spectral and Physical Data for Isolated Compounds
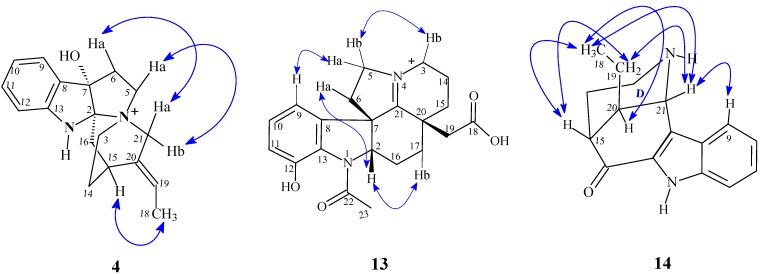
From the leaf EtOH extract (LEE) of A. ulei, ursolic acid (1), a white solid, m.p. 296.5–297.6 °C and = +26.0° (c. 0.33, MeOH) was isolated for the first time from this species [18,19]. Stem bark EtOH extracts (SBEE) exhibited a precipitate, methyl-chiro-inositol (2), an amorphous solid, m.p. 150.3–152.2 °C that could be identified based on comparison of its spectral data with that of the literature [20]. Based on acquired spectral data and comparison with data in the literature [21] one of the substances was identified as (+)-3,4,5,6-tetradehydro-β-yohimbine (3, 25.0 mg), a yellow solid, m.p. 260.0–264.0 °C and = +42.3° (c. 0.06, MeOH). In the accurate mass spectrum of this compound there is an H+ adduct signal ([M+H]+) at m/z 351.1748, that is compatible with the molecular formula C21H22N2O3 (theoretical [M+H]+ m/z 351.1709, Δ = 11 ppm). The indole alkaloid β-yohimbine (5, 11.0 and 5.6 mg, respectively) was isolated from SBEE and the root wood EtOH extract basic fraction (RWEEBF) as light yellow-colored needles, m.p. 191.0–192.0 °C, that are soluble in MeOH and CHCl3, = +12.6° (c. 0.03, MeOH). The MS exhibited a molecular ion adduct ([M+H]+) m/z 355.28 that is compatible with the molecular formula C21H26N2O3. This formula has four Hs more than the quaternary β-carboline 3. The indole alkaloid yohimbine (6, 19.0 and 9.8 mg, respectively) was isolated from SBEE and RWEEBF as an amorphous solid, m.p. 226.0–228.0 °C and = +57.8° (c 0.90, MeOH). Differences in chemical shifts and coupling constants were observed for H signals assigned to the stereogenic centers and confirmed by 1H and 13C-NMR and literature data [22], together with data for β-yohimbine (5) [22]. Alkaloids 5 and 6 were evaluated for in vitro antiplasmodial activity against the chloroquine-resistant Fc M29-Cameroon strain of Plasmodium falciparum and found to present IC50 values > 1 μg/mL [23]. Several alkaloids, including 6, were cited in a patent on new antimicrobial agents that included antimalarials [24]. The alkaloid 19,20-dehydro-17α-yohimbine (7, 4.0 mg) could be identified by comparison of its NMR data to literature data [25]. It was isolated as an amorphous solid, m.p. 143.2–144.4 °C, = +16.8° (c 0.06, MeOH) and exhibited HRMS with signal at m/z 353.1892 ([M+H]+), that is compatible with the molecular formula C21H24N2O3 (theoretical [M+H]+ m/z 353.1865; Δ = 8 ppm). The aspidospermatane-type indole alkaloid 19E-hunteracine (4, 5.0 mg) has not been previously isolated from a species of Aspidosperma. The IR spectrum of this compound exhibited intense partially overlapped bands centered at 2,924 and 2,853 cm−1 that are characteristic of the O-H and N-H stretching band and at 1,673 cm−1 a characteristic C=C stretching band. In the 1H-NMR spectrum, a signal at δH 5.50 (H-19) was assigned to a vinylic H of a trisubstituted double bond (exocyclic ethylidene group) that was coupled to the H-atoms of a CH3 group with signal at δH 1.74 (H-18). H-atoms with signals at δH 3.50 (H-5a and/or H-3b) correlated over two or three bonds with deshielded C-atoms with signals at δC 101.6 (C-2), 88.1 (C-7), 60.1 (C-21) and 43.2 (C-6) are consistent with the presence of a quaternary N-atom bonded to these carbons. Analysis of 2D 1H-NMR and 1H-NOESY NMR dipolar coupling of H-18 CH3 and H-15 (CH) allowed for assignment of the E-configuration to the ethylidene group (Figure 2). There are few 1H-NMR data available in the literature [26,27,28] for 19E-hunteracine which has previously been isolated from Hunteria eburnea Pichon (Apocynaceae).
Figure 2.
Correlations in the NOESY spectrum for indole alkaloids 4, 13 and 14 isolated from A. ulei.
Fractions 4–9 (331.0 mg) and 5–7 (135.0 mg) of the root bark EtOH extract (RBEE) after purification by HPLC furnished a boonein δ-lactone as a brown resin (9, 10.0 mg) [29] and the alkaloids uleine (10, 40.0 mg) and N-demethyluleine (15, 28.0 mg), m.p. 123.0–125.0 °C and 139.9–140.6 °C, respectively. The latter two compounds have been isolated previously from Aspidosperma ulei. The structural difference between demethyluleine and uleine is the absence of the N-CH3 in the former compound that, according to the literature [30,31,32] leads to differences of ca. 2 ppm in chemical shifts depending on the solvents used.
The alkaloids 20-epi-dasycarpidone (11, 26.0 mg) and 20-epi-N-nor-dasycarpidone (14, 13.0 mg), m.p. 164.3–165.3 and 220.3–221.4 °C, = −96.0° (c. 0.02, CHCl3) and +42.7° (c. 0.05, MeOH), respectively, exhibit some differences regarding the chemical shift of C-7 (located on the indole ring). According to literature sources [30,33,34], this carbon should be more deshielded (δc 124.4, 123.8), while in compounds 11 and 14 these carbons are more shielded (δc 113.5 and 116.2). Based on the 1H-NMR and 1H-NOESY spectra of 14 it was possible to assign the equatorial and axial Hs of the D ring of 14 (Figure 2) that exhibit important dipolar couplings between δH 5.22 (H-21) and 7.85 (H-9), δH 1.35 (H-19) and 0.97 (H-18), couplings at δH 2.87 (H-15), 1.35 (H-19) and δH 0.97 (H-18), besides coupling of δH 2.47 (H-20) and δH 0.97 (3H-18). These data corroborate the epimeric structure of the ethyl side chain in axial position in the piperidine ring. The NMR data obtained for uleine (10) and 20-epi-dasycarpidone (11) provided evidence for the difference of the normal series and epi series. In the piperidine ring of uleine the ethyl side chain is in the equatorial position and in 20-epi-uleine this side chain is in the axial position. In the spectra of 11, this difference is evidenced by a 1,3-diaxial γ-effect by the ethyl group on the axial H of C-14, resulting in steric compression of C-14, C-20 and to a lesser extent C-18 and C-19. These C-atoms are more shielded than in the normal series. Olivacine (8, 5,0 mg) was isolated from the root bark through precipitation of the root bark EtOH extract acidic fraction (RBEEAF) and exhibited 1H and 13C-NMR data consistent with those found in the literature [35].
