Abstract
New series of N-(arylidene)hydrazinoacetyl sulfonamides 4a1–6, 4b1–6 and N-(4-aryl-3-chloro-2-oxoazetidin-1-yl)aminoacetyl sulfonamides 5a1–6, 5b1–6 were synthesized. The structures of the new derivatives was confirmed using spectral methods (FT-IR, 1H-NMR, 13C-NMR). The antibacterial activities of these compounds against Gram positive (Staphyloccoccus aureus ATCC 6583, Staphyloccoccus epidermidis ATCC 12228, Enterococcus faecalis ATCC 25912) and Gram negative (Klebsiella pneumoniae CIP 53153, Proteus vulgaris CIP 104989, Citrobacter freundii CIP 5732, Enterobacter cloacae CIP 103475, Escherichia coli ATCC 25922, Pseudomonas aeruginosa CIP 82118) bacterial strains were evaluated using the broth micro-dilution method. Compound 4a2 displayed the highest antibacterial activity, especially against Staphyloccoccus epidermidis, Enterococcus faecalis and Pseudomonas aeruginosa. The antioxidant potential of the synthesized compounds was also investigated according to ferric reducing power, total antioxidant activity and DPPH radical scavenging assays. All tested compounds showed excellent antioxidant activity in comparison with sulfadiazine and sulfisoxazole which were used as parent sulfonamides. Moreover, some of them showed an antioxidant activity comparable with that of ascorbic acid. In general, the compounds designed based on a sulfadiazine skeleton (compounds 4a1–6, 5a1–6) are more active than those obtained from sulfisoxazole (compounds 4b1–6, 5b1–6), and the N-(arylidene)hydrazinoacetyl sulfonamide derivatives 4a1–6, 4b1–6 are more active than their azetidionone analogues 5a1–6, 5b1–6.
Keywords: sulfonamide, azetidinone, synthesis, antimicrobial activity, antioxidant effect
1. Introduction
The 2-azetidinone skeleton, otherwise known as the β-lactam ring, has been recognized as a useful building block in the synthesis of biologically important compounds. Azetindin-2-one derivatives display interesting biological activities such as antifungal, antimicrobial [1,2,3,4], antitubercular [5,6], analgesic, anti-inflammatory [7,8], chymase inhibitory [9], antitumoral [10,11,12], antiviral, antidiabetic and cholesterol absorption inhibitory properties [13]. The activity of famous antibiotic classes such as the penicillins, cephalosporins, carumonam, aztreonam, thienamicine, nocardicins and carbapenems is attributed to the presence of an 2-azetidinone ring [2]. Unfortunately, the most widely used of them exert selective pressure on bacteria and permit the proliferation of resistant organisms. Several synthetic and semi-synthetic β-lactam antibiotics were developed due to the growing resistance of bacteria towards the classical β-lactam antibiotics and the need for drugs with a more specific antibacterial activity [1]. The biological activity of sulfonamides is also well documented. They have be found to be useful in a variety of applications, including antibacterial, antifungal, antitumor agents, diuretics, carbonic anhydrase inhibitors, hypoglycemic agents, thyroid inhibitors, anticonvulsants and protease inhibitors [14,15]. Among antibacterial sulfonamides, sulfadiazine and its silver and cerium salts have an important place. They are widely used as topical agents for the management of burns where they prevent infections and promote rapid healing with minimal scarring [15].
Wounds are physical injuries that result in an opening of the skin. Proper healing of wounds is essential for the restoration of disrupted anatomical continuity and disturbed functional status of the skin [16]. Normal healing of wounds is a dynamic process following three phases: inflammation, granulation (tissue formation) and re-epithelization (tissue remodeling), which overlap in time [17]. It was proven that reactive oxygen species (ROS) and bacterial infections are deleterious to the wound healing process due to their harmful effects on cells and tissues [18]. ROS are produced in high amounts at wound sites as a defense mechanism against invading bacteria. At the same time, the process of wound healing may be hampered by the presence of free radicals, which can damage the cells surrounding the wound, or by microbial infection [19] and recent data has proved the beneficial effects of antioxidants in the wound healing process [20,21,22]. In the present study, we are reporting the design, synthesis and biological evaluation of some new 2-azetidinone derivatives of sulfadiazine and sulfisoxazole with potential use in wound healing processes.
2. Results and Discussion
2.1. Chemistry
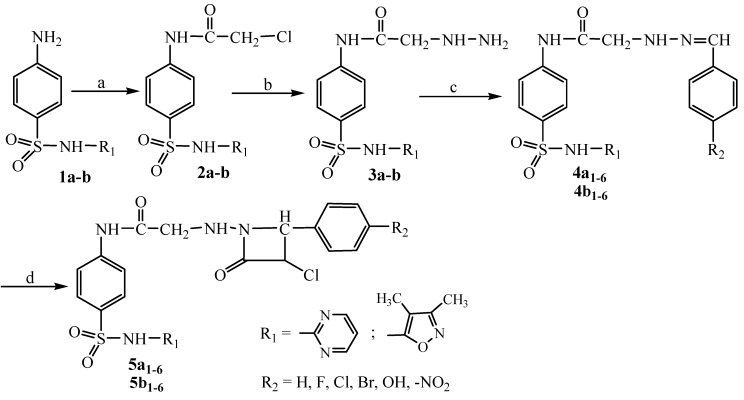
Azetidinone derivatives 5a1–6, 5b1–6 were prepared using the method summarized in Scheme 1. First, sulfadiazine (4-amino-N-pyrimidin-2-yl-benzensulfonamide, 1a) and sulfisoxazole [4-amino-N-(3,4-dimethyl-1,2-oxazol-5-yl)benzensulfonamide, 1b] were reacted with chloroacetyl chloride whereby the corresponding chloracetyl derivatives 2a–b were obtained. Compounds 2a–b on amination with hydrazine hydrate afforded hydrazinoacetyl sulfonamide derivatives 3a–b [23]. The condensation reaction of compounds 3a–b with various aromatic aldehydes yielded N-(arylidene)hydrazinoacetyl sulfonamide derivatives 4a1–6, 4b1–6. Finally, the compounds 4a1–6, 4b1–6 upon reaction with chloracetyl chloride in the presence of triethylamine afforded N-(4-aryl-3-chloro-2-oxoazetidin-1-yl)aminoacetyl sulfonamides 5a1–6, 5b1–6.
Scheme 1.
Synthesis of azetidinone derivatives (5a1–6, 5b1–6).
Reagents and Conditions: (a) chloracetyl chloride, dry acetone, anhydrous K2CO3, heating 12 h; (b) hydrazine hydrate 99%, ethanol, heating 10 h; (c) aromatic aldehydes, acetic acid, ethanol 50%, heating 8 h; (d) chloracetyl chloride, anhydrous 1,4-dioxane, triethylamine, room temperature, stirrer 3 h.
The structure of the compounds was assigned on the basis of spectral (IR, 1H-NMR, 13C-NMR) data. The IR spectra of compounds 4a1–6 (sulfadiazine series) showed absorption bands for the -CH2-NH- group in the range of 2830–2853 cm−1, for the NH-CO group in the range of 1622–1623 cm−1 and for the characteristic azomethine group (CH=N) in the 1534–1539 cm−1 range. In the spectra of the sulfisoxazole derivatives 4b1–6 the characteristic absorption bands were observed in the region of 2842–2870 cm−1 (-CH2-NH-), 1622–1629 cm−1 (NH-CO) and 1507–1540 cm−1 (CH=N). In the 1H-NMR spectra of the N-(arylidene)hydrazinoacetyl sulfonamides 4a1–6, 4b1–6 the -CH2-NH methylene protons resonated as a doublet in the 3.56–3.79 ppm region, while the proton of the azomethine group (N=CH) appeared as a singlet in the 8.06–8.22 ppm region. In the IR spectra of the azetidinone derivatives 5a1–6, 5b1–6 the carbonyl group of the β-lactam ring appeared as a characteristic absorption band in the range of 1739–1745 cm−1 and 1739–1752 cm−1, respectively. The IR absorption bands and 1H-NMR signals characteristic of the azomethine group disappeared from the spectra of the azetidinone derivatives, which confirms that the cyclization reaction with chloracetyl chloride took place. The 1H-NMR spectra of 5a1–6 (sulfadiazine series) and 5b1–6 (sulfisoxazole series) showed two doublets, which are characteristic for N-CH and CH-Cl that appear in the range of 5.32–5.45 and 5.02–5.23 ppm, respectively. In 13C-NMR spectra of the azetidinone derivatives, the characteristic signals for a β-lactam ring (CH-NH, CH-Cl, CO cyclic) appeared in the range of 67.8–76.1, 61.04–64.3 and 160.3–162.8 ppm (5a1–6 series) and 75.9–79.1, 64.2–67.0 and 160.3–168.2 ppm (5b1–6 series) respectively. The spectral data lend strong support to the proposed structures of all the synthesized compounds.
