Abstract
Headspace-solid phase microextraction-gas chromatography-mass spectrometry (HS-SPME-GC-MS) was used to identify the volatile organic compounds (VOCs) of the different flower development stages of Cananga odorata for the evaluation of floral volatile polymorphism as a basis to determine the best time of harvest. Electronic nose results, coupled with discriminant factor analysis, suggested that emitted odors varied in different C. odorata flower development stages, including the bud, display-petal, initial-flowering, full-flowering, end-flowering, wilted-flower, and dried flower stages. The first two discriminant factors explained 97.52% of total system variance. Ninety-two compounds were detected over the flower life, and the mean Bray–Curtis similarity value was 52.45% among different flower development stages. A high level of volatile polymorphism was observed during flower development. The VOCs were largely grouped as hydrocarbons, esters, alcohols, aldehydes, phenols, acids, ketones, and ethers, and the main compound was β-caryophyllene (15.05%–33.30%). Other identified compounds were β-cubebene, d-germacrene, benzyl benzoate, and α-cubebene. Moreover, large numbers of VOCs were detected at intermediate times of flower development, and more hydrocarbons, esters, and alcohols were identified in the full-flowering stage. The full-flowering stage may be the most suitable period for C. odorata flower harvest.
Keywords: Cananga odorata, volatile organic compound, flower development, HS-SPME-GC-MS
1. Introduction
Cananga odorata (Lam.) Hook. f. & Thomson, commonly known as ylang-ylang, belongs to the Annonaceae family and the Cananga genus. This perennial tropical tree, native to the Indonesian archipelago, is cultivated primarily in Comoros, Mayotte, and Madagascar. The tree is also grown in the Philippines, Reunion, Thailand, Vietnam, and China [1,2]. The aroma emitted by its flowers has caused ylang-ylang to be widely utilized for garlands, headdresses, and other personal adornments. Moreover, the heavily scented flowers are used in the cosmetics industry. In particular, ylang-ylang essential oil, obtained from fresh flowers of C. odorata through water steam distillation or chemical extraction, is one of the most important raw materials used in the perfume industry in a variety of applications, from high-grade perfume production to the soap industry. Moreover, this oil has applications in aromatherapy and is approved for food use by the US Food and Drug Administration [3,4]. Therefore, C. odorata has high economic and ornamental value because for its use as fragrance and food flavor properties.
Numerous studies have already been conducted to detect the odoriferous molecules released by C. odorata [5,6,7]. As reported in the literature, the identified volatile compounds in ylang-ylang oil are largely grouped as monoterpenes, terpenic alcohols, sesquiterpenic alcohols, sesquiterpene hydrocarbons, acetates, benzoates, and phenols [8,9]. Moreover, the major oil components are p-cresyl methyl ether, methyl benzoate, linalool, benzyl acetate and geranyl acetate, β-caryophyllene, d-germacrene, and (E,E)-α-farnesene [10]. According to the measurement and comparison of 15 major compounds of ylang-ylang oil from Comoros, Mayotte and Madagascar, the ISO 3063: 2004 (E) norm was published by the French standardization system (AFNOR, French standard) [11]. However, most studies on this subject have only focused on analyzing the volatile organic compounds (VOCs) emitted by ylang-ylang oil. Limited information is available on the floral volatile polymorphism of C. odorata at different flower development stages. Moreover, the volatile compounds of different flower development stages sever as indicators that determine the time of harvest, quality of essential oil, and flavor of food, among others. Therefore, this study aims to investigate the floral volatiles in C. odorata and to evaluate the volatile polymorphism of different flower development stages to determine the best time of harvest.
2. Results and Discussion
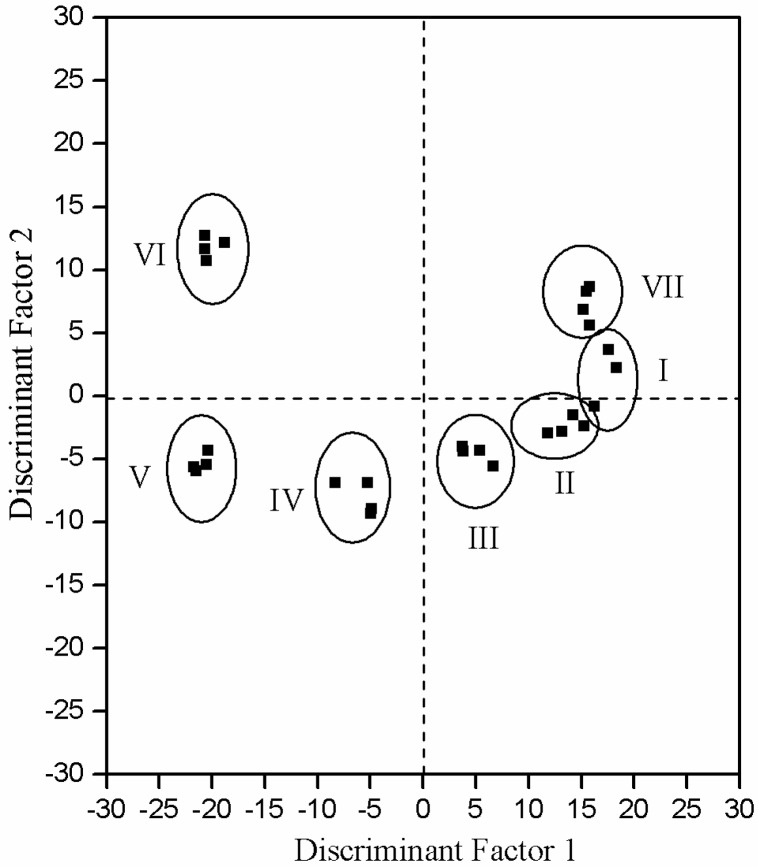
2.1. Discrimination of Different Flower Stages by Electronic Nose
C. odorata flowers were selected on the basis of their botanical characteristics to evaluate floral volatile polymorphisms according to different development stages: bud, display-petal, initial-flowering, full-flowering, end-flowering, wilted-flower, and dried flower stages [12] (Figure 1). To discriminate the different flower stages better, an electronic nose was coupled with discriminant factor analysis (DFA) [13,14] to classify the aroma emitted by C. odorata flowers. The process was conducted by using signals corresponding to four repeated exposures of each stage, as shown in Figure 2. The DFA score plot in Figure 2 shows that the electronic nose effectively discriminates each of the different flower stages of C. odorata. The first two discriminant factors, DF1 (81.16%) and DF2 (16.36%), explain 97.52% of total system variance. DFA is as valid as the multivariate statistical model if the percentage of recognition is higher than 90% [14]. In this case, the percentage of recognition was 97.52% (maximum 100%), which indicates that a certain degree of discrimination was achieved. For instance, the initial-flowering, full-flowering, and end-flowering stages were clearly distinguishable from one group to another, although a small overlap was observed between the bud and display-petal stages. These results show that the odors emitted at different stages of C. odorata flower development evidently differed. As Wilson and Baietto [13] suggested, electronic nose analysis based on botanical characteristics is a useful technique to discriminate flower aromas at different stages.