20(E)-nor-subincanadine E (12, 36.0 mg) was isolated from the stem bark of A. ulei and its spectral data were similar to those found in the literature [36]. It has been reported as an intermediate in syntheses of Strychnos alkaloids [37,38].
The new indole alkaloid 12-hydroxy-N-acetyl-21(N)-dehydroplumeran-18-oic acid (13, 4.4 mg) was isolated as a resin from the root wood EtOH extract (RWEE) of A. ulei. The IR spectrum exhibited overlapped broad O-H and N-H stretching bands at 3,440 cm−1 and characteristic C=O bands of a conjugated acid and an amide, 1,683 and 1,631 cm−1, respectively. In the 1H and 13C spectra, only three aromatic H signals and three aromatic CH signals were observed. Through long-distance couplings evidenced in the HMBC spectrum it was concluded that the OH group was at the C-12 (δc 149.2) position thus confirming the monosubstitution of the aromatic ring. Analyses of the HMBC spectrum confirmed the presence of a quaternary N-atom and the C-atom of the iminium (C=N+) group (signal at δc 190.0, C-21) and long-range correlations of δH 4.43 (H-5a), δH 4.07 (H-3a), δH 3.93 (H-3b) and δH 2.29 (H-6b) and δC 190.0 (C-21).
The 1H,1H-NOESY spectrum of 13 evidenced dipolar couplings between protons at δH 4.68 (H-2) and δH 2.70 (H-6a) and permitted the assignment of the relative stereochemistry of H-2/H-6 as β. Also, evident from this spectrum were correlations of signals at δH 3.93 (H-3b) and δH 4.25 (H-5b), at δH 4.68 (H-2) and δH 1.81 (H-17b), and at δH 6.78 (H-9) and δH 4.43 (H-5a) in compound 13, as shown in Figure 2. A structural similarity search allowed for models to be obtained for comparison of data [39] together with 1H and 13C data from the literature [40].
2.3. In Vitro Inhibition of P. falciparum and Cytotoxicity
Compounds 1, 5, 6, 8, 10 and 15 have been evaluated for antiplasmodial activity in previous reports [4,11,14,23,24] and were not tested herein. Antiplasmodial tests were performed on indole alkaloids 3, 4, 11, 12 and 13 herein. Indole alkaloids 7 and 14 were not tested for lack of available material. The results of the evaluation of the inhibitory potential of compounds 3, 4, 11, 12 and 13 in vitro against P. falciparum are presented in Table 1. To our knowledge this is the first time that the antiplasmodial activity of these compounds was studied. Compounds 3, 4, 12 and 13 were inactive (IC50 ≥ 11 µg/mL. Compound 11 exhibited antiplasmodial activity [IC50 = 4.5 ± 0.2 µg/mL (16.7 μM)]. The cytotoxicity of 3, 4, 11, 12 and 13 was evaluated against NIH3T3 murine fibroblasts. None of these compounds inhibited the growth of fibroblasts (IC50 > 50 μg/mL).
Table 1.
Inhibition of the in vitro growth of P. falciparum K1 strain by isolated indole alkaloids.
| Nº | Name | IC50 ± SD | IC50 ± SD | Result |
| µg/mL | μM | |||
| 3 | 3,4,5,6-tetradehydro-β-yohimbine | 14.0 ± 2.7 | 39.9 ± 7.7 | I |
| 4 | 19E-hunteracine | > 50.0 | > 176 | I |
| 11 | 20-epi-dasycarpidone | 4.5 ± 0.2 | 16.7 ± 0.7 | MA |
| 12 | 20(E)-nor-subincanadine E | 14.5 ± 2.8 | 54.3 ± 10.5 | I |
| 13 | 12-hydroxy-N-acetyl-21(N)-dehydroplumeran-18-oic acid | > 50.0 | > 135 | I |
| DS | chloroquine diphosphate | 0.17 ± 0.1 | 0.33 ± 0.19 | A |
| DS | quinine sulphate | 0.12 ± 0.05 | 0.30 ± 0.15 | A |
SD = Standard deviation, DS = drug standard.. IC50 ≤ 0.1 µM = highly active (HA); 0.1 < IC50 < 5 µM = active (A); IC50 5–20 µM = moderately active (MA); IC50 > 20.0 µM = inactive (I).
3. Experimental
3.1. General Procedures
Melting points were determined on a Digital Microdetermination apparatus (Mettler Toledo) equipped with a FP82HT heating plate and FP90 processing unit. Determinations were performed at a heating velocity of 2 °C/min and were not corrected. IR spectra were acquired on a Perkin-Elmer Spectrum 100 FT-IR spectrometer using a Universal Attenuated Total Reflectance Accessory (UATR) in the range of 400 to 4,000 cm−1. HPLC analysis of calibration solutions and those of extracts and fractions of A. ulei was performed on a Waters modular chromatograph. This system was controlled by Empower software. The system consisted of a Waters-1525 binary pump and a photo diode array detector (PDA) model 2996. HPLC separations were performed on a Phenomenex RP-18 column (4.6 × 250 mm, 5 μm) and a Phenomenex RP-18 (10 × 250 mm, 10 μm). The samples were eluted with ACN, MeOH and a solution containing ultrapure H2O (Milli-Q, Millipore) and trifluoroacetic acid (TFA, 0.1–0.3%). High-resolution mass spectra (ESI-HRMS) were obtained by dissolving samples in suitable solvents and infusing the resulting solutions directly into the electrospray ionizer of a Shimadzu LCMS-IT-TOF (225-07100-34) mass spectrometer. 1D and 2D 1H and 13C-NMR spectra such as COSY, HSQC, HMBC and NOESY were obtained on a Bruker Avance DRX500 instrument.
3.2. Collection, Botanic Identification and Processing of Plant Materials
Aspidosperma ulei is commonly known as pitiá or piquiá. It was collected in Garapa in the City of Acarape in Ceará State, Brazil. Voucher specimens (registry numbers 30823, 32630 and 34813) were deposited in the Prisco Bezerra Herbarium of the University of Ceará. Botanic identification was performed by Prof. Edson P. Nunes of the Department of Biology of the Federal University of Ceará, Fortaleza, Ceará. Leaves, stem bark, heartwood, root bark and root wood were separately dried and milled. Powdered plant materials were weighed and then extracted as described below.
3.3. Preparation of Extracts of A. ulei and Isolation Procedures
Extraction of dry, powdered plant materials was carried out by maceration in EtOH at r.t. for 72 h. The mass of each plant material was extracted a total of three times (3 × 10 L). The EtOH solutions obtained from each extraction were rotary evaporated under reduced pressure and combined to provide each extract (Table 2).
Table 2.
Data for Aspidosperma ulei EtOH extract preparation by maceration and evaporation.
| Dry, powdered plant material | Dry plant extract | |||||
|---|---|---|---|---|---|---|
| Part | Mass extracted (kg) | Name | Yield (g) | % Yield | Description | |
| Heartwood | 3.0 | HWEE | 52 | 1.7 | Yellow powder | |
| Leaf | 1.0 | LEE | 98 | 9.8 | Green powder | |
| Root bark | 3.0 | RBEE | 274 | 9.1 | Viscous residue | |
| Root wood | 3.0 | RWEE | 122 | 4.1 | Viscous residue | |
| Stem bark | 2.0 | SBEE | 173 | 8.7 | Viscous residue | |
HWEE: heartwood EtOH extract, LEE: leaf EtOH extract, RBEE: root bark EtOH extract, RWEE: root wood EtOH extract, SBEE: stem bark EtOH extract.
