Abstract
Because plants are estimated to produce over 200,000 metabolites, research into new natural substances that can be used in the pharmaceutical, agrochemical and agro-industrial production of drugs, biopesticides and food additives has grown in recent years. The global market for plant-derived drugs over the last decade has been estimated to be approximately 30.69 billion USD. A relevant specific example of a plant that is very interesting for its numerous pharmacological properties, which include antidiabetic, anticarcinogenic, and neuroprotective effects is Gymnema sylvestre, used as a medicinal plant in Asia for thousands of years. Its properties are attributed to triterpenoidic saponins. In light of the considerable interest generated in the chemistry and pharmacological properties of G. sylvestre triterpenes and their analogues, we have undertaken this review in an effort to summarise the available literature on these promising bioactive natural products. The review will detail studies on the isolation, chemistry and bioactivity of the triterpenoids, which are presented in the tables. In particular the triterpenoids oxidised at C-23; their isolation, distribution in different parts of the plant, and their NMR spectral data; their names and physico-chemical characterisation; and the biological properties associated with these compounds, with a focus on their potential chemotherapeutic applications.
Keywords: Gymnema sylvestre, triterpenoids, oleanes, pharmacological activities, phytochemistry
1. Introduction: Medicinal Plants and Their Secondary Metabolites
Plants form an important part of our diet, and plant constituents and their nutritional value have been intensively studied for decades. In addition to primary metabolites (carbohydrates, lipids and amino acids), plants are able to synthesise a wide variety of compounds referred to as secondary metabolites. These are defined as compounds that do not have a well-recognised role in the essential processes of the plant but play an important role in the interactions of the plant with the environment [1]. Despite the term “secondary”, these compounds confer selective advantages to plant species by suppressing the growth of weeds; providing protection from predators, pathogens and abiotic stress; attracting pollinators, providing benefits with respect to animals and microorganisms; and serving as signals in interactions with other plants [2,3,4,5,6,7,8]. In addition, they play a role at the cellular level as growth regulators, modulators of gene expression and signal transducers [9]. Because plants are estimated to produce over 200,000 metabolites [10], research into new natural substances that can be used in the pharmaceutical, agrochemical and agro-industrial production of drugs, biopesticides, and food additives has grown in recent years. The yield of these compounds is often low (less than 1% dry weight) and depends greatly on the physiological and developmental stage of the plant [11]. Of the more than 400,000 species of plants, the phytochemicals of only a small percentage have been studied, and of these phytochemicals, only a small percentage has been examined for their biological properties because this research is complex and expensive. Two-thirds of medicinal plants are collected from the wild, but in Europe, only approximately 10% of plants used for commercial purposes are cultivated [12]. Currently one fourth of all prescribed pharmaceuticals in industrialised countries contains compounds that are directly or indirectly, via semi-synthesis, derived from plants. Furthermore, 11% of the 252 drugs considered as basic and essential by WHO are exclusively derived from flowering plants [13]. The global market for plant-derived drugs has been estimated to be approximately 30.69 billion USD annually over the last decade, and phytochemicals such as terpenes and steroids represent the most significant fraction with estimated annual sales of 12.4 billion USD [14].
Higher plants are rich source of bioactive constituents or phyto-pharmaceuticals used in the pharmaceutical industry. Many of these pharmaceuticals are still in use today, and often, useful synthetic substitutes have been found that possess the same efficacy and pharmacological specificity [15]. In addition to the field of pharmacology, secondary metabolites from plant extracts can also be used in the agrochemical sector. The recent interest in chemical to be used as defences for agricultural crops with less impact on the environment from operators in the sector and consumers has stimulated research into the isolation of new molecules of natural origin for use as biopesticides [9].
Over 60% of cancer drugs and 66% of antimicrobial compounds on the market today (including antibacterial, antifungal and antiviral compounds) are natural products or are derived from them [16]. Despite the difficulties associated with the study and isolation of phytochemicals, they have the advantage of greater efficacy than synthetic drugs for some diseases and a lower incidence of side effects. Additionally, a large percentage of the world’s population has no access to conventional pharmacological treatments. The different reasons mentioned above describe a situation in which research has ample space to improve and simplify extraction protocols and increase the levels of metabolites in plants of interest, through classical techniques of genetic improvement or innovative biotechnological approaches aimed at modifying biosynthetic pathways, in addition to testing substances already isolated for the widest possible spectrum of biological activities.