2.2. Biological Evaluation
2.2.1. Antibacterial Activity
The antibacterial activities of the title compounds were evaluated using the broth micro-dilution method [24] and the results are listed Table 1 and Table 2.
Table 1.
MIC values (µg/mL) of the sulfadiazine derivatives 4a1–6, 5a1–6.
| Sample | MICs * values (µg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SA | SE | EF | KP | PV | CF | EC a | EC b | PA | |
| 4a1 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | 256 |
| 4a2 | >512 | 128 | 256 | >512 | >512 | >512 | >512 | >512 | 128 |
| 4a3 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 |
| 4a4 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | 128 |
| 4a5 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 |
| 4a6 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 |
| 5a1 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 |
| 5a2 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 |
| 5a3 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 |
| 5a4 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 |
| 5a5 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 |
| 5a6 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 |
| S | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | 800 |
| A | 35.8 | 3 | 3 | 16 | - | - | 8 | 35.8 | 128 |
* Mean values (n = 3); SA: Staphyloccoccus aureus ATCC 6583; SE: Staphyloccoccus epidermidis ATCC 12228; EF: Enterococcus faecalis ATCC 25912; KP: Klebsiella pneumoniae CIP 53153; PV: Proteus vulgaris CIP 104989; CF: Citrobacter freundii CIP 5732; EC a: Enterobacter cloacae CIP 103475; EC b: Escherichia coli ATCC 25922; PA: Pseudomonas aeruginosa CIP 82118; S: Sulfanilamide; A: Ampicillin.
Table 2.
MIC values (µg/mL) of the sulfisoxazole derivatives 4b1–6, 5b1–6.
| Sample | MICs * values (µg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SA | SE | EF | KP | PV | CF | EC a | EC b | PA | |
| 4b1 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 |
| 4b2 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 |
| 4b3 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 |
| 4b4 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 |
| 4b5 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | 256 |
| 4b6 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 |
| 5b1 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 |
| 5b2 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 |
| 5b3 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 |
| 5b4 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 |
| 5b5 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 |
| 5b6 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 |
| S | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | 800 |
| A | 35.8 | 3 | 3 | 16 | - | - | 8 | 35.8 | 128 |
* Mean values (n = 3); SA: Staphyloccoccus aureus ATCC 6583; SE: Staphyloccoccus epidermidis ATCC 12228; EF: Enterococcus faecalis ATCC 25912; KP: Klebsiella pneumoniae CIP 53153; PV: Proteus vulgaris CIP 104989; CF: Citrobacter freundii CIP 5732; EC a: Enterobacter cloacae CIP 103475; EC b: Escherichia coli ATCC 25922; PA: Pseudomonas aeruginosa CIP 82118; S: Sulfanilamide; A: Ampicillin.
The minimum inhibitory concentrations (MICs) of almost all compounds were more than 512 μg/mL. The N-(arylidene)hydrazinoacetyl derivative of sulfadiazine (compound 4a2) was the most active compound, as it was active on Staphyloccoccus epidermidis ATCC 12228 (128 μg/mL), Enterococcus faecalis ATCC 25912 (256 μg/mL) and Pseudomonas aeruginosa CIP 82118 (128 μg/mL). The compounds 4a1 (256 μg/mL), 4a4 (128 μg/mL) and 4b5 (256 μg/mL) were active against Pseudomonas aeruginosa. All tested compounds are more active than sulfanilamide, but less active than ampicillin used as positive controls.
2.2.2. Antioxidant Activity
2.2.2.1. Ferric Reducing Power
The measurement of reducing power defines an important aspect of the antioxidant activity of the compounds. In this assay, the presence of a reducing agent in the sample results in reducing of the ferric/ferricyanide complex to its ferrous (Fe2+) form. The amount of Fe2+ is then quantitatively monitored by measuring the intensity of Perl’s Prussian blue colour complex at 695 nm [25]. The results expressed as EC50 values (mg/mL) are presented in Table 3 and Table 4. The small value of the EC50 indicates a higher ferric reducing power.
Table 3.
Ferric reducing power (EC50 mg/mL) of the sulfadiazine derivatives 4a1–6, 5a1–6.
| Sample | EC50 mg/mL | Sample | EC50 mg/mL |
|---|---|---|---|
| 4a1 | 0.0663 ± 0.0056 | 5a1 | 0.1553 ± 0.0152 |
| 4a2 | 0.0756 ± 0.0040 | 5a2 | 0.1376 ± 0.0002 |
| 4a3 | 0.0856 ± 0.0051 | 5a3 | 0.1745 ± 0.0125 |
| 4a4 | 0.0790 ± 0.0026 | 5a4 | 0.1798 ± 0.0018 |
| 4a5 | 0.0510 ± 0.0036 | 5a5 | 0.0945 ± 0.0085 |
| 4a6 | 0.0503 ± 0.0025 | 5a6 | 0.2277 ± 0.0037 |
| 1a | 2.6140 ± 0.0301 | AA | 0.0075 ± 0.0002 |
Data are mean ± SD (n = 3, p < 0.05).
Table 4.
Ferric reducing power (EC50 mg/mL) of the sulfisoxazole derivatives 4b1–6, 5b1–6.
| Sample | EC50 mg/mL | Sample | EC50 mg/mL |
|---|---|---|---|
| 4b1 | 0.0756 ± 0.0055 | 5b1 | 0.1450 ± 0.0003 |
| 4b2 | 0.2043 ± 0.0055 | 5b2 | 0.1164 ± 0.0025 |
| 4b3 | 0.0610 ± 0.0036 | 5b3 | 0.1915 ± 0.0216 |
| 4b4 | 0.0612 ± 0.0040 | 5b4 | 0.3182 ± 0.0411 |
| 4b5 | 0.0210 ± 0.0065 | 5b5 | 0.0935 ± 0.0098 |
| 4b6 | 0.1173 ± 0.0066 | 5b6 | 0.1106 ± 0.0149 |
| 1b | 0.9640 ± 0.0443 | AA | 0.0075 ± 0.0002 |
Data are mean ± SD (n = 3, p < 0.05).
As it can be seen both N-(arylidene)hydrazinoacetyl and azetidinone derivatives are more active than their sulfonamide parents, sulfadiazine (1a) and sulfizoxazole (1b). In the N-(arylidene) hydrazinoacetyl series of sulfadiazine (compounds 4a1–6) it was observed that the most active compounds were those which resulted from reaction of condensation with 4-hydroxybenzaldehyde (compound 4a5) and 4-nitrobenzaldehyde (compound 4a6). The values of EC50 for these compounds were 0.0510 ± 0.0036 (compound 4a5) and 0.0503 ± 0.0025 (compound 4a6), which means that they are about 50 times more active than sulfadiazine (EC50 = 2.6140 ± 0.0301). Concerning the azetidinone series the most active compound was 5a5, which is the analogue of 4a5 in the azetidinone series. This compound is approximately 28 time more active (EC50 = 0.0945 ± 0.0085) then sulfadiazine (EC50 = 2.6140 ± 0.0301) (Table 3). The ferric reducing power of the compounds resulting through modulation of sulfisoxazole is lower than that of the the analogues of the sulfadiazine series. In reference to sulfisoxazole (1b), all tested compounds 4b1–6, 5b1–6 are more active. The most active compounds were 4b5 [N-(arylidene)hydrazinoacetyl series] and 5b5 (azetidinone series), which have in their structure the 4-hydroxyphenyl radical. These compounds are 46 times (4b5, EC50 = 0.0210 ± 0.0065) and 10 times (5b5, EC50 = 0.0935 ± 0.0098) more active, respectively, than sulfisoxazole (1b, EC50 = 0.9640 ± 0.0443) (Table 4).
The chemical modulation of the parent sulfonamides improve their ferric reducing power and all tested compounds are more active than sulfadiazine and sulfisoxazole, respectively, but they are less active than ascorbic acid (AA) at the same concentration.
2.2.2.2. Total Antioxidant Activity
The total antioxidant activity was determined using phophomolybdenum blue complex with a maximum absorption at 695 nm [26]. The data presented in Table 5 and Table 6 show that the tested compounds are more active than sulfadiazine and sulfisoxazole, respectively, and moreover, the sulfadiazine derivatives are more active than sulfisoxazole compounds.
Table 5.
Total antioxidant activity (EC50 mg/mL) of the sulfadiazine derivatives 4a1–6, 5a1–6.
| Sample | EC50 mg/mL | Sample | EC50 mg/mL |
|---|---|---|---|
| 4a1 | 0.0180 ± 0.0044 | 5a1 | 0.0398 ± 0.0022 |
| 4a2 | 0.0280 ± 0.0067 | 5a2 | 0.0498 ± 0.0015 |
| 4a3 | 0.0110 ± 0.0007 | 5a3 | 0.0330 ± 0.0098 |
| 4a4 | 0.0360 ± 0.0089 | 5a4 | 0.0507 ± 0.0037 |
| 4a5 | 0.0440 ± 0.0050 | 5a5 | 0.0563 ± 0.0009 |
| 4a6 | 0.0220 ± 0.0072 | 5a6 | 0.0341 ± 0.0055 |
| 1a | 6.6483 ± 0.0180 | AA | 0.0067 ± 0.0003 |
Data are mean ± SD (n = 3, p < 0.05).