3.3.1. Isolation of Chemical Components from Leaf Extracts
LEE (10 g) was continuously extracted with Hex, followed by DCM, EtOAc and MeOH providing four fractions after evaporation of solvents. The EtOAc fraction (1.5 g), after normal phase CC (🛇 = 2.5 cm, 8 g of silica gel) using a gradient of increasing polarity of MeOH in CHCl3 yielded 37 fractions (10 mL each) of which fraction 7 (59.3 mg) was a finely divided white solid, soluble in CHCl3 and MeOH. Spectrometric analysis of NMR, MS and other data revealed this compound to be the pentacyclic triterpene ursolic acid (1) reported herein for the first time for this species.
3.3.2. Isolation of Chemical Components from Stem Bark Extracts
SBEE (50 g) was completely dissolved in distilled H2O (150 mL) using an ultrasound bath (20 min). Then, DCM (150 mL) was added to yield a 2-phase system. MeOH (100 mL) was added to the H2O phase and this mixture was refrigerated for 24 h and yielded a white precipitate (3.35 g) after decantation, that was determined through spectrometric analysis to be the H2O soly methyl-chiro-inositol (2), m.p. 150.3–152.2 °C. CC on the evaporated mother liquor using a gradient of MeOH (5, 10, then 100%) in DCM as eluents yielded 17 fractions (50 mL each). Fractions were combined based on TLC and further purified by preparative HPLC using ACN and 0.2% aq. TFA (70:30), resulting in the isolation of the alkaloid 3,4,5,6-tetradehydro-β-yohimbine (3, 25.0 mg).
SBEE (1 g) was dissolved in MeOH and adsorbed onto silica gel (0.5 g) by total evaporation of solvent. The resulting dry silica-sample mixture was fractionated by CC (5.0 g of silica gel, 🛇 = 2,0 cm) by sequential elution with Hex, DCM, EtOAc and MeOH (100 mL of each solvent) followed by total evaporation of fractions. The MeOH fraction (776 mg) was chromatographed on Sephadex LH-20. MeOH was used as eluent. Alkaloids were detected by TLC stained with dragendorff reagent. After sequential chromatographies and purification by semi-preparative reverse-phase HPLC (4.6 × 250 mm, 5 μm) using elution with 0.1% aq. TFA and MeOH (55:45), flow 4.72 mL/min, run time 10 min, detection wavelength 254 nm. 4 fractions were collected that contained, respectively, the alkaloids hunteracine (4, 5.0 mg), β-yohimbine (5, 11.0 mg), yohimbine (6, 19.0 mg) and 19,20-dehydro-17α-yohimbine (7, 4.0 mg).
3.3.3. Acid-Base Fractionation of EtOH Extracts
Heartwood EtOH extract (HWEE), RWEE and RBEE (20 g of each) were separately dissolved in 2M HCl (200 mL) with stirring (30 min). Each resulting solution was extracted with DCM (3 × 300 mL). The combined organic phases were dried over anhydrous Na2SO4, evaporated to dryness and gave rise to the acidic alkaloid fractions of the heartwood, root wood and root bark EtOH extracts (HWEEAF (255 mg), RWEEAF (287 mg) and RBEEAF (384 mg), respectively). Conc. NH4OH was added dropwise to each acid fraction until each was pH 9 (Merck 0-14 Indicator Paper). Each fraction was then extracted with DCM (3 × 200 mL). The organic layers were combined, dried over anhydrous Na2SO4, filtered and totally evaporated to yield basic alkaloid fractions of the heartwood, root wood and root bark EtOH extracts (HWEEBF (363 mg), RWEEBF (302 mg) and RBEEBF (792 mg), respectively).
3.3.3.1. Isolation of Chemical Components from Acidic Fractions
RBEEAF was subjected to normal-phase CC (10 g silica gel, 🛇 = 2.5 cm) using a gradient of increasing polarity of MeOH in DCM as eluents and resulting in 12 chromatographic fractions. Chromatographic fractions 4–9 (331 mg) were combined. The alkaloid olivacine (8, 5.0 mg) was obtained by precipitation from the combined fraction. The combined fraction was further separated by HPLC using a reverse-phase, semi-preparative column (10.0 × 250 mm, 5 μm) that was eluted using 0.1% aq. TFA and MeOH (45:55). The run time was 15 min at a flow rate of 4.5 mL/min. Six fractions were collected using a detector wavelength of 323 nm. This procedure yielded a boonein lactone (9, 10.0 mg) and the alkaloids uleine (10, 40.0 mg) and 20-epi-dasycarpidone (11, 26.0 mg).
The fraction HWEEAF was separated by reverse-phase, semi-preparative HPLC (4.6 × 250 mm, 5 μm) using 0.1% aq. TFA and MeOH (70:30) at a flow rate of 3.0 mL/min, a total run time of 20 min and detector running at a wavelength of 300 nm. Four fractions were collected and fraction 4 (43.0 mg) was sufficiently pure for full spectrometric characterization by 1D and 2D 1H and 13C-NMR techniques and its structure proved to be that of an indole alkaloid, 20(E)-nor-subincanadine E (12), derived from the stemmadenine skeleton.
3.3.3.2. Isolation of Chemical Components from Basic Fractions
RWEEBF was separated by CC (10 g of silica gel, 🛇 = 2.5 cm) using a gradient elution of increasing polarity of MeOH and DCM. 12 fractions were obtained. The combined fraction RWEEBF5-8 (58.0 mg) was separated by semi-preparative, reverse-phase HPLC (10.0 × 250 mm, 5 μm) with elution using 0.1% aq. TFA and MeOH (60:40) at a flow rate of 4.0 mL/min, a run time of 15 min and the detector set at 254 nm. Six fractions were collected that contained the indole alkaloids β-yohimbine (5, 5.6 mg) and yohimbine (6, 9.8 mg) and the new compound 12-hydroxy-N-acetyl-21(N)-dehydro-plumeran-18-oic acid (13, 4.4 mg).
RBEEBF was dissolved in MeOH and adsorbed on 0.5 g of silica gel by pulverization with a mortar and pestle and total evaporation of solvent. The silica-sample mixture was fractionated by CC (2.5 g of silica gel, 🛇 = 2.5 cm) using elution with these solvents: DCM (100%) and then 1, 6 and 100% MeOH in DCM. 10 fractions (25 mL each) resulted. Combined fraction RBEEBF5-7 (135.0 mg) was re-chromatographed using semi-preparative, reverse-phase HPLC (4.6 × 250 mm, 5 μm), elution with 0.1% aq. TFA and MeOH (55:45), and detector operating at 254 nm. The alkaloids 20-epi-N-nor-dasycarpidone (14, 13.0 mg) and N-demethyluleine (15, 28.0 mg) were obtained from this procedure.
