Abstract
Phytochemical constituents of Vitis wenchowensis were investigated for the first time. A new resveratrol trimer, wenchowenol (1), was isolated from the roots and stems of Vitis wenchowensis along with four known stilbenoids 2–5. The structure and relative configuration of 1 were established on the basis of spectral evidence, especially HMBC and NOESY experiments. It showed potent antioxidant activity against DPPH (1,1-diphenyl-2-picrylhydrazyl) radical.
Keywords: Vitis wenchowensis, resveratrol oligomer, wenchowenol, Vitaceae, antioxidant, DPPH
1. Introduction
Resveratrol, a phytoalexin isolated from diverse plant families, has become one of the most extensively studied natural products, since it was involved in the health benefits associated with a moderate consumption of red wine (the so-called “French paradox”) in the early 1990s [1,2]. Numerous in vitro and in vivo studies have demonstrated its great potential to prevent or treat a wide variety of diseases, including cardiovascular disease, cancer, and neurodegenerative diseases, as well as to extend lifespan [3].
Nevertheless, in some particular families of plants (e.g., Vitaceae, the grapevine family), a large number of phytoalexins were polymerized as resveratrol oligomers, arising from the oxidative coupling of two to eight units of resveratrol monomer [4]. They have attracted considerable attention not only for their structural diversity and biological activities, but also owing to their vital roles in plant defense mechanisms [5].
Our previous phytochemical investigations on Vitaceae plants have led to discovery of novel bioactive resveratrol oligomers [6,7,8]. Our continued research on chemical constituents of the roots and stems of Vitis wenchowensis has now resulted in the isolation of a new resveratrol trimer, wenchowenol (1) together with four known stilbenoids 2–5. In this paper, we report the structural characterization of 1 and its antioxidant activity against DPPH (1,1-diphenyl-2-picrylhydrazyl) radicals.
2. Results and Discussion
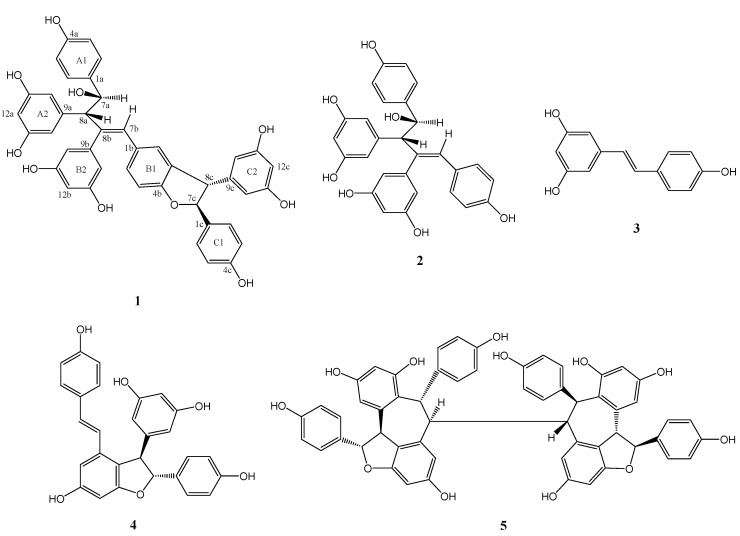
The roots and stems of V. wenchowensis were extracted with methanol at room temperature to yield a crude extract. This crude extract was then partitioned between ethyl acetate and water. The ethyl acetate solubles were separated by silica gel column chromatography (CC), followed by preparative HPLC to afford compounds 1–5 (Figure 1).
Figure 1.
Chemical structures of stilbenoids from V. wenchowensis.
Wenchowenol (1) was obtained as colorless amorphous powder, which was determined to have a molecular formula of C42H34O10 from its HR-ESI-MS, which corresponded to a hydroxylated resveratrol trimer, since the typical formula of resveratrol trimers is C42H32O9. The 1H-NMR and 1H,1H-COSY spectra of 1 showed the presence of two sets of ortho-coupled aromatic H-atoms assignable to two 4-hydroxyphenyl groups, three sets of 3,5-dihydroxyphenyl groups, a set of H-atom forming a ABX system on a 1,3,4-trisubstituted benzene ring, an olefinic H-atom, and two sets of mutually coupled methine H-atoms.