Table 6.
Total antioxidant activity (EC50 mg/mL) of the sulfisoxazole derivatives 4b1–6, 5b1–6.
| Sample | EC50 mg/mL | Sample | EC50 mg/mL |
|---|---|---|---|
| 4b1 | 0.0481 ± 0.0042 | 5b1 | 0.0433 ± 0.0009 |
| 4b2 | 0.0612 ± 0.0078 | 5b2 | 0.0756 ± 0.0033 |
| 4b3 | 0.0330 ± 0.0009 | 5b3 | 0.0574 ± 0.0025 |
| 4b4 | 0.0332 ± 0.0047 | 5b4 | 0.0385 ± 0.0078 |
| 4b5 | 0.0551 ± 0.0086 | 5b5 | 0.0718 ± 0.0008 |
| 4b6 | 0.0794 ± 0.0091 | 5b6 | 0.0825 ± 0.0045 |
| 1b | 21.658 ± 0.0224 | AA | 0.0067 ± 0.0003 |
Data are mean ± SD (n = 3, p < 0.05).
The most favorable influence seems to be the presence of halogen on the phenyl ring, especially the presence of chlorine in the sulfadiazine series (compounds 4a3, 5a3) and the presence of chlorine and bromine in the sulfisoxazole series (compounds 4b3–4, 5b3–4). The compound 4a3 (EC50 = 0.0110 ± 0.0007) is about 600 times more active than sulfadiazine (1a) (EC50 = 6.6483 ± 0.0180) and its antioxidant activity is comparable with the activity of ascorbic acid (AA) (EC50 = 0.0067 ± 0.0003). Although its azetidinone analogue 5a3 has a lower activity, it remains significant in reference with sulfadiazine (Table 5). In the sulfisoxazole series the compounds 4b3 (EC50 = 0.0330 ± 0.0009) and 4b4 (EC50 = 0.0332 ± 0.0047) are approximately 650 time more active than sulfisoxazole (1b) (EC50 = 21.658 ± 0.0224). Their azetidinone analogues are 380 times (5b3, EC50 = 0.0574 ± 0.0025) and 560 times (5b4, EC50 = 0.0385 ± 0.0078) more active than sulfisoxazole.
2.2.2.3. DPPH Radical Scavenging Assay
DPPH is a well-know radical which demonstrates a strong absorption band centered at about 517 nm, and it becomes colorless or pale yellow when it is neutralized. DPPH radical is scavenged by antioxidants through the donation of proton forming the reduced DPPH, and it is commonly used to evaluate the radical scavenging capacity of antioxidants [27]. The scavenging activities of the N-(arylidene)hydrazinoacetyl sulfonamides 4a1–6, 4b1–6 and N-(4-aryl-3-chloro-2-oxoazetidin-1-yl)aminoacetyl sulfonamides 5a1–6, 5b1–6, are presented in Table 7 and Table 8. All tested compounds are more active than their parent (sulfadiazine and sulfisoxazole) sulfonamides and some of them have a scavenging ability comparable with the scavenging ability of ascorbic acid.
Table 7.
DPPH radical scavenging ability of sulfadiazine derivatives 4a1–6, 5a1–6.
| Sample | Scavenging ability (%) | Sample | Scavenging ability (%) |
|---|---|---|---|
| 4a1 | 87.73 ± 0.69 | 5a1 | 67.18 ± 0.14 |
| 4a2 | 92.22 ± 0.89 | 5a2 | 78.25 ± 0.49 |
| 4a3 | 73.09 ± 0.50 | 5a3 | 80.94 ± 0.74 |
| 4a4 | 85.66 ± 0.89 | 5a4 | 71.52 ± 0.48 |
| 4a5 | 93.17 ± 0.64 | 5a5 | 61.28 ± 0.13 |
| 4a6 | 77.28 ± 0.83 | 5a6 | 71.61 ± 0.33 |
| 1a | 11.15 ± 0.24 | AA | 97.08 ± 0.52 |
Table 8.
DPPH radical scavenging ability of sulfisoxazole derivatives 4b1–6, 5b1–6.
| Sample | Scavenging ability (%) | Sample | Scavenging ability (%) |
|---|---|---|---|
| 4b1 | 48.17 ± 0.63 | 5b1 | 64.28 ± 0.49 |
| 4b2 | 6.43 ± 0.41 | 5b2 | 44.03 ± 0.13 |
| 4b3 | 67.39 ± 0.52 | 5b3 | 44.01 ± 0.86 |
| 4b4 | 42.95 ± 0.23 | 5b4 | 35.52 ± 0.48 |
| 4b5 | 61.20 ± 0.68 | 5b5 | 61.28 ± 0.13 |
| 4b6 | 82.65 ± 0.18 | 5b6 | 33.81 ± 0.09 |
| 1b | 36.59 ± 0.08 | AA | 97.08 ± 0.52 |
The compounds obtained starting from sulfadiazine (compounds 4a1–6, 5a1–6, Table 7) are more active than sulfisoxazole derivatives 4b1–6, 5b1–6 (Table 8). In reference with sulfadiazine (1a), its N-(arylidene)hydrazinoacetyl derivatives 4a1–6 are 6.5–8.4 times more active. Under similar conditions the azetidinone derivatives 5a1–6 are slightly less active, being 5.5–7.3 more active than sulfadiazine. The most active compound is 4a5 [N4-(4-hydroxybenzylidene)hydrazinoacetylamino-N1-(pyrimidin-2-yl)benzensulfonamide]; its scavenging ability (93.17 ± 0.64) being 8.4 time higher than sulfadiazine (11.15 ± 0.24) and comparable with ascorbic acid (97.08 ± 0.52).
3. Experimental
3.1. General Procedures
Melting points were measured using a Buchi Melting Point B-540 apparatus and are uncorrected. The FT-IR spectra were recorded on an ABB Bomen MB3000 spectrometer, over a 500–4000 cm−1 range, after 32 scans at a resolution of 4 cm−1. The spectra processing was carried out with Horizon MBTM FTIR Software. The 1H-NMR (300 MHz) and 13C-NMR (75 MHz) spectra were obtained on a Bruker Avance ARX-300 spectrometer using tetramethylsilane as internal standard and DMSO-d6 as solvent. The chemical shifts are shown in δ values (ppm). The progress of the reaction was monitored on TLC, using pre-coated Kieselgel 60 F254 plates (Merck) and the compounds were visualized by UV light.
3.2. Synthetic Procedures
3.2.1. Preparation of N-(arylidene)hydrazinoacetyl Sulfonamides 4a1–6; 4b1–6
To a solution of hydrazinoacetyl sulfonamide derivatives (10 mmol) in ethanol 50% (200 mL), glacial acetic acid (0.5 mL) and the appropriate aldehyde (10 mmol) were added. The mixture was heated under reflux for 8 h, and then it was cooled at room temperature. The solid was filtered off, dried and recrystallized from isopropyl alcohol.
N4-(Benzylidene)hydrazinoacetylamino-N1-(pyrimidin-2-yl)benzensulfonamide (4a1). Yield 87%, m.p. 200–202 °C; IR (KBr, cm−1): 3450 (-NH), 2853 (CH2-NH), 2924 (=CH- pyrimidine ring), 1693 (C=O), 1623 (HN-CO), 1591 (-C=C- aromatic ring), 1534 (N=CH), 1448 (-C=C- pyrimidine ring), 1313 (C-O), 1128 (-NH-SO2), 1097 (CH aromatic ring), 947 (S-N), 840 (S-C); 1H-NMR δ: 8.72–8.91 (dm, 3H, pyrimidine ring), 8.27 (s, 1H, CO-NH), 8.08 (s, 1H, N=CH), 7.15–7.90 (dm, 9H, Ar-H), 4.19 (s, 1H, -NH-SO2), 3.60 (d, 2H, CH2), 3.43 (m, 1H, HN-N).
N4-(4-Fluorobenzylidene)hydrazinoacetylamino)-N1-(pyrimidin-2-yl)benzensulfonamide (4a2). Yield 90%, m.p. 20–210 °C; IR (KBr, cm−1): 3350 (-NH), 2833 (CH2-NH), 2933 (=CH- pyrimidine ring), 1693 (C=O), 1622 (HN-CO), 1592 (-C=C- aromatic ring), 1535 (N=CH), 1455 (-C=C- pyrimidine ring), 1314 (C-O), 1229 (C-F), 1130 (-NH-SO2), 1099 (CH aromatic ring), 928 (S-N), 836 (S-C); 1H-NMR δ: 8.63-8.90 (dm, 3H, pyrimidine ring), 8.29 (s, 1H, CO-NH), 8.10 (s, 1H, N=CH), 7.1–7.95 (d, 8H, Ar-H), 4.20 (s, 1H, -NH-SO2), 3.79 (d, 2H, CH2), 3.45 (m, 1H, HN-N).
