Abstract
The palladium (II)-catalysed reactions of alkenols and aminoalkenols such as oxycarbonylations or bicyclisations are powerful methods for the construction of oxygen and nitrogen-containing heterocyclic compounds. This review highlights recent progress in the development of the asymmetric palladium(II)-catalysed Wacker-type cyclisations of unsaturated polyols and aminoalcohols. The scope, limitations, and applications of these reactions are presented.
Keywords: palladium, asymmetric catalysis, Wacker-type reaction, heterocyclisation, oxycarbonylation, lactonisation, chiral ligands
1. Introduction
Palladium-catalysed functionalisations of alkenes have become a powerful tool in organic synthesis [1,2,3,4]. Today, there are numerous applications of these transformations in the preparation of a large array of the useful products. Among them, an intramolecular oxidative cyclisation, referred to as the Wacker-type cyclisation, is one of the most versatile methods for the preparation of heterocycles [5,6,7]. Particularly, palladium(II)-catalysed reactions of unsaturated alcohols and amino alcohols such as oxy-/aminocarbonylations [8,9,10,11,12,13,14,15], bicyclisations [16,17,18] and domino cyclisation-cross couplings [19,20,21,22,23] serve as a potent stereoselective methods for the construction of oxa-/azaheterocyclic structures found in many natural or biologically relevant compounds [19,20,21,24,25]. The key intermediate of these domino transformations is an alkyl-σ-Pd(II) complex A, which is trapped by carbon monoxide and/or by cyclisation with a second hydroxyl function specifically placed in the substrate to give bicyclic products B, C (Scheme 1). Trapping of the intermediate A by other nucleophilic species provides oxa/azaheterocyclic compounds D linked with various substituents. Although a number of reviews on palladium-catalysed oxidative cyclisation and issues of stereochemical control already exist [3,4,5,6,7,22,23,24,26,27,28,29,30,31,32,33,34], no general summary on the asymmetric versions of the Pd(II)-catalysed Wacker-type cyclisation of unsaturated alcohols and aminoalcohols has been published. Considering the great potential for application of these methods to the synthesis of biologically active targets and for the extension to the synthesis of optically pure saturated heterocycles, we report here a summary of the main achievements in this area.
Scheme 1.
Palladium-catalysed intramolecular Wacker-type cyclisations of unsaturated alcohols and amino alcohols.
2. Asymmetric Wacker-Type Oxidative Heterocyclisations
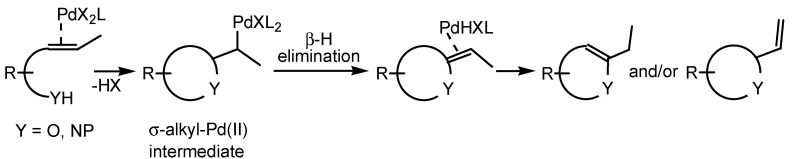
The intramolecular Wacker-type cyclisation using oxygen or nitrogen nucleophiles is one of the most important processes for the preparation of O- and N-heterocycles [4,5,6]. The palladium(II) coordinates to the alkene C−C double bond and activates it towards nucleophilic attack. Subsequent β-hydride elimination leads to the cyclised product in its thermodynamically stable form (Scheme 2).
Scheme 2.
Pd(II)-catalysed Wacker-type oxidative heterocyclisations.
2.1. Oxidative Wacker Cyclisation of o-Allylphenols
Compared to the impressive development of asymmetric reactions with chiral palladium(0) catalysts, asymmetric oxidative reactions with palladium(II) species have received only scant attention. The first attempts to accomplish an asymmetric version of Wacker-type cyclisation were described by Hosokawa and Murahashi [35,36]. However, the truly effective ligands for this transformation were developed by Uozomi and Hayashi [37,38,39]. 2-(2,3-Dimethylbut-2-enyl)phenol (1a) was cyclised to the corresponding dihydrobenzofuran (S)-2a using (S,S)-boxax {(S,S)-2,2′-bis[4-(alkyl)oxazolyl]-1,1′-binaphthyl} ligands in the presence of p-benzoquinone in methanol (Scheme 3). The best selectivity and efficiency gave (S,S)-iPr-boxax, the cyclised product was formed with 96% ee in 75% yield. It is noteworthy that the diastereomeric isomer (R,S)-iPr-boxax (R1 = H, R2 = iPr) was much less active and less enantioselective (18% ee, 3% yield).
Scheme 3.
AsymmetricPd(II)-catalysed Wacker-type cyclisation of allylphenol 1a using (S,S)-boxax ligands.
Another significant feature of this transformation is the strong dependence of catalytic activity on the nature of the anionic ligands attached to the palladium. The reaction of 1a was much faster with the palladium catalyst generated from palladium bis(trifluoroacetate) than that from palladium diacetate or dichlorobis(acetonitrile)palladium. Furthermore, this reaction was not catalysed by chloride complex PdCl2{(S,S)-iPr-boxax} at all. Thus, it was expected that a cationic palladium/boxax complex was generated as the active species by dissociation of palladium bis(trifluoroacetate) to the relatively stable trifluoroacetate anion in polar solvent. Indeed, a cationic palladium(II)/boxax species generated by addition of 2 equiv. of (S,S)-iPr-boxax to Pd(CH3CN)4(BF4)2 was found to be catalytically much more active than the Pd(OCOCF3)2{(S,S)-iPr-boxax} complex. The reaction of 1a in the presence of mentioned cationic species was complete in 50 min, giving 91% yield of (S)-2a with 97% ee. Generation of cationic species by abstraction of chloride from PdCl2{(S,S)-iPr-boxax} through treatment with 2 equiv. of a silver(I) salt (AgBF4, AgPF6 or AgSbF6) was also successful (full conversions of 1a were achieved in 1 hour to give the product in 86%–91% yield with 95%–98% ee).
The Stoltz laboratory has developed an enantioselective Pd(II)-catalysed oxidative phenol cyclisation in nonpolar organic solvents with molecular oxygen using (−)-sparteine as the chiral ligand [40,41] (Scheme 4).
Scheme 4.
Pd(II)-catalysed asymmetric aerobic oxidative cyclisation of allylphenol 3.
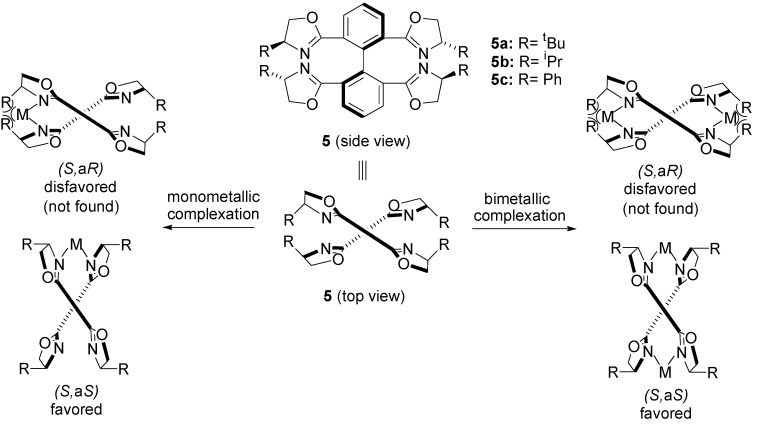
From recent works on enantioselective Wacker-type cyclisation of the o-allylphenols [42,43,44,45,46], the one worthy of emphasis, published by Zhang [44,45], reports a new family of tetraoxazoline ligands 5 for the construction of chelation-induced axially chiral catalytic systems (Figure 1). The axially achiral tetraoxazoline ligands 5, in which four identical chiral oxazoline groups are induced into the four ortho positions of a biphenyl axis, may produce only one of two possible diastereomeric metal complexes during the coordinating process. As it can be seen in Figure 1, the metal complexes (S,aS) are sterically more favorable compared with their diastereomers (S,aR). Hence, it is expected that only one diastereomeric metal complex with (S)-axial configuration is formed during the chelation-induced process.
