Abstract
Fractionation of the chloroform extract of Wikstroemia coriacea led to the isolation of two new compounds, oleodaphnoic acid (1), a guaiane-type sesquiterpenoid, and coriaceol (2), an 1,5-diphenyl-1-pentanone analogue, together with nine known compounds. The structures of 1 and 2 were elucidated by extensive spectroscopic data analysis. The known compounds were oleodaphnal (3), indicanone (4), (5R,8R,8aR)-3,8-dimethyl-4,5,6,7,8,8a-hexahydro-5-(1-methylethenyl)-2(1H)-azulenone, (5), 1,5 diphenyl-1-pentanone (6), (+)-3-hydroxy-1,5-diphenyl-1-pentanone (7), umbelliferone (8), daphnoretin (9), β-sitostenone (10) and (−)-hinokinin (11).
Keywords: oleodaphnoic acid; coriaceol; Wikstroemia coriaceae; Thymelaeaceae; guaiane; 1,5-diphenyl-1-pentanone
1. Introduction
Wikstroemia (Thymelaeaceae) is a genus consisting of about 70 species indigenous to Asia, Malaysia, Australia and Pacific Islands [1]. Wikstroemia coriacea B.C. Seemann [2] is an endemic plant, widely distributed in Eastern Polynesia, which is used in folk medicine for its emetic, purgative, narcotic and vesicant properties [3]. Moreover, leaves and stems were used for the treatment of syphilis, gonorrhea, urethritis and leucorrhea [4]. Although there are many phytochemical reports on the genus Wikstroemia focused on sesquiterpenes [5,6], diterpenes [7,8,9,10], triterpenes [7,11], coumarins [12,13,14], flavonoids [15,16,17] and lignans [7,18,19,20], no study has been carried out on the chemistry of Wikstroemia coriacea. This paper reports our phytochemical discovery of two new natural compounds, oleodaphnoic acid (1), a guaiane-type sesquiterpenoid, and coriaceol (2), an 1,5-diphenyl-1-pentanone (6) analogue, along with nine known compounds including oleodaphnal (3), indicanone (4), (5R,8R,8aR)-3,8-dimethyl-4,5,6,7,8,8a-hexahydro-5-(1-methylethenyl)-2(1H)-azulenone, (5), 1,5-diphenyl-1-pentanone (6), (+)-3-hydroxy-1,5-diphenyl-1-pentanone (7), umbelliferone (8), daphnoretin (9), β-sitostenone (10) and (−)-hinokinin (11) (Figure 1). In addition, (−)-hinokinin (11) was isolated for the first time in the genus Wikstroemia.
Figure 1.
Chemical structures of compounds 1–11.
2. Results and Discussion
A total of eleven compounds were identified from the stem bark of W. coriacea. A new sesquiterpenoid oleodaphnoic acid (1, Figure 1) was isolated as a colorless powder. Its molecular formula C15H18O3 was assigned on the basis of HR-ESI-MS (m/z 247.1326 [M+H]+, calcd. 247.1329), implying seven degrees of unsaturation. The IR spectrum displayed significant bands for an unsaturated ketone (1681 cm−1) and an α,β,γ,δ unsaturated carboxylic acid (3373 and 1646 cm−1). The 1H-NMR spectrum showed two tertiary methyl groups as singlets (δ 1.85 and 1.77), and an exo-methylene resonance as two broad singlets (δ 4.76 and 4.75) (Table 1). The 13C-DEPTQ NMR spectrum exhibited 15 carbon resonances, including two methyls, four methylenes, one exo-methylene, one ketone, one carboxylic function and two double bonds (Table 1). From the COSY and HSQC spectra, the occurrence of both partial structures, CH3C=CH2 and CH2CH2CHCH2, was suggested. In the HMBC diagram, cross-peak observed between the methyl protons H-13 and the methine carbon C-7 was indicative of the combination of the above partial structures. Further analysis of the other significant long-range 1H-13C correlations (Figure 2) suggested that NMR data were typical of a guaiane-type skeleton [21]. NMR data close similarity regarding the C-7 carbons of oleodaphnal (3) [22], indicanone (4) [6] and (5R,8R,8aR)-3,8-dimethyl-4,5,6,7,8,8a-hexahydro-5-(1-methylethenyl)-2(1H)-azulenone (5) [23], respectively, allowed to infer the R stereochemistry of C-7. Finally, the carboxylic group was assigned to be at the C-10 position based on the HMBC relationship between H-9 and C-14. From the above results the structure of oleodaphnoic acid was formulated as 1.
