Abstract
Polymethylmethacrylate–graphene (PMMA/RGO) nanocomposites were prepared via in situ bulk polymerization using two different preparation techniques. In the first approach, a mixture of graphite oxide (GO) and methylmethacrylate monomers (MMA) were polymerized using a bulk polymerization method with a free radical initiator. After the addition of the reducing agent hydrazine hydrate (HH), the product was reduced via microwave irradiation (MWI) to obtain R-(GO-PMMA) composites. In the second approach, a mixture of graphite sheets (RGO) and MMA monomers were polymerized using a bulk polymerization method with a free radical initiator to obtain RGO-(PMMA) composites. The composites were characterized by FTIR, 1H-NMR and Raman spectroscopy and XRD, SEM, TEM, TGA and DSC. The results indicate that the composite obtained using the first approach, which involved MWI, had a better morphology and dispersion with enhanced thermal stability compared with the composites prepared without MWI.
Keywords: graphene, graphene oxide, polymer, methyl methacrylate, microwave synthesis
1. Introduction
Graphene (GR) is the thinnest two-dimensional graphitic carbon (sp2-bonded carbon sheet) material known, being one atom in thickness [1,2,3]. GR has recently attracted much interest as a filler for the development of new nanocomposites [4,5,6,7,8,9,10,11,12,13,14]. Its extraordinary structural, mechanical, thermal, optical and electrical properties make GR an excellent two-dimensional filler material for polymer composites for applications in many technological fields [4,5,6,7,8].
One of the greatest challenges for this is achieving good dispersion of the nanoscale filler in the composites. Good dispersion is crucial for achieving the desired enhancement in the final physical and chemical properties of the composites [2], especially for GR, which has a strong tendency to agglomerate due to intrinsic van der Waals forces [15]. Various techniques have been developed for the synthesis of these nanocomposite structures, including solution mixing, melt blending, in-situ microwave irradiation (MWI) and in-situ polymerization [16,17,18,19,20,21].
One of the advantages of graphene oxide (GO) is that it can be easily dispersed in water and physiological environments due to its abundant hydrophilic groups, which include hydroxyl, epoxide and carboxylic groups on its large surface [22,23]. Recently, the El-Shall group demonstrated a novel approach for the production of GR sheets (RGO) prepared via the Hoffman Hummers method via reduction of GO using hydrazine hydrate (HH) facilated by using MWI. The resulting product is composed of graphene sheets with polar functional groups, even after the reduction [24]. GO has an affinity for polar solvents and polymers [25]. This affinity makes GO an important intermediate in the preparation of RGO polymer composites via chemical reduction [21]. In this approach, oxygen functional groups, such as carboxyl, carbonyl and hydroxyl groups, were introduced into the carbon skeleton of the GO [25,26], enabling it to interact with polymers through those oxygenated functionalities [27,28,29,30,31]. This interaction has been demonstrated during the preparation of polymer nanocomposites, in which the dispersion of chemically reduced GO is maintained in the presence of the polymer [23,24,25,26,27,28,29]. We are interested in investigating the use as a filler of RGO nanosheets prepared via the reduction of GO facilated by MWI, which offers a fast and easy method for synthesizing polymer nanocomposites [16]. In MWI, dielectric heating energy is transferred directly to the reactants, and the energy is supplied to the molecules faster than they are able to relax, creating high instantaneous temperatures that typically increase the yield and quality of the product [13].
Polymethylmethacrylate (PMMA) is an important polymeric material that has been used in medicine (bone cement), dentistry, the paper, paint and automobile industries and in many other applications [22,23,24,25,26,32]. Several studies have reported the successful incorporation of GR nanosheets into the PMMA matrix with different preparation techniques using various methods of GR preparation, such as from reduced GO, functionalized RGO sheets or chemical vapor deposition [17,18,19,33]. Herein, we report two different preparation techniques for polymethylmethacrylate (PMMA/RGO) nanocomposites. The first technique involves preparing the RGO nanosheets filler via the MWI reduction of GO in the presence of HH. The second technique involves in situ reduction of GO/PMMA via MWI in the presence of HH. Our goal is to understand the effect of nanocomposite preparation techniques on the homogeneity and thermal behavior of RGO-polymer nanocomposites. The general approach focused on a facile method for in situ bulk polymerization of polymethylmethacrylate containing RGO nanosheets (RGO/PMMA). In the first approach, a mixture of GO and methylmethacrylate monomers (MMA) were polymerized using a bulk polymerization method. After the addition of a reducing agent, HH, the product was reduced using conventional microwave irradiation. In the second approach, a mixture of RGO, which was produced via MWI and MMA monomers were polymerized using a bulk polymerization method using a free radical initiator to obtain RGO-(PMMA) composites. The composites were characterized by FTIR, 1H-NMR and Raman spectroscopy and XRD, SEM, TEM, TGA and DSC. The results indicate that the composite obtained using the first approach, which involved MWI, had a better morphology and dispersion with enhanced thermal stability compared with the composites prepared without MWI.
2. Results and Discussion
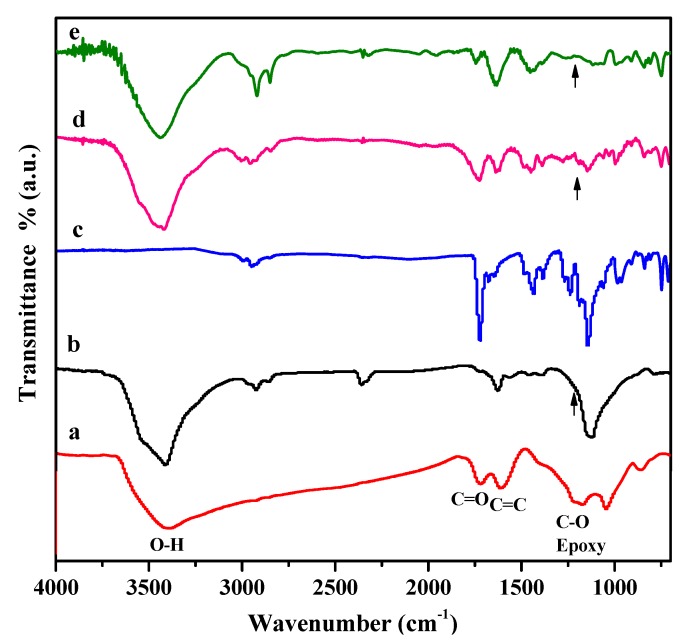
FTIR spectral analysis was performed to confirm the chemical structure of all of the RGO/poly(methyl methacrylate) (PMMA) nanocomposites. Figure 1 summarizes the FTIR spectra of the GO, RGO, neat PMMA, RGO-(PMMA) and R-(GO-PMMA) nanocomposites. The characteristic FTIR features of GO (Figure 1a) include the presence of different types of oxygen functionalities, which have been confirmed by the band at 3420 cm−1, which corresponds to the O-H group, the bands at 1720 and 1618 cm−1, which correspond to the C=O carbonyl/carboxyl and C=C aromatic groups, respectively, and the band at 1220 cm−1, which corresponds with the C-O in the epoxide group [30,34]. For the RGO, Figure 1b indicates that the O-H band at 3430 cm−1 was reduced in intensity due to the deoxygenation of the GO-oxygenated functionalities. The spectrum of RGO also contains bands at 1627 cm−1 and 1139 cm−1, which correspond to C=C and C-O groups, respectively. For the neat PMMA (Figure 1c), the spectrum showed characteristic bands at 2965, 2847 and 1730 cm−1, which correspond to the aliphatic C-H, -CH2 and C=O groups, respectively. The spectrum also had a band at 1150 cm−1 that has been assigned to the C-O-C group. The bands between 1270 cm−1 and 990 cm−1 originate from the C-O group. For the RGO-(PMMA) nanocomposites, Figure 1d shows the characteristic bands at 3420, 1726 and 1620 cm−1 that correspond to the O–H, C=O and C=C groups, respectively [13]. When MWI was employed in the preparation of R-(GO-PMMA) nanocomposites (Figure 1e), there was an increase in the intensity of the C=C bands and a decrease in the intensity of the C=O bands. In addition, strong characteristic bands associated with aliphatic C-H and -CH2 groups were observed at 2921 and 2854 cm−1, respectively, in the spectrum of the R-(GO-PMMA) nanocomposites compared with that obtained by the in situ method (Figure 1d), which indicates the presence of polymer chains despite the use of HH. In summary, the FTIR results suggest that all of the nanocomposites exhibit the characteristic peaks for PMMA chains and RGO sheets.