Figure 1.
Model figures of diastereomeric monometallic and bimetallic complexes with tetraoxazoline ligands.
The generality of the chelation-induced axially chiral Pd-catalyst, 5c-Pd(OCOCF3)2, has been successfully demonstrated through the Wacker-type cyclisation of a series of o-allylphenols 1a–h and o-allylnaphtol 1i. As shown in Scheme 5 a wide array of chiral 2,3-dihydrobenzofurans 2a–h and dihydronaphto[1,2-b]furan 2i was obtained with excellent enantioselectivities (up to 99% ee), regardless of the steric or electronic properties of the aromatic moiety on the substrate 2.
Scheme 5.
Asymmetric intramolecular Wacker-type cyclisation of 1 using tetraoxazoline 5c-Pd(OCOCF3)2 catalyst.
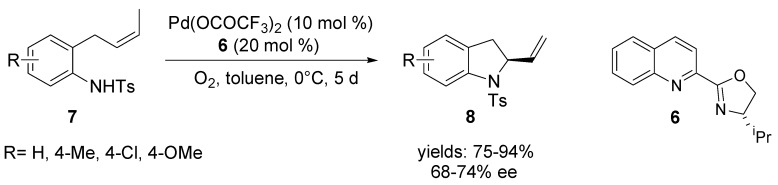
The first Pd-catalysed asymmetric aza-Wacker-type cyclisation of the olefinic tosylamides was published by the same group in 2010 [46]. By using a chiral quinolineoxazoline ligand 6 in the presence of Pd(II)-trifluoroacetate and oxygen at 0 °C, o-allylanilines 7 were cyclised to enantiomerically enriched dihydroindoles 8 in good yields with up to 74% ee (Scheme 6).
Scheme 6.
Aza-Wacker-type cyclisation reaction of o-allylanilines 7.
2.2. Oxidative Wacker Cyclisation of Alkeneols
The papers of Sasai and co-workers dealing with the development and design of new chiral ligands constitute an outstanding contribution in the field of asymmetric oxidative Wacker-type cyclisation [47,48,49,50,51,52]. Novel spirobis(isoxazoline) 9 and spiro(isoxazole-isoxazoline) ligands 10 were successfully applied in the asymmetric cyclisation of geranylphenols [42], alkenyl alcohols [48,49,50], 2-alkenyl-1,3-diketones [43], 4-alkenoic acids [51] and alkene amides [52]. These transformations effect stereo-selective construction of useful heterocycles (Scheme 7). Dihydropyrans 12 were obtained from alkenyl alcohols 11 with good enantioselectivity (up to 86% ee). Enantioselective 6-endo-trig Wacker-type cyclisation of 2-alkenyl-1,3-diketones 13 promoted by Pd-(M,S,S)-iPr-SPRIX catalyst provided optically active chromene derivatives 14. Recently, the same group published the enantioselective cyclisation of 4-alkenoic acids 15 [51] in this catalytic system. The reaction proceeded via a π-alkyl Pd-intermediate by an allylic C-H activation to give γ-lactone derivatives 16 with moderate to good selectivity.
Scheme 7.
PdII-SPRIX-catalysed cyclisation reaction of alkenyl alcohols 11, 2-alkenyl-1,3-diketones 13 and 4-alkenoic acids 15.
3. Asymmetric Domino Wacker-Type Cyclisation/Coupling Reactions
3.1. Coupling with Alkenes via Heck Vinylation
In 1993, Semmelhack showed that an organo-Pd(II) intermediate, formed by intramolecular oxypalladation of hydroxyalkenes, can be trapped by alkenes in the process of Heck vinylation reaction using stoichiometric amount of Pd(OAc)2 [53]. From the screening of reoxidation systems for the catalytic version of this transformation, the use of CuCl (1 equiv.)/O2 system turned out to be most effective (Wacker conditions). However, this method is limited to substrates that cannot undergo β-hydride elimination from organo-Pd(II) intermediate.
The utility of this domino Wacker-Heck reaction was illustrated by the research group of Tietze in the enantioselective Pd(II)-catalysed total synthesis of vitamin E and stereoselective synthesis of 4-dehydroxydiversonol [19,20,21]. The reaction of alkenyl phenol 17 and methyl vinyl ketone (18) dissolved in dichloromethane in the presence of catalytic amount of Pd(OCOCF3)2, the chiral ligand (S,S)-Bn-boxax and p-benzoquinone as a reoxidant afforded chromane 19 with 97% ee in 84% yield (Scheme 8). The non-asymmetric synthesis of 2,3-dihydrobenzo[1,4]dioxins, -oxazins, 1,4-dioxanes and perhydro-1,4-oxazines using analogous strategy was also described [54,55].
Scheme 8.
Asymmetric domino Wacker-Heck reaction of alkenyl phenol 17 and methyl vinyl ketone 18 using (S,S)-Bn-boxax ligand.
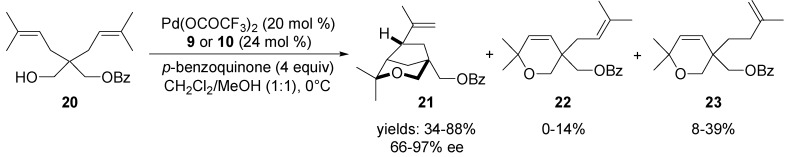
The first enantioselective domino intramolecular Wacker-intramolecular Heck reaction, in which a dialkenyl alcohol was converted into bicyclic ether, was described by Sasai and co-workers [48,49]. In the presence of spirobis(isoxazoline) 9 or spiro(isoxazole-isoxazoline) 10 ligands, the substrate 20 was converted to the domino product 21, along with dihydropyranes 22 and 23, products of β-hydride elimination (Scheme 9). Interestingly, previously used catalysts, such as Pd(OCOCF3)2-bis(oxazolinyl) propane, Pd(OCOCF3)2-(S,S)-iPr-boxax or Pd(OCOCF3)2-(−)-sparteine did not promote this reaction. This fact was reasoned by a stronger coordinating ability of all these ligands compared with spiro- bis(isooxazolines) 9 and thus, by suppressed Lewis acidity of the catalysts. On the other hand, the chiral spirobis(isoxazole) ligands, with even weaker coordinating ability than SPRIX, were also ineffective in this domino process. Therefore, the spiro(isoxazole-isoxazoline) ligands 10 were designed as a combination of two different coordinating units.
Scheme 9.
Asymmetric intramolecular domino Wacker-Heck reaction of dialkenyl alcohol 20 using spiro bis(isoxazoline) and spiro (isoxazole-isoxazoline) ligands.
Yang and co-workers [56] reported the enantioselective Pd(II)-catalysed domino Wacker-Heck bicyclisation using nitrogen atom-based nucleophiles and molecular oxygen as the sole reoxidant. Using the chiral Pd(II)-(−)-sparteine complex, 2-allylanilide substrates were converted to corresponding indoline domino products (Scheme 10).
