Abstract
A new class of multifunctionalized sarcophagine derivatives was synthesized for 64Cu chelation. The platform developed in this study could have broad applications in 64Cu-radiopharmaceuticals.
Keywords: positron emission tomography (PET), sarcophagine, 64Cu, bifunctional chelator (BFC)
1. Introduction
Positron emission tomography (PET) is a powerful imaging modality to obtain quantitative molecular and biochemical information about physiological processes in vivo. With a half life of 12.7 h, copper-64 is well suited for the radiolabeling of proteins, antibodies, and peptides. Moreover, copper-64 decays by β+ (17.8%) and β− emission (38.4%), making it an attractive radioisotope for both PET imaging (β+) and therapy (β+ and β−). Because direct conjugation of 64Cu onto bioligands is not practical, various bifunctional chelators (BFCs) have been developed for radiolabeling. Among all BFCs, macrocyclic chelators have demonstrated enhanced in vivo stability over acyclic chelators such as ethylenediaminetetraacetic acid (EDTA) and diethylenetriaminepentaacetic acid (DTPA). Up to now, a number of macrocyclic BFCs have been developed and applied in PET probe designs, including 1,4,7,10-tetraazacyclododecane-N,N',N'',N'''-tetraacetic acid (DOTA) [1,2], 1,4,7-triaza-cyclononane-1,4,7-triacetic acid (NOTA) derivatives [3,4], cross-bridged 1,4,8,11-tetraazacyclo-tetradecane-1,4,8,11-tetraacetic acid (CB-TETA) [2,5], and 1,4,8,11-tetraazabicyclo[6.6.2]hexadecane (CB-TE2A) [6,7,8]. In particular, various studies have demonstrated that the hexaazamacrobicyclic sarcophagines (denoted as “Sar”, Scheme 1) could form extraordinarily stable Cu complexes under mild conditions with good in vivo stability [9,10,11,12,13,14,15,16].
Scheme 1.
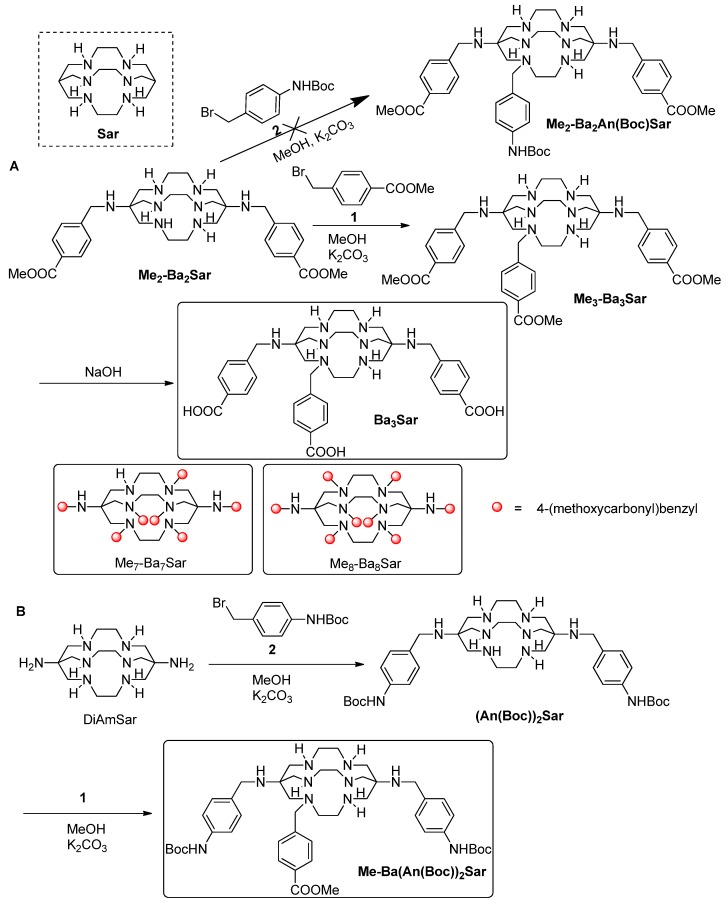
Synthesis of Ba3Sar, Ba7Sar, Ba8Sar and Me-Ba(An(Boc))2Sar.