A comparison between the NMR data of 1 and those of the co-occurring amurensin A (2), which has been previously isolated from V. amurensis [9], revealed that 1 contained 2 as partial structure (resveratrol units A and B). Compound 1 showed signals (Table 1, signals from 1c to 14c) corresponding to an additional resveratrol unit C, which was confirmed by HMBC data (Figure 2). The HMBCs H-8c/C-3b, C-4b, and C-10c(14c) and H-7c/C-2c(6c) and C-9c indicated that the resveratrol unit C formed a dihydrofuran ring fused with the aromatic ring B1, while the 4-hydroxyphenyl group (ring C1) was attached at C-7c. Thus the structure of 1 was determined as shown in Figure 1. Its 1H and 13C-NMR data were assigned in Table 1.
Table 1.
1H and 13C-NMR Data of wenchowenol (1) in acetone-d6a.
| position | δH (mult., J Hz) | δ C | position | δH (mult., J Hz) | δ C | |
|---|---|---|---|---|---|---|
| 1a | 135.7 | 7b | 6.73 b | 128.2 | ||
| 2a (6a) | 7.03 (2H, d, 8.5) | 129.5 | 8b | 142.3 | ||
| 3a (5a) | 6.56 (2H) b | 115.6 | 9b | 144.8 | ||
| 4a | 157.4 | 10b (14b) | 6.01 b | 109.2 | ||
| 7a | 5.02 (1H, d, 10.4) | 76.0 | 11b (13b) | 159.4 | ||
| 8a | 3.68 (1H, d, 10.5) | 64.6 | 12b | 6.12 (1H, t, 2.0) | 102.3 | |
| 9a | 144.0 | 1c | 132.9 | |||
| 10a (14a) | 5.94 (2H, d, 2.0) | 109.2 | 2c (6c) | 7.10 (2H, d, 8.5) | 128.6 | |
| 11a (13a) | 158.5 | 3c (5c) | 6.74 (2H, d, 8.9) | 116.3 | ||
| 12a | 5.91 (1H, t, 1.9) | 101.5 | 4c | 158.8 | ||
| 1b | 132.3 | 7c | 5.27 (1H, d, 8.2) | 94.5 | ||
| 2b | 6.86 (1H, d, 8.2) | 130.6 | 8c | 4.23 (1H, d, 8.0) | 58.8 | |
| 3b | 6.55 b | 109.3 | 9c | 145.3 | ||
| 4b | 159.6 | 10c (14c) | 6.01 b | 107.7 | ||
| 5b | 131.0 | 11c (13c) | 159.9 | |||
| 6b | 6.76 b | 127.7 | 12c | 6.16 (1H, t, 2.5) | 102.5 |
a 1H-NMR spectra were measured at 500 MHz, and 13C-NMR spectra were run at 125 MHz; b Overlapping (in the same column).
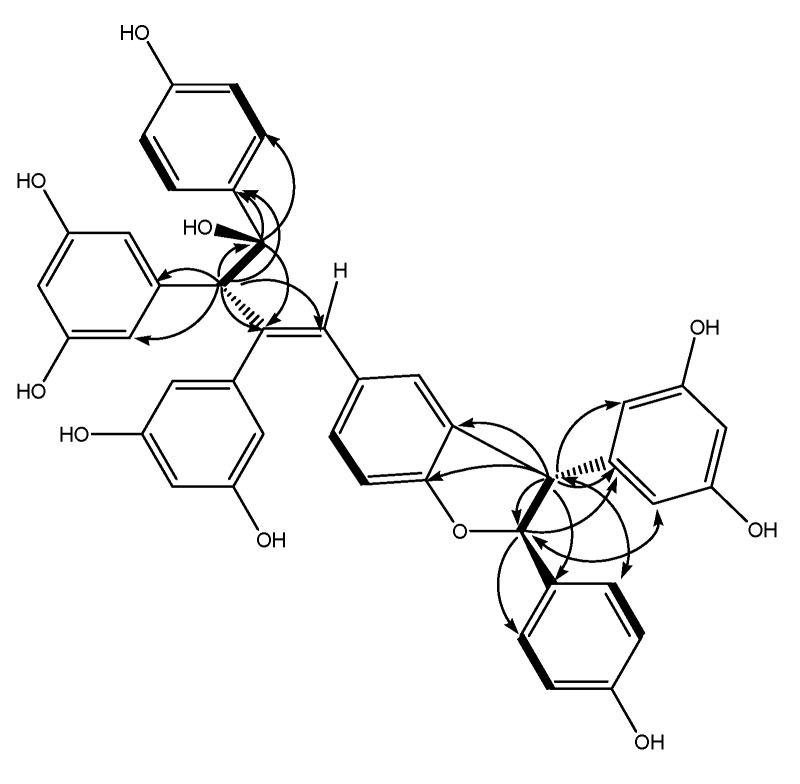
Figure 2.