Table 1.
1H-NMR (500 MHz,) and 13C-NMR (125 MHz,) data of oleodaphnoic acid (1) (CDCl3) and coriaceol (2) (CD3OD).
| Position | 1 | Position | 2 | ||
|---|---|---|---|---|---|
| 1H (δ) | 13C (δ) | 1H (δ) | 13C (δ) | ||
| 1 | - | 149.5 | 1 | - | 203.2 |
| 2 | 3.38 (brs, 2H) | 42.2 | 2 | 5.10 (dd, 8.4; 3.3, 1H) | 74.1 |
| 3 | - | 204.6 | 3 | 1.83 (m, 1H) | 35.4 |
| 4 | - | 144.7 | 1.59 (m, 1H) | ||
| 5 | - | 166.1 | 4 | 1.83 (m, 1H) | 27.9 |
| 6 | 2.81 (m, 2H) | 32.1 | 1.74 (m, 1H) | ||
| 7 | 2.54 (quint, 7.3, 1H) | 42.5 | 5 | 2.65 (dt, 14.0; 7.3, 1H) | 36.3 |
| 8 | 2.01 (m, 1H) | 32.0 | 2.60 (dt, 14.0; 7.3, 1H) | ||
| 1.81 (m, 1H) | 1' | - | 136.0 | ||
| 9 | 2.80 (m, 2H) | 25.6 | 2', 6' | 7.90 (m, 2H) | 129.5 |
| 10 | - | 127.7 | 3', 5' | 7.48 (m, 2H) | 129.9 |
| 11 | - | 148.7 | 4' | 7.61 (m, 1H) | 134.6 |
| 12 | 4.76 (brs, 1H) | 110.1 | 1'' | - | 143.2 |
| 4.75 (brs, 1H) | 2'', 6'' | 7.13 (m, 2H) | 129.3 | ||
| 13 | 1.77 (s, 3H) | 20.9 | 3'', 5'' | 7.22 (m, 2H) | 129.4 |
| 14 | - | 172.7 | 4'' | 7.13 (m, 1H) | 126.8 |
| 15 | 1.85 (s, 3H) | 9.0 | |||
Figure 2.
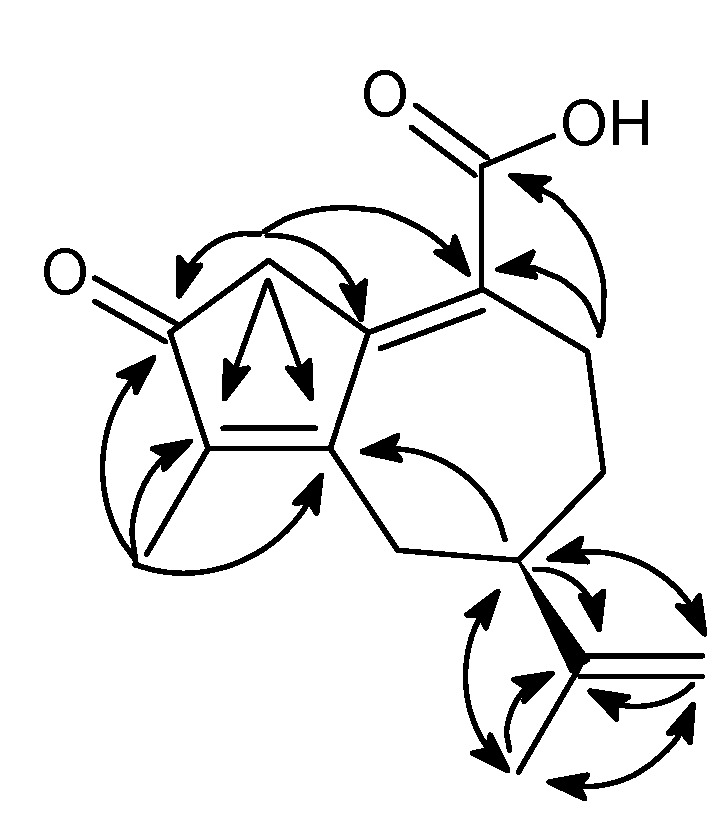
Key HMBC Correlations (H→C) of oleodaphnoic acid (1).
