Abstract
Several new substituted sulfonamide compounds were synthesized and their structures were confirmed by 1H-NMR, 13C-NMR, FT-IR, and mass spectroscopy. The antibacterial activities of the synthesized compounds were screened against standard strains of six Gram positive and four Gram negative bacteria using the microbroth dilution assay. Most of the compounds studied showed promising activities against both types of bacteria.
Keywords: sulfonamide, 4-methylbenzenesulfonamide, 4-nitrobenzenesulfonamide, 4-methoxybenzenesulfonamide, imidazole, benzimidazole, antibacterial activity
1. Introduction
Heterocycles containing sulfonamido moieties have attracted obvious attention due to their significant biological properties and their role as pharmacophores [1,2,3,4,5,6]. Studies have shown that sulfonamide compounds were used as antibacterial agents [7,8,9], anticancer [10,11,12], anti-inflammatory, analgesic agents [13,14,15], antifungal agents [9,16] and antiviral agents [17]. Imidazole and its derivatives have been reported to be bioactive molecules in many important biological systems with a wide range of pharmacological activity. In general, they are well known as proton donors and/or acceptors in enzymatic systems, coordination system ligands and as the basis of charge–transfer processes [18,19], as well as antibacterial [20,21,22], anti-parasitic [23], antiepileptic [24], anti-inflammatory and anticancer agents [25,26,27].
In our study, new promising bioactive compounds based on the sulfonamide moiety were designed and synthesized by a simple and efficient method, followed by the evaluation of their biological activities. The synthesis emphasizes a strategy that combines two or more pharmacologically compatible moieties in one molecule by attaching a sulfonamide moiety to an imidazole, benzimidazole or another sulfonamide moiety. We believe this route has a wide range of applications and we have high expectations for the future development of new compounds.
2. Results and Discussion
2.1. Synthesis
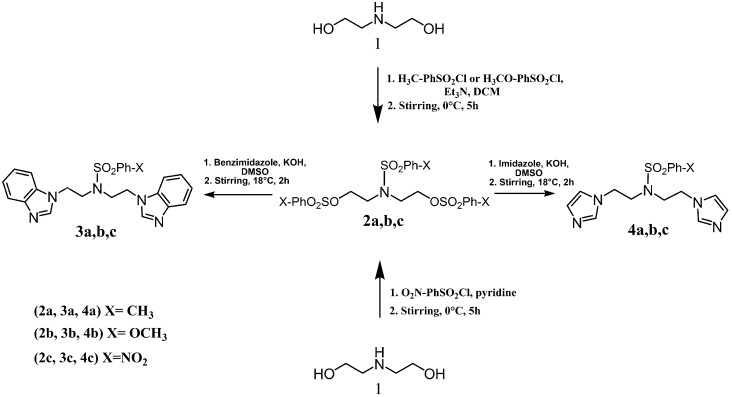
Bis-benzimidazole and bis-imidazole sulfonamides were synthesized from the diol 1 as shown in Scheme 1.
Scheme 1.
Synthesis of bis-benzimidazole sulfonamides and bis-imidazole sulfonamides 3a–c, 4a–c.
Three bis-benzimidazole sulfonamides and three bis-imidazole sulfonamides compounds were obtained by treating imidazole (or benzimidazole) with tris-(4-substituted benzensulfonate)- diethanolamine under basic conditions to form the corresponding bis-imidazole (or bis-benzimidazole) sulfonamides. The reaction of tris-(4-substituted benzensulfonate) with either imidazole or benzimidazole has produced symmetrical products and it is in agreement with literature [28] to synthesize tris-(4-substituted benzensulfonate)-diethanolamine. In the current study, this intermediate was applied as a reagent to synthesize symmetric bis-imidazole (or bis-benzimidazole) sulfonamide compounds. In this reaction proton abstraction from the nitrogen of imidazoles rings by potassium hydroxide [29] is considered the key step. The resulting imidazolide (or benzimidazolide) anions attack the carbon bearing the 4-substituted benzensulfonate in both sides of diethanolamine with the nitrogen atom being protected via the third 4-substituted benzenesulfonyl group.
The FTIR spectra for compounds 3a–c and 4a–c showed absorption bands at 1351–1375 cm−1 and 1150–1185 cm−1 which were assigned to the O=S=O group. The same compounds showed stretching absorption bands at 3100–3047 cm−1, 2975–2855 cm−1, 1590–1457 cm−1, and 1666–1584 cm−1 attributed to (C-H)Aromatic, (C-H)Aliphatic, (C=C)Aromatic, and (C=N), respectively. The target compounds 3b and 4b showed characteristic stretching absorption bands at 1,220 cm−1 and 1,238 cm−1 which were assigned to C-O-C, while the bands at 1529–1520 cm−1 and 1355–1340 cm−1 for compounds 3c and 4c were assigned to Ar-NO2.
The 1H-NMR spectra of compounds 3a, 4a, 3b, and 4b showed singlets at δ 2.33 ppm and δ 2.80 ppm which were assigned to the 4-methyl and 4-methoxy protons of the arylsulfonyl groups, respectively. A triplet recorded at δ 3.31–3.54 ppm and δ 3.95–4.27 ppm was assigned to the two methylene groups protons of compounds 3a–c and 4a–c. The aromatic protons of compounds 3a–c and 4a–c were recorded as multiplets in the δ 6.89–7.83 ppm range. A singlet which was observed at δ 7.42–8.16 ppm corresponds to the isolated C-H of the imidazole and benzimidazole rings. The 13C-NMR spectra of compounds 3a–c and 4a–c showed characteristic peaks in the δ 163.23–163.34 ppm, δ 149.03–153.12 ppm and δ 129.78–144.28 ppm ranges which were assigned to CAr-O, CAr-NO2 and CAr-S, respectively. The peaks recorded at δ 43.09–45.77 ppm, δ 48.20–50.37 ppm and δ 56.18–56.24 ppm were attributed to the methylene and methoxy carbon atoms, correspondingly.
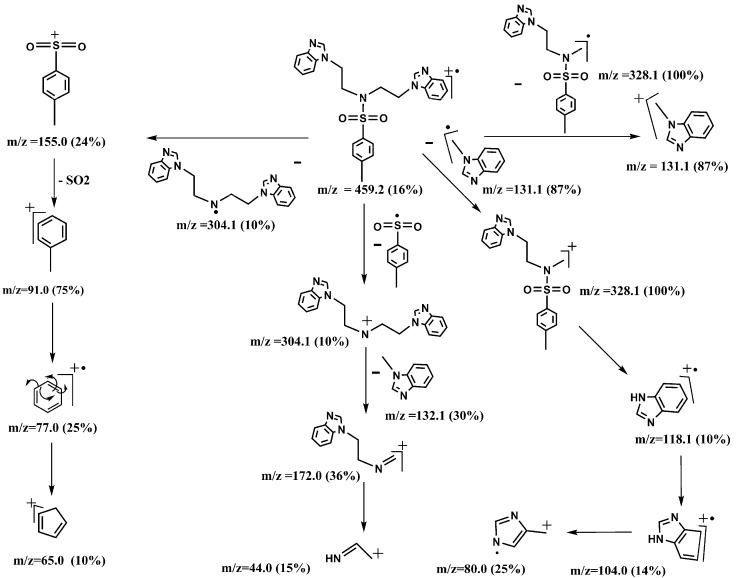
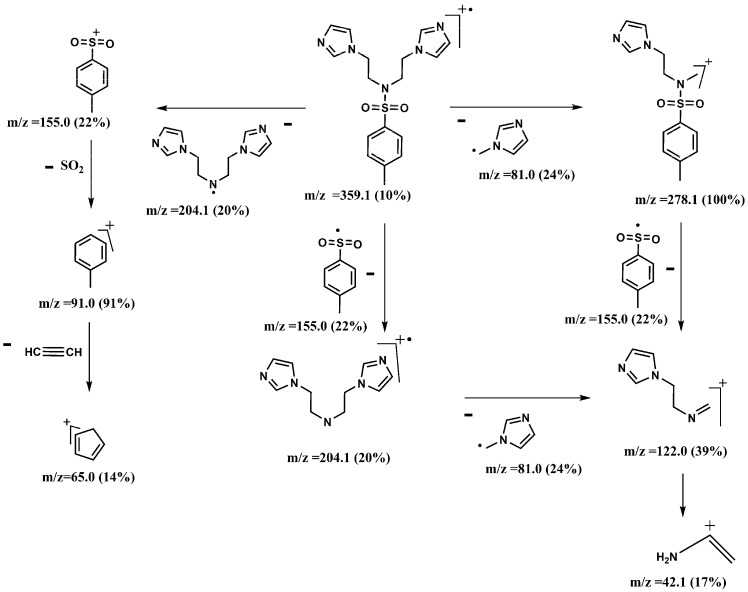
The mass spectra of compounds 3a and 4a showed various characteristic peaks. Those at m/z 459.2 and 359.1 were assigned to the molecular ions of 3a and 4a, respectively. The base peak of 3a at m/z 328.1 was assigned to the N-(benzimidazol-1-yl)ethyl-N-4-dimethylbenzenesulfonamido radical, while the base peak of 4a at m/z 278.1 was assigned to the N-(imidazole-1-yl)ethyl-(4-methylbenzene) sulfonamide-methyliumyl ion. The characteristic peaks at m/z 155.0 and 91.0 for both 3a and 4a were due to (4-methylphenyl)dioxosulfanium and 4-methylbenzene-1-ylium ions, respectively Scheme 2 and Scheme 3 show the fragmentation patterns for 3a and 4a, respectively.