N4-(4-Chlorobenzylidene)hydrazinoacetylamino-N1-(pyrimidin-2-yl)benzensulfonamide (4a3). Yield 83% m.p. 20–207 °C; IR (KBr, cm−1): 3448 (-NH), 2830 (CH2-NH), 2930 (=CH- pyrimidine ring), 1695 (C=O), 1623 (HN-CO), 1591 (-C=C- aromatic ring), 1536 (N=CH), 1496 (-C=C- pyrimidine ring), 1312 (C-O), 1173 (-NH-SO2), 1084 (CH aromatic ring), 940 (S-N), 839 (S-C), 751 (C-Cl); 1H-NMR δ: 8.7–8.89 (dm, 3H, pyrimidine ring), 8.22 (s, 1H, CO-NH), 8.15 (s, 1H, N=CH), 7.0–7.76 (d, 8H, Ar-H), 4.17 (s, 1H, -NH-SO2), 3.61 (d, 2H, CH2), 3.40 (m, 1H, HN-N).
N4-(4-Bromobenzylidene)hydrazinoacetylamino-N1-(pyrimidin-2-yl)benzensulfonamide (4a4). Yield 81%, m.p. 212–213 °C; IR (KBr, cm−1): 3446 (-NH), 2850 (CH2-NH), 2940 (=CH- pyrimidine ring), 1690 (C=O), 1623 (HN-CO), 1591 (-C=C- aromatic ring), 1535 (N=CH), 1454 (-C=C- pyrimidine ring), 1314 (C-O), 1130 (-NH-SO2), 1079 (CH aromatic ring), 959 (S-N), 840 (S-C), 550 (C-Br); 1H-NMR δ: 8.71–8.89 (dm, 3H, pyrimidine ring), 8.21 (s, 1H, CO-NH), 8.18 (s, 1H, N=CH), 7.19–7.84 (d, 8H, Ar-H), 4.16 (s, 1H, -NH-SO2), 3.56 (d, 2H, CH2), 3.40 (m, 1H, HN-N).
N4-(4-Hydroxybenzylidene)hydrazinoacetylamino-N1-(pyrimidin-2-yl)benzensulfonamide (4a5). Yield 86%, m.p. 208–209 °C; IR (KBr, cm−1): 3505 (OH), 3335 (-NH), 2850 (CH2-NH), 2932 (=CH- pyrimidine ring), 1677 (C=O), 1622 (HN-CO), 1590 (-C=C- aromatic ring), 1539 (N=CH), 1514 (-C=C- pyrimidine ring), 1304 (C-O), 1168 (-NH-SO2), 1078 (CH aromatic ring), 965 (S-N), 839 (S-C); 1H-NMR δ: 8.56–8.89 (dm, 3H, pyrimidine ring), 8.24 (s, 1H, CO-NH), 8.07 (s, 1H, N=CH), 7.35–7.89 (d, 8H, Ar-H), 5.05 (s, 1H, Ar-OH), 4.17 (s, 1H, -NH-SO2), 3.63 (d, 2H, CH2), 3.44 (m, 1H, HN-N).
N4-(4-Nitrobenzylidene)hydrazinoacetylamino-N1-(pyrimidin-2-yl)benzensulfonamide (4a6). Yield 75%, m.p. 227–230 °C; IR (KBr, cm−1): 3445 (-NH), 2851 (CH2-NH), 2932 (=CH- pyrimidine ring), 1689 (C=O), 1623 (HN-CO), 1592 (-C=C- aromatic ring), 1536 (N=CH), 1514 (-C=C- pyrimidine ring), 1343 (C-NO2), 1316 (C-O), 1174 (-NH-SO2), 1078 (CH aromatic ring), 951 (S-N), 837 (S-C); 1H-NMR δ: 8.50–8.87 (dm, 3H, pyrimidine ring), 8.21 (s, 1H, CO-NH), 8.06 (s, 1H, N=CH), 7.33–7.90 (d, 8H, Ar-H), 4.16 (s, 1H, -NH-SO2), 3.70 (d, 2H, CH2), 3.41 (m, 1H, HN-N).
N4-(Benzylidene)hydrazinoacetylamino-N1-(3,4-dimethyl-1,2-oxazol-5-yl)benzensulfonamide (4b1). Yield 87%, m.p. 118–120 °C; IR (KBr, cm−1): 3465 (-NH), 2864 (CH2-NH), 1686 (C=O), 1625 (HN-CO), 1592 (-C=C- aromatic ring), 1518 (N=CH), 1496 (-C=C- oxazole ring), 1310 (C-O), 1151 (-NH-SO2), 1093 (CH aromatic ring), 956 (S-N), 830 (S-C); 1H-NMR δ: 8.41 (s, 1H, CO-NH), 8.12 (s, 1H, N=CH), 7.21–7.93 (dm, 9H, Ar-H), 4.62 (s, 1H, -NH-SO2), 3.59 (d, 2H, CH2), 3.24 (m, 1H, HN-N), 2.01–2.38 (s, 6H, 2CH3).
N4-(4-Fluorobenzylidene)hydrazinoacetylamino-N1-(3,4-dimethyl-1,2-oxazol-5-yl)benzensulfonamide (4b2). Yield 68%, m.p. 187–199 °C; IR (KBr, cm−1): 3452 (-NH), 2863 (CH2-NH), 1697 (C=O), 1631 (HN-CO), 1601 (-C=C- aromatic ring), 1507 (N=CH), 1482 (-C=C- oxazole ring), 1320 (C-O), 1225 (C-F), 1153 (-NH-SO2), 1097 (CH aromatic ring), 962 (S-N), 824 (S-C); 1H-NMR δ: 8.36 (s, 1H, CO-NH), 8.21 (s, 1H, N=CH), 7.18–7.91 (d, 8H, Ar-H), 4.65 (s, 1H, -NH-SO2), 3.62 (d, 2H, CH2), 3.38 (m, 1H, HN-N), 2.08–2.34 (s, 6H, 2CH3).
N4-(4-Chlorobenzylidene)hydrazinoacetylamino-N1-(3,4-dimethyl-1,2-oxazol-5-yl)benzensulfonamide (4b3). Yield 62%, m.p. 175–178 °C; IR (KBr, cm−1): 3451 (-NH), 2850 (CH2-NH), 1702 (C=O), 1622 (HN-CO), 1586 (-C=C- aromatic ring), 1540 (N=CH), 1483 (-C=C- oxazole ring), 1312 (C-O), 1169 (-NH-SO2), 1086 (CH aromatic ring), 957 (S-N), 830 (S-C), 814 (C-Cl); 1H-NMR δ: 8.31 (s, 1H, CO-NH), 8.16 (s, 1H, N=CH), 7.25–7.98 (d, 8H, Ar-H), 4.74 (s, 1H, -NH-SO2), 3.64 (d, 2H, CH2), 3.39 (m, 1H, HN-N), 2.08–2.40 (s, 6H, 2CH3).
N4-(4-Bromobenzylidene)hydrazinoacetylamino-N1-(3,4-dimethyl-1,2-oxazol-5-yl)benzensulfonamide (4b4). Yield 57%, m.p. 219 °C; IR (KBr, cm−1): 3439 (-NH), 2870 (CH2-NH), 1669 (C=O), 1625 (HN-CO), 1591 (-C=C- aromatic ring), 1522 (N=CH), 1481 (-C=C- oxazole ring), 1315 (C-O), 1156 (-NH-SO2), 1095 (CH aromatic ring), 940 (S-N), 832 (S-C), 555 (C-Br); 1H-NMR δ: 8.32 (s, 1H, CO-NH), 8.18 (s, 1H, N=CH), 7.36-7.94 (d, 8H, Ar-H), 4.61 (s, 1H, -NH-SO2), 3.65 (d, 2H, CH2), 3.39 (m, 1H, HN-N), 2.10–2.32 (s, 6H, 2CH3).
N4-(4-Hydroxybenzylidene)hydrazinoacetylamino-N1-(3,4-dimethyl-1,2-oxazol-5-yl)benzensulfonamide (4b5). Yield 63%, m.p. 190–192 °C; IR (KBr, cm−1): 3495 (C-OH), 3479 (-NH), 2859 (CH2-NH), 1659 (C=O), 1622 (HN-CO), 1593 (-C=C- aromatic ring), 1530 (N=CH), 1476 (-C=C- oxazole ring), 1323 (C-O), 1150 (-NH-SO2), 1094 (CH aromatic ring), 962 (S-N), 825 (S-C); 1H-NMR δ: 8.36 (s, 1H, CO-NH), 8.22 (s, 1H, N=CH), 7.31–7.84 (d, 8H, Ar-H), 4.92 (s, 1H, Ar-OH), 4.62 (s, 1H, -NH-SO2), 3.58 (d, 2H, CH2), 3.42 (m, 1H, HN-N), 2.08–2.32 (s, 6H, 2CH3).
