Abstract
Characterzation of polyphenolic compounds in the stems of P. multiflorum was conducted using HPLC, high resolution LC-MS and LC-MSn. Proanthocyanidins in particular were isolated in 4.8% yield using solvent extraction followed by Sephadex LH-20 fractionation. HPLC analysis using a diol column revealed oligomers (from dimer to nonamer) as minor components, with (epi)catechin monomeric units predominating, and oligomers with higher degree of polymerization being dominant. Thiolysis treatment of the proanthocyanidins using mercaptoacetic acid produced thioether derivatives of (epi)catechin as the major product and a mean value of the degree of polymerization of 32.6 was estimated from the ratio of terminal and extension units of the (epi)catechin. The isolated proanthocyanidins were shown to strongly inhibit α-amylase with an acarbose equivalence (AE) value of 1,954.7 µmol AE/g and inhibit α-glucosidase with an AE value of 211.1 µmol AE/g.
Keywords: P. multiflorum, proanthocyanidins, α-amylase, (epi)catechin
1. Introduction
The root of Foti or Polygonum multiflorum Thunb has found broad use as a tonic and herb in China for many traditional uses, including treatment of hair loss [1], replenishing the vital essence of blood, curing malaria, and clearing away toxins [2]. The main active constituents in the roots have been characterized as polyphenolic compounds, including hydroxyanthraquinones, stilbenoids and proanthocyanidins [3,4], which are responsible for the radical scavenging activity of the roots [5]. The mice fed with root extracts of Polygonum multiflorum Thunb were shown to possess improved learning and memory ability and reduced oxidative stress status as measured by malondialdehyde concentrations [6]. Consistent with this work, it was found that antioxidants in Polygonum multiflorum Thunb roots were mostly extractable using water [7], indicating the active components are hydrophilic compounds including 2,3,5,4'-tetrahydroxystilbene 2-O-β-d-glucopyranoside, which is the main antioxidant in the root [5]. In addition to the radical scavenging activity, Sang and workers have shown that stilbene glucoside from Polygonum multiflorum Thunb can trap methylglyoxal, the toxic reactive carbonyl species that can react with proteins and lead to the formation of advanced glycation endproducts (AGES) [8]. Since methylglyoxal toxicity is a major cause of diabetic complications [9], mitigation of its harmful effect through a herbal medicine would be a potential useful application of Polygonum multiflorum Thunb extracts.
The existing literature on bioactive components strongly substantiates the health promotion effects of the roots of Polygonum multiflorum Thunb. In addition to the roots, the stems, or Fleeceflower Stem tuber of the plant have been used as traditional herb for over a thousand years for health promotion purposes. In contrast to the roots, much less research has been carried out on characterization of bioactive components in the stems. Although it has been reported that the stem of Polygonum multiflorum Thunb, exhibited even more potent antioxidant capacity than the root of Polygonum multiflorum Thunb [10], its polyphenolic profile is not fully characterized. Reported herein are the results of our attempts to elucidate the polyphenolic compound profiles in the stems of Polygonum multiflorum Thunb. In particular, using a high throughput assay as a guide, we identified and characterized the proanthocyanidins in the stems as potent starch hydrolase inhibitors, which has potential as functional ingredients in reducing postprandial hyperglycemia.
2. Results and Discussion
2.1. HPLC and High Resolution Mass Spectra Characterization of Polyphenolic Compounds Extract
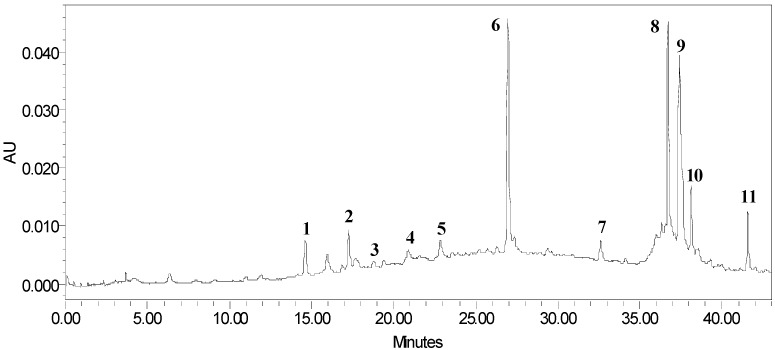
The HPLC chromatogram of a methanolic extract of P. multiflorum Thunb is shown in Figure 1. Eleven peaks were characterized based on the HR-LC-MS results and MSn fragmentation behaviors (Table 1). Three classes of compounds were identified.
