Abstract
Chemical investigation of Croton pullei (Euphorbiaceae) collected in the Brazilian Amazon region was revisited. The chemical composition of the essential oils of leaves and stems was analyzed by GC/MS. It was found that both the oils comprise mainly terpenes, among which linalool was the major one (24.90 and 39.72%, respectively). Phytochemical investigation of the stem methanol extract led to the isolation of a new natural product from the glutarimide alkaloid group named N-[2,6-dioxo-1-(2-phenylethyl)-3-piperidinyl]-acetamide, confirming that C. pullei is a rich source of this class of alkaloids. The hexane and methanol extracts of the stems of C. pullei showed moderate antibacterial and antifungal activity and the highest inhibition was observed when the methanol extract was tested against Staphylococcus aureus CCMB 262 and CCMB 263.
Keywords: Croton pullei, essential oils, glutarimide alkaloid, antibacterial activity, antifungal activity
1. Introduction
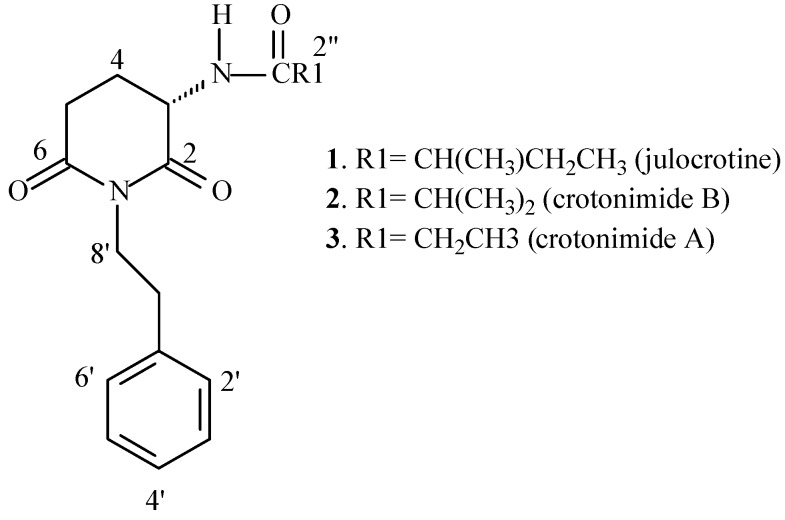
Croton L. is one of the largest genera of Euphorbiaceae, with about 1,200 species, mostly distributed in the West Indies and South America with some in North America [1,2], Africa and Madagascar [3]. Their species are trees, shrubs, herbs and lianas that occur in the most variable tropical ecosystems [4,5]. Croton pullei Lanj. is a small tree or a woody liana with restricted distribution, occurring in Guyana, French Guyana and Brazil (States of Pará and Maranhão) [5]. Some Croton species produce essential oils containing mostly terpenoids and phenylpropanoids, while some species produce only terpenoids [6]. Among the monoterpenes, α- and β-pinene, linalool and 1,8-cineole are often found; the most frequently found sesquiterpenes are β-caryophyllene and germacrene D, and among the phenylpropanoids, methyleugenol and related compounds are the most common; some other compounds, such as fatty acids, aliphatic esters and diterpenes are seldom cited [6]. Glutarimide alkaloids, among them julocrotine (1) and crotonimides B (2) and A (3) (Figure 1), terpenoids, flavonoids and a ferulamide derivative were previously isolated from C. pullei [7,8]. The structure of julocrotine was confirmed by X-ray analysis [9]. These glutarimide alkaloids were restricted to a small group of Euphorbiaceae, including Julocroton and some Croton species [10,11,12,13,14,15], but recently, julocrotine together with two other compounds of this class of substances were isolated from Cordia (Boraginaceae) [16]. Biological studies indicated that julocrotine has antiproliferative effects against the amastigote and promastigote forms of Leshmania (L.) amazonensis [17]. Several biological activities of Croton species extracts, essential oils and isolated compounds, including antibacterial and antifungal activities were reported [6]. A survey of the literature revealed that no studies on the volatile compounds or antimicrobial activity of C. pullei have been published to date. The aim of this study was to continue the chemical investigation of the non-volatile compounds of C. pullei, as it is a rich source of glutarimide alkaloids, and also to evaluate the chemical composition of the essential oil and the possible antimicrobial effects of this species.
Figure 1.
Structures of the glutarimide alkaloids from Croton pullei.