1.1. Terpenes and Terpenoids
Among the many biologically active substances of plant origin, a great deal of attention has been paid to terpenes, which are composed of isoprene subunits. More than 30,000 terpenes have been isolated thus far [17]. Terpenes are classified as mono-, sesqui-, di-, ses-, tri-, and tetraterpenes according to the number of isoprenoid units. The carbon skeleton may be acyclic, or they may possess mono-, bi-, tri-, tetra-, and pentacyclic structures [18,19,20,21,22,23,24]. Terpenes are of widespread and can be found in all organisms, both prokaryotic and eukaryotic. However, most bioactive terpenes have been detected in higher plants. Whereas mono- and sesquiterpenes are predominantly found in essential plant oils, the higher terpenes, including triterpenes, are found in balsams and resins [25,26]. Triterpenoids in their free form (sapogenins), bound to glycosides (saponins) or acetylated are particularly important and are ubiquitous throughout the plant kingdom; both in vitro and in vivo studies have shown that they also have important biological functions. Because of their relatively complex structures, terpenoids are usually referred to by trivial names instead of using systematic IUPAC nomenclature.
1.2. Classification of Terpenoids and Their Biological Effects
Based on the number of sugars present, saponins are classified as monodesmosides, with a single saccharide chain generally linked to C-3; bidesmosides, with two sugar chains linked to C-3 and C-28; and tridesmosides, with three saccharide chains. The sugars can be linear or branched; the most widespread saccharides in triterpenoid saponins are d-glucose, d-galactose, l-arabinose, l-rhamnose, d-xylose, d-fucose and glucuronic acid [27]. The biological effects of such terpenoids are very different, and can be summarised as follows: antitumor, antiviral, antidiabetic, anti-inflammatory, hepatoprotective, analgesic, antimicrobial, antimycotic, virostatic, immunomodulatory, and tonic activities [17]. A few compounds, such as corosolic acid, a dietary supplement against diabetes, are already on the market, and several others are in clinical trials or are to be launched soon. Many triterpenes exhibit significant biological activity, but several triterpenoids possess haemolytic and cytostatic properties that restrict their pharmaceutical use. To overcome these limitations and to expand the range of usable triterpenes, transformation of the compound by chemical or biotechnological techniques is possible. They are also of extreme interest to the agrochemical field because they are involved in the defence mechanisms of plants. Their biological activities are mainly related to interactions with cell membranes. The main mechanism of action of saponins against fungi appears to be related to their ability to form complexes with sterols in fungal membranes by altering the membrane integrity [28].
2. Triterpenoids from Gymnema sylvestre and Their Pharmacological Activities
The triterpenoidic saponins also act against insect pests by forming an insoluble complex with cholesterol, which is the precursor in the biosynthesis of the hormone ecdysone. Because cholesterol is the only source of sterols for the majority of the insects and a main energy supply, the cholesterol linked to saponins cannot be used, which leads to insect death [29].
A relevant specific example of a plant that is very interesting for its numerous pharmacological properties, which include antidiabetic, anticarcinogenic, and neuroprotective effects is Gymnema sylvestre, used as a medicinal plant in Asia for thousands of years. Its properties are attributed to triterpenoidic saponins. In light of the considerable interest generated in the chemistry and pharmacological properties of G. sylvestre triterpenes and their analogues, we have undertaken this review in an effort to summarise the available literature on these promising bioactive natural products.
The review will detail studies on the isolation, chemistry and bioactivity of the triterpenoids, which are presented in the following tables: triterpenoids oxidised at C-23 (Table 1 and Figure 1); their isolation, distribution in different parts of the plant, and their NMR spectral data (Table 2); their names and physico-chemical characterisation (Table 3 and Table 4, respectively); and, finally, the biological properties associated with these compounds, with a focus on their potential chemotherapeutic applications (Table 5).