Scheme 10.
Asymmetric domino Wacker-Heck bicyclisation of 2-allylanilide substrates using (−)-sparteine.
Afterwards, Sasai et al. successfully utilised the Pd(II)-SPRIX catalyst in the same reaction for the construction of pyrrolizines/pyrroloindoles [52]. Under the optimised conditions, which is 10 mol % of Pd(OCOCF3)2, 15 mol % of (M,S,S)-iPr-SPRIX 9c and 3Å MS in toluene at 70 °C under O2 atmosphere for 120 h, substrates with gem-dialkyl groups, cyclopentyl- or cyclohexyl-substituted substrates (Scheme 11, Equation a), and N-(2-allylphenyl) cinnamamide, (E)-N-(2-allylphenyl)-3-(2-chlorophenyl)-acrylamide and (E)-N-(2-allylphenyl)-3-(4-fluorophenyl)acrylamide substrates (Scheme 11, Equation b) were tested.
Scheme 11.
Asymmetric domino Wacker-Heck reaction in the synthesis of pyrrolizines/ pyrroloindoles using (M,S,S)-iPr-SPRIX ligand 9c.
3.2. Coupling with Aryl Halides
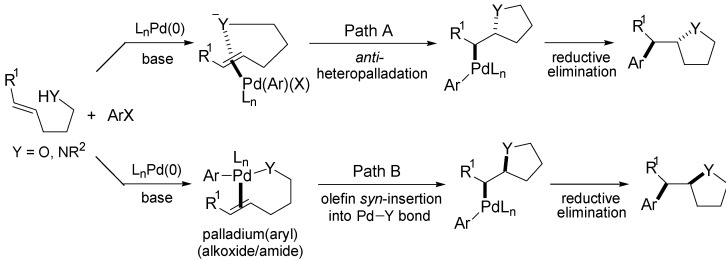
Wolfe and co-workers developed a general strategy for synthesis of saturated heterocycles via palladium-catalysed carboetherification and carboamination reactions between aryl or alkenyl halides and alkenes bearing pendant heteroatoms [22,23,57]. These transformations effect the stereoselective construction of synthetically interesting heterocycles, such as tetrahydrofurans, pyrrolidines, imidazolidin-2-ones, isoxazolidines, oxazolidines, pyrazolidines, piperazines, morpholines and diazepines.
One of the possible mechanisms involves the coordination of the alkene to the Pd(Ar)(X) species [generated upon oxidative addition of the aryl halide to Pd(0)], which activates the double bond toward nucleophilic attack (Scheme 12, Path A). The alkyl-σ-Pd(II) complex formed in the process of anti-heteropalladation is subsequently converted to the product through the well-known carbon–carbon bond forming reductive elimination. anti-Heteropalladation reactions are well-established with relatively electrophilic PdX2 complexes, however, these processes are not as common with less-electrophilic Pd(Ar)(X) intermediates [58,59]. Another plausible mechanism could proceed through oxidative addition of the aryl halide to Pd(0) followed by substrate deprotonation and substitution to provide alkene-bound palladium(aryl)(alkoxide/amide) complexes (Scheme 12, Path B). The alkyl-σ-Pd(II) complex is then formed in the process of syn-1,2-migratory insertion into the Pd–O/N bond and converted to the product by reductive elimination [22,23,57].
Scheme 12.
Mechanism of palladium-catalysed carboetherification and carboamination reactions.
The first asymmetric variant of this methodology was developed by Wolfe and Mai for the synthesis of enantioenriched pyrrolidines [60]. The substrates 24a–c were coupled with several different aryl or alkenyl bromides and iodides using (R)-Siphos-PE as ligand to give the desired products in moderate to good yields with 72%–94% ee (Scheme 13). Interestingly, little or no stereocontrol was observed with chiral bidentate ligands.
Scheme 13.
Asymmetric palladium-catalysed carboamination reaction for the synthesis of enantiomerically enriched pyrrolidines using (R)-Siphos-PE ligand.
The asymmetric carboamination method was applied towards a concise enantioselective synthesis of (–)-tylophorine [60]. Aryl bromide 25 was coupled with N-boc-pent-4-enylamine (24a) using the Pd/(R)-Siphos-PE catalyst (Scheme 14). The desired pyrrolidine 26 was formed in 69% yield and 88% ee, and converted to (–)-tylophorine in two steps and nearly quantitative yield.
Scheme 14.
Synthesis of (–)-tylophorine.
The utility of asymmetric carboamination method was also illustrated in an enantioconvergent synthesis of the benzomorphan alkaloid (+)-aphanorphine [61]. Racemic γ-aminoalkene derivative 27 and 4-bromoanisole were transformed into a 1:1 mixture of enantiomerically enriched diastereomers 28 (Scheme 15). The treatment of this mixture with trifluoroacetic acid led to cleavage of both the N-Boc and O-TMS groups, and tosylation of resulting pyrrolidine derivative provided 29a,b in 83% yield (1:1 dr) over two steps. The enantioconvergent intramolecular Friedel-Crafts alkylation of this diastereomeric mixture provided 30 in 63% yield with 81% ee.
Scheme 15.
Synthesis of (+)-aphanorphine.
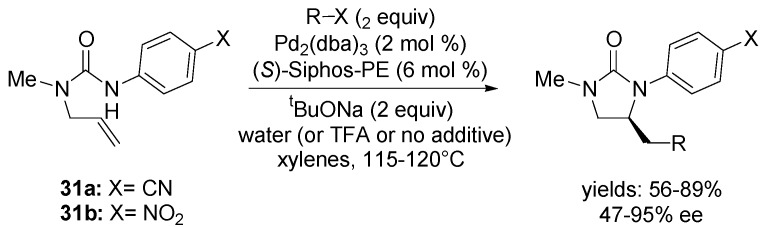
Most recently, Wolfe and Hopkins described the first asymmetric alkene carboamination reaction between N-allyl urea derivatives and aryl halides [62]. The authors initially examined the effect of nitrogen nucleophilicity on asymmetric induction using (S)-Siphos-PE ligand (Scheme 16). The level of asymmetric induction increased with increasing electron-withdrawing ability of the p-substituent on N-aryl moiety, however, the chemical yield decreased due to the diminished reactivity of these substrates. The enhancement of the reaction temperature (120 °C in xylenes) solved the problem with reactivity and the desired products were generated in 81%–87% yield with 86%–92% ee.
Scheme 16.
Asymmetric carboamination reaction of N-allyl urea derivatives with 1-bromo-4-tert-butylbenzene using (S)-Siphos-PE ligand.
Afterwards, the substrates 31a,b were coupled with a range of different aryl halide derivatives (Scheme 17). It was found that addition of 2 equiv of water (or 40 mol % of TFA) to the reaction mixtures in some cases significantly improved the enantioselectivities. Efforts to employ alkenyl halides as coupling partners were mostly unsuccessful.
Scheme 17.
Asymmetric carboamination reaction of substrates 31a,b with different aryl halides using (S)-Siphos-PE ligand.
4. Asymmetric Domino Wacker-Type Cyclisation/Carbonylation Reactions
Palladium(II)-catalysed cylisations of unsaturated alcohols, amines and other suitable substrates accompanied by the insertion of carbon monoxide provide a simple and straightforward access to one-carbon homologated esters, lactones and amides [1,2,6,29,30,31,32,33,34]. These domino processes have proven particularly useful for stereoselective construction of a range of oxygen and nitrogen-containing heterocyclic compounds [7,8,9,10,11,12,13,14,15]. Such a transformation of enantiomerically pure substrates has found numerous applications as the key step in the total syntheses of natural compounds [17,18,25,26,27,28,29].
