Abstract
Five xanthone derivatives and one flavanol were isolated from the dichloromethane extract of Garcinia mangostana. Dichloromethane, ethyl acetate extract and the major xanthone (α-mangostin) were evaluated in vitro against erythrocytic schizonts of Plasmodium falciparum, intracellular amastigotes of Leishmania infantum and Trypanosoma cruzi and free trypomastigotes of T. brucei. The major constituent α-mangostin was also checked for antimicrobial potential against Candida albicans, Escherichia coli, Pseudomonas aeruginosa, Bacillius subtilis, Staphylococcus aureus, Mycobacterium smegmatis, M. cheleneoi, M. xenopi and M. intracellulare. Activity against P. falciparum (IC50 2.7 μg/mL) and T. brucei (IC50 0.5 μg/mL) were observed for the dichloromethane extract, however, with only moderate selectivity was seen based on a parallel cytotoxicity evaluation on MRC-5 cells (IC50 9.4 μg/mL). The ethyl acetate extract was inactive (IC50 > 30 µg/mL). The major constituent α-mangostin showed rather high cytotoxicity (IC50 7.5 µM) and a broad but non-selective antiprotozoal and antimicrobial activity profile. This in vitro study endorses that the antiprotozoal and antimicrobial potential of prenylated xanthones is non-conclusive in view of the low level of selectivity.
Keywords: Garcinia mangostana, α-mangostin, in vitro, antiplasmodial, antileishmanial, antitrypanosomal
1. Introduction
The genus Garcinia (Guttiferae, syn. Clusiaceae) contains well-known fruit trees with about 35 genera and up to 800 species of which the fruits of many are edible and serve as a substitute for tamarinds in curries [1]. Garcinia mangostana Linn., known as mangosteen, is cultivated in the tropical rainforest of Southeast Asian nations like Indonesia, Malaysia, Sri Lanka, Philippines and Thailand where traditional medicine uses the pericarp for the treatment of abdominal pain, diarrhea, cystitis, eczema, dysentery, wound suppuration and chronic ulcers [2,3]. In vitro and in vivo laboratory studies have demonstrated that extracts of G. mangostana have very diverse pharmacological activities including anti-inflammatory, cytotoxic, antioxidant, antitumoral, immunomodulatory, neuroprotective, anti-allergic, antibacterial and antiviral properties [4,5,6,7]. Phytochemical investigation of the pericarp of G. mangostana revealed the presence of prenylated xanthones, benzophenones, bioflavonoids and triterpenes [8,9,10]. Over 68 xanthone-type constituents were reported [11], of which the prenylated cage-type is particularly encouraging for further biological and chemical studies. The most studied xanthones are the α-, β-, and γ-mangostins, garcinone E, 8-deoxygartanin and gartanin [7,12].
The present study evaluated the in vitro antileishmanial, antiplasmodial and antitrypanosomal potential of the dichloromethane and ethyl acetate extracts of G. mangostana, as well as the isolation and characterization of its xanthone constituents.
2. Results and Discussion
2.1. Phytochemical Study
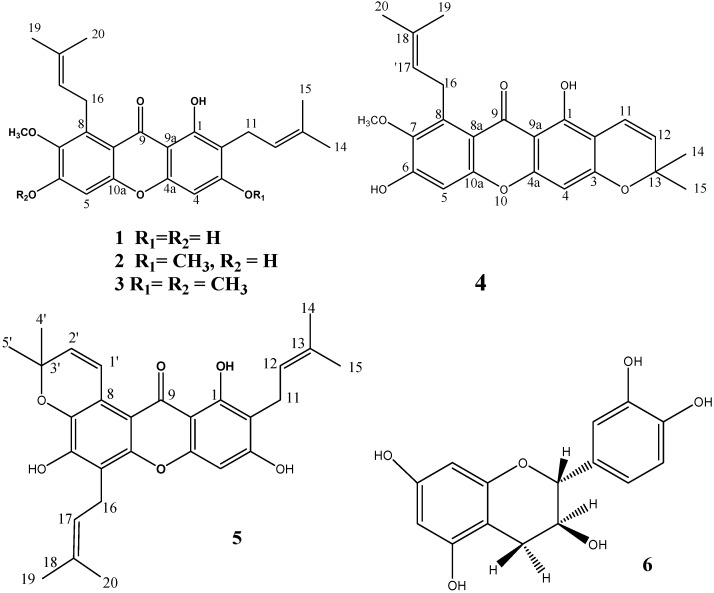
Chromatographic separation and purification of the dichloromethane extract of G. mangostana pericarp produced the compounds 1–6 (Figure 1). NMR-data (Table 1 and Table 2) and comparison with reported data led to the identification of α-mangostin (1) [13], β-mangostin (2) [14], 1-hydroxy-3,6,7-trimethoxy-2,8-bis (3-methylbut-2-enyl) xanthone (3) [15], 9-hydroxycalabaxanthone (4) [16,17], tovophyllin A (5) [18,19] and catechin (6) [20]. α-Mangostin was the major compound isolated from these series, enabling in vitro antiprotozoal and antimicrobial evaluation.