3.4. Spectrometric Data for Isolated Compounds
(+)-3,4,5,6-Tetradehydro-β-yohimbine (3). Yellow solid, m.p. 260.0–264.0 °C, = + 42.3° (c. 0.06, MeOH); IR (MeOH) υ max 3344, 3060, 1733, 1637, 1321, 1278, 1166 cm−1; 1H-NMR (CD3OD, 500 MHz): δ 8.36 (bs, H-6), 8.25 (d, 6.5 Hz, H-9), 8.24 (d, 6.7 Hz, H-5), 7.71 (dd, 6.5 and 7.0 Hz, H-11), 7.66 (d, 8.0 Hz, H-12), 7.38 (dd, 6.5 and 7.0 Hz, H-10), 4.79 (d, 12.0 Hz, H-21b), 4.32 (t, 12.0 Hz, H-21a), 3.84 (s, 3H, OCH3), 3.82 (m, H-17), 3.55 (dd, 3.8 and 18.0 Hz, H-14b), 3.18 (dd, 10.5 and 18.0 Hz, H-14a), 2.35 (t, 10.5 Hz, H-16), 2.21 (m, H-15), 1.54 (m, H-18a), 2.14 (m, H-18b), 2.12 (m, H-20), 2.03 (m, H-19b), 1.40 (m, H-19a). 13C-NMR (CD3OD, 125 MHz): δ 175.5 (C=O, C-22), 145.4 (C, C-13), 140.5 (C, C-3), 135.5 (C, C-2), 134.0 (CH, C-5), 132.9 (CH, C-11), 132.6 (C, C-7), 124.0 (CH, C-9), 123.2 (CH, C-10), 121.4 (C, C-8), 116.9 (CH, C-6), 113.9 (CH, C-12), 72.3 (CH, C-17), 60.9 (CH2, C-21), 58.3 (CH, C-16), 52.7 (OMe, C-23), 36.9 (CH, C-15), 36.5 (CH, C-20), 34.4 (CH2, C-18), 31.1 (CH2, C-14), 28.2 (CH2, C-19).
19(E)-Hunteracine (4). Yellow solid, m.p. 343.0–343.3 °C; −26.6°, (c. 0.06, MeOH); IV (KBr pellet) υ max 3249, 2924, 1268, 1134, 1673, 1470, 800, 720 cm−1. 1H-NMR (MeOH, 500 MHz): δH 7.30 (d, 7.0 Hz, H-9), 7.20 (t, 7.7 Hz, H-11), 6.92 (t, 7.7 Hz, H-10), 6.75 (d, 7.7 Hz, H-12), 5.50 (q, 9.0 Hz, H-19), 4.55 (dd, 2.4, 2.7 Hz, H-21α), 3.95 (d, 14.5 Hz, H-21β), 3.75 (t, 10.5 Hz. H-3α), 3.50 (m, H-5α), 3.37 (m, H-5β), 3.37 (m, H-15), 3.0 (m, H-3β), 2.71 (d, 14.0 Hz, H-16α), 2.63 (m, H-6α), 2.50 (m, H-6β), 2.42 (m, H-14α), 2.07 (dd, 4.8 Hz, H-16β), 1.98 (m, H-14β), 1.74 (d, 6.7 Hz, H-18). 13C-NMR (MeOH, 125 MHz): δc 147.3 (C, C-13), 133.2 (C, C-8), 131.7 (C, C-20), 131.2 (CH, C-11), 123.9 (CH, C-9), 122.4 (CH, C-10), 119.8 (CH, C-19), 112.2 (CH, C-12), 101.5 (C, C-2), 88.3 (C, C-7), 60.1 (CH2, C-21), 57.6 (CH2, C-5), 53.7 (CH2, C-3), 43.2 (CH2, C-6), 34.5 (CH2, C-16), 28.0 (CH, C-15), 24.5 (CH2, C-14), 12.9 (CH3, C-18). ESI-HRMS found: m/z 283.1800 [M+H]+ (C18H23N2O requires m/z 283.1810 [M+H]+, Δ = 4 ppm).
(+)-β-Yohimbine (5). Colorless crystals, m.p. 191.0-192.0 °C; = +12.6° (c. 0.03, MeOH); IR (MeOH) υmax 3419, 1726, 1325, 1271, 1060, 742 cm−1; 1H-NMR (CDCl3+CD3OD, 500 MHz) δ 7.39 (d, 7.8 Hz, H-9), 6.95 (dt, 8.0 Hz, H-10), 7.05 (dt, 8.0 Hz, H-11), 7.28 (d, 8.0 Hz, H-12), 2.90 (m, H-6a), 2.74 (ddd, 3.2 and 1.6 Hz, H-6b), 3.12 (dd, 5.1 and 11.5 Hz, H-5a), 2.63 (dt, 4.6 and 11.5 Hz, H-5b), 3.35 (bd, 11.0 Hz, H-3), 2.18 (m, H-14a), 1.35 (m, H-14b), 1.55 (dt, 3.0 and 11.0 Hz, H-15), 2.15 (m, H-16), 3.81 (s, 3H, OCH3), 3.78 (m, H-17), 2.05 (ddd, 3.0, 7.0 and 12.0 Hz, H-18a), 1.40 (m, H-18b), 1.70 (ddd, 3.0, 7.0 and 12.0 Hz. H-19a), 1.20 (m, H-19b), 1.50 (m, H-20), 2.97 (dd, 3.0 and 11.0 Hz, H-21a), 2.20 (q, 11.0 Hz, H-21b).13C-NMR (CDCl3+CD3OD, 125 MHz): δ 177.0 (C=O, C-22), 52.4 (OMe, C-23), 118.8 (CH, C-9), 120.0 (CH. C-10), 122.2 (CH, C-11), 112.2 (CH, C-12), 128.4 (C, C-8), 138.3 (C, C-13), 108.0 (C, C-7), 135.1 (C, C-2), 22.4 (CH2, C-6), 54.3 (CH2, C-5), 61.5 (CH, C-3), 34.5 (CH2, C-14), 43.6 (CH, C-15), 58.9 (CH, C-16), 73.1 (CH, C-17), 35.2 (CH2, C-18), 29.2 (CH2, C-19), 40.7 (CH, C-20), 61.9 (CH2, C-21).
Yohimbine (6). Amorphous solid, m.p. 226.0–228.0 °C; IR (MeOH) υ max 3404, 3228, 1671, 1370, 1296, 1200, 1124, 739, 719 cm−1; 1H-NMR (CD3OD, 500 MHz): δ 7.49 (d, 8.0 Hz, H-9), 7.38 (d, 8.0 Hz, H-12), 7.17 (t, 7.5 Hz, H-11), 7.07 (t, 7.5 Hz, H-10), 4.60 (d, 11.4 Hz, H-3), 4.33 (s, H-17), 3.82 (s, 3H, OCH3), 3.76 (m, H-5a), 3.50 (m, H-5b), 3.50 (m, H-21a), 3.24 (m, H-6a), 3.08 (d, 11.9 Hz, H-6b), 3.08 (d, 11.9 Hz, H-21b), 2.85 (d, 13.5 Hz, H-14a), 2.46 (d, 1.7 Hz, H-16), 2.27 (m, H-15), 2.00 (d, 2.0 Hz, H-18a), 1.74 (m, H-20), 1.74, (m, H-18b), 1.64 (m, H-19a), 1.59 (m, H-14b), 1.59 (m, H-19b). 13C- NMR (CD3OD, 125 MHz): δ 174.7 (C=O, C-22), 138.7 (C, C-13), 130.1 (C, C-2), 127.5 (C, C-8), 123.6 (CH, C-11), 120.8 (CH, C-10), 119.2 (CH, C-9), 112.7 (CH, C-12), 107.0 (C, C-7), 68.3 (CH, C-17), 62.7 (CH, C-3), 59.6 (CH2, C-21), 53.8 (CH2, C-5), 53.0 (CH, C-16), 52.5 (OMe, C-23), 39.3 (CH, C-20), 35.8 (CH, C-15), 33.2 (CH2, C-18), 33.1 (CH2, C-14), 23.6 (CH2, C-19), 20.4 (CH2, C-6).