2. Results and Discussion
2.1. Volatile Compounds
In total, 87 compounds were identified, accounting for 91–99.5% of the volatiles. The percentage of the compounds identified in the leaf and stem oils of C. pullei is listed along with their retention indices in Table 1. The yields of the essential oils from leaves and stems were 0.50% and 0.06%, respectively. The 57 compounds listed account for 87.20% and 91.95% of the volatiles of the leaves and stems, respectively. Except for the presence of 1-nonen-3-ol in the leaves and methyleugenol in the leaves and stems, terpenes made up the majority of the components, including monoterpenes (44.51% in leaves and 62.17% in stems) and sesquiterpenes (41.61% in leaves and 26.69% in stems). Oxygenated monoterpenes (42.56%) were major components in stem oil, while sesquiterpene hydrocarbons were predominant in the leaves. Similar amounts of monoterpene hydrocarbons and oxygenated sesquiterpenes were detected in both oils. The main compound identified in the leaf and stem oils was linalool (24.90 and 39.72%, respectively), followed by α-pinene (8.08 and 8.23%, respectively) and β-pinene (6.63 and 9.81%, respectively). Comparing the chemical composition of the two oils, it is clear that there are only quantitative differences between the major compounds. On the other hand, the two oils showed a great qualitative difference, with the presence of twenty-three compounds detected only in the leaf oil, and fourteen in the stem oil.
Table 1.
Volatiles (%) identified in the leaf and stem oils of Croton pullei.
| Constituents | RI * | Leaf | Stem |
|---|---|---|---|
| α-pinene | 937 | 8.08 | 8.23 |
| β-pinene | 981 | 6.63 | 9.81 |
| Myrcene | 994 | 1.07 | |
| 1,8-cineole | 1033 | 3.72 | |
| γ-terpinene | 1060 | 0.50 | |
| cis-linalool furanoxide | 1074 | 0.26 | |
| 1-nonen-3-ol | 1080 | 0.60 | 0.71 |
| trans-linalool furanoxide | 1091 | 0.32 | |
| Linalool | 1105 | 24.90 | 39.72 |
| α-campholenal | 1129 | 0.67 | |
| α-terpineol | 1193 | 0.60 | |
| trans-pinocarveol | 1142 | 0.49 | |
| trans-sabinol | 1148 | 0.40 | |
| terpinen-4-ol | 1180 | 0.68 | |
| Myrtenal | 1200 | 0.60 | |
| β-elemene | 1395 | 1.24 | |
| γ-elemene | 1340 | 0.96 | |
| α-cubebene | 1352 | 0.36 | |
| α-copaene | 1379 | 0.31 | |
| β-elemene | 1388 | 0.44 | |
| Methyleugenol | 1408 | 0.48 | 0.45 |
| cis-α-bergamotene | 1419 | 0.30 | |
| β-caryophyllene | 1424 | 3.96 | 1.24 |
| trans-α-bergamotene | 1439 | 0.27 | |
| γ-elemene | 1437 | 0.73 | |
| Aromadendrene | 1444 | 0.15 | |
| α-humulene | 1459 | 1.84 | |
| allo-aromadendrene | 1466 | 0.29 | |
| γ-gurjunene | 1481 | 0.90 | 0.43 |
| germacrene D | 1486 | 1.19 | |
| β-selinene | 1492 | 2.91 | 1.25 |
| α-selinene | 1500 | 2.67 | 1.21 |
| bicyclogermacrene | 1502 | 2.67 | |
| α-muurolene | 1505 | 0.15 | |
| (Z)-α-bisabolene | 1510 | 0.46 | |
| γ-cadinene | 1519 | 1.50 | |
| δ-cadinene | 1527 | 1.12 | 0.78 |
| cis-calamenene | 1537 | 1.15 | 0.69 |
| trans-cadina-1,4-diene | 1545 | 0.08 | |
| α-calacorene | 1545 | 0.08 | 0.45 |
| (E)-nerolidol | 1564 | 3.91 | 4.62 |
| Spathulenol | 1580 | 2.72 | 2.38 |
| caryophyllene oxide | 1585 | 1.80 | 1.71 |
| Viridiflorol | 1604 | 0.16 | |
| Ledol | 1616 | 0.78 | |
| Junenol | 1625 | 1.00 | |
| iso-spathulenol | 1631 | 0.40 | |
| epi-cadinol | 1642 | 0.57 | |
| α-muurolol | 1650 | 0.57 | |
| Pogostol | 1660 | 3.58 | 4.65 |
| (Z)-α-santalol | 1685 | 1.63 | 2.05 |
| Acorenone | 1697 | 0.53 | 1.13 |
| (Z)-epi-β-santalol | 1703 | 0.80 | 0.57 |
| cyclocolorenone | 1759 | 0.09 | 0.87 |
| manoyl oxide | 2002 | 0.87 | |
| 13-epi-manoyl oxide | 2025 | 0.39 | |
| 2-oxo-manoyl oxide | 2243 | 0.68 |
* RI on Rtx-5MS.
