Abstract
An efficient synthesis of novel dispirooxindoles has been achieved through three-component 1,3-dipolar cycloaddition of azomethine ylides generated in situ by the decarboxylative condensation of isatin and an α-amino acid with the dipolarophile 5-benzylideneimidazolidine-2,4-dione. The improved procedure features mild reaction conditions, high yields, high diastereoselectivities, a one-pot procedure and operational simplicity.
Keywords: multicomponent reactions; 1,3-dipolarcycloaddition; azomethine ylide; dispirooxindole
1. Introduction
Nowadays, conventional one-pot, multicomponent reactions (MCRs) are considered to be one of the most efficient strategies in organic and medical chemistry for synthesizing structurally diverse compounds and biologically active natural products, usually in a stereoselective-manner [1]. The highly effective one-pot procedure of MCRs exhibits many advantages, including atom economy, facile synthesis, convergence, productivity and easy execution [2]. MCRs were a common method for building molecular diversities with complex scaffolds and had broad applications in combinatorial chemistry, which allowed a rapid access to identify a promising lead molecule in drug candidate discovery [3,4,5].
The 1,3-dipolar cycloaddition of azomethine ylides with olefinic and acetylenic dipolarophiles is one of the most useful MCRs for providing an useful approach to building nitrogen-containing five-membered ring heterocycles, such as pyrroline, pyrrolidine, pyrrolizidine or spirooxindole derivatives [6,7,8,9], which served as useful molecular scaffolds for the exploration and exploitation of pharmacophore space via diversity-oriented synthesis [10,11,12,13]. Among of them, dispirooxindole ring systems possess more interesting structural properties and have been reported to exhibit strong bioactivity profiles including antimicrobial [14], antitumoral [15], anti-inflammatory [16], anti-HIV [17] and potent non-peptide inhibition of the p53–MDM2 interaction [18].
Hydantoin derivatives are widely used in malignant hyperthermia, neuroleptic malignant syndrome, spasticity, and anticonvulsants [19,20,21,22], especially the spirohydantoins, which are considered to be a novel aldose reduetase inhibitor to treat for diabetes [23]. Significant efforts have been focused on developing a general synthetic route to access those compounds. However, to the best of our knowledge, only a few methods were reported to synthesize the spiropyrrolidine bisoxindoles [24,25], and a general method to prepare dispirooxindole hydantoin derivatives is still lacking. Thus, in order to extend our interest in cycloaddition reactions of novel spiro compounds and nitrogen heterocycles with biological activities [26,27,28], we report herein the efficient synthesis in excellent yields of a series of novel dispirooxindole derivatives by the three-component 1,3-dipolar cycloaddition reaction of nonstabilized azomethine ylides generated in situ by the decarboxylative condensation of isatin and primary α-amino acid with the Knoevenagel adduct derivatives (preformed by reaction of hydantoin with substituted benzaldehydes).
2. Results and Discussion
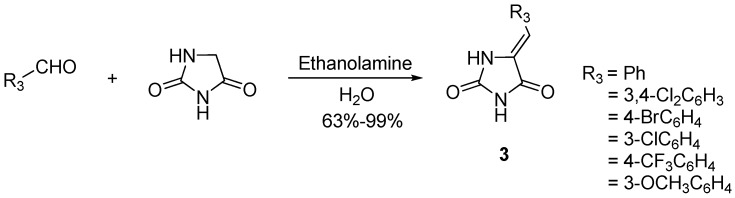
In our initial endeavor, the Knoevenagel adducts 3 were synthesized via a method involving condensation of commercially available hydantoin and substituted benzaldehydes in water using ethanolamine as catalyst. After work-up, the crude reaction mixtures were purified by recrystallization in ethanol/water (40:60) to afford 63-99% total yields of the target products (Scheme 1). Melting point, NMR and mass spectrometry data were consistent with those reported in the literature [29].
Scheme 1.
The synthetic route to compounds 3.
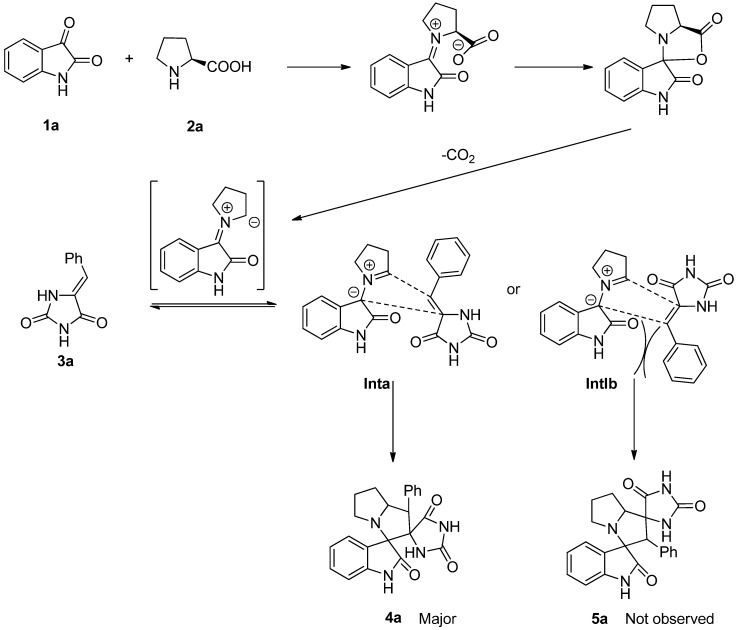
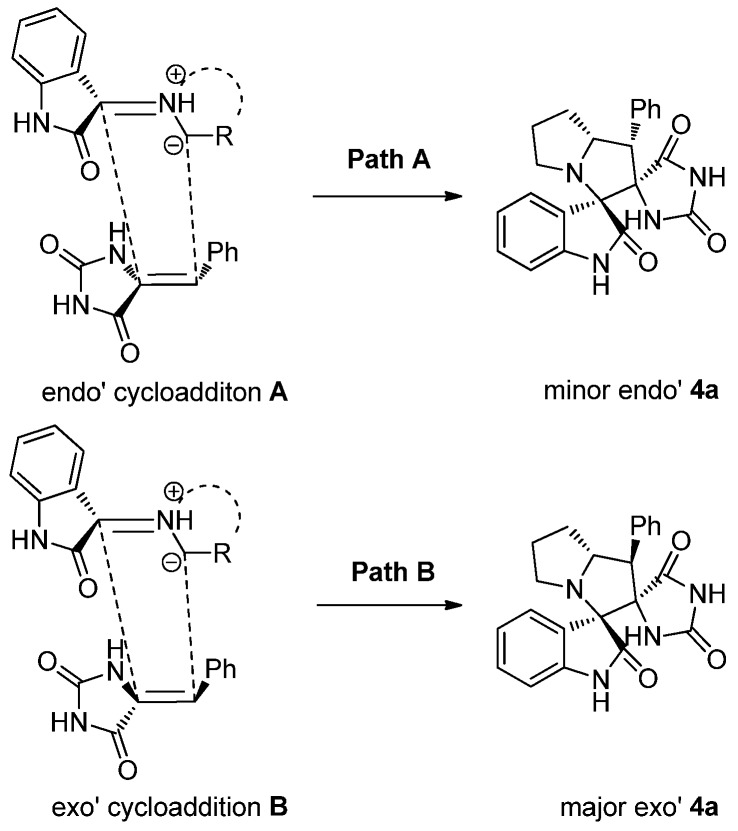