Figure 1.
Structures of compounds 1–6.
Table 1.
1H-NMR (500 MHz) spectral data of xanthones 1–5.
| Position | Compound 1 1 | Compound 2 2 | Compound 3 2 | Compound 4 2 | Compound 5 2 |
|---|---|---|---|---|---|
| 1 | 13.72, s | 13.42, s | 13.44, s | 13.72, s | 13.79, s |
| 4 | 6.28, s | 6.24, s | 6.30, s | 6.26, s | 6.37, s |
| 5 | 6.80, s | 6.74, s | 6.75, s | 6.85, s | − |
| 11 | 3.35, d (J = 7.3 Hz) | 3.37, d (J = 7.2 Hz) | 3.36, d (J = 7.1 Hz) | 6.74, d (J = 10.0 Hz) | 3.48, d (J = 6.0 Hz) |
| 12 | 5.17, t (J = 7.3 Hz) | 5.17, t (J = 7.2 Hz) | 5.26, t (J = 7.1 Hz) | 5.58, d (J = 10.0 Hz) | 5.31, t (J = 7.0 Hz) |
| 14 | 1.77, s | 1.75, s | 1.70, s | 1.27, s | 1.79, s |
| 15 | 1.63, s | 1.62, s | 1.71, s | 1.28, s | 1.71, s |
| 16 | 4.04, d (J = 7.0 Hz) | 4.09, d (J = 7.2 Hz) | 4.15, d (J = 7.2, Hz) | 4.10, d (J = 7.0 Hz) | 3.59, d (J = 6.0 Hz) |
| 17 | 5.17, t (J = 7.3 Hz) | 5.18, t (J = 7.2 Hz) | 5.26, t (J = 7.2 Hz) | 5.27, t (J = 7.3 Hz) | 5.31, t (J = 7.0 Hz) |
| 19 | 1.71, s | 1.61, s | 1.70, s | 1.71, s | 1.87, s |
| 20 | 1.73, s | 1.72, s | 1.82, s | 1.82, s | 1.89, s |
| 3-OMe | 3.82, s | 3.91, s | |||
| 6-OMe | 3.97, s | ||||
| 7-OMe | 3.71, s | 3.80, s | 3.82, s | 3.83, s | |
| 1' | 8.00, d (J = 10.0 Hz) | ||||
| 2' | 5.79, d (J = 10.0 Hz) | ||||
| 4' | 1.51, s | ||||
| 5' | 1.51, s |
1 DMSO-d6, 2 CDCl3.
Table 2.