N4-(4-Nitrobenzylidene)hydrazinoacetylamino-N1-(3,4-dimethyl-1,2-oxazol-5-yl)benzensulfonamide (4b6). Yield 71%, m.p. 280 °C; IR (KBr, cm−1): 3433 (-NH), 2842 (CH2-NH), 1683 (C=O), 1629 (HN-CO), 1593 (-C=C- aromatic ring), 1516 (N=CH), 1480 (-C=C- oxazole ring), 1342 (C-NO2), 1329 (C-O), 1161 (-NH-SO2), 1106 (CH aromatic ring), 951 (S-N), 838 (S-C); 1H-NMR δ: 8.28 (s, 1H, CO-NH), 8.21 (s, 1H, N=CH), 7.27–7.88 (d, 8H, Ar-H), 4.64 (s, 1H, -NH-SO2), 3.56 (d, 2H, CH2), 3.21 (m, 1H, HN-N), 2.11–2.34 (s, 6H, 2CH3).
3.2.2. Preparation of N-(4-aryl-3-chloro-2-oxoazetidin-1-yl)aminoacetyl Sulfonamides 5a1–6; 5b1–6
To a solution of N-(arylidene)hydrazinoacetyl sulfonamides 4a1–6; 4b1–6 (2 mmol) in anhydrous 1,4-dioxane (50 mL), chloracetyl chloride (3 mmol) and triethylamine (2 mmol) were added dropwise at 0–5 °C. The mixture of reaction was stirred at room temperature for 3 h and the solid (triethylamine hydrochloride) was removed. The solution was heated under reflux for 5 h and then the solvent was evaporated under reduced pressure. The solid product was washed with water (20 mL), filtered off, dried and recrystallized from absolute ethanol. The progress of the reaction was monitored by silica gel coated TLC plates.
N4-(2-Phenyl-3-chloro-4-oxoazetidin-1-yl)aminoacetylamino-N1-(pyrimidin-2-yl)benzensulfonamide (5a1). Yield 75%, m.p. 218–220 °C; IR (KBr, cm−1): 3450 (-NH), 2869 (CH2-NH), 2945 (=CH- pyrimidine ring), 1745 (CO β-lactam), 1622 (HN-CO), 1591 (-C=C- aromatic ring), 1449 (-C=C- pyrimidine ring), 1314 (C-O), 1131 (-NH-SO2), 1078 (CH aromatic ring), 945 (S-N), 841 (S-C), 635 (C-Cl); 1H-NMR δ: 8.49–8.72 (dm, 3H, pyrimidine ring), 8.25 (s, 1H, CO-NH), 7.21–7.96 (dm, 9H, Ar-H), 5.45 (d, 1H, CH-Ar, azetidinone ring), 5.18 (d, 1H, CH-Cl), 4.27 (s, 1H, -NH-SO2), 3.75 (d, 2H, CH2), 3.39 (m, 1H, HN-N); 13C-NMR δ: 170.4 (CONH), 162.8 (CO, β-lactam), 126.7–140.9 (12 aromatic carbons), 118.9 (1C pyrimidine ring), 154.7 (3C pyrimidine ring), 67.8 (CH), 62.7 (CH-Cl), 57.7 (CH2); Anal. calcd for C21H19O4N6S: C 55.87, H 4.24, N 18.61; found: C 56.02, H 4.38, N 18.48.
N4-[2-(4-Fluoro)phenyl-3-chloro-4-oxoazetidin-1-yl]aminoacetylamino-N1-(pyrimidin-2-yl)benzen-sulfonamide (5a2). Yield 90%, m.p. 213–215 °C; IR (KBr, cm−1): 3355 (-NH), 2831 (CH2-NH), 2943 (=CH- pyrimidine ring), 1744 (CO β-lactam), 1624 (HN-CO), 1593 (-C=C- aromatic ring), 1508 (-C=C- pyrimidine ring), 1315 (C-O), 1230 (C-F), 1131 (-NH-SO2), 1100 (CH aromatic ring), 926 (S-N), 836 (S-C), 613 (C-Cl); 1H-NMR δ: 8.47–8.70 (dm, 3H, pyrimidine ring), 8.22 (s, 1H, CO-NH), 7.23–7.97 (d, 8H, Ar-H), 5.35 (d, 1H, CH-Ar, azetidinone ring), 5.04 (d, 1H, CH-Cl), 4.37 (s, 1H, -NH-SO2), 3.79 (d, 2H, CH2), 3.39 (m, 1H, HN-N); 13C-NMR δ: 169.2 (CONH), 162.5 (CO, β-lactam), 124.9–141.06 (12 aromatic carbons), 118.8 (1C pyrimidine ring), 151.8 (3C pyrimidine ring), 69.2 (CH), 61.04 (CH-Cl), 55.6 (CH2); Anal. calcd for C21H18O4N6SF: C 53.73, H 3.86, N 17.90; found: C 53.96, H 4.06, N 18.14.
N4-[2-(4-Chloro)phenyl-3-chloro-4-oxoazetidin-1-yl]aminoacetylamino-N1-(pyrimidin-2-yl)benzen-sulfonamide (5a3). Yield 85%, m.p. 201–203 °C; IR (KBr, cm−1): 3355 (-NH), 2830 (CH2-NH), 2944 (=CH- pyrimidine ring), 1745 (CO β-lactam), 1624 (HN-CO), 1592 (-C=C- aromatic ring), 1490 (-C=C- pyrimidine ring), 1314 (C-O), 1174 (-NH-SO2), 1088 (CH aromatic ring), 958 (S-N), 825 (S-C), 610 (C-Cl); 1H-NMR) δ: 8.48–8.74 (dm, 3H, pyrimidine ring), 8.24 (s, 1H, CO-NH), 7.19–7.96 (d, 8H, Ar-H), 5.38 (d, 1H, CH-Ar, azetidinone ring), 5.00 (d, 1H, CH-Cl), 4.4 (s, 1H, -NH-SO2), 3.75 (d, 2H, CH2), 3.39 (m, 1H, HN-N); 13C-NMR δ: 167.6 (CONH), 160.5 (CO, β-lactam), 126.7–142.6 (12 aromatic carbons), 120.5 (1C pyrimidine ring), 155.1 (3C pyrimidine ring), 68.7 (CH), 62.8 (CH-Cl), 54.4 (CH2); Anal. calcd for C21H18O4N6SCl: C 51.91, H 3.73, N 17.29; found: C 52.14, H 3.95, N 17.04.
N4-[2-(4-Bromo)phenyl-3-chloro-4-oxoazetidin-1-yl]aminoacetylamino-N1-(pyrimidin-2-yl)benzen-sulfonamide (5a4). Yield 88%, m.p. 206–208 °C; IR (KBr, cm−1): 3445 (-NH), 2848 (CH2-NH), 2943 (=CH- pyrimidine ring), 1739 (CO β-lactam), 1624 (HN-CO), 1590 (-C=C- aromatic ring), 1485 (-C=C- pyrimidine ring), 1315 (C-O), 1130 (-NH-SO2), 1067 (CH aromatic ring), 961 (S-N), 840 (S-C), 630 (C-Cl), 572 (C-Br); 1H-NMR δ: 8.47–8.71 (dm, 3H, pyrimidine ring), 8.22 (s, 1H, CO-NH), 7.16–7.85 (d, 8H, Ar-H), 5.36 (d, 1H, CH-Ar, azetidinone ring), 5.05 (d, 1H, CH-Cl), 4.27 (s, 1H, -NH-SO2), 3.79 (d, 2H, CH2), 3.39 (m, 1H, HN-N); 13C-NMR δ: 165.3 (CONH), 160.7 (CO, β-lactam), 126.6–136.6 (12 aromatic carbons), 118.7 (1C pyrimidine ring), 156.6 (3C pyrimidine ring), 68.2 (CH), 62.09 (CH-Cl), 57.5 (CH2); Anal. calcd for C21H18O4N6SBr: C 47.56, H 3.42, N 15.85; found: C 47.28, H 3.71, N 15.67.