19,20-Dehydro-17α-yohimbine (7). Amorphous solid, m.p. 143.2–144.4 °C; IR (UATR) υ max 3228, 2924, 2854, 1726, 1673, 1455, 1199, 749 cm−1, 1H-NMR (CD3OD, 500 MHz): δH 7.47 (d, 7.8 Hz, H-9), 7.35 (d, 7.8 Hz, H-12), 7.15 (t, 7.4 Hz, H-11), 7.06 (t. 7.4 Hz, H-10), 5.88 (s, H-19), 4.45 (s, H-17), 4.07 (m, H-21a), 3.98 (m, H-21b), 3.81 (s, 3H, OCH3), 3.23 (m, H-6a), 3.16 (m, H-15), 3.07 (m, H-6b), 3.07 (m, H-14a), 2.29 (m, H-18b), 2.52 (m, H-16), 2.52 (m, H-18a), 1.52 (m, H-14b . 13C-NMR (CD3OD, 125 MHz): δC 174.7 (C=O, C-22), 138.0 (C, C-13), 127.4 (C, C-8), 126.8 (CH, C-19), 123.8 (CH, C-11), 120.9 (CH, C-10), 119.3 (CH, C-9), 112.7 (CH, C-12), 106.8 (C, C-7), 66.7 (CH, C-17), 60.1 (CH2, C-21), 52.8 (OMe, C-23), 51.4 (CH, C-16), 34.9 (CH2, C-14), 34.6 (CH2, C-18), 32.9 (CH, C-15), 20.3 (CH2, C-6). ESI-HRMS found m/z 353.1892 [M+H]+ (C21H24N2O3 requires m/z 353.1865 [M+H]+, Δ = 8 ppm).
Olivacine (8). Yellow solid, m.p. 314.8–315.2 °C; IR (UATR) υmax 3082, 2918, 2851, 1599, 1467, 1409, 1339, 1243, 866, 740 cm−1. 1H-NMR (MeOH, 500 MHz): δH 8.84 (s, H-18), 8.26 (d, 7.4 Hz, H-9), 8.15 (d, 6.3 Hz, H-3), 7.90 (d, 6.3 Hz, H-14), 7.52 (m, H-11/H-12), 7.26 (m, H-10), 3.07 (s, H-21), 2.81 (s, H-17). 13C-NMR (MeOH, 125 MHz): δc 160.1 (C, C-20), 144.4 (C, C-13), 141.5 (C, C-2), 137.3 (CH, C-3), 134.6 (C, C-15), 129.3 (CH, C-11), 127.7 (C, C-7), 124.4 (C, C-19), 123.4 (C, C-8), 122.4 (CH, C-9), 120.9 (CH, C-10), 117.2 (CH, C-14), 116.6 (CH, C-18), 112.8 (C, C-16), 112.1(CH, C-12), 21.9 (CH3, C-21), 12.6 (CH3, C-17).
Uleine (10). Amorphous solid, m.p. 123.0–125.0 °C; = + 9.0° (c. 0.33, CDCl3); IR (KBr pellet) υmax 3386, 2926, 1669, 1459, 1198, 1130, 745 cm−1. 1H-NMR (CDCl3, 500 MHz): δH 8.39 (s, N-1), 7.56 (d, 10.0 Hz, H-9), 7.35 (d, 5.0 Hz, H-12), 7.19 (dd, 5.0 and 10.0 Hz, H-11), 7.11 (dd, 5.0 and 10.0 Hz, H-10), 5.28 (s, H-17a), 5.00 (s, H-17b), 4.10 (d, 2.0 Hz, H-21), 2.70 (m, H-15), 2.49 (m, H-3a), 2.30 (s, H-5), 2.08 (m, H-14a, H-3b, H-20), 1.69 (m, H-14b), 1.12 (q, 7.0 Hz, H-19), 0.86 (t, 7.0 Hz, 3H). 13C-NMR (CDCl3, 125 MHz): δc 138.9 (C, C-16), 136.8 (C, C-13), 135.4 (C, C-2), 129.6 (C, C-8), 122.9 (CH, C-11), 120.1 (CH, C-10), 119.7 (CH, C-9), 110.9 (CH, C-12), 107.9 (C, C-7), 107.0 (CH2, C-17), 56.8 (CH, C-21), 46.5 (CH2, C-3), 46.3 (CH, C-20), 44.5 (CH3, C-5), 39.7 (CH, C-15), 34.9 (CH2, C-14), 24.6 (CH2, C-19), 12.0 (CH3, C-18). ESI-HRMS m/z 267.1877 [M+H]+ (C18H22N2 requires m/z 267.1861 [M+H]+, Δ = 6 ppm).
(−)-20-Epi-dasycarpidone (11). Amorphous solid, m.p. 164.3–165.3 °C; = −96.0° (c. 0.02, CDCl3); IV (MeOH) υmax 3383, 3234, 1664, 1466, 1330, 1181, 799, 750, 721 cm−1; 1H-NMR (CDCl3, 500 MHz): δH 9.88 (s, N-H), 7.74 (d, 8.0 Hz, H-9), 7.60 (d, 8.0 Hz, H-12), 7.51 (t, 7.0 Hz, H-11), 7.39 (t, 7.0 Hz, H-10), 5.09 (s, H-21), 3.37 (d, 8.7 Hz, H-3β), 2.88 (m, H-3α), 2.86 (m, H-15), 2.80 (s, N-CH3), 2.72 (m, H-20), 2.55 (m, H-14β), 2.06 (d, 15.0 Hz, H-14α), 1.36 (m, H-19), 0.91 (t, 7.3 Hz, H-18). 13C-NMR (CDCl3, 125 MHz): δc 190.9 (C=O, C-16), 138.2 (C, C-13), 134.0 (C, C-2), 128.4 (CH, C-11), 126.7 (C, C-8), 123.9 (CH, C-10), 120.7 (CH, C-9), 113.8 (CH, C-12), 113.5 (C, C-7), 58.1 (CH, C-21), 54.3 (CH3, C-5), 46.5 (CH2, C-3), 46.4 (CH, C-20), 44.2 (CH, C-15), 42.1 (N-CH3, C-5), 26.6 (CH2, C-14), 24.7 (CH2, C-19), 11.3 (CH3, C-18) ESI-HRMS found m/z 269.1669 [M+H]+ (C17H21N2O requires m/z 269.1654 [M+H]+, Δ = 6 ppm).