Scheme 2.
Mass fragmentation pattern of 3a.
Scheme 3.
Mass fragmentation pattern of 4a.
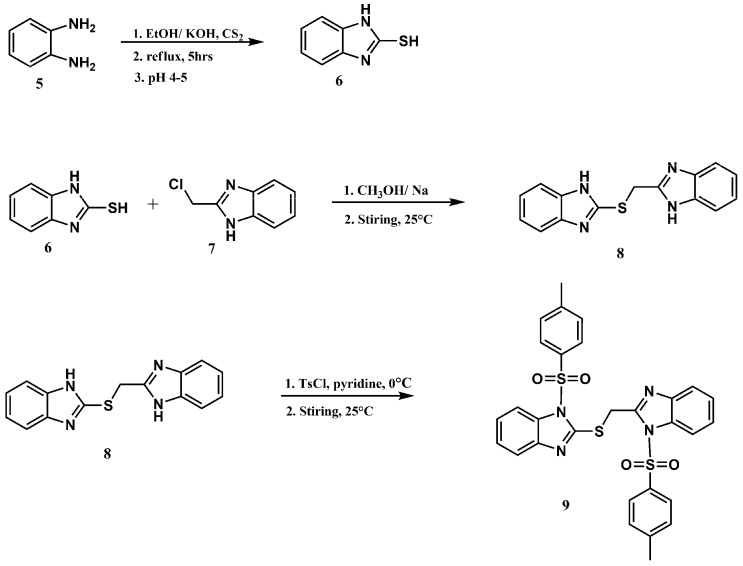
Scheme 4 shows the preparation of compounds 9 from 2-((benzimidazol-2-yl)methylthio)-benzimidazole. A simple method was adopted to synthesize a pure heterocyclic product in good yield. 2-Mercaptobenzimidazole and sodium methoxide were stirred with 2-chloromethylbenzimidazole. A pale-yellow solid precipitated instantly due to the reactivity of -SH group then it was treated with tosyl chloride in pyridine.
Scheme 4.
Synthesis of N-4-methylbenzenesulfonyl ((N-(4-methylbenzenesulfonyl)-benzimida zol-2-yl)methylthio)-benzimidazole (9).
The infrared spectrum of compound 8 indicated the absence of a free -SH absorption band and the appearance of S-C stretching at 748 cm−1, while the absorption bands at 1,365 and 1,170 cm−1 were assigned to the O=S=O group. The 1H-NMR spectrum of compound 9 showed two singlet peaks at δ 2.31, and 2.37 ppm integrating for six protons for the two methyl protons of the sulfonamido moieties. The protons of the methylene group appeared as a singlet at δ 5.18 ppm, whereas the aromatic protons appeared as multiplets and doublet peaks in the δ 7.19–7.98 ppm range. The 13C-NMR of compound 9 showed peaks at δ 21.77 and 21.80 ppm which was assigned to two non-corresponding methyls for two tosyl groups. The CH2-S carbon atom was observed at δ 31.20 ppm.
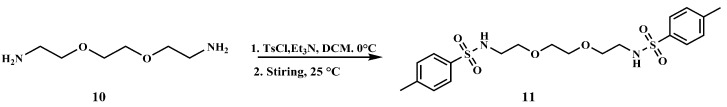
Scheme 5 shows the preparation of compound 11 from 2,2-(ethylenedioxy)bis(ethylamine) by treating with tosyl chloride and triethylamine in dry dichloromethane that produced a significant yield of pure bis-sulfonamide compound 11.
Scheme 5.
Synthesis of 4-methyl-N-(2-{2-[2-(4-methylbenzenesulfonamido)ethoxy]ethoxy} ethyl)-benzenesulfonamide (11).
The FTIR spectrum for compound 11 showed absorption bands at 3276 cm−1 indicating the presence of a N-H group, 1088 cm−1 for a C-O-C group, 1124 cm−1 for C-C-O vibrations, and the peaks at 1317, 1152 cm-1 were assigned to the O=S=O group. The 1H-NMR spectrum of compound 11 showed characteristic doublet peaks at δ 7.27 ppm and 7.73 ppm which were assigned to the aromatic protons. The methylene groups were recorded as a quartet at δ 3.09 ppm and a triplet at δ 3.50 ppm integrating for eight and four protons, respectively. The amino protons of compound 11 appeared as a triplet at δ 5.50 ppm. The corresponding methyls for two tosyl groups were observed as a single peak at δ 2.39 ppm integrating for six protons. The 13C-NMR of compound 11 showed aromatic carbon peaks at δ 143.45, 137.07, 129.78 and 127.18 ppm. The peak at δ 21.59 ppm was assigned to the two corresponding methyl groups while, peaks of 4 × CH2-O were observed at δ 69.78 and 70.43 ppm. The structures of compounds 3a, 4a, 9 and 11 were further characterized by single crystal X-ray diffraction which indicated tetragonal and triclinic crystal systems for 3a and 4a, respectively, while the crystal structures of 9 and 11 have been reported [30,31]. Crystallographic data for compounds 3a and 4a have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication numbers CCDC 923664 and CCDC 923665, respectively. Copies of the data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB21EZ, UK; fax: (+44)-1223-336-033; or [e-mail: deposit@ccdc.cam.ac.uk].
2.2. Antibacterial Activities
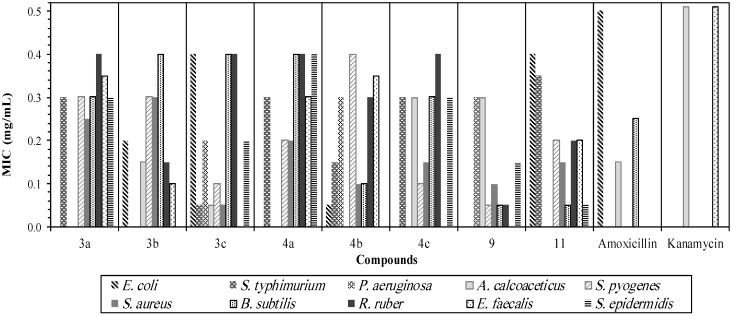
In an in vitro antibacterial bioassay, the eight compounds 3a–c, 4a–c, 9 and 11 were evaluated by microbroth dilution assays using representative standard strains of Gram-positive and Gram-negative bacteria, and the results are listed in Table 1. Minimum inhibitory concentrations (mg/mL) of the compounds against the test microorganisms were determined. It was shown (Figure 1) that the majority of the compounds studied possessed significant antibacterial activity towards most of the selected microorganisms. The highest activities were observed for compounds 9 and 11, followed by 3c and 4c then 3b and 4b. Compounds 3a and 4a showed the least antibacterial activity for the selected concentration range, as shown in Figure 1. In the structure-activity relationships (SAR) studies, it has been reported that the incorporation of two different pharamacophores in a single structure enhanced the resulting compounds’ biological activities [32,33,34]. The presence of substituents on aromatic rings also affects the antibacterial activities of compounds. Compounds with resonance electron-withdrawing substitution (nitro) showed greater antibacterial activities than those with electron-donating substituent groups (methyl and methoxy) [32,33,34] and this is clearly shown in the case of compounds 3a–c and 4a–c. Studies have also shown that the presence of a sulfur atom as a sulfide in drugs provides a greater stability to the three dimensional structure of the molecule [35]. It is observed that the presence of sulfur in compound 9 has a significant contribution to the antibacterial activities against Gram positive and Gram negative bacteria. This is believed to due to the existence of a toxophoric (-N=C-S-) group [36,37,38]. Furthermore, the attachment of two toxophoric groups (amine) with benzensulfonyl moieties in compound 11 enhanced the antibacterial activity against most of both kinds of bacteria [39,40,41].