4.1. Intramolecular Alkoxylation/Methoxycarbonylation
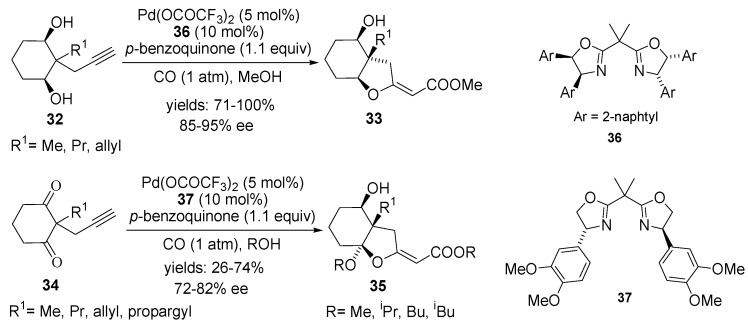
The first asymmetric version of the domino Pd-catalysed cyclisation-carbonylation reaction reported Kato, Akita et al. accomplishing desymmetrisation of cyclic meso-2-methyl-2-propargyl-cyclohexane-1,3-diols 32 [63] and -1,3-diones 34 [64] using palladium(II)-complex, bearing chiral bis(oxazoline) ligands (Scheme 18). Based on a ligand screening, by using Pd(OCOCF3)2-{2,2′-isopropylidenebis[4S,5R)-4,5-di(2-naphtyl)-2-oxazoline] (36)} catalyst in the presence of p-benzoquinone in methanol at −45 °C to −50 °C under carbon monoxide atmosphere, the cyclic meso-2-alkyl-2-propargyl-1,3-cyclohexane-diols 32 were carbonylated to bicyclic-alkoxyacrylates 33 in good yields with high enantioselectivities (85%–95% ee) [65]. The best results for desymmetrisation of cyclohexane-diones 34 were achieved with Pd(OCOCF3)2-{2,2′-isopropylidenebis[(4R)-4-(3,4-dimethoxyphenyl)-2-oxazoline] (37)} affording enantiomerically enriched bicyclic products 35 in 28%–74% yields with 72%–82% ee [66].
Scheme 18.
Asymmetric cyclisation-carbonylation of meso-2-alkyl-2-propargyl-1,3-cyclohexane-diols 32 and -1,3-diones 34 using Pd(II)-[box] catalysts.
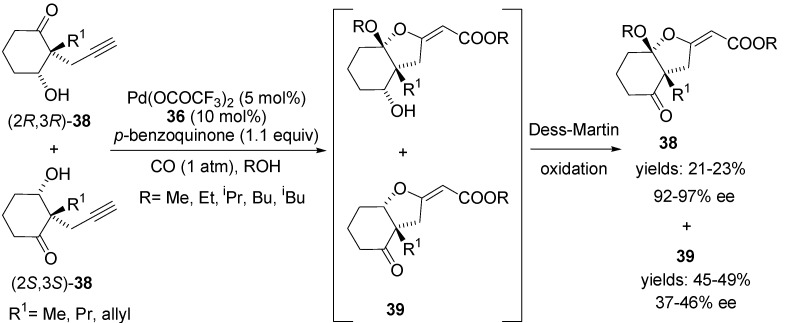
Further expansion of this oxidative cyclisation-carbonylation domino process into asymmetric transformations has been demonstrated in the parallel kinetic resolution of propargyl ketols 38 (Scheme 19). The 2S,3S enantiomer of (±)-38 was preferentially converted to 39 (45%–49% yields, 37%–46% ee), and the 2R,3R enantiomer of (±)-38 was transformed into 40 by subsequent Dess-Martin oxidation (21%–23% yields, 92%–97% ee) [67].
Scheme 19.
Parallel kinetic resolution of propargyl ketols 38 using Pd(II)-[box] catalyst.
In 2007, Tietze and co-workers described an enantioselective palladium-catalysed domino reaction of alkenes 41 and 42 as well as allyl phenyl ethers 45 affording the corresponding chromans 43, 44 and benzodioxins 46 (Scheme 20) [68]. In some cases, better results were obtained when the reaction was performed in dichloromethane with only 1 equiv of ROH. In other few cases, the use of molybdenium hexacarbonyl as CO-source instead of carbon monoxide at ambient pressure was advantageous.
Scheme 20.
Asymmetric domino reaction of alkenes 41–42 and phenyl ethers 45 employing (S,S)-Bn-boxax.
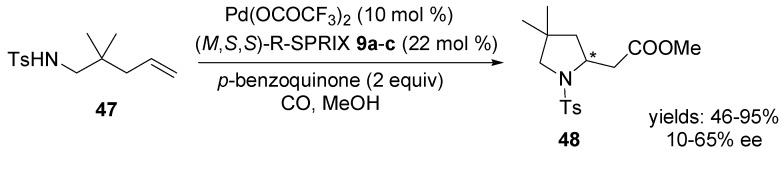
The first enantioselective aminocarbonylation was published by Sasai and co-workers [69]. The reaction of N-(2,2-dimethylpent-4-enyl)-p-toluenesulfonamide 47 in the presence of Pd(II)-SPRIX catalysts and p-benzoquinone in methanol under a carbon monoxide atmosphere afforded the corresponding pyrrolidinyl acetic acid methyl ester 48 in a good yield and moderate enantioselectivity (Scheme 21).
Scheme 21.
Asymmetric aminocarbonylation of N-(2,2-dimethylpent-4-enyl)-p-toluenesulfonamide 47 using Pd(II)-[SPRIX] catalysts.
4.2. Intramolecular Alkoxylation/Lactonisation
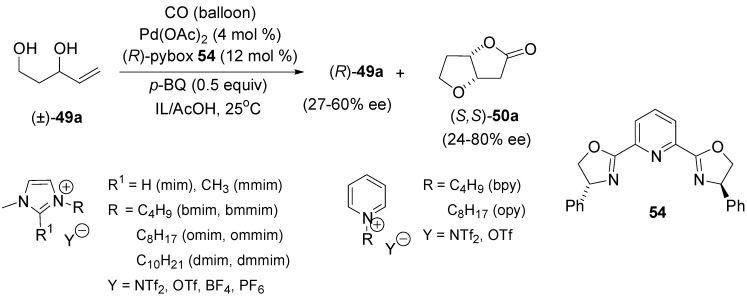
In 2008, Gracza and co-workers [70,71] disclosed an enantioselective variant of another Wacker-type Pd(II)-catalysed cyclisation. The oxycarbonylative annulations of unsaturated polyols and amino alcohols, either diastereoselective or enantioselective, proceeded to bicyclic lactones using chiral palladium(II) complexes. In the initial report [70], a method for the kinetic resolution of alkene-α,γ-1,3-diols 49 via asymmetric oxycarbonylative bicyclisation has been investigated. The conversion was controlled by the amount of p-benzoquinone. Besides the chiral ligand, the efficiency of this process depends on the anionic part of the catalyst and the solvent. Based on a ligand screening, the box-type N,N-bidentate ligands 2,6-bis[(R)-4-phenyloxazolin-2-yl]pyridine 51, {(3aR,8aS)-bis(8,8a-dihydro-3aH-indeno[1,2-d]oxazol-2-yl)}methane (52) and {(3aS,8aR)-bis(8,8a-dihydro-3aH-indeno[1,2-d]-oxazol-2-yl)}isopropane (53) have been identified as the most suitable ligands for Pd-catalysed oxidative lactonisation of unsaturated diols (Scheme 22). Sulphur and/or phosphorus-containing ligands have been proven to be incompatible with the oxidative catalytic system. Under optimum conditions, the kinetic resolution of pent-4-ene-diol (±)-49a using Pd(II)-[(R,S)-indabox] (51) and Pd(II)-[(S,R)-indabox] (53) provided both enantiomerically enriched lactones (R,R)-50a (29% yield, 62% ee) and (S,S)-50a (22% yield, 61% ee), respectively [71]. Similarly, the syn-diols (±)-49b and (±)-49c afforded the corresponding natural Hagen’s glands exo-lactones (R,R,R)-50b and (R,R,R)-50c, with high diastereoselectivity and good yields, however with low enantioselectivities. It should be noted that, contrary to CuCl2, the regeneration of the active Pd(II) species with p-benzoquinone is carried out in the absence of AcONa.