A new natural phenylphenalenone named coriaceol (2, Figure 1) was obtained as an yellowish oil and showed an accurate [M+H]+ ion at m/z 255.1381 (calcd. 255,1379) in the HR-ESI-MS corresponding to the empirical molecular formula C17H18O2 and implying nine degrees of unsaturation. The IR spectrum of 2 suggested the presence of hydroxyl group (3440 cm−1), conjugated ketone carbonyl (1683 cm−1) and aromatic rings (1603–1480 cm−1). The 13C-NMR spectrum gave a total of 17 separate resonances and the 13C-DEPTQ sequence showed three methylene, eleven methine groups and three quaternary carbons, including a conjugated ketone carbonyl at δ 203.2 (Table 1). The 1H-NMR spectrum exhibited two AA’MM’X spin systems, typical of two monosubstituted aromatic rings and an oxygen-bearing methine signal at δ 5.10 (dd, J = 8.4, 3.3 Hz) (Table 1). With the aid of COSY experiments, a -OCH(CH2)3 subunit was identified by further analysis of the remaining 1H resonances. Finally, the location of the two phenyl moieties was supported by the HMBC correlations observed between the ortho H-2' (d 7.90) and H-2'' (d 7.13) signals and carbons C-1 and CH2-5, respectively. From the above spectral data, the structure of coriaceol (2) was established as 2-hydroxy-1,5-diphenylpentan-1-one. This compound, which was previously reported with no NMR data as a synthetic intermediate [24], had not yet previously isolated from a natural source. Attempt to determine the stereochemistry at C-2 was not successful due to the decomposition of the compound.
In addition to these two new structures, we isolated three sesquiterpenoids: oleodaphnal (3) [22], indicanone (4) [6], (5R,8R,8aR)-3,8-dimethyl-4,5,6,7,8,8a-hexahydro-5-(1-methylethenyl)-2(1H)-azulenone, (5) [23]; two phenylphenalenones: 1,5-diphenyl-1-pentanone (6) [25], (+)-3-hydroxy-1,5-diphenyl-1-pentanone (7) [26]; two coumarins: umbelliferone (8) [27], daphnoretin (9) [28]; one triterpenoid: β-sitostenone (10) [29] and one lignan: (−)-hinokinin (11) [30] (Figure 1). The identifications of these nine known compounds were confirmed by comparison of their physical and spectroscopic data (UV, 1H, 13C-NMR, MS and [α]) with the corresponding authentic samples or with values described in the literature.
Literature data reported that most of the isolated compounds are known to possess interesting biological activities such as: indicanone (4) for its anti-inflammatory activity [6]; (5R,8R,8aR)-3,8-dimethyl-4,5,6,7,8,8a-hexahydro-5-(1-methylethenyl)-2(1H)-azulenone (5) for its cytotoxic activity on the P-388 cell line [5,31]; (+)-3-hydroxy-1,5-diphenyl-1-pentanone (7) for its anti-HIV activity [26]; umbelliferone (8) for its anti-inflammatory activities [27], antioxidant [32,33], antihyperlipidemic [34] and anticancer [35,36,37], daphnoretin (9) for its antifungal [38], anticancer [15], inhibition of various sites in DNA synthesized voice [14], activation of protein kinase C (platelet aggregation) [39,40], antiviral hepatitis B [41] and respiratory syncytial virus (RSV) properties [42]; the triterpenoid β-sitostenone (10) for its interesting biological activities as a strong hypoglycemic [43] and antiarrhythmic [44,45], anti-emetic [46], vasodilator agent [47], and also for its antituberculosis activity [45,48] and anti-inflammatory activity [49]; (−)-hinokinine for its antiparasitic, antifungal [50], antigenotoxic and antioxidant activities [30].