Table 1.
Antibacterial activities of compounds studied.
| No. | Structure of samples | Bacteria/MICs (mg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gram-negative bacteria | Gram-positive bacteria | ||||||||||
| Escherichia coli | Salmonella typhimurium | Pseudomonas aeruginosa | Acinetobacter calcoaceticus | Streptococcus pyogenes | Staphylococcus aureus | Bacillus subtilis | Rodococcus ruber | Enterococcus faecalis | Staphylococcus epidermidis | ||
| 3a | 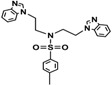 |
>0.5 | 0.30 | >0.5 | >0.5 | 0.30 | 0.25 | 0.30 | 0.40 | 0.35 | 0.30 |
| 3b | 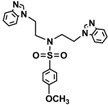 |
0.2 | >0.5 | >0.5 | 0.15 | 0.30 | 0.30 | 0.40 | 0.15 | 0.10 | >0.5 |
| 3c | 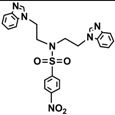 |
0.40 | 0.05 | 0.20 | 0.05 | 0.10 | 0.05 | 0.40 | 0.40 | >0.5 | 0.20 |
| 4a | 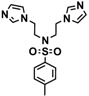 |
>0.5 | 0.30 | >0.5 | >0.5 | 0.20 | 0.20 | 0.40 | 0.40 | 0.30 | 0.40 |
| 4b | 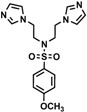 |
0.05 | 0.15 | 0.30 | >0.5 | 0.40 | 0.10 | 0.10 | 0.30 | 0.35 | >0.5 |
| 4c | 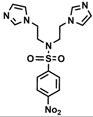 |
>0.5 | 0.30 | >0.5 | 0.30 | 0.10 | 0.15 | 0.30 | 0.40 | >0.5 | 0.30 |
| 9 | 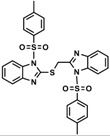 |
>0.5 | >0.5 | 0.30 | 0.30 | 0.05 | 0.10 | 0.05 | 0.05 | >0.5 | 0.15 |
| 11 | 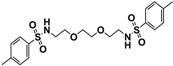 |
0.40 | 0.35 | >0.5 | >0.5 | 0.20 | 0.15 | 0.05 | 0.20 | 0.20 | 0.05 |
| AM |  |
<0.05 | <0.05 | Nd | 0.15 | 0.05 | <0.05 | 0.25 | <0.05 | <0.05 | nd |
| KA |  |
<0.05 | <0.05 | <0.05 | >0.5 | <0.05 | <0.05 | <0.05 | <0.05 | >0.5 | <0.05 |
MIC: Minimum inhibitory concentration, AM: Amoxicillin, KA: Kanamycin, nd: not detected.
Figure 1.
MIC’s Histogram for synthesized compounds (0.05–0.40 mg/mL concentration) versus ten strains of bacteria.
It is obvious from the overall antibacterial results that different compounds reacted in different ways against bacteria. In these compounds, strains of Gram-positive bacteria seem to be more sensitive than Gram negative micro-organisms.
Thus, comparing the results of both the synthesized compounds and amoxicillin against a β-lactam resistant Gram-positive bacteria (Staphylococcus epidermidis) demonstrated interesting antibacterial inhibitory values for most of the synthesized compounds. The MIC values are between 0.05 mg/mL (for compound 11) to 0.4 mg/mL (for compound 4a) whereas the β-lactam antibiotic amoxicillin was inactive against this strain of Gram-positive bacteria.
In addition, compounds 3c, 4b, and 9 showed significant activities, with MIC values (0.2, 0.3 and 0.3 mg/mL, respectively) toward a β-lactam resistant Gram-negative bacterium (Pseudomonas aeruginosa) when compared to the antibiotic amoxicillin. Compounds 4b, 9 and 11 with MIC values of 0.10, 0.05 and 0.05 mg/mL, respectively showed interesting antibacterial activities against Bacillus subtilis, which required a high dose of amoxicillin (0.25 mg/mL) [42]. Compounds 3a–b, 4a,b, and 11 demonstrated inhibitory effects ranging between 0.1–0.35 mg/mL against the Gram-positive bacterium Enterococcus faecalis, however, the antibiotic kanamycin was inactive toward the samples at the concentration range of this study (0.05–0.5 mg/mL). Both commercial antibiotics amoxicillin and kanamycin exhibited MIC values (0.15 mg/mL and >0.5 mg/mL, sequentially) against Acinetobacter calcoaceticus, while compound 3c exhibited a significant antibacterial inhibitory effect at 0.05 mg/mL against the mentioned Gram-negative bacteria.
3. Experimental
3.1. General
The IR spectra were obtained with a Perkin Elmer 400 Fourier Transform Infrared (FTIR) spectrometer. 1H and 13C-NMR spectra were recorded on Jeol Lambda and ECA DELTA spectrometers at 400 MHz. The mass spectra were recorded using an Agilent 5975 system for EI/MS and a Finnigan TSQ7000 for HREI/MS (NUS, Singapore). Melting points were measured on a Gallenkamp melting point apparatus in open-end capillary tubes and are uncorrected. Thin layer chromatography was carried out on pre-coated silica gel plates (0.25 mm, 20 × 20 cm, 60F254, E. Merck). Flash column chromatography on silica gel 60 (230–400 mesh, E. Merck).General grade solvents and reagents were purchased from commercial suppliers and used without further purification.
3.2. Synthesis N-(4-Methylbenzenesulfonyl)-bis((4-methylbenzenesulfonyl(oxy))ethyl)amine (2a) and N-(4-Methoxybenzenesulfonyl)-bis((4-methoxybenzenesulfonyl(oxy))-ethyl)amine (2b)
These compounds were prepared according to the modified procedure described in [28]. Diethanolamine (5.5 g, 0.0524 mol) was dissolved in distilled dichloromethane (100 mL). The solution was cooled to 0 °C and then triethylamine (24.4 mL, 17.78 g, 0.176 mol) was added. With the temperature maintained at 0 °C, solid p-toluenesulfonyl chloride (31.4 g, 0.164 mol) or (4-methoxybenzenesulfonyl chloride (34.1 g, 0.165 mol) were added in portions with vigorous stirring over the course of 5 h to obtain compounds 2a or 2b, respectively. The reaction mixture was stirred at room temperature overnight. A pale yellow filtrate was produced from Et3NHCl filtration, washed three times with 1 mol/L HCl, followed by 5 × 40 mL portions of water and 5 × 40 mL portions of saturated NaHCO3 solution. The organic layer was dried over anhydrous magnesium sulfate and evaporated to obtain yellow viscous liquid that solidified after 5–7 days.
