Abstract
Swietenia macrophylla King (Meliaceae) is an endangered and medicinally important plant indigenous to tropical and subtropical regions of the World. S. macrophylla has been widely used in folk medicine to treat various diseases. The review reveals that limonoids and its derivatives are the major constituents of S. macrophylla. There are several data in the literature indicating a great variety of pharmacological activities of S. macrophylla, which exhibits antimicrobial, anti-inflammatory, antioxidant effects, antimutagenic, anticancer, antitumor and antidiabetic activities. Various other activities like anti-nociceptive, hypolipidemic, antidiarrhoeal, anti-infective, antiviral, antimalarial, acaricidal, antifeedant and heavy metal phytoremediation activity have also been reported. In view of the immense medicinal importance of S. macrophylla, this review aimed at compiling all currently available information on its ethnomedicinal uses, phytochemistry and biological activities of S. macrophylla, showing its importance.
Keywords: Swietenia macrophylla, mahogany, limonoids, biological activity, phytochemicals
1. Introduction
Plants besides having the critical role of photosynthesis are also a source of numerous phytochemicals used through a variety of herbal remedies and foodstuffs with curative properties that help mankind sustain its health. Thereby, the development of pharmaceutical products necessitates a comprehensive investigation of medicinal plants to enhance our knowledge about their biological activities and the phytoconstituents responsible for them [1]. Additionally, the fact that only a limited number of medicinal plant species have received complete scientific inspection makes the need for comprehensive investigations in this area more evident [2]. Plants with a long history of use in ethno- medicine can be a rich source of substances for the treatment of various chronic or infectious diseases [3]. The big-leaved mahogany tree (Swietenia macrophylla), well known in the timber industry for its wood quality, is one such plant [4]. As S. macrophylla is important for the economy of many neotropical countries due to its high-quality timber [5,6], nuclear microsatellites and DNA-fingerprinting methods were established to verify the geographic origin of S. macrophylla in order to prevent illegal logging of this plant [7]. However, large areas of former S. macrophylla forests have been converted to other uses, and the remaining forests are few [8,9]. The depletion of S. macrophylla populations has led to concern for the future of the species and its commercial trade. In 2002, S. macrophylla was listed in Appendix II (species that may face extinction if trade is not controlled) of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) [10]. Recent studies showed that S. macrophylla, besides its timber industry uses, also has noteworthy value and benefits in phytomedicine because of the variety of biological activities it presents. This review summarizes the botany, ethnomedicinal uses, phytochemistry, biological activities and mechanisms underlying the bioactivities of S. macrophylla.
2. Botany
2.1. Botanical Name
Swietenia macrophylla King.
2.2. Synonyms
Swietenia belizensis Lundell, Swietenia candollei Pittier, Swietenia krukovii Gleason, Swietenia macrophylla King var. marabaensis Ledouxet Lobato, Swietenia tessmannii Harms.
2.3. Common Names
Mahoni (all parts of Indonesia); baramahauni, bara-mahagoni, mahagni (Bangladesh); mahogany, big- or large-leaved mahogany, bastard mahogany, Brazilian mahogany tree, Colombian mahogany tree, Dominican mahogany, Honduras mahogany, Mexican mahogany tree, Peruvian mahogany tree, Spanish mahogany, West Indian mahogany (England); acajoudu Honduras, acajou du Venezuela, acajou étranger (France); Echtes mahagoni (Germany); mogano (Italy); cheria mahogany (Malaysia); mahok, mahonie (Netherland); mogno (Portugal); caoba, caoba de Honduras, caoba de Santo, caoba del Atlántico, caobahondureña, domingo, (Spain); mahokkani-baiyani, mahokkani-bailek (Thailand) [11,12].
2.4. Botanical Description and Distribution
The mahogany tree Swietenia macrophylla, also called “sky fruit” due to upward trend of its fruits towards the sky, is a beautiful, lofty, evergreen large tropical tree with a height of 30–40 m and girth of 3–4 m [13]. However, sometimes the height of this long-lived deciduous tree with an umbrella-shaped crown reaches a height of up to 50 m. S. macrophylla belongs to the Melicaceae family, with 50 genera and 1,400 species [14]. It grows natively throughout the tropical regions of the Americas, in particular Central and South American countries such as Mexico and Bolivia [15,16,17] and in west India, Malaysia, and southern China [18].
3. Ethnomedicinal Uses
Swietenia macrophylla has been used in Asia and many other countries to treat diverse ailments based on its antimicrobial, anti-inflammatory, antioxidant effects, antimutagenic, anticancer, antitumor and antidiabetic activities. Almost all parts of the plant are used in traditional medicine for the treatment of various human ailments. The fruit of S. macrophylla has been used commercially in health care products for the improvement of blood circulation and skin condition. The seed of S. macrophylla in particular has significant medicinal properties. In Malaysia, the seeds are used traditionally to treat hypertension, diabetes, and relieve pain [17]. The seeds of S. macrophylla have been reported to have anti-inflammatory, antimutagenicity and antitumor activity [19]. An Bolivian Amazonian ethnic group has used the seeds for leishmaniasis and as an abortion medicine [20]. In Indonesia, S. macrophylla seeds have been used as a folk medicine for treatment of diabetes, hypertension, and malaria [21]. The bark extract has been used as an astringent for wounds and used occasionally for tanning because of the rich red color if provides [22].