Recently, we successfully improved the functionalization approach of the Sar cage through a direct alkylation (SN2) reaction and synthesized BaBaSar that has two pendant carboxylate groups at either end of the Sar cage. This novel multivalency bifunctional chelator could be further conjugated to multiple targeting ligands (such as RGD peptides) via biologically stable amide bonds. Unlike monovalent ligands, the polyvalent ligands could lead to increased target binding affinity and tumor uptake [17,18,19,20,21,22,23]. For example, dimeric, tetrameric and octameric RGD peptides (integrin αvβ3 targeting) have been developed to increase tumor uptake based on polyvalency principle [18,19,24]. Clearly, a Sar cage with multiple functional groups for further modification could serve as an important platform for the construction of multivalent probes. Herein, we report a streamlined synthetic approach to the multi-functionalized Sar cage suitable for the design and syntheses of polyvalent probes. We also explore the in vitro and in vivo stability of 64Cu labeled Ba3Sar.
2. Results and Discussion
Our initial syntheses are shown in Scheme 1. The precursor Me2-Ba2Sar was synthesized as reported [23]. Further alkylation of Me2-Ba2Sar was realized using methyl 4-bromomethylbenzoate (1) in the presence of Na2CO3 as base. MeOH was used as the solvent due to its good solubilization of Sar cages. Due to the low selectivity of the multiple amino groups on Me2-Ba2Sar, the reaction mixture was complicated, as indicated by HPLC. However, we could control the ratio of Me2-Ba2Sar and 1 to achieve reasonable yields for the desired products. For example, in the synthesis of Me3-Ba3Sar, we used a 1.2:1 ratio of 1 to Me2-Ba2Sar, and Me3-Ba3Sar was obtained in the yield of 32%. When 10 equivalent of 1 was used for the synthesis, Me7-Ba7Sar and Me8-Ba8Sar could be obtained in the yields of 15% and 12%, respectively. The methyl protecting groups were readily removed in 0.2 N NaOH to afford Ba3Sar, Ba7Sar, and Ba8Sar in almost quantitative yields. The free benzoic acids are useful for the conjugation with terminal or lysine side chain amino groups of peptides or proteins. In addition to the homo-functionalized Sar cages, it would be interesting to introduce different functional groups to the Sar cages. However, our initial test on modifying Me2-Ba2Sar with tert-butyl(4-(bromomethyl)phenyl) carbamate (2) failed to provide us the heterofunctionalized Me2-Ba2An(Boc)Sar (Scheme 1), which may be attributed to the reactivity difference between methyl 4-bromomethylbenzoate and tert-butyl(4-(bromomethyl)phenyl)carbamate. In order to obtain a heterofunctionalized sarcophagine, we synthesized the protected (An(Boc))2Sar as our previous report (Scheme 1). The purified (An(Boc))2Sar was then further alkylated with 1 to give Me-Ba(An(Boc))2Sar with Boc and methyl protective groups on it. The overall yield for Me-Ba(An(Boc))2Sar from DiAmSar was 22%.
As a proof of principle experiment, we chose Ba3Sar as an example to test the radiolabeling efficacy of the synthesized chelators. As shown in Figure 1, Ba3Sar can be efficiently labeled with 64Cu at pH 5.5 in sodium acetate buffer after 20 min incubation at 40 °C. Even without purification, radio trace HPLC showed greater than 95% purity of 64Cu-Ba3Sar. Furthermore, to broaden the application of Ba3Sar, we tested the 64Cu labeling in basic conditions, potentially useful for bioligands sensitive to acids. In phosphate buffer (pH 7.4) and borate buffer (pH 8.5), the radiolabeling of Ba3Sar could give 71% and 81% yield after 30 min incubation at 40 °C, respectively. To estimate the highest achievable limit of the specific activity of the product, we gradually decreased the amount of Ba3Sar added to the reaction. When 2 µg Ba3Sar was loaded into 2 mCi (74 MBq) 64Cu solution, the labeling yield was still as high as 75%. The specific activity of the radiolabeled conjugate was 500 mCi/µmol.