Figure 1.
The morphological characteristics of C. odorata flower in seven different stages. (I) bud stage; (II) display-petal stage; (III) initial-flowering stage; (IV) full-flowering stage; (V) end-flowering stage; (VI) wilted-flower stage; and (VII) dried flower stage.
Figure 2.
Two-dimensional (2D) DFA plots of flower from different stages by electronic nose. (I) bud stage; (II) display-petal stage; (III) initial-flowering stage; (IV) full-flowering stage; (V) end-flowering stage; (VI) wilted-flower stage; and (VII) dried flower stage.
2.2. Analysis of C. odorata Floral Volatile Polymorphism by Headspace-Solid Phase Microextraction-Gas Chromatography-Mass Spectrometry (HS-SPME-GC-MS)
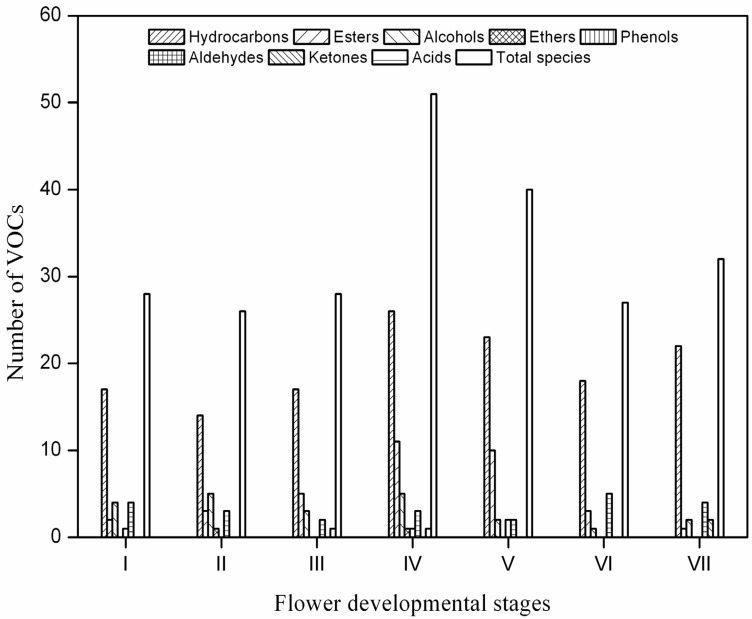
Previous researchers [7,8,9,10] have extensively investigated the volatile compositions of essential oils from C. odorata flower by using the hydrodistillation method [10,15]. However, distillation has several disadvantages, including time consumption, and loss of target compounds because of thermal degradation [16,17,18,19,20]. Studies on the volatile compounds present at different stages of C. odorata flower development have never been conducted elsewhere. Therefore, the volatile compounds in C. odorata flowers were analyzed by using HS-SPME coupled with GC-MS. Table 1 shows the 92 components identified over the flower-life. These components include 47 hydrocarbons, 17 esters, 14 alcohols, seven aldehydes, two phenols, 1 acid, three ketones, and one ether. In accordance with the report by Stashenko et al. [9], Burdock and Carabin [3], the total profile of the volatile compounds in all studied stages reveals the predominance of hydrocarbons, esters, alcohols, and aldehydes (Figure 3). Of these 92 compounds, 11 were particularly identified in the full-flowering stage on the basis of 15 AFNOR (ISO 3063: 2004 (E)). This finding is considered as characteristic of ylang-ylang essential oil. Additionally, volatile compositions varied considerably among the seven life-flower stages of C. odorata flowers. The mean Bray–Curtis similarity (BCS) value was 52.45% ± 11.61% (range: 32.84%–77.34%, n = 21 comparisons, Table 2). The full-flowering stage was more similar to the initial-flowering stage (BCS = 59.68%) than to the dried flower stage (BCS = 42.63%), and was largely dissimilar to the wilted-flower stage (BCS = 35.02%). As regards the comparison among the studied stages, 10 volatiles among the total volatile constituents notably existed in all life-flower stages, whereas 11 more volatile compounds were present in four of the seven stages. The results showed low similarity among the seven stages, although several constitutes [α-cadinol, farnesol isomer a, nerolidol, α-pinene, etc.] were exclusively identified in different stages. The pattern may indicate a relatively high volatile diversity variation at a full life-flower scale.
Table 1.