N4-[2-(4-Hydroxy)phenyl-3-chloro-4-oxoazetidin-1-yl]aminoacetylamino-N1-(pyrimidin-2-yl)benzen-sulfonamide (5a5). Yield 80%, m.p. 231–233 °C; IR (KBr, cm−1): 3497 (C-OH), 3355 (-NH), 2843 (CH2-NH), 2943 (=CH- pyrimidine ring), 1740 (CO β-lactam), 1622 (HN-CO), 1590 (-C=C- aromatic ring), 1495 (-C=C- pyrimidine ring), 1315 (C-O), 1175 (-NH-SO2), 1067 (CH aromatic ring), 961 (S-N), 840 (S-C), 636 (C-Cl), 1H-NMR δ: 8.54–8.75 (dm, 3H, pyrimidine ring), 8.26 (s, 1H, CO-NH), 7.30–7.90 (d, 8H, Ar-H), 5.34 (d, 1H, CH-Ar, azetidinone ring), 5.06 (d, 1H, CH-Cl), 4.95 (s, 1H, Ar-OH), 4.25 (s, 1H, -NH-SO2), 3.64 (d, 2H, CH2), 3.40 (m, 1H, HN-N); 13C-NMR δ: 167.7 (CONH), 160.3 (CO, β-lactam), 114.7–132.1 (12 aromatic carbons), 119.5 (1C pyrimidine ring), 153.4 (3C pyrimidine ring), 72.9 (CH), 63.3 (CH-Cl), 54.2 (CH2); Anal. calcd for C21H19O5N6S: C 53.95, H 4.10, N 17.98; found: C 54.21, H 4.32, N 18.19.
N4-[2-(4-Nitro)phenyl-3-chloro-4-oxoazetidin-1-yl]aminoacetylamino-N1-(pyrimidin-2-yl)benzen-sulfonamide (5a6). Yield 86%, m.p. 200–203 °C; IR (KBr, cm−1): 3350 (-NH), 2846 (CH2-NH), 2942 (=CH- pyrimidine ring), 1743 (CO β-lactam), 1624 (HN-CO), 1593 (-C=C- aromatic ring), 1496 (-C=C- pyrimidine ring), 1343 (C-NO2), 1316 (C-O), 1131 (-NH-SO2), 1079 (CH aromatic ring), 951 (S-N), 839 (S-C), 635 (C-Cl); 1H-NMR) δ: 8.36–8.87 (dm, 3H, pyrimidine ring), 8.29 (s, 1H, CO-NH), 7.19–7.99 (d, 8H, Ar-H), 5.33 (d, 1H, CH-Ar, azetidinone ring), 5.02 (d, 1H, CH-Cl), 4.24 (s, 1H, -NH-SO2), 3.62 (d, 2H, CH2), 3.37 (m, 1H, HN-N); 13C-NMR δ: 168.3 (CONH), 161.9 (CO, β-lactam), 118.5–137.2 (12 aromatic carbons), 118.6 (1C pyrimidine ring), 155.8 (3C pyrimidine ring), 76.1 (CH), 64.3 (CH-Cl), 58.0 (CH2); Anal. calcd for C21H18O6N7S: C 50.80, H 3.65, N 19.75; found: C 50.64, H 3.89, N 19.97.
N4-(2-Phenyl-3-chloro-4-oxoazetidin-1-yl)aminoacetylamino-N1-(3,4-dimethyl-1,2-oxazol-5-yl)benzen-sulfonamide (5b1). Yield 51%, m.p. 212–213 °C; IR (KBr, cm−1): 3342 (-NH), 2860 (CH2-NH), 1743 (CO β-lactam), 1624 (HN-CO), 1591 (-C=C- aromatic ring), 1495 (-C=C- oxazole ring), 1318 (C-O), 1153 (-NH-SO2), 1092 (CH aromatic ring), 957 (S-N), 836 (S-C), 663 (C-Cl); 1H-NMR δ: 8.34 (s, 1H, CO-NH), 7.13–7.86 (dm, 9H, Ar-H), 5.37 (d, 1H, CH-Ar, azetidinone ring), 5.19 (d, 1H, CH-Cl), 4.57 (s, 1H, -NH-SO2), 3.51 (d, 2H, CH2), 3.11 (m, 1H, HN-N), 1.95–2.30 (s, 6H, 2CH3); 13C-NMR δ: 168.5 (CONH), 164.0 (CO, β-lactam), 151.4–155.8 (2C oxazole ring), 101.05 (1C oxazole ring), 118.8–135.3 (12 aromatic carbons), 77.2 (CH), 66.3 (CH-Cl), 59.2 (CH2), 8.48 (2CH3); Anal. calcd for C22H22O5N5S: C 56.40, H 4.73, N 14.95; found: C 56.63, H 4.94, N 15.18.
N4-[2-(4-Fluorophenyl)-3-chloro-4-oxoazetidin-1-yl]aminoacetylamino-N1-(3,4-dimethyl-1,2-oxazol-5-yl)benzensulfonamide (5b2). Yield 76%, m.p. 209–211 °C; IR (KBr, cm−1): 3450 (-NH), 2861 (CH2-NH), 1739 (CO β-lactam), 1633 (HN-CO), 1591 (-C=C- aromatic), 1497 (-C=C- oxazole ring), 1322 (C-O), 1232 (C-F), 1153 (-NH-SO2), 1094 (CH aromatic ring), 961 (S-N), 836 (S-C), 662 (C-Cl); 1H-NMR δ: 8.24 (s, 1H, CO-NH), 7.10–7.85 (d, 8H, Ar-H), 5.38 (d, 1H, CH-Ar, azetidinone ring), 5.23 (d, 1H, CH-Cl), 4.57 (s, 1H, -NH-SO2), 3.57 (d, 2H, CH2), 3.41 (m, 1H, HN-N), 2.02–2.27 (s, 6H, 2CH3); 13C-NMR δ: 169.6 (CONH), 160.3 (CO, β-lactam), 118.6–139.9 (12 aromatic carbons), 99.56 (1C oxazole ring), 151.8–153.3 (2C oxazole ring), 75.9 (CH), 64.2 (CH-Cl), 56.2 (CH2), 10.84 (2CH3); Anal. calcd for C22H21O5N5SF: C 54.31, H 4.35, N 14.40; found: C 54.09, H 4.58, N 14.68.
N4-[2-(4-Chlorophenyl)-3-chloro-4-oxoazetidin-1-yl]aminoacetylamino-N1-(3,4-dimethyl-1,2-oxazol-5-yl)benzensulfonamide (5b3). Yield 72%, m.p. 211–213 °C; IR (KBr, cm−1): 3361 (-NH), 2869 (CH2-NH), 1750 (CO β-lactam), 1620 (HN-CO), 1591 (-C=C- aromatic), 1495 (-C=C oxazole ring), 1318 (C-O), 1153 (-NH-SO2), 1092 (CH aromatic ring), 957 (S-N), 836 (S-C), 663 (C-Cl); 1H-NMR δ: 8.23 (s, 1H, CO-NH), 7.22–7.92 (d, 8H, Ar-H), 5.35 (d, 1H, CH-Ar, azetidinone ring), 5.20 (d, 1H, CH-Cl), 4.68 (s, 1H, -NH-SO2), 3.52 (d, 2H, CH2), 3.33 (m, 1H, HN-N), 2.05–2.36 (s, 6H, 2CH3); 13C-NMR (DMSO) δ in ppm: 170.3 (CONH), 163.6 (CO, β-lactam), 118.8–132.7 (12 aromatic carbons), 100.97 (1C oxazole ring), 150.4–152.7 (2C oxazole ring), 77.4 (CH), 66.3 (CH-Cl), 53.1 (CH2), 8.50 (2CH3); Anal. calcd for C22H21O5N5SCl: C 52.54, H 4.21, N 13.92; found: C 52.38, H 4.08, N 13.71.
N4-[2-(4-Bromophenyl)-3-chloro-4-oxoazetidin-1-yl]aminoacetylamino-N1-(3,4-dimethyl-1,2-oxazol-5-yl)benzensulfonamide (5b4). Yield 80%, m.p. 212–214 °C; IR (KBr, cm−1): 3386 (-NH), 2866 (CH2-NH), 1748 (CO β-lactam), 1626 (HN-CO), 1589 (-C=C- aromatic ring), 1483 (-C=C- oxazole ring), 1315 (C-O), 1158 (-NH-SO2), 1095 (CH aromatic ring), 960 (S-N), 859 (S-C), 660 (C-Cl), 556 (C-Br); 1H-NMR δ: 8.21 (s, 1H, CO-NH), 7.34–7.98 (d, 8H, Ar-H), 5.33 (d, 1H, CH-Ar, azetidinone ring), 5.19 (d, 1H, CH-Cl), 4.56 (s, 1H, -NH-SO2), 3.60 (d, 2H, CH2), 3.36 (m, 1H, HN-N), 2.08–2.28 (s, 6H, 2CH3); 13C-NMR δ: 171.3 (CONH), 160.7 (CO, β-lactam), 128.8–142.7 (12 aromatic carbons), 102.37 (1C oxazole ring), 151.7–153.5 (2C oxazole ring), 78.8 (CH), 67.0 (CH-Cl), 55.0 (CH2), 8.50 (2CH3); Anal. calcd for C22H21O5N5SBr: C 48.27, H 3.87, N 12.79; found: C 48.54, H 3.94, N 12.97.