From the mechanistic perspective, the azomethine ylides, a powerful class of reagents, have featured in a number of 1,3-dipolar cycloaddition reactions. In combination with the experiences from our previous work, we envisaged that an azomethine ylide could be generated in situ from isatin (1a) and L-proline (2a), and then trapped with Knoevenagel adduct 5-benzylideneimidazolidine-2,4-dione (3a) acting as dipolarophile, to afford spiropyrrolizidine oxindole 4a. Hence, the 1,3-dipolar cycloaddition reaction would be facilitated in one-pot with two steps. Although the azomethine ylides, generated from the reaction of isatin and L-proline, have two nucleophilic carbons potentially resulting in two regioisomers. However, high regioselectivity was observed in the formation of the product (see below). It may resulted from the more stable transition state (Inta) leading to the observed products (4a). Meanwhile, the other possible one (Intb) would be less stable because of steric interactions between the aryl ring of Knoevenagel adducts and isatin backbone (Scheme 2).
Scheme 2.
Possible reaction mechanism for the synthesis of dispirooxindole hydantoin derivatives.
In an effort to identify the reaction parameters of this one-pot process, the three-component reaction of isatin, L-proline and 5-benzylideneimidazolidine-2,4-dione was carried out as model reaction. Firstly, various solvents were examined under 80 °C,and the results were summarized in Table 1. Acetonitrile and ether solvents such as dioxane and THF gave moderate yields (entries 1–3), and with toluene as solvent, a poor yield resulted (entry 4). To our delight, the alcohol solvent methanol gave promising yields, and ethanol afforded even better results (entries 5–6). Ethanol may facilitate the production of the azomethine ylide by accelerating the formation of an iminium species between isatin and L-proline. Then the reaction temperature, mixed solvent and time were further investigated, the desired product was finally obtained as a single regioisomer in almost quantitative yield (95%) after 10 hours at 50 °C (entries 7–9). Consequently, we chose these conditions for the rest of our studies.
Table 1.
Optimization of reaction conditions a.
| Entry | Solvent | Temp (°C) | Yield b (%) |
|---|---|---|---|
| 1 | 1,4-dioxane | 80 | 33 |
| 2 | THF | 80 | 42 |
| 3 | CH3CN | 80 | 31 |
| 4 | Toluene | 80 | 17 |
| 5 | Methanol | reflux | 57 |
| 6 | Ethanol | 80 | 68 |
| 7 | Ethanol/H2O | 100 | 55 |
| 8 | Ethanol | 50 | 74 |
| 9 c | Ethanol | 50 | 95 |
a Unless indicated otherwise, the reaction was performed with 3a (0.5 mmol), 1a isatin (0.5 mmol), and L-proline (0.5 mmol) in different solvents (10.0 mL) and temperatures for 5 h. b Isolated yield based on isatin. c 10 h.
With the optimized reaction conditions in hand, various structurally diverse 5-benzyli- deneimidazolidine-2,4-diones 3 were investigated. Gratifyingly, the corresponding spiro-pyrrolidine products 4a–4m were obtained as single diastereoisomers in high yields. As shown in Table 2, different substitutents on the aryl ring, such as bromo, chloro, CF3 and OCH3 groups at the meta or para positons all gave corresponding products 4e, 4i, 4l and 4m in high yields ranging from 85% to 90% (entries 5, 9, 12 and 13). Furthermore, different substituents in the isatin such as 5-F, -Cl or -Br also reacted smoothly to generate the desired products in high yields (entries 3, 6, 7 and 10). More interestingly, the unprotected isatin gave even better results (entry 1 vs. 2), which opens a door to further functionalize the products in future medicinal chemistry studies.
Table 2.
Scope of the reaction.
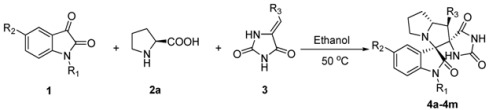
| Entry | 1 | 3 | 4 | Yield (%) |
|---|---|---|---|---|
| 1 | R1 = R2 = H | R3 = Ph | 4a | 93 |
| 2 | R1 = CH2C6H4, R2 = H | R3 = Ph | 4b | 89 |
| 3 | R1 = H, R2 = Br | R3 = Ph | 4c | 92 |
| 4 | R1 = CH2C6H4, R2 = H | R3 = 3,4-Cl2C6H3 | 4d | 83 |
| R1 = R2 = H | ||||
| 5 | R1 = H, R2 = F | R3 = 4-BrC6H4 | 4e | 90 |
| 6 | R1 = H, R2 = Cl | R3 = Ph | 4f | 91 |
| 7 | R1 = CH3, R2 = H | R3 = Ph | 4g | 84 |
| 8 | R1 = R2 = H | R3 = Ph | 4h | 95 |
| 9 | R1 = H, R2 = Cl | R3 = 3-ClC6H4 | 4i | 88 |
| 10 | R1 = CH2C6H4, R2 = H | R3 = 3-ClC6H4 | 4j | 93 |
| 11 | R1 = H R2 = H | R3 = 3-ClC6H4 | 4k | 89 |
| R1 = H R2 = H | ||||
| 12 | R3 = 4-CF3C6H4 | 4l | 85 | |
| 13 | R3 = 4-OCH3C6H4 | 4m | 82 |
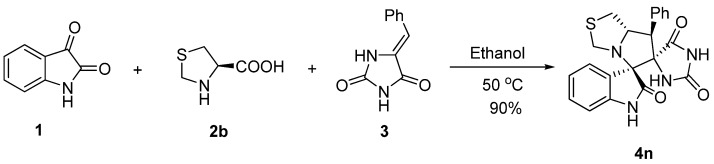
We also studied the cycloaddition reaction of the different amino acid thioproline 2b, wbereby the reaction between isatin 1, 2b and 3 happened smoothly to afford the desired spirothiopyrrolidine 4n as a single diastereoisomer in 90% yield (Scheme 3).