4. Phytochemistry
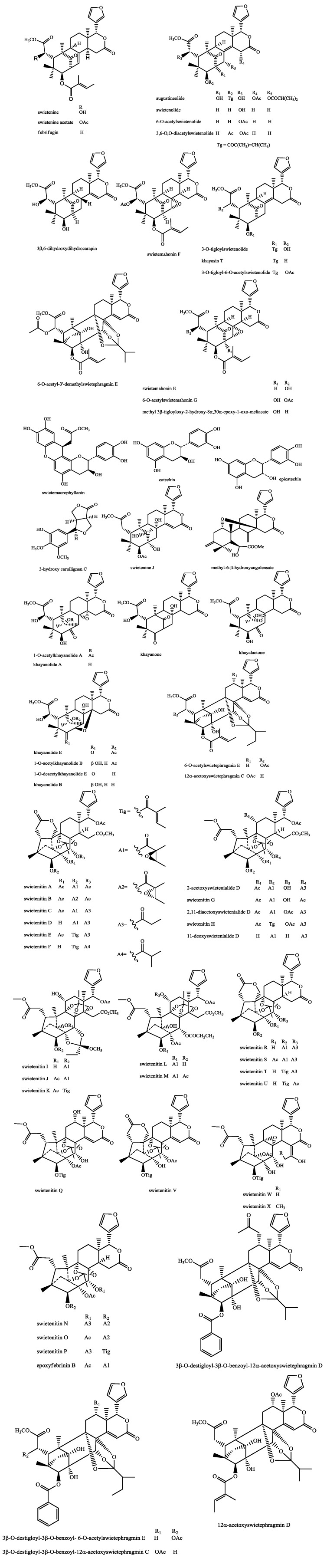
Phytochemical investigations have shown that limonoids and their derivatives are the major constituents of S. macrophylla [23,24,25]. Limonoids are derived from tetracyclic triterpenes similar to euphol (H-20β) or tirucallol (H-20α) by a series of oxidative changes, interspersed with molecular rearrangements. Tetranortriterpenoids with a 4,4,8-trimethyl-17-furanyl steroidal skeleton is an alternative name for limonoids because in the process of oxidative changes, the side chain is eventually oxidised to a β-substituted furan ring by the loss of four carbon atoms [26]. During the last few years, there has been an increasing trend and awareness in S. macrophylla research. Quite a significant amount of research has already been carried out during the past few decades in exploring the chemistry of different parts of S. macrophylla. A wide array of isolated pure compounds with a plethora of pharmacological activities has been identified from different parts of S. macrophylla, as summarized in Table 1. The chemical structures of the major compounds isolated from this plant are presented in Figure 1.
Table 1.
Compounds isolated from Swietenia macrophylla.
| Plant part used | Compounds | Class | References |
|---|---|---|---|
| Seed | swietenine | Limonoid | [27] |
| swietenolide | Limonoid | [23,28,29] | |
| 8,30-epoxyswietenine acetate | Limonoid | ||
| swietenine acetate | Limonoid | ||
| swielenolidetiglate | Limonoid | ||
| swietenolidediacetate | Limonoid | [30,31] | |
| augustineolide | Limonoid | ||
| 3β,6-dihydroxydihydrocarapin | Limonoid | [24] | |
| 7-deacetoxy-7-oxogedunin | Limonoid | ||
| andirobin | Limonoid | ||
| proceranolide | Limonoid | [32] | |
| 6-O-acetylswietenolide | Limonoid | [33,34] | |
| 3,6-O,O-diacetylswietenolide | Limonoid | [35] | |
| khayasin T | Limonoid | ||
| swietemahonin E | Limonoid | [36] | |
| swietemahonin F | Limonoid | [21] | |
| swietemahonin G | Limonoid | ||
| 2-hydroxyswietenine | Limonoid | ||
| 6-deoxyswietenine (febrifugin) | Limonoid | [37] | |
| methyl 3β-tigloyloxy-2,6-dihydroxy-1-oxo-meliac-8(30)-enate | Limonoid | [25] | |
| methyl 3β-tigloyloxy-2-hydroxy-1-oxo-meliac-8(30)-enate | Limonoid | ||
| methyl 3β-tigloyloxy-2-hydroxy-8α,30α-epoxy-1-oxo-meliacate | Limonoid | ||
| methyl 3β-acetoxy-2,6-dihydroxy-8α,30α-epoxy-1-oxo-meliacate | Limonoid | ||
| methyl 3β-isobutyryloxy-2,6-dihydroxy-8α,30α-epoxy-1-oxo-meliacate | Limonoid | [38] | |
| 6-O-acetyl-3′-demethylswietephragmin E | Limonoid | ||
| 3-O-tigloylswietenolide | Limonoid | ||
| 3-O-tigloyl-6-O-acetylswietenolide | Limonoid | [38,39] | |
| 6-O-acetylswietemahonin G | Limonoid | [38,40] | |
| scopoletin | Coumarin | [38,41] | |
| stearic acid methyl ester | Fatty acid ester | ||
| β-sitostenone | Steroid | [38,42] | |
| 3β-hydroxystigmast-5-en-7-one | Steroid | ||
| β-sitosterol | Steroid | [38,43] | |
| stigmasterol | Steroid | ||
| Bark | swietemacrophyllanin | Polyphenol | [22] |
| catechin | Polyphenol | [22,44] | |
| epicatechin | Polyphenol | [22,45,46] | |
| Leaves | swietenolide monohydrate | Limonoid | [47] |
| swietephragmin H | Limonoid | [48] | |
| swietephragmin I | Limonoid | ||
| swietephragmin J | Limonoid | ||
| swietemacrophine | Limonoid | ||
| γ-himachalene | Essential Oil | [49] | |
| germacrene D | Essential Oil | ||
| germacrene A | Essential Oil | ||
| cadina-1,4-diene | Essential Oil | ||
| hexadecanoic acid | Essential Oil | ||
| ethylhexadecanoate | Essential Oil | ||
| swietenine J | Limonoid | [50] | |
| methyl-6-β-hydroxyangolensate | Limonoid | [50,51] | |
| 1-O-acetylkhayanolide A | Limonoid | [50,52] | |
| khayanolide E | Limonoid | [50,53] | |
| khayalactone | Limonoid | [50,54] | |
| khayanone | Limonoid | ||
| 1-O-acetylkhayanolide B | Limonoid | [50,55] | |
| 1-O-deacetylkhayanolide E | Limonoid | ||
| khayanolide A | Limonoid | ||
| khayanolide B | Limonoid | ||
| 6-O-acetylswietephragmin E | Limonoid | [50,56] | |
| 3β-O-destigloyl-3β-O-benzoyl- 6-O-acetylswietephragmin E | Limonoid | ||
| 12α-acetoxyswietephragmin C | Limonoid | ||
| 3β-O-destigloyl-3β-O-benzoyl-12α-acetoxyswietephragmin C | Limonoid | ||
| 12α-acetoxyswietephragmin D | Limonoid | ||
| 3β-O-destigloyl-3β-O-benzoyl-12α-acetoxyswietephragmin D | Limonoid | ||
| Twig | swietenitin A | Limonoid | [57] |
| swietenitin B | Limonoid | ||
| swietenitin C | Limonoid | ||
| swietenitin D | Limonoid | ||
| swietenitin E | Limonoid | ||
| swietenitin F | Limonoid | ||
| swietenitin G | Limonoid | ||
| swietenitin H | Limonoid | ||
| swietenitin I | Limonoid | ||
| swietenitin J | Limonoid | ||
| swietenitin K | Limonoid | ||
| swietenitin L | Limonoid | ||
| swietenitin M | Limonoid | ||
| Twig | swietenitin N | Limonoid | [58] |
| swietenitin O | Limonoid | ||
| swietenitin P | Limonoid | ||
| swietenitin Q | Limonoid | ||
| swietenitin R | Limonoid | ||
| swietenitin S | Limonoid | ||
| swietenitin T | Limonoid | ||
| swietenitin U | Limonoid | ||
| swietenitin V | Limonoid | ||
| swietenitin W | Limonoid | ||
| swietenitin X | Limonoid | ||
| 2-acetoxyswietenialide D | Limonoid | ||
| 2,11-diacetoxyswietenialide D | Limonoid | ||
| 11-deoxyswietenialide D | Limonoid | ||
| epoxyfebrinin B | Limonoid | ||
| Stem | 3-hydroxycaruilignan C | Lignan | [59] |
Figure 1.