N-(4-Methylbenzenesulfonyl)-bis((4-methylbenzenesulfonyl(oxy))ethyl)amine (2a). White solid; Yield: (87%) ; m.p. 98–100 °C; FTIR (cm−1): 3098, 3045 (C-H)Ar, 2967 (C-H)Aliph, 1498, 1472 (C=C)Ar, 1360, 1155 (O=S=O); 1H-NMR (CDCl3) δ ppm: 7.77–7.65 (m, 6H, Ar-H), 7.54–7.48 (m, 4H, Ar-H ), 7.44–7.40 (m, 2H, Ar-H), 4.45 (t, J = 6.23 Hz, 4H, CH2-OTs), 3.23 (t, J = 6.23 Hz, 4H, CH2-N-Ts), 2.33 (two singlets, 9H, Ar-CH3). 13C-NMR (CDCl3) δ ppm: 144.60 (2 × CAr-S-O), 142.92 (CAr-S-N), 140.22 (2 × CAr-CH3), 138.55 (CAr-CH3), 131.04 (4 × CHAr), 129.40 (2 × CHAr), 127.23 (4 × CHAr), 127.10 (2 × CHAr), 55.79 (2 × CH2-OTs), 46.66 (2 × CH2-N-Ts), 22.30 (2 × CH3-Ar), 20.52 (CH3-Ar).
N-(4-Methoxybenzenesulfonyl)-bis((4-methoxybenzenesulfonyl(oxy))ethyl)amine (2b) Off-white solid; Yield: 92%; m.p 157–158 °C; FTIR (cm−1): 3096, 3050 (C-H)Ar, 2922 (C-H)Aliph, 1494, 1466 (C=C)Ar, 1366, 1160 (O=S=O), 1220 (C-O-C); 1H-NMR (DMSO-d6) δ ppm: 7.80–7.76 (m, 4H, Ar-H), 7.65–7.51 (m, 2H, Ar-H), 7.19–7.16 (m, 4H, Ar-H), 7.06–7.04 (m, 2H, Ar-H), 3.98 (t, J = 5.77 Hz, 4H, CH2-OTs), 3.28 (t, J = 5.77 Hz, 4H, CH2-N-Ts), 3.85 (two singlets, 9H, Ar-O-CH3); 13C-NMR (DMSO-d6) δ ppm: 164.18 (2 × CAr-OCH3), 163.30 (CAr-OCH3), 130.48 (4 × CHAr), 129.94 (CAr-S-O), 129.73 (2 × CHAr), 127.58 (2 × CHAr), 126.62 (2 × CAr-S-O), 115.50 (4 × CHAr), 115.13 (2 × CHAr), 68.51 (2 × CH2-OTs), 56.41, 56.25 (3 × OCH3), 47.80 (2 × CH2-N-Ts).
3.3. Synthesis N-(4-Nitrobenzenesulfonyl)-bis((4-nitrobenzenesulfonyl(oxy))ethyl)amine (2c)
A solution of 4-nitrobenzenesulfonyl chloride (21 g, 0.095 mol) in pyridine (40 mL) was added dropwise to a solution of diethanolamine (3.5 g, 0.03 mol) in pyridine (10 mL) while maintaining the temperature at 0 °C. The mixture was stirred at room temperature overnight and then poured into a beaker containing 400 mL of ice water. The mixture was then stirred for another 30 minutes, extracted with dichloromethane and washed with distilled water (3 × 50 mL). The organic layer was dried with anhydrous magnesium sulfate and the solvent evaporated under reduced pressure to give a green viscous liquid which solidified after five days to give a greenish-brown solid; Yield: 57%; m.p 184–186 °C; FTIR (cm−1): 3095, 3048 (C-H)Ar, 2950 (C-H)Aliph, 1518, 1476 (C=C)Ar, 1360, 1174 (O=S=O), 1532, 1347 (Ar-NO2); 1H-NMR (DMSO-d6) δ ppm: 8.43–8.39 (m, 4H, Ar-H), 8.35–8.32 (m, 2H, Ar-H), 8.14–7.11 (m, 6H, Ar-H), 3.55 (t, J = 6.25 Hz, 4H, CH2-OTs), 3.32 (t, J = 6.25 Hz, 4H, CH2-N-Ts); 13C-NMR (DMSO-d6) δ ppm: 159.20 (2 × CAr-NO2), 158.78 (2 × CAr-S-O), 149.08 (CHAr-NO2), 141.23 (CAr-S-O), 130.88 (4 × CHAr), 127.30 (2 × CHAr), 125.43 (4 × CHAr), 123.84 (2 × CHAr), 63.53 (2 × CH2-OTs), 53.44 (2 × CH2-N-Ts).
3.4. General Procedure for Synthesis of 3a, 3b, 3c and 4a, 4b, 4c
Potassium hydroxide (1.85 g, 0.033 mol) was added to a solution of imidazole or benzimidazole (0.022 mol) in DMSO (20 mL) and the mixture was stirred for 30 min at 20 °C, and the corresponding 2a, 2b or 2c (0.01 mol; 5.67 g, 6.15 g and 6.60 g respectively) was added portionwise under vigorous stirring in a water bath. The stirring was continued for another 2 h, the water (200 mL) was then added to the mixture which was extracted with chloroform (6 × 25 mL). The combined extracts were washed with water and dried over anhydrous magnesium sulfate. The solvent was evaporated off and the product was recrystallized from methanol.
N,N-bis[(Benzimidazol-1-yl)ethyl]-4-methylbenzenesulfonamide (3a). White solid; Yield: 94%; a single crystal was obtained properly for X-ray structural determination by using DMF; m.p.192–194 °C; FTIR (cm−1): 3098, 3047 (C-H)Ar, 2927 (C-H)Aliph, 1666, 1615, 1598 (C=N)Ar, 1498, 1460 (C=C)Ar, 1362, 1150 (O=S=O); 1H-NMR (DMSO-d6) δ ppm: 8.09 (s, 2H, C-HBImidazole), 7.64–7.60 (m, 4 H, 2 × C-HAr, 2 × C-HBImidazole), 7.44 (d, 2H, J = 8.15 Hz, C-HAr), 7.30–7.19 (m, 6H, C-HBImidazole), 4.27 (t, J = 6.80 Hz, 4H, 2 × CH2-NAr), 3.49 (t, J = 6.80 Hz, 4H, 2 × CH2-N), 2.33 (s, 3H, -CH3); 13C-NMR (DMSO-d6) δ ppm: 144.57 (2 × CHBImidazole), 144.20 (CAr-S), 143.84 (2 × CBImidazole), 135.36 (CAr-CH3), 134.04 (2 × CBImidazole), 130.43 (2 × CHAr), 127.49 (2 × CHBImidazole), 123.07 (2 × CHBImidazole), 122.15 (2 × CHAr), 120.06 (2 × CHBImidazole), 110.59 (2 × CHBImidazole), 48.71 (2 × CH2-N), 43.77 (2 × CH2-NAr), 21.51 (CH3); EIMS (m/z): 459 (16%, M+), 278 (100%), 304 (10%), 278 (13%), 172 (36%), 155 (24%), 131 (87%), 91 (75%).
N,N-bis[2-(Benzimidazol-1-yl)ethyl]-4-methoxybenzenesulfonamide (3b). White solid; Yield: 84%; m.p. 138–140 °C; FTIR (cm−1): 3096, 3055 (C-H)Ar, 2910 (C-H)Aliph, 1666, 1615, 1597 (C=N)Ar, 1494, 1460 (C=C)Ar, 1375, 1164 (O=S=O), 1220 (C-O-C); 1H-NMR (DMSO-d6) δ ppm: 8.09 (s, 2H, C-HBImidazole), 7.68–7.62 (m, 4H, 2 × C-HAr, 2 × C-HBImidazole), 7.45 (d, 2H, J = 8.15 Hz, C-HAr), 7.28–7.15 (m, 4H, C-HBImidazole), 7.00–6.96 (m, 2H, C-HBImidazole), 4.27 (t, J = 6.80 Hz, 4H, 2 × CH2-N), 3.80 (s, 3H, OCH3), 3.48 (t, J = 6.80 Hz, 4H, 2 × CH2-N); 13C-NMR (DMSO-d6) δppm 163.23 (CAr-O), 144.51 (2 × CHBImidazole), 143.83 (2 × CBImidazole), 134.07 (2 × CBImidazole), 129.78 (CAr-S), 129.70 (2 × CHAr), 123.05 (2 × CHBImidazole), 122.19 (2 × CHBImidazole), 120.08 (2 × CHBImidazole), 115.10 (2 × CHAr), 110.60 (2 × CHBImidazole), 56.18 (O-CH3), 48.66 (2 × CH2-N), 43.75 (2 × CH2-N); EIMS (m/z): 475 (20%, M+), 344 (100%), 304 (10%), 278 (13%), 172 (44%), 107 (35%), 131(75%), 91(80%).