Volatile compounds identified in seven different stages of C. odorata flower development using HS-SPME-GC-MS. (I) bud stage; (II) display-petal stage; (III) initial-flowering stage; (IV) full-flowering stage; (V) end-flowering stage; (VI) wilted-flower stage; and (VII) dried flower stage.
| Peak | RT * (min.) | LRI * | Compounds | Relative Content (%) ± SD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | IV | VII | ||||
| Acids | ||||||||||
| 1 | 9.001 | 1020 | (S)-α-methoxybenzeneacetic acid | - | - | 6.631 ± 0.601 | 11.711 ± 0.571 | - | - | - |
| Esters | ||||||||||
| 2 | 8.460 | 1002 | (Z)-3-hexen-1-ol acetate | - | - | - | 0.070 ± 0.012 | - | - | - |
| 3 | 8.675 | 1009 | hexyl acetate | - | - | - | 0.026 ± 0.001 | - | - | - |
| 4 | 8.746 | 1011 | (E)-2-hexen-1-ol acetate | - | - | - | 0.044 ± 0.019 | - | - | - |
| 5 | 11.219 | 1094 | methyl benzoate | - | 0.067 ± 0.011 | 0.308 ± 0.002 | 2.605 ± 0.673 | 5.604 ± 0.129 | - | - |
| 6 | 13.240 | 1162 | benzyl acetate | - | - | - | 0.149 ± 0.001 | 0.126 ± 0.030 | - | - |
| 7 | 13.495 | 1170 | benzoic acid, ethyl ester | - | - | - | 0.314 ± 0.260 | 0.226 ± 0.085 | - | - |
| 8 | 15.481 | 1238 | linalyl acetate | - | - | - | - | - | 0.139 ± 0.380 | - |
| 9 | 17.191 | 1298 | methyl 2-methoxybenzoate | - | - | 0.050 ± 0.012 | - | - | - | - |
| 10 | 18.810 | 1358 | lavandulyl acetate | - | - | - | 0.079 ± 0.016 | 0.063 ± 0.014 | - | - |
| 11 | 19.258 | 1374 | 1,2-ethanediol 1-benzoate | - | - | - | 0.304 ± 0.239 | - | - | - |
| 12 | 19.369 | 1378 | neryl acetate | - | - | 7.882 ± 0.227 | 11.740 ± 0.718 | - | - | - |
| 13 | 19.380 | 1379 | geraniol acetate | - | 0.075 ± 0.051 | - | - | 13.777 ± 1.311 | - | - |
| 14 | 21.092 | 1445 | cinnamyl acetate | - | - | - | 0.073 ± 0.008 | 0.161 ± 0.015 | - | - |
| 15 | 24.231 | 1572 | (Z)-3-hexenyl benzoate | 0.123 ± 0.013 | - | 0.115 ± 0.042 | - | 0.118 ± 0.014 | 0.144 ± 0.001 | - |
| 16 | 28.647 | 1768 | benzyl benzoate | 1.638 ± 0.054 | 2.498 ± 0.004 | 2.239 ± 0.802 | 2.146 ± 1.489 | 6.150 ± 0.896 | 2.893 ± 0.817 | 0.668 ± 0.226 |
| 17 | 30.801 | 1871 | benzyl salicylate | - | - | - | 0.066 ± 0.014 | 0.113 ± 0.030 | - | - |
| 18 | 32.626 | 1963 | geranyl benzoate | - | - | - | - | 0.044 ± 0.018 | - | - |
| Alcohols | ||||||||||
| 19 | 4.919 | 854 | 3-hexen-1-ol | - | 0.086 ± 0.025 | - | - | - | - | - |
| 20 | 9.349 | 1031 | α-toluenol | - | - | - | 0.071 ± 0.048 | 0.086 ± 0.033 | - | - |
| 21 | 9.365 | 1032 | (±)-1,2-ethanediol, 1,2-diphenyl-, (R*, R*) | 0.159 ± 0.017 | - | - | - | - | - | - |
| 22 | 9.425 | 1034 | p-tolualcohol | - | - | - | 0.061 ± 0.006 | - | - | - |
| 23 | 11.364 | 1099 | β-linalool | - | - | 0.053 ± 0.010 | 0.062 ± 0.001 | - | - | - |
| 24 | 15.819 | 1250 | β-geraniol | 1.396 ± 0.101 | 2.262 ± 0.102 | - | - | - | - | - |
| 25 | 23.698 | 1550 | elemol | 0.310 ± 0.045 | - | - | - | - | - | - |
| 26 | 24.402 | 1579 | germacrene D-4-ol | - | 0.387 ± 0.106 | 0.335 ± 0.042 | - | - | - | - |
| 27 | 25.815 | 1640 | 10,10-dimethyl-2,6-dimethylenebicyclo[7.2.0]undecan-5-β-ol | - | - | - | - | - | - | 0.425 ± 0.108 |
| 28 | 25.939 | 1646 | t-cadinol | - | - | - | 0.090 ± 0.010 | - | - | - |
| 29 | 26.214 | 1658 | α-cadinol | - | 0.146 ± 0.049 | - | - | - | - | - |
| 30 | 27.500 | 1715 | geranylgeraniol | - | - | - | 0.736 ± 0.129 | - | 0.027 ± 0.000 | - |
| 31 | 27.527 | 1716 | farnesyl alcohol | 0.406 ± 0.043 | 1.238 ± 0.038 | 0.383 ± 0.000 | - | 1.227 ± 0.319 | - | - |
| 32 | 29.988 | 1832 | (E)-nerolidol | - | - | - | - | - | - | 0.113 ± 0.000 |
| Hydrocarbons | ||||||||||
| 33 | 6.558 | 931 | α-pinene | 0.196 ± 0.009 | - | - | - | - | - | - |