Scheme 3.
MCRs of thioproline 2b.
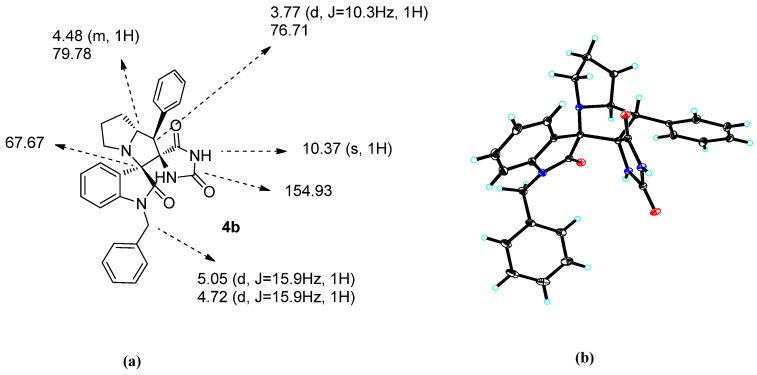
To further confirm the structure, diastereoselectivity and regioselectivity, detailed NMR spectral and X-ray analyses were carried out. The structures proposed for all products were in agreement with their NMR spectra, as discussed for compound 4b as an example In the 1H-NMR spectrum of 4b, the pyrrolidine ring proton of C-5 exhibited a multiplet (m) peak at δ 4.48 (m, 1H). The C-4 proton which was attached to the aryl group appeared as a doublet at δ 3.77 (d, J = 10.3 Hz, 1H). The aromatic protons were distributed in the δ 7.41–6.24 region. The NH proton appeared as a singlet at δ 8.37 and δ 10.37. Based on the calculation of the coupling constant (J-based configuration analysis, J > 10 Hz), the relative configuration of this structure should be as same as compound 4b shown in Figure 1 and the configuration was further confirmed by the X-ray study of a single crystal of compound 4b (Figure 1b). The results revealed that the pyrrolidine ring adopted an envelope form with the spiro carbon being out of plane. The 13C-NMR of compound 4b supported the proposed structure as well. The pyrrolidine ring carbons resonated in the δ 67.67 ppm region. The carbonyl carbon resonated at δ 154.93 ppm, respectively.
Figure 1.
(a) Selected 1H- and 13C-NMR chemical shifts of 4b. (b) Single crystal X-ray diffraction study of compound 4b.
The regioselectivity in formation of 4 can be explained by considering the secondary orbital interaction (SOI) mechanism proposed in Scheme 4 [30]. The reaction proceeds through the generation of azomethine ylide via the condensation of isatin with L-proline and decarboxylation. The dipolarophile 3 regioselectively reacts with azomethine ylides in ethanol to give the desired products compounds 4. The X-ray structure of the product 4b reflects that the cycloaddition proceeds via an exo’-transition state (Scheme 4, path B). This can be explained by the fact that the corresponding endo’-transition state (A) would require more free energy of activation than the exo’-transition state (B) leading to 4a as the former would result in electrostatic repulsion between the cis carbonyls increasing the free energy of activation. Accordingly, the observed regioisomer 4 via path B is more favorable because of the SOI which is not possible in path A [31].
Scheme 4.
Plausible mechanism for the formation of compound 4.
3. Experimental
3.1. General
All reagents were purchased from commercial sources and used without further purification. Melting points are corrected. 1H-NMR spectra were determined on a Bruker Avance III 400MHz spectrometer in DMSO-d6 solution. J values are in Hz. Chemical shifts are expressed in ppm downfield from internal standard TMS. HRMS data were obtained using Bruker micrOTOF-Q instrument or TOF-MS instrument. The starting compounds 3 were prepared according to the previously reported procedures.
3.2. General Procedure for the Synthesis of Dispirooxindoles 4
A dry 50 mL flask was charged with istain derivatives 1(0.5 mmol), L-proline, L-thioproline 2a or 2b (0.5 mmol), and imidazolidin-2-one derivatives 3 (0.5 mmol), and ethanol (10 mL). The mixture was stirred at 50 °C for 10 h. After completion of the reaction (monitored by TLC), the solvent was cooled, then was filtrated and washed by 10 mL of ethanol twice to give solid. The solid was dried at 80 °C for 4h under vacuum to give compounds 4. The structures of the products were identified by 1H-NMR, 13C{1H}-NMR and HRMS spectra. The structure and regiochemistry of the products were assigned on the basis of their spectroscopic analysis.
1-Phenylhexahydro-1H-pyrrolizine-2-spiro-5'-imidazolidine-2',4'-dione-3-spiro-3''-indoline-2''-one (4a). White solid; m.p. 196–198 °C; 1H-NMR (DMSO-d6): δ (ppm) 1.56–1.69 (m, 1H, CH2), 1.73–2.06 (m, 3H, CH2), 2.81–2.95 (m, 1H, CH2), 3.70 (d, J = 10.3 Hz, 1H, CH), 4.31–4.52 (m, 1H, CH), 6.80 (d, J = 7.6 Hz, 1H, ArH), 6.98–7.07 (m, 1H, ArH), 7.19–7.34 (m, 4H, ArH), 7.36 (d, J = 7.1 Hz, 2H, ArH), 7.50 (d, J = 7.5 Hz, 1H, ArH), 8.10 (s, 1H, NH), 10.33 (s, 1H, NH), 10.42 (s, 1H, NH); 13C{1H}-NMR (DMSO-d6): δ 27.19, 29.56, 46.43, 55.99, 56.79, 67.50, 76.79, 79.47, 109.78, 120.98, 124.21, 127.36, 128.20, 128.32, 129.31, 129.66, 134.80, 154.93, 172.39, 176.38; HRMS: calcd. for C22H20N4O3+ [M+H]+: 389.1613, found: 389.1614.