N,N-bis[(Benzimidazol-1-yl)ethyl]-4-nitrobenzenesulfonamide (3c). Pale green solid; Yield: 62%; m.p. 80–82 °C; FTIR (cm−1): 3102, 3055 (C-H)Ar, 2950 (C-H)Aliph, 1660, 1615, 1598 (C=N)Ar, 1510, 1485 (C=C)Ar, 1360, 1178 (O=S=O), 1529, 1340 (Ar-NO2); 1H-NMR (DMSO-d6) δ ppm: 8.16 (s, 2H, C-HBImidazole), 7.78–7.73 (m, 4H, 2 × C-HAr, 2 × C-HBImidazole), 7.52 (d, 2H, J = 8.15 Hz, 2 × C-HAr), 7.31–7.22 (m, 4H,C-HBImidazole), 6.99–6.94 (m, 2H, C-HBImidazole), 4.23 (t, J = 6.80 Hz, 4H, 2 × CH2-N), 3.54 (t, J = 6.80 Hz, 4H, 2 × CH2-N); 13C-NMR (DMSO-d6) δ ppm: 153.12 (CAr-NO2), 145.57 (2 × CHBImidazole), 143.65 (2 × CBImidazole), 134.12 (2 × CBImidazole), 130.04 (CAr-S), 128.44 (2 × CHAr), 124.95 (2 × CHBImidazole), 120.34 (2 × CHBImidazole), 118.88 (2 × CHBImidazole), 117.10 (2 × CH), 111.07 (2 × CHBImidazole), 50.37 (2 × CH2-N), 44.75 (2 × CH2-N); EIMS (m/z): 490 (16%, M+), 359 (100%), 304 (20%), 278 (30%), 186 (45%), 22 (75%).
N,N-bis[(Imidazol-1-yl)ethyl]-4-methylbenzenesulfonamide (4a). White solid; Yield: 82%; a single crystal was obtained for X-ray analysis by using acetonitrile; m.p. 92–94 °C; FTIR (cm−1) 3093 (C-H)Ar, 2975 (C-H)Aliph, 1597 (C=N)Ar, 1510, 1457 (C=C)Ar, 1351, 1159 (O=S=O); 1H-NMR (DMSO-d6) δ ppm: 7.72 (d, J = 8.15 Hz, 2 × C-HAr), 7.54 (s, 2 × C-HImidazole), 7.41(d, J = 8.15 Hz, 2 × C-HAr), 7.12 (s, 2H, C-HImidazole), 6.89 (s, 2H, C-HImidazole), 3.96 (t, J = 6.80 Hz, 4H, 2 × CH2-N), 3.32 (t, J = 6.80 Hz, 4H, 2 × CH2-N), 2.39 (s, 3H, CH3); 13C-NMR (DMSO-d6) δ ppm 144.28 (CAr-S), 137.87 (2 × CHImidazole), 135.53 (CAr-CH3), 130.49 (2 × CHAr), 128.99 (2 × CHImidazole), 127.56 (2 × CHAr), 119.95 (2 × CHImidazole), 50.30 (2 × CH2-N), 45.75 (2 × CH2-N), 21.49 (-CH3); EIMS (m/z): 359 (10%, M+), 278 (100%), 204 (20%), 155 (22%), 122 (39%), 91 (97%).
N,N-bis[2-(Imidazol-1-yl)ethyl]-4-methoxybenzenesulfonamide (4b). Off-white solid; Yield: 90%; m.p. 66–68 °C; FTIR (cm−1): 3100 (C-H)Ar, 2855 (C-H)Aliph, 1584 (C=N)Ar, 1530, 1460 (C=C)Ar, 1360, 1170 (O=S=O), 1238 (C-O-C); 1H-NMR (DMSO-d6) δ ppm: 7.76 (d, J = 8.61 Hz, 2H, C-HAr), 7.55 (s, 2H, C-HImidazole), 7.14–7.10 (m, 4H, 2 × C-HAr, 2 × C-HImidazole), 6.89 (s, 2H, C-HImidazole), 3.97 (t, J = 6.80 Hz, 4H, 2 × CH2-N), 3.82 (s, 3H, O-CH3), 3.31 (t, J = 6.80 Hz, 4H, 2 × CH2-N); 13C-NMR (DMSO-d6) δ ppm: 163.34 (CAr-O), 137.92 (2 × CHImidazole), 129.95 (2 × CHImidazole), 129.86 (CAr-S), 129.01 (2 × CHAr), 120.06 (2 × CHImidazole), 115.23 (2 × CHAr), 56.24 (O-CH3), 50.24 (2 × CH2-N), 45.67 (2 × CH2-N); EIMS (m/z): 375 (22% , M+), 294 (100% ), 204 (20%), 171 (36%), 122 (35%), 107 (35%).
N,N-bis[(Imidazol-1-yl)ethyl]-4-nitrobenzenesulfonamide (4c). Pale green solid; Yield: 54%; m.p. 46–48 °C; FTIR (cm−1): 3080 (C-H)Ar, 2890 (C-H)Aliph, 1620 (C=N)Ar, 1574, 1460 (C=C)Ar, 1375, 1185 (O=S=O), 1520, 1355 (Ar-NO2); 1H-NMR (DMSO-d6) δ ppm 7.96 (d, J = 8.60 Hz, 2H, C-HAr), 7.78 (s, 2H, C-HImidazole), 7.36–7.32 (m, 4H, 2 × C-HAr, 2 × C-HIMi), 6.95 (s, 2H, C-HImidazole), 3.95 (t, J = 6.80 Hz, 4H, 2 × CH2-N), 3.55(t, J = 6.80 Hz, 4H, 2 × CH2-N). 13C-NMR (DMSO-d6) δ ppm 149.03 (CAr-NO2), 138.23 (2 × CHImidazole), 130.11 (2 × CHImidazole), 129.78 (CAr-S), 128.12 (2 × CHAr), 122.06 (2 × CHImidazole), 112.14 (2 × CHAr), 48.20 (2 × CH2-N), 43.09 (2 × CH2-N); EIMS (m/z): 390 (20%, M+), 309 (60%), 204 (20%), 186 (30%), 122 (100%), 81 (75%).
3.5. Synthesis 2-Mercaptobenzimidazole (6)
Prepared according to the modified procedure in [43]. o-Phenylenediamine (7 g, 0.065 mol) was dissolved in absolute ethanol (40 mL) in a 250 mL flask. Carbon disulfide (10 mL) was then added to the solution followed by the addition of a solution of potassium hydroxide (4.35 g, 0.077 mol) in water (25 mL). The reaction mixture was thoroughly stirred and refluxed for 5 h. It was initially yellow, then turned to brown as the reaction progressed. Evolution of hydrogen sulfide gas was observed. After completion of the reaction, the mixture was poured into a beaker with ice-water and acidified with 4N hydrochloric acid to pH 4–5 to obtain a white precipitate. The precipitate was then filtered and recrystallized from ethanol. White solid; Yield 84%; m.p. 303–305 °C; FTIR (cm−1): 3,250 (N-H), 1,557 (C=C)Ar, 1,633 (C=O), 1,517 (C=N).