Figure 1.
FTIR spectra of (a) GO; (b) RGO; (c) neat PMMA; (d) RGO-(PMMA) nanocomposites and (e) R-(GO-PMMA) nanocomposites.
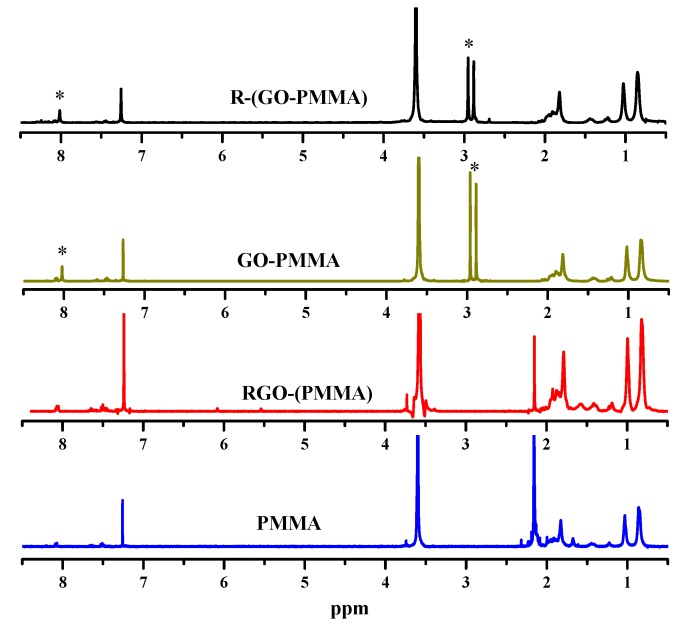
Additional structural evidence to verify the success of polymerization and preparation of the RGO/poly(methyl methacrylate) (PMMA) nanocomposites can be obtained from 1H-NMR data. The 1H-NMR spectra of the neat PMMA, RGO-(PMMA), GO-PMMA and R-(GO-PMMA) nanocomposites are shown in Figure 2. The peaks at δ = ~0.6–1.0 ppm correspond to -CH3, and those between δ = ~1.3 and 2.0 ppm correspond to -CH2. It has been reported that PMMA exhibits a band at δ = 2.8–3.6 ppm that corresponds to the methoxy ester linkage (–COOCH3) [13,35] these peaks were observed for the polymer and its RGO nanocomposites using the different preparation methods.
Figure 2.
1H-NMR spectra of neat PMMA and all of the nanocomposites prepared from in situ bulk polymerization and MWI [* indicates residual solvent (DMF) peaks].
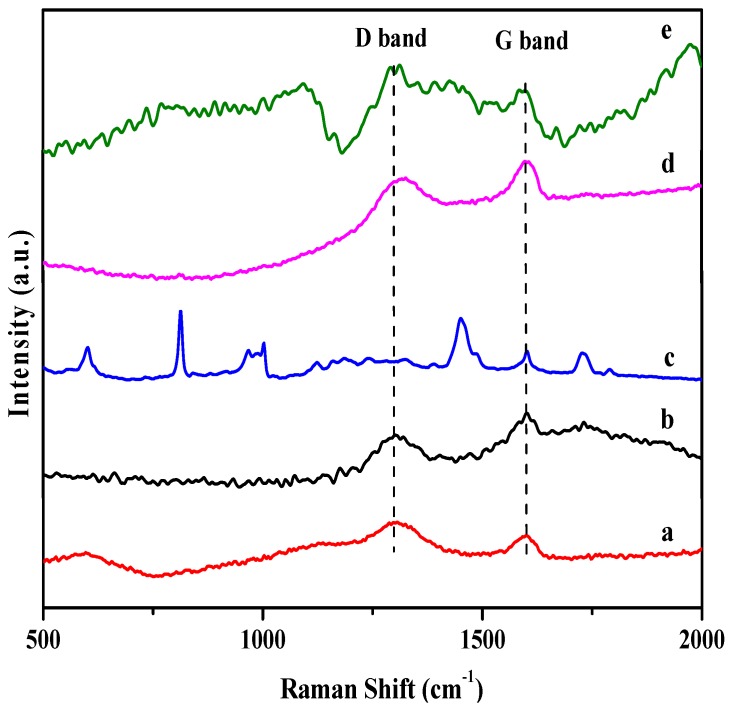
Raman spectroscopy was also employed to characterize the RGO/PMMA nanocomposites. Raman spectroscopy is a very powerful tool that provides essential information for evaluating the covalent modification of RGO nanosheets [19]. Figure 3 displays the Raman spectra of GO, RGO, neat PMMA and PMMA prepared in situ bulk polymerization and MWI. A comparison of the intensity of the characteristic D band at 1300 cm−1 and the G band at 1600 cm−1 is a well-accepted method for estimating the quality and structural order of graphitic structures [7,8,13]. The D band is related to the sp3 states of carbon and is used as proof of disruption in the aromatic π-electron system in RGO. In addition, the G band at 1600 cm−1 is related to the sp2 state of carbon [19]. The D/G ratio in the GO, RGO, RGO-(PMMA) and R-(GO-PMMA) nanocomposites is summarized in Table 1. The ratio of the intensities of the two bands (D/G) should increase as a result of the interaction between the π network in RGO and the ester linkage group -COOCH3 in PMMA [9,13]. The results clearly showed an increase in the intensities of the (D/G) band ratios of RGO (0.66), RGO-(PMMA) (0.93) and R-(GO-PMMA) (1.31). This result indicates that the sp2-hybridized carbons were converted to sp3 hybridized carbons, which may be due to the covalent attachment of the RGO sheets to the PMMA [36]. The ratio was the highest for the R-(GO-PMMA) nanocomposites, indicating a stronger covalent interaction than in the RGO-(PMMA) nanocomposite, which was prepared via in situ bulk polymerization.