Figure 1.
HPLC chromatogram of phenolic compounds in P. multiflorum Thunb.
Table 1.
LC-HR-MS and LC-MSn data for compounds.
| Peak No. | Compound identity | Measured HRMS m/z [M−H]− | Predicted HRMS m/z [M−H]− | Mass Error (ppm) | MSn fragmentations [M−H]− |
|---|---|---|---|---|---|
| 1 | Dimer, (epi)C2 | 577.1366 | 577.1351 | −2.5 | 451,425,287 |
| Molecular formula | C30H26O12 | ||||
| 2 | Trimer, (epi)C3 | 865.2010 | 865.1985 | −2.8 | 575,407 |
| Molecular formula | C45H38O18 | ||||
| 3 | Trimer, (epi)C2+(epi)CG | 1017.2071 | 1017.2095 | 2.4 | 847,729, 677,577,287 |
| Molecular formula | C52H42O22 | ||||
| 4 | Ester of phenylpropanoid and phenylethanoid glucoside | 839.2396 | 839.2404 | 1.0 | 677,641,515 |
| Molecular formula | C41H43O19 | ||||
| 5 | Tetramer, (epi)C4 | 1153.2572 | 1153.2619 | 4.1 | 863,575,407 |
| Molecular formula | C60H50O24 | ||||
| 6 | Tetrahydroxystilbene glucoside | 405.1190 | 405.1191 | 0.3 | 243,137 |
| Molecular formula | C20H22O9 | ||||
| 7 | Methyl-O-digalloyl-glucoside | 497.0934 | 497.0937 | 0.6 | 345,327 |
| Molecular formula | C21H22O14 | ||||
| 8 | Emodin-O-glucoside | 431.0982 | 431.0984 | 0.3 | 269,241 |
| Molecular formula | C21H20O10 | ||||
| 9 | Physcion-O-glucoside | 445.1149 | 445.1140 | −1.9 | 283,268 |
| Molecular formula | C22H22O10 | ||||
| 10 | Physcion | 283.0599 | 283.0612 | 4.5 | 268,240 |
| Molecular formula | C16H12O5 | ||||
| 11 | Emodin | 269.0445 | 269.0455 | 3.9 | 241,224 |
| Molecular formula | C15H10O5 |
2.1.1. Flavan-3-ols
Compounds in peaks 1, 2, 3 and 5 were tentatively assigned as proanthocyanidins according to the accurate [M−H]− anion mass and the fragmentation patterns of the ESI-MSn (anion mode). Compounds 1, 2 and 5 have accurate m/z [M−H]− values of 577.1366, 865.2010 and 1153.2572, respectively, and were tentatively assigned as dimeric, trimeric and tetrameric proanthocyanidins. The compound in peak 3 has an accurate m/z [M−H]− of 1017.2071 and was tentatively assigned as a proanthocyanidin trimer with two (epi)catechin units and one (epi)catechin gallate unit.
The secondary mass of peak 1 gave three daughter ion peaks. A peak at m/z 451 arises from heterocyclic ring fission (HRF) of the heterocyclic rings; a peak at m/z 425 arises from retro-Diels-Alder fission (RDA-F) of the heterocyclic rings, and, lastly, a peak m/z 287 fragment is formed via quinone-methide (QM) cleavage of the interflavanic bond [11].