1-Phenylhexahydro-1H-pyrrolizine-2-spiro-5'-imidazolidine-2',4'-dione-3-spiro-3''-N-benzylindoline-2''-one (4b). White solid; m.p. 187–189 °C; 1H-NMR (DMSO-d6): δ (ppm) 1.51–1.73 (m, 1H, CH2), 1.75–2.10 (m, 3H, CH2), 2.81–2.92 (m, 1H, CH2). 3.77 (d, J = 10.3 Hz, 1H, CH), 4.33–4.41 (brs, 1H, CH2), 4.43–4.53 (m, 1H, CH), 4.72 (d, J = 15.9 Hz, 1H, CH2), 5.05 (d, J = 15.9 Hz, 1H, CH2), 6.82 (d, J = 7.7 Hz, 1H, ArH), 7.01–7.14 (m, 1H, ArH), 7.18–7.35 (m, 9H, ArH), 7.36–7.46 (d, J = 6.9 Hz, 2H, ArH), 7.59 (d, J = 7.2 Hz, 1H, ArH), 8.37 (s, 1H, NH), 10.37 (s, 1H, NH); 13C{1H}-NMR (DMSO-d6): δ 27.47, 29.67, 42.75, 46.49, 56.06, 67.67, 76.71, 79.78, 109.35, 121.77, 123.69, 126.96, 127.35, 127.51, 128.28, 128.31, 129.4, 129.78, 134.67, 136.12, 142.46, 154.93, 172.15, 174.95; HRMS: calcd. for C29H26N4O3+ [M+H]+: 479.2080, found: 479.2083.
1-Phenylhexahydro-1H-pyrrolizine-2-spiro-5'-imidazolidine-2',4'-dione-3-spiro-3''-5-bromoindoline-2''-one (4c). White solid; m.p. 175–177 °C; 1H-NMR (DMSO-d6): δ (ppm) 1.57–1.70 (m, 1H, CH2), 1.75–2.08 (m, 3H, CH2), 2.73–2.90 (m, 1H, CH2 ), 3.66 (d, J = 10.5 Hz, 1H, CH), 4.34–4.46 (m, 1H, CH), 6.75 (d, J = 8.3 Hz, 1H, ArH), 7.23–7.34 (m, 3H, ArH), 7.37 (d, J = 7.0 Hz, 2H, ArH), 7.45 (dd, J = 8.3, 1.5 Hz, 1H, ArH), 7.60 (s, 1H, ArH), 8.29 (s, 1H, NH), 10.44 (s, 1H, NH), 10.59 (s, 1H, NH); 13C{1H}-NMR (DMSO-d6): δ 27.35, 29.52, 46.45, 56.74, 67.50, 76.87, 79.65, 111.79, 112.98, 126.70, 127.48, 128.24, 129.71, 130.67, 132.28, 134.55, 141.52, 154.85, 172.34, 175.93; HRMS: calcd. for C22H19BrN4O3+ [M+H]+: 467.0728, found: 467.0719.
1-(3,4-Dichloro)phenylhexahydro-1H-pyrrolizine-2-spiro-5'-imidazolidine-2',4'-dione-3-spiro-3''-N-benzylindoline-2''-one (4d). White solid; m.p. 160–162 °C; 1H-NMR (DMSO-d6): δ (ppm) 1.53–1.67 (m, 1H, CH2), 1.68-1.80 (m, 1H, CH2), 1.95–2.11 (m, 2H, CH2), 2.62 (t, J = 7.2 Hz, 1H, CH2), 3.45–3.55 (m, 1H, CH2), 4.25 (d, J = 8.0 Hz, 1H, CH), 4.66–4.79 (m, 2H, 1/2CH2, CH), 5.05 (d, J = 15.7 Hz, 1H, CH2), 6.84 (d, J = 7.8 Hz, 1H, ArH), 7.01–7.10 (m, 1H, ArH), 7.21–7.31 (m, 4H, ArH), 7.34 (d, J = 7.1 Hz, 2H, ArH), 7.53 (dd, J = 8.4, 2.3 Hz, 2H, ArH), 7.63 (d, J = 2.2 Hz, 1H, ArH), 7.87 (s, 1H, ArH), 7.98 (d, J = 8.6 Hz, 1H, ArH), 10.68 (s, 1H, NH); 13C{1H}-NMR (DMSO-d6): δ 25.05, 29.22, 42.62, 47.58, 53.38, 68.27, 76.75, 78.47, 109.57, 122.27, 123.31, 127.42, 127.84, 128.47, 128.64, 130.04, 132.49, 132.63, 133.20, 135.48, 135.83, 143.65, 155.35, 174.80, 175.40; HRMS: calcd. for C29H24Cl2N4O3+ [M+H]+: 547.1300, found: 547.1304.
1-(4-Bromo)phenylhexahydro-1H-pyrrolizine-2-spiro-5'-imidazolidine-2',4'-dione-3-spiro-3''-indoline-2''-one (4e). White solid; m.p. 222–224 °C; 1H-NMR (DMSO-d6): δ (ppm) 1.59–1.70 (m, 1H, CH2), 1.72–2.05 (m, 3H, CH2), 2.83–2.96 (m, 1H, CH2), 3.70 (d, J = 10.3 Hz, 1H, CH), 4.32–4.40 (m, 1H, CH), 6.80 (d, J = 7.6 Hz, 1H, ArH), 6.97–7.06 (m, 1H, ArH), 7.19–7.28 (m, 1H, ArH), 7.33 (d, J = 8.5 Hz, 2H, ArH), 7.43–7.59 (m, 3H, ArH), 8.21 (s, 1H, NH), 10.41 (s, 1H, NH), 10.45 (s, 1H, NH); 13C{1H}-NMR (DMSO-d6): δ 27.13, 29.46, 46.52, 56.26, 67.60, 76.78, 79.40, 109.86, 120.86, 121.06, 124.13, 128.37, 129.44, 131.16, 131.92, 134.34, 142.22, 155.01, 172.46, 176.34; HRMS: calcd. for C22H19BrN4O3+ [M+H]+: 467.0718, found: 467.0719.