20(E)-17-nor-subincanadine E (12). Dark solid, m.p. 230.1–231.2 °C; = +76.6° (c. 0.03, MeOH); IV (KBr pellet) υ max 3400, 1677, 1461, 1203, 1132, 800, 721 cm −1. 1H-NMR (MeOH, 500 MHz): δH 12.08 (s, N-H), 7.63 (d, 8.0 Hz, H-12), 7.54 (d, 8.0 Hz, H-9), 7.29 (dd, 1.0, 8.0 Hz, H-11), 7.25 (dd, 1.0, 8.0 Hz. H-10), 5.58 (d, 7.0 Hz, H-19), 4.21 (d, 15.0 Hz, H-21α), 3.78 (d, 15.0 Hz, H-21β), 3.68 (dt, 3.0, 14.0 Hz, H-5α), 3.55 (ddd, 3.0, 16.0 Hz, H-6α), 3.44 (t, 13.0 Hz, H-5β), 3.25 (m, H-16α/β), 3.23 (dd, 6.0, 9.0 Hz, H-3α), 3.17 (m, H-15), 3.13 (m, H-6β), 2.33 (m, H-3β), 1.91 (hept, 6.0 Hz, H-14α), 1.54 (d, 6.0 Hz, H-18), 1.52 (m, H-14β). 13C-NMR (MeOH, 125 MHz): δC 136.6 (C, C-13), 136.3 (C, C-2), 135.1 (C, C-20), 128.2 (C, C-8), 124.5 (CH, C-19), 121.8 (CH, C-11), 120.0 (CH, C-10), 118.1 (CH, C-9), 111.9 (CH, C-12), 107.8 (C, C-7), 57.5 (CH2, C-5), 52.6 (CH2, C-21), 44.8 (CH2, C-3), 32.7 (CH2, C-16), 30.9 (CH, C-15), 23.1 (CH2, C-14), 20.7 (CH2, C-6), 13.7 (CH3, C-18). ESI-HRMS found m/z 267.1900 [M+H]+ (C18H22N2 requires m/z 267.1861 [M+H]+, Δ = 15 ppm).
12-Hydroxy-N-acetyl-21(N)-dehydroplumeran-18-oic acid (13). Resin, = −16.4° (c. 0.05, MeOH); IV (pellet, KBr) υ max 3440, 2943, 1683, 1631, 1475, 1201, 802 cm−1. 1H-NMR (MeOH, 500 MHz): δH 7.13 (d, 7.9 Hz, H-10), 6.94 (d, 7.9 Hz, H-11), 6.78 (d, 7.9 Hz, H-9), 4.68 (m, H-2), 4.43 (m, H-5α), 4.25 (m, H-5β), 4.07 (m, H-3α), 3.93 (m, H-3β), 2.70 (q, 10.0 Hz, H-6α), 2.57 (d, 16.0 Hz, H-19α), 2.42 (s, CH3CO-N, H-2’), 2.40 (m, H-19β), 2.34 (m, H-16α), 2.29 (m, H-15α), 2.29 (m, H-6β), 2.27 (m, H-14α), 2.19 (m, H-17α), 2.11 (m, H-14β), 1.83 (m, H-16β), 1.81 (m, H-17β), 1.62 (m, H-15β). 13C-NMR (MeOH, 125 MHz): δC 190.0 (C, C-21), 173.5 (C, C-18), 171.9 (C, C-22), 149.2 (C, C-12), 137.0 (C, C-8), 129.7 (CH, C-10), 127.5 (C, C-13), 121.1 (CH, C-11), 115.5 (CH, C-9), 73.2 (CH, C-2), 63.4 (C, C-7), 59.4 (CH2, C-5), 51.1 (CH2, C-3), 41.7 (C, C-20), 40.9 (CH2, C-19), 37.6 (CH2, C-6), 33.1 (CH2, C-17), 31.1 (CH2, C-15), 25.6 (CH2, C-16), 22.9 (CH3, C-23), 19.3 (CH2, C-14). ESI-HRMS found m/z 369.1832 [M+H]+ (C21H25N2O4 requires m/z 369.1814 [M+H]+, Δ = 5 ppm).
(+)-20-Epi-N-nor-dasycarpidone (14). Amorphous solid, m.p. 220.3–221.4 °C; = +42.7° (c. 0.05, MeOH); IV (pellet, UATR) υmax 3,260, 2,922, 2,852, 1,646, 1,464, 747 cm−1; 1H-NMR (MeOH, 300 MHz): δH 7.85 (d, 8.0 Hz, H-9), 7.55 (d, 7.0 Hz, H-12), 7.44 (t, 7.0 Hz, H-11), 7.26 (t, 8.0 Hz, H-10), 5.22 (s, H-21), 3.17 (m, H-3β), 2.92 (m, H-3β), 2.87 (s, H-15), 2.47 (t, H-20), 2.26 (m, H-14β), 2.05 (bd, 14.5 Hz, H-14α), 1.35 (m, H-19), 0.97 (t, 7.0 Hz, H-18). 13C-NMR (MeOH3, 75 MHz): δc 191.7 (C=O, C-16), 140.4 (C, C-13), 135.5 (C, C-2), 128.6 (CH, C-11), 126.4 (C, C-8), 122.9 (CH, C-10), 122.0 (CH, C-9), 116.2 (C, C-7), 114.5 (CH, C-12), 50.3 (CH, C-21), 47.3 (CH, C-20), 46.6 (CH, C-15), 37.4 (CH2, C-3), 26.6 (CH2, C-14), 25.6 (CH2, C-19), 11.6 (CH3, C-18). ESI-HRMS found m/z 255.1527 [M+H]+ (C16H18N2O requires m/z 255.1497 [M+H]+, Δ = 12 ppm).
N-demethyluleine (15). Amorphous solid, m.p. 139.9–140.6 °C; = +35.8° (c. 0.05, MeOH); IR (UATR) υmax 3200, 2922, 2853, 1632, 1452, 1321, 737 cm−1. 1H-NMR (MeOH, 500 MHz): δH 7.53 (d, 7.0 Hz, H-9), 7.34 (d, 7.0 Hz, H-12), 7.11 (t, 7.0 Hz, H-11), 7.00 (t, 7.0 Hz, H-10), 5.53 (s, H-17b), 5.01 (s, H-17a), 4.36 (d, 2.0 Hz, H-21), 2.76 (sl, H-15), 2.62 (m, 2H-3), 1.99 (m, H-14b, H-20), 1.68 (m, H-14a), 1.14 (m, 2H-19), 0.89 (t, 7.7 Hz, 3H-18). 13C-NMR (MeOH, 125 MHz): δc 140.0 (C, C-13), 139.2 (C, C-2), 137.2 (C, C-16), 128.0 (C, C-8), 123.7 (CH, C-11), 120.4 (CH, C-10), 119.5 (CH, C-9), 112.2 (CH, C-12), 110.2 (C, C-7), 108.7 (CH2, C-17), 50.6 (CH, C-21), 46.7 (CH, C-20), 41.8 (CH, C-15), 37.9 (CH2, C-3), 35.7 (CH2, C-14), 25.7 (CH2, C-19), 12.1 (CH3, C-18). ESI-HRMS found m/z 253.1709 [M+H]+ (C17H20N2 requires m/z 253.1705 [M+H]+, Δ = 2 ppm).
3.5. Biological activity of isolated compounds from Aspidosperma ulei
3.5.1. In Vitro Culture of Plasmodium Falciparum and in Vitro Antiplasmodial Assay
The multi-drug resistant K1 strains of P. falciparum (Thailand, MRA-159, MR4-ATCC) were maintained in continuous culture [41]. The in vitro antiplasmodial test was performed as previously described [14]. Briefly the substances were diluted in DMSO to a stock concentration of 5 mg/mL and subsequently diluted in complete culture medium to obtain sample solutions having concentrations in the range 100-0.14 µg/mL. Sample solutions were applied to the wells of 96-well test plates containing red blood cell suspension having initial parasitemia of 1.5%. Each sample concentration was tested in triplicate and each test plate was incubated for 48 h at 37 °C. After incubation, the contents of the wells were evaluated by optical microscopy. The inhibition of the growth of parasites (IGP%) was evaluated as a percentage by comparison with controls:
| IGP% = 100 × [1 – (parasitemia with sample/parasitemia of untreated controls)] |
3.5.2. Cell Culture and Cytotoxicity Test Using the Alamar BlueTM Assay
The NHI-3T3 cell line of mouse fibroblasts was grown in DMEN medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 µg/mL streptomycin and 100 U/mL penicillin, and incubated at 37 °C with a 5% atmosphere of CO2. For assays, the cells were plated in 96-well plates (104 cells per well) and the Alamar BlueTM assay was performed using previously described procedures [42,43]. Briefly, after 24 h, the compounds were dissolved in DMSO and added to each well to give final concentrations of 50 µg/mL. Plates were incubated for 48 h. Control groups had final well concentrations of 0.1% DMSO. Two hours before the end of the incubations, 10 µL of Alamar BlueTM was added to each well. The fluorescent signal was monitored with a multiplate reader using 530–560 nm excitation and 590 nm emission wavelengths.