| 34 | 8.034 | 986 | bicyclo[3.1.1]hept-2-ene, 3,6,6-trimethyl | - | - | - | 0.138 ± 0.025 | - | - | - |
| 35 | 8.057 | 987 | β-pinene | 0.132 ± 0.024 | - | 4.061 ± 0.000 | 2.861 ± 0.277 | 6.872 ± 1.055 | - | - |
| 36 | 8.067 | 988 | β-myrcene | - | 0.106 ± 0.005 | 0.118 ± 0.010 | - | - | - | 0.097 ± 0.000 |
| 37 | 9.262 | 1029 | α-limonene | - | - | - | 0.078 ± 0.009 | - | 0.029 ± 0.008 | 0.030 ± 0.000 |
| 38 | 9.283 | 1030 | 3-ethylidenecycloheptene | - | - | - | - | 0.072 ± 0.012 | - | - |
| 39 | 10.961 | 1086 | isoterpinolene | - | - | - | 0.022 ± 0.003 | - | - | - |
| 40 | 19.348 | 1376 | α-ylangene | 4.093 ± 0.099 | - | - | - | - | - | - |
| 41 | 19.357 | 1378 | α-copaene | - | 6.019 ± 0.361 | - | - | - | 2.669 ± 0.298 | - |
| 42 | 19.516 | 1384 | p-anisyl acetate | - | - | - | - | 0.107 ± 0.028 | - | - |
| 43 | 19.522 | 1384 | cyclohexene, 1-methyl-5-(1-methylethenyl) | - | - | - | 0.058 ± 0.009 | - | - | - |
| 44 | 19.587 | 1386 | α-bourbonene | - | - | - | - | - | - | 0.118 ± 0.000 |
| 45 | 19.715 | 1391 | β-elemen | 1.545 ± 0.076 | - | - | 0.736 ± 0.088 | - | 0.648 ± 0.228 | - |
| 46 | 20.591 | 1425 | β-caryophyllene | 33.296 ± 0.710 | 32.526 ± 0.456 | 26.866 ± 1.198 | 17.013 ± 0.233 | 15.048 ± 1.629 | 21.154 ± 0.230 | 27.904 ± 0.462 |
| 47 | 20.789 | 1432 | β-cubebene | 0.382 ± 0.022 | 7.139 ± 0.617 | 7.347 ± 0.619 | 11.790 ± 1.428 | 12.914 ± 1.285 | 0.691 ± 0.052 | 10.012 ± 0.883 |
| 48 | 21.014 | 1439 | α-selinene | - | - | - | - | 0.025 ± 0.005 | - | - |
| 49 | 21.015 | 1441 | γ-caryophyllene | - | - | - | - | - | - | 0.038 ± 0.002 |
| 50 | 21.021 | 1442 | L-alloaromadendrene | - | - | 0.037 ± 0.000 | 12.792 ± 1.004 | - | - | - |
| 51 | 21.179 | 1448 | isoledene | - | - | - | 0.088 ± 0.001 | - | - | - |
| 52 | 21.288 | 1452 | α-cubebene | 1.095 ± 0.173 | 0.448 ± 0.196 | 0.417 ± 0.036 | 0.147 ± 0.015 | 0.183 ± 0.019 | 0.159 ± 0.016 | 0.574 ± 0.167 |
| 53 | 21.312 | 1453 | (E)-β-farnesene | - | - | - | 0.284 ± 0.243 | 0.522 ± 0.040 | 0.931 ± 0.000 | 0.895 ± 0.000 |
| 54 | 21.473 | 1460 | α-caryophyllene | 13.975 ± 0.545 | 12.974 ± 0.157 | 10.189 ± 0.042 | 5.966 ± 0.416 | 5.220 ± 0.598 | 8.366 ± 0.017 | 9.037 ± 0.228 |
| 55 | 22.106 | 1485 | D-germacrene | 10.857 ± 0.191 | 13.732 ± 0.000 | 7.018 ± 0.627 | 1.067 ± 0.008 | 0.462 ± 0.053 | 19.498 ± 1.054 | 9.027 ± 0.792 |
| 56 | 22.366 | 1495 | (+)-epi-bicyclosesquiphellandrene | 1.457 ± 0.213 | 0.592 ± 0.422 | - | 0.320 ± 0.022 | 0.327 ± 0.070 | - | - |
| 57 | 22.449 | 1498 | bicyclogermacrene | 1.767 ± 0.593 | 1.994 ± 0.386 | 0.752 ± 0.030 | 0.511 ± 0.051 | 0.538 ± 0.078 | 0.723 ± 0.079 | 0.418 ± 0.000 |
| 58 | 22.500 | 1500 | α-muurolene | - | 0.522 ± 0.000 | 0.516 ± 0.000 | 0.576 ± 0.072 | 0.274 ± 0.012 | - | - |
| 59 | 22.589 | 1501 | (Z,E)-α-farnesene | 5.144 ± 0.249 | - | - | 6.913 ± 1.857 | - | - | - |
| 60 | 22.602 | 1505 | [S-(R*,S*)]-5-(1,5-dimethylhexen-4-yl)-2-methyl-1,3-cyclohexa-1,3-diene | - | - | - | - | - | - | 14.114 ± 0.000 |
| 61 | 22.639 | 1507 | α-farnesene | - | - | 11.185 ± 0.000 | 0.070 ± 0.001 | 5.935 ± 0.306 | 15.687 ± 0.000 | 0.117 ± 0.025 |
| 62 | 22.793 | 1512 | (−)-β-bisabolene | - | - | - | 0.196 ± 0.113 | 0.267 ± 0.050 | 0.570 ± 0.000 | 0.423 ± 0.000 |
| 63 | 22.931 | 1518 | bicyclo[4.4.0]dec-1-ene 2-isopropyl-5-methyl-9-methylene | - | 0.751 ± 0.000 | - | - | - | - | 0.768 ± 0.000 |
| 64 | 22.982 | 1520 | δ-cadinene | 3.361 ± 0.118 | 2.426 ± 0.394 | 2.405 ± 0.087 | 2.405 ± 0.117 | 1.315 ± 0.112 | 1.723 ± 0.186 | 2.132 ± 0.005 |
| 65 | 23.067 | 1524 | l-calamenene | 1.078 ± 0.075 | - | - | - | - | - | 0.726 ± 0.000 |