Scheme 22.
Kinetic resolution of alkene-1,3-diols (±)-49 in an asymmetric Pd(II)-catalysed oxycarbonylation.
Efforts from Vo-Thanh’s and Gracza’s group improved the efficiency of the process [71]. By application of ionic liquids and/or microwave activation, noticeable propitious enhancements in both reaction rate and enantiomeric excess (up to 80% ee) were observed (Scheme 23).
Scheme 23.
Kinetic resolution of pent-4-ene-1,3-diol (±)-49a by Pd(II)-catalysed oxycarbonylation in ionic liquids.
In a later study of the transformation of symmetric substrates [72], the same authors reported Pd-catalysed oxycarbonylation of the meso-diols xylo-55, ribo-57 and pseudo-C2-symmetric d-arabino-derivative 56. The Pd(II)-initiated oxycarbonylative bicyclisation of meso-diols 55, 57 in the presence of chiral Pd-catalysts using ligands with opposite asymmetric induction [(R,S)-indabox 52, (S,S)-bis(4-isopropyloxazolin-2-yl)methane (62)] afforded bicylic lactones 58 and 61 in good yields and with excellent 2,3-threo-diastereoselectivity (Scheme 24). In the reaction of the of the pseudo-C2-symmetric enitol 56, the diastereomer d-gluco-59 was isolated as a major product (65% yield, resulting from the intramolecular Si-attack of nucleophilic hydroxyl group to the Pd(II)-activated double bond) along with its minor diastereomer d-galacto-60 (9%). Such product distribution is most probably due to the endo-positions of two attached substituents on fused rings of lactone d-galacto-60, which in consequence increased the steric hindrance, and therefore its formation is slowed down. In fact, simply raising of temperature to 60 °C led to the complete consumption of enitol 56 in only 30 min to form energetically favorable diastereomer d-gluco-59 as the sole product in 78% yield.
Scheme 24.
Palladium(II)-catalysed oxycarbonylation of meso-diols 55 and 57, and pseudo-C2-symmetric enitol 56.
Recently, asymmetric Pd(II)-catalysed carbonylative bicyclisation of amino alcohols have been disclosed [73,74]. Gracza and co-workers reported the kinetic resolution of racemic N-protected 1-amino-pent-4-ene-3-ols 63 catalysed by prior to use prepared chiral palladium(II) complexes [73]. The N-protected 2-oxa-6-azabicyclo[3.3.0]octan-3-ones (R,R)-64 (derivatives of the natural Geissman-Waiss lactone) were obtained in 20%–40% yields with 60%–73% ee. (Scheme 25a). Sasai and co-workers succeeded in constructing the tetrahydropyrrolo[1,2-c]pyrimidine skeleton 66 via Pd(II)-catalysed amidocarbonylation of alkenylureas 65 (Scheme 25b) [74]. By using the chiral spiro bis(isoxazoline) ligands, SPRIXs, the desired products of carbonylative bicyclisation were produced in good yields and with moderate to good enantioselectivities.
Scheme 25.
Kinetic resolution of N-protected 1-aminopent-4-ene-3-ols (±)-63 in the Pd(II)-catalysed amidocarbonylation and Pd(II)-catalysed amidocarbonylation of alkenylureas 65.
5. Conclusions
The palladium(II)-catalysed Wacker-type cyclisations of alkenes have evolved into a highly useful methodology in synthetic organic chemistry. The domino and/or multicatalytic processes involving intramolecular oxidative cyclisation reactions, employing oxygen/nitrogen-containing nucleophiles, can provide various heterocyclic compounds. In many cases, the chemo-, regio- and diastereoselective Pd(II)-mediated cyclisations have succeeded in building of complex structures. This overview summarised the asymmetric versions of these palladium(II)-catalysed processes. The main issues include: (1) asymmetric Wacker-type oxidative cyclisations of substrates having both a carbon-carbon double bond and an amino/hydroxylated tether; (2) asymmetric domino Pd-catalysed N/O-cyclisation/coupling reactions; (3) asymmetric intramolecular alkoxylation/ methoxycarbonylation; (4) asymmetric intramolecular alkoxylation/lactonisation. Although considerable progress has been made in this area, some future trends are easy to predict: new catalytic systems will be developed to make Pd(0)-reoxidation more efficient, and a larger variety of chiral ligands will be available for enantioselective transformations. There are also many opportunities for the development of further domino processes and new modifications of the reaction system, omitting carbon monoxide atmosphere for extended use in medicinal chemistry exploiting automated workflows for liquid-phase parallel synthesis. In addition, there is a great potential for the application of these methods for the synthesis of natural products and biologically active heterocycles.