13C-NMR (125 MHz) spectral data of xanthones 1–5.
| Position | Compound 1 1 | Compound 2 2 | Compound 3 2 | Compound 4 2 | Compound 5 2 |
|---|---|---|---|---|---|
| 1 | 159.9 | 159.7 | 157.9 | 157.8 | 160.44 |
| 2 | 109.9 | 111.5 | 109.6 | 104.4 | 108.4 |
| 3 | 162.3 | 163.5 | 161.5 | 159.8 | 161.6 |
| 4 | 92.3 | 88.8 | 86.7 | 94.0 | 93.4 |
| 4a | 154.5 | 154.4 | 153.4 | 156.1 | 155.3 |
| 5 | 101.8 | 101.5 | 96.3 | 101.6 | 115.2 |
| 6 | 156.6 | 155.6 | 156.1 | 154.5 | 151.0 |
| 7 | 143.4 | 142.5 | 142.1 | 142.7 | 135.8 |
| 8 | 136.3 | 137.0 | 135.2 | 136.9 | 136.5 |
| 8a | 112.2 | 112.3 | 112.9 | 112.1 | |
| 9 | 181.3 | 181.9 | 180.0 | 181.8 | 182.9 |
| 9a | 103.6 | 103.8 | 102.0 | 103.6 | 117.2 |
| 10a | 154.1 | 155.2 | 153.4 | 155.6 | |
| 11 | 21.3 | 21.3 | 20.4 | 115.6 | 21.4 |
| 12 | 122.4 | 122.3 | 121.6 | 126.9 | 121.4 |
| 13 | 130.3 | 132.0 | 129.6 | 77.8 | 132.6 |
| 14 | 25.5 | 25.8 | 25.8 | 28.3 | 25.8 |
| 15 | 17.9 | 18.2 | 16.3 | 25.6? | 17.9 |
| 16 | 25.6 | 31.2 | 24.1 | 26.5 | 22.6 |
| 17 | 123.7 | 123.2 | 120.5 | 123.1 | 121.0 |
| 18 | 130.3 | 131.7 | 129.7 | 131.8 | 131.3 |
| 19 | 17.7 | 17.8 | 15.8 | 18.1 | 17.9 |
| 20 | 25.7 | 26.7 | 24.1 | ? | 25.8 |
| 3-OMe | − | 55.8 | 54.9 | ||
| 6-OMe | − | − | 53.9 | ||
| 7-OMe | 60.1 | 62.0 | 60.9 | ||
| 8-OMe | 7-OMe | ||||
| 1' | 121.0 | ||||
| 2' | 131.3 | ||||
| 3' | 77.1 | ||||
| 4' | 27.4 | ||||
| 5' | 27.4 |
1 DMSO-d6, 2 CDCl3.
2.2. In Vitro Antiprotozoal and Antimicrobial Activity
The dichloromethane and ethyl acetate extracts of G. mangostana were evaluated in an integrated in vitro screen for their antiplasmodial, antileishmanial and antitrypanosomal potential (Table 3). While the ethyl acetate extract showed no antiprotozoal activity at all, a pronounced inhibitory effect (IC50) was obtained with the dichloromethane extract against Plasmodium falciparum (IC50 2.7 µg/mL) and Trypanosoma brucei (IC50 0.5 µg/mL), but only with acceptable selectivity (SI) for T. brucei (SI 18.8). Some side activity was also noted against T. cruzi and Leishmania infantum (IC50 7.6 and 7.5 µg/mL), but with low selectivity.
Table 3.
Antiprotozoal activity of G. mangostana extracts and α-mangostin.
| Sample | P. falciparum | L. infantum | T. cruzi | T. brucei | MRC-5 | ||||
|---|---|---|---|---|---|---|---|---|---|
| IC50 | SI | IC50 | SI | IC50 | SI | IC50 | SI | IC50 | |
| Dichloromethane extract | 2.7 µg | 3.5 | 7.5 µg | 3.5 | 7.6 µg | 1.2 | 0.5 µg | 18.8 | 9.4 µg |
The major constituent α-mangostin was also checked for antimicrobial potential against Candida albicans, Escherichia coli, Pseudomonas aeruginosa, Bacillius subtilis, Staphylococcus aureus, Mycobacterium smegmatis, M. cheleneoi, M. xenopi and M. intracellulare (Table 4). Although inhibitory activity could be indicated against B. subtilis and S. aureus (MIC 1.6 and 3.2 µg/mL) and the Mycobacterium species (MIC 1.5 µg/mL), selectivity was quite low in view of the observed cytotoxicity on MRC-5 cells (IC50 7.5 µM) (Table 3). No activity at all was found against C. albicans, E. coli and P. aeruginosa (IC50 >200 µg/mL).