| 66 | 23.101 | 1525 | 4,9-cadinadiene | - | - | - | - | 0.271 ± 0.027 | - | 0.311 ± 0.000 |
| 67 | 23.176 | 1528 | cis-α-bisabolene | - | - | - | - | 0.081 ± 0.005 | - | - |
| 68 | 23.333 | 1534 | α-cedrene | - | - | - | - | 0.111 ± 0.011 | 0.163 ± 0.000 | 0.199 ± 0.006 |
| 69 | 23.334 | 1535 | 1,4-cadinadiene | - | 0.133 ± 0.030 | 0.568 ± 0.014 | - | - | - | - |
| 70 | 23.335 | 1536 | α-funebrene | - | - | - | 0.189 ± 0.039 | - | - | - |
| 71 | 23.434 | 1538 | bicyclo[2.2.1]heptane 2-cyclopropylidene-1,7,7-trimethyl | - | - | - | 0.440 ± 0.102 | 0.102 ± 0.005 | 0.272 ± 0.149 | - |
| 72 | 23.697 | 1550 | (E,Z)-α-farnesene | - | - | 0.036 ± 0.019 | - | - | 0.040 ± 0.000 | - |
| 73 | 23.818 | 1554 | α-bergamotene | - | - | 0.061 ± 0.030 | - | - | - | - |
| 74 | 23.819 | 1555 | β-gurjurene | 1.277 ± 0.165 | 0.036 ± 0.001 | 0.131 ± 0.000 | 1.159 ± 0.041 | 0.131 ± 0.001 | 0.531 ± 0.247 | 0.939 ± 0.249 |
| 75 | 24.401 | 1578 | β-bourbonene | - | - | - | 0.125 ± 0.009 | - | - | - |
| 76 | 24.548 | 1585 | β-caryophyllene oxide | - | - | - | 0.042 ± 0.015 | - | - | 3.659 ± 0.365 |
| 77 | 25.590 | 1630 | (+)-α-longipinene | 0.123 ± 0.013 | - | - | - | - | - | - |
| 78 | 25.937 | 1646 | copaene | 0.229 ± 0.028 | - | 0.069 ± 0.000 | 0.179 ± 0.117 | 0.297 ± 0.232 | 0.434 ± 0.060 | 3.339 ± 0.206 |
| 79 | 26.210 | 1659 | elixene | - | - | - | - | 0.247 ± 0.212 | - | - |
| Phenols | ||||||||||
| 80 | 10.516 | 1071 | p-kresol | - | - | - | 0.174 ± 0.107 | 0.048 ± 0.013 | - | - |
| 81 | 19.918 | 1399 | 1,2-dimethoxy-4-(2-propenyl)-benzen | 0.087 ± 0.020 | - | - | - | 0.138 ± 0.056 | - | - |
| Ethers | ||||||||||
| 82 | 14.346 | 1099 | estragole | - | 0.137 ± 0.008 | - | 0.125 ± 0.009 | - | - | - |
| Aldehydes | ||||||||||
| 83 | 7.313 | 959 | benzaldehyde | 0.142 ± 0.014 | 0.072 ± 0.021 | - | - | - | 4.193 ± 0.413 | 1.600 ± 0.000 |
| 84 | 9.684 | 1043 | benzeneacetaldehyde | - | - | - | - | - | 0.177 ± 0.094 | - |
| 85 | 15.479 | 1237 | citral b | - | - | 0.202 ± 0.000 | - | 0.059 ± 0.006 | - | 0.538 ± 0.000 |
| 86 | 15.486 | 1239 | β-citral | 0.623 ± 0.111 | 0.512 ± 0.512 | - | 0.194 ± 0.086 | - | - | 0.081 ± 0.025 |
| 87 | 16.322 | 1268 | α-citral | 2.226 ± 0.269 | 2.166 ± 0.221 | 1.756 ±0.085 | 1.685 ± 0.314 | 1.377 ± 0.046 | 1.021 ± 0.093 | 1.009 ± 0.005 |
| 88 | 27.365 | 1709 | farnesal | - | - | - | - | - | 1.128 ± 0.014 | - |
| 89 | 27.965 | 1736 | (E,E)-farnesal | 0.192 ± 0.014 | - | - | 0.034 ± 0.023 | - | 3.131 ± 1.136 | - |
| Ketones | ||||||||||
| 90 | 7.892 | 981 | methyl heptenone | - | - | - | - | - | - | 0.218 ± 0.013 |
| 91 | 14.153 | 1192 | 2-hydroxy-3-cyanopyridine | - | - | - | 0.047 ± 0.001 | 0.101 ± 0.055 | - | 0.089 ± 0.007 |
| 92 | 25.197 | 1613 | junenol | - | - | - | - | - | - | 0.665 ± 0.000 |
* RT = Retention time (minutes); * LRI = Linear retention indices to C7-C30 n-alkanes on DB-5MS column, LRI literatures were according to the results of Benini et al. [2], the database of VCF 15.1, and the Pherobase.
Figure 3.
The composition of volatile compounds in different stages of C. odorata flower development by HS-SPME-GC-MS. (I) bud stage; (II) display-petal stage; (III) initial-flowering stage; (IV) full-flowering stage; (V) end-flowering stage; (VI) wilted-flower stage; and (VII) dried flower stage.
Table 2.
The Bray-Curtis similarity values (%) among different stages of C. odorata flower development. (I) bud stage; (II) display-petal stage; (III) initial-flowering stage; (IV) full-flowering stage; (V) end-flowering stage; (VI) wilted-flower stage; and (VII) dried flower stage.