Acknowledgments
The authors gratefully thank the Slovak Grant Agencies (APVV, Bratislava, project No. APVV-0203-10 and ASFEU, Bratislava, ITMS projects No. 26240120001, 26240120025).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Tsuji J. Palladium Reagents and Catalysis. John Wiley & Sons Ltd.; Chichester, UK/Hoboken, NJ, USA: 2004. [Google Scholar]
- 2.Malleron J.-L., Fiaud J.-C., Legros J.-Y. Handbook of Palladium-Catalyzed Organic Reactions. Academic Press; London, UK: 1997. [Google Scholar]
- 3.Tietze L.F., Ila H., Bell H.P. Enantioselective palladium-catalyzed transformations. Chem. Rev. 2004;104:3453–3516. doi: 10.1021/cr030700x. [DOI] [PubMed] [Google Scholar]
- 4.McDonald R.I., Liu G., Stahl S.S. Palladium(II)-catalyzed alkene functionalization via nucleopalladation: stereochemical pathways and enantioselective catalytic applications. Chem. Rev. 2011;111:2981–3019. doi: 10.1021/cr100371y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeni G., Larock R.C. Synthesis of heterocycles via palladium π-olefin and π-alkyne chemistry. Chem. Rev. 2004;104:2285–2310. doi: 10.1021/cr020085h. [DOI] [PubMed] [Google Scholar]
- 6.Beccalli E.M., Broggini G., Martinelli, Sottocornola S. C–C, C–O, C–N Bond formation on sp2 carbon by Pd(II)-catalyzed reactions involving oxidant agents. Chem. Rev. 2007;107:5318–5365. doi: 10.1021/cr068006f. [DOI] [PubMed] [Google Scholar]
- 7.Jäger V., Gracza T., Dubois E., Hasenohrl T., Hümmer W., Kautz U., Kirschbaum B., Lieberknecht A., Remen L., Shaw D., et al. Pd(II)-Catalyzed Carbonylation of Unsaturated Polyols and Aminopolyols. In: Helmchen G., Dibo J., Flubacher D., Wiese B., editors. Organic Synthesis via Organometallics OSM 5. Vieweg; Braunschweig, Germany: 1997. pp. 331–360. [Google Scholar]
- 8.Semmelhack M.F., Bodurow C. Intramolecular alkoxypalladation/carbonylation of alkenes. J. Am. Chem. Soc. 1984;106:1469–1498. doi: 10.1021/ja00317a059. [DOI] [Google Scholar]
- 9.Semmelhack M.F., Kim C., Zhang N., Bodurow C., Sanner M., Dobler W., Meier M. Intramolecular alkoxy-carbonylation of hydroxy alkenes promoted by Pd(II) Pure Appl. Chem. 1990;62:2035–2040. doi: 10.1351/pac199062102035. [DOI] [Google Scholar]
- 10.Semmelhack M.F., Zhang N. Stereoselective formation of tetrahydrofuran rings via intramolecular alkoxycarbonylation of hydroxyalkenes. J. Org. Chem. 1989;54:4483–4485. doi: 10.1021/jo00280a003. [DOI] [Google Scholar]
- 11.Tamaru Y., Kobayashi T., Kawamura S.-I., Ochiai H., Hojo M., Yoshida Z.-I. Palladium catalyzed oxycarbonylation of 4-penten-1,3-diols: Efficient stereoselective synthesis of cis 3-hydroxytetrahydrofuran 2-acetic acid lactones. Tetrahedron Lett. 1985;26:3207–3210. doi: 10.1016/S0040-4039(00)98153-X. [DOI] [Google Scholar]
- 12.Gracza T., Hasenöhrl T., Stahl U., Jäger V. Synthesis of 3,6-Anhydro-2-deoxy-1,4-glyconolactones by Pd(II)-catalyzed, regioselective oxycarbonylation of C5- and C6-enitols, ω-homologation of aldoses to produce intermediates for C-glycoside/C-nucleoside synthesis. Synthesis. 1991:1108–1118. doi: 10.1055/s-1991-28400. [DOI] [Google Scholar]
- 13.Babjak M., Zálupský P., Gracza T. Regiocontrol in the palladium(II)-catalysed oxycarbonylation of unsaturated polyols. ARKIVOC. 2005;v:45–57. [Google Scholar]
- 14.Tamaru Y., Yoshida Z. Heterocyclic synthesis by the use of the oxidizing potential of palladium(II) J. Organomet. Chem. 1987;334:213–223. doi: 10.1016/0022-328X(87)80051-7. [DOI] [Google Scholar]
- 15.Tamaru Y., Hojo M., Yoshida Z. Palladium(2+)-catalyzed intramolecular aminocarbonylation of 3-hydroxy-4-pentenylamines and 4-hydroxy-5-hexenylamines. J. Org. Chem. 1988;53:5731–5741. doi: 10.1021/jo00259a024. [DOI] [Google Scholar]
- 16.Babjak M., Remeň Ľ., Szolcsányi P., Zálupský P., Miklóš M., Gracza T. Novel bicyclisation of unsaturated polyols in PdCl2-CuCl2-AcOH catalytic system. J. Organomet. Chem. 2006;691:928–940. doi: 10.1016/j.jorganchem.2005.10.036. [DOI] [Google Scholar]
- 17.Szolcsányi P., Gracza T. Novel Pd(II)-catalysed N,O-bicyclisation as an efficient route to the 6-oxa-2-azabicyclo[3.2.1]octane skeleton. Chem. Commun. 2005;2005:3948–3950. doi: 10.1039/b506731f. [DOI] [PubMed] [Google Scholar]
- 18.Palík M., Karlubíková O., Lásiková A., Kožíšek J., Gracza T. Total synthesis of (+)-varitriol. Eur. J. Org. Chem. 2009;2009:709–715. doi: 10.1002/ejoc.200801070. [DOI] [Google Scholar]
- 19.Tietze L.F., Sommer K.M., Zinngrebe J., Stecker F. Palladium-catalyzed enantioselective domino reaction for the efficient synthesis of vitamin E. Angew. Chem. Int. Ed. 2005;44:257–259. doi: 10.1002/anie.200461629. [DOI] [PubMed] [Google Scholar]
- 20.Tietze L.F., Stecker F., Zinngrebe J., Sommer K. Enantioselective palladium-catalyzed total synthesis of vitamin E by employing a domino Wacker-Heck reaction. Chem. Eur. J. 2006;12:8770–8776. doi: 10.1002/chem.200600849. [DOI] [PubMed] [Google Scholar]
- 21.Tietze L.F., Spiegl D.A., Stecker F., Major J., Raith C., Große C. Stereoselective synthesis of 4-dehydroxydiversonol employing enantioselective palladium-catalysed domino reactions. Chem. Eur. J. 2008;14:8956–8963. doi: 10.1002/chem.200800967. [DOI] [PubMed] [Google Scholar]
- 22.Wolfe J.P. Palladium-catalyzed carboetherification and carboamination reactions of γ-hydroxy- and γ-aminoalkenes for the synthesis of tetrahydrofurans and pyrrolidines. Eur. J. Org. Chem. 2007:571–582. doi: 10.1002/ejoc.200600767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolfe J.P. Stereoselective synthesis of saturated heterocycles via palladium-catalyzed alkene carboetherification and carboamination reactions. Synlett. 2008:2913–2937. doi: 10.1055/s-0028-1087339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gracza T. Intramolecular Oxycarbonylation in Stereoselective Synthesis. In: Andrushko V., Andrushko N., editors. Stereoselective Synthesis of Drugs &Natural Products. Wiley; Hoboken, NJ, USA: 2013. Chapter 15. [Google Scholar]
- 25.Szolcsányi P., Gracza T., Špánik I. Short racemic syntheses of calvine and epicalvine. Tetrahedron Lett. 2008;49:1357–1360. doi: 10.1016/j.tetlet.2007.12.099. [DOI] [Google Scholar]