2.1.2. Phenylpropanoids and Stilbenoids
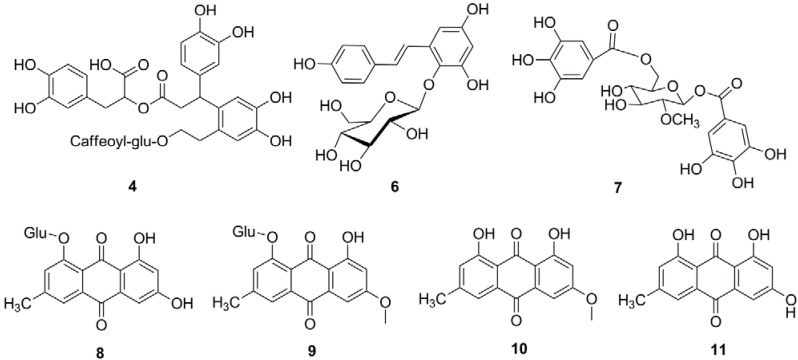
Peak 4 has an accurate m/z [M−H]− of 839.2396. Its corresponding formula is C41H43O19. The same compound was found in Meehania urticifolia [12]. The fragmentation by MS2 gave a peak at m/z [M−H]− of 677 due to loss of one caffeoyl group from the molecular ion. The fragment ion at m/z [M−H]− 515 might arise from the further loss of one glucose moiety. Therefore, peak 4 was tentatively assigned as the complex of a phenylpropanoid ester and phenylethanoid glucoside (Figure 2). The compound in peak 6 has the accurate m/z [M−H]− of 405.1190. Its secondary ion at m/z 243 arises from the loss of one glucose moiety. Its secondary ion at m/z 137 might be derived from alkene bond cleavage, therefore, it was tentatively assigned as tetrahydroxystilbene glucoside. Based on this result, we can conclude that, the stems of the Polygonum multiflorum Thunb also contain significant amounts of the stilbene derivatives found in the roots.
Figure 2.
Structures of phenolic compounds identified in P. multiflorum Thunb. G, galloyl group; Glu, glucose.
2.1.3. Anthraquinone Derivatives
Four compounds of this class were detected. Peaks 8 and 9 have accurate m/z [M−H]− at 431.0982 and 445.1149. Their secondary ions are 269 and 283, respectively, which arises from the loss of one glucose moiety. Therefore, they were tentatively assigned as emodin glucoside and physcion glucoside. Peak 10 has an accurate m/z [M−H]− of 283.0599. Its secondary ion at m/z 268 [M−H−15]− was is suggested to be formed by elimination of a methyl group. The peak at m/z 240 [M−H−15−28]− was suggested to be formed by further eliminating a CO group [13]. This is consistent with the reported fragmentation pattern of physcion [13]. The compound of peak 11 has an accurate m/z [M−H]− of 269.0445. Its secondary ion at m/z 241 [M−H−28]− arises from loss of a CO group. Its secondary ion at m/z 224 [M−H−28−17]− arises from further loss of a hydroxyl group. Therefore, compound 11 was tentatively identified as emodin. The chemical structures were shown in Figure 2.
In addition to the three class of compounds, a hydrolysable tannin (peak 7) was identified as methyl-O-digalloyl glucoside, with accurate [M−H]− at m/z 497.0934. Its secondary ion at m/z 345 arises from the loss of one galloyl moiety. Its fragment ion at m/z 327 might be derived from further loss of water.
2.2. HPLC and LC-ESI-MSn Characterization of Proanthocyanidins
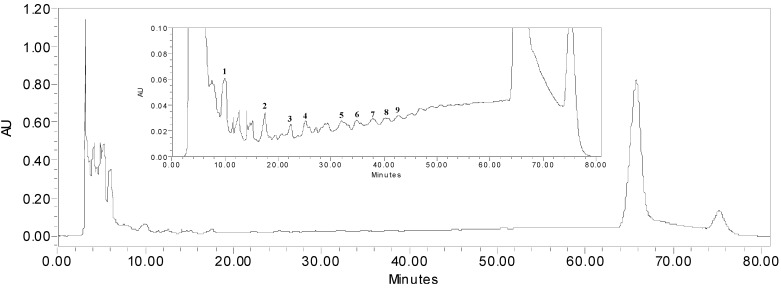
Aqueous acetone extraction of P. multiflorum Thunb yielded crude proanthocyanidins. To fully characterize the compounds present in this extract, HPLC and LC-MSn analysis were carried out using a diol column that was reported to be particularly effective in separating proanthocyanidins [11,12,13]. This worked nicely as shown by the resulting HPLC chromatogram (Figure 3).