Despite the occurrence of linalool in the leaves and stems of C. pullei, their oxides were detected only in the leaf oil. Croton species containing high amounts of linalool were C. cajucara Benth. [18], C. lanjouwensis Jabl. [19], C. aubrevillei J. Léonard [20] and C. micradenus Urb. [21]. High amounts of α-pinene were found in some Croton species, such as C. adenocalyx Baill. [22], C. antanosiensis Leandri [23], C. matourensis Aubl. [24] and C. micradenus Urb. [21]. This is the first report on the chemical composition of the essential oils of these species.
2.2. Novel Non-Volatile Compound
Substance 4 was isolated from the methanol extract of the stems of C. pullei and identified as the glutarimide alkaloid N-[2,6-dioxo-1-(2-phenylethyl)-3-piperidinyl]-acetamide (Figure 2). This is the first time that compound 4 is isolated as a natural product; until now it has only been obtained by synthesis [25]. It was isolated as a light brown amorphous solid, soluble in methanol, with = −11° (c 0.03, CH3OH) ( −8.2° in CHCl3 [25]) and IR absorptions at 3333, 2901, 2852, 1726, 1678, 746, 695 cm−1. The molecular formula of substance 4 was suggested to be C15H18O3N2 from the HRMS with M+ 274.1317 Da (calculated 274.131743). The 1H-NMR spectrum (CD3OD) displayed signals of a phenylethyl group (H-2'-H-8') similar to those of julocrotine and crotonimides A and B [7] and signals of a glutarimide unit with the H-3 signal unfolding in a double doublet at δH 4.60 (J = 12.6 and 5.6 Hz) and the H-4 and H-5 signals in multiplets at δH 2.13–1.86 and 2.75–2.71, respectively; the signal of hydrogen in the group N-H was not observed or it was rather weak and the signal of the acetyl side chain was observed as a singlet at δH 2.02. The 13C-NMR spectrum (CD3OD) showed the signals of a phenylethyl and glutarimide moieties and of an N-acetyl group with three carbonyl signals at δC 173.3, 173.4 and 172.9. All assignments were based on 2D-NMR data of 1H-1H COSY (300 MHz), HETCOR (13C-1H COSY, 1J(CH), 75 MHz), HMBC [1H-13C COSY, 2,3J(CH)] experiments. Figure 2 shows the most important HMBC correlations of substance 4. The NMR data of 4 reported before were partial and recorded in CDCl3 and those reported here were recorded in CD3OD and are now fully assigned (Table 2).
Figure 2.
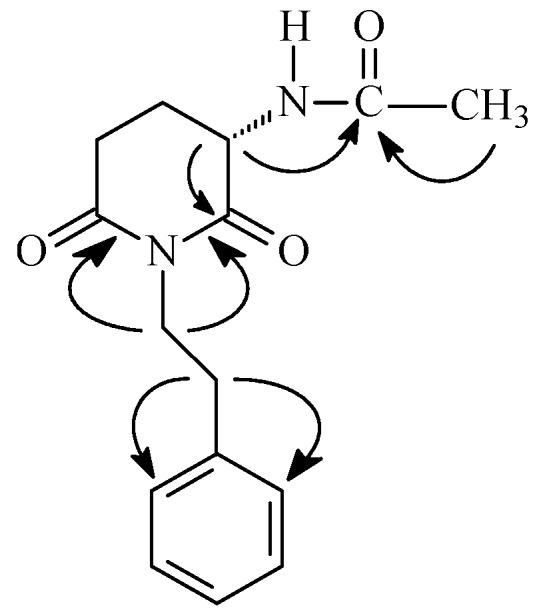
Structure and correlations of the HMBC spectrum of substance 4.
Table 2.