Chemical structures of various phytochemicals isolated from S. macrophylla.
5. Biological Activities
5.1. Antiviral Activity
A bioactive compound, 3-hydroxycaruilignan C (3-HCL-C) was isolated from the most active ethyl acetate fraction of S. macrophylla stems and the chemical structure was identified using 1D and 2D nuclear magnetic resonance spectroscopy and confirmed using mass spectrometry. 3-HCL-C showed anti-HCV (Hepatitis C virus) activity at either the protein or RNA level at nontoxic concentrations, with an EC50 value of 10.5 ± 1.2 µM. The suppression of HCV RNA replication was increased by the combinations of 3-HCL-C, interferon-α (IFN-α), and HCV NS5B polymerase inhibitor (2′-C-methylcytidine; NM-107) or HCV NS3/4A protease inhibitor (telaprevir; VX-950). By inducing IFN-stimulated response element transcription and IFN-dependent antiviral gene expression, 3-HCL-C interfered with HCV replication and through this pathway it was demonstrated to be a potential antiviral agent [59].
5.2. Anti-Inflammatory Activity
Treatment of mice with S. macrophylla ethanolic seed extract (1,000 mg/kg body weight) reduced carrageenan-induced inflammation by 79%. The hexane and methanol fractions of S. macrophylla ethanol extract exhibited lower efficacies of 23% and 60% inflammatory inhibition, respectively [19]. In 2010 Chen and coworkers [38] isolated seventeen compounds by chromatographic purification of the ethyl acetate-soluble fraction of the S. macrophylla fruit methanolic extract on a silica gel column.
An assay of the seventeen compounds on inhibition of superoxide anion generated by human neutrophils in response to fMet-Leu-Phe showed anti-inflammatory effects (IC50 ≤ 35.7 µM) for six limonoid compounds: 6-O-acetyl-3′-demethylswietephragmin E, 3,6-O,O-diacetylswietenolide, 3-O-tigloylswietenolide, 3-O-tigloyl-6-O-acetylswietenolide, swietemahonin E and 6-O-acetyl-swietemahonin G. The structure of the compounds was supported by 1H-1H COSY and NOESY experiments, and 13C-NMR assignments were confirmed by DEPT, HSQC, and HMBC techniques.
5.3. Anti-Infective Activity
Control of infections by Pseudomonas aeruginosa is critical and important due to the widespread antibiotic resistance reported for this pathogen [60]. The existence of anti-infective compounds in the seeds of S. macrophylla was proved by using a host-pathogen screening assay on Caenorhabditis elegans infected by Pseudomonas aeruginosa which is the common cause of nosocomial contamination in medical care facilities, leading to unwanted secondary infections in patients [61]. Although the ethyl acetate extract did not show any antibacterial activity in vitro, it enhanced the survival rate of the worms infected with P. aeruginosa. The extract boosted induction of gene pivotal for innate immunity of C. elegans such as defense gene lys-7 which encodes for a lysozyme-like antimicrobial factor [62].
5.4. Anticancer and Antitumor Activity
The antitumor activity of the ethanol extract of S. macrophylla seeds and its hexane and methanol fractions were investigated using the Epstein-Barr virus early-antigen (EBV_EA) activation, with 12-0-tetradecanoylphorbol-13-acetate (TPA) as the tumor promoter. The results revealed considerable inhibitory activity on EBV_EA activation, indicating an antitumor-promoting effect [19]. The cytotoxic activity of the crude ethanol extract of the seeds of S. macrophylla and its fractions was assessed against selected human cancer cell lines, namely, HCT116, KB, Ca Ski and MCF-7 by using MTT assay. The S. macrophylla ethyl acetate fraction (SMEAF) showed the most potent activity against HCT116 cell line (IC50 = 35.35 ± 0.50 μg/mL). The induction of apoptosis was confirmed both by DNA fragmentation using TUNEL assay and the externalization of phosphatidylserine using annexin V/PI staining. The cell cycle analysis revealed a prominent increase in sub-G1 population at concentrations of 0.05 mg/mL and above. Results also showed that SMEAF induced collapse of the mitochondrial membrane potential after 24 h and caused depletion in total intracellular glutathione [17].
5.5. Antimutagenic Activity
A micronucleus test was used for studying the antimutagenicity of the ethanol extract of S. macrophylla seeds. Treatment of mice with S. macrophylla ethanol seed extract (20 mg/kg body weight) reduced the number of micronucleated polychromatic erythrocytes induced by mitomycin C, a known mutagen, by roughly 50%. The result demonstrated a significant antimutagenicity of the S. macrophylla crude extract [19].
5.6. Antidiabetic Activity
Treatment of streptozotocin- and nicotinamide-induced type 2 diabetic rats with S. macrophylla seed methanol extract (300 mg/kg body weight) for 12 consecutive days reduced the fasting blood glucose level by 32.78% [63].The extract at the same dose also significantly reduced the elevated level of serum total cholesterol (18.56%) and triglyceride (10.41%), and increased the reduced liver glycogen level by 46.27% [63]. In oral glucose tolerance test (OGTT), the methanol extract of S. macrophylla seeds exhibited a 59.69% reduction in blood glucose level after 12 consecutive days of oral treatment with 300 mg/kg body weight demonstrating the potent antidiabetic activity of S. macrophylla [64].
Swietenine, a tetranortriterpenoid, was chromatographed from the ethanol soluble fraction of S. macrophylla seed extract by medium-pressure chromatography system employing gradient elution technique with hexane, chloroform (0–100%) and characterized by 1H- and 13C-NMR, MS and mp. This compound was found to possess considerable dose-dependent hypoglycemic effect in type 2 diabetic rats. Oral administration of swietenine (25 and 50 mg/kg body weight) significantly decreased fasting blood glucose level of type 2 diabetic rats in a dose-dependent manner. Treatment with swietenine significantly reduced the elevated cholesterol, triglyceride and liver glycogen level to the normal level in a dose-dependent manner when compared with non-diabetic control group [65]. Oral treatment with swietenine (25 and 50 mg/kg body weight) for 5 days reduced the fasting glucose level by 47.34 mg/dL and 55.85 mg/dL, respectively [65]. Oral administration of alcoholic seed extract of S. macrophylla and glibenclamide in streptozotocin-induced diabetic rats showed an improvement in body weight and decreased blood glucose level and the effect of S. macrophylla extract was more pronounced at 100 mg/kg body weight. Oral treatment with S. macrophylla extract and glibenclamide significantly increased the haemoglobin, glycosylated haemoglobin and serum insulin levels. The liver glycogen level in diabetic rats treated with S. macrophylla was elevated to the normal level obtained with glibenclamide [66]. Oral administration with the aqueous extract of S. macrophylla seeds (2 g/kg body weight) on streptozotocin-induced diabetic rats showed hypoglycaemic activity with a significant reduction of fasting plasma glucose by 98.66 ± 9.26 mg/dL [67].