3. Experimental
3.1. General
HPLC was performed using an Agilent 1100 pump equipped with a Varian Dynamax Microsorb Si column (250 × 21.4 mm i.d., 5 μm, 100 Å) and a Varian Dynamax Microsorb C18 column (250 × 10 mm i.d., 5 μm, 100 Å), respectively, a refractomeric and a Diode Array Detector (DAD) detector. Optical rotations were measured on a Perkin-Elmer model 241 polarimeter equipped with a sodium lamp (589 nm) and a 1 dm cell. HRMS experiments were performed with a QStar Elite mass spectrometer (Applied Biosystems SCIEX) equipped with an ESI source operated in the positive ion mode. IR spectra were obtained with cell composed of two calcium fluoride windows separated by 0.21-mm thick PTFE spacer A145 using a Bruker FTIR Vertex 70 spectrometer. NMR spectra were recorded at 300 K for ~1 mg samples using a Bruker Avance DRX 500 spectrometer, equipped with a Bruker Cryoplateform and a 5 mm TXI cryoprobe. NMR spectra were referenced to CDCl3 (δH = 7.26 ppm and δC = 77.16 ppm) or to CD3OD (δH = 3.31 ppm and δC = 49.00 ppm) [51]. Standard Bruker pulse sequences were used for homonuclear and heteronuclear two-dimensional experiments.
3.2. Plant Material
The stem bark of W. coriacea was collected from Nuku Hiva, Marquesas Islands, and was identified by Dr Jean-François Butaud. A voucher specimen (CM 1725) has been deposited in the Herbarium of French Polynesia [52].
3.3. Extraction and Isolation
Air-dried and powdered stem bark of W. coriacea (150 g) was extracted with chloroform (3 × 350 mL, 3h rt.) for 10 h. After concentration in vacuo, the remaining solid (7.75 g) was subjected to low pressure chromatography (SiO2; CHCl3/MeOH, 1:0 to 9:1, v:v, then MeOH) to yield three fractions F1 (3.2 g), F2 (2.8 g) and F3 (1.5 g). F1 (79 mg) was chromatographed by semi-preparative HPLC (SiO2; hexane/isopropanol, 99:1, v:v, 10 mL/min) to provide oleodaphnal (3, 4.4 mg), (5R,8R,8aR)-3,8-dimethyl-4,5,6,7,8,8a-hexahydro-5-(1-methylethenyl)-2(1H)-azulenone, (5, 4.5 mg), 1,5-diphenyl-1-pentanone (6, 4.6 mg), 2-hydroxy-1,5-diphenyl-1-pentanone (2, 2.8 mg), and (+)-3-hydroxy-1,5-diphenyl-1-pentanone (7, 1.9 mg), β-sitostenone (10, 1 mg) and (−)-hinokinin (11, 0.6 mg). F2 (1.34 g) was fractioned using LH20 (CH2Cl2/MeOH, 1:1, v:v) to isolate umbelliferone (8, 68 mg), daphnoretin (9, 40 mg) and indicanone (4, 7.7 mg). Oleodaphnoic acid (1, 11 mg) was obtained from F3 (108 mg) through semi-preparative HPLC (C18; H2O /EtOH, 7:3 to 0:1 for 30 min then 0:1 10 min, v:v, 2.3 mL/min).
Oleodaphnoic acid (1). Colorless powder; C15H18O3; 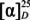 +8 (c 0.02 CHCl3); HR-ESI-MS m/z 247.1326 [M+H]+, calcd. 247.1329; FTIR (CCl4) νmax 3373, 1681, 1646, 1599, 1516, 1448, 1384 cm−1; 1H- and 13C-NMR (CDCl3) data, see Table 1.
+8 (c 0.02 CHCl3); HR-ESI-MS m/z 247.1326 [M+H]+, calcd. 247.1329; FTIR (CCl4) νmax 3373, 1681, 1646, 1599, 1516, 1448, 1384 cm−1; 1H- and 13C-NMR (CDCl3) data, see Table 1.
Coriaceol (2). Yellowish oil; C17H18O2; 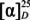 −12 (c 0.003 MeOH); HR-ESI-MS m/z 255.1381 [M+H]+, calcd. 255.1379; FTIR (CCl4) νmax 3440, 1683, 1646, 1593, 1578, 1490, 1362 cm−1; 1H- and 13C-NMR (CD3OD) data, see Table 1.
−12 (c 0.003 MeOH); HR-ESI-MS m/z 255.1381 [M+H]+, calcd. 255.1379; FTIR (CCl4) νmax 3440, 1683, 1646, 1593, 1578, 1490, 1362 cm−1; 1H- and 13C-NMR (CD3OD) data, see Table 1.