1-Phenylhexahydro-1H-pyrrolizine-2-spiro-5'-imidazolidine-2',4'-dione-3-spiro-3''-5-fluoroindoline-2''-one (4f). White solid; m.p. 196–197 °C; 1H-NMR (DMSO-d6): δ (ppm) 1.59–1.74 (m, 1H, CH2), 1.76–1.94 (m, 2H, CH2), 1.95–2.071 (m, 1H, CH2), 2.72–2.83 (m, 1H, CH2), 3.71 (d, J = 10.4 Hz, 1H, CH), 4.33–4.43 (m, 1H, CH), 6.79 (dd, J = 8.5, 4.6 Hz, 1H, ArH), 7.06–7.14 (m, 1H, ArH), 7.22–7.33 (m, 3H, ArH), 7.34–7.43 (m, 3H, ArH), 8.35 (s, 1H, NH), 10.37 (s, 1H, NH), 10.43 (s, 1H, NH); 13C{1H}-NMR (DMSO-d6): δ 27.74, 29.63, 46.26, 56.35, 67.52, 77.11, 79.72, 110.45, 110.53, 115.71, 115.95, 116.21, 126.02, 126.10, 127.48, 128.19, 129.73, 134.60, 138.25, 154.89, 156.72, 158.62, 171.89, 176.34; HRMS: calcd. for C22H19FN4O3+ [M+H]+: 407.1520, found: 407.1519.
1-Phenylhexahydro-1H-pyrrolizine-2-spiro-5'-imidazolidine-2',4'-dione-3-spiro-3''-5-chloroindoline-2''-one (4g). White solid; m.p. 193–195 °C; 1H-NMR (DMSO-d6): δ (ppm) 1.56–1.71 (m, 1H, CH2), 1.76–1.95 (m, 2H, CH2), 1.96–2.06 (m, 1H, CH2), 2.76–2.87(m, 1H, CH2), 3.67 (d, J = 10.4 Hz, 1H, CH), 4.32–4.44 (m, 1H, CH), 6.82 (d, J = 8.3 Hz, 1H, ArH), 7.22–7.34 (m, 4H, ArH), 7.33–7.41 (m, 2H, ArH), 7.49 (d, J = 1.9 Hz, 1H, ArH), 8.30 (s, 1H, NH), 10.42 (s, 1H, NH), 10.57 (s, 1H, NH); 13C{1H}-NMR (DMSO-d6): δ 27.50, 29.58, 46.42, 56.67, 67.53, 76.94, 79.70, 111.29, 125.25, 126.33, 127.51, 128.09, 128.25, 129.43, 129.73, 134.56, 141.09, 154.89, 172.24, 176.10; HRMS: calcd. for C22H19ClN4O3+ [M+H]+: 423.1225, found: 423.1224.
1-Phenylhexahydro-1H-pyrrolizine-2-spiro-5'-imidazolidine-2',4'-dione-3-spiro-3''-N-methylindoline-2''-one (4h). White solid; m.p. 199–201 °C; 1H-NMR (DMSO-d6): δ (ppm) 1.58–1.71 (m, 1H, CH2), 1.73–2.09 (m, 3H, CH2), 2.80–2.93 (m, 1H, CH2), 3.10 (s, 3H,CH3), 3.74 (d, J = 10.4 Hz, 1H, CH), 4.38–4.50 (m, 1H, CH), 7.00 (d, J = 7.8 Hz, 1H, ArH), 7.06–7.16 (m, 1H, ArH), 7.22–7.43 (m, 6H, ArH), 7.57 (d, J = 7.5 Hz, 1H, ArH), 8.18 (s, 1H, NH), 10.34 (s, 1H, NH), 13C{1H}-NMR (DMSO-d6): δ 26.29, 27.28, 29.61, 46.53, 57.04, 67.59, 76.57, 79.58, 108.79, 121.74, 123.65, 127.48, 128.03, 128.28, 129.54, 129.74, 134.78, 143.63, 154.92, 172.40, 174.69; HRMS: calcd. for C23H22N4O3+ [M+H]+: 403.1767, found: 403.1770.
1-(3-Chloro)phenylhexahydro-1H-pyrrolizine-2-spiro-5'-imidazolidine-2',4'-dione-3-spiro-3''-indoline-2''-one (4i). White solid; m.p. 156–128 °C; 1H-NMR (DMSO-d6): δ (ppm) 1.58–1.71 (m, 1H, CH2), 1.74–7.97 (m, 3H, CH2), 2.79–2.91 (m, 1H, CH2), 3.74 (d, J = 10.3 Hz, 1H, CH), 4.31–4.44 (m, 1H, CH), 6.80 (d, J = 7.7 Hz, 1H, ArH), 6.95–7.07 (m, 1H, ArH), 7.15–7.28 (m, 1H, ArH), 7.29–7.40 (brs, 3H, ArH), 7.45 (s, 1H, ArH), 7.52 (d, J = 7.5 Hz, 1H, ArH), 8.32 (s, 1H, NH), 10.40 (s, 1H, NH), 10.43 (s, 1H, NH); 13C{1H}-NMR (DMSO-d6): δ 27.29, 29.48, 46.44, 56.19, 67.57, 76.76, 79.47, 109.85, 121.03, 124.07, 127.50, 128.46, 129.40, 129.49,130.02, 132.89, 137.46, 142.13, 154.98, 172.21, 176.27; HRMS: calcd. for C22H19ClN4O3+ [M+H]+: 423.1224, found: 423.1224.
1-(3-Chloro)phenylhexahydro-1H-pyrrolizine-2-spiro-5'-imidazolidine-2',4'-dione-3-spiro-3''-5-chloroindoline-2''-one (4j). White solid; m.p. 161–163 °C; 1H-NMR (DMSO-d6): δ (ppm) 1.64–1.75 (m, 1H, CH2), 1.76–1.94 (m, 2H, CH2), 1.95–2.06 (m, 1H, CH2), 2.71–2.83 (m, 1H, CH2), 3.74 (d, J = 10.4 Hz, 1H, CH), 4.31–4.38 (m, 1H, CH), 6.82 (d, J = 8.3 Hz, 1H, ArH), 7.28–7.39 (m, 4H, ArH), 7.46 (s, 1H, ArH), 7.55 (d, J = 1.7 Hz, 1H, ArH), 8.50 (s, 1H, NH), 10.47 (s, 1H, NH), 10.57 (s, 1H, NH); 13C{1H}-NMR (DMSO-d6): δ 27.73, 29.87, 46.88, 56.56, 68.02, 77.02, 80.05, 109.77, 122.27, 123.78, 127.26, 128.77, 129.08, 129.88, 130.52, 133.36, 136.29, 137.46, 142.70, 155.46, 172.43, 175.26; HRMS: calcd. for C22H18Cl2N4O3+ [M+H]+: 457.0831, found: 457.0834.