Figure 3.
HPLC chromatogram of proanthocyanidins in P. multiflorum Thunb. The identities of the compounds are listed in Table 2.
We were able to assign the structures of the proanthocyanidins from peak 1 to 9 according to [M−H]− anion mass and the fragmentation pattern of the ESI-MSn (anionic mode). Compounds in peaks 1, 2, 4, 6 and 8 have negative molecular ions [M−H]− for dimer (m/z 577), trimer (865), tetramer (1153), pentamer (1441), and hexamer (1729) (epi)catechins with B type linkages, respectively. The type of linkage in proanthocyanidins can be identified readily by MS as A-type linkage (2 Da less) or B-type linkage. Peaks 3, 5, 7 and 9 have [M−H]− of m/z 1017 (trimer), 1305 (tetramer), 1593 (pentamer) and 1881 (hexamer) derivatives of one gallate unit based on the molecular anion of 152 (galloyl) larger than the respective (epi)catechin oligomers (Table 2). The MS2 fragmentation patterns are in agreement with those of typical proanthocyanidins. Compared to the higher oligomer peaks at 66 min (Figure 3), these oligomer contents are insignificant. In addition, the amount of proanthocyanidins in the extract was estimated from HPLC chromatograms using epicatechin as a reference standard. The proanthocyanidins content was estimated to be 37 mg epicatechin equivalent per 100 mg extracts.
Table 2.
Observed ions of proanthocyanidins in P. multiflorum Thunb by ESI-MS (anionic mode).
| Peak number | Compound identity | m/z [M−H]− | MS2 fragmentation ions |
|---|---|---|---|
| 1 | Dimer, (epi)C2 | 577 | 451,425,287 |
| 2 | Trimer, (epi)C3 | 865 | 575,407 |
| 3 | Trimer, (epi)C2-(epi)CG | 1017 | 847,729,577,287 |
| 4 | Tetramer, (epi)C4 | 1153 | 863,575,407 |
| 5 | Tetramer, (epi)C3-(epi)CG | 1305 | 1179,1017,847,729,577,451 |
| 6 | Pentamer, (epi)C5 | 1441 | 1315,1151,865 |
| 7 | Pentamer, (epi)C4-(epi)CG | 1593 | 1467,1441,1305,1017,727,575,447 |
| 8 | Hexamer, (epi)C6 | 1729 | 1603,1577,1441,1151,1027,863.737,576 |
| 9 | Hexamer, (epi)C5-(epi)CG | 1881 | 1729,1593,1305,1177,1015,865,739 |
(epi)C, (epi)CG are the abbreviation for (epi)catechin, (epi)catechin gallate, respectively. The number in subscript indicates that the number of monomers.
2.3. Thiolysis of Proanthocyanidins for HPLC and LC-ESI-MS Analysis
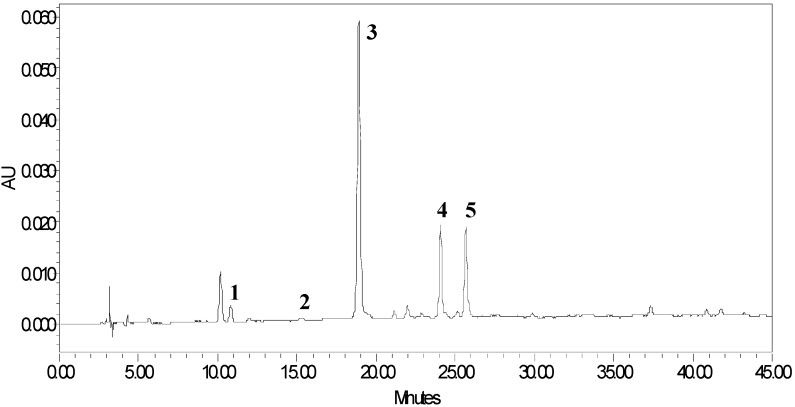
To determine the mean degree of polymerization, thiolysis reaction using mercaptoacetic acid as the nucleophilic agent were conducted to investigate the main components of the proanthocyanidins and the resulting HPLC profile of the thiolysis reaction products is given in Figure 4 [14].