Figure 3.
Raman spectra of (a) GO; (b) RGO; (c) neat PMMA; (d) RGO-(PMMA) nanocomposites and (e) R-(GO-PMMA) nanocomposites.
Table 1.
Summary of the D/G ratio determined from the Raman spectroscopy.
| Sample | D band 1299 cm−1 | G band 1600 cm−1 | D/G ratio |
|---|---|---|---|
| GO | 0.052 | 0.036 | 1.44 |
| RGO | 0.016 | 0.025 | 0.64 |
| PMMA | --- | --- | --- |
| RGO-(PMMA) | 0.988 | 1.066 | 0.93 |
| R-(GO-PMMA) | 0.021 | 0.016 | 1.31 |
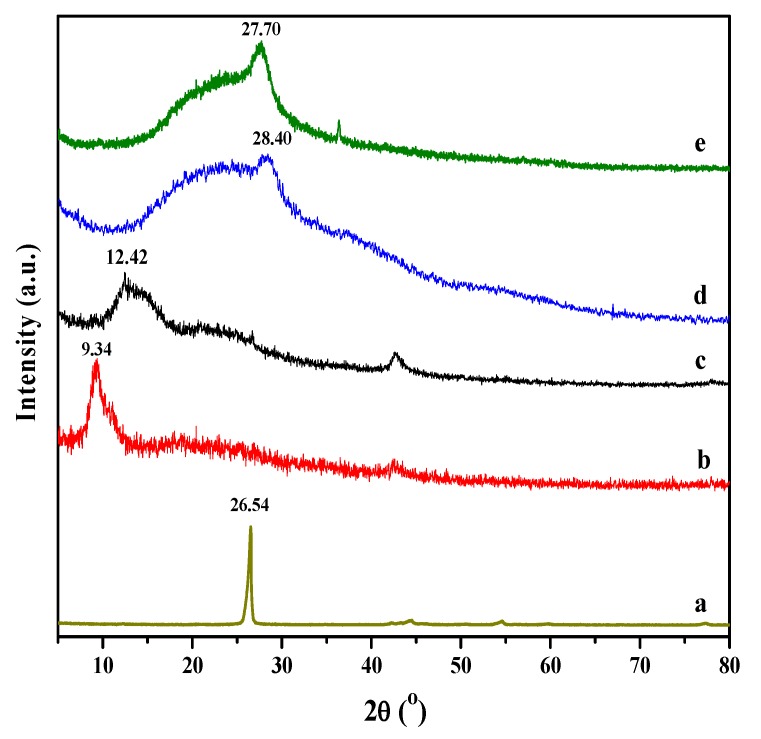
The presence, intercalation and/or exfoliation of RGO sheets in the PMMA matrix can be evaluated using XRD. Figure 4 displays the XRD patterns for graphite, GO, RGO, neat PMMA, RGO-(PMMA) and R-(GO-PMMA). The XRD pattern of the graphite displayed in Figure 4a showed a strong characteristic peak at 2θ = 26.54°, with a d-spacing of 0.34 nm. The XRD pattern of GO displayed in Figure 4b showed a characteristic peak (2θ) at approximately 9.34°, which corresponds with a d-spacing of 0.95 nm. After GO was reduced by HH (Figure 4c), the d-spacing decreased. In addition, the peak appeared at 2θ = 12.42°, with a d-spacing of 0.71 nm. This result confirms the chemical reduction of GO and formation of RGO via the HH reducing agent [37,38]. In addition, this result also indicates the removal of large number of oxygen-containing groups and the formation of much more exfoliated RGO sheets, as well as a change in the hybridization of the reduced carbon atoms from tetrahedral sp3 to planar sp2 [39,40]. The XRD pattern of the RGO-(PMMA) nanocomposites (Figure 4d) had a broad peak, indicating an amorphous structure, which corresponds primarily with the PMMA with a 2θ of 28.40° and a d-spacing of 0.32 nm. The XRD pattern of the prepared R-(GO-PMMA) nanocomposites prepared via MWI (Figure 4e) showed an increase in the d-spacing, with a band at 2θ = 27.70° and a d-spacing of 0.33 nm. The shift in the d-spacing in the R-(GO-PMMA) nanocomposites indicates that the interlayer spacing increased due to various degrees of intercalation, which suggest that various oxygen-containing functional groups (i.e., carboxyl, epoxy and hydroxyl) on the RGO sheet planes were introduced in a larger quantity by MWI. These oxygen-containing functional groups enhanced the interaction along the edges of the nanosheets resulting in an enlargement in the interlayer spacing of RGO/PMMA. The broad peaks near 19° in the R-(GO-PMMA) spectrum showed less broadening compared with the RGO-(PMMA) nanocomposite, indicating agglomeration in these nanocomposites. This agglomeration may arise from strong van der Waals interactions between the reduced GO sheets [41]. In addition, the characteristic peaks of RGO and GO do not appear in the patterns of the nanocomposites, which indicates that the RGO layers were exfoliated in the composites [39,42,43].
Figure 4.
XRD patterns of (a) Graphite; (b) GO; (c) RGO; (d) RGO-(PMMA) nanocomposites and (e) R-(GO-PMMA) nanocomposites.