3.6. Synthesis 2-((Benzimidazol-2-yl)methylthio)-benzimidazole (8)
Prepared according to modified procedure in [44]. Sodium (0.85 g, 0.037 mol) was added to a solution of 2-mercaptobenzimidazole (5 g, 0.033 mol) in anhydrous methanol (60 mL) and the mixture was vigorously stirred for 20 minutes. 2-Chloromethylbenzimidazole (5.55 g, 0.033 mol) was added portion-wise to the mixture and left to stir for 2 h. A yellow precipitate was formed, filtered and washed with methanol, cold water and dried in an oven. The crude product was recrystallized from tetrahydrofuran to give a white solid of the title compound. White solid; Yield: 96%; m.p. 255–257 °C; FTIR (cm−1): 3372 (N-H), 3090 (C-H)Ar, 2975 (C-H)Aliph, 1621 (C=N)Ar, 1590 (C=C)Ar, 748 (C-S); 1H-NMR (DMSO-d6) δ ppm: 12.52 (s, H, N-H), 12.23 (s, H, N-H), 7.41–7.32 (m, 4H, C-HAr), 6.97–6.90 (m, 4H, C-HAr), 4.93 (s, 2H, CH2-S). 13C-NMR (DMSO-d6) δ ppm: 153.11 (CAr-S), 149.73 (CAr-S-CH2), 140.32, 139.66 (4 × CAr), 125.34, 123.92 (4 × CHAr), 114.53, 116.03 (4 × CHAr) 33.12 (CH2).
3.7. Synthesis N-4-Methylbenzenesulfonyl ((N-(4-methylbenzenesulfonyl)benzimidazol-2-yl)methylthio)-benzimidazole (9)
A solution of p-toluenesulfonyl chloride (7.44 g, 0.018 mol) in pyridine (25 mL) was added dropwise to a solution of 2-((benzimidazol-2-yl)methylthio)-benzimidazole (8) (5 g, 0.018 mol) in pyridine (25 mL) at 0 °C, within 3 h. The mixture was stirred at room temperature and left overnight. It was then quenched with ice-water, stirred for another 20 minutes, extracted with dichloromethane (5 × 30 mL) and washed with distilled water. The organic layer was dried over anhydrous magnesium sulfate and solvent evaporated off. The crude product was purified by using flash chromatography with hexane-ethyl acetate (4:1, v/v) as eluent. The obtained solid was recrystallized from acetonitrile to give colorless crystals of compound (9). Colorless crystals; Yield 52%; m.p. 204–206 °C; FTIR (cm−1) 3087 (C-H)Ar, 2990 (C-H)Aliph, 1660, 1,616 (C=N)Ar, 1593, 1462 (C=C)Ar, 1365, 1170 (O=S=O), 752 (C-S); 1H-NMR (CDCl3) δ ppm 7.98–7.89 (m, 6H, C-HAr), 7.64–7.49 (m, 2H, C-HAr), 7.38–7.24 (m, 6H, C-HAr), 7.19 (d, J = 8.61, 2H, C-HAr), 5.18 (s, 2H, CH2), 2.31, 2.37 (two singlets, 6H, 2 × CH3); 13C-NMR (CDCl3) δ ppm 151.81 (CBImidazole-S), 149.43 (CBImidazole-CH2-S), 146.42, 146.18 (2 × CAr-S), 143.22, 141.80 (2 × CBImidazole-N), 135.04, 134.57 (2 × CAr-CH3), 133.98, 133.16 (2 × CBImidazole-N), 130.38, 130.15 (4 × CHAr), 127.54, 127.45 (4 × CHAr), 125.52, 124.91, 124.69, 124.14, 120.56, 118.95, 113.56, 112.95 (8×CHBImidazole), 31.20 (CH2-S), 21.80, 21.77 (2 × CH3); EIMS (m/z): 588 (45%, M+), 433 (80%), 278 (35%), 155 (85%), 148 (12%), 91 (100%).
3.8. Synthesis 4-Methyl-N-(2-{2-[2-(4-ethylbenzenesulfonamido)ethoxy]ethoxy}ethyl)benzenesulfon-amide (11)
p-Toluenesulfonyl chloride (5.66 g, 0.029 mol) was dissolved in dry dichloromethane (25 mL) and added dropwise to a stirring solution of 1,8-diamino-3,6-dioxaoctane(2,2′-(ethylenedioxy)bis-(ethyl amine) (2 g, 0.013 mol) and triethylamine (4.42 mL, 0.031 mol) in dichloromethane (25 mL) at 0 °C. The mixture was stirred further at room temperature for overnight and extracted with water and saturated solution of NaHCO3 (3 × 15 mL). The organic layer was dried over anhydrous magnesium sulfate and the solvent was evaporated off. Colorless crystals were obtained through slow evaporation of methanolic solution at room temperature. Yield: 92%; m.p. 88–90 °C; FTIR (cm−1) 3276 (NH), 3090 (C-H)Ar, 2921, 2898, 2872 (C-H)Aliph, 1596 (C=C)Ar, 1317, 1152 (O=S=O), 1088 (C-O-C), 1124 (C-C-O); 1H-NMR (CDCl3) δ ppm: 7.73 (d, J = 8.61 Hz, 4H, C-HAr), 7.27 (d, J = 8.61 Hz, 4H, C-HAr), 5.50 (t, J = 5.89 Hz, 2H, 2 × NH), 3.50 (t, J = 5.44 Hz, 8H, 4 × CH2-O), 3.09 (q, J = 8.15 Hz, 4H, 2 × CH2-NH), 2.39 (s, 6H, 2 × CH3); 13C-NMR (CDCl3) δ ppm: 143.45 (2 × CAr-S), 137.07 (2 × CAr-CH3), 129.78 (4 × CHAr), 127.18 (4 × CHAr), 70.43 (2 × CH2-O), 69.78 (2 × CH2-O), 42.99 (2 × CH2-N), 21.59 (2 × CH3); EIMS (m/z): 456 (25%, M+), 301 (100%), 155 (70%), 146 (35%), 91 (90%).
3.9. Antibacterial Evaluation
The antibacterial activity of synthesized compounds 3a–c, 4a–c, 9 and 11 was tested against standard strains of ten bacteria. They were obtained from the collection of the School of Biosciences and Biotechnology, Faculty of Science and Technology, University Kebangsaan, Malaysia. These strains included Gram positive bacteria: Streptococcus pyogenes ATCC19615, Staphylococcus aureus ATCC 29213, Bacillus subtilis ATCC6051, Rodococcus Ruber ATCC27863, Enterococcus faecalis ATCC 29212, Staphylococcus epidermidis ATCC12228 and Gram negative bacteria: Escherichia coli ATCC10538, Salmonella typhimurium ATCC14028, Pseudomonas aeruginosa ATCC15442, Acinetobacter calcoaceticus ATCC 23055.
The antibacterial activity was assessed in terms of minimum inhibitory concentrations (MICs) by using microbroth dilution assays according to the CLSI guidelines [45]. All the tested compounds were dissolved in dimethylsulfoxide (DMSO) which was used as negative control with concentrations range from 0.05 to 0.5 mg/mL. Commercial antibiotics amoxicillin and kanamycin in the same range of concentrations were used as a positive control. The bacterial stock cultures were maintained on nutrient agar plates. A loopful of bacterial cells from the nutrient agar plates was inoculated into 100 mL nutrient broth in 250 mL side arm Erlenmeyer flask and incubated at 37 °C for 16 h with vigorous shaking. After incubation, the culture was diluted with fresh media to give an O.D600 nm of 0.1. Fifty μL of standardized 18 h incubated bacterial culture was introduced into test tubes containing 5 mL media followed by the addition of various concentration of the compounds studied. The MIC was recorded as the lowest concentration that inhibits the growth of the bacterial strains. All assays were performed in triplicate and MIC’s values are given in mg/mL.
4. Conclusions
Novel imidazole and benzimidazole compounds with a sulfonamido moiety as substituent, i.e., 3a–c, 4a–c, and 9 in addition to novel bis-sulfonamide compound i.e., 11 were successfully synthesized through simple methods. The structures for the synthesized compounds were confirmed by FTIR, NMR, and HRMS studies. These compounds were evaluated for in vitro antibacterial activities against ten strains of bacteria. Compounds 3c, 9 and 11 demonstrated the highest bioactivities among the compounds, however, most of them showed significant activities for both Gram-positive and Gram-negative bacteria. Such results are encouraging for synthesis of promising new complexes from these compounds with several metals to evaluate their biological activity in the near future. Further studies of these new synthesized sulfonamide derivatives by the same authors are in progress related to their use as ligands and their complexes.