4. Conclusions
This work is part of our ongoing phytochemical studies on Polynesian endemic plants aiming at a better knowledge of Polynesian plant biodiversity. We report herein the first phytochemical assessment of the stem bark of W. coriacea with the occurrence of two new natural compounds oleodaphnoic acid (1) and and coriaceol (2), a 1,5-diphenyl-1-pentanone analogue, beside nine known ones. The identified components belong to different secondary metabolite classes including guaiane-type sesquiterpenoids (compounds 1, 3, 4, 5), triterpenoids (10), phenylphenalenones (2, 6, 7), coumarins (8, 9) and a lignan (11) which raises questions about the complexity of the biosynthetic routes to yield such less common chemodiversity exhibited by the same plant. Most of the isolated compounds are known for their relevant biological activities, which add more interest to this endemic Polynesian plant. We will follow up phytochemical analysis along with phylogenetic studies of all endemic species belonging to Wikstroemia genus grown in Polynesia aiming at a biodiversity assessment regarding insular plant adaptation and evolution.
Acknowledgments
The authors are thankful to the “Ministère de l’Enseignement, de la Recherche et de la Technologie” of France for N. Ingert PhD Grant, to the “Délégation à la Recherche de la Polynésie Française” for financial support (Marquesas Research program), and to J.F. Butaud for botanical identification and plant localisation.
Footnotes
Sample Availability: Samples of the compounds 1–11 are available from the authors.
References
- 1.Wang Y., Gilbert M.G. Flora of China. Volume 13. Science Press and Missouri Botanical Garden; Beijing, China and St. Louis, MO, USA: 2007. WIKSTROEMIA Endlicher, Prodr. Fl. Norfolk; pp. 215–229. [Google Scholar]
- 2.Butaud J.-F., Cartier C.L., Florence J., Perlman S.P., Sachet M.-H., Wood K.R. Herbier de la Polynésie française: Wikstroemia coriacea B.C. Seemann. [(accessed on 26 November 2012)]. Available online: http://www.herbier-tahiti.pf/Selection_Taxonomie.php?id_tax=10079/
- 3.Pétard P. Plantes utiles de la Polynésie-Raau Tahiti. Haere Po No Tahiti; Papeete, French Polynesia: 1986. [Google Scholar]
- 4.Nadeaud J. Plantes usuelles des Tahitiens. J. Martel; Montpellier, French Polynesia: 1864. [Google Scholar]
- 5.Lin R.-W., Tsai I.-L., Duh C.-Y., Lee K.-H., Chen I.-S. New Lignans and Cytotoxic Constituents from Wikstroemia lanceolata. Planta Med. 2004;70:234–238. doi: 10.1055/s-2004-815540. [DOI] [PubMed] [Google Scholar]
- 6.Wang L.Y., Unehara T., Kitanaka S. Anti-inflammatory activity of new guaiane type sesquiterpene from Wikstroemia indica. Chem. Pharm. Bull. 2005;53:137–139. doi: 10.1248/cpb.53.137. [DOI] [PubMed] [Google Scholar]
- 7.Borris R.P., Blaskó G., Cordell G.A. Ethnopharmacologic and phytochemical studies of the Thymelaeaceae. J. Ethnopharmacol. 1988;24:41–91. doi: 10.1016/0378-8741(88)90138-9. [DOI] [PubMed] [Google Scholar]
- 8.Wu D., Sorg B., Adolf W., Opferkuch H.J., Seip E.H., Hecker E. Oligo- and macrocyclic diterpenes in thymelaeaceae and euphorbiaceae occurring and utilized in yunnan (Southwest China) 4. Tigliane type diterpene esters (phorbol-12,13-diesters) from Wikstroemia canescens. Phytother. Res. 1993;7:194–196. doi: 10.1002/ptr.2650070220. [DOI] [Google Scholar]