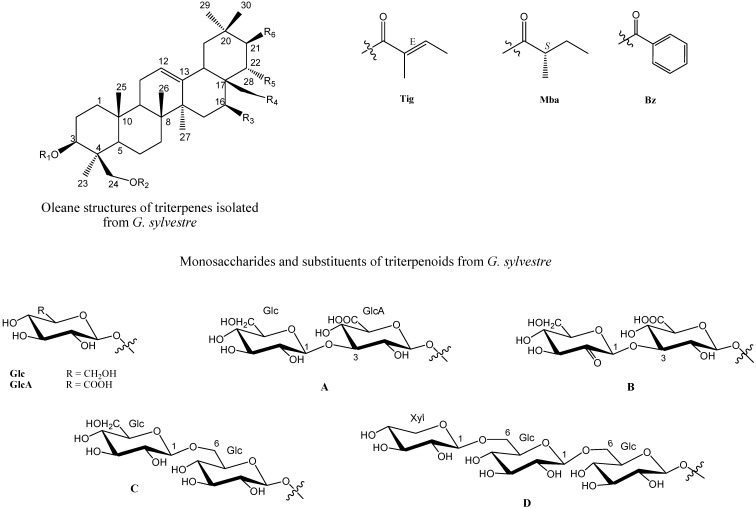
Table 1.
Common name and relative substituents of triterpenes with olean-12-ene skeleton.
| No. | Common Name | R1 | R2 | R3 | R4 | R5 | R6 |
|---|---|---|---|---|---|---|---|
| 1 | Gymnemic acid I/3-O-β-d-Glucuronopyranosyl-21-O-tigloyl-28-O-acetyl gymnemagenin | GlcA | H | OH | OAc | OH | OTig |
| 2 | Gymnemic acid II/3-O-β-d-Glucuronopyranosyl-21-[S(+)-2-methyl-butyloyl]-28-O-acetyl gymnemagenin | GlcA | H | OH | OAc | OH | OMba |
| 3 | Gymnemic acid III/3-O-β-d-Glucuronopyranosyl-21-[S(+)-2-methyl-butyloyl]-gymnemagenin | GlcA | H | OH | OH | OH | OMba |
| 4 | Gymnemic acid IV/3-O-β-d-Glucuronopyranosyl-21-O-tigloyl-gymnemagenin | GlcA | H | OH | OH | OH | OTig |
| 5 | Gymnemic acid V/3-O-β-d-Glucuronopyranosyl-21,22-bis-tigloyl gymnemagenin | GlcA | H | OH | OH | OTig | OTig |
| 6 | Gymnemic acid VI/3-O-[β-d-Glucuronopyranosyl(1→3)-β-D-glucuronopyranosyl]-21-O-tigloyl gymnemagenin | A | H | OH | OH | OH | OTig |
| 7 | Gymnemic acid VII/3-O-β-d-Glucuronopyranosylgymnestrogenin | GlcA | H | OH | OH | H | OH |
| 8 | Gymnemic acid VIII | B | H | OH | OH | OH | OMba |
| 9 | Gymnemic acid IX | B | H | OH | OH | OH | OTig |
| 10 | Gymnemic acid X/3-O-β-d-Glucuronopyranosyl-28-O-acethyl gymnemagenin | GlcA | H | OH | OAc | OH | OH |
| 11 | Gymnemic acid XI/3-O-β-d-Glucuronopyranosyl-21,28-bis-O-tigloyl gymnemagenin | GlcA | H | OH | OTig | OH | OTig |
| 12 | Gymnemic acid XII/3-O-β-d-Glucuronopyranosyl (1→3)-O-β-d-glucopyranosyl-21-O-tigloyl-28-O-acetyl gymnemagenin | A | H | OH | OAc | OH | OTig |
| 13 | Gymnemic acid XIII | GlcA | H | OH | OMba | OH | OH |
| 14 | Gymnemic acid XIV | GlcA | H | OH | OTig | OH | OH |
| 15 | Gymnemic acid XV/3-O-β-d-Glucuronopiranosyl-21-O-2-methylbutyryl-22-O-2-methylcrotonoylgymnemagenin | GlcA | H | OH | OH | OTig | OMba |
| 16 | Gymnemic acid XVI/3-O-β-d-Glucuronopiranosyl 16,22-O-bis-2-methylcrotonoylgymnemagenin | GlcA | H | Tig | OH | OTig | OH |
| 17 | Gymnemic acid XVII/3-O-β-d-Glucuronopiranosyl-21-O-benzoyl gymnemagenin | GlcA | H | OH | OH | OH | OBz |
| 18 | Gymnemic acid XVIII/3-O-β-d-Glucuronopiranosyl-28-O-benzoyl gymnemagenin | GlcA | H | OH | OBz | OH | OH |