1-(3-Chloro)phenylhexahydro-1H-pyrrolizine-2-spiro-5'-imidazolidine-2',4'-dione-3-spiro-3''-N-benzylindoline-2''-one (4k). White solid; m.p. 216–218 °C; 1H-NMR (DMSO-d6): δ (ppm) 1.62–1.75 (m, 1H, CH2), 1.76–2.04 (m, 3H, CH2), 2.79–2.91 (m, 1H, CH2), 3.82 (d, J = 10.4 Hz, 1H, CH), 4.40–4.50 (m, 1H, CH), 4.72 (d, J = 15.9 Hz, 1H, CH2), 5.05 (d, J = 15.9 Hz, 1H, CH2), 6.83 (d, J = 7.8 Hz, 1H, ArH), 7.04–7.13 (m, 1H, ArH), 7.12–7.42 (m, 9H, ArH), 7.50 (s, 1H, ArH), 7.62 (d, J = 7.5 Hz, 1H, ArH), 8.55 (s, 1H, NH), 10.46(s, 1H, NH); 13C{1H}-NMR (DMSO-d6): δ 27.68, 29.84, 43.13, 46.86, 56.63, 68.00, 77.00, 80.02, 109.74, 122.25, 123.76, 127.23, 127.78, 128.03, 128.67, 128.82, 129.06, 129.78, 129.92, 130.49, 133.35, 136.27, 137.45, 142.69, 155.43, 172.43, 175.24; HRMS: calcd. for C29H25ClN4O3+ [M+H]+: 513.1693, found: 513.1693.
1-(4-Trifluoromethy)phenylhexahydro-1H-pyrrolizine-2-spiro-5'-imidazolidine-2',4'-dione-3-spiro-3''-indoline-2''-one (4l). White solid; m.p. 239–241 °C; 1H-NMR (DMSO-d6): δ 1.60–1.72 (m, 1H, CH2), 1.73–1.87 (m, 1H, CH2), 1.87–2.06 (m, 2H, CH2), 2.87–2.98 (m, 1H, CH2), 3.82 (d, J = 10.3 Hz, 1H, CH), 4.41–4.51 (m, 1H, CH), 6.81 (d, J = 7.7 Hz, 1H, ArH), 6.98–7.06 (m, 1H, ArH), 7.21–7.30 (m, 1H, ArH), 7.52 (d, J = 7.6 Hz, 1H, ArH), 7.60 (d, J = 8.2 Hz, 2H, ArH), 7.69 (d, J = 8.2 Hz, 2H, ArH), 8.30 (s, 1H, NH), 10.45 (s, 1H, NH), 10.48 (s, 1H, NH); 13C{1H}-NMR (DMSO-d6): δ 27.02, 29.38, 46.55, 56.55, 67.65, 76.82, 79.50, 109.87, 121.08, 122.92, 124.05, 125.06, 125.62, 127.86, 128.18, 128.35, 129.50, 129.81, 130.59, 139.88, 142.27, 154.97, 172.52, 176.28; HRMS: calcd. for C23H19F3N4O3+ [M+H]+: 457.1486, found: 457.1488.
1-(3-Methoxy)phenylhexahydro-1H-pyrrolizine-2-spiro-5'-imidazolidine-2',4'-dione-3-spiro-3''-indoline-2''-one (4m). Yellow solid; m.p. 177–179 °C; 1H-NMR (400 MHz, DMSO-d6): δ (ppm) 1.57–1.70 (m, 1H, CH2), 1.72–1.87 (m, 1H, CH2), 1.87–2.04 (m, 2H, CH2), 2.83–2.95 (m, 1H, CH2), 3.67 (d, J = 10.3 Hz, 1H, CH), 4.35–4.46 (m, 1H, CH), 6,77–6.86 (m, 2H, ArH), 6.89 (d, J = 7.6 Hz, 1H, ArH), 6.97–7.06 (m, 2H, ArH), 7.16–7.28 (m, 2H, ArH), 7.49 (d, J = 7.5 Hz, 1H, ArH), 8.07 (s, 1H, NH), 10.34 (s, 1H, NH), 10.40 (s, 1H, NH); 13C{1H}-NMR (100 MHz, DMSO-d6): δ 27.16, 29.63, 46.50, 54.93, 56.85, 59.75, 67.50, 76.83, 79.45, 109.82, 112.82, 115.22, 121.01, 121.95, 124.25, 128.34, 129.25, 136.42, 142.21, 155.00, 159.07, 172.55, 176.43; HRMS: calcd. for C23H22N4O4+ [M+H]+: 419.1718, found: 419.1719.
7-Phenyl-5-spiro-3'-indoline-2'-one-hexahydropyrrolo[1,2-c]thiazole-6-spiro-5''-imidazolidine-2'',4''-dione (4n). White solid; m.p. 200–202 °C; 1H-NMR (DMSO-d6): δ (ppm) 2.87 (dd, J = 11.2, 4.1 Hz, 1H, CH2). 3.07 (dd, J = 11.1, 6.8 Hz, 1H, CH2), 3.54 (d, J = 9.4 Hz, 1H, CH), 3.71–3.80 (m, 2H, CH2), 4.63 (m, 1H, CH), 6.79 (d, J = 7.7 Hz, 1H, ArH), 7.01 (m, 1H, ArH), 7.21–7.35 (m, 4H, ArH), 7.40 (d, J = 6.6 Hz, 1H, ArH), 7.73 (d, J = 7.6 Hz, 1H, ArH), 8.58 (s, 1H, NH), 10.30 (s, 1H, NH), 10.48 (s, 1H, NH); 13C{1H}-NMR (DMSO-d6): δ 34.80, 51.90, 55.13, 70.18, 77.11, 78.29, 109.70, 120.70, 122.78, 127.84, 128.31, 129.21, 129.67, 129.83, 133.78, 141.79, 154.84, 170.67, 175.45; HRMS: calcd. for C21H18N4O3S+ [M+H]+: 407.1176, found: 407.1178.