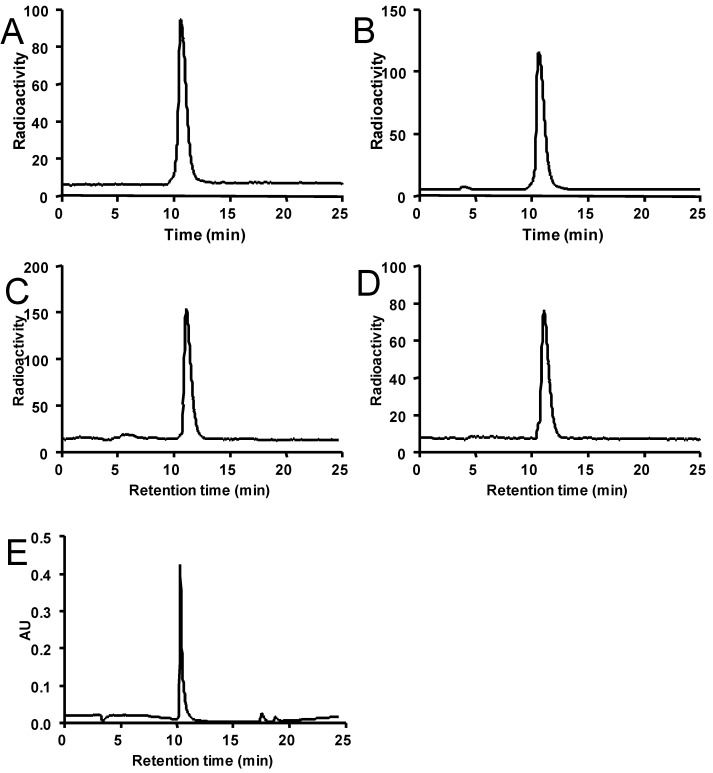
Figure 1.
In vitro stability. (A) The radiotrace standard of 64Cu-Ba3Sar. (B) The radiotrace of 64Cu-Ba3Sar after 20 h incubation in 1 × PBS. (C) The radiotrace of 64Cu-Ba3Sar after 3 h incubation in mouse serum. (D) The radiotrace of 64Cu-Ba3Sar after 24 h incubation in mouse serum. (E) The UV trace of standard 64Cu-Ba3Sar.
The in vitro stability of 64Cu-Ba3Sar was evaluated after incubation in 1 × PBS and 10% mouse serum by radio HPLC (Figure 1). No significant amount of free 64Cu was detected by radio HPLC up to 20 h post incubation. We also like to point out that less than 5% of 64Cu was trapped on NanoSep 10 K filter, suggesting minimum activity was trapped in serum proteins. These data are consistent with the previously published stability results [9,10,11,12,13]. The cross-bridged and cage-like configuration of the Sar structure could lead to the high stability of the complex.
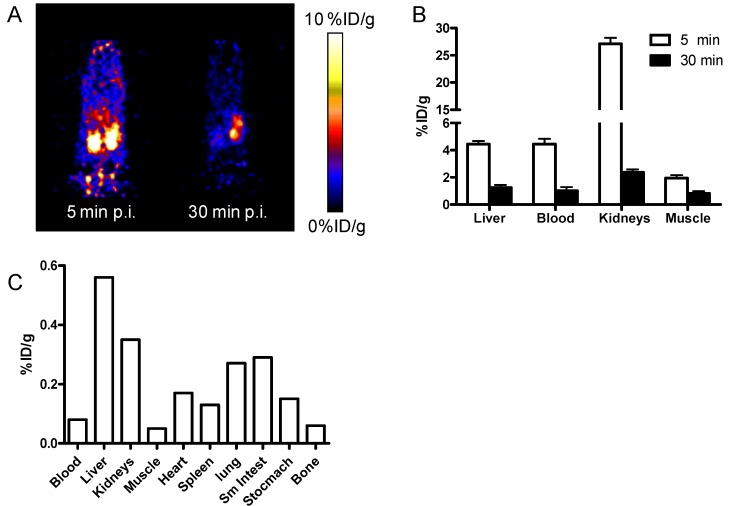
The in vivo distribution and stability of 64Cu-Ba3Sar were evaluated by static microPET scans at 5 min and 30 min after injection of 64Cu-Ba3Sar via tail vain into 6–7 weeks old nude mice (Figure 2). microPET images show that the activity is fast cleared from kidneys. At 5 min post injection, the liver uptake is 4.45 ± 0.40%ID/g, which has no significant difference compared with blood uptake (4.38 ± 0.67%ID/g). At 30 min p.i., the liver uptake (1.26 ± 0.32%ID/g) is only slightly higher than the blood (1.02 ± 0.47%ID/g) and muscle (0.83 ± 0.28%ID/g). The low liver uptake and fast clearance from body indirectly suggested the high in vivo stability of 64Cu-Ba3Sar since the released free 64Cu would be easily accumulated in liver [25]. To further investigate the localization of 64Cu-Ba3Sar in normal athymic nude mice, we also performed biodistribution study at 24 h after injection. As can be seen in Figure 2C, the highest uptake was found in liver (0.56%ID/g). The low uptake in normal organs also suggests the high in vivo stability of 64Cu-Ba3Sar. Overall, the circulation of 64Cu-Ba3Sar is similar to our previous investigated sarcophagine derivatives.