Table 4.
Antimicrobial activity (IC50) of α-mangostin.
| B. subtilis | C. albicans | E. coli | P. aeruginosa | S. aureus | Mycobacterium | ||||
|---|---|---|---|---|---|---|---|---|---|
| smegmatis | cheleneoi | xenopi | intracellulare | ||||||
| MIC (µM) | 3.9 | >200 | 7.8 | 3.7 | 3.7 | 3.7 | 3.7 | ||
To the best of our knowledge, no data exist in the literature regarding the antiprotozoal activity and potential significance of G. mangostana as a source of antitrypanosomal and antiplasmodial compounds. G. parvifolia (Miq) has been used as a herbal remedy to treat malaria [21] and α-mangostin was found active against P. falciparum with IC50 values of 5.1 and 17 µM [22,23]. In our study, α-mangostin was found slightly more potent (IC50 2.2 µM), but also cytotoxic to MRC-5 cells (IC50 7.5 µM), hence suggesting a non-specific inhibition. The latter also explains the observed activity against L. infantum, T. brucei and T. cruzi, with IC50 values between 8.0 and 9.0 µM (Table 3). Another illustration of non-selectivity are several studies quoting the antimicrobial potential of G. mangostana extract [24,25]. However, the observed IC50 values may still justify the claimed (topical) uses of G. mangostana to treat infections in the traditional medicine.
This study clearly illustrates that interpretation of the antiprotozoal and antimicrobial potential of prenylated xanthones proves to be far from easy in view of the low level of selectivity. Available data in literature must be interpreted with great caution, particularly when parallel cytotoxicity data are not available. One route of further research on xanthones could be through structural modification with the sole option to maximize efficacy and reduce toxicity, e.g., non-selectivity.
3. Experimental Section
3.1. General
The UV and IR spectra were recorded on Hitachi-UV-3200 and JASCO 320-A spectrometers. The 1H-, 13C-NMR and 2D-NMR spectra were recorded on a Bruker AMX-500 spectrometer with tetramethylsilane (TMS) as internal standard. Chemical shifts are given in ppm (δ) relative to tetramethylsilane internal standard and scalar coupling constants (J) are reported in Hertz. FAB and HRFABMS (neg. ion mode, matrix: glycerol) were registered on a JEOL JMS-HX110 mass spectrometer. Thin layer chromatography (TLC) was performed on precoated silica gel F254 plates (E. Merck, Darmstadt, Germany); detection was done at 254 nm and by spraying with p-anisaldehyde/H2SO4 reagent. All chemicals were purchased from Sigma Chemical Company (St. Louis, MO, USA).
3.2. Plant Material
The fruits of G. mangostana Linn. were purchased from a local market at Riyadh city in 2009.
3.3. Extraction and Isolation
The air-dried pericarp (500 gm) was extracted by maceration with 70% ethanol (3 × 2 L) at room temperature. After filtration and evaporation of the solvent under vacuum, the combined ethanolic extract (70 gm) was suspended in water (200 mL) and successively partitioned with n-hexane (3 × 400 mL), dichloromethane (3 × 400 mL) and ethyl acetate (3 × 400 mL) to deliver the corresponding extracts. Based on pattern of separation and close similarity of compounds on TLC examination for both n-hexane and dichloromethane extracts, they were pooled together. The combined fractions were further purified by application onto the top of a silica gel packed column (Merck), eluted with n-hexane/ethyl acetate, followed by ethyl acetate/methanol solvent system gradient to give five fractions A–F. Fractions A, B and C were separately purified by chromatotron (Harrison Research, Palo Alto, California, CA, USA) using 5%, 15% and 20% ethyl acetate/n-hexane to give compound 3 (12 mg), 4 (8 mg) and 5 (20 mg). Direct crystallization of fractions D and E eluted by 30% and 40% ethyl acetate/n-hexane gave compound 2 (10 mg) and 1 (300 mg), while direct crystallization of fraction F eluted by 5% methanol/ethyl acetate gave compound 6 (20 mg).