1H (300 MHz) and 13C (75.5 MHz) NMR data of substance 4 (CD3OD).
| δH | δC | |
|---|---|---|
| 2 | - | 173.3 * |
| 3 | 4.60 (dd, J = 12.6 and 5.6 Hz) | 51.8 |
| 4 | 2.13–1.86 (m) | 24.8 |
| 5 | 2.75–2.71 (m) | 32.4 |
| 6 | - | 172.9 * |
| 1' | - | 139.9 |
| 2' | 7.29–7.18 (m) | 129.9 |
| 3' | 7.29–7.18 (m) | 129.5 |
| 4' | 7.29–7.18 (m) | 127.3 |
| 5' | 7.29–7.18 (m) | 129.5 |
| 6' | 7.29–7.18 (m) | 129.9 |
| 7' | 2.77 (t, J =7.8 Hz) | 34.8 |
| 8' | 3.95–3.80 (m) | 42.6 |
| 1'' | - | 173.7 |
| 2'' | 2.02 (s) | 22.5 |
* Signals can be interchanged.
2.3. Antimicrobial Activity
The methanol and hexane extracts of C. pullei were evaluated for their antimicrobial activity against Gram-negative bacteria, Gram-positive bacteria and yeast species responsible for various forms of acquired infections in humans and, more often, antimicrobial resistance. The MIC and MCC data on the hexane and methanol extracts obtained from the stems of C. pullei are shown in Table 3.
Table 3.
Results of Minimum Inhibitory Concentration (MIC) and Minimum Microbicidal Concentration (MMC) in mg mL−1, of the hexane and methanol extracts of C. pullei stems.
| Organism | Hexane | MeOH | Control | |||
| MIC | MMC | MIC | MMC | Nist/Chlorf | DMSO | |
| Escherichia coli CCMB 261 | 5 | - | 5 | 5 | R | 5.00 |
| Pseudomonas aeruginosa CCMB 268 | 5 | 10 | 1.25 | 2.5 | 0.31 | 5.00 |
| Salmonella sp. CCMB 281 | 2.5 | 10 | 1.25 | 10 | 0.16 | 5.00 |
| Staphylococcus aureus CCMB 262 | 0.156 | 5 | 0.078 | 2.5 | 0.31 | 5.00 |
| S. aureus CCMB 263 | 1.25 | 10 | 0.078 | 5 | 0.31 | 10.00 |
| S. aureus CCMB 285 | 10 | 10 | 2.5 | 5 | R | 10.00 |
| Bacillus cereus CCMB 282 | 2.5 | 2.5 | 1.25 | 1.25 | 0.16 | 5.00 |
| Candida albicans CCMB 286 | 2.5 | 5 | 2.5 | 5 | 0.63 | 10.00 |
| C. albicans CCMB 266 | 2.5 | 10 | 2.5 | 5 | 0.08 | 10.00 |
| C. parapsilosis CCMB 288 | 2.5 | 10 | 2.5 | 10 | R | 10.00 |
R: resistent, Nyst: nystatin, Chlorf: chloramphenicol.
Both extracts showed inhibition of the tested microorganisms and the effect of the methanol extract was higher than the hexane extract. The highest inhibition effect of the methanol extract was observed against the Gram-negative bacterium Pseudomonas aeruginosa. CCMB 268 (MIC = 1.25 mg mL−1), and the Gram-positive bacteria S. aureus CCMB 262 (MIC = 0.078 mg mL−1), S. aureus CCMB 263 (MIC = 0.078 mg mL−1) and B. cereus CCMB 282 (MIC = 1.25 mg mL−1). The hexane extract was more active against S. aureus CCMB 262 (MIC = 0.156 mg mL−1) and S. aureus CCMB 263 (MIC = 1.25 mg mL−1).
According to Fontanay et al. [26] MIC values below 10 μg mL−1 are considered good and those around 50 μg mL−1 moderate for antibacterial activity. MIC values that equal hundreds of μg mL−1 indicate that the compound has no activity. Thus, it can be considered that the methanol extract of C. pullei has moderate activity against both S. aureus tested strains.
In similar a study, Selowa and coworkers [27] using extracts of three Croton species (C. megalobotrys, C. steenkampianus and C. salvaticus) observed that the methanol extract of C. megalobotrys was the most active extract inhibiting S. aureus at 0.625 mg mL−1, P. aeruginosa at 0.313 mg mL−1 against and E. coli at 0.125 mg mL−1. The methanol extracts of C. campestris [28] and C. membranaceus showed antimicrobial activity against S. aureus.