N4-[2-(4-Hydroxyphenyl)-3-chloro-4-oxoazetidin-1-yl]aminoacetylamino-N1-(3,4-dimethyl-1,2-oxazol-5-yl)benzensulfonamide (5b5). Yield 55%, m.p. 210–212 °C; IR (KBr, cm−1): 3561 (C-OH), 3461 (-NH), 2855 (CH2-NH), 1746 (CO β-lactam), 1621 (HN-CO), 1590 (-C=C- aromatic), 1480 (-C=C- oxazole ring), 1308 (C-O), 1151 (-NH-SO2), 1093 (CH aromatic ring), 989 (S-N), 835 (S-C), 659 (C-Cl); 1H-NMR δ in ppm: 8.31 (s, 1H, CO-NH), 7.28–7.81 (d, 8H, Ar-H), 5.32 (d, 1H, CH-Ar, azetidinone ring), 5.10 (d, 1H CH-Cl), 4.84 (s, 1H, Ar-OH), 4.57 (s, 1H, -NH-SO2), 3.55 (d, 2H, CH2), 3.39 (m, 1H, HN-N), 2.04–2.28 (s, 6H, 2CH3); 13C-NMR δ: 175.2 (CONH), 168.2 (CO, β-lactam), 115.8–126.8 (12 aromatic carbons), 101.79 (1C oxazole ring), 148.9–150.5 (2C oxazole ring), 77.3 (CH), 64.8 (CH-Cl), 55.2 (CH2), 8.51 (2CH3); Anal. calcd for C22H22O6N5S: C 54.54, H 4.58, N 14.45; found: C 54.73, H 4.63, N 14.71.
N4-[2-(4-Nitrophenyl)-3-chloro-4-oxoazetidin-1-yl]aminoacetylamino-N1-(3,4-dimethyl-1,2-oxazol-5-yl)benzensulfonamide (5b6). Yield 59%, m.p. 218–220 °C; IR (KBr, cm−1): 3415 (-NH), 2863 (CH2-NH), 1752 (CO β-lactam), 1624 (HN-CO), 1591 (-C=C- aromatic ring), 1490 (-C=C- oxazole ring), 1337 (C-O), 1329 (C-NO2) 1154 (-NH-SO2), 1092 (CH aromatic ring), 987 (S-N), 836 (S-C), 662 (C-Cl); 1H-NMR δ: 8.18 (s, 1H, CO-NH), 7.19–7.86 (d, 8H, Ar-H), 5.36 (d, 1H, CH-Ar, azetidinone ring), 5.20 (d, 1H, CH-Cl), 4.55 (s, 1H, -NH-SO2), 3.49 (d, 2H, CH2), 3.11 (m, 1H, HN-N); 2.09–2.27 (s, 6H, 2CH3); 13C-NMR δ: 170.7 (CONH), 165.1 (CO, β-lactam), 118.7–130.6 (12 aromatic carbons), 101.20 (1C oxazole ring), 148.8–150.4 (2C oxazole ring), 79.1 (CH), 65.4 (CH-Cl), 60.7 (CH2), 9.54 (2CH3); Anal. calcd for C22H21O7N6S: C 51.46, H 4.12, N 16.37; found: C 51.29, H 4.36, N 16.09.
3.3. Biological Evaluation
3.3.1. Antibacterial Assay
In order to evaluate the antibacterial activity of the synthesized compounds 4a1–6, 4b1–6, 5a1–6 and 5b1–6, a panel of three Gram positive (Staphyloccoccus aureus ATCC 6583, Staphyloccoccus epidermidis ATCC 12228, Enterococcus faecalis ATCC 25912) and six Gram negative (Klebsiella pneumoniae CIP 53153, Proteus vulgaris CIP 104989, Citrobacter freundii CIP 5732, Enterobacter cloacae CIP 103475, Escherichia coli ATCC 25922, Pseudomonas aeruginosa CIP 82118) bacterial strains were used. Minimum inhibitory concentrations (MICs) were assessed according to the guidelines of EUCAST Def. 3.1 [24]. Briefly, stock solutions were prepared by solving the substances mentioned above (200 mg) in dimethyl sulfoxide (DMSO, 19.5 mL). Using these solutions, series of two-fold dilutions were subsequently obtained. In a 9 cm diameter Petri dish, one milliliter of each dilution was mixed thoroughly with Mueller-Hinton agar (19 mL), sterilized by autoclaving and cooled to 50 °C. After this, the concentrations of the substances inside the medium were 512, 256, 128, 64, 32, 16, 8, 4, 2, and 1 μg/mL respectively. A blank plate (control of growth) was also prepared by mixing DMSO (1 mL) with molten agar (19 mL). From each bacterial strain, a 0.5 McFarland suspension was prepared in 0.85% saline solution and after that, the inoculum was standardized in order to assure 104 colony-forming units (CFU) per spot (5 μL). All inoculated plates were incubated for 18 h at 36 °C. The MIC was interpreted as the lowest concentration of the substance that completely inhibits the growth of bacteria in the spot area. Each determination was performed in triplicate in order to accurately confirm the MIC values.
3.3.2. Antioxidant Assays
The antioxidant activity was estimated using in vitro tests: ferric reducing power, total antioxidant capacity and radical scavenging ability.
3.3.2.1. Ferric Reducing Power
The ferric reducing power of the compounds was quantified by the method described by [25] with slight modifications. The sample solution (1 mL, 5 mg/mL in DMSO) was mixed with sodium phosphate buffer (1 mL, 0.2 M, pH 6.6) and potassium ferricyanide (1 mL, 1% w/v) in a test tube. The reaction mixture was incubated at 50 °C for 20 min in a water bath and then the reaction was stopped by adding trichloroacetic acid (1 mL, 10% w/v). After centrifugation of the mixture at 4500 rpm for 15 min, the upper layer of the solution (1mL) was collected and diluted further by adding deionised water (1 mL) and ferric chloride (0.2 mL, 0.1% w/v). After 5 min of incubation, the absorbance was measured at 700 nm against a blank (the mixture of DMSO with the reagents). A higher absorbance indicates a higher reducing power. For each sample it was calculated the effective concentration (EC50) and the reducing power was expressed in reference with ascorbic acid (AA) in the same concentration.
3.3.2.2. Total Antioxidant Activity
The antioxidant activity of tested compounds was evaluated using the phosphomolybdenum method according to the procedure of [26] with minor modifications. The method is based on the reduction of Mo(VI) to Mo(V) by the tested compounds followed by the formation of a green phosphate/Mo(V) complex at acid pH. An aliquot of sample solution (50 µL, 5 mg/mL in DMSO) is mixed with the reagent solution (2 mL, 0.6 M sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The samples were incubated at 95 °C for 90 min and then were cooled to room temperature. The absorbance was measured at 695 nm against a blank (DMSO mixed with reagent solution). For each sample the effective concentration (EC50) was calculated and the antioxidant activity was expressed in reference with ascorbic acid (AA) in the same concentration.
3.3.2.3. DPPH Radical Scavenging Assay
The radical scavenging activity of the tested compounds towards the radical 1,1-diphenyl-2-picrylhydrazyl (DPPH) was measured as described by [27] with slight modifications. The sample solution (50 µL, 20 mg/mL in DMSO) was mixed thoroughly with a solution of DPPH in methanol (2.95 mL, 0.1 mM). The sample was left for 30 min at room temperature, in the dark, and after that the absorbance was measured at 517 nm (As). A methanol solution of DPPH was used as control sample (Ac). The ability to scavenge the DPPH radical was calculated using the following formula:
| % Inhibition = 100 × (Ac−As)/Ac |
and it was expressed in reference with the radical scavenging activity of ascorbic acid (AA) in the same concentration.
3.3.3. Statistical Analysis
All assays (antimicrobial and antioxidant) were carried out in triplicate. Data were analysed by an analysis of variance (ANOVA) (p < 0.05) and were expressed as means ± SD. The total antioxidant antivity (EC50 values) were calculated by linear interpolation between values above and below 50% activity.
4. Conclusions
In this study new N-(arylidene)hydrazinoacetyl and new 2-azetidionone derivatives have been designed and synthesized starting from sulfadiazine and sulfizoxazole. The structures of all new compounds were proved using spectral methods. The compounds were evaluated for their antimicrobial and antioxidant activity. Although their antimicrobial potential was reduced, they shown excellent antioxidant properties; for some of them the potential is comparable with the antioxidant activity of ascorbic acid. These results support the antioxidant potential of the synthesized compounds and their applications in several disease mediated by reactive oxygen species (ROS) including the healing of the wounds.
Acknowledgments
This work was supported in part by the project “Doctoral Scholarships for increasing competitiveness in the medical and pharmaceutical field” (POSDRU/88/1.5/S/58965) and the grant of the Romanian National Authority for Scientific Research, CNCS – UEFISCDI, project number PN-II-ID-PCE-2011-3-0906 (Contract No. 274/31.10.2011). The authors thank Polymer Research Group, Department of Organic Chemistry, University of Ghent, Belgium for their help to perform NMR analyses.