4. Conclusions
This work represents a significant contribution to the knowledge of the chemical composition of A. ulei. This included the structural elucidation of a new indole alkaloid, identification of two indole alkaloids not previously reported in Aspidosperma spp. and identification of seven known compounds for the first time in A. ulei. Isolated indole alkaloid 20-epi-dasycarpidone (11) was shown to exhibit moderate inhibitory activity against the K1 strain of P. falciparum. Furthermore, the presence of highly active antimalarial indole alkaloids olivacine and uleine in A. ulei extracts was confirmed in the present study as was the absence of in vitro cytotoxicity of several isolated compounds. Taken together, these results lend further support to earlier reports regarding the antimalarial potential of botanicals prepared from A. ulei and isolated antiplasmodial and antimalarial components.
Acknowledgments
This research was financed through grants from the Brazilian National Council for Scientific Development and Technology (CNPq, National Malaria Network, Bionorth Network), the Amazonas State Foundation for the Advancement of Research (NOSSAPLAM Project, FAPEAM/ PRONEX). A.M.P. would like to recognize the PQ bursary received from CNPq.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: In general, samples of the compounds isolated herein are unavailable from the authors due to their isolation on a small scale. They are readily isolated using the procedures described.
References
- 1.World Malaria Report. World Health Organization; Geneva, Switzerland: 2011. [Google Scholar]
- 2.Willcox M.L., Bodeker G. Clinical Review. Traditional herbal medicines for malaria. Med. J. 2004;329:1156–1159. doi: 10.1136/bmj.329.7475.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milliken W. Plants for Malaria. Plants for Fever. The Royal Botanic Gardens; Kew, UK: 1997. [Google Scholar]
- 4.Dolabela M.F., Oliveira S.G., Peres J.M., Nascimento J.M.S., Póvoa M.M., Oliveira A.B. In vitro antimalarial activity of six Aspidosperma species from the state of Minas Gerais (Brazil) An. Acad. Bras. Ciên. 2012;84:899–910. doi: 10.1590/S0001-37652012000400005. [DOI] [PubMed] [Google Scholar]
- 5.Pereira M.M., Jácome L.R.P., Alcântara A.F.C., Alves R.B., Raslan D.S. Alcalóides indólicos isolados de espécies do gênero Aspidosperma (Apocynaceae) Quím. Nova. 2007;30:970–983. [Google Scholar]
- 6.Oliveira V.B., Freitas M.S.M., Mathias L., Braz-Filho R., Vieira I.J.C. Atividade biológica e alcalóides indólicos do gênero Aspidosperma (Apocynaceae): uma revisão. Rev. Bras. Plantas Med. 2009;11:92–99. doi: 10.1590/S1516-05722009000100015. [DOI] [Google Scholar]
- 7.Henrique M.C., Nunomura S.M., Pohlit A.M. Alcalóides indólicos das cascas de Aspidosperma vargasii e A. desmanthum. Quím. Nova. 2010;33:2284–2287. [Google Scholar]
- 8.Büchi G., Warnhoff E.W. The structure of uleine. J. Am. Chem. Soc. 1959;81:4433–4434. [Google Scholar]
- 9.Büchi G., Gould S.J., Näf F. Stereospecific syntheses of uleine and epiuleine. J. Am. Chem. Soc. 1971;93:2492–2501. doi: 10.1021/ja00739a022. [DOI] [Google Scholar]
- 10.Mitaine-Offer A.C., Sauvain M., Valentin A., Callapa J., Mallié M., Zèches-Hanrot M. Antiplasmodial activity of Aspidosperma indole alkaloids. Phytomedicine. 2002;9:142–145. doi: 10.1078/0944-7113-00094. [DOI] [PubMed] [Google Scholar]
- 11.Dominique M., Roland M. Use of uleine for the prevention and/or the treatment of infectious diseases. WO 2011160684 A1 20111229. PCT Int. Appl. :2011.
- 12.Oliveira A.B. Standardized extracts and fractions of husks of Aspidosperma parvifolium and/or ulein for pharmaceutical use. PI BR 20095584 A2 20110823. Brazilian Patent. 2011
- 13.Oliveira A.B., Dolabela M., Póvoa M., Santos C.A.M., Varotti F.P. Antimalarial activity of ulein and proof of its action on the Plasmodium falciparum digestive vacuole. Malaria J. 2010;9 Oral Presentation O9. [Google Scholar]
- 14.Andrade-Neto V.F., Pohlit A.M., Pinto A.C., Silva E.C., Nogueira K.L., Melo M.R., Henrique M.C., Amorim R.C., Silva L.F., Costa M.R., et al. In vitro inhibition of Plasmodium falciparum by substances isolated from Amazonian antimalarial plants. Mem. Inst. Oswaldo Cruz. 2007;102:359–365. doi: 10.1590/s0074-02762007000300016. [DOI] [PubMed] [Google Scholar]
- 15.Rocha e Silva L.F., Montoia A., Amorim R.C.N., Melo M.R., Henrique M.C., Nunomura S.M., Costa M.R.F., Andrade Neto V.F., Costa D.S., Dantas G., et al. Comparative in vitro and in vivo antimalarial activity of the indole alkaloids ellipticine, olivacine, cryptolepine and a synthetic cryptolepine analog. Phytomedicine. 2012;20:71–76. doi: 10.1016/j.phymed.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Uchoa D.E.A. Ph.D. Thesis. Universidade Federal do Ceará; Fortaleza, Brazil: 2006. Aplicação de técnicas contemporâneas de ressonância magnética nuclear no estudo fitoquímico de Aspidosperma ulei Markgf. [Google Scholar]
- 17.Ondetti M.A., Deulofeu V. Alkaloids from Aspidosperma australe Mull Argov. Relationship of olivacine to u-alkaloid C. The structure of olivacine and u-alkaloid C (guatambuine) Tetrahedron Lett. 1959;7:1–4. doi: 10.1016/S0040-4039(01)82723-4. [DOI] [Google Scholar]
- 18.Seebacher W., Simic N., Weis R., Saf R., Kunert O. Spectral assignments and reference data. Magn. Reson. Chem. 2003;41:636–638. doi: 10.1002/mrc.1214. [DOI] [Google Scholar]
- 19.Lemes G.F., Ferri P.H., Lopes M.N. Constituintes químicos de Hyptidendron canun (Pohl ex Benth.) R. Harley (Lamiaceae) Quím. Nova. 2011;34:39–42. doi: 10.1590/S0100-40422011000100008. [DOI] [Google Scholar]