5.7. Anti-Nociceptive Activity
Oral treatment of mice with ethanol and aqueous extracts of S. macrophylla fruits (200 mg/kg body weight) showed a reduction in the number of writhing, tail immersion and hot plate response exhibiting a powerful anti-nociceptive activity. Tail-flick and hot-plate responses at the dose of 200 mg/kg body weight also corroborated the considerable analgesic effect of the ethanol extract of S. macrophylla. The analgesic activity produced through a mechanism partially connected to either lipooxygenase and/or cyclooxygenase via the arachidonic acid cascade and/or opioid receptors producing analgesia in thermal and chemical pain models. The total results confirmed anti-nociceptive activity of S. macrophylla and substantiated its traditional consumption as pain killer [68].
5.8. Hypolipidemic Activity
Oral treatment with swietenine (25 and 50 mg/kg body weight) in neonatal-streptozotocin induced type 2 diabetic rats significantly reduced the elevated cholesterol and triglyceride level. After 5 days of treatment with swietenine (25 mg/kg body weight) the level of cholesterol and triglyceride was reduced by 17.25 mg/dL and 29.12 mg/dL, respectively. For 50 mg/kg of swietenine, this reduction increased to 24.35 mg/dL and 31.58 mg/dL, respectively [65]. The treatment with the methanol extract of S. macrophylla (300 mg/kg body weight) in streptozotocin- and nicotinamide-induced type 2 diabetic rats for 12 consecutive days caused reduction in the elevated level of serum total cholesterol (18.56%) and triglyceride (10.41%), respectively [63]. Under the same experimental condition the extract showed 45.41% and 37.78% reduction in cholesterol and triglyceride levels in streptozotocin-induced diabetic rats, respectively [64].
5.9. Antioxidant Activity
Three compounds, namely, catechin, epicatechin, and swietemacrophyllanin were isolated from the fractionation of the acetone extract of S. macrophylla bark with n-hexane, diethyl ether and ethyl acetate, followed by subsequent chromatographic separation of the fractions. The structure of the compounds was elucidated by spectroscopic data and by comparison of the NMR data with those of catiguanins A and B, phenylpropanoid-substituted epicatechins [22]. These compounds from the polyphenols or flavan-3-ols class exhibited antioxidant activity using the DPPH [1,1-diphenyl-2-picrylhydrazyl] free radical scavenging assay. The study showed that swietemacrophyllanin had the strongest antioxidant activity, with an IC50 value of 56 µg/mL compared with Trolox used as a reference. The ethanol extract of S. macrophylla seeds also showed antioxidant activity in the streptozotocin-induced diabetic rats. Antioxidants such as vitamins C and E levels in the plasma, and reduced glutathione level in the plasma, kidney, and liver increased in rats treated with the extract [69].
5.10. Antimicrobial Activity
The antibacterial and antifungal efficacy of the methanol and aqueous extracts of S. macrophylla seeds was evaluated on selected pathogenic bacterial and fungal strains by disc diffusion and micro dilution assay methods. The methanol and aqueous extract of S. macrophylla exhibited activity against Pseudomonas aeruginosa MTCC 424, Klebsiella pneumonia MTCC 109, Bacillus cereus MTCC 430, Staphylococcus aureus MTCC 96, and Escherichia coli MTCC 443 [70]. The extracts showed antifungal activity against Candida albicans MTCC 183, Aspergillus niger MTCC 16404, Cryptococcus albidus MTCC 2661, and Aspergillus flavus MTCC 1973. E. coli MTCC 443 and C. albidus MTCC 2661 were found to be the most susceptible bacteria and fungi, respectively. The methanol extract demonstrated greater efficacy than aqueous extract of S. macrophylla. The results showed that the seeds of S. macrophylla possessed marked antibacterial and antifungal activity [70].
5.11. Antidiarrhoeal Activity
The petroleum ether extract of S. macrophylla seeds was investigated for antidiarrhoeal effect in Wister albino rats and the results showed a significant antidiarrhoeal activity proved by the reduction in the rate of defecation and consistency of faeces in castor oil induced diarrhoea rats at various extract doses of 25, 50 and 100 mg/kg body weight. A reduction of 4.45% to 34.60% in intestinal transit and significant inhibition of castor oil induced enterpooling exhibited profound anti-diarrhoea activity of the extract. The in vivo study revealed that seeds of S. macrophylla may be a source of antidiarrhoeal drug in the future [71].
5.12. Antimalarial Activity
A decoction of S. macrophylla seeds was reported as an antimalarial remedy [21]. The antimalarial effect of the S. macrophylla seed methanol extract was investigated against Plasmodium falciparum [72]. The bark extract of S. macrophylla showed strong antimalarial activity (78% inhibition at 100 µg/mL) in vitro on chloroquine resistant P. falciparum strain (Indo). The in vivo study of the bark extract at 250 mg/kg body weight exhibited 73% inhibition on the rodent malaria P. vinckeipetteri 279BY [73]. The aqueous extract of S. macrophylla seeds showed antibabesial activity besides the antimalarial activity against P. falciparum [74].
5.13. Antifeedant Activity
In an antifeedant study, four out of fifteen limonoids isolated from the acetone extract of S. macrophylla fruits washed with petroleum ether (60–80 °C) and triturated with ethyl acetate for silica gel column chromatography using CHCl3 with increasing amount of ethyl acetate, showed good antifeedant activity against Spodoptera frugiperda. Swietenolide, 6-O-acetylswietenolide, 3,6-O,O-diacetylswietenolide, and swietemahonin F were subjected to a bioassay on the final instar larvae of S. frugiperda at the concentration of 1,000 ppm. The results revealed that swietenolide exerted the greatest antifeedant activity (antifeedant index of 94.1 ± 2.90). The other compounds; 6-O-acetylswietenolide, 3,6-O,O-diacetylswietenolide, and swietemahonin F, exhibited antifeedant indices of 72.2 ± 19.60, 72.0 ± 9.38, and 70.2 ± 8.90, respectively. The compounds were identified by detailed analysis of their respective high resolution 1H- and 13C-NMR spectra including COSY, HETCOR and HMBC correlations [32].