Acknowledgments
The authors thank the University of Malaya for financial support by HIR UM-MOHE (F00004–21001, FP001/2010A and UMRG RG233-12AFR grants, and Dr. Hamid Khaledi for his assistance in solving the crystal structures of compounds 3a and 4a.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/18/10/11978/s1.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the final compounds 3a–c, 4a–c, 9 and 11 are available from the authors.
References
- 1.Lu X., Zhang H., Li X., Chen G., Li Q.S., Luo Y., Ruan B.F., Chen X.W., Zhu H.L. Design, synthesis and biological evaluation of pyridine acyl sulfonamide derivatives as novel COX-2 inhibitors. Bioorg. Med. Chem. 2011;19:6827–6832. doi: 10.1016/j.bmc.2011.09.034. [DOI] [PubMed] [Google Scholar]
- 2.Luo Y., Qiu K.M., Lu X., Liu K., Fu J., Zhu H.L. Synthesis, biological evaluation, and molecular modeling of cinnamic acyl sulfonamide derivatives as novel antitubulin agents. Bioorg. Med. Chem. 2011;19:4730–4738. doi: 10.1016/j.bmc.2011.06.088. [DOI] [PubMed] [Google Scholar]
- 3.Chandak N., Bhardwaj J.K., Sharma R.K., Sharma P.K. Inhibitors of apoptosis in testicular germ cells: Synthesis and biological evaluation of some novel IBTs bearing sulfonamide moiety. Eur. J. Med. Chem. 2013;59:203–208. doi: 10.1016/j.ejmech.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Kamal A., Swapna P., Shetti R.V., Shaik A.B., Narasimha Rao M.P., Gupta S. Synthesis, biological evaluation of new oxazolidino-sulfonamides as potential antimicrobial agents. Eur. J. Med. Chem. 2013;62:661–669. doi: 10.1016/j.ejmech.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 5.Akurathi V., Dubois L., Lieuwes N.G., Chitneni S.K., Cleynhens B.J., Vullo D., Supuran C.T., Verbruggen A.M., Lambin P., Bormans G.M. Synthesis and biological evaluation of a 99mTc-labelled sulfonamide conjugate for in vivo visualization of carbonic anhydrase IX expression in tumor hypoxia. Nucl. Med. Biol. 2010;37:557–564. doi: 10.1016/j.nucmedbio.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Andrighetti-Fröhner C.R., de Oliveira K.N., Gaspar-Silva D., Pacheco L.K., Joussef A.C., Steindel M., Simões C.M.O., de Souza A.M.T., Magalhaes U.O., Afonso I.F., Rodrigues C.R., Nunes R.J., Castro H.C. Synthesis, biological evaluation and SAR of sulfonamide 4-methoxychalcone derivatives with potential antileishmanial activity. Eur. J. Med. Chem. 2009;44:755–763. doi: 10.1016/j.ejmech.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 7.Gadad A.K., Mahajanshetti C.S., Nimbalkar S., Raichurkar A. Synthesis and antibacterial activity of some 5-guanylhydrazone/thiocyanato-6-arylimidazo[2,1-b]-1,3,4-thiadiazole-2-sulfonamide derivatives. Eur. J. Med. Chem. 2000;35:853–857. doi: 10.1016/S0223-5234(00)00166-5. [DOI] [PubMed] [Google Scholar]
- 8.Azab M., Youssef M., El-Bordany E. Synthesis and antibacterial evaluation of novel heterocyclic compounds containing a sulfonamido moiety. Molecules. 2013;18:832–844. doi: 10.3390/molecules18010832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ezabadi I.R., Camoutsis C., Zoumpoulakis P., Geronikaki A., Soković M., Glamočilija J., Ćirić A. Sulfonamide-1,2,4-triazole derivatives as antifungal and antibacterial agents: Synthesis, biological evaluation, lipophilicity, and conformational studies. Bioorg. Med. Chem. 2008;16:1150–1161. doi: 10.1016/j.bmc.2007.10.082. [DOI] [PubMed] [Google Scholar]
- 10.Ghorab M.M., Ragab F.A., Heiba H.I., Arafa R.K., El-Hossary E.M. In vitro anticancer screening and radiosensitizing evaluation of some new quinolines and pyrimido[4,5-b]quinolines bearing a sulfonamide moiety. Eur. J. Med. Chem. 2010;45:3677–3684. doi: 10.1016/j.ejmech.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Ghorab M.M., Ragab F.A., Hamed M.M. Design, synthesis and anticancer evaluation of novel tetrahydroquinoline derivatives containing sulfonamide moiety. Eur. J. Med. Chem. 2009;44:4211–4217. doi: 10.1016/j.ejmech.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 12.Bano S., Javed K., Ahmad S., Rathish I.G., Singh S., Alam M.S. Synthesis and biological evaluation of some new 2-pyrazolines bearing benzene sulfonamide moiety as potential anti-inflammatory and anti-cancer agents. Eur. J. Med. Chem. 2011;46:5763–5768. doi: 10.1016/j.ejmech.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Sondhi S.M., Johar M., Singhal N., Dastidar S.G., Shukla R., Raghubir R. Synthesis and anticancer, antiinflammatory and analgesic activity evaluation of some sulfa drug and acridine derivatives. Monatsh. Chem. 2000;131:511–520. doi: 10.1007/s007060050332. [DOI] [Google Scholar]
- 14.El-Araby M., Omar A., Hassanein H.H., El-Helby A.G.H., Abdel-Rahman A.A. Design, synthesis and in vivo anti-inflammatory activities of 2,4-diaryl-5-4H-imidazolone derivatives. Molecules. 2012;17:12262–12275. doi: 10.3390/molecules171012262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nanthakumar R., Muthumani P., Girija K. Anti-inflammatory and antibacterial activity study of some novel quinazolinones. Arab. J. Chem. 2011 doi: 10.1016/j.arabjc.2010.12.035. [DOI] [Google Scholar]
- 16.Zoumpoulakis P., Camoutsis C., Pairas G., Soković M., Glamočlija J., Potamitis C., Pitsas A. Synthesis of novel sulfonamide-1,2,4-triazoles, 1,3,4-thiadiazoles and 1,3,4-oxadiazoles, as potential antibacterial and antifungal agents. Biological evaluation and conformational analysis studies. Bioorg. Med. Chem. 2012;20:1569–1583. doi: 10.1016/j.bmc.2011.12.031. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z., Xu W., Liu K., Yang S., Fan H., Bhadury P.S., Huang D.Y., Zhang Y. Synthesis and antiviral activity of 5-(4-chlorophenyl)-1,3,4-thiadiazole sulfonamides. Molecules. 2010;15:9046–9056. doi: 10.3390/molecules15129046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atia A.J.K. Synthesis and antibacterial activities of new metronidazole and imidazole derivatives. Molecules. 2009;14:2431–2446. doi: 10.3390/molecules14072431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kipp B.H., Faraj C., Li G., Njus D. Imidazole facilitates electron transfer from organic reductants. Bioelectrochemistry. 2004;64:7–13. doi: 10.1016/j.bioelechem.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Khalafi-Nezhad A., Soltani R.M.N., Mohabatkar H., Asrari Z., Hemmateenejad B. Design, synthesis, antibacterial and QSAR studies of benzimidazole and imidazole chloroaryloxyalkyl derivatives. Bioorg. Med. Chem. 2005;13:1931–1938. doi: 10.1016/j.bmc.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Jain A.K., Ravichandran V., Sisodiya M., Agrawal R.K. Synthesis and antibacterial evaluation of 2-substituted-4,5-diphenyl-N-alkyl imidazole derivatives. Asian Pac. J. Trop. Med. 2010;3:471–474. doi: 10.1016/S1995-7645(10)60113-7. [DOI] [Google Scholar]