3.3. Crystallographic Data and Molecular Structure of Compound 4b [32]
C29H26N4O3•C2H6O, M = 524.61, triclinic, a = 9.8656(7) Å, b = 11.0707(8) Å, c = 13.5643(10) Å, α = 98.6770(10)°, β = 110.6190(10)°, γ = 103.4270(10)°, V = 1303.90(16) Å3, T = 100(2) K, space group P1, Z = 2, μ(MoKα) = 0.090 mm-1, 18612 reflections measured, 7266 independent reflections (Rint = 0.0225). The final R1 values were 0.0436 (I > 2σ(I)). The final wR(F2) values were 0.1160 (I > 2σ(I)). The final R1 values were 0.0560 (all data). The final wR(F2) values were 0.1250 (all data). The goodness of fit on F2 was 1.021.
4. Conclusions
In conclusion, we have successfully developed an efficient method for the synthesis of potentially biologically active a series of novel dispirocycloadducts via a three-component 1,3-dipolar cycloaddition reaction of azomethine ylides. This method has the advantages of convenient operation, the availability of starting materials, mild reaction conditions employed, high yields and high efficiency, as well as the complete regio- and stereoselectivity observed. Further studies to acquire more information about the pharmacological activity of these compounds are in progress in our laboratory.
Acknowledgments
We are grateful to Zhiyan Huang (Nanyang Technological University) for his critical reviews on this manuscript. We also thank Xiaonian Li (Kunming Institute of Botany) and Jian Yang (Kunming University of Science and Technology) for their helpful assistance with the single crystal X-ray diffraction analysis. Financial support from Natural Science Foundation of China (No. 81102325), China Postdoctoral Science Foundation (No. 2012T50781), Young Teachers Fund of Sichuan University (No. 2011SCU11108) and Sichuan Provincial Health Department Research Project (No. 110173) is gratefully acknowledged.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/18/5/5142/s1.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of all the compounds are available from the authors.
References and Notes
- 1.Pellissier H. Stereocontrolled domino reactions. Chem. Rev. 2013;113:442–524. doi: 10.1021/cr300271k. [DOI] [PubMed] [Google Scholar]
- 2.Slobbe P., Ruijter E., Orru R.V.A. Recent applications of multicomponent reactions in medicinal chemistry. Medchemcomm. 2012;3:1189–1218. [Google Scholar]
- 3.Domling A., Wang W., Wang K. Chemistry and biology of multicomponent reactions. Chem. Rev. 2012;112:3083–3135. doi: 10.1021/cr100233r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonne D., Constantieux T., Coquerel Y., Rodriguez J. Stereoselective Multiple Bond-Forming Transformations (MBFTs): The Power of 1,2-and 1,3-Dicarbonyl Compounds. Chem. Eur. J. 2013;19:2218–2231. doi: 10.1002/chem.201204018. [DOI] [PubMed] [Google Scholar]
- 5.Burrell A.J.M., Coldham I., Watson L., Oram N., Pilgram C.D., Martin N.G. Stereoselective formation of fused tricyclic amines from acyclic aldehydes by a cascade process involving condensation, cyclization, and dipolar cycloaddition. J. Org. Chem. 2009;74:2290–2300. doi: 10.1021/jo8019913. [DOI] [PubMed] [Google Scholar]
- 6.Singh G.S., Desta Z.Y. Isatins as privileged molecules in design and synthesis of spiro-fused cyclic frameworks. Chem. Rev. 2012;112:6104–6155. doi: 10.1021/cr300135y. [DOI] [PubMed] [Google Scholar]
- 7.Nair V., Suja T.D. Intramolecular 1,3-dipolar cycloaddition reactions in targeted syntheses. Tetrahedron. 2007;63:12247–12275. doi: 10.1016/j.tet.2007.09.065. [DOI] [Google Scholar]
- 8.Presset M., Mohanan K., Hamann M., Coquerel Y., Rodriguez J. 1,3-Dipolar Cycloaddition of Hydrazones with alpha-Oxo-ketenes: A Three-Component Stereoselective Entry to Pyrazolidinones and an Original Class of Spirooxindoles. Org. Lett. 2011;13:4124–4127. doi: 10.1021/ol2016669. [DOI] [PubMed] [Google Scholar]
- 9.Lashgari N., Ziarani G.M. Synthesis of heterocyclic compounds based on isatin through 1, 3-dipolar cycloaddition reactions. ARKIVOC. 2012:277–320. doi: 10.3998/ark.5550190.0013.108. [DOI] [Google Scholar]
- 10.Liu H., Dou G.L., Shi D.Q. Regioselective synthesis of novel spiropyrrolidines and spirothiapyrrolizidines through multicomponent 1,3-Dipolar cycloaddition reaction of azomethine ylides. J. Comb. Chem. 2010;12:633–637. doi: 10.1021/cc100035q. [DOI] [PubMed] [Google Scholar]
- 11.Liu H., Dou G.L., Shi D.Q. Regio- and stereoselective synthesis of novel dispiropyrrolidine bisoxindole derivatives via multicomponent reactions. J. Comb. Chem. 2010;12:292–294. doi: 10.1021/cc900195t. [DOI] [PubMed] [Google Scholar]
- 12.Hu Y., Zou Y., Wu H., Shi D.Q. A facile and efficient ultrasound-assisted synthesis of novel dispiroheterocycles through 1,3-dipolar cycloaddition reactions. Ultrason. Sonochem. 2012;19:264–269. doi: 10.1016/j.ultsonch.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Huang Z.B., Zhao Q., Chen G., Wang H.Y., Lin W., Xu L.X., Liu H.T., Wang J.X., Shi D.Q., Wang Y.C. An efficient synthesis of novel dispirooxindole derivatives via one-pot three-component 1,3-Dipolar cycloaddition reactions. Molecules. 2012;17:12704–12717. doi: 10.3390/molecules171112704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arun Y., Bhaskar G., Balachandran C., Ignacimuthu S., Perumal P.T. Facile one-pot synthesis of novel dispirooxindole-pyrrolidine derivatives and their antimicrobial and anticancer activity against A549 human lung adenocarcinoma cancer cell line. Bioorgan. Med. Chem. Lett. 2013;23:1839–1845. doi: 10.1016/j.bmcl.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 15.Tian Y., Nam S., Liu L., Yakushijin F., Yakushijin K., Buettner R., Liang W., Yang F., Ma Y.L., Horne D., Jove R. Spirooxindole derivative SOID-8 induces apoptosis associated with inhibition of JAK2/STAT3 signaling in melanoma cells. PLOS One. 2012;7:e49306. doi: 10.1371/journal.pone.0049306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chowdhury S., Liu S.F., Cadieux J.A., Hsieh T., Chafeev M., Sun S.Y., Jia Q., Sun J.Y., Wood M., Langille J., et al. Tetracyclic spirooxindole blockers of hNa(V)1.7: Activity in vitro and in CFA-induced inflammatory pain model. Med. Chem. Res. 2013;22:1825–1836. [Google Scholar]