5.14. Acaricidal Activity
Varroatosis is a disease caused by Varroa destructor mites (Acari: Varroidae) which has become a serious pest problem for honeybees, Apis mellifera and Apis cerana (Hymenoptera: Apidae) worldwide. The mites cause damage to immature and adult bees by feeding on hemolymph, thus, greatly weakening or killing the bees [75]. The ethanol extract of the stem bark and leaves of S. macrophylla showed acaricidal activity in the honeybee colonies infested by V. destructor mites. The rate of infestation after 12 days of treatment with 500 ppm of S. macrophylla bark and leaf extract decreased to 1.08% and 2.41%, respectively. These extracts, which proved to be safe to the environment and harmless to the bees, are promising as safe natural products for the control of Varroa mites [76].
5.15. Heavy Metal Phytoremediation Activity
In the study on the phytoremediation potential of S. macrophylla, hydroponic experiments were performed with cadmium concentration gradients at concentrations of 0, 7.5, 15, and 30 mg/L to identify cadmium accumulation and tolerance of S. macrophylla seedlings as well as their potential for phytoextraction. The results indicated that cadmium inhibited S. macrophylla seedling growth at the highest exposure concentration of 30 mg/L and demonstrated great potential for phytoextraction at concentrations of 7.5 and 15 mg/L. S. macrophylla seedlings accumulated up to 154 mg/L cadmium in twigs at the concentration of 15 mg/L. These results suggest that S. macrophylla can be used as a potential candidate for remediating cadmium contaminated sites in tropical regions, because of cadmium uptake capacity and high biomass production of mahogany shoots [77,78].
6. Conclusions
Besides being a good source for timber products and reforestation programmes in the tropics, S. macrophylla exhibits a wide spectrum of significant biological activities. A review of the phytochemistry of S. macrophylla has revealed a large number of limonoids, the principle bioactive compounds, which have not been investigated for their bioactivities. In view of the medicinal importance of S. macrophylla greater attention is warranted towards the discovery of their potential pharmaceutical uses.
Acknowledgments
The authors would like to thank the University of Malaya for providing the research grant (RP001-2012C) and PPP grant (PV022-2011B). The generosity of Chan Gomathi in providing the technical support is also greatly appreciated.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Jothy S.L., Torey A., Darah I., Choong Y.S., Saravanan D., Chen Y., Latha L.Y., Deivanai S., Sasidharan S. Cassia spectabilis (DC) Irwin et Barn: A promising traditional herb in health improvement. Molecules. 2012;17:10292–10305. doi: 10.3390/molecules170910292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazumder P.M., Percha V., Farswan M., Upaganlawar A. Cassia: A wonder gift to medical sciences. Int. J. Clin. Pharm. 2008;1:16–38. [Google Scholar]
- 3.Duraipandiyan V., Ayyanar M., Ignacimuthu S. Antimicrobial activity of some ethno medicinal plants used by Paliyar tribe from Tamil Nadu, India. BMC Compl. Altern. Med. 2006;6:e35. doi: 10.1186/1472-6882-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemes M.R., Gribel R., Proctor J., Grattapaglia D. Population genetic structure of mahogany (Swietenia macrophylla King, meliaceae) across the Brazilian Amazon, based on variation at microsatellite loci: Implications for conservation. Mol. Ecol. 2003;12:2875–2883. doi: 10.1046/j.1365-294X.2003.01950.x. [DOI] [PubMed] [Google Scholar]
- 5.Newton A., Baker P., Ramnarine S., Mesen J., Leakey R. The mahogany shoot borer: Prospects for control. Forest Ecol. Manag. 1993;57:301–328. doi: 10.1016/0378-1127(93)90179-Q. [DOI] [Google Scholar]
- 6.Perez J., Eigenbrode S.D., Hilje L., Tripepi R.R., Aguilar M.E., Mesen F. Use of grafting to prevent Hypsipyla grandella (Zeller)(Lepidoptera: Pyralidae) damage to new world meliaceae species. Neotrop. Entomol. 2010;39:618–625. doi: 10.1590/S1519-566X2010000400024. [DOI] [PubMed] [Google Scholar]
- 7.Degen B., Ward S., Lemes M., Navarro C., Cavers S., Sebbenn A. Verifying the geographic origin of mahogany (Swietenia macrophylla King) with DNA-fingerprints. Forensic. Sci. Int. Gen. 2013;7:55–62. doi: 10.1016/j.fsigen.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Lemes M.R., Esashika T., Gaoue O.G. Microsatellites for mahoganies: Twelve new loci for Swietenia macrophylla and its high transferability to Khaya senegalensis. Am. J. Bot. 2011;98:e207–e209. doi: 10.3732/ajb.1100074. [DOI] [PubMed] [Google Scholar]
- 9.Paiva E.A. Anatomy, ultrastructure, and secretory activity of the floral nectaries in Swietenia macrophylla (meliaceae) Am. J. Bot. 2012;99:1910–1917. doi: 10.3732/ajb.1200122. [DOI] [PubMed] [Google Scholar]
- 10.Grogan J., Barreto P. Big-leaf mahogany on cites appendix II: Big challenge, big opportunity. Conserv. Biol. 2005;19:973–976. [Google Scholar]
- 11.AgroForestry Tree Datadase. A Tree Species Reference and Selection Guide. [(accessed on 1 May 2013)]. Available online: http://www.worldagroforestry.org.