Figure 4.
HPLC chromatographic profile of thiolysis products of proanthocyanidins. Catechin, 1; epicatechin, 2; catechin thioether, 3; epicatechin thioether, 4; (epi)catechin gallate thioether, 5.
The peaks were identified according to the retention times of the standard compounds and LC-ESI-MS results. The major product observed was the 4β-(carboxymethyl)sulphanyl-(−)-catechin methyl ester, followed by 4β-(carboxymethyl)sulphanyl-(−)-epicatechin methyl ester and 4β-(carboxymethyl)sulphanyl-(−)-(epi)catechin gallate methyl ester. These results showed that the extension units of proanthocyanidins in P. multiflorum Thunb consist of catechin, epicatechin and (epi)catechin gallate. For the terminal units, catechin and epicatechin were both observed from the thiolysis mixtures. By comparing the peak areas of the individual curves obtained from the HPLC, the mean DP of P. multiflorum Thunb proanthocyanidins was calculated to be 32.6 based on Equation (1):
| (1) |
It should be pointed out that, due to the different molar absorbance coefficient of thiolytic products 1–5, the actual mean degree of polymerization is different.
2.4. Starch Hydrolase Inhibition Activity
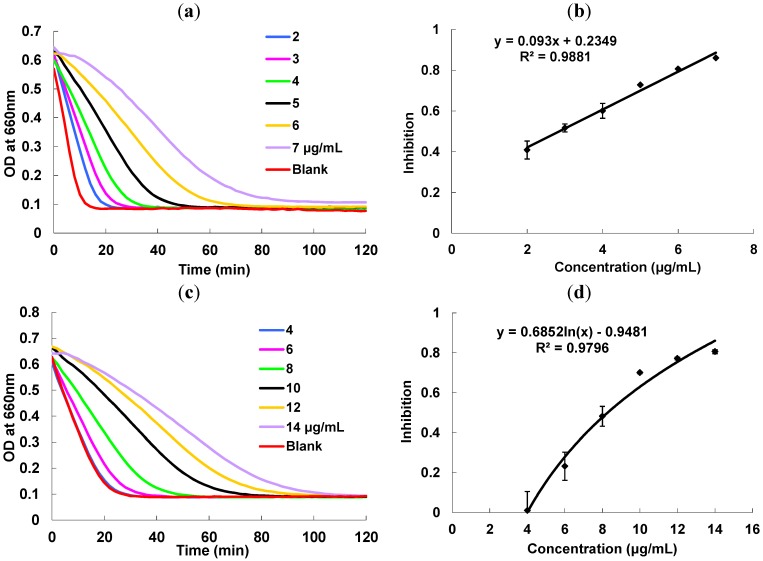
P. multiflorum proanthocyanidins extract showed strong inhibitory activity towards starch hydrolase as measured using a high throughput assay developed in our lab based on turbidity changes as a means to monitor starch hydrolysis kinetics [15]. The speed of the hydrolysis by α-amylase and α-glucosidase decreases with increasing proanthocyanidin concentrations (Figure 5). A linear dose-dependent correlation effect could be observed and the IC50 values were calculated as 2.9 and 7.4 µg/mL, respectively. By comparing with acarbose as a reference standard, the acarbose equivalent (AE) values of P. multiflorum proanthocyanidins are 1,954.7 ± 104.2 µmol AE/g for α-amylase (IC50 = 2.9 ± 0.15 µg/mL) and 211.1 ± 10.1 µmol AE/g for α-glucosidase (IC50 = 7.4 ± 0.35 µg/mL). Therefore, the proanthocyanidins are more selective towards α-amylase compared to acarbose. This warrants further study on how this selectivity would impact the side effects in human subjects in the future.
Figure 5.
(a) Kinetic curves of α-amylase under different concentration ofproanthocyanidins extract; (b) α-amylase inhibition activity vs. concentrations of proanthocyanidins extract; (c) Kinetic curves of α-glucosidase under different concentration of proanthocyanidins extract; (d) α-glucosidase inhibition activity vs. concentrations of proanthocyanidins extract.