| 19 | Gymnemagenin/3β,16β,21β,22α,23,28-hexahydroxyolean-12-ene | H | H | OH | OH | OH | OH |
| 20 | Prosapogenin/3-O-β-d-Glucuronopyranosyl gymnemagenin | GlcA | H | OH | OH | OH | OH |
| 21 | 12-Oleanene-3β,16β,23,28-tetrol/23-Hydroxylongispinogenin | H | H | OH | OH | H | H |
| 22 | 3,16,23,28-O-Tetraacetyl 3β,16β,23,28-tetrahydroxyolean-12-ene | OAc | OAc | OAc | OAc | H | H |
| 23 | 21-O-(2S)-Methylbutanoyl 3β,16β,21β,22α,23,28-hexahydroxyolean-12-ene | H | H | OH | OH | OH | OMba |
| 24 | 28-O-acetyl 21-O-(2S)-methylbutanoyl 3β,16β,21β,22α,23,28-hexahydroxyolean-12-ene | H | H | OH | OAc | OH | OMba |
| 25 | 3,16,22,23,28-O-Pentaacetyl 21-O-(2S)-methylbutanoyl 3β,16β,21β,22α,23,28-hexahydroxyolean-12-ene | OAc | OAc | OAc | OAc | OAc | OMba |
| 26 | 21-O-Tigloyl 3β,16β,21β,22α,23,28-hexahydroxyolean-12-ene | H | H | OH | OH | OH | OTig |
| 27 | Gymnemanol/3β,16β,22α,23,28-pentahydroxyolean-12-en | H | H | OH | OH | OH | H |
| 28 | Gymnemasin A/3-O-[β-d-Gluconopyranosyl(1→3)-β-d-glucuronopyranosyl]-22-O-tigloyl gymnemanol | A | H | OH | OH | OTig | H |
| 29 | Gymnemasin B/3-O-[β-d-Gluconopyranosyl(1→3)-β-d-glucuronopyranosyl]-gymnemanol | A | H | OH | OH | OH | H |
| 30 | Gymnemasin C/3-O-β-d-glucuronopyranosyl-22-tigloyl gymnemanol | GlcA | H | OH | OH | OTig | H |
| 31 | Gymnemasin D/3-O-β-d-glucuronopyranosylgymnemanol | GlcA | H | OH | OH | OH | H |
| 32 | Gymnemoside-a/21-O-Tigloyl-22-O-acetylgymnemagenin 3-O-β-d-glucupyranosiduronic acid | GlcA | H | OH | OH | OAc | OTig |
| 33 | Gymnemoside-b/16-O-Acetyl-21-O-tigloyl-gymnemagenin 3-O-β-d-glucupyranosiduronic acid | GlcA | H | OAc | OH | OH | OTig |
| 34 | Gymnemoside-c/21-O-Benzoyl-28-O-acetylgymnemagenin 3-O-β-d-glucupyranosiduronic acid | GlcA | H | OH | OAc | OH | OBz |
| 35 | Gymnemoside-d/23-O-[β-d-Xylopyranosyl (1→6)-β-d-glucopyranosyl (1→6)-β-d-glucopyranosyl] gymnestrogenin | H | D | OH | OH | H | OH |
| 36 | Gymnemoside-e/23-O-[β-d-Xylopyranosyl(1→6)-β-d-glucopyranosyl (1→6)-β-d-glucopyranosyl]-28-O-[β-d-glucopyranosyl(1→6)-β-d-glucopyranosy] 23-hydroxylongispinogenin | H | D | OH | C | H | H |