| I | II | III | IV | V | VI | VII | |
|---|---|---|---|---|---|---|---|
| I | 100 | ||||||
| II | 77.34 | 100 | |||||
| III | 58.37 | 66.78 | 100 | ||||
| IV | 41.61 | 41.90 | 59.68 | 100 | |||
| V | 32.84 | 42.16 | 52.26 | 50.98 | 100 | ||
| VI | 54.90 | 60.00 | 61.08 | 35.02 | 41.49 | 100 | |
| VII | 59.99 | 65.60 | 61.02 | 42.63 | 42.33 | 53.44 | 100 |
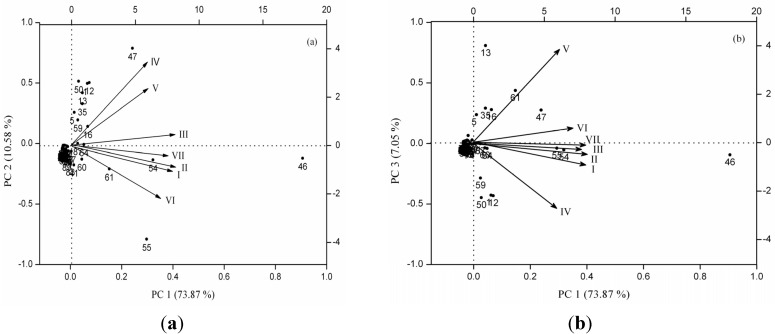
Variations of the volatile compositions emitted by C. odorata flowers significantly depend on the stage of maturity. The same phenomena are also observed in other plant species, such as Michelia alba [21], and Michelia champaca flowers [16]. In this study, the highest floral volatile polymorphism was detected at the intermediate levels of flower development, and the richness of volatile compounds showed a unimodal or hump-shaped pattern between the number of VOCs and times of flower development. To identify which volatiles contributed the most to the differences among the seven flower stages, the data on 92 volatile compounds identified in C. odorata at a full life-flower scale were analyzed by using principal component analysis (PCA). The first three components of PCA explained 73.87%, 10.58%, and 7.05% of the variation, explaining ~92% of combined variance (Figure 4). Hereinto, volatiles that had high positive scores on PC 1 include α-caryophyllene, d-germacrene, α-farnesene, δ-cadinene, α-citral, and β-caryophyllene, which are highly positively related to the bud, display-petal, and initial-flowering stages. Volatiles with high positive scores on PC 2 include benzyl benzoate, methyl benzoate, (S)-α-methoxybenzeneacetic acid, neryl acetate, geraniol acetate, β-pinene, β-cubebene, (Z,E)-α-farnesene, and l-alloaromadendrene, which are negatively correlated with the wilted-flower stage. The remaining 77 volatiles components, which include common components, α-cubebene, bicyclogermacrene, β-gurjurene, and copaene, as well as relatively rare volatile compounds, including 3-hexen-1-ol, β-myrcene, α-ylangene, do not exhibit any association with the first three components.
Figure 4.
Principal components analysis biplot showing relationship between the different stages of C. odorata flower development and the volatile compounds: PC1 vs. PC2 plots (a) and PC 1 vs. PC 3 plots (b). (I) bud stage, (II) display-petal stage, (III) initial-flowering stage, (IV) full-flowering stage, (V) end-flowering stage, (VI) wilted-flower stage, and (VII) dried flower stage. Black dots represent distribution of 92 volatile compounds in C. odorata flower (numbers correspond to those in Table 1).
As for the bud stage, 28 volatile compounds belonging to different chemical classes were identified: hydrocarbons (80.01%), esters (1.76%), aldehydes (3.18%), and alcohols (2.27%). The most abundant compound was β-caryophyllene, accounting for about 35% of the total GC peak area, followed by α-caryophyllene (13.98%), d-germacrene (10.86%), (Z,E)-α-farnesene (5.14%), and α-ylangene (4.09%). With respect to other flower stages, the bud stage was characterized by higher hydrocarbons.
As for the display-petal stage, 26 volatile compounds belonging to different chemical classes were identified: hydrocarbons (79.40%), esters (2.64%), alcohols (4.12%), and aldehydes (2.75%). The most abundant compound was β-caryophyllene, accounting for about 32% of the total GC peak area, followed by d-germacrene (13.73%), α-caryophyllene (12.97%), β-cubebene (7.14%), and α-copaene (6.02%). With respect to other flower stages, the display-petal stage was characterized by higher alcohols.
As for the initial-flowering stage, 28 volatile compounds belonging to different chemical classes were identified: hydrocarbons (71.78%), esters (10.59%), acids (6.63%), alcohols (0.77%), and aldehydes (1.96%). The most abundant compound was β-caryophyllene, accounting for about 26.87% of the total GC peak area, followed by α-farnesene (11.86%), α-caryophyllene (10.19%), neryl acetate (7.89%), β-cubebene (7.35%), and d-germacrene (7.02%). With respect to other flower stages, the initial-flowering stage was characterized by higher acids.
As for the full-flowering stage, 51 volatile compounds belonging to different chemical classes were identified: hydrocarbons (66.17%), esters (17.62%), acides (11.72%), aldehydes (3.83%), and alcohols (1.02%). The most abundant compound was β-caryophyllene, accounting for about 17.03% of the total GC peak area, followed by l-alloaromadendrene (12.79%), β-cubebene (11.79%), neryl acetate (11.74%), (S)-α-methoxybenzeneacetic acid (11.71%), and (Z,E)-α-farnesene (6.91%). With respect to other flower stages, the full-flowering stage was characterized by higher esters and acids.
As for the end-flowering stage, 40 volatile compounds belonging to different chemical classes were identified: hydrocarbons (51.07%), esters (26.38%), alcohols (1.13%), and aldehydes (2.87%). The most abundant compound was β-caryophyllene, accounting for about 15.05% of the total GC peak area, followed by geraniol acetate (13.78%), β-cubebene (12.91%), β-pinene (6.87%), benzyl benzoate (6.15%), and α-farnesene (5.94%). With respect to other flower stages, the end-flowering stage was characterized by higher esters.
As for the wilted-flower stage, 27 volatile compounds belonging to different chemical classes were identified: hydrocarbons (74.29%), aldehydes (19.30%), and esters (3.18%). The most abundant compound was β-caryophyllene, accounting for about 21.15% of the total GC peak area, followed by d-germacrene (19.50%), β-cubebene (0.69%), α-farnesene (15.69%), α-caryophyllene (8.37%), and benzaldehyde (4.19%). With respect to other flower stages, the wilted-flower stage was characterized by higher aldehydes.
As for the dried flower stage, 32 volatile compounds belonging to different chemical classes were identified: hydrocarbons (84.88%), aldehydes (6.46%), and esters (0.69%). The most abundant compound was β-caryophyllene, accounting for about 27.90% of the total GC peak area, followed by [S-(R*,S*)]-5-(1,5-dimethylhexen-4-yl)-2-methyl-1,3-cyclohexa-1,3-diene (14.11%), β-cubebene (10.01%), α-caryophyllene (9.04%), and d-germacrene (9.03%). With respect to other flower stages, the dried flower stage was characterized by higher hydrocarbons.