3. Experimental
3.1. Reagents and Instruments
α-Amylase (A3176, type VI-B, from porcine pancreas), corn starch (S4126), acarbose, α-glucosidase in the form of rat intestine acetone powder, mercaptoacetic acid were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). The stems of Polygonum multiflorum Thunb came from a cultivated product obtained in July 2011 from Jiuzhihe Township, Luotian County, Hubei, China. The voucher specimen was deposited in the Singapore Arboretum (number: SING-2011-553).
LC/MS spectra were acquired using a Bruker Amazon ion trap mass spectrometer (Billerica, MA, USA) equipped with a Dionex Ultimate 3000RS HPLC system (Bannockburn, IL, USA). The heated capillary and spray voltage were maintained at 250 °C and 4.5 kV, respectively. Nitrogen was operated at 80 psi for sheath gas flow rate and 20 psi for auxiliary gas flow rate. The full scan mass spectra from m/z 50–2,000 were acquired in negative ion mode with a scan speed of one second per scan. The MSn collision gas was helium with collision energy of 30% of the 5V end cap maximum tickling voltage.
3.2. Extraction of Polyphenolic Compounds from P. multiflorum Thunb
The dried P. multiflorum Thunb (50 g) was meshed and extracted with 90% methanol (200 mL) for 4 h at room temperature. Filtration was performed to remove any insoluble solids. The supernatant was collected and the volatiles were evaporated by rotary evaporator and the dried residue was further dissolved in methanol for HPLC analysis.
3.3. Isolation of Proanthocyanidins
The proanthocyanidins were extracted based on an earlier report with a slight modification [16]. P. multiflorum Thunb (50 g) was dissolved in acetone-H2O (7:3, v/v) (3 × 500 mL), shaked for 4 h, and filtered to remove any insoluble solids. The filtrate was evaporated to dryness by rotary evaporation. The residue was dried to give 7.4 g of solid (14.8%). The solid were then purified on a LH-20 column. After the sample was loaded, the column was flushed with methanol-water (1:1, v/v) until the eluent turned colorless. The adsorbed proanthocyanidins were then eluted with acetone-H2O (7:3, v/v). The collected solution was evaporated to dryness to give 2.4 g (4.8%) of sample.
3.4. HPLC and Tandem Mass Spectrometry
The acetone-H2O (7:3) extract (10 mg) from P. multiflorum Thunb was dissolved in one mL methanol. Five μL of the solution was filtered through a 0.45 μm filter before injection into the LC/MSn system equipped with a Develosil® diol column (250 mm × 4.6 mm i.d., 5 μm, Seto, Aichi Japan). The elution conditions were as follows: flow rate, 1.0 mL/min; column temperature 35 °C; mobile phase A, 2% acetic acid in acetonitrile; mobile phase B, acidic aqueous methanol (CH3OH: H2O: HOAc, 95:3:2 v/v/v). The starting mobile phase condition is 7% B and held isocratic for 3 min. Subsequently, solvent B was ramped to 37.6% over 57 min and to 100% B 3 min thereafter. The condition was held at 100% B for seven min prior to returning to starting conditions (7% B) over six min. UV-VIS detector were set at 280 nm and epicatechin standard were run under the same condition for estimation of proanthocyanidins contents.
The 90% methanol extract (10 mg) from P. multiflorum Thunb was dissolved in one mL methanol. One µL of the solution was filtered through a 0.45 μm filter before injection into the LC/MSn system equipped with a Atlantis® T3 column (150 mm × 3.0 mm i.d., 3 μm, Ireland). The elution conditions were as follows: flow rate, 0.3 mL/min; column temperature 25 °C; mobile phase A, water (0.1% acetic acid); mobile phase B, acetonitrile. The starting mobile phase condition is 5% B; hold isocratic for 30 min. Subsequently, ramp solvent B to 80% over 5 min and to 100% B 5 min thereafter. Hold the conditions at 100% B for 5 min prior to returning to starting conditions (5% B) over 3 min. The wavelength of UV detector was set at 280 nm.