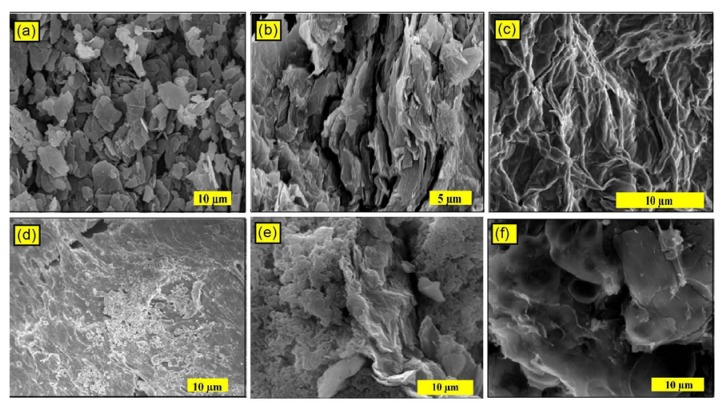
Direct evidence for the exfoliation of the RGO in the final polymer nanocomposites can be obtained from SEM and TEM, which can also provide images of the dispersion in the RGO layers in the PMMA matrix. Figure 5a shows the SEM image of the graphite. The particles have a plate-like shape with average sizes of 1–10 µm. The prepared GO (Figure 5b) was not fully exfoliated and had a flaky texture. This result suggests a partially exfoliated structure and reflects its layered microstructure containing large interlayer spacing and thick multilayer stacks, which is in agreement with the literature [44]. Figure 5c shows the SEM image of RGO, which reveals that the RGO consisted of randomly aggregated thin crumpled sheets that are closely associated with each other, forming a disordered solid [45]. The SEM image of the RGO-(PMMA) nanocomposites prepared via the in situ method (Figure 5e) shows that RGO is stacked up, and not well dispersed in the PMMA matrix. For the R-(GO-PMMA) nanocomposites (Figure 5f), the wrinkled and crumpled profile of RGO was observed with significant changes in the morphology compared with neat PMMA (Figure 5d) and RGO-(PMMA) (Figure 5e).
Figure 5.
The SEM micrographs of (a) graphite; (b) GO; (c) RGO; (d) neat PMMA; (e) RGO-(PMMA) nanocomposites; and (f) R-(GO-PMMA) nanocomposites after reduction by MWI.
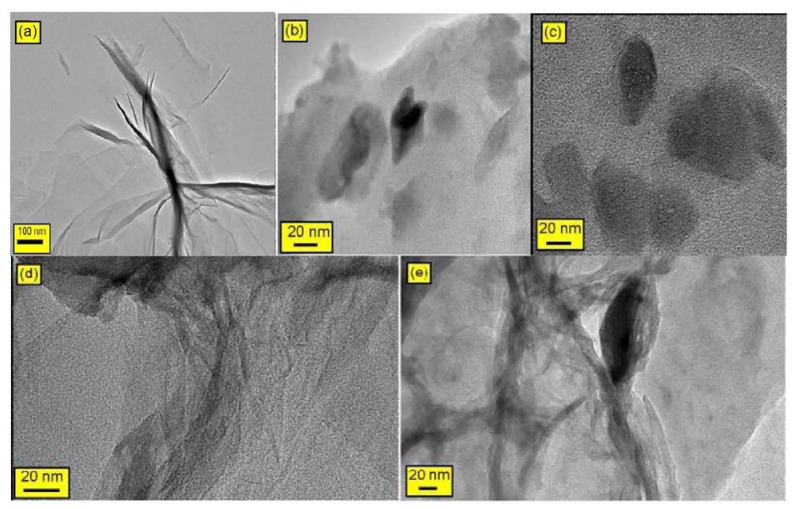
Because SEM cannot spatially resolve the thickness of an individual RGO-based sheet, transmission electron microscopy (TEM) was employed to determine if the RGO-based sheets were indeed present in the composites as single exfoliated sheets or as multi-layered sheets [6]. TEM offers direct evidence for the formation of the RGO nanosheets on the PMMA nanocomposites. Figure 6 displays the TEM images of all of the composites and confirm the presence of RGO sheets in the PMMA matrix. In the case of R-(GO-PMMA), Figure 6e, it is clearly shown that RGO is better dispersed into the PMMA matrix compared to the case of RGO-(PMMA) (Figure 6d). The XRD, SEM and TEM results suggest that the RGO sheets were dispersed in the PMMA matrix, which indicates good compatibility between the nanosheet and polymer matrix. This result may be attributed to the covalent interactions between the RGO nanosheet and PMMA matrix [18,19,46,47]. The de-lamination, intercalation and exfoliation of the RGO sheets was enhanced and improved with MWI and showed better dispersion of the RGO filler in the PMMA matrix, which should enhance the thermal properties of the RGO-(PMMA) nanocomposites.
Figure 6.
The TEM micrographs of (a) RGO; (b) neat PMMA; (c) GO-PMMA; (d) RGO-(PMMA) nanocomposites; and (e) R-(GO-PMMA) nanocomposites.
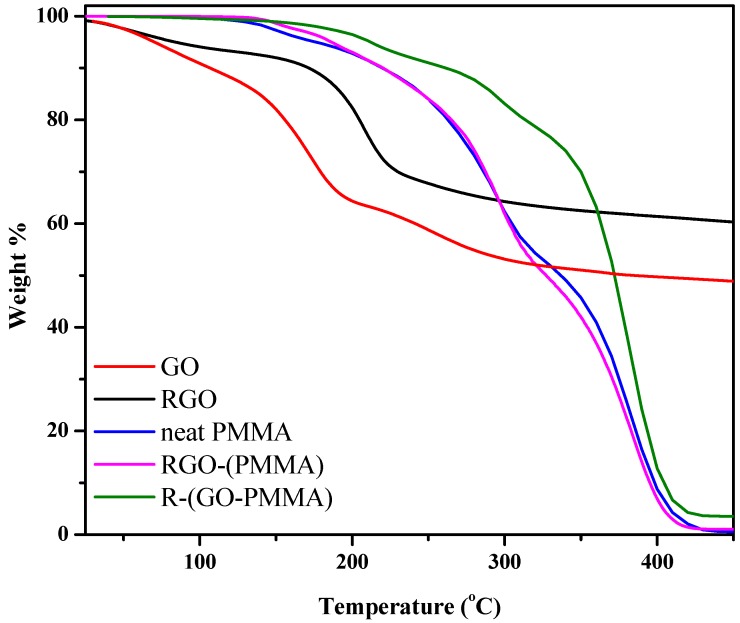
To investigate the effect of the extent of interaction between the PMMA matrix and RGO on the composite properties, we compared the thermal degradation of the polymer itself with the composites. The thermal degradation studies of the GO, RGO, neat PMMA, RGO-(PMMA) and R-(GO-PMMA) nanocomposites were performed using thermogravimetric analysis (TGA) under a N2 atmosphere. The results are displayed in Figure 7 and summarized in Table 2. The results indicated that GO (Figure 7a) was not thermally stable and exhibited a significant mass loss (22%) at 160 °C due to the pyrolysis of the oxygen-containing functional groups generating CO, CO2 and steam [48,49]. However, the TGA curve of the RGO (Figure 7b) showed enhanced thermal stability compared with GO due to the removal of oxygen-containing functional groups by hydrazine reduction.
Figure 7.
TGA thermograms of neat PMMA, RGO-(PMMA) and R-(GO-PMMA) nanocomposites.
Table 2.