Figure 2.
(A) Decay-corrected whole-body coronal microPET images of athymic female nude mice from a static scan at 5 min, and 30 min after injection of 64Cu-Ba3Sar. (B) microPET quantification of major organs at 5 min, and 30 min after injection of 64Cu-Ba3Sar. The data are expressed as average ± standard deviation, n = 3. (C) Biodistribution studies of 64Cu-Ba3Sar in normal female nude mouse at 24 h after injection.
3. Experimental
3.1. General
All chemicals obtained commercially were of analytic grade and used without further purification. The syringe filter and polyethersulfone membranes (pore size, 0.22 μm; diameter, 13 mm) were obtained from Nalge Nunc International (Rochester, NY, USA). The semi-preparative reversed-phase HPLC using a Vydac protein and peptide column (218TP510; 5 µm, 250 × 10 mm) was performed on a Dionex 680 chromatography system with a UVD 170U absorbance detector (Sunnyvale, CA, USA) and model 105S single-channel radiation detector (Carroll & Ramsey Associates, Berkeley, CA, USA). With a flow rate of 4 mL/min, the mobile phase stayed at 95% solvent A [0.1% trifluoroacetic acid (TFA) in water] and 5% B [0.1% TFA in acetonitrile (MeCN)] at 0–2 min and was changed from 95% solvent A and 5% B at 2 min to 35% solvent A and 65% solvent B at 32 min. Analytical HPLC had the same gradient with flow rate of 1 mL/min using a Vydac protein and peptide column (218TP510; 5 µm, 250 × 4.6 mm). The UV absorbance was monitored at 218 nm and the identification of the peptides was confirmed based on the UV spectrum using a PDA detector. Copper-64 was purchased from University of Wisconsin. It was dissolved in 0.1 N HCl solution and the specific activity > 1 Ci/µmol. For all the solvents used in the labeling study, Chelex 100 resin was used to remove heavy metal. MicroPET scans were performed on a microPET R4 rodent model scanner (Siemens Medical Solutions USA, Inc., Knoxville, TN, USA). The scanner has a computer-controlled bed and 10.8-cm transaxial and 8-cm axial fields of view (FOVs). It has no septa and operates exclusively in the 3-dimensional (3D) list mode. Animals were placed near the center of the FOV of the scanner. 1H and 13C NMR spectra were obtained on a 300 MHz superconducting NMR spectrometer (Bruker, Billerica, MA, USA). The mass spectra were recorded by Electrospray Ionization Mass spectrum (Shimadzu, Pleasanton, CA, USA).