It is known that the stem extracts of C. pullei are a rich source of glutarimide alkaloids, but despite the high amounts of julocrotine (1) in this species, it seems that this compound does not contribute to the antimicrobial activity of the test extracts, since according to Bayor and coworkers julocrotine exhibited no significant activity against S. aureus, Bacillu subtilis and P. aeruginosa [29] and that’s why it was not tested again. On the other hand, among the isolated compounds from C. pullei in prior chemical investigations [7], the diterpenes kaurenoic acid and ribenone and the triterpene lupeol are known for their antimicrobial activities [30,31,32,33] and could contribute for the observed activity of the extracts.
3. Experimental
3.1. Material and Isolation and Identification of Non-Volatile and Distillation of the Volatile Constituents
Samples of C. pullei were taken from the wild plant in a secondary forest in the municipality of Peixe-Boi, State of Pará, Brazil (October, 2008). A voucher specimen (# 188,908) was kept in the Herbarium MG of the Museu Paraense Emílio Goeldi (MPEG).
3.2. Extraction of Volatile Compounds
The samples were dried for 7 days in an air-conditioned room (at low humidity) and then ground. Leaves (100 g) and stems (80 g) were hydrodistilled for 3 h using a Clevenger-type apparatus with the refrigeration water maintained at 15 °C. The oils obtained were centrifuged for 5 min (3,000 rpm), dried over Na2SO4, centrifuged again, and immediately submitted to GC/FID and GC/MS analysis. The solution containing 2 µL of the oil in 1 mL of hexane was immediately prepared to gas chromatography analysis. The total oil yield was expressed in percentage (volume/mass) on the basis of dried material.
3.3. Analysis of the Volatiles
The oils were analyzed using a Shimadzu GC/MS Model QP 2010 Plus, equipped with a Rtx-5MS (30 m × 0.25 mm; 0.25 μm film thickness) fused silica capillary column. Helium was used as carrier gas adjusted to 1.2 mL.min−1; with splitless injection of 1 μL of a hexane solution; injector and interface temperature were 250 °C; oven temperature programmed was 60–240 °C at 3 °C.min−1. EIMS: electron energy, 70 eV; ion source temperature was 200 °C. Identification of the compounds were made by comparison of their GC mass and retention data with those in NIST-05 library and cited in the literature data [34]. Retention indices were calculated using n-alkane standard solutions (C8-C26) available from Fluka S. A. (Steinheim, Switzerland), in the same chromatographic conditions. Quantitative data were obtained from the electronic integration of the total ion chromatogram (TIC) peak areas.
3.4. Isolation and Identification of Compound 4
The stems of C. pullei were extracted as in [8]. The dichloromethane phase of the methanol extract was fractionated by column chromatography on silica using mixtures of hexane, ethyl acetate and methanol in gradients of increasing polarities as eluents. The fraction eluted with hexane-EtOAc 70% was purified by column chromatography on Sephadex LH-20 using methanol as eluent leading to the isolation of 41 mg of compound 4. Spectrometric methods were used for structural determination. NMR spectra were recorded on a Varian 300 MHz NMR spectrometer (300 MHz and 75 MHz for 1H and 13C, respectively) using TMS as internal standard; the IR spectrum was recorded on a Thermo electron IR 100 spectrometer; optical rotation was measured at the sodium D line (589 nm) on a Perkin Elmer 341 and the HRMS was recorded on a VG Auto Spec-300.
3.5. Antimicrobial Assays
3.5.1. Microbial Strains
The following bacteria and yeasts were used for the experiments: Escherichia coli CCMB 261 (sensitive to trimetoprime and resistant to sulphonamide), Pseudomonas aeruginosa CCMB 268, Salmonella sp. CCMB 281, Staphylococcus aureus CCMB 262 (resistant to streptomycin and dihydrostreptomycin), Staphylococcus aureus CCMB 263, Staphylococcus aureus CCMB 285, Bacillus cereus CCMB 282, Candida albicans CCMB 286, Candida albicans CCMB 266 and Candida parapsilosis CCMB 288 (resistant to amphoterycin-B). All microorganisms were cultured on Müeller-Hinton agar (MHA). The bacterial strains were cultured at 37 °C for 24h and yeasts at 28 °C for 48h. All the microbial tests were performed in triplicate.