Key HMBC (indicated by arrows from 1H to 13C), 1H–1H COSY correlations (indicated by bold lines), and NOESY correlations (indicated by double-headed arrows between two protons) for 1.
In order to clarify the stereochemistry of 1, a NOESY (Figure 2) experiment was carried out. The NOEs between H-8c and H-2c(6c), H-7c and H-10c(14c) illustrated that H-8c and H-7c should be trans oriented. A comparison of NMR data between molecules 1 and 2 [1] suggested that H-8a and H-7a has a trans orientation, which was identical with 2. Thus the stereochemistry was as shown in Figure 1. Compound 1 showed potent scavenging activity against DPPH radical with IC50 = 43 μM, which was comparable with resveratrol (IC50 = 38 μM).
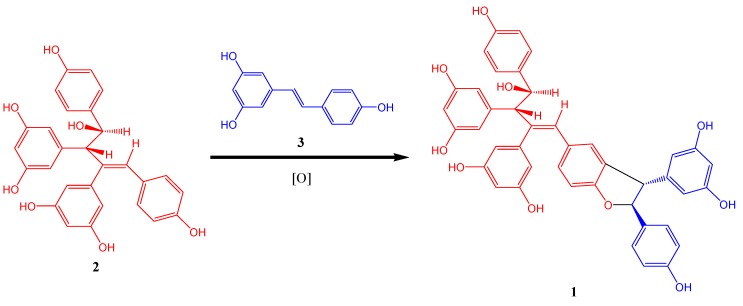
Although oligomerization of stilbenes could generate various skeleton types, formation of dihydrofuran ring usually occurs during oxidative coupling [8]. Therefore 1 was probably generated by oxidative coupling between 2 and resveratrol. A proposed biogenetic pathway is shown in Figure 3.
Figure 3.
Postulated Biogenetic Pathway of 1.
3. Experimental
3.1. General Methods
1H-NMR spectra were recorded at 500 MHz, and 13C-NMR spectra were recorded at 125 MHz with tetramethylsilane (TMS) and solvent signals as internal references. HR-ESI-MS data were acquired with a FT-ICR mass spectrometer. IR spectra were measured on a FT-IR spectrometer as KBr pellets. Column chromatography (CC) was carried out on silica gel (200–300 mesh from Qingdao Marine Chemical Co. Ltd., Qingdao, China).
3.2. Chemicals
1,1-Diphenyl-2-picrylhydrazyl (DPPH) were purchased from Sigma (St. Louis, MO, USA). Solvents (analytical grade) for extraction and CC were purchased from Huadong Chemicals (Hangzhou, China).
3.3. Plant Material
The roots and stems of V. wenchowensis were collected in June 2007 in Wenzhou, Zhejiang Province, China. The material was identified by Dr. Yunpeng Zhao (College of Life Sciences, Zhejiang University, Hangzhou, China). A voucher specimen (No. P070674) was deposited at the Department of Biology, Zhejiang University, China.
3.4. Extraction and Isolation
The plant material (1.8 kg) was extracted three times with MeOH (3 × 20 L) at room temperature. The solvent was evaporated in vacuo to produce a concentrated MeOH extract (155 g), which was then diluted with H2O (1 L) to give an aqueous solution (1 L). The aqueous solution was extracted with EtOAc three times (3 × 2.0 L). The combined EtOAc layers were condensed in vacuo to provide an EtOAc extract (82 g), which was then subjected to silica gel CC (1,000 g, 5 cm diameter column) eluted with light petroleum-EtOAc mixtures (10:1 to 1:10) to yield eight fractions. Fraction 3 (1.3 g) was subjected to preparative HPLC (column YMC-C18, 250 × 20 mm i.d.; solvent MeOH-H2O, 40%:60%; flow rate 8 mL/min; detection 280 nm) to afford two pure isolates 3 (tR = 28 min, 75 mg), 2 (tR = 51 min, 40 mg) and 4 (tR = 84 min, 120 mg). Fraction 5 (1.6 g) was separated by preparative HPLC under similar conditions except for the ratio of MeOH: H2O (45%:55%) to give compounds 1 (tR = 48 min, 24 mg), and 5 (tR = 79 min, 150 mg).