3.2. Preparation of Ba3Sar, Ba7Sar, and Ba8Sar
Me2-Ba2Sar was synthesized as reported in the literature [9,10,11]. To a solution of Me2-Ba2Sar (38.9 mg, 63.7 µmol) in MeOH (5 mL), was added 1.2 equiv. of methyl 4-(bromomethyl)benzoate (1, 17.0 mg, 76.4 µmol) in tetrahydrofuran (THF, 2.5 mL) and sodium carbonate (Na2CO3, 31.8 mg, 300 µmol). The reaction was refluxed under stirring for 5 h. After cooling down to room temperature, 5% acetic acid (5 mL) was added the crude mixture. Semipreparative HPLC afforded the Me3-Ba3Sar as off-white solid (32%, 15.4 mg, 20.4 µmol). The retention time of Me3-Ba3Sar on analytical HPLC is 20.6 min. The molecular weight was determined by the Electrospray Ionization Mass spectrum (ESI-MS) to be 759.4 for [M+H]+ (chemical formula: C41H59N8O6, calculated molecular weight: 759.5). To remove the methyl protection groups from Me3-Ba3Sar, Me3-Ba3Sar (5 mg, 6.6 µmol) was dissolved in 0.2 N NaOH (1 mL) and the reaction mixture was kept at 60 °C for 1 h. After cooling down to room temperature, 5% acetic acid (5 mL) was added the crude mixture. Semipreparative HPLC afforded Ba3Sar as white powder (98%, 4.6 mg, 6.4 µmol). The retention time of Ba3Sar on analytical HPLC is 10.4 min. The molecular weight of Ba3Sar was determined by the Electrospray Ionization Mass spectrum (ESI-MS) to be 717.3 for [M+H]+ (chemical formula: C38H53N8O6, calculated molecular weight: 717.4). 1H-NMR (300 MHz, DMSO-d6): δ = 13.1 (s, 3H), 7.94 (d, J = 8.0 Hz, 8H), 7.51 (d, J = 7.3 Hz, 4H), 3.92–3.32 (m, 18H), 3.21–2.07 (m, 18H). 13C-NMR (75 MHz, DMSO-d6): δ = 167.2, 167.1, 158.6, 158.2, 158.0, 144.0, 130.1, 130.0, 129.4, 118.2, 115.2, 59.1, 57.2, 55.6, 45.1, 44.3. The molecular weight of Ba7Sar was determined by the Electrospray Ionization Mass spectrum (ESI-MS) to be 1253.7 for [M+H]+ (chemical formula: C70H77N8O14, calculated molecular weight: 1253.6). The molecular weight of Ba8Sar was determined by the Electrospray Ionization Mass spectrum (ESI-MS) to be 1387.8 for [M+H]+ (chemical formula: C78H83N8O16, calculated molecular weight: 1387.6).
3.3. Prepareation of Ba(An(Boc))2Sar
To the solution of DiAmSar (20 mg, 63.7 µmol) in MeOH (20 mL) was added 4-(bromomethyl)benzoate (1, 43.5 mg, 191.1 µmol) solution in THF (10 mL) and Na2CO3 (21.2 mg, 200 µmol). The reaction was refluxed under stirring for 10 h. After cooling down to room temperature, 5% acetic acid (25 mL) was added the crude mixture. Semipreparative HPLC afforded the (An(Boc))2Sar (44%, 20.3 mg, 28.0 µmol) as a white solid. The molecular weight for An2Sar was determined by the Electrospray Ionization Mass spectrum (ESI-MS) to be 725.3 for [M+H]+ (chemical formula: C38H65N10O4, calculated molecular weight: 725.5).
To further alkylate (An(Boc))2Sar to Me-Ba(An(Boc))2Sar, (An(Boc))2Sar (5 mg, 6.9 µmol) dissolved in MeOH (0.5 mL) was added to methyl 4-(bromomethyl)benzoate (1, 3.1 mg, 13.8 µmol) in THF (1 mL) and Na2CO3 (1.6 mg, 15 µmol). The reaction mixture was refluxed for 5 h. After cooling down to room temperature, 5% acetic acid (5 mL) was added the crude mixture. Semipreparative HPLC afforded the Me-Ba(An(Boc))2Sar as white powder (50%, 3.0 mg, 3.4 µmol). The retention time of Me-Ba(An(Boc))2Sar on analytical HPLC is 12.7 min. The molecular weight was determined by the Electrospray Ionization Mass spectrum (ESI-MS) to be 873.7 for [M+H]+ (chemical formula: C47H73N10O6, calculated molecular weight: 873.6).
3.4. Radiochemistry
Acidic conditions:64CuCl2 (20 µL, 74 MBq in 0.1 N HCl) was diluted in 0.1 N sodium acetate (80 µL, pH 5.5) and added to Ba3Sar (20 µg). The reaction mixture was kept at 40 °C for 20 min. 64Cu-labeled product was subsequently purified by analytical HPLC and the radioactive peak containing the desired product was collected. After removal of the solvent by rotary evaporation, the 64Cu-Ba3Sar tracer was reconstituted in PBS (1 mL) and passed through a 0.22 µm syringe filter for in vivo animal experiments. The decay-corrected radiochemical yield (RCY) was 95%.