3.5.2. Minimum Inhibitory Concentration (MIC)
The minimum inhibitory concentration (MIC) of the methanol extract of the leaves of C. pullei was determined based on a micro dilution method in a 96 multi-well microtiter plates [35]. All microbial tests were performed in MHA. The extracts were dissolved DMSO-water solution (1:1) and sterilized by filtration through cellulose acetate membrane (0.22 mm). Serial dilutions from 10 to 0.078 10 to 0.078 mg mL−1 of the extracts were prepared. Each well received 10 μL of suspension of each micro-test. The purity of the suspension of the inoculums was verified in a simultaneous incubation. After the period of incubation, 50 μL of triphenyltetrazolium chloride 2-3-5 (TTC) was added to a final concentration of 0.40 mg mL−1 (final concentration; assays with yeasts) and 30 μL of rezasurine (RZ, assays with bacteria) to a final concentration of 0.01% for qualitative analysis of microbial growth in the wells in order to determine the antimicrobial activity of each dilution of the samples. Nistatine (20 mg mL−1) and cloramphenicol (10 mg mL−1) were used as positive controls. Controls were performed to test the viability of microorganisms and the sterility of the culture medium. The MIC was considered the lowest extract concentration where there was no visible microbial growth after color indicator (TTC and RZ) step.
3.5.3. Minimal Microbicidal Concentration (MMC)
Petri dishes containing MHA were used for this assay; 5 µL of each MIC well were transferred to MHA and cultured at 28 °C for 48 h (yeasts) and at 37 °C for 24 h (bacteria). The MMC was considered the lowest extract concentration where there was no cellular growth.
4. Conclusions
The essential oils of the leaves and stems of Croton pullei were predominantly composed of terpenes, and the major constituent of both oils (linalool) showed only small quantitative variations (24.90 and 39.72%, respectively). The isolation of the new natural product substance 1 confirms the ability of this species to produce glutarimide alkaloids. The hexane and methanol extracts of the stems of C. pullei showed moderate antibacterial and antifungal activity and the highest inhibition was observed against Staphylococcus aureus CCMB 262 and CCMB 263 for both extracts. The presence of kaurenoic acid, ribenone and lupeol in the stems extracts of C. pullei, but not julocrotine, can explain in part the observed antimicrobial activity.
Acknowledgments
The authors are grateful to MCT/PPG-7/USAID, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) and Fundação de Amparo à Pesquisa no Estado do Pará (FAPESPA/PA) for the financial support, to Osvaldo Cardoso do Nascimento from the Museu Paraense Emílio Goeldi for the vegetal sample collection, to Coleção de Culturas de Microrganismos da Bahia (CCMB) and Universidade Estadual de Feira de Santana (Bahia, Brazil) for the microbial strains, to LAPAC from the Universidade Federal do Pará for the IR spectrum, to Laboratório de Espectrometria de massas (Unicamp) for the HRMS spectra, to Maria da Conceição Ferreira de Oliveira (PPGQ-UFC) and Fátima Nunes (ICB-UFPA) for the . R.N.S.P. is grateful to CNPq for the fellowship.
Footnotes
Sample Availability: Samples of the compounds 1–4 and the essential oils are available from the author.
References
- 1.Webster G.L. Synopsis of the genera and suprageneric tax of Euphorbiaceae. Ann. Mo. Bot. Gard. 1994;81:33–144. doi: 10.2307/2399909. [DOI] [Google Scholar]
- 2.Govaerts R., Frodin D.G., Radcliffe-Smith A. World Checklist and Bibliography of Euphorbiaceae (and Pandaceae) 1st ed. Royal Botanic Gardens; Kew, UK: 2000. pp. 1, 661. [Google Scholar]
- 3.Webster G.L. A provisional synopsis of the section of the genus Croton (Euphorbiaceae) Taxon. 1993;42:793–823. doi: 10.2307/1223265. [DOI] [Google Scholar]