- 9.Abe F., Iwase Y., Yamauchi T., Kinjo K., Yaga S., Ishii M., Iwahana M. Minor daphnane-type diterpenoids from Wikstroemia retusa. Phytochemistry. 1998;47:833–837. doi: 10.1016/S0031-9422(97)00529-3. [DOI] [PubMed] [Google Scholar]
- 10.Abe F., Iwase Y., Yamauchi T., Kinjo K., Yaga S. Daphnane diterpenoids from the bark of Wikstroemia retusa. Phytochemistry. 1997;44:643–647. doi: 10.1016/S0031-9422(96)00602-4. [DOI] [PubMed] [Google Scholar]
- 11.Chen C.C., Lin Y.C., Chen Y.P., Hsu H.Y. A study on the constituents of Wikstroemia indica. Taiwan Yao Hsueh Tsa Chih. 1981;33:28–29. [Google Scholar]
- 12.Nakabayashi T. Studies on the coumarin derivatives. VII. Constituents of the bark of Daphne Kiusiana Miquel and others (Thymelaeaceae) J. Pharm. Biomed. Anal. 1954;74:192–194. [Google Scholar]
- 13.Tandon S., Rastogi R.P. Wikstrosin, a tricoumarin from Wikstroemia viridiflora. Phytochemistry. 1977;16:1991–1993. doi: 10.1016/0031-9422(77)80110-6. [DOI] [Google Scholar]
- 14.Hall I.H., Tagahara K., Lee K.H. Antitumor agents LIII: The effects of daphnoretin on nucleic acid and protein synthesis of ehrlich ascites tumor cells. J. Pharm. Sci. 1982;71:741–744. doi: 10.1002/jps.2600710706. [DOI] [PubMed] [Google Scholar]
- 15.Lee K.-H., Tagahara K., Suzuki H., Wu R.-Y., Haruna M., Hall I.H., Huang H.-C., Ito K., Iida T., Lai J.-S. Antitumor Agents. 49. Tricin,Kaempferol-3-O-β-d-Glucopyranoside and (+)-Nortrachelogenin, Antileukemic Principles From Wikstroemia indica. J. Nat. Prod. 1981;44:530–535. doi: 10.1021/np50017a003. [DOI] [PubMed] [Google Scholar]
- 16.Niwa M., Jiang P.-F., Hirata Y. Two New C-3/C-3"-Biflavanones from Wikstroemia sikokiana. Chem. Pharm. Bull. 1986;34:3631–3634. doi: 10.1248/cpb.34.3631. [DOI] [Google Scholar]
- 17.Baba K., Taniguchi M., Kozawa M. Three biflavonoids from Wikstroemia sikokiana. Phytochemistry. 1994;37:879–883. doi: 10.1016/S0031-9422(00)90376-5. [DOI] [Google Scholar]
- 18.Tandon S., Rastogi R.P. Wikstromol, a new lignan from Wikstroemia viridiflora. Phytochemistry. 1976;15:1789–1791. doi: 10.1016/S0031-9422(00)97493-4. [DOI] [Google Scholar]
- 19.Torrance S.J., Hoffmann J.J., Cole J.R. Wikstromol, antitumor lignan from Wikstroemia foetida var. oahuensis gray and Wikstroemia uva-ursi gray (thymelaeaceae) J. Pharm. Sci. 1979;68:664–665. doi: 10.1002/jps.2600680545. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki H., Lee K.-H., Haruna M., Iida T., Ito K., Huang H.-C. (+)-Arctigenin, a lignan from Wikstroemia indica. Phytochemistry. 1982;21:1824–1825. [Google Scholar]
- 21.Rahman A.-U., Ahmad V.U. 13C-NMR of Natural Products. Volume 1. Plenum Publishing; New York, NY, USA: 1992. p. 984. [Google Scholar]
- 22.Taninaka H., Takaishi Y., Honda G., Imakura Y., Sezik E., Yesilada E. Terpenoids and aromatic compounds from Daphne oleoides ssp. oleoides. Phytochemistry. 1999;52:1525–1529. doi: 10.1016/S0031-9422(99)00305-2. [DOI] [Google Scholar]
- 23.Zdero C., Bohlmann F., Niemeyer H.M. Isocedrene and Guaiane Derivatives from Pleocarphus revolutus. J. Nat. Prod. 1988;51:509–512. doi: 10.1021/np50057a009. [DOI] [PubMed] [Google Scholar]
- 24.Vitale A.A., Doctorovich F., Sbarbati Nudelman N. One-pot synthesis of diarylalkylcarbinols and substituted derivatives through carbon monoxide insertion reactions into aryllithiums. J. Organomet. Chem. 1987;332:9–18. doi: 10.1016/0022-328X(87)85117-3. [DOI] [Google Scholar]
- 25.Martínez R., Ramón D.J., Yus M. Easy α-alkylation of ketones with alcohols through a hydrogen autotransfer process catalyzed by RuCl2(DMSO)4. Tetrahedron. 2006;62:8988–9001. doi: 10.1016/j.tet.2006.07.013. [DOI] [Google Scholar]