Basic conditions: 64CuCl2 (20 µL, 74 MBq in 0.1 N HCl) was diluted in 0.1 N phosphate (80 µL, pH 7.4) or 0.1 N borate buffer (80 µL, pH 8.5) and added to Ba3Sar (20 µg). The reaction mixture was kept at 40 °C for 30 min. 64Cu-labeled product was subsequently purified by analytical HPLC and the radioactive peak containing the desired product was collected. The decay-corrected radiochemical yields (RCY) were 71% (pH 7.4) and 81% (pH 8.5).
3.5. In Vitro Stability
20 MBq 64Cu-Ba3Sar was incubated in 1× PBS (1 mL, pH 7.4) at 40 °C for 24 h. Then, the stability was measured by analytical radio HPLC with the above mentioned program.
3.6. Serum Stability of 64Cu-Ba3Sar
The in vitro stability of 64Cu-Ba3Sar was evaluated by incubation of 7.4 MBq (200 μCi) of 64Cu-Ba3Sar with mouse serum (10%, 1 mL) at 37 °C. At 3 h and 24 h, the solution was filtered through a NanoSep 10 K centrifuge (Pall Corp., Port Washington, NY, USA) to isolate the low-molecular-weight metabolites. The NanoSep 10 K filter was washed with PBS (200 µL) two more times. The filtrates were combined and analyzed by reverse-phase HPLC using conditions identical to those used for the standard 64Cu-Ba3Sar analysis.
3.7. MicroPET Imaging and Biodistribution
Animal procedures were performed according to a protocol approved by the University of Southern California Institutional Animal Care and Use Committee. For static microPET scans, the mice were injected with approximately 3.7 MBq (100 μCi) of 64Cu-Ba3Sar via the tail vein (n = 3 for each group). At 5 min and 30 min post injection (p.i.), the mice were anesthetized with isoflurane (5% for induction and 2% for maintenance in 100% O2) using a knock-down box. With the help of a laser beam attached to the scanner, the mice were placed in the prone position and near the center of the field of view of the scanner. The 3-min static scans were then obtained. Images were reconstructed by use of a 2-dimensional ordered-subsets expectation maximization (OSEM) algorithm. No background correction was performed. Regions of interest (ROIs; 5 pixels for coronal and transaxial slices) were drawn over the organs of interest on decay-corrected whole-body coronal images. The maximum counts per pixel per minute were obtained from the ROI and converted to counts per milliliter per minute by using a calibration constant. With the assumption of a tissue density of 1 g/mL, the ROIs were converted to counts per gram per min. Image ROI-derived %ID/g values were determined by dividing counts per gram per minute by injected dose. No attenuation correction was performed. The biodistribution study at 24 h post injection of 64Cu-Ba3Sar was performed in a normal female nude mouse as reported [26].
4. Conclusions
Novel homo-/hetero-functionalized sarcophagine chelators for 64Cu radiopharmaceuticals have been developed. In our initial evaluation, good in vitro and in vivo stability was observed for 64Cu-Ba3Sar. These multifunctional chelators could serve as a versatile building platform for multivalent/multimodaltity imaging probe construction, which would have a broad application in both imaging and therapy related research involving copper and other radiometals.
Acknowledgments
This work was financially supported by the American Cancer Society (MSRG-12-034-01-CCE).