Conflicts of Interest
The authors declared no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 4a1–6, 4b1–6, 5a1–6, 5b1–6 are available from the authors.
References
- 1.Chavan A.A., Pai N.R. Synthesis and biological activity of N-substituted-3-chloro-2-azetidinones. Molecules. 2007;12:2467–2477. doi: 10.3390/12112467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishwar B.K., Mishra S.K., Jainey P.J., Shastry C.S. Antimicrobial studies of synthesized azetidinone derivatives from sulfamethoxazole moiety. J. Chem. Pharm. Res. 2011;3:114–118. [Google Scholar]
- 3.Jarrahpour A., Zarei M. Synthesis of novel N-sulfonyl monocyclic β-lactams as potential antibacterial agents. Molecules. 2006;11:49–58. doi: 10.3390/11010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cerić H., Šindler-Kulyk M., Kovačević M., Perić M., Živković A. Azetidinone-isothiazolidinones: Stereoselective synthesis and antibacterial evaluation of new monocyclic beta-lactams. Bioorg. Med. Chem. 2010;18:3053–3058. doi: 10.1016/j.bmc.2010.03.045. [DOI] [PubMed] [Google Scholar]
- 5.Sharma R., Samadhiya P., Srivastava S.D., Srivastava S.K. Synthesis and pharmaceutical importance of 2-azetidinone derivatives of phenothiazine. J. Chem. Sci. 2012;124:633–637. doi: 10.1007/s12039-012-0257-x. [DOI] [Google Scholar]
- 6.Samadhiya P., Sharma R., Srivastava S.K., Srivastava S.D. Synthesis of 2-azetidinone derivatives of 6-nitro-1H-indazole and their biological importance. Quím. Nova. 2012;35:914–919. [Google Scholar]
- 7.Kumar A., Rajput C.S., Bhati S.K. Synthesis of 3-[4'-(p-chlorophenyl)-thiazol-2'-yl]-2-[(substituted azetidinone/thiazolidinone)-aminomethyl]-6-bromoquinazolin-4-ones as anti-inflammatory agent. Bioorg. Med. Chem. 2007;15:3089–3096. doi: 10.1016/j.bmc.2007.01.042. [DOI] [PubMed] [Google Scholar]
- 8.Smith E.M., Sorota S., Kim H.M., McKittrick B.A., Nechuta T.L., Bennett C., Knutson C., Burnett D.A., Kieselgof J., Tan Z., et al. T-type calcium channel blockers: Spiro-piperidine azetidines and azetidinones-optimization, Design and synthesis. Bioorg. Med. Chem. Lett. 2010;20:4602–4606. doi: 10.1016/j.bmcl.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Aoyama Y., Uenaka M., Kii M., Tanaka M., Konoike T., Hayasaki-Kajiwara Y., Naya N., Nakajima M. Design, Synthesis and pharmacological evaluation of 3-benzylazetidine-2-one-based human chymase inhibitors. Bioorg. Med. Chem. 2001;9:3065–3075. doi: 10.1016/S0968-0896(01)00209-7. [DOI] [PubMed] [Google Scholar]
- 10.O’Boyle N.M., Greene L.M., Bergin O., Fichet J.-B., McCabe T., Lloyd D.G., Zisterer D.M., Meegan M.J. Synthesis, Evaluation and structural studies of antiproliferative tubulin-targeting azetidin-2-ones. Bioorg. Med. Chem. 2011;19:2306–2325. doi: 10.1016/j.bmc.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 11.Galletti P., Quintavalla A., Ventrici C., Giannini G., Cabri W., Penco S., Gallo G., Vincenti S., Giacomini D. Azetidinones as zinc-binding groups to design selective HDAC8 inhibitors. Chem. Med. Chem. 2009;4:1991–2001. doi: 10.1002/cmdc.200900309. [DOI] [PubMed] [Google Scholar]
- 12.Tripodi F., Pagliarin R., Fumagalli G., Bigi A., Fusi P., Orsini F., Frattini M., Coccetti P. Synthesis and biological evaluation of 1,4-diaryl-2-azetidinones as specific anticancer agents: activation of adenosine monophosphate activated protein kinase and induction of apoptosis. J. Med. Chem. 2012;55:2112–2124. doi: 10.1021/jm201344a. [DOI] [PubMed] [Google Scholar]
- 13.Mehta P.D., Sengar N.P., Pathak A.K. 2-Azetidinone-a new profile of various pharmacological activities. Eur. J. Med. Chem. 2010;45:5541–5560. doi: 10.1016/j.ejmech.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 14.Gavernet L., Barrios I.A., Cravero M.S., Bruno-Blanch L.E. Design, Synthesis, and anticonvulsant activity of some sulfamides. Bioorg. Med. Chem. 2007;15:5604–5614. doi: 10.1016/j.bmc.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 15.Chohan Z.H. Metal-based antibacterial and antifungal sulfonamides: Synthesis, Characterization, and biological properties. Transit. Metal Chem. 2009;34:153–161. doi: 10.1007/s11243-008-9171-y. [DOI] [Google Scholar]
- 16.Sasidharan S., Logeswaran S., Latha L.Y. Wound healing activity of Elaeis Guineensis leaf extract ointment. Int. J. Mol. Sci. 2012;13:336–347. doi: 10.3390/ijms13010336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schönfelder U., Abel M., Wiegand C., Klemm D., Elsner P., Hipler U.-C. Influence of selected wound dressings on PMN elastase in chronic wound fluid and their antioxidative potential in vitro. Biomaterials. 2005;26:6664–6673. doi: 10.1016/j.biomaterials.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 18.Melo-Silveira R.F., Fidelis G.P., Costa M.S., Telles C.B., Dantas-Santos N., de Oliveira Elias S., Ribeiro V.B., Barth A.L., Macedo A.J., Leite E.L., et al. In vitro antioxidant, Anticoagulant and antimicrobial activity and in inhibition of cancer cell proliferation by xylan extracted from Corn Cobs. Int. J. Mol. Sci. 2012;13:409–426. doi: 10.3390/ijms13010409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roy P., Amdekar S., Kumar A., Singh R., Sharma P., Singh V. In vivo antioxidative property, antimicrobial and wound healing activity of flower extracts of Pyrostegia venusta (Ker Gawl) Miers. J. Ethnopharmacol. 2012;140:186–192. doi: 10.1016/j.jep.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Ghaisas M.M., Kshirsagar S.B., Sahane R.S. Evaluation of wound healing activity of ferulic acid in diabetic rats. Int. Wound. J. 2012 doi: 10.1111/j.1742-481X.2012.01119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chigurupati S., Mughal M.R., Chan S.L., Arumugam T.V., Baharani A., Tang S.C., Yu Q.S., Holloway H.W., Wheeler R., Poosala S., et al. A synthetic uric acid analog accelerates cutaneous wound healing in mice. PLoS One. 2010;5:e10044. doi: 10.1371/journal.pone.0010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naik H.R., Naik H.S., Naik T.R., Naika H.R., Gouthamchandra K., Mahmood R., Ahamed B.M. Synthesis of novel benzo[h]quinolines: Wound healing, Antibacterial, DNA binding and in vitro antioxidant activity. Eur. J. Med. Chem. 2009;44:981–989. doi: 10.1016/j.ejmech.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Parasca O.M., Lupascu F., Vasile C., Mares M., Nastasa V., Profire L. New hydrazynes with sulfonamidic structure: Synthesis, Characterization and biological activity. Rev. Med. Chir. 2013 in press. [PubMed] [Google Scholar]
- 24.European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society for Clinical Microbiology and Infectious Diseases (ESCMID) EUCAST Definitive Document E. Def 3.1.: Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by agar dilution. Clin. Microbiol. Infect. 2000;6:509–515. doi: 10.1046/j.1469-0691.2000.00142.x. [DOI] [PubMed] [Google Scholar]
- 25.Cacic M., Molnar M., Sarkanj B., Has-Schon E., Rajkovic V. Synthessis and antioxidant activity of some new coumarinyl-1,3-Thiazolidine-4-ones. Molecules. 2010;15:6795–6809. doi: 10.3390/molecules15106795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cervellati R., Galletti P., Greco E., Cocuzza C.E., Musumeci R., Bardini L., Paolucci F., Pori M., Soldati R., Giacomini D. Monocyclic β-lactams as antibacterial agents: Facing antioxidant activity of N-methylthio-azetidinones. Eur. J. Med. Chem. 2012;60:340–349. doi: 10.1016/j.ejmech.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 27.Osorio M., Aravena J., Vergara A., Taborga L., Baeza E., Catalán K., González C., Carvajal M., Carrasco H., Espinoza L. Synthesis and DPPH radical scavenging activity of prenylated phenol derivatives. Molecules. 2012;17:556–570. doi: 10.3390/molecules17010556. [DOI] [PMC free article] [PubMed] [Google Scholar]