Summary of the thermal behavior data obtained from TGA and DSC measurements.
| Sample |
Td
a (°C) |
Tmax
b (°C) |
Tg (°C) |
|---|---|---|---|
| GO | 107 | 160 | --- |
| RGO | 172 | 210 | --- |
| PMMA | 290 | 335 | 117 |
| RGO-(PMMA) | 295 | 326 | 94 |
| R-(GO-PMMA) | 299 | 372 | 120 |
a: The degradation temperature at a 10% weight loss in the decomposition stage. b: The maximum degradation temperature at a 50% weight loss in the decomposition stage.
The TGA results of the RGO-(PMMA) nanocomposites showed a decrease of 9 °C in the maximum degradation temperature (temperature at 50% mass loss, Tmax) from 335 to 326 °C compared with the neat PMMA. For the R-(GO-PMMA) nanocomposites (Figure 7e), the curve indicated that they were more thermally stable, with a maximum degradation temperature (Tmax = 372 °C) that was 37 °C higher than that of neat PMMA. We attributed this result to the homogenous dispersion of the RGO filler within the PMMA matrix, and this homogeneity was enhanced by MWI.
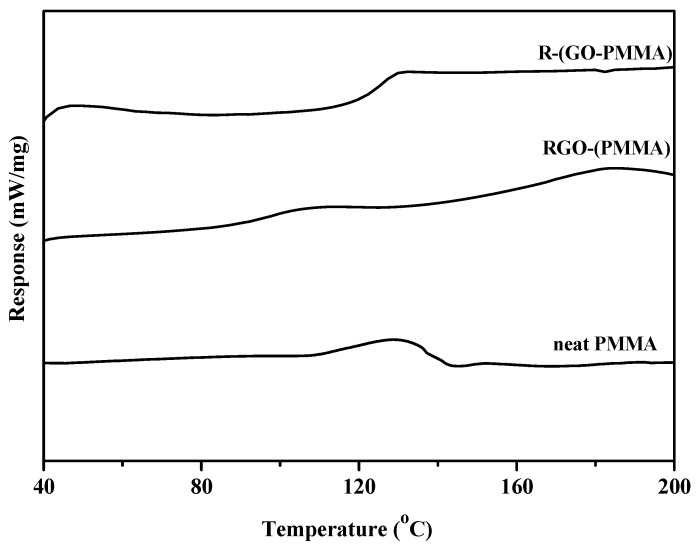
To further understand the thermal behavior and homogeneity of the nanocomposites prepared by the two different methods, differential scanning calorimetry (DSC) of the neat PMMA, RGO-(PMMA) and R-(GO-PMMA) nanocomposites was employed to compare the glass transition temperature (Tg) of the polymer itself and with the nanocomposites.
The Tg values obtained from the DSC thermograms are displayed Figure 8 and summarized in Table 2. For the RGO-(PMMA) nanocomposites (Figure 8b), the results showed that the Tg value of the nanocomposites after loading the RGO decreased significantly (by 23 °C). This value is lower than that of the neat PMMA (Figure 8a). However, when we used the second approach in which the nanocomposites were prepared using MWI, the R-(GO-PMMA) nanocomposites (Figure 8c) showed a significantly improved thermal stability, with a Tg of 120 °C, which 26 °C higher than that of the RGO-(PMMA) nanocomposites and 3 °C than that of the neat PMMA. This result suggests a strong interaction between the PMMA chains and RGO.
Figure 8.
DSC thermograms of neat PMMA, RGO-(PMMA) and reduced R-(GO-PMMA) nanocomposites.
Previous work has shown that the interfacial strength between nanofillers and polymers and consequently, the thermal properties of nanocomposites, can be altered by varying the sample preparation method [37,50]. In this work, when MWI was used, the Tg increased upon addition of the RGO nanofiller, which may be due to either restriction in the chain mobility resulting from the confinement effect of the 2D-layered RGO incorporated into the matrix or strong nanofiller-polymer interaction [51,52,53]. Therefore, good dispersion without agglomeration of RGO may result from the fast thermal reduction process that is offered by MWI. When we employed the in situ bulk polymerization approach to prepare the RGO-(PMMA) nanocomposites, the Tg decreased, which may be associated with the agglomeration of the RGO nanosheets due to the van der Waals forces between the RGO layers, leading to an increase in the mobility of the polymer chain resulting from an increase in the free volume, which has been observed with the addition of a plasticizer [50,53,54].
3. Experimental
3.1. Materials
Extra pure graphite powder (>99.5%) was purchased from Merck (Darmstadt, Germany), and hydrazine hydrate (HH, 80%) was obtained from Loba Chemi. Pvt. Ltd (Mumbai, India). Methyl methacrylate (MMA, Acros Chemical Co., Loughborough, UK, 99%) was kept in a refrigerator and used as received. Benzoyl peroxide (BP, BDH Chemicals Ltd., East Yorkshire, UK) was used as an initiator. Potassium permanganate (KMNO4, >99%) and hydrogen peroxide (H2O2, 30%) were obtained from Merck. Other solvents and chemicals were of analytical grade and used without further purification.
3.2. Preparation of Graphite Oxide (GO)
GO was synthesized by the oxidation of graphite powder via the Hummers and Offeman method [55]. Natural graphite (3.5 g) was added to 98% H2SO4 (100 mL) under vigorous stirring. Next KMNO4 (10 g) was slowly added, and the temperature was maintained below 20 °C. The stirring was continued for 2 h at 35 °C. Then, the content of the flask was poured into deionized water (500 mL), and a sufficient amount of H2O2 (ca. 20 mL of a 30% aqueous solution) was added to destroy any excess permanganate. Upon treatment with the peroxide, the suspension turned bright yellow. GO was isolated by filtration through a sintered glass filter. The product was thoroughly washed with dilute HCl and then hot water to remove the residual sulfate ions yielding a yellow-brown filter cake. After repeated washing of the resulting yellowish-brown cake with hot water, the GO was dried at 80 °C.
3.3. Preparation of RGO
The dried GO (400 mg) was stirred and sonicated in deionized water (20 mL) until a homogeneous yellow dispersion was obtained. The GO can be dispersed easily in water due to the presence of a variety of hydrophilic oxygen groups (OH, O and COOH) on the basal planes and edges. The solution was placed inside a conventional microwave after the addition of HH reducing agent (400 μL). The microwave oven (KenWood MW740) was operated at full power (900 W) in 30 s cycles (on for 10 s and off and stirring for 20 s) for a total reaction time of 2 min [16]. The yellow dispersion of GO gradually changed to a black color indicating the completion of the chemical reduction to RGO. The RGO sheets were separated using a centrifuge (Centurion Scientific Ltd., West Sussex, UK) operated at 5,000 rpm for 15 min and dried at 80 °C overnight.
3.4. Preparation of the RGO-(PMMA) Nanocomposites via the in Situ Method
RGO powder [2.0 (wt./wt.%)] was added to the MMA monomer, stirred and sonicated for 1 h. The benzoyl peroxide (BP) initiator (5.0 wt.%) was added to the suspension and stirred until the initiator dissolved. Then, the mixture was heated to 60 °C to initiate the polymerization using a shaking-water bath (GFL, Burgwedel, Germany). The reaction mixture was maintained at 60 °C for 20 h. After the polymerization finished, the product was poured into an excess of methanol, stirred for 15 min and washed with hot water to remove the MMA monomers. Then, the product was filtered and dried at 80 °C overnight.