- 4.Secco R.S. Notas sobre as lianas do gênero Croto L. (Euphorbiaceae) Bol. Mus. Para. Emílio Goeldi. Sér. Bot. 1992;8:265–281. [Google Scholar]
- 5.Secco R.S. Sinopse das espécies de Croton L. (Euphorbiaceae) na Amazônia brasileira: um ensaio taxonômico. 1st ed. Museu Paraense Emílio Goeldi; Belém, Brazil: 2008. pp. 119–123. [Google Scholar]
- 6.Salatino A., Salatino M.L.F., Negri G. Traditional uses, Chemistry and Pharmacology of Croton species (Euphorbiaceae) J. Braz. Chem. Soc. 2007;18:11–33. doi: 10.1590/S0103-50532007000100002. [DOI] [Google Scholar]
- 7.Barbosa P.S., Abreu A.S., Batista E.F., Guilhon G.M.S.P., Muller A.H., Arruda M.S.P., Santos L.S., Arruda A.C., Secco R.S. Glutarimide alkaloids andterpenoids from Croton pullei var. glabrior Lanj. Biochem. Syst. Ecol. 2007;35:887–890. doi: 10.1016/j.bse.2007.04.006. [DOI] [Google Scholar]
- 8.Silva S.O., Peixoto R.N., Silva J.R.A., Alves C.N., Guilhon G.M.S.P., Santos L.S., Brasil D.S. Identification of (−)(E)-N-[2(S)-Hydroxy-2-(4-hydroxyphenyl) ethyl]ferulamide, a Natural Product Isolated from Croton pullei: Theoretical and Experimental Analysis. Int. J. Mol. Sci. 2011;12:9389–9403. doi: 10.3390/ijms12129389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreira R.Y.O., Brasil D.S.B., Alves C.N., Guilhon G.M.S.P., Santos L.S., Arruda M.S.P., Muller A.H., Barbosa P.S., Abreu A.S., Silva E.O., et al. Crystal structure and theoretical calculations of julocrotine, a natural product with antileishmanial activity. Int. J. Quantum Chem. 2008;108:513–520. doi: 10.1002/qua.21355. [DOI] [Google Scholar]
- 10.Nakano T., Djerassi C., Corral R.A., Orazi O.O. Structure of julocrotine. J. Org. Chem. 1961;26:1184–1191. doi: 10.1021/jo01063a051. [DOI] [Google Scholar]
- 11.Aboagye F.A., Sam G.H., Massiot G., Lavaud C. Julocrotine, a glutarimide alkaloid from Croton membranaceus. Fitoterapia. 2000;71:461–462. doi: 10.1016/S0367-326X(00)00141-6. [DOI] [PubMed] [Google Scholar]
- 12.Cuong N.M., Sung T.V., Ahn B.-Z. Cytotoxic compounds from Croton cascarilloides. Saengyak Hakhoechi. 2002;33:207–210. [Google Scholar]
- 13.Suárez A.I., Blanco Z., Delle Monache F., Compagnone R.S., Arvelo F. Three new glutarimide alkaloids from Croton cuneatus. Nat. Prod. Res. 2004;18:421–426. doi: 10.1080/14786410310001622004. [DOI] [PubMed] [Google Scholar]
- 14.Stuart K.L., McNeill D., Kutney J.P., Eigendorf G., Klein P.K. Isolation and synthesis of glutamine and glutarimide derivatives from Croton humilis. Tetrahedron. 1973;29:4071–4075. doi: 10.1016/0040-4020(73)80239-X. [DOI] [Google Scholar]
- 15.Kapingu M.C., Mbwambo Z.H., Moshi M.J., Magadula J.J. Brine shrimp lethality of a glutarimide alkaloid from Croton sylvaticus Hochst. East Cent. Afr. J. Pharm. Sci. 2005;8:3–5. [Google Scholar]
- 16.Parks J., Gyeltshen T., Prachyawarakorn V., Mahidol C., Ruchirawat S., Kittakoop P. Glutarimide alkaloids and a terpenoid benzoquinone from Cordia globifera. J. Nat. Prod. 2010;73:992–994. doi: 10.1021/np100078s. [DOI] [PubMed] [Google Scholar]
- 17.Guimarães L.R.C., Rodrigues A.P.D., Marinho P.S.B., Muller A.H., Guilhon G.M.S.P., Santos L.S., Nascimento J.L.M., Silva E.O. Activity of the julocrotine, a glutarimide alkaloid from Croton pullei var. glabrior, on Leishmania (L.) amazonensis. Parasitol. Res. 2010;107:1075–1081. doi: 10.1007/s00436-010-1973-0. [DOI] [PubMed] [Google Scholar]
- 18.Lopes D., Bizzo H.R., Sobrinho A.F.S., Pereira M.V.G. Linalool-rich essential oil from leaves of Croton cajucara Benth. J. Essent. Oil Res. 2000;12:705–708. doi: 10.1080/10412905.2000.9712196. [DOI] [Google Scholar]