- 20.Endringer D.C., Pezzuto J.M., Soares C.M., Braga F.C. L-(+)-Bornesitol. Acta Crystallogr. Sect. E. 2007;63:1067–1068. doi: 10.1107/S1600536806037019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waschsmuth R, Matusch R. Anhydronium bases from Rauvolfia serpentina. Phytomedicine. 2002;61:705–709. doi: 10.1016/s0031-9422(02)00372-2. [DOI] [PubMed] [Google Scholar]
- 22.Wenkert E., Chang C.-J., Chawla H.P.S., Cochran D.W., Hagaman E.W., King J.C., Orito K. General methods of synthesis on indole alkaloids. 14. Short routes of construction of yohimboid and ajmalicinoid alkaloid systems and their 13C nuclear magnetic resonance spectral analysis. J. Am. Chem. Soc. 1976;98:3645–3655. doi: 10.1021/ja00428a044. [DOI] [Google Scholar]
- 23.Passemar C., Saléry M., Soh P.N., Linas M.-D., Ahond A., Poupat C., Benoit-Vical F. Indole and aminoimidazole moieties appear as key structural units in antiplasmodial molecules. Phytomedicine. 2011;18:1118–1125. doi: 10.1016/j.phymed.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Hu Y., Coates A.R.M. Novel combination for treatment of microbial infections. WO 2012032360 CA 2809203. Patent PCT Int. Appl. 2012
- 25.Pascale C., Richard B., Deverre J.R., Sevenet T., Zeches M., Le Men-Oliver L. Alkaloids from leaves and root bark of Ervatamia hirta. Phytomedicine. 1991;30:3785–3792. [Google Scholar]
- 26.Bartlett M.F., Korzun B., Sklar R., Smith A.F., Taylor W.I. The alkaloids of Hunteria eburnea Pichon. II: The quaternary bases. J. Org. Chem. 1963;28:1445–1449. doi: 10.1021/jo01041a001. [DOI] [Google Scholar]
- 27.Burnell R.H., Chapelle A., Khalil M.F. Hunteracine: The crystal structure of a quaternary alkaloid from Hunteria eburnea Pichon. J. Chem. Soc. Chem. Comm. 1970;12:722–723. [Google Scholar]
- 28.Burnell R.H., Chapelle A., Khalil M.F. Quaternary bases from Hunteria eburnea Pichon. Can. J. Chem. 1974;52:2327–2330. doi: 10.1139/v74-335. [DOI] [Google Scholar]
- 29.Marini-Bettolo G.B., Nicoletti M., Messana I., Patamia M., Galeffi C., Oguakwa J.U., Portalone G., Vaciago A. Research on Africa medicinal plants-IV: Boonein, a new C-9 terpenoid lactone from Alstonia boonei: A possible precursor in the indole alkaloid biogenesis. Tetrahedron. 1983;39:323–329. doi: 10.1016/S0040-4020(01)91827-7. [DOI] [Google Scholar]
- 30.Borris R.P., Larris D.C., Cordell G.A. Studies on the uleine alkaloids I. Carbon-13-NMR studies on uleine, 20-epiuleine and (4S)-uleine-Nb-oxide. J. Nat. Prod. 1983;46:200–205. doi: 10.1021/np50026a012. [DOI] [Google Scholar]
- 31.Jácome R.L.R.P., Oliveira A.B., Raslan D.S., Wagner H. Estudo químico e perfil cromatográfico de Aspidosperma parvifolium A. DC. (“pau-pereira”) Quím. Nova. 2004;27:897–900. [Google Scholar]
- 32.Amat M., Pérez M., Llor N., Escolano C., Luque F.J., Molins E., Bosch J. Conjugate additions to phenylglycinol-derived unsaturated δ-lactams enantioselective synthesis of uleine alkaloids. J. Org. Chem. 2004;69:8681–8693. doi: 10.1021/jo0487101. [DOI] [PubMed] [Google Scholar]
- 33.Forns P., Diez A., Rubiralta M. Synthetic applications of 2-(1,3-dithian-2-yl) indoles VI. Synthesis of 20-epidasycarpidone. Tetrahedron. 1996;52:3564–3574. doi: 10.1021/jo9609900. [DOI] [PubMed] [Google Scholar]
- 34.Gràcia J., Casamitjana N., Bonjoch J., Bosch J. Total synthesis of uleine-type and Strychnos alkaloids through a common intermediate. J. Org. Chem. 1994;59:3939–3951. doi: 10.1021/jo00093a028. [DOI] [Google Scholar]
- 35.Figueiredo E.R., Vieira I.J.C., Souza J.J., Braz-Filho R., Mathias L., Kanashiro M.M., Côrtes F.H. Isolamento, identificação e avaliação da atividade antileucêmica de alcalóides indólicos monoterpênicos de Tabernaemontana salzmannii (A. DC.), Apocynaceae. Rev. Bras. Farmacogn. 2010;20:675–681. doi: 10.1590/S0102-695X2010005000019. [DOI] [Google Scholar]
- 36.Kobayashi J., Sekiguchi M., Shimamoto S., Shigemori H., Ishiyama H., Ohsaki A. Subicanadines A–C, novel quaternary índole alkaloids from Aspidosperma subincanum. J. Org. Chem. 2002;67:6449–6455. doi: 10.1021/jo025854b. [DOI] [PubMed] [Google Scholar]
- 37.Amat M., Coll M.-D., Passarella D., Bosch J. An enantioselective synthesis of the Strychnos alkaloid (−)-tubifoline. Tetrahedron-Asymmetry. 1996;7:2775–2778. doi: 10.1016/0957-4166(96)00359-X. [DOI] [Google Scholar]
- 38.Amat M., Coll M.D., Bosch J., Espinosa E., Molins E. Total synthesis of the Strychnos indole alkaloids (−)-tubifoline, (−)-tubifolidine and (−)-19,20-dihydroakuammicine. Tetrahedron-Asymmetr. 1997;8:935–948. doi: 10.1016/S0957-4166(97)00071-2. [DOI] [Google Scholar]
- 39.Milborrow B.V., Djerassi C. Alkaloids studies: Part LXI: The structure of twelve new alkaloids from Aspidosperma cylindrocarpon. J. Chem. Soc. C. 1969:417–424. doi: 10.1039/j39690000417. [DOI] [Google Scholar]
- 40.Guimarães A.H., Braz-Filho R., Vieira I.J.C. 1H and 13C-NMR data of the simplest plumeran indole alkaloids isolated from Aspidosperma species. Molecules. 2012;17:3025–3043. doi: 10.3390/molecules17033025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trager W., Jensen J.B. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 42.Rocha e Silva L.F., Lima E.S., Vasconcellos M.C., Aranha E.S.P., Costa D.S., Santos E.V.M., Silva T.C.M., Morais S.K.R., Quignard E.L.J., Alecrim M.G.C., et al. In vitro and in vivo antimalarial activity and cytotoxicity of extracts, fractions and a substance isolated from the Amazonian plant Tachia grandiflora (Gentianaceae) Mem. Inst. Oswaldo Cruz. 2013;108 doi: 10.1590/0074-0276108042013017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahmed S.A., Gogal R.M., Jr., Walsh J.E. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: An alternative to [3H] thymidine incorporation assay. J. Immunol. Meth. 1994;15:15211–15224. doi: 10.1016/0022-1759(94)90396-4. [DOI] [PubMed] [Google Scholar]