Author Contributions
Conception and design: Z.L. and P.S.C. Achieving Data and its analysis: S.L. and Z.L. Writing, review, and/or revision of the manuscript: S.L, Z.L, and P.S.C.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Niu G., Li Z., Cao Q., Chen X. Monitoring therapeutic response of human ovarian cancer to 17-DMAG by noninvasive PET imaging with (64)Cu-DOTA-trastuzumab. Eur. J. Nucl. Med. Mol. Imaging. 2009;36:1510–1519. doi: 10.1007/s00259-009-1158-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boswell C.A., Sun X., Niu W., Weisman G.R., Wong E.H., Rheingold A.L., Anderson C.J. Comparative in vivo stability of copper-64-labeled cross-bridged and conventional tetraazamacrocyclic complexes. J. Med. Chem. 2004;47:1465–1474. doi: 10.1021/jm030383m. [DOI] [PubMed] [Google Scholar]
- 3.Chong H.S., Mhaske S., Lin M., Bhuniya S., Song H.A., Brechbiel M.W., Sun X. Novel synthetic ligands for targeted PET imaging and radiotherapy of copper. Bioorg. Med. Chem. Lett. 2007;17:6107–6110. doi: 10.1016/j.bmcl.2007.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prasanphanich A.F., Nanda P.K., Rold T.L., Ma L., Lewis M.R., Garrison J.C., Hoffman T.J., Sieckman G.L., Figueroa S.D., Smith C.J. 64Cu-NOTA-8-Aoc-BBN(7-14)NH2] targeting vector for positron-emission tomography imaging of gastrin-releasing peptide receptor-expressing tissues. Proc. Natl. Acad. Sci. USA. 2007;104:12462–12467. doi: 10.1073/pnas.0705347104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sprague J.E., Peng Y., Fiamengo A.L., Woodin K.S., Southwick E.A., Weisman G.R., Wong E.H., Golen J.A., Rheingold A.L., Anderson C.J. SSynthesis, characterization and in vivo studies of Cu(II)-64-labeled cross-bridged tetraazamacrocycle-amide complexes as models of peptide conjugate imaging agents. J. Med. Chem. 2007;50:2527–2535. doi: 10.1021/jm070204r. [DOI] [PubMed] [Google Scholar]
- 6.Sun X., Wuest M., Weisman G.R., Wong E.H., Reed D.P., Boswell C.A., Motekaitis R., Martell A.E., Welch M.J., Anderson C.J. Radiolabeling and in vivo behavior of copper-64-labeled cross-bridged cyclam ligands. J. Med. Chem. 2002;45:469–477. doi: 10.1021/jm0103817. [DOI] [PubMed] [Google Scholar]
- 7.Woodin K.S., Heroux K.J., Boswell C.A., Wong E.H., Weisman G.R., Niu W.J., Tomellini S.A., Anderson C.J., Zakharov L.N., Rheingold A.L. Kinetic inertness and electrochemical behavior of copper(II) tetraazamacrocyclic complexes: Possible implications for in vivo stability. Eur. J. Inorg. Chem. 2005;23:4829–4833. [Google Scholar]
- 8.Liu W., Hao G., Long M.A., Anthony T., Hsieh J.T., Sun X. Imparting multivalency to a bifunctional chelator: A scaffold design for targeted PET imaging probes. Angew. Chem. Int. Ed. Engl. 2009;48:7346–7349. doi: 10.1002/anie.200903556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai H., Fissekis J., Conti P.S. Synthesis of a novel bifunctional chelator AmBaSar based on sarcophagine for peptide conjugation and 64Cu radiolabelling. Dalton Trans. 2009;27:5395–5400. doi: 10.1039/b902210d. [DOI] [PubMed] [Google Scholar]
- 10.Cai H., Li Z., Huang C.W., Park R., Shahinian A.H., Conti P.S. An improved synthesis and biological evaluation of a new cage-like bifunctional chelator, 4-((8-amino-3,6,10,13,16,19-hexaazabicyclo[6.6.6]icosane-1-ylamino)methyl) benzoic acid, for 64Cu radiopharmaceuticals. Nucl. Med. Biol. 2010;37:57–65. doi: 10.1016/j.nucmedbio.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Cai H., Li Z., Huang C.W., Shahinian A.H., Wang H., Park R., Conti P.S. Evaluation of copper-64 labeled AmBaSar conjugated cyclic RGD peptide for improved microPET imaging of integrin alphavbeta3 expression. Bioconjug. Chem. 2010;21:1417–1424. doi: 10.1021/bc900537f. [DOI] [PubMed] [Google Scholar]
- 12.Di Bartolo N.M., Sargeson A.M., Donlevy T.M., Smith S.V. Synthesis of a new cage ligand, SarAr, and its complexation with selected transition metal ions for potential use in radioimagin. J. Chem. Soc. Dalton. 2001;15:2303–2309. [Google Scholar]