- 26.Heumann A., Réglier M. The stereochemistry of palladium catalysed cyclisation reactions. Part C: Cascade reactions. Tetrahedron. 1996;52:9289–9346. doi: 10.1016/0040-4020(96)00545-5. [DOI] [Google Scholar]
- 27.Muzart J. Palladium-catalysed reactions of alcohols. Part B: Formation of C–C and C–N bonds from unsaturated alcohols. Tetrahedron. 2005;61:4179–4212. doi: 10.1016/j.tet.2005.02.026. [DOI] [Google Scholar]
- 28.Muzart J. Palladium-catalysed reactions of alcohols. Part C: Formation of ether linkages. Tetrahedron. 2005;61:5955–6008. doi: 10.1016/j.tet.2005.04.002. [DOI] [Google Scholar]
- 29.Muzart J. Palladium-catalysed reactions of alcohols. Part D: Rearrangements, carbonylations, carboxylations and miscellaneous reactions. Tetrahedron. 2005;61:9423–9463. doi: 10.1016/j.tet.2005.06.103. [DOI] [Google Scholar]
- 30.Hyland C. Cyclisations of allylic substrates via palladium catalysis. Tetrahedron. 2005;61:3457–3471. doi: 10.1016/j.tet.2005.01.112. [DOI] [Google Scholar]
- 31.Muzart J. Pd0- and PdII-catalyzed oxaheterocyclization of substrates having both an allylic leaving group and a hydroxylated tether. J. Mol. Cat. A. 2010;319:1–29. doi: 10.1016/j.molcata.2009.12.001. [DOI] [Google Scholar]
- 32.Beccalli E.M., Broggini G., Fasana A., Rigamonti M. Palladium-catalyzed C–N bond formation via direct C–H bond functionalization. Recent developments in heterocyclic synthesis. J. Organomet. Chem. 2011;696:277–295. doi: 10.1016/j.jorganchem.2010.09.078. [DOI] [Google Scholar]
- 33.Wu X.-F., Neumann H., Beller M. Synthesis of heterocycles via palladium-catalyzed carbonylations. Chem. Rev. 2013;113:1–35. doi: 10.1021/cr300100s. [DOI] [PubMed] [Google Scholar]
- 34.Wu X.-F., Neumann H., Beller M. Palladium-catalyzed oxidative carbonylation reactions. ChemSusChem. 2013;6:229–241. doi: 10.1002/cssc.201200683. [DOI] [PubMed] [Google Scholar]
- 35.Hosokawa T., Uno T., Inui S., Murahashi S.-I. Palladium(II)-catalyzed asymmetric oxidative cyclization of 2-allylphenols in the presence of copper(II) acetate and molecular oxygen. Study of the catalysis of the Wacker-type oxidation. J. Am. Chem. Soc. 1981;103:2318–2323. doi: 10.1021/ja00399a030. [DOI] [Google Scholar]
- 36.Hosokawa T., Okuda C., Murahashi S.-I. Substituent effects on palladium(II)-catalyzed enantioselective cyclization of a series of 2-(2-butenyl)phenols. J. Org. Chem. 1985;50:1282–1287. doi: 10.1021/jo00208a025. [DOI] [Google Scholar]
- 37.Uozumi Y., Kato K., Hayashi T. Catalytic asymmetric Wacker-type cyclization. J. Am. Chem. Soc. 1997;119:5063–5064. doi: 10.1021/ja9701366. [DOI] [Google Scholar]
- 38.Uozumi Y., Kato K., Hayashi T. Cationic palladium/boxax complexes for catalytic asymmetric Wacker-type cyclization. J. Org. Chem. 1998;63:5071–5075. doi: 10.1021/jo980245i. [DOI] [Google Scholar]
- 39.Uozumi Y., Kyota H., Kato K., Ogasawara M., Hayashi T. Design and preparation of 3,3′-disubstituted 2,2′-bis(oxazolyl)-1,1′-binaphtyls (boxax): New chiral bis(oxazoline) ligands for catalytic asymmetric Wacker-type cyclization. J. Org. Chem. 1999;64:1620–1625. doi: 10.1021/jo982104m. [DOI] [PubMed] [Google Scholar]
- 40.Trend R.M., Ramtohul Y.K., Ferreira E.M., Stoltz B.M. Palladium-catalyzed oxidative Wacker cyclization in nonpolar organic solvents with molecular oxygen: A stepping stone to asymmetric aerobic cyclizations. Angew. Chem. Int. Ed. 2003;42:2892–2895. doi: 10.1002/anie.200351196. [DOI] [PubMed] [Google Scholar]
- 41.Trend R.M., Ramtohul Y.K., Stoltz B.M. Oxidative cyclizations in a nonpolar solvent using molecular oxygen and studies on the stereochemistry of oxypalladation. J. Am. Chem. Soc. 2005;127:17778–17788. doi: 10.1021/ja055534k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takenaka K., Tanigaki Y., Patil M. L., Rao C.V.L., Takizawa S., Suzuki T., Sasai H. Enantioselective 6-endo-trig Wacker-type cyclization of 2-geranylphenols: Application to a facile synthesis of (−)-cordiachromene. Tetrahedron: Asymmetry. 2010;21:767–770. doi: 10.1016/j.tetasy.2010.04.060. [DOI] [Google Scholar]
- 43.Takenaka K., Mohanata S.C., Patil M.L., Rao C.V.L., Takizawa S., Suzuki T., Sasai H. Enantioselective Wacker-type cyclization of 2-alkenyl-1,3-diketones promoted by Pd-SPRIX catalyst. Org. Lett. 2010;12:3480–2010. doi: 10.1021/ol1013069. [DOI] [PubMed] [Google Scholar]
- 44.Wang F., Zhang Y.J., Yang G., Zhang W. Highly enantioselective Pd(II)-catalyzed Wacker-type cyclization of 2-allylphenols by use of bisoxazoline ligands with axis-unifixed biphenyl backbone. Tetrahedron Lett. 2007;48:4179–4182. doi: 10.1016/j.tetlet.2007.04.064. [DOI] [Google Scholar]
- 45.Zhang Y.J., Wang F., Zhang W. Chelation-induced axially chiral palladium complex system with tetraoxazoline ligands for highly enantioselective Wacker-type cyclization. J. Org. Chem. 2007;72:9208–9213. doi: 10.1021/jo701469y. [DOI] [PubMed] [Google Scholar]
- 46.Jiang F., Wu Z., Zhang W. Pd-catalyzed asymmetric aza-Wacker-type cyclization reaction of olefinic tosylamides. Tetrahedron Lett. 2010;51:5124–5126. doi: 10.1016/j.tetlet.2010.07.084. [DOI] [Google Scholar]
- 47.Bajracharya G.B., Arai M.A., Koranne P.S., Suzuki T., Takizawa S., Sasai H. Development of chiral spiro ligands for metal-catalyzed asymmetric reactions. Bull. Chem. Soc. Jpn. 2009;82:285–302. doi: 10.1246/bcsj.82.285. [DOI] [Google Scholar]
- 48.Arai M.A., Kuraishi M., Arai T., Sasai H. A new asymmetric Wacker-type cyclization and tandem cyclization promoted by Pd(II)-spiro bis(isoxazoline) catalyst. J. Am. Chem. Soc. 2001;123:2907–2908. doi: 10.1021/ja005920w. [DOI] [PubMed] [Google Scholar]
- 49.Koranne P.S, Tsujihara T., Arai M.A., Bajracharya G.B., Suzuki S., Onitsuka K., Sasai H. Design and synthesis of chiral hybrid spiro (isoxazole–isoxazoline) ligands. Tetrahedron: Asymmetry. 2007;18:919–923. doi: 10.1016/j.tetasy.2007.04.010. [DOI] [Google Scholar]
- 50.Takenaka K., Nagano T., Takizawa S., Sasai H. Asymmetric synthesis of chiral spiro bis(isoxazoline) and spiro (isoxazole–isoxazoline) ligands. Tetrahedron: Asymmetry. 2010;21:379–381. doi: 10.1016/j.tetasy.2010.03.006. [DOI] [Google Scholar]