Wenchowenol (1): colorless amorphous powder; [α]20D +21 (c 0.25, MeOH); UV (MeOH) λmax (log ε) 228 (4.5), 284 (3.2), 315 (3.2) nm; IR (KBr) νmax 3387, 1611, 1516, 1444, 1332, 1236, 1174, 1001, and 834 cm−1; 1H and 13C NMR data, see Table 1; HR-ESI-MS m/z [M - H]− 697.2078 (calcd for C42H33O10, 697.2074).
Compounds 2–5 were determined as amurensin A [9], resveratrol [10], ε-viniferin [11], and hopeaphenol [12], respectively, according to the spectroscopic data in the literature.
3.5. Determination of Antioxidant Activities
The antioxidant activities of 1 were determined by the DPPH assay as previously described [8]. Briefly, the reaction mixture containing sample solution (20 μL) and DPPH (180 μL, 150 μM) in ethanol was placed in a 96-well microplate and incubated at 37 °C for 30 min. The absorbance was measured at 517 nm by a microplate reader. IC50 value represents the concentration of a compound to scavenge 50% of DPPH radicals. Resveratrol was used as positive control.
4. Conclusions
In present study, phytochemical investigation on the roots and stems of Vitis wenchowensis has led to the isolation of a new resveratrol trimer, named wenchowenol (1), which is probably the oxidative coupling product of amurensin A and resveratrol. DPPH assay demonstrated that 1 is a potent antioxidant comparable to the famous red wine polyphenol resveratrol.
Acknowledgments
This study was supported by 863 Program of China (2013AA092902), Qianjiang Talent Plan (2012R10068), Zhejiang Natural Science Foundation (LQ13B020004) and K.C. Wong Magna Fund in Ningbo University.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/18/7/7486/s1.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Frankel E.N., Waterhouse A.L., Kinsella J.E. Inhibition of human LDL oxidation by resveratrol. Lancet. 1993;341:1103–1104. doi: 10.1016/0140-6736(93)92472-6. [DOI] [PubMed] [Google Scholar]
- 2.Renaud S., de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-F. [DOI] [PubMed] [Google Scholar]
- 3.Baur J.A., Sinclair D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 4.Chong J., Poutaraud A., Hugueney P. Metabolism and roles of stilbenes in plants. Plant Sci. 2009;177:143–155. doi: 10.1016/j.plantsci.2009.05.012. [DOI] [Google Scholar]
- 5.He S., Yan X. From resveratrol to its derivatives: new sources of natural antioxidant. Curr. Med. Chem. 2013;20:1005–1017. [PubMed] [Google Scholar]
- 6.He S., Jiang L., Wu B., Li C., Pan Y. Chunganenol: an unusual antioxidative resveratrol hexamer from Vitis chunganensis. J. Org. Chem. 2009;74:7966–7969. doi: 10.1021/jo901354p. [DOI] [PubMed] [Google Scholar]
- 7.He S., Jiang L., Wu B., Zhou J., Pan Y.J. Two novel antioxidative stilbene tetramers from Parthenocissus laetevirens. Helv. Chim. Acta. 2009;92:1260–1267. doi: 10.1002/hlca.200800371. [DOI] [Google Scholar]
- 8.He S., Wu B., Pan Y., Jiang L. Stilbene oligomers from Parthenocissus laetevirens: isolation, biomimetic synthesis, absolute configuration, and implication of antioxidative defense system in the plant. J. Org. Chem. 2008;73:5233–5241. doi: 10.1021/jo8001112. [DOI] [PubMed] [Google Scholar]
- 9.Huang K.-S., Lin M. Oligostilbenes from the roots of Vitis amurensis. J. Asian Nat. Prod. Res. 1999;2:21–28. doi: 10.1080/10286029908039886. [DOI] [PubMed] [Google Scholar]
- 10.Jayatilake G.S., Jayasuriya H., Lee E.-S., Koonchanok N.M., Geahlen R.L., Ashendel C.L., McLaughlin J.L., Chang C.-J. Kinase Inhibitors from Polygonum cuspidatum. J. Nat. Prod. 1993;56:1805–1810. doi: 10.1021/np50100a021. [DOI] [PubMed] [Google Scholar]
- 11.Langcake P., Pryce R. A new class of phytoalexins from grapevines. Experientia. 1977;33:151–152. doi: 10.1007/BF02124034. [DOI] [PubMed] [Google Scholar]
- 12.Reniero F., Rudolph M., Angioni A., Bernreuther A., Cabras P., Mattivi F. Identification of two stilbenoids from Vitis roots. Vitis. 1996;35:125–127. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.