3.4. Spectral Data (Table 1 and Table 2)
Trihydroxy-7-methoxy-2,8-diprenylxanthone (α-mangostin) (1). Yellow amorphous powder; m.p. 180–182 °C; HREIMS: m/z = 410.1729 (calc. for C24H26O6, 410.46). 1H-NMR (DMSO, 500 MHz); 13C-NMR (d-DMSO, 125 MHz): see Table 1 and Table 2.
1,6-Dihydroxy-3,7-dimethoxy-2,8-diprenylxanthone- (β-mangostin) (2). Pale yellow crystal; m.p. 162–163 °C; HREIMS: m/z = 424 calc. for C25H28O6, 424.46. 1H-NMR (CDCl3, 500 MHz); 13C-NMR (CDCl3, 125 MHz): see Table 1 and Table 2.
1-Hydroxy-3,6,7-trimethoxy-2,8-bis(3-methylbut-2-enyl) xanthone (3). Pale yellow gum; m.p. 152–154 °C; HREIMS: m/z = 438.5128 (calc. for C26H30O7, 438.5128). 1H NMR (CDCl3, 500 MHz); 13C-NMR (CDCl3, 125 MHz): see Table 1 and Table 2.
9-Hydroxycalabaxanthone (4). Bright yellow needles; m.p. 152–154 °C; HREIMS: m/z = 408.1572 (calc. for C24H24O6, 408.45). 1H-NMR (CDCl3, 500 MHz); 13C-NMR (CDCl3, 125 MHz): see Table 1 and Table 2.
1,3,6-Trihydroxy-2,5-diprenyl-6',6'-dimethylpyrano(2',3':7,8) xanthone (tovophyllin A) (5). Yellow needles; m.p. 218–220 °C; HREIMS: m/z = 462.541 (calc. for C28H30O6, 462.2042). 1H-NMR (CDCl3, 500 MHz); 13C-NMR (CDCl3, 125 MHz): see Table 1 and Table 2.
3,5,7,3',4'-Pentahydroxyflavan (Catechin) (6). Cryst.; m.p. 175–177 °C; HREIMS: m/z = 290.0790 (calc. for C15H14O6, 290.272). 1H-NMR (CDCl3, 500 MHz): 2.74 (dd, J = 16.5, 4.5 Hz, H-4a) 2.86 (dd, J = 16.5, 4.5 Hz, H-4b), 4.19 (m, H-3), 4.83 (brs, H-2), 5.94 (d, J = 1.5 Hz, H-6), 5.99 (d, J = 1.5 Hz, H-8), 6.78 (dd, J = 8.0, 1.0 Hz, H-6'), 6.81 (d, J = 8.0 Hz, H-5'),7.00 (d, J = 1.5 Hz, H-2'); 13C-NMR (CD3OD, 125 MHz): 80.2 (C-2), 67.5 (C-3), 29.5 (C-4), 157.7 (C-5), 96.5 (C-6), 158.0 (C-7), 96.0 (C-8), 157.4 (C-9), 100.2 (C-10), 132.3 (C-1'), 115.4 (C-2'), 146.0 (C-3'), 145.8 (C-4'), 116.0 (C-5'), 119.5 (C-6').
3.5. Reference Drugs
For the different tests, appropriate reference drugs were used as positive control: tamoxifen for MRC-5, chloroquine for P. falciparum, miltefosine for L. infantum, benznidazole for T. cruzi and suramin for T. brucei. All reference drugs were either obtained from the fine chemical supplier Sigma-Aldrich (Taufkirchen, Germany; tamoxifen, suramin) or from WHO-TDR (Geneva, Switzerland; chloroquine, miltefosine, benznidazole).
3.6. Biological Assays
The integrated panel of microbial screens and standard screening methodologies were adopted as previously described [26]. All assays were performed in triplicate at the Laboratory of Microbiology, Parasitology and Hygiene at the University of Antwerp (Antwerp, Belgium). Extracts were tested at five concentrations (64, 16, 4, 1 and 0.25 μg/mL) to establish a full dose-titration and determination of the IC50 (inhibitory concentration 50%). The final in-test concentration of DMSO did not exceed 0.5%, which is known not to interfere with the different assays [26]. The selectivity of activity was assessed by simultaneous cytotoxicity evaluation on the MRC-5 fibroblast cell line. The criterion for activity was an IC50 <10 μg/mL and a selectivity index (SI) of >4.