3.5. Preparation of the R-(GO-PMMA) Nanocomposites via the MWI Method
GO powder [2.0 (wt./wt.%)] was added to the MMA monomer, stirred and sonicated for 1 h. The benzoyl peroxide (BP) initiator (5.0 wt.%) was added to the suspension and stirred until the initiator dissolved. Then, the reaction mixture was maintained at 60 °C for 20 h to promote polymerization using a shaking-water bath (GFL). After the polymerization finished, the product was poured into an excess of methanol, stirred for 15 min and washed with hot water to remove MMA monomers. Then, the product was filtered and dried at 80 °C overnight. Four hundred milligrams of the dried composite of GO-PMMA was dissolved in DMF, stirred and sonicated for 1 h. Then, the composite was placed inside a conventional microwave oven (Kenwood MW740) following the addition of HH (400 µL). The microwave oven was operated at full power (900 W) in 30 s cycles (on for 10 s and off and stirring for 20 s) for a total reaction time of 2 min [16]. Then, the composites were separated using a centrifuge (Centurion Scientific Ltd.) operated at 5,000 rpm for 15 min and dried in an oven at 80 °C overnight. For comparison, the neat PMMA was prepared via a similar procedure in the absence of the RGO and GO.
3.6. Characterization and Instrumentation
The FTIR (Thermo Scientific Nicolet-iS10, Madison, WI, USA) spectra of the composites were recorded in the range of 4000–500 cm−1. The 1H-NMR of the solutions was recorded on a Bruker Avance (III) instrument (Bruker, Milton-Ontario, Canada) at 200 MHz using CDCl3 as the solvent, and the composites were macerated in the solvent for 1 day. The Raman spectra of composites were measured with a Bruker Equinox 55 FT-IR spectrometer equipped with an FRA106/S FT-Raman module and a liquid N2-cooled Ge detector using the 1064 nm line of a Nd:YAG laser with an output laser power of 200 mW. The thermogravimetric analyses (TGA) of the composites were studied using a NETZCH 209 F1 thermogravimetric analyzer (Netzsch, Selb, Germany). The X-ray diffraction (Model PW 1729, Philips, Amsterdam, The Netherlands) of the composites were investigated with Cu radiation [30 kV, 40 mA, Kα radiation (λ = 1.54430 Å)] between 2θ of 5° and 100°. The decomposition temperature measurements using TGA were performed under an N2 atmosphere at a heating rate of 10 °C per minute from 25 °C to 800 °C. Differential scanning calorimetry (DSC, NETZCH 204 F1) measurements were employed to estimate the glass-transition temperature (Tg) of each composite. The composites were heated from −25 °C to 100 °C at a heating rate of 10 °C per min. Then, a double run was performed after cooling at a heating rate of 2 °C per min from 25 °C to 350 °C. The Tg was taken as the midpoint of the transition. A scanning electron microscope (SEM, FEI Quanta 200, FEI, Hillsboro, OR, USA) was employed to study the morphology of the composites after they were mounted on the composite slabs and coated with gold via sputtering system (Polaron E6100, Bio-Rad, Birmingham, UK). Ultrathin sections of the composites were prepared for transmission electron microscopy (TEM) studies; the transmission electron microscope (JEOL JSM-2100F, JEOL, Tokyo, Japan) was operated at 200 kV.
4. Conclusions
In summary, we have successfully prepared R-(GO-PMMA) and RGO-(PMMA) using in situ bulk polymerization facilitated by MWI. Thermal analysis showed an enhancement in the thermal properties of the RGO/PMMA nanocomposite prepared using MWI, which indicates that the RGO sheets efficiently reinforced the PMMA matrix. Therefore, our approach is promising for the development of a new class of graphene–polymer nanocomposites.
Acknowledgments
This study is part of research Project No. 405010901101. The authors gratefully acknowledge the continued support from Alfaisal University and its Office of Research. In addition, the authors wish to thank King Abdul Aziz City of Science and Technology (KACST) for the XRD, SEM and TEM characterization.
Footnotes
Sample Availability: Samples of the compounds are available from the coressponding author.
References
- 1.Geim A.K., Novoselov K.S. The rise of graphene. Nat. Mater. 2007;6:183–191. doi: 10.1038/nmat1849. [DOI] [PubMed] [Google Scholar]
- 2.Paul D.R., Robeson L.M. Polymer nanotechnology: Nanocomposites. Polymer. 2008;49:3187–3204. doi: 10.1016/j.polymer.2008.04.017. [DOI] [Google Scholar]
- 3.Dresselhaus M.S., Dresselhaus G. Intercalation compounds of graphite. Adv. Phys. 2002;51:1–186. doi: 10.1080/00018730110113644. [DOI] [Google Scholar]
- 4.Balandin A.A., Ghosh S., Bao W., Calizo I., Teweldebrhan D., Miao F., Lau C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008;8:902–907. doi: 10.1021/nl0731872. [DOI] [PubMed] [Google Scholar]
- 5.Park S., Ruoff R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009;4:217–224. doi: 10.1038/nnano.2009.58. [DOI] [PubMed] [Google Scholar]
- 6.Stankovich S., Dikin D.A., Dommett G.H.B., Kohlhaas K.M., Zimney E.J., Stach E.A., Piner R., Nguyen S.T., Ruoff R.S. Graphene-based composite materials. Nature. 2006;442:282–286. doi: 10.1038/nature04969. [DOI] [PubMed] [Google Scholar]
- 7.Ramanathan T., Abdala A.A., Stankovich S., Dikin D.A., Herrera-Alonso M., Piner R.D., Adamson D.H., Schniepp H.C., Chen X., Ruoff R.S., et al. Functionalized graphene sheets for polymer nanocomposites. Nat. Nanotechnol. 2008;3:327–331. doi: 10.1038/nnano.2008.96. [DOI] [PubMed] [Google Scholar]
- 8.Dikin D.A., Stankovich S., Zimney E.J., Piner R., Dommett G.H.B., Evmenenko G., Nguyen S.T., Ruoff R.S. Preparation and characterization of graphene oxide paper. Nature. 2007;448:457–460. doi: 10.1038/nature06016. [DOI] [PubMed] [Google Scholar]