- 22.González-Chávez M.M., Méndez F., Martínez R., Pérez-González C., Martínez-Gutiérrez F. Design and synthesis of anti-MRSA benzimidazolylbenzene-sulfonamides. QSAR studies for prediction of antibacterial activity. Molecules. 2010;16:175–189. doi: 10.3390/molecules16010175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernández-Núñez E., Tlahuext H., Moo-Puc R., Torres-Gómez H., Reyes-Martínez R., Cedillo-Rivera R., Nava-Zuazo C., Navarrete-Vazquez G. Synthesis and in vitro trichomonicidal, giardicidal and amebicidal activity of N-acetamide(sulfonamide)-2-methyl-4-nitro-1H-imidazoles. Eur. J. Med. Chem. 2009;44:2975–2984. doi: 10.1016/j.ejmech.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Karakurt A., Dalkara S., Özalp M., Özbey S., Kendi E., Stables J.P. Synthesis of some 1-(2-naphthyl)-2-(imidazole-1-yl)ethanone oxime and oxime ether derivatives and their anticonvulsant and antimicrobial activities. Eur. J. Med. Chem. 2001;36:421–433. doi: 10.1016/S0223-5234(01)01223-5. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki F., Kuroda T., Tamura T., Soichiro S., Ohmori K., Ichikawa S. New antiinflammatory agents. 2, 5-Phenyl-3H-imidazo[4,5-c][1,8]naphthyridin-4(5H)-ones: A new class of nonsteroidal antiinflammatory agents with potent activity like glucocorticoids. J. Med. Chem. 1992;35:2863–2870. doi: 10.1021/jm00093a020. [DOI] [PubMed] [Google Scholar]
- 26.Vijesh A.M., Isloor A.M., Telkar S., Arulmoli T., Fun H.K. Molecular docking studies of some new imidazole derivatives for antimicrobial properties. Arab. J. Chem. 2013;6:197–204. doi: 10.1016/j.arabjc.2011.10.007. [DOI] [Google Scholar]
- 27.Bhatnagar A., Sharma P.K., Kumar N. A review on “Imidazoles”: Their chemistry and pharmacological potentials. Int. J. PharmTech Res. 2011;3:268–282. [Google Scholar]
- 28.Chak B.C., McAuley A. The synthesis and characterization of the pendant-armed ligand N,N′-bis(2′-pyridylmethyl)-1,7-dithia-4,11-diazacyclotetradecane (L4) and crystal structures of L4 and the copper(II) complex [Cu(L4)](ClO4)2 Crystal structure of the nickel(II) complex of N-(2′-pyridylmethyl)-1,4,7-trithia-11-azacyclotetradecane (L2), [Ni(L2)(CH3CN)](ClO4)2·CH3CN. Can. J. Chem. 2006;84:187–195. doi: 10.1139/v05-241. [DOI] [Google Scholar]
- 29.Starikova O.V., Dolgushin G.V., Larina L.I., Ushakov P.E., Komarova T.N., Lopyrev V.A. Synthesis of 1,3-dialkylimidazolium and 1,3-dialkylbenzimidazolium salts. Russ. J. Org. Chem. 2003;39:1467–1470. doi: 10.1023/B:RUJO.0000010563.79902.47. [DOI] [Google Scholar]
- 30.Al-Mohammed N.N., Alias Y., Abdullah Z., Khaledi H. 1-Tosyl-2-[(1-tosyl-1H-benzimidazol-2-yl)methylsulfanyl]-1H-benzimidazole. Acta Crystallogr. Sect. E. 2011;67:o1043. doi: 10.1107/S1600536811011822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Mohammed N.N., Alias Y., Abdullah Z., Khaledi H. N,N′-{[Ethane-1,2-diylbis(oxy)]bis- (ethane-2,1-diyl)}bis(4-methylbenzenesulfonamide) Acta Crystallogr. Sect. E. 2012;68:o1983. doi: 10.1107/S1600536812024324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plech T., Wujec M., Siwek A., Kosikowska U., Malm A. Synthesis and antimicrobial activity of thiosemicarbazides, s-triazoles and their Mannich bases bearing 3-chlorophenyl moiety. Eur. J. Med. Chem. 2011;46:241–248. doi: 10.1016/j.ejmech.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 33.Kossakowski J., Krawiecka M., Kuran B., Stefańska J., Wolska I. Synthesis and preliminary evaluation of the antimicrobial activity of selected 3-benzofurancarboxylic acid derivatives. Molecules. 2010;15:4737–4749. doi: 10.3390/molecules15074737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gu W., Wu R., Qi S., Gu C., Si F., Chen Z. Synthesis and antibacterial evaluation of new N-acylhydrazone derivatives from dehydroabietic acid. Molecules. 2012;17:4634–4650. doi: 10.3390/molecules17044634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cecil R. The Role of Sulfur in Proteins. In: Neurath H., editor. The Proteins. 2nd ed. Volume 1. Academic Press; New York, NY, USA: 1963. p. 379. [Google Scholar]
- 36.Mamolo M.G., Falagiani V., Zampieri D., Vio L., Banfi E. Synthesis and antimycobacterial activity of [5-(pyridin-2-yl)-1,3,4-thiadiazol-2-ylthio]acetic acid arylidene-hydrazide derivatives. Farmaco. 2001;56:587–592. doi: 10.1016/S0014-827X(01)01097-7. [DOI] [PubMed] [Google Scholar]
- 37.Mazzone G., Bonina F., Puglisi G., Reina R.R., Arrigo C., Cosentino C., Blandino G. Synthesis and biological evaluation of some 5-aryl-2-amino-1,3,4-oxa(thia)diazoles. Farmaco. 1982;37:685–700. [PubMed] [Google Scholar]
- 38.Alwan S.M. Synthesis and preliminary antimicrobial activities of new arylideneamino-1,3,4-thiadiazole-(thio/dithio)-acetamido cephalosporanic acids. Molecules. 2012;17:1025–1038. doi: 10.3390/molecules17011025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greim H., Bury D., Klimisch H.J., Oeben-Negele M., Ziegler-Skylakakis K. Toxicity of aliphatic amines: Structure-activity relationship. Chemosphere. 1998;36:271–295. doi: 10.1016/S0045-6535(97)00365-2. [DOI] [PubMed] [Google Scholar]
- 40.Ouyang L., Huang Y., Zhao Y., He G., Xie Y., Liu J., He J., Liu B., Wei Y. Preparation, antibacterial evaluation and preliminary structure–activity relationship (SAR) study of benzothiazol- and benzoxazol-2-amine derivatives. Bioorg. Med. Chem. Lett. 2012;22:3044–3049. doi: 10.1016/j.bmcl.2012.03.079. [DOI] [PubMed] [Google Scholar]
- 41.Matos M., Vazquez-Rodriguez S., Santana L., Uriarte E., Fuentes-Edfuf C., Santos Y., Muñoz-Crego A. Synthesis and structure-activity relationships of novel amino/nitro substituted 3-arylcoumarins as antibacterial agents. Molecules. 2013;18:1394–1404. doi: 10.3390/molecules18021394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daniel M., Harry L., Mai V. Guide to Antimicrobials. San Francisco VA Medical Center Infectious Diseases Section; San Francisco, CA, USA: 2012. [Google Scholar]
- 43.Wang M.L., Liu B.L. Synthesis of 2-mercaptobenzimidazole from the reaction of o-phenylene diamine and carbon disulfide in the presence of potassium hydroxide. J. Chinese Inst. Chem. Engineers. 2007;38:161–167. doi: 10.1016/j.jcice.2007.01.003. [DOI] [Google Scholar]
- 44.Satyanarayana S., Nagasundara K.R. Synthesis and spectral properties of the complexes of cobalt(II), nickel(II), copper(II), zinc(II), and cadmium(II) with 2-(Thiomethyl-2′-benzimidazolyl)-benzimidazol. Synth. React. Inorg. Met. Org. Chem. 2004;34:883–895. doi: 10.1081/SIM-120037514. [DOI] [Google Scholar]
- 45.Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard M7-A7. Volume 26 Wayne, PA, USA: 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.