- 26.Huang S.Z., Zhang X.J., Li X.Y., Jiang H.Z., Ma Q.Y., Wang P.C., Liu Y.Q., Hu J.M., Zheng Y.T., Zhou J., et al. Phenols with Anti-HIV Activity from Daphne acutiloba. Planta Med. 2012;78:182–185. doi: 10.1055/s-0031-1280263. [DOI] [PubMed] [Google Scholar]
- 27.Kim J.S., Kim J.C., Shim S.H., Lee E.J., Jin W., Bae K., Son K.H., Kim H.P., Kang S.S., Chang H.W. Chemical constituents of the root of Dystaenia takeshimana and their anti-inflammatory activity. Arch. Pharm. Res. 2006;29:617–623. doi: 10.1007/BF02968244. [DOI] [PubMed] [Google Scholar]
- 28.Navarro-Garcia V.M., Herrera-Ruiz M., Rojas G., Zepeda L.G. Coumarin derivatives from Loeselia mexicana. Determination of the anxiolytic effect of daphnoretin on elevated plus-maze. J. Mex. Chem. Soc. 2007;51:193–197. [Google Scholar]
- 29.Seca A.M.L., Silva A.M.S., Silvestre A.J.D., Cavaleiro J.A.S., Domingues F.M.J., Neto C.P. Chemical composition of the light petroleum extract of Hibiscus cannabinus bark and core. Phytochem. Anal. 2000;11:345–350. doi: 10.1002/1099-1565(200011/12)11:6<345::AID-PCA540>3.0.CO;2-T. [DOI] [Google Scholar]
- 30.Medola J.F., Cintra V.P., Pesqueira e Silva É.P.C., de Andrade Royo V., da Silva R., Saraiva J., Albuquerque S., Bastos J.K., Andrade e Silva M.L., Tavares D.C. (−)-Hinokinin causes antigenotoxicity but not genotoxicity in peripheral blood of Wistar rats. Food Chem. Toxicol. 2007;45:638–642. doi: 10.1016/j.fct.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 31.Blay G., Garcia B., Molina E., Pedro J.R. Synthesis of all 7[alpha]H-guaia-4,11-dien-3-one diastereomers from (+)-dihydrocarvone. Tetrahedron. 2005;61:11156–11162. doi: 10.1016/j.tet.2005.09.026. [DOI] [Google Scholar]
- 32.Hoult J.R.S., Payá M. Pharmacological and biochemical actions of simple coumarins: Natural products with therapeutic potential. Gen. Pharmacol. 1996;27:713–722. doi: 10.1016/0306-3623(95)02112-4. [DOI] [PubMed] [Google Scholar]
- 33.Singh R., Singh B., Singh S., Kumar N., Kumar S., Arora S. Umbelliferone—An antioxidant isolated from Acacia nilotica (L.) Willd. Ex. Del. Food Chem. 2010;120:825–830. doi: 10.1016/j.foodchem.2009.11.022. [DOI] [Google Scholar]
- 34.Ramesh B., Pugalendi K.V. Antihyperglycemic effect of Umbelliferone in streptozotocin-diabetic rats. J. Med. Food. 2006;9:562–566. doi: 10.1089/jmf.2006.9.562. [DOI] [PubMed] [Google Scholar]
- 35.Sharifi S., Lotterer E., Michaelis H.C., Bircher J. Pharmaco-kinetics of coumarin and its metabolites. Preliminary results in three healthy volunteers. J. Irish Coll. Phys. Surg. 1992;22:29–32. [Google Scholar]
- 36.Marshall M.E., Kervin K., Benefield C., Umerani A., Albainyjenei S., Zhao Q., Khazaeli M.B. Growth-inhibitory effects of coumarin (1,2-benzopyrone) and 7-hydroxycoumarin on human malignant cell lines in vitro. J. Cancer Res. Clin. Oncol. 1994;120:S3–S10. doi: 10.1007/BF01377114. [DOI] [PubMed] [Google Scholar]
- 37.Maucher A., Vonangerer E. Antitumour activity of coumarin and 7-hydroxycoumarin against 7, 12-dimethylbenz[a]anthracene-induced rat mammary carcinomas. J. Cancer Res. Clin. Oncol. 1994;120:502–504. doi: 10.1007/BF01191806. [DOI] [PubMed] [Google Scholar]
- 38.Hu K., Kobayashi H., Dong A., Iwasaki S., Yao X.S. Antifungal, antimitotic and anti-HIV-1 agents from the roots of Wikstroemia indica. Planta Med. 2000;66:564–567. doi: 10.1055/s-2000-8601. [DOI] [PubMed] [Google Scholar]