3.6.1. Antiplasmodial Activity
Chloroquine-resistant P. falciparum K 1-strain was cultured in human erythrocytes O+ at 37 °C under a low oxygen atmosphere (3% O2, 4% CO2, and 93% N2) in RPMI-1640, supplemented with 10% human serum. Infected human red blood cells (200 μL, 1% parasitaemia, 2% haematocrit) were added to each well and incubated for 72 h. After incubation, test plates were frozen at −20 °C. Parasite multiplication was measured using the Malstat assay, a colorimetric method based on the reduction of 3-acetyl pyridine adenine dinucleotide (APAD) by parasite-specific lactate-dehydrogenase (pLDH) [26,27].
3.6.2. Antileishmanial Activity
L. infantum MHOM/MA (BE)/67 amastigotes were collected from the spleen of an infected donor hamster and used to infect primary peritoneal mouse macrophages. To determine in vitro antileishmanial activity, 3 × 104 macrophages were seeded in each well of a 96-well plate. After 2 days outgrowth, 5 × 105 amastigotes/well, were added and incubated for 2 h at 37 °C. Pre-diluted plant extracts were subsequently added and the plates were further incubated for 5 days at 37 °C and 5% CO2. Parasite burdens (mean number of amastigotes/macrophage) were microscopically assessed on 500 cells after Giemsa staining of the testplates, and expressed as a percentage of the blank controls without plant extract.
3.6.3. Antitrypanosomal Activity
Trypanosoma brucei Squib-427 strain (suramin-sensitive) was cultured at 37 °C and 5% CO2 in Hirumi-9 medium, supplemented with 10% fetal calf serum (FCS) [28]. About 1.5 × 104 trypomastigotes/well were added to each well and parasite growth was assessed after 72 h at 37 °C by adding resazurin [29]. For Chagas disease, T. cruzi Tulahuen CL2 (benznidazole-sensitive, LacZ-reporter strain) [30] was maintained on MRC-5 cells in minimal essential medium (MEM) supplemented with 20 mM L-glutamine, 16.5 mM sodium hydrogen carbonate and 5% FCS. In the assay, 4 × 103 MRC-5 cells and 4 × 104 parasites were added to each well. After incubation at 37 °C for 7 days, parasite growth was assessed by adding the substrate chlorophenol red α-D-galactopyranoside. The color reaction was read at 540 nm after 4 h and absorbance values were expressed as a percentage of the blank controls.
3.6.4. Antimicrobial Activity
Samples were tested for antimicrobial activity according to the Clinical Laboratory Standard Institution using American type of Culture Collection (ATCC) standard [31].
3.6.5. Cytotoxicity Assay
MRC-5 SV2 cells were cultivated in MEM, supplemented with L-glutamine (20 mM), 16.5 mM sodium hydrogen carbonate and 5% FCS. For the assay, 104 MRC-5 cells/well were seeded onto the test plates containing the pre-diluted sample and incubated at 37 °C and 5% CO2 for 72 h. Cell viability was assessed fluorimetrically after 4 h of addition of resazurin. Fluorescence was measured (excitation 550 nm, emission 590 nm) and the results were expressed as % reduction in cell viability compared to control [26].
4. Conclusions
Interpretation of the antiprotozoal and antimicrobial potential of prenylated xanthones proves to non-conclusive in view of the low level of selectivity. One route of further research on this subject could be through structural modification with the sole option to maximize efficacy and avoid non-selectivity.
Acknowledgments
The authors thank An Matheeussen and Margot Desmet for performing the antiprotozoal in vitro assays. The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the research group project No. (RGP-VPP-073).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 1–6 are available from the authors.