- 9.Park S., Lee K.-S., Bozoklu G., Cai W., Nguyen S.T., Ruoff R.S. Graphene oxide papers modified by divalent ions-enhancing mechanical properties via chemical cross-linking. ACS Nano. 2008;2:572–578. doi: 10.1021/nn700349a. [DOI] [PubMed] [Google Scholar]
- 10.Chen H., Muller M.B., Gilmore K.J., Wallace G.G., Li D. Mechanically strong, Electrically Conductive, And biocompatible graphene paper. Adv. Mater. 2008;20:3557–3561. doi: 10.1002/adma.200800757. [DOI] [Google Scholar]
- 11.Singh S., Joung D., Zhai L., Das S., Khondaker S.I., Seal S. Graphene based materials: past, present and future. Prog. Mater. Sci. 2011;56:1178–1271. doi: 10.1016/j.pmatsci.2011.03.003. [DOI] [Google Scholar]
- 12.Huang X., Zongyou Y., Shixin W., Xiaoying Q., Qiyuan H., Qichun Z. Graphene-based materials: synthesis, characterization, properties and applications. Small. 2011;7:1876–1902. doi: 10.1002/smll.201002009. [DOI] [PubMed] [Google Scholar]
- 13.Matusinovic Z., Rogosic M., Sipusic J. Synthesis and characterization of poly(styrene-co-methyl methacrylate)/layered double hydroxide nanocomposites via in situ polymerization. Polym. Degrad. Stabil. 2009;94:95–101. doi: 10.1016/j.polymdegradstab.2008.10.001. [DOI] [Google Scholar]
- 14.Aerdts A.M., Haan J.W., German A.L. Proton and carbon NMR spectra of alternating and statistical styrene-methyl methacrylate copolymers revisited. Macromolecules. 1993;26:1965–1971. doi: 10.1021/ma00060a025. [DOI] [Google Scholar]
- 15.Potts J.R., Dreyer D.R., Bielawski C.W., Ruoff R.S. Graphene-based polymer nanocomposites. Polymer. 2011;52:5–25. doi: 10.1016/j.polymer.2010.11.042. [DOI] [Google Scholar]
- 16.Hassan H., Abdelsayed V., Khder A., AbouZeid K., Terner J., El-Shall M., Al-Resayes S., El-Azhary A. Microwave synthesis of graphene sheets supporting metal nanocrystals in aqueous and organic media. J. Mater. Chem. 2009;19:3832–3837. doi: 10.1039/b906253j. [DOI] [Google Scholar]
- 17.Jang J.Y., Kim M.S., Jeong H.M., Shin C.M. Graphite oxide/poly(methyl methacrylate) nanocomposites prepared by a novel method utilizing macroazoinitiator. Comp. Sci. Tech. 2009;69:186–191. doi: 10.1016/j.compscitech.2008.09.039. [DOI] [Google Scholar]
- 18.Yuan X.Y., Zou L.L., Liao C.C., Dai J.W. Improved properties of chemically modified graphene/poly(methyl methacrylate) nanocomposites via a facile in-situ bulk polymerization. Express Polym. Lett. 2012;6:847–858. doi: 10.3144/expresspolymlett.2012.90. [DOI] [Google Scholar]
- 19.Wang J., Hu H., Wang X., Xu C., Zhang M., Shang X. Preparation and mechanical and electrical properties of graphene nanosheets–poly(methyl methacrylate) nanocomposites via in situ suspension polymerization. J. App. Poly. Sci. 2011;122:1866–1871. doi: 10.1002/app.34284. [DOI] [Google Scholar]
- 20.Lee C., Wei X., Kysar J., Hone J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science. 2008;321:385–388. doi: 10.1126/science.1157996. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y., Tan Y.-W., Stormer H.L., Kim P. Experimental observation of the quantum Hall effect and Berry’s phase in graphene. Nature. 2005;438:201–204. doi: 10.1038/nature04235. [DOI] [PubMed] [Google Scholar]
- 22.Pasupuleti S., Medras G. Ultrasonic degradation of poly (styrene-co-alkyl methacrylate) copolymers. Ultrason. Sonochem. 2010;17:819–826. doi: 10.1016/j.ultsonch.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Mehmet C., Seven P. Synthesis, Characterization and investigation of dielectric properties of two-armed graft copolymers prepared with methyl methacrylate and styrene onto PVC using atom transfer radical polymerization. Reac. Funct. Polym. 2011;71:395–401. doi: 10.1016/j.reactfunctpolym.2010.12.012. [DOI] [Google Scholar]
- 24.El-Shall M.S., Abdelsayed V., Khder A.S., Hassan H., El-Kaderi H.M., Reich T.E. Metallic and bimetallic nanocatalysts incorporated into highly porous coordination polymer MIL-101. J. Mater. Chem. 2009;19:7625–7631. doi: 10.1039/b912012b. [DOI] [Google Scholar]
- 25.Potts J.R., Lee S.H., Alam T.M., An J., Stoller M.D., Piner R.D., Ruoff R.S. Thermomechanical properties of chemically modified graphene/poly(methyl methacrylate) composites made by in situ polymerization. Carbon. 2011;49:2615–2623. doi: 10.1016/j.carbon.2011.02.023. [DOI] [Google Scholar]
- 26.Priestley R.D., Ellison C.J., Broadbelt L.J., Torkelson J.M. Structural relaxation of polymer glasses at surfaces, interfaces, and in between. Science. 2005;309:456–459. doi: 10.1126/science.1112217. [DOI] [PubMed] [Google Scholar]
- 27.Pavia D.L., Lampman G.M., Kriz G.S. A Guide for Students for Organic Chemistry. Introduction of Spectroscopy. 3rd ed. Saunder College Publishing; Philadelphia, PA, USA: 1979. [Google Scholar]
- 28.Socrates G. Infrared Characteristic Group Frequencies. 2nd ed. John Wiley & Sons; Chichester, UK: 2003. [Google Scholar]
- 29.Titelman G.I., Gelman V., Bron S., Khalfin R.L., Cohen Y., Bianco-Peled H. Characteristics and microstructure of aqueous colloidal dispersions of graphite oxide. Carbon. 2005;43:641–649. doi: 10.1016/j.carbon.2004.10.035. [DOI] [Google Scholar]
- 30.Li D., Müller M.B., Gilje S., Kaner R.B., Wallace G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008;3:101–105. doi: 10.1038/nnano.2007.451. [DOI] [PubMed] [Google Scholar]
- 31.Sikka M., Pellegrini N.N., Schmitt E.A., Winey K.I. Modifying a polystyrene/poly(methyl methacrylate) interface with poly(styrene-co-methyl methacrylate) random copolymers. Macromolecules. 1997;30:445–455. doi: 10.1021/ma961302h. [DOI] [Google Scholar]