- 39.Ko F.N., Chang Y.L., Kuo Y.H., Lin Y.L., Teng C.M. Daphnoretin, a new protein kinase C activator isolated from Wikstroemia indica C. A. Mey. Biochem. J. 1993;295:321–327. doi: 10.1042/bj2950321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J.P., Raung S.L., Kuo Y.H., Teng C.M. Daphnoretin-induced respiratory burst in rat neutrophils is, probably, mainly through protein kinase C activation. Eur. J. Pharmacol. Mol. Pharmacol. Sect. 1995;288:341–348. doi: 10.1016/0922-4106(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 41.Chen H.C., Chou C.K., Kuo Y.H., Yeh S.F. Identification of a protein kinase C (PKC) activator, daphnoretin, that suppresses hepatitis B virus gene expression in human hepatoma cells. Biochem. Pharmacol. 1996;52:1025–1032. doi: 10.1016/0006-2952(96)00420-0. [DOI] [PubMed] [Google Scholar]
- 42.Ho W.S., Xue J.Y., Sun S.S.M., Ooi V.E.C., Li Y.L. Antiviral Activity of Daphnoretin Isolated from Wikstroemia indica. Phytother. Res. 2010;24:657–661. doi: 10.1002/ptr.2935. [DOI] [PubMed] [Google Scholar]
- 43.Alexander-Lindo R.L., Morrison E.Y.S.A., Nair M.G., McGrowder D.A. Effect of the Fractions of the Hexane Bark Extract and Stigmast-4-en-3-one Isolated from Anacardium occidentale on Blood Glucose Tolerance Test in an Animal Model. Int. J. Pharmacol. 2007;3:41–47. doi: 10.3923/ijp.2007.41.47. [DOI] [Google Scholar]
- 44.Kiyoshi H., Yukari N., Masakazu M., Sansei N., Katsuyuki U., Kimiko S., Tetsuya K., Akinobu S. Antiarrhythmic agent. 2003–113107. Japan Patent. 2003 Apr 18;
- 45.Prachayasittikul S., Suphapong S., Worachartcheewan A., Lawung R., Ruchirawat S., Prachayasittikul V. Bioactive Metabolites from Spilanthes acmella Murr. Molecules. 2009;14:850–867. doi: 10.3390/molecules14020850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Y., Kinoshita K., Koyama K., Takahashi K., Tai T., Nunoura Y., Watanabe K. Anti-emetic principles of Pogostemon cablin (Blanco) Benth. Phytomedicine. 1999;6:89–93. doi: 10.1016/S0944-7113(99)80041-5. [DOI] [PubMed] [Google Scholar]
- 47.Kolak U., Arı Ş., Birman H., Hasançebi S., Ulubelen A. Cardioactive Diterpenoids from the Roots of Salvia amplexicaulis. Planta Med. 2001;67:761–763. doi: 10.1055/s-2001-18359. [DOI] [PubMed] [Google Scholar]
- 48.Saludes J.P., Garson M.J., Franzblau S.G., Aguinaldo A.M. Antitubercular constituents from the hexane fraction of Morinda citrifolia L. (Rubiaceae) Phytother. Res. 2002;16:683–685. doi: 10.1002/ptr.1003. [DOI] [PubMed] [Google Scholar]
- 49.Tewtrakul S., Tansakul P., Daengrot C., Ponglimanont C., Karalai C. Anti-inflammatory principles from Heritiera littoralis bark. Phytomedicine. 2010;17:851–855. doi: 10.1016/j.phymed.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 50.Silva M.L., Martins C.H., Lucarini R., Sato D.N., Pavanb F.R., Freitas N.H., Andrade L.N., Pereira A.C., Bianco T.N., Vinholis A.H., et al. Antimycobacterial activity of natural and semi-synthetic lignans. Z. Naturforsch. C. 2009;64:779–784. doi: 10.1515/znc-2009-11-1204. [DOI] [PubMed] [Google Scholar]
- 51.Gottlieb H.E., Kotlyar V., Nudelman A. NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities. J. Org. Chem. 1997;62:7512–7515. doi: 10.1021/jo971176v. [DOI] [PubMed] [Google Scholar]
- 52.The Herbarium of French Polynesia. [(accessed on 25 February 2013)]. Available online: http://www.herbier-tahiti.pf.