- 12.Soerianegara I., Lemmens R. Plant Resources of South-East Asia No. 5(1) Timber Trees: Major Commercial Timbers. Centre for Agricultural Publishing and Documentation (PUDOC); 1993. p. 610. [Google Scholar]
- 13.Rastogi R.P., Mehrotra B. Compendium of Indian Medicinal Plants. Central Drug Research Institute; New Delhi, India: 1990. p. 397. [Google Scholar]
- 14.Arumugasamy K., Latha K. Studies on some pharmacognostic profiles of Swietenia macrophylla. King. Anc. Sci. Life. 2004;24:97–102. [PMC free article] [PubMed] [Google Scholar]
- 15.Pennington T.D. Mahogany carving a future. Biologist (London) 2002;49:204–208. [PubMed] [Google Scholar]
- 16.Paiva É.A.S., Lemos-Filho J.P., Oliveira D.M.T. Imbibition of Swietenia macrophylla (Meliaceae) seeds: The role of stomata. Ann. Bot. 2006;98:213–217. doi: 10.1093/aob/mcl090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goh B.H., Kadir A. In vitro cytotoxic potential of Swietenia macrophylla King seeds against human carcinoma cell lines. J. Med. Plants Res. 2011;5:1395–1404. [Google Scholar]
- 18.Mulholland D.A., Parel B., Coombes P.H. The chemistry of the Meliaceae and Ptaeroxylaceae of Southern and Eastern Africa and Madagascar. Curr. Org. Chem. 2000;4:1011–1054. doi: 10.2174/1385272003375941. [DOI] [Google Scholar]
- 19.Guevara A., Apilado A., Sakurai H., Kozuka M., Takuda H. Anti-inflammatory, antimutagenicity, and antitumor-promoting activities of mahogany seeds, Swietenia macrophylla (Meliacea) Philipp. J. Sci. 1996;125:271–277. [Google Scholar]
- 20.Bourdy G., DeWalt S., Chávez de Michel L., Roca A., Deharo E., Muñoz V., Balderrama L., Quenevo C., Gimenez A. Medicinal plants uses of the Tacana, an Amazonian Bolivian ethnic group. J. Ethnopharmacol. 2000;70:87–109. doi: 10.1016/S0378-8741(99)00158-0. [DOI] [PubMed] [Google Scholar]
- 21.Kadota S., Marpaung L., Kikuchi T., Ekimoto H. Constituents of the seeds of Swietenia mahagoni Jacq. II. Structures of swietemahonin A, B, C, D, E, F, and G and swietemahonolide. Chem. Pharm. Bull. 1990;38:894–901. doi: 10.1248/cpb.38.894. [DOI] [Google Scholar]
- 22.Falah S., Suzuki T., Katayama T. Chemical constituents from Swietenia macrophylla bark and their antioxidant activity. Pak. J. Biol. Sci. 2008;11:2007–2012. doi: 10.3923/pjbs.2008.2007.2012. [DOI] [PubMed] [Google Scholar]
- 23.Connolly J.D., Labbé C. Tetranortriterpenoids and related compounds. Part 24. The interrelation of swietenine and swietenolide, the major tetranortriterpenoids from the seeds of Swietenia macrophylla (meliaceae) J. Chem. Soc. Perkin Trans. 1. 1998:529–530. [Google Scholar]
- 24.Taylor A.R., Taylor D.A. Limonoid extractives from Swietenia macrophylla. Phytochemistry. 1983;22:2870–2871. doi: 10.1016/S0031-9422(00)97721-5. [DOI] [Google Scholar]
- 25.Kojima K., Isaka K., Ogihara Y. Tetranortriterpenoids from Swietenia macrophylla. Chem. Pharm. Bull. 1998;46:523–525. doi: 10.1248/cpb.46.523. [DOI] [Google Scholar]
- 26.Kipassa N.T., Iwagawa T., Okamura H., Doe M., Morimoto Y., Nakatani M. Limonoids from the stem bark of Cedrela odorata. Phytochemistry. 2008;69:1782–1787. doi: 10.1016/j.phytochem.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Solomon K.A., Malathi R., Rajan S., Narasimhan S., Nethaji M. Swietenine. Acta Crystallogr. E. 2003;59:o1519–o1521. doi: 10.1107/S1600536803020476. [DOI] [Google Scholar]
- 28.Connolly J., McCrindle R., Overton K., Warnock W. Swietenolide. Tetrahedron Lett. 1965;6:2937–2940. doi: 10.1016/S0040-4039(00)90240-5. [DOI] [Google Scholar]
- 29.Chakrabartty T., Connolly J., McCrindle R., Overton K., Schwarz J. Tetranortriterpenoids—VI:[Bicyclononanolides VI] Swietenolide—the functional groups. Tetrahedron. 1968;24:1503–1506. doi: 10.1016/0040-4020(68)88103-7. [DOI] [Google Scholar]
- 30.Chan K., Tang T., Toh H. Isolation of swietenolide diacetate from Swietenia macrophylla. Phytochemistry. 1976;15:429–430. doi: 10.1016/S0031-9422(00)86843-0. [DOI] [Google Scholar]
- 31.Goh B.H., Abdul Kadir H., Abdul Malek S., Ng S.W. Swietenolide diacetate from the seeds of Swietenia macrophylla. Acta Crystallogr. E. 2010;66:o1396–o1396. doi: 10.1107/S1600536810017733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mootoo B.S., Ali A., Motilal R., Pingal R., Ramlal A., Khan A., Reynolds W.F., McLean S. Limonoids from Swietenia macrophylla and S. Aubrevilleana. J. Nat. Prod. 1999;62:1514–1517. doi: 10.1021/np990199x. [DOI] [PubMed] [Google Scholar]
- 33.Ollis W., Ward A., de Oliveira H.M., Zelnik R. Andirobin. Tetrahedron. 1970;26:1637–1645. doi: 10.1016/S0040-4020(01)93014-5. [DOI] [Google Scholar]
- 34.Goh B.H., Kadir H.A., Malek S.N.A., Ng S.W. (αR,4R,4aR,6aS,7R,8S,10R,11S)-Methylα-acetoxy-4-(3-furanyl)-10-hydroxy-4a,7,9,9-tetramethyl-2,13-dioxo-1,4,4a,5,6,6a,7,8,9,10,11,12-dodecahydro-7,11-methano-2H-cycloocta[f][2]benzopyran-8 acetate(6-O-acetylswietenolide) from the seeds of Swietenia macrophylla. Acta Crystallogr. E. 2010;E63:o2802–o2803. doi: 10.1107/S1600536810039942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fowles R.G., Mootoo B., Ramsewak R., Reynolds W., Lough A. 3, 6-di-O-acetylswietenolide 0.25-hydrate. Acta Crystallogr. E. 2007;63:o660–o661. doi: 10.1107/S1600536806054432. [DOI] [Google Scholar]