- 32.Marrs B., Andrews R., Pienkowski D. Multiwall carbon nanotubes enhance the fatigue performance of physiologically maintained methyl methacrylate–styrene copolymer. Carbon. 2007;45:2098–2104. doi: 10.1016/j.carbon.2007.05.013. [DOI] [Google Scholar]
- 33.Zeng X., Yang J., Yuan W. Preparation of a poly(methyl methacrylate)-reduced graphene oxide composite with enhanced properties by a solution blending method. Eur. Polym. J. 2012;48:1674–1682. doi: 10.1016/j.eurpolymj.2012.07.011. [DOI] [Google Scholar]
- 34.Gudarzi M., Sharif F. Enhancement of dispersion and bonding of graphene-polymer through wet transfer of functionalized graphene oxide. Express Polym. Lett. 2012;6:1017–1031. doi: 10.3144/expresspolymlett.2012.107. [DOI] [Google Scholar]
- 35.Morejón L., Mendizábal E., Delgado J.A., Davidenko N., Dellamary L.F., Manríquez R., Ginebra M.P., Gil F.J., Planell J.A. Synthesis and characterization of poly (methyl methacrylate-styrene) copolymeric beads for bone cements. Lat. Am. Appl. Res. 2005;35:175–182. [Google Scholar]
- 36.Fang M., Wang K., Lu H., Yang Y., Nutt S. Exfoliated graphite oxide decorated by PDMAEMA chains and polymer particles. J. Mater. Chem. 2010;20:1982–1992. doi: 10.1039/b919078c. [DOI] [PubMed] [Google Scholar]
- 37.Gilije S., Han S., Wang M., Wang K.L., Kaner R.B. A chemical route to graphene for device applications. Nano Lett. 2007;7:3394–3398. doi: 10.1021/nl0717715. [DOI] [PubMed] [Google Scholar]
- 38.Fan B., Peng W., Li Y., Li X., Wang S., Zhang G., Zhang F. Deoxygenation of exfoliated graphite oxide under alkaline conditions: A green route to graphene preparation. Adv. Mater. 2008;20:4490–4493. doi: 10.1002/adma.200801306. [DOI] [Google Scholar]
- 39.McAllister M., Li J., Adamson D., Schniepp H., Abdala A., Herrera-Alonso M., Milius D., Car R., Prudhomme R., Aksay I. Single sheet functionalized graphene by oxidation and thermal expansion of graphite. Chem. Mater. 2007;19:4396–4404. doi: 10.1021/cm0630800. [DOI] [Google Scholar]
- 40.Subrahmanyam K., Vivekchand S., Govindaraj A., Rao C. A study of graphenes prepared by different methods: characterization, properties and solubilization. J. Mater. Chem. 2008;18:1517–1523. doi: 10.1039/b716536f. [DOI] [Google Scholar]
- 41.Zhang R., Hu Y., Xu J., Fan W., Chen Z. Flammability and thermal stability studies of styrene–butyl acrylate copolymer/graphite oxide nanocomposite. Polym. Degrad. Stabil. 2004;85:583–588. doi: 10.1016/j.polymdegradstab.2004.01.020. [DOI] [Google Scholar]
- 42.Fang M., Wang K., Lu H., Yang Y., Nutt S. Covalent polymer functionalization of graphene nanosheets and mechanical properties of composites. J. Mater. Chem. 2009;19:7098–7105. doi: 10.1039/b908220d. [DOI] [Google Scholar]
- 43.Cao Y., Feng J., Wu P. Preparation of organically dispersible graphene nanosheet powders through a lyophilization method and their poly(lactic acid) composites. Carbon. 2010;48:3834–3839. doi: 10.1016/j.carbon.2010.06.048. [DOI] [Google Scholar]
- 44.Oh W.C., Chen M.L., Zhang K., Zhang F.J. The Effect of thermal and ultrasonic treatment on the formation of graphene-oxide nanosheets. J. Korean Phys. Soc. 2010;56:1097–1102. doi: 10.3938/jkps.56.1097. [DOI] [Google Scholar]
- 45.Stankovich S., Dikin D., Piner R., Kohlhaas K., Kleinhammes A., Jia Y., Wu Y., Nguyen S., Ruoff R. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon. 2007;45:1558–1565. doi: 10.1016/j.carbon.2007.02.034. [DOI] [Google Scholar]
- 46.Ramanathan T., Stankovich S., Dikin D.A., Liu H., Shen H., Nguyen S.T., Brinson L.C. Graphitic nanofillers in PMMA nanocomposites-an investigation of particle size and dispersion and their influence on nanocomposite properties. J. Polym. Sci. Pol. Phys. 2007;45:2097–2112. doi: 10.1002/polb.21187. [DOI] [Google Scholar]
- 47.Yuan X. Enhanced interfacial interaction for effective reinforcement of poly(vinyl alcohol) nanocomposites at low loading of graphene. Polym. Bull. 2011;67:1785–1797. doi: 10.1007/s00289-011-0506-z. [DOI] [Google Scholar]
- 48.Salavagione H.J., Martínez G., Ellis G. Recent advances in the covalent modification of graphene with polymers. Macromol. Rapid Comm. 2011;32:1771–1789. doi: 10.1002/marc.201100527. [DOI] [PubMed] [Google Scholar]
- 49.Lerf A., He H., Forster M., Klinowski J. Structure of graphite oxide revisited. J. Phys. Chem. B. 1998;102:4477–4482. doi: 10.1021/jp9731821. [DOI] [Google Scholar]
- 50.Jeong H.M., Choi M.Y., Kim M.S., An J.H., Jung J.S., Kim J.H., Kim B.K., Cho S.M. Styrenic polymer/organoclay nanocomposite prepared via in-situ polymerization with an azoinitiator linked to an epoxy oligomer. Macromol. Res. 2006;14:610–616. doi: 10.1007/BF03218732. [DOI] [Google Scholar]
- 51.Gao W., Alemany L.B., Ci L., Ajayan P.M. New insights into the structure and reduction of graphite oxide. Nat. Chem. 2009;1:403–408. doi: 10.1038/nchem.281. [DOI] [PubMed] [Google Scholar]
- 52.Uthirakumar P., Nahm K.S., Hahn Y.B., Lee Y.-S. Preparation of polystyrene/montmorillonite nanocomposites using a new radical initiatormontmorillonite hybrid via in situ intercalative polymerization. Eur. Polym. J. 2004;40:2437–2444. doi: 10.1016/j.eurpolymj.2004.06.013. [DOI] [Google Scholar]
- 53.Pramoda K.P., Hussain H., Koh H.M., Tan H.R., He C.B. Covalent bonded polymer–graphene nanocomposites. J. Polym. Sci. Pol. Chem. 2010;48:4262–4267. doi: 10.1002/pola.24212. [DOI] [Google Scholar]
- 54.Ellison C.J., Torkelson J.M. The distribution of glass-transition temperature in nanoscopically confined glass formers. Nat. Mater. 2003;2:695–700. doi: 10.1038/nmat980. [DOI] [PubMed] [Google Scholar]
- 55.Hummers W.S., Offeman R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958;80:1339–1339. doi: 10.1021/ja01539a017. [DOI] [Google Scholar]