- 51.Takenaka K., Akita M., Tanigaki Y., Takizawa S., Sasai H. Enantioselective cyclization of 4-alkenoic acids via an oxidative allylic C–H esterification. Org. Lett. 2011;13:3506–3509. doi: 10.1021/ol201314m. [DOI] [PubMed] [Google Scholar]
- 52.Ramalingan C., Takenaka K., Sasai H. Pd(II)-SPRIX catalyzed enantioselective construction of pyrrolizines/pyrroloindoles employing molecular oxygen as the sole oxidant. Tetrahedron. 2011;67:2889–2894. doi: 10.1016/j.tet.2011.02.057. [DOI] [Google Scholar]
- 53.Semmelhack M.F., Epa W.R. Catalytic tandem oxy-palladation and vinylation. Tetrahedron Lett. 1993;34:7205–7208. doi: 10.1016/S0040-4039(00)79288-4. [DOI] [Google Scholar]
- 54.Tietze L.F., Wilckens K.F., Yilmaz S., Stecker F., Zinngrebe J. Synthesis of 2,3-dihydrobenzo[1,4]dioxins and -oxazins via a domino Wacker-Heck reaction. Heterocycles. 2006;70:309–319. doi: 10.3987/COM-06-S(W)24. [DOI] [Google Scholar]
- 55.Tietze L.F., Heins A., Soleiman-Beigi M., Raith C. Synthesis of anulated 1,4-dioxanes and perhydro-1,4-oxazines by domino-Wacker-carbonylation and domino-Wacker-Mizoroki-Heck reactions. Heterocycles. 2009;77:1123–1146. doi: 10.3987/COM-08-S(F)91. [DOI] [Google Scholar]
- 56.Yip K.-T., Yang M., Law K.-L., Zhu N.-Y., Yang D. Pd(II)-Catalyzed enantioselective oxidative tandem cyclization reactions. Synthesis of indolines through C–N and C–C bond formation. J. Am. Chem. Soc. 2006;128:3130–3131. doi: 10.1021/ja060291x. [DOI] [PubMed] [Google Scholar]
- 57.Schultz D.M., Wolfe J.P. Recent developments in palladium-catalyzed alkene aminoarylation reactions for the synthesis of nitrogen heterocycles. Synthesis. 2012:351–361. doi: 10.1002/chin.201216239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hosokawa T., Murahashi S.-I. New aspects of oxypalladation of alkenes. Acc. Chem. Res. 1990;23:49–54. doi: 10.1021/ar00170a006. [DOI] [Google Scholar]
- 59.Hegedus L.S. Transition metals in the synthesis and functionalization of indoles. Angew. Chem. Int. Ed. 1988;27:1113–1126. doi: 10.1002/anie.198811133. [DOI] [Google Scholar]
- 60.Mai D.N., Wolfe J.P. Asymmetric palladium-catalyzed carboamination reactions for the synthesis of enantiomerically enriched 2-(arylmethyl)- and 2-(alkenylmethyl)pyrrolidines. J. Am. Chem. Soc. 2010;132:12157–12159. doi: 10.1021/ja106989h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mai D.N., Rosen B.R., Wolfe J.P. Enantioconvergent synthesis of (+)-aphanorphine via asymmetric Pd-catalyzed alkene carboamination. Org. Lett. 2011;13:2932–2935. doi: 10.1021/ol2009895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hopkins B.A., Wolfe J.P. Synthesis of enantiomerically enriched imidazollidin-2-ones through asymmetric palladium-catalyzed alkene carboamination. Angew. Chem. Int. Ed. 2012;51:9886–9890. doi: 10.1002/anie.201205233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kato K., Tanaka M., Yamamoto Y., Akita H. Asymmetric cyclization-carbonylation of cyclic-2-methyl-2-propargyl-1,3-diols. Tetrahedron Lett. 2002;43:1511–1513. doi: 10.1016/S0040-4039(02)00049-7. [DOI] [Google Scholar]
- 64.Kato K., Tanaka M., Yamamura S., Yamamoto Y., Akita H. Asymmetric cyclization-carbonylation of 2-propargyl-1,3-dione. Tetrahedron Lett. 2003;44:3089–3092. doi: 10.1016/S0040-4039(03)00547-1. [DOI] [Google Scholar]
- 65.Kato K., Matsuba Ch., Kusakabe T., Takayama H., Yamamura S., Mochida T., Akita H., Peganova T.A., Vologdin N.V., Gusev O.V. 2,2´-Isopropylidene-bis[(4S,5R)-4,5-di(2-naphtyl)-2-oxazoline] ligand for asymmetric cyclization-carbonylation of meso-2-alkyl-2-propargylcyclohexane-1,3-diols. Tetrahedron. 2006;62:9988–9999. doi: 10.1016/j.tet.2006.08.004. [DOI] [Google Scholar]
- 66.Kusakabe T., Kato K., Takaishi S., Yamamura S., Mochida T., Akita H., Peganova T.A., Vologdin N.V., Gusev O.V. Asymmetric cyclization-carbonylation of 2-alkyl-2-propargylcyclohexane-1,3-diones: facile access to optically active hydradanes. Tetrahedron. 2008;64:319–327. doi: 10.1016/j.tet.2007.10.106. [DOI] [Google Scholar]
- 67.Kato K., Motodate S., Takaishi S., Kusakabe T., Akita H. Parallel kinetic resolution of propargyl ketols: formal synthesis of (+)-bakkenolide A. Tetrahedron. 2008;64:4627–4636. doi: 10.1016/j.tet.2008.02.101. [DOI] [Google Scholar]
- 68.Tietze L.F., Zinngrebe J., Speigl D.A., Stecker F. Palladium-catalyzed domino-Wacker-carbonylation reaction for the enantioselective synthesis of chromans and benzodioxins. Heterocycles. 2007;74:473–789. doi: 10.3987/COM-07-S(W)67. [DOI] [Google Scholar]
- 69.Shinohara T., Arai M.A., Wakita K., Arai T., Sasai H. The first enantioselective intramolecular aminocarbonylation of alkenes promoted by Pd(II)-spiro bis(isoxazoline) catalyst. Tetrahedron Lett. 2003;44:711–714. doi: 10.1016/S0040-4039(02)02650-3. [DOI] [Google Scholar]
- 70.Kapitán P., Gracza T. Asymmetric intramolecular Pd(II)-catalysed oxycarbonylation of alkene-1,3-diols. ARKIVOC. 2008;viii:8–17. [Google Scholar]
- 71.Doháňošová J., Lásiková A., Toffano M., Gracza T., Vo-Than G. Kinetic resolution of pent-4-ene-1,3-diol by Pd(II)-catalysed oxycarbonylation in ionic liquids. New J. Chem. 2012;36:1744–1750. doi: 10.1039/c2nj40170c. [DOI] [Google Scholar]
- 72.Kapitán P., Gracza T. Stereocontrolled oxycarbonylation of 4-benzaloxyhepta-1,6-diene-3,5-diols promoted by chiral palladium(II) complexes. Tetrahedron: Asymmetry. 2008;19:38–44. doi: 10.1016/j.tetasy.2007.12.003. [DOI] [Google Scholar]
- 73.Koóš P., Špánik I., Gracza T. Asymmetric intramolecular Pd(II)-catalysed amidocarbonylation of unsaturated amino alcohols. Tetrahedron: Asymmetry. 2009;20:2720–2723. doi: 10.1016/j.tetasy.2009.10.024. [DOI] [Google Scholar]
- 74.Tsujihara T., Shinohara T., Takenaka K., Takizawa S., Onitsuka K., Hatanaka M., Sasai H. Enantioselective intramolecular oxidative aminocarbonylation of alkenylureas catalyzed by palladium-spiro bis(isoxazoline) complexes. J. Org. Chem. 2009;74:9274–9279. doi: 10.1021/jo901778a. [DOI] [PubMed] [Google Scholar]