References
- 1.Chen L.G., Yang L.L., Wang C.C. Anti-inflammatory activity of Mangostins from Garcinia mangostana. Food Chem. Toxicol. 2008;46:688–693. doi: 10.1016/j.fct.2007.09.096. [DOI] [PubMed] [Google Scholar]
- 2.Chin Y.W., Kinghorn A.D. Structural characterization, biological effects, and synthetic studies on xanthones from mangosteen (Garcinia mangostana), a popular botanical dietary supplement. Min. Rev. Org. Chem. 2008;5:355–364. doi: 10.2174/157019308786242223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balasubramanian K., Rajagopalan K. Novel xanthones from Garcinia mangostana, structures of BR-xanthone-A and BR-xanthone-B. Phytochemistry. 1988;27:1552–1554. doi: 10.1016/0031-9422(88)80242-5. [DOI] [Google Scholar]
- 4.Pedraza-Chaverri J., Cárdenas-Rodríguez N., Orozco-Ibarra M., Pérez-Rojas J.M. Medicinal properties of mangosteen (Garcinia mangostana) Food Chem. Toxicol. 2008;46:3227–3239. doi: 10.1016/j.fct.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 5.Moongkarndi P., Kosem N., Kaslungka S., Luanratana O., Pongpan N., Neungton N. Antiproliferation, antioxidation and induction of apoptosis by Garcinia mangostana (Mangosteen) on SKBR3 human breast cancer cell line. J. Ethnopharmacol. 2004;90:161–166. doi: 10.1016/j.jep.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 6.Kaomongkolgit R., Chaisomboon N., Pavasant P. Apoptotic effect of alpha-mangostin on head and neck squamous carcinoma cells. Arch. Oral. Biol. 2011;56:483–490. doi: 10.1016/j.archoralbio.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 7.Yua L., Zhao M., Yang B., Bai W. Immunomodulatory and anticancer activities of phenolics from Garcinia mangostana fruit pericarp. Food Chem. 2009;116:969–973. doi: 10.1016/j.foodchem.2009.03.064. [DOI] [Google Scholar]
- 8.Ji X., Avula B., Khan I.A. Quantitative and qualitative determination of six xanthones in Garcinia mangostana L. by LC-PDA and LC-ESI-MS. J. Pharm. Biomed. Anal. 2007;43:1270–1276. doi: 10.1016/j.jpba.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Peres V., Nagem T.J., Faustino de Oliveira F. Tetraoxygenated naturally occurring xantones. Phytochemistry. 2000;55:683–710. doi: 10.1016/S0031-9422(00)00303-4. [DOI] [PubMed] [Google Scholar]
- 10.Williams R.B., Hoch J., Glass T.E., Evans R., Miller J.S., Wisse J.H., Kingston D.G.I. A novel cytotoxic guttiferone analogue from Garcinia macrophylla from the Surinam rainforest. Planta Med. 2003;69:864–866. doi: 10.1055/s-2003-43204. [DOI] [PubMed] [Google Scholar]
- 11.Shan T., Ma Q., Guo K., Liu J., Li W., Wang F., Wu E. Xanthones from mangosteen extracts as natural chemopreventive agents: Potential anticancer drugs. Curr. Mol. Med. 2011;11:666–677. doi: 10.2174/156652411797536679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett G.J., Lee H.H. Xanthones from Guttiferae. Phytochemistry. 1989;28:967–998. doi: 10.1016/0031-9422(89)80170-0. [DOI] [Google Scholar]
- 13.Sen A.K., Sarkar K.K., Mazumder P.C., Banerji N., Uusvuori R., Hase T.A. The structure of garcinones A, B and C: Three new xanthones from Garcinia mangostana. Phytochemistry. 1982;21:1747–1750. [Google Scholar]
- 14.Ghazali S.I.S., Lian G.E., Abd Ghani K.D. Chemical constituent from roots of Garcinia mangostana (Linn.) Int. J. Chem. 2010;2:134–142. [Google Scholar]
- 15.Nilar, Harrison L.J. Xanthones from the heartwood of Garcinia mangostana. Phytochemistry. 2002;60:541–548. doi: 10.1016/S0031-9422(02)00142-5. [DOI] [PubMed] [Google Scholar]