- 19.Leão I.M.S., Andrade C.H.S., Pinheiro M.L.B., Machado M.I.L., Rocha A.F.I., Craveiro A.A., Alencar J.W., Matos F.J.A. Essential oil of Croton lanjouwensis Jablonski from Brazilian Amazonian region. J. Essent. Oil Res. 1998;10:643–644. doi: 10.1080/10412905.1998.9700995. [DOI] [Google Scholar]
- 20.Menut C., Lamarty G., Bessière J.M., Seuleiman A.M., Fendero P., Maidou E., Dénamganai J. Aromatics plants of Tropical Central Africa. XXII. Volatile constituents of Croton aubrevillei J. Léonard and C. zambesicus Muell. Argic. J. Essent. Oil Res. 1995;4:419–422. [Google Scholar]
- 21.Pino J.A., Marbot R., Payo A., Herrera P., Marti M.P. Chemical composition of the leaf oil of Croton micradenus Urb. from Cuba. J. Essent. Oil Bear. Pl. 2005;8:1–5. doi: 10.1080/0972060X.2005.10643411. [DOI] [Google Scholar]
- 22.Craveiro A.A., Alencar J.W., Matos F.J.A., Machado M.I.L. The essential oil of Croton adenocalyx A. DC. J. Essent. Oil Res. 1990;2:145–146. doi: 10.1080/10412905.1990.9697845. [DOI] [Google Scholar]
- 23.Radulović N., Mananjarasoa E., Harinantenaina L., Yoshinori A. Essential oil composition of four Croton species from Madagascar and their chemotaxanomy. Biochem. System. Ecol. 2006;34:648–653. doi: 10.1016/j.bse.2006.02.005. [DOI] [Google Scholar]
- 24.Gottlieb O.R., Koketsu M., Magalhaes M.T., Maia J.G.S., Mendes P.H., da Rocha A.I., da Silva M.L., Wilberg V.C. Óleos essenciais da Amazônia VII. Acta Amazon. 1981;11:143–148. [Google Scholar]
- 25.Teng B., Zheng J., Huang H., Huang P. Enantioselective synthesis of glutarimide alkaloids cordiarimides A, B, crotonimides A, B, and julocrotine. Chin. J. Chem. 2011;29:1312–1318. doi: 10.1002/cjoc.201180248. [DOI] [Google Scholar]
- 26.Fontanay S., Grare M., Mayer J., Finance C., Duval R.M. Ursolic, oleanolic and betulinic acids: Antibacterial spectra and selectivity indexes. J. Ethnopharmacol. 2008;120:272–276. doi: 10.1016/j.jep.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Selowa S.C., Shai L.J., Masoko P., Mokgotho M.P., Magano S.R. Antibacterial activity of extracts of three Croton species collected in Mpumalanga region in South Africa. Afr. J. Trad. 2010;7:98–103. doi: 10.4314/ajtcam.v7i2.50861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matias E.F.F., Santos K.K.A., Almeida T.S., Costa J.G.M., Coutinho H.D.M. Atividade antibacteriana in vitro de Croton campestris A., Ocimum gratissimum L. e Cordia verbenacea DC. Rev. Bras. Bioci. 2010;8:294–298. [Google Scholar]
- 29.Bayor M., Gbedema S.Y., Annan K. The antimicrobial activity of Croton membranaceous, a species used in formulations for measles in Ghana. J. Pharmacogn. Phytother. 2009;1:47–51. [Google Scholar]
- 30.Okoye T.C., Akah P.A., Omeje E.O., Okoli C.O., Nworu S.C., Hamman M. Antibacterial and anticancer activity of kaurenoic acid from root bark extract of Annona senegalensis . Planta Medica. 2011;77:PF-11. [Google Scholar]
- 31.Boeck P., Sá M.M., Souza B.S., Cercená R., Escalante A.M., Zachino S.A., Cechinel Filho V., Yunes R. A simple synthesis of kaurenoic esters and other derivatives and evaluation of their antifungal activity. J. Braz. Chem. Soc. 2005;16:1360–1366. doi: 10.1590/S0103-50532005000800009. [DOI] [Google Scholar]
- 32.Murthy Y.L.N., Devarapalli M., Reddy G.D., Varahalarao V. Phytochemical investigation of the bark of Excoecaria agallocha Linn. J. Pharm. Res. 2010;3:1–5. [Google Scholar]
- 33.Gallo M.B.C., Sarachine M.J. Biological activities of lupeol. Int. J. Biomedical Pharm. Sci. 2009;3:46–66. [Google Scholar]
- 34.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th ed. Allured Pub. Corp.; Carol Stream, IL, US: 2007. p. 804. [Google Scholar]
- 35.Sarker S.D., Nahar L., Kumarasamy Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the vitro antibacterial screening of phytochemicals. Methods. 2007;42:321–324. doi: 10.1016/j.ymeth.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]