3. Experimental
3.1. Plant Materials
Fresh C. odorata flowers came from populations grown at the Spice and Beverage Research Institute, CATAS in Hainan, China. The flowers were classified into seven groups according to their botanical characteristics (Figure 1): (I) bud stage: buds emerged from scaly bracts, yellowish green, completely closed; (II) display-petal stage: completely open calyxes, petals spreading, pubescent, and green; (III) initial-flowering stage: semi-open petals, light green; (IV) full-flowering stage: completely open petals, green, observable pistils and stamens; (V) end-flowering stage: fully matured petals of deep yellow coloration; (VI) wilted-flower stage: petals and calyxes withered and yellow; and (VII) dried- flower stage: petals dried.
3.2. Methods
The inflorescence of C. odorata is a raceme that always exhibits inconsistent flowering [12]. Approximately 20 g of raw flower material of C. odorata was collected at 8:00 a.m.–10:00 a.m., 8–10 August 2013, depending on the different stages of flower development. Following collections, the flowers were moisturized and sent back to the laboratory immediately. In all experiments, flowers were sliced by using a knife into similarly thin slices to be able to place the sample in a headspace bottle (volume ~5 mL) while maintaining uniform flower sample structure and weight. Thereafter, 1.5 g of sliced materials were weighted and allowed to stand for 30 min at ambient room temperature 22 ± 3 °C. The assayed fibers used in this study were DVB-CAR-PDMS with a 50/30 μm film thickness (Supelco, Bellefonte, PA, USA). The SPME device was inserted into the sealed vial by manually penetrating the silicone septum, and the fiber was exposed to the headspace of the sliced material after 40 min. After extraction, the needle on the SPME manual holder was set to 0.5 cm in the GC injector. The fiber was then directly desorbed for 10min. Each sampling and analysis step was performed in triplicate. Empty bottles were used as a control in the analyses.
An HP-7890A/5975C GC-MS system (Agilent Technologies, Wilmington, USA), with a DB-5MS column (30 m × 0.25 mm I.D. × 0.25 μm microns, Agilent Technologies) was used under the following conditions: MS transfer line heater of 280 °C, injector temperature of 250 °C, and operation in the splitless mode. Initial oven temperature was held at 50 °C for 5 min., then programmed from 50 °C to 80 °C at 10 °C/min, from 80 °C to 220 °C at 5 °C/min, from 220 °C to 280 °C at 10 °C/min, and finally maintained for 6 min at 280 °C. Helium gas was used as a carrier gas at a flow rate of 1.0 mL/min. An Agilent 5975 C mass spectrometer was operated in the electron ionization mode at 70 eV with a source temperature of 230 °C, a quadrupole set to 150 °C, and a scan from an m/z 30 to 500 in the full-scan mode.
Linear retention indices (LRI) were determined on the basis of alkanes series (C7–C30). It is defined as:
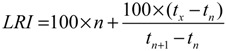 |
(1) |
where tn and tn+1 are retention times of the reference n-alkanes hydrocarbons eluting immediately before and after chemical compound “x”, tx is the retention time of compound “x”. Volatile compounds were identified on the basis of their LRI and by comparing their mass spectra with a computerized MS-database using NIST 2008 library, Volatile Compounds in Food 15.1, and the Pherobase (database of pheromones and semi-chemicals). We also compared the fragmentation patterns in the mass spectra with those reported in the literatures [2,9]. The concentrations of the component (relative contents were done by calculating percentage of peak area in GC chromatograms), computerized by normalization method from the equation:
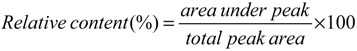 |
(2) |
Additionally, a Bray-Curtis similarity (BCS), signifying the compositional similarity different flower developmental stages, was used to examine whether the floral volatiles in C. odorata were different. This index incorporates the presence as well as relative content of the component [22] It is defined as:
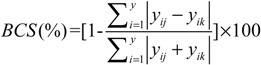 |
(3) |
where yij and yik are the relative content of component i in group j and k, respectively, and p is the total number of components in both groups.
3.3. Data Analysis
Volatile compounds identified in C. odorata at a full life-flower scale were analyzed by using principal component analysis (PCA). PCA was carried out using R package version 2.12.2 for Windows.
4. Conclusions
The odors emitted by C. odorata flowers evidently differed among flower development stages. Ninety-two volatile compounds were identified in all flower-life stages, and the main compounds were caryophyllene, α-caryophyllene, β-cubebene, d-germacrene, benzyl benzoate, δ-cadinene, β-gurjurene, α-citral, and α-cubebene. The relative content of benzyl benzoate and β-cubebene became more obvious during the expansion of bud and full opening of flowers. However, when the flower developed further, the emissions of these major components decreased, whereas those of β-caryophyllene and d-germacrene increased. Moreover, the volatile compounds emitted by C. odorata flowers largely depend on the stage of maturity. Large numbers of VOCs emerged at intermediate times of flower development. More hydrocarbon, esters, and alcohols compounds were detected in the full-flowering stage than in other flower stages, which may have contributed to the aroma profile of the flowers. Thus, a high level of floral volatile polymorphism was observed in C. odorata at a full life-flower scale. Under these circumstances, measures that introduce timely harvest, such as selective harvesting at intermediate levels of flower development, are recommended when the high level of volatile compounds and the aroma quality of flowers are a concern.
Acknowledgments
Financial support from the Ministry of Agriculture of the People’s Republic of China (No. 2014NWB044) is gratefully acknowledged.
Author Contributions
X.-W.Q., C.-Y.H., L.-H.T. conceived and designed research; S.-Z.H., F.X., R.-S.H. performed research and analyzed the data; X.-W.Q. and L.-H.T. wrote the paper; G.W. performed research and contributed materials. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Not available.