- 17.Nicolaou K.C., Sanchini S., Sarlah D., Lu G., Wu T.R., Nomura D.K., Cravatt B.F., Cubitt B., de la Torre J.C., Hessell A.J., et al. Design, synthesis, and biological evaluation of a biyouyanagin compound library. Proc. Natl. Acad. Sci. USA. 2011;108:6715–6720. doi: 10.1073/pnas.1015258108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Millard M., Pathania D., Grande F., Xu S.L., Neamati N. Small-Molecule Inhibitors of p53-MDM2 Interaction: The 2006-2010 Update. Curr. Pharm. Des. 2011;17:536–559. doi: 10.2174/138161211795222649. [DOI] [PubMed] [Google Scholar]
- 19.Palnitkar S.S., Bin B., Jimenez L.S., Morimoto H., Williams P.G., Paul-Pletzer K., Parness J. H-3 Azidodantrolene: Synthesis and use in identification of a putative skeletal muscle dantrolene binding site in sarcoplasmic reticulum. J. Med. Chem. 1999;42:1872–1880. doi: 10.1021/jm9805079. [DOI] [PubMed] [Google Scholar]
- 20.Byrtus H., Obniska J., Czopek A., Kaminski K., Pawlowski M. Synthesis and anticonvulsant activity of new N-Mannich bases derived from 5-cyclopropyl-5-phenyl- and 5-cyclopropyl-5-(4-chlorophenyl)-imidazolidine-2,4-diones. Bioorg. Med. Chem. 2011;19:6149–6156. doi: 10.1016/j.bmc.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 21.Blanco-Ania D., Hermkens P.H.H., Sliedregt L., Scheeren H.W., Rutjes F. Synthesis of hydantoins and thiohydantoins spiro-fused to pyrrolidines: Druglike molecules based on the 2-Arylethyl amine scaffold. J. Comb. Chem. 2009;11:527–538. doi: 10.1021/cc800190z. [DOI] [PubMed] [Google Scholar]
- 22.Basappa, Kumar C.S.A., Swamy S.N., Sugahara K., Rangappa K.S. Anti-tumor and anti-angiogenic activity of novel hydantoin derivatives: Inhibition of VEGF secretion in liver metastatic osteosarcoma cells. Bioorg. Med. Chem. 2009;17:4928–4934. doi: 10.1016/j.bmc.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Khanfar M.A., Hill R.A., Kaddoumi A., El Sayed K.A. Discovery of novel GSK-3 beta inhibitors with potent in vitro and in vivo activities and excellent brain permeability using combined ligand- and structure-based virtual screening. J. Med. Chem. 2010;53:8534–8545. doi: 10.1021/jm100941j. [DOI] [PubMed] [Google Scholar]
- 24.Zhang D.Y., Ye D.J., Feng E.G., Wang J.F., Shi J.M., Jiang H.L., Liu H. Highly alpha-selective synthesis of sialyl spirohydantoins by regiospecific domino condensation/O -> N acyl migration/N-sialylation of carbodiimides with peracetylated sialic acid. J. Org. Chem. 2010;75:3552–3557. doi: 10.1021/jo100016k. [DOI] [PubMed] [Google Scholar]
- 25.Merino-Montiel P., Lopez O., Alvarez E., Fernandez-Bolanos J.G. Synthesis of conformationally-constrained thio(seleno)hydantoins and alpha-triazolyl lactones from D-arabinose as potential glycosidase inhibitors. Tetrahedron. 2012;68:4888–4898. [Google Scholar]
- 26.Xie Y.M., Yao Y.Q., Sun H.B., Yan T.T., Liu J., Kang T.R. Facile synthesis of functionalized spiropyrrolizidine oxindoles via a three-component tandem cycloaddition reaction. Molecules. 2011;16:8745–8757. doi: 10.3390/molecules16108745. [DOI] [Google Scholar]
- 27.Liu J., Sun H.B., Liu X.J., Ouyang L., Kang T.R., Xie Y.M., Wang X.Y. Direct construction of novel exo'-selective spiropyrrolidine bisoxindoles via a three-component 1,3-dipolar cycloaddition reaction. Tetrahedron Lett. 2012;53:2336–2340. [Google Scholar]
- 28.Wu G.S., Ouyang L., Liu J., Zeng S., Huang W., Han B., Wu F.B., He G., Xiang M.L. Synthesis of novel spirooxindolo-pyrrolidines, pyrrolizidines, and pyrrolothiazoles via a regioselective three-component [3+2] cycloaddition and their preliminary antimicrobial evaluation. Mol. Divers. 2013;17:271–283. doi: 10.1007/s11030-013-9432-3. [DOI] [PubMed] [Google Scholar]
- 29.Thenmozhiyal J.C., Wong P.T.H., Chui W.K. Anticonvulsant activity of phenylmethylenehydantoins: A structure-activity relationship study. J. Med. Chem. 2004;47:1527–1535. doi: 10.1021/jm030450c. [DOI] [PubMed] [Google Scholar]
- 30.Pardasani R.T., Pardasani P., Chaturvedi V., Yadav S.K., Saxena A., Sharma I. Theoretical and synthetic approach to novel spiroheterocycles derived from isatin derivatives and L-proline via 1,3-dipolar cycloaddition. Heteroatom Chem. 2003;14:36–41. doi: 10.1002/hc.10063. [DOI] [Google Scholar]
- 31.Lakshmi N.V., Thirumurugan P., Perumal P.T. An expedient approach for the synthesis of dispiropyrrolidine bisoxindoles, spiropyrrolidine oxindoles and spiroindane-1,3-diones through 1,3-dipolar cycloaddition reactions. Tetrahedron Lett. 2010;51:1064–1068. doi: 10.1016/j.tetlet.2009.12.079. [DOI] [Google Scholar]
- 32.Crystallographic data of compooud 4b reported in this manuscript have been deposited with Cambridge Crystallographic Data Centre as supplement ary publication no. CCDC-927973. [(accessed on 3 May 2013)]. Available online: http://www.ccdc. cam.ac.uk/conts/retrieving.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.