3.5. Thiolysis of the Proanthocyanidins for HPLC Analysis
This was performed following a reported method [13]. In a small glass vial, P. multiflorum Thunb proanthocyanidins (50 μL, 2 mg/mL in methanol) was mixed with methanol acidified with concentrated HCl (50 μL, 3.3%, v/v) and 100 μL of mercaptoacetic acid (5% v/v in methanol). The vial was sealed with an inert Teflon cap. The reaction was carried out at 40 °C for 30 min and then kept at room temperature for 10 h and kept in the freezer (−20 °C) until it was filtered through 0.45 μm filter and analyzed by HPLC. The thiolyzed proanthocyanidins were analyzed using LC/MS with a C18 column (250mm × 4.6mm i.d., 3 μm, Waters). The binary mobile phases consisted of A (2% acetic acid in water, v/v) and B (methanol), which were delivered in a linear gradient of B from 15 to 80% (v/v) in 45 min. The flow rate was set at 1.0 mL/min. The wavelength of UV detector was set at 280 nm.
3.6. α-Amylase and α-Glucosidase Inhibition Assay
The inhibition activities of P. multiflorum proanthocyanidins on α-amylase and α-glucosidase were monitored and quantified based on turbidity measurements according to previous work [15]. Inhibition assay solution consisted of enzyme solution (20 μL), inhibitor solution (20 μL), and 1% starch solution (60 μL). Acarbose was prepared in sodium phosphate buffer (0.1 M, pH 6.9). Proanthocyanidins were first dissolved in methanol and then diluted with sodium phosphate buffer to appropriate concentrations.
In a 96-well microplate, enzyme solution (20 μL) was preincubated with a series of concentrations of proanthocyanidin solution (20 μL) in a microplate reader for 15 min at 37 °C. The reaction was started by injecting starch solution (60 μL) using a 12-channel multichannel pipet. The turbidity change was immediately monitored at 660 nm for 2 h. The change of optical density (OD) was used as an indication of turbidity level. The percentage of inhibition was defined by Equation (2):
| (2) |
where AUCsample is the area under the curve (AUC) of inhibitor; AUCcontrol is the area under the curve without inhibitors. The IC50 can be defined as the concentration of inhibitor that produces 50% inhibition of enzyme activity under the specified assay condition. It was obtained from interpolation of percentage of inhibition against inhibitor concentration curve.
3.7. Statistical Analysis
Statistical analysis was performed using Microsoft Excel. The data were expressed as mean of quintuple runs ± SD with replicate analysis.
4. Conclusions
We have shown that stems of P. multiflorum Thunb contain similar polyphenolic compounds to those found in roots, which are well studied and utilized as herbal medicine. It is remarkable that the stems contain high amounts of proanthocyanidins, predominantly composed of catechin and/or epicatechin units and with a very large mean degree of polymerization of 32.5. This is an ideal feature for converting the proanthocyanidins into value-added epicatechin derivatives [17]. The proanthocyanidins exhibit potent α-amylase and moderate α-glucosidase inhibitory activity. Stems of P. multiflorum Thunb are readily available and economical, and are under-utilized compared to the roots. Our results highlights the need for further research and application of polyphenolic compounds in the stems as starting materials for the preparation of epicatechin derivatives and as functional food ingredients.
Acknowledgments
The funding of the research is provided by Jing Brand Company (Hubei, China).
Footnotes
Sample Availability: Not available.