- 36.Kadota S., Marpaung L., Kikuchi T., Ekimoto H. Constituents of the seeds of Swietenia mahagoni JACQ. I, Isolation, structures, and 1H- and 13C-nuclear magnetic resonance signal assignments of new tetranortriterpenoids related to swietenine and swietenolide. Chem. Pharm. Bull. 1990;38:639–651. doi: 10.1248/cpb.38.639. [DOI] [Google Scholar]
- 37.Govindachari T., Suresh G., Banumathy B., Masilamani S., Gopalakrishnan G., Kumari G.K. Antifungal activity of some B, D-secolimonoids from two meliaceous plants. J. Chem. Ecol. 1999;25:923–933. doi: 10.1023/A:1020809204288. [DOI] [Google Scholar]
- 38.Chen J.-J., Huang S.-S., Liao C.-H., Wei D.-C., Sung P.-J., Wang T.-C., Cheng M.-J. A new phragmalin-type limonoid and anti-inflammatory constituents from the fruits of Swietenia macrophylla. Food Chem. 2010;120:379–384. doi: 10.1016/j.foodchem.2009.09.093. [DOI] [Google Scholar]
- 39.Chen J.-J., Huang S.-Y., Duh C.-Y., Chen I.-S., Wang T.-C., Fang H.-Y. A new cytotoxic amide from the stem wood of Hibiscus tiliaceus. Planta Med. 2006;72:935–938. doi: 10.1055/s-2006-931604. [DOI] [PubMed] [Google Scholar]
- 40.Sy L.-K., Brown G.D., Haynes R. A novel endoperoxide and related sesquiterpenes from Artemisia annua which are possibly derived from allylic hydroperoxides. Tetrahedron. 1998;54:4345–4356. [Google Scholar]
- 41.Chen J.-J., Chou T.-H., Peng C.-F., Chen I.-S., Yang S.-Z. Antitubercular dihydroagarofuranoid sesquiterpenes from the roots of Microtropis fokienensis. J. Nat. Prod. 2007;70:202–205. doi: 10.1021/np060500r. [DOI] [PubMed] [Google Scholar]
- 42.Chen J.-J., Cho J.-Y., Hwang T.-L., Chen I.-S. Benzoic acid derivatives, acetophenones, and anti-inflammatory constituents from Melicope semecarpifolia. J. Nat. Prod. 2007;71:71–75. doi: 10.1021/np0704349. [DOI] [PubMed] [Google Scholar]
- 43.Chen J.-J., Lin W.-J., Liao C.-H., Shieh P.-C. Anti-inflammatory benzenoids from Antrodia camphorata. J. Nat. Prod. 2007;70:989–992. doi: 10.1021/np070045e. [DOI] [PubMed] [Google Scholar]
- 44.Davis A.L., Cai Y., Davies A.P., Lewis J. 1H and 13C-NMR assignments of some green tea polyphenols. Magn. Reson. Chem. 1996;34:887–890. doi: 10.1002/(SICI)1097-458X(199611)34:11<887::AID-OMR995>3.0.CO;2-U. [DOI] [Google Scholar]
- 45.Sun J., Jiang Y., Wei X., Shi J., You Y., Liu H., Kakuda Y., Zhao M. Identification of (−)-epicatechin as the direct substrate for polyphenol oxidase isolated from litchi pericarp. Food Res. Int. 2006;39:864–870. doi: 10.1016/j.foodres.2006.05.001. [DOI] [Google Scholar]
- 46.Fan P., Lou H., Yu W., Ren D., Ma B., Ji M. Novel flavanol derivatives from grape seeds. Tetrahedron Lett. 2004;45:3163–3166. [Google Scholar]
- 47.Tan S.-K., Osman H., Wong K.-C., Fun H.-K., Chantrapromma S. Swietenolide monohydrate. Acta Crystallogr. E. 2008;64:o1267–o1268. doi: 10.1107/S1600536808017431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tan S.-K., Osman H., Wong K.-C., Boey P.-L. New phragmalin-type limonoids from Swietenia macrophylla king. Food Chem. 2009;115:1279–1285. doi: 10.1016/j.foodchem.2009.01.043. [DOI] [Google Scholar]
- 49.Soares M.G., Batista-Pereira L.G., Fernandes J.B., Corrêa A.G., da Silva M.F.G., Vieira P.C., Rodrigues Filho E., Ohashi O.S. Electrophysiological responses of female and male Hypsipyla grandella (zeller) to Swietenia macrophylla essential oils. J. Chem. Ecol. 2003;29:2143–2151. doi: 10.1023/A:1025694720727. [DOI] [PubMed] [Google Scholar]
- 50.Liu J.-Q., Wang C.-F., Chen J.-C., Qiu M.-H. Limonoids from the leaves of Swietenia macrophylla. Nat. Prod. Res. 2012;26:1887–1891. doi: 10.1080/14786419.2011.625499. [DOI] [PubMed] [Google Scholar]
- 51.Narender T., Khaliq T. Shweta; 13C-NMR spectroscopy of D and B, D-ring seco-limonoids of Meliaceae family. Nat. Prod. Res. 2008;22:763–800. doi: 10.1080/14786410701628812. [DOI] [PubMed] [Google Scholar]
- 52.Nakatani M., Abdelgaleil S.A., Saad M.M., Huang R.C., Doe M., Iwagawa T. Phragmalin limonoids from Chukrasia tabularis. Phytochemistry. 2004;65:2833–2841. doi: 10.1016/j.phytochem.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 53.Olmo L.R., da Silva M., Rodrigues Fo E., Vieira P.C., Fernandes J.B., Pinheiro A.L., Vilela E.F. Limonoids from leaves of Khaya senegalensis. Phytochemistry. 1997;44:1157–1161. doi: 10.1016/S0031-9422(96)00571-7. [DOI] [Google Scholar]
- 54.Tchuendem M.-H., FoyereAyafor J., Connolly J.D., Sterner O. Khayalactone, a novel limonoid from Khaya grandifoliola. Tetrahedron Lett. 1998;39:719–722. [Google Scholar]
- 55.Olmo L.R., Silva M., Rodrigues Fo E., Vieira P.C., Fernandes J.B., Marsaioli A.J., Pinheiro A.L., Vilela E.F. Rearranged limonoids from Khaya senegalensis. Phytochemistry. 1996;42:831–837. doi: 10.1016/0031-9422(95)00093-3. [DOI] [Google Scholar]
- 56.da Silva M.N., Arruda M.S.P., Castro K.C.F., da Silva M.F.d.G., Fernandes J.B., Vieira P.C. Limonoids of the phragmalin type from Swietenia macrophylla and their chemotaxonomic significance. J. Nat. Prod. 2008;71:1983–1987. doi: 10.1021/np800312h. [DOI] [PubMed] [Google Scholar]
- 57.Lin B.-D., Zhang C.-R., Yang S.-P., Zhang S., Wu Y., Yue J.-M. D-ring-opened phragmalin-type limonoid orthoesters from the twigs of Swietenia macrophylla. J. Nat. Prod. 2009;72:1305–1313. doi: 10.1021/np900139c. [DOI] [PubMed] [Google Scholar]
- 58.Lin B.-D., Zhang C.-R., Yang S.-P., Wu Y., Yue J.-M. Phragmalin-type limonoid orthoesters from the twigs of Swietenia macrophylla. Chem. Pharm. Bull. 2011;59:458–465. doi: 10.1248/cpb.59.458. [DOI] [PubMed] [Google Scholar]