- 13.Voss S.D., Smith S.V., DiBartolo N., McIntosh L.J., Cyr E.M., Bonab A.A., Dearling J.L., Carter E.A., Fischman A.J., Treves S.T., et al. Positron emission tomography (PET) imaging of neuroblastoma and melanoma with 64Cu-SarAr immunoconjugates. Proc. Natl. Acad. Sci. USA. 2007;104:17489–17493. doi: 10.1073/pnas.0708436104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Z., Jin Q., Huang C.W., Dasa S., Chen L., Yap L.P., Liu S., Cai H., Park R., Conti P.S. Trackable and Targeted Phage as Positron Emission Tomography (PET) Agent for Cancer Imaging. Theranostics. 2011;1:371–380. doi: 10.7150/thno/v01p0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper M.S., Ma M.T., Sunassee K., Shaw K.P., Williams J.D., Paul R.L., Donnelly P.S., Blower P.J. Comparison of 64Cu-Complexing Bifunctional Chelators for Radioimmunoconjugation: Labeling Efficiency, Specific Activity, and in Vitro/in Vivo Stability. Bioconjug. Chem. 2012;23:1029–1039. doi: 10.1021/bc300037w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paterson B.M., Roselt P., Denoyer D., Cullinane C., Binns D., Noonan W., Jeffery C.M., Price R.I., White J.M., Hicks R.J., et al. PET imaging of tumours with a 64Cu labeled macrobicyclic cage amine ligand tethered to Tyr3-octreotate. Dalton Trans. 2014;43:1386–1396. doi: 10.1039/c3dt52647j. [DOI] [PubMed] [Google Scholar]
- 17.Mammen M., Choi S.K., Whitesides G.M. Polyvalent interactions in biological systems: Implications for design and use of multivalent ligands and inhibitors. Angew. Chem. Int. Edit. 1998;37:2755–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 18.Li Z.B., Cai W., Cao Q., Chen K., Wu Z., He L., Chen X. 64Cu-labeled tetrameric and octameric RGD peptides for small-animal PET of tumor αvβ3 integrin expression. J. Nucl. Med. 2007;48:1162–1171. doi: 10.2967/jnumed.107.039859. [DOI] [PubMed] [Google Scholar]
- 19.Li Z.B., Chen K., Chen X. 68Ga-labeled multimeric RGD peptides for microPET imaging of integrin αvβ3 expression. Eur J. Nucl. Med. Mol. Imaging. 2008;35:1100–1108. doi: 10.1007/s00259-007-0692-y. [DOI] [PubMed] [Google Scholar]
- 20.Li Z.B., Wu Z., Chen K., Ryu E.K., Chen X. 18F-labeled BBN-RGD heterodimer for prostate cancer imaging. J. Nucl. Med. 2008;49:453–461. doi: 10.2967/jnumed.107.048009. [DOI] [PubMed] [Google Scholar]
- 21.Liu Z., Liu S., Wang F., Chen X. Noninvasive imaging of tumor integrin expression using (18)F-labeled RGD dimer peptide with PEG (4) linkers. Eur. J. Nucl. Med. Mol. Imaging. 2009;36:1296–1307. doi: 10.1007/s00259-009-1112-2. [DOI] [PubMed] [Google Scholar]
- 22.Wu Z., Li Z.B., Cai W., He L., Chin F.T., Li F., Chen X. 18F-labeled mini-PEG spacered RGD dimer (18F-FPRGD2): Synthesis and microPET imaging of alphavbeta3 integrin expression. Eur. J. Nucl. Med. Mol. Imaging. 2007;34:1823–1831. doi: 10.1007/s00259-007-0427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu S., Li Z., Yap L.P., Huang C.W., Park R., Conti P.S. Efficient preparation and biological evaluation of a novel multivalency bifunctional chelator for 64Cu radiopharmaceuticals. Chemistry. 2011;17:10222–10225. doi: 10.1002/chem.201101894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X., Tohme M., Park R., Hou Y., Bading J.R., Conti P.S. Micro-PET imaging of αvβ3-integrin expression with 18F-labeled dimeric RGD peptide. Mol. Imaging. 2004;3:96–104. doi: 10.1162/1535350041464892. [DOI] [PubMed] [Google Scholar]
- 25.Bass L.A., Wang M., Welch M.J., Anderson C.J. In vivo transchelation of copper-64 from TETA-octreotide to superoxide dismutase in rat liver. Bioconjug. Chem. 2000;11:527–532. doi: 10.1021/bc990167l. [DOI] [PubMed] [Google Scholar]
- 26.Wu Y., Zhang X., Xiong Z., Cheng Z., Fisher D.R., Liu S., Gambhir S.S., Chen X. microPET imaging of glioma integrin {alpha}v{beta}3 expression using (64)Cu-labeled tetrameric RGD peptide. J. Nucl. Med. 2005;46:1707–1718. [PubMed] [Google Scholar]