References
- 1.Kristiawan M., Sobolik V., Allaf K. Isolation of Indonesian cananga oil using multi-cycle pressure drop process. J. Chromatogr. A. 2008;1192:306–318. doi: 10.1016/j.chroma.2008.03.068. [DOI] [PubMed] [Google Scholar]
- 2.Benini C., Ringuet M., Wathelet J.P., Lognay G., du Jardin P., Fauconnier M.L. Variations in the essential oils from ylang-ylang (Cananga odorata [Lam.] Hook f. & Thomson forma genuina) in the Western Indian Ocean islands. Flavour Frag. J. 2012;27:356–366. doi: 10.1002/ffj.3106. [DOI] [Google Scholar]
- 3.Burdock G.A., Carabin L.G. Safety assessment of ylang-ylang oil as a food ingredient. Food Chem. Toxicol. 2008;46:433–445. doi: 10.1016/j.fct.2007.09.105. [DOI] [PubMed] [Google Scholar]
- 4.Jung D.J., Cha J.Y., Kim S.E., Ko I.G., Jee Y.S. Effect of ylang-ylang aroma on blood pressure and heart rate in healthy men. J. Exerc. Rehabil. 2013;9:250–255. doi: 10.12965/jer.130007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benini C., Danflous J.P., Wathelet J.P., du Jardin P., Fauconnier M.L. Ylang-ylang [Cananga odorata (Lam.) Hook. f. & Thomson]: An unknown essential oil plant in an endangered sector. Biotechnol. Agron. Soc. 2010;14:693–705. [Google Scholar]
- 6.Kristiawan M., Sobolik V., Allaf K. Yield and composition of Indonesian cananga oil obtained by steam distillation and organic solvent extraction. Int. J. Food Eng. 2012;8:1–18. [Google Scholar]
- 7.Brokl M., Fauconnier M.L., Benini C., Lognay G., du Jardin P., Focant J.F. Improvement of Ylang-Ylang essential oil characterization by GC-GC-TOFMS. Molecules. 2013;18:1783–1797. doi: 10.3390/molecules18021783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaydou E.M., Randriamiharisoa R., Bianchini J.P. Composition of the essential oil of Ylang-Ylang (Cananga odorata Hook Fil. & Thomson forma genuina) from Madagascar. J. Agric. Food Chem. 1986;34:481–487. doi: 10.1021/jf00069a028. [DOI] [Google Scholar]
- 9.Stashenko E.E., Torres W., Morales J.R.M. A study of the compositional variation of the essential oil of ylang-ylang (Cananga odorata Hook Fil. & Thomson, forma genuina) during flower development. J. High Resolut. Chromatogr. 1995;18:101–104. doi: 10.1002/jhrc.1240180206. [DOI] [Google Scholar]
- 10.Benini C., Mahy G., Bizoux J.P., Wathelet J.P., du Jardin P., Brostaux Y., Fauconnier M.L. Comparative chemical and molecular variability of Cananga odorata (Lam.) Hook. f. & Thomson forma genuina (Ylang-Ylang) in the Western Indian Ocean islands: Implication for valorization. Chem. Biodivers. 2012;9:1389–1402. doi: 10.1002/cbdv.201100306. [DOI] [PubMed] [Google Scholar]
- 11.AFNOR . Oil of Ylang-Ylang [Cananga odorata (Lam.) Hook. f. & Thomson forma genuina] AFNOR; Paris, France: 2005. [Google Scholar]
- 12.Editorial Committee of Flora Reipublicae Popularis Sinicae . Flora Reipublicae Popularis Sinicae. Science Press; Beijing, China: 1979. p. 119. [Google Scholar]
- 13.Wilson A.D., Baietto M. Advances in electronic-nose technologies development for biomedical applications. Sensors. 2011;11:1105–1176. doi: 10.3390/s110101105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang M.X., Wang X.C., Liu Y., Xu X.L., Zhou G.H. Species discrimination among three kinds of puffer fish using an electronic nose combined with olfactory sensory evaluation. Sensors. 2012;12:12562–12571. doi: 10.3390/s120912562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olivero J., Gracia T., Payares P., Vivas R., Diaz D., Daza E., Paul G. Molecular structure and gas chromatographic retention behavior of the components of Ylang-Ylang oil. J. Pharm. Sci. 1997;86:625–630. doi: 10.1021/js960196u. [DOI] [PubMed] [Google Scholar]
- 16.Jiang D.Y., Li Y.H., He F., Lin Q.P., Pan H.T. The components and changes of VOCs of Michelia champaca L. flower at different developmental stages. Sci. Agric. Sin. 2012;45:1215–1225. [Google Scholar]
- 17.Agah M., Najafian S. Essential oil content and composition of Lippa citriodora as affected by drying method before flowering stages. Eur. J. Exp. Biol. 2012;2:1771–1777. [Google Scholar]
- 18.Chen H.C., Chi H.S., Lin L.Y. Headspace solid-phase microextraction analysis of volatile components in Narcissus tazetta var. chinensis Roem. Molecules. 2013;18:13723–13734. doi: 10.3390/molecules181113723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L., Zhao J.C. Determination of the volatile composition of Rhodobryum giganteum (Schwaegr.) Par. (Bryaceae) using solid-phase microextraction and gas chromatography/mass spectrometry (GC/MS) Molecules. 2009;14:2195–2201. doi: 10.3390/molecules14062195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J., Liu X.G., Dong F.S., Xu J., Zheng Y.Q., Shan W.L. Determination of the volatile composition in essential oil of Descurainia sophia (L.) Webb ex Prantl (Flixweed) by gas chromatography/mass spectrometry (GC/MS) Molecules. 2010;15:233–240. doi: 10.3390/molecules15010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanimah S., Suri R., Azizun R.N., Hazniza A., Radzali M., Rusli I., Hassan M.D. Volatile compounds of essential oil from different stages of Michelia alba (cempaka putih) flower development. J. Trop. Agric. Food Sci. 2008;36:109–119. [Google Scholar]
- 22.Clarke K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993;18:117–143. [Google Scholar]