- 59.Wu S.F., Lin C.K., Chuang Y.S., Chang F.R., Tseng C.K., Wu Y.C., Lee J.C. Anti-hepatitis C virus activity of 3-hydroxy caruilignan C from Swietenia macrophylla stems. J. Viral Hepat. 2012;19:364–370. doi: 10.1111/j.1365-2893.2011.01558.x. [DOI] [PubMed] [Google Scholar]
- 60.Bodey G.P., Bolivar R., Fainstein V., Jadeja L. Infections caused by Pseudomonas aeruginosa. Clin. Infect. Dis. 1983;5:279–313. doi: 10.1093/clinids/5.2.279. [DOI] [PubMed] [Google Scholar]
- 61.Richards M.J., Edwards J.R., Culver D.H., Gaynes R.P. Nosocomial infections in medical intensive care units in the united states. Crit. Care Med. 1999;27:887–892. doi: 10.1097/00003246-199905000-00020. [DOI] [PubMed] [Google Scholar]
- 62.Dharmalingam K., Tan B.-K., Mahmud M.Z., Sedek S.A.M., Majid M.I.A., Kuah M.-K., Sulaiman S.F., Ooi K.L., Khan N.A.K., Muhammad T.S.T. Swietenia macrophylla extract promotes the ability of Caenorhabditis elegans to survive Pseudomonas aeruginosa infection. J. Ethnopharmacol. 2012;139:657–663. doi: 10.1016/j.jep.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 63.Maiti A., Dewanjee S., Jana G., Mandal S.C. Hypoglycemic effect of Swietenia macrophylla seeds against type II diabetes. Int. J. Green Pharm. 2008;2:224–227. doi: 10.4103/0973-8258.44738. [DOI] [Google Scholar]
- 64.Maiti A., Dewanjee S., Kundu M., Mandal S.C. Evaluation of antidiabetic activity of the seeds of Swietenia macrophylla in diabetic rats. Pharm. Biol. 2009;47:132–136. doi: 10.1080/13880200802436703. [DOI] [Google Scholar]
- 65.Dewanjee S., Maiti A., Das A.K., Mandal S.C., Dey S.P. Swietenine: A potential oral hypoglycemic from Swietenia macrophylla seed. Fitoterapia. 2009;80:249–251. doi: 10.1016/j.fitote.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 66.Kalaivanan K., Pugalendi K.V. Antihyperglycemic effect of the alcoholic seed extract of Swietenia macrophylla on streptozotocin-diabetic rats. Pharmacognosy Res. 2011;3:67–71. doi: 10.4103/0974-8490.79119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Biswas U.K., Chakraborty R., Banerjee P., Maji D., Mondal M.C., Raychaudhuri U. Antidiabetic and antioxidant effect of Swietenia macrophylla seeds in experimental type 2 diabetic rats. Int. J. Diabetes Dev. Ctries. 2013;33:60–65. doi: 10.1007/s13410-012-0109-8. [DOI] [Google Scholar]
- 68.Das A., JebaSunilson J., Gopinath R., Radhamani S., Nilugal K. Anti-nociceptive activity of the fruits of Swietenia macrophylla king. J. Pharm. Res. 2009;2:1367–1369. [Google Scholar]
- 69.Kalpana K., Pugalendi K.V. Antioxidative and hypolipidemic efficacy of alcoholic seed extract of Swietenia macrophylla in streptozotocin diabetic rats. J. Basic Clin. Physiol. Pharmacol. 2011;22:11–21. doi: 10.1515/jbcpp.2011.001. [DOI] [PubMed] [Google Scholar]
- 70.Maiti A., Dewanjee S., Mandal S.C., Annadurai S. Exploration of antimicrobial potential of methanol and water extract of seeds of Swietenia macrophylla (family: Meliaceae), to substantiate folklore claim. Iran. J. Pharm. Therap. 2007;6:99–102. [Google Scholar]
- 71.Maiti A., Dewanjee S., Mandal S.C. In vivo evaluation of antidiarrhoeal activity of the seed of Swietenia macrophylla king (meliaceae) Trop. J. Pharm. Res. 2007;6:711–716. [Google Scholar]
- 72.Soediro I., Padmawinata K., Wattimena J.R., Rekita S. Study of the active antimalarial methanolic extract of Swietenia macrophylla king (meliaceae) Acta Pharm. Indones. 1990;15:1–13. [Google Scholar]
- 73.Munoz V., Sauvain M., Bourdy G., Callapa J., Rojas I., Vargas L., Tae A., Deharo E. The search for natural bioactive compounds through a multidisciplinary approach in Bolivia. Part II. Antimalarial activity of some plants used by Mosetene Indians. J. Ethnopharmacol. 2000;69:139–155. doi: 10.1016/S0378-8741(99)00096-3. [DOI] [PubMed] [Google Scholar]
- 74.Murnigsih T., Matsuura H., Takahashi K., Yamasaki M., Yamato O., Maede Y., Katakura K., Susuki M., Kobayashi S., Yoshihara T. Evaluation of the inhibitory activities of the extracts of Indonesian traditional medicinal plants against Plasmodium falciparum and Babesia gibsoni. J. Vet. Med. Sci. 2005;67:829–831. doi: 10.1292/jvms.67.829. [DOI] [PubMed] [Google Scholar]
- 75.Weinberg K.P., Madel G. The influence of the mite Varroa jacobsoni oud. On the protein concentration and the haemolymph of the brood of worker bees and drones of the honey bee Apis mellifera I. Apidologie. 1985;16:421–436. doi: 10.1051/apido:19850407. [DOI] [Google Scholar]
- 76.El Zalabani S.M., El-Askary H.I., Mousa O.M., Issa M.Y., Zaitoun A.A., Abdel-Sattar E. Acaricidal activity of Swietenia mahogani and Swietenia macrophylla ethanolic extracts against Varroa destructorin honeybee colonies. Exp. Parasitol. 2012;130:166–170. doi: 10.1016/j.exppara.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 77.Fan K.-C., Hsi H.-C., Chen C.-W., Lee H.-L., Hseu Z.-Y. Cadmium accumulation and tolerance of mahogany (Swietenia macrophylla) seedlings for phytoextraction applications. J. Environ. Manag. 2011;92:2818–2822. doi: 10.1016/j.jenvman.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 78.Ahmadpour P., Ahmadpour F., Mahmud T., Abdu A., Soleimani M., Tayefeh F.H. Phytoremediation of heavy metals: A green technology. Afr. J. Biotechnol. 2012;11:14036–14043. [Google Scholar]