- 16.Sen A.K., Sarkar K.K., Majumder P.C., Banerji N. Minor xanthones of Garcinia mangostana. Phytochemistry. 1981;20:183–185. [Google Scholar]
- 17.Trisuwan K., Ritthiwigrom T. Benzophenone and xanthone derivatives from the inflorescences of Garcinia cowa. Arch. Pharm. Res. 2012;35:1733–1738. doi: 10.1007/s12272-012-1004-z. [DOI] [PubMed] [Google Scholar]
- 18.Antônio A.A.L., De Oliveira W.G., Taveira Neiva R.M. Xanthones from Tovomita pyrifolium. Phytochemistry. 1975;14:803–806. doi: 10.1016/0031-9422(75)83040-8. [DOI] [Google Scholar]
- 19.Bennett G.J., Harrison L.J., Sia G.L., Sim K.Y. Triterpenoids, tocotrienols and xanthones from the bark of Cratoxylum cochinchinense. Phytochemistry. 1993;32:1245–1251. doi: 10.1016/S0031-9422(00)95100-8. [DOI] [Google Scholar]
- 20.Ejele A.E., Iwu I.C., Enenebeaku C.K., Ukiwe L.N., Okolue B.N. Bioassay-guided isolation, purification and partial characterization of antimicrobial compound from basic metabolite of Garcinia Kola. J. Emerg. Trends Eng. Appl. Sci. 2012;3:668–672. [Google Scholar]
- 21.Faizatun S., Rahayu L. HPLC analysis and pharmacokinetic study of mangostin after orally administration in rats. Int. J. Pharm. Bio. Sci. 2009;2:43–49. [Google Scholar]
- 22.Riscoe M., Kelly J.X., Winter R. Xanthones as antimalarial agents: Discovery, mode of action, and optimization. Curr. Med. Chem. 2005;12:2539–2549. doi: 10.2174/092986705774370709. [DOI] [PubMed] [Google Scholar]
- 23.Mahabusarakam W., Kuaha K., Wilairat P., Taylor W.C. Prenylated xanthones as potential antiplasmodial substances. Planta Med. 2006;72:912–916. doi: 10.1055/s-2006-947190. [DOI] [PubMed] [Google Scholar]
- 24.Priya V., Jainu M., Mohan S.K., Saraswathi P., Gopan S.C. Antimicrobial activity of pericarp extract of Garcinia mangostana Linn. Int. J. Pharm. Sci. Res. 2010;1:278–281. [Google Scholar]
- 25.Chomnawang M.T., Sakagami S.S., Nukoolkarn V.S., Gritsanapan W. Antimicrobial effects of Thai medicinal plants against acne-inducing bacteria. J. Ethnopharmacol. 2005;101:330–333. doi: 10.1016/j.jep.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 26.Cos P., Vlietinck A.J., Berghe D.V., Maes L. Anti-infective potential of natural products: How to develop a stronger In vitro proof-of-concept. J. Ethnopharmacol. 2006;106:290–302. doi: 10.1016/j.jep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Makler M.T., Ries J.M., Williams J.A., Bancroft J.E., Piper R.C., Hinrichs D.J. Parasite lactate dehydrogenase as an assay for Plasmodium falciparum drug sensitivity. Am. J. Trop. Med. Hyg. 1993;48:739–741. doi: 10.4269/ajtmh.1993.48.739. [DOI] [PubMed] [Google Scholar]
- 28.Hirumi H., Hirumi K. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J. Parasitol. 1989;75:985–989. doi: 10.2307/3282883. [DOI] [PubMed] [Google Scholar]
- 29.Raz B., Iten M., Grether-Buhler Y., Kaminsky R., Brun R. The Alamiar Blue assay to determine drug sensitivity of African trypanosomes (T. b. rhodesiense, T. b. gambiense) in vitro. Acta Trop. 1997;68:139–147. doi: 10.1016/S0001-706X(97)00079-X. [DOI] [PubMed] [Google Scholar]
- 30.Buckner F.S., Verlinde C.L., la Flamme A.C., van Voorhis W.C. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing beta-galactosidase. Antimicrob. Agents Chemother. 1996;40:2592–2597. doi: 10.1128/aac.40.11.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferraro M.J. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. NCCLS; Viallanova, PA, USA: 1997. National committee for Clinical Laboratory Standards. [Google Scholar]