References
- 1.China Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China. China Chemical Industry Press; Beijing, China: 2004. p. 122. [Google Scholar]
- 2.Ou M. Chinese English Dictionary of Traditional Chinese Medicine. The People’s Publishing House; Beijing, China: 1996. p. 509. [Google Scholar]
- 3.Nanaka G.I., Miwa N., Nishioka I. Stilbene glycoside gallates and proanthocyanidins from Polygonum multiflorum. Phytochemistry. 1982;21:429–432. doi: 10.1016/S0031-9422(00)95282-8. [DOI] [Google Scholar]
- 4.Yao S., Li Y., Kong L. Preparative isolation and purification of chemical constituents from the root of Polygonum multiflorum by high-speed counter-current chromatography. J. Chromatogr. A. 2006;1115:64–71. doi: 10.1016/j.chroma.2006.02.071. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y., Wang M., Rosen R.T., Ho C.T. 2,2-diphenyl-1-picrylhydrazyl radical-scavenging active components from Polygonum multiflorum Thunb. J. Agric. Food Chem. 1999;47:2226–2228. doi: 10.1021/jf990092f. [DOI] [PubMed] [Google Scholar]
- 6.Chan Y.-C., Wang M.-F., Chang H.-C. Polygonum multiflorum Extracts improve cognitive performance in senescence accelerated mice. Am. J. Chin. Med. 2003;31:171–179. doi: 10.1142/S0192415X03000862. [DOI] [PubMed] [Google Scholar]
- 7.Chan E., Wong C.Y.-K., Wan C.-W., Kwok C.-Y., Wu J.-H., Ng K.-M., So C.-H., Au A.L.-S., Poon C.C.W., Seto S.-W., et al. Evaluation of anti-oxidant capacity of root of Scutellaria baicalensis Georgi, in comparison with roots of Polygonum multiflorum Thunb and Panax ginseng CA Meyer. Am. J. Chin. Med. 2010;38:815–827. doi: 10.1142/S0192415X10008263. [DOI] [PubMed] [Google Scholar]
- 8.Lv L., Shao X., Wang L., Huang D., Ho C.-T., Sang S. tilbene glucoside from Polygonum multiflorum Thunb. A novel natural inhibitor of advanced glycation end product formation by trapping of methylglyoxal. J. Agric. Food Chem. 2010;58:2239–2245. doi: 10.1021/jf904122q. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed N., Thornalley P.J. Advanced glycation endproducts: What is their relevance to diabetic complications? Diabetes Obes. Metab. 2007;9:233–245. doi: 10.1111/j.1463-1326.2006.00595.x. [DOI] [PubMed] [Google Scholar]
- 10.Wong C.C., Li H.B., Cheng K.W., Chen F. A systematic survey of antioxidant activity of 30 Chinese medicinal plants using the ferric reducing antioxidant power assay. Food Chem. 2006;97:705–711. doi: 10.1016/j.foodchem.2005.05.049. [DOI] [Google Scholar]
- 11.Wang H.Y., Liu T.T., Song L.X., Huang D.J. Profiles and alpha-amylase inhibition activity of proanthocyanidins in unripe Manilkara zapota (Chiku) J. Agric. Food Chem. 2012;60:3098–3104. doi: 10.1021/jf204715q. [DOI] [PubMed] [Google Scholar]
- 12.Murata T., Miyase T., Yoshizaki F. Hyaluronidase inhibitory rosmarinic acid derivatives from Meehania urticifolia. Chem. Pharm. Bull. 2011;59:88–95. doi: 10.1248/cpb.59.88. [DOI] [PubMed] [Google Scholar]
- 13.Ye M., Han J., Chen H., Zheng J., Guo D. Analysis of Phenolic compounds in rhubarbs using liquid chromatography coupled with electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 2007;18:82–91. doi: 10.1016/j.jasms.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Chen W., Fu C., Qin Y., Huang D. One-pot depolymerizative extraction of proanthocyanidins from mangosteen pericarps. Food Chem. 2009;114:874–880. doi: 10.1016/j.foodchem.2008.10.056. [DOI] [Google Scholar]
- 15.Liu T., Song L., Wang H., Huang D. A high-throughput assay for quantification of starch hydrolase inhibition based on turbidity measurement. J. Agric. Food Chem. 2011;59:9756–9762. doi: 10.1021/jf202939d. [DOI] [PubMed] [Google Scholar]
- 16.Fu C., Loo A.E.K., Chia F.P.P., Huang D. Oligomeric proanthocyanidins from mangosteen pericarps. J. Agric. Food Chem. 2007;55:7689–7694. doi: 10.1021/jf071166n. [DOI] [PubMed] [Google Scholar]
- 17.Fu C.L., Chen W., Quek Y.L., Ni R.Y., Ghani A.B.A., Leong W.W.Y., Zeng H.Q., Huang D.J. Sustainability from agricultural waste: Chiral ligands from oligomeric proanthocyanidins via acid-mediated depolymerization. Tetrahedron Lett. 2010;51:6322–6324. [Google Scholar]