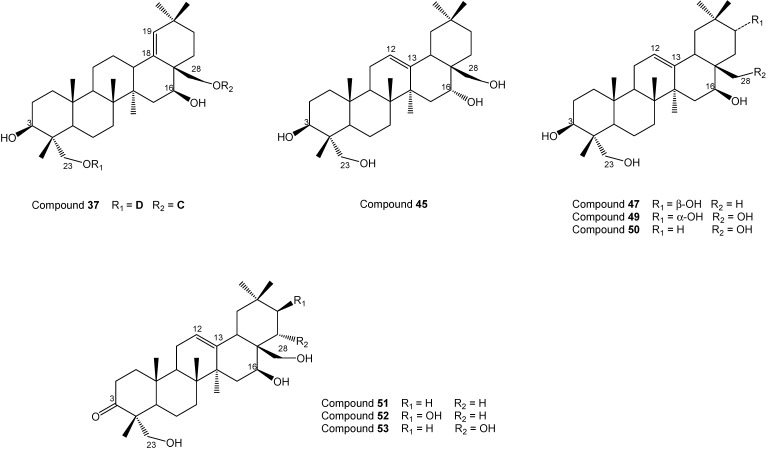
| 37 | Gymnemoside-f/23-O-[β-d-Xylopyranosyl(1→6)-β-d-glucopyranosyl (1→6)-β-d-glucopyranosyl]-28-O-[9-d-glucopyranosyl (1→6)-β-d-glucopyarnosyl] 3β,16β,23,28-tetrahydroxyolean-18-ene | See Figure 2 | |||||
| 38 | 23-O-[β-d-Glucopyranosyl (1→6)-β-d-glucopyranosyl]-oleanene-3β,16β,23,28-tetrol/(+)-28-O-Desglucosylgymnemasaponin IV | H | C | OH | OH | H | H |
| 39 | Gymnemasaponin I | H | H | OH | Glc | H | H |
| 40 | Gymnemasaponin II | H | Glc | OH | Glc | H | H |
| 41 | Gymnemasaponin III | H | Glc | OH | C | H | H |
| 42 | Gymnemasaponin IV | H | C | OH | Glc | H | H |
| 43 | Gymnemasaponin V | H | C | OH | C | H | H |
| 44 | Gymnestrogenin/3β,16β,21β,23,28-Pentahydroxyolean-12-ene | H | H | OH | OH | H | OH |
| 45 | 3β,16α,23,28-Tetrahydroxyolean-12-ene | See Figure 2 | |||||
| 46 | 3β,23,28-Trihydroxyolean-12-ene | H | H | H | OH | H | H |
| 47 | 3β,16β,21β,23-Tetrahydroxyolean-12-ene | See Figure 2 | |||||
| 48 | 3β,16β,21β,23,28-Pentahydroxyolean-12-ene | H | H | OH | OH | H | OH |
| 49 | 3β,16β,21α,23,28-Pentahydroxyolean-12-ene | See Figure 2 | |||||
| 50 | 3β,16β,23,28-Tetrahydroxyolean-13(18)-ene | See Figure 2 | |||||
| 51 | 16β,23,28-Trihydroxyolean-12-en-3-one | See Figure 2 | |||||
| 52 | 16β,21β,23,28-Tetrahydroxyolean-12-en-3-one | See Figure 2 | |||||
| 53 | 16β,21β,22α,23,28-Pentahydroxyolean-12-en-3-one |
For partial structures Glc, GlcA, A, B, C, and D see Figure 1.
Figure 1.
Chemical structures of triterpenes isolated from G. sylvestre.
Table 2.
Isolation and distribution of triterpenes in the different parts of the plants and their NMR spectral data.
| No. | Part of the Plant | Extract | Ref. | Aspect | Solvents of NMR Spectra | ||
|---|---|---|---|---|---|---|---|
| 1H-NMR | 13C-NMR | Ref. | |||||
| 1 | Leaves | H2O CH3OH |
[31] [46] |
/ | C5D5N | C5D5N | [31,35] |
| 2 | Leaves | H2O CH3OH |
[31] [46] |
/ | C5D5N | C5D5N | [31] |
| 3 | Leaves | H2O CH3OH |
[31] [46] |
Colourless powder | C5D5N | C5D5N | [30,31,56] |
| 4 | Leaves | H2O CH3OH |
[31] [46] |
Colourless powder | C5D5N | C5D5N | [30,31,56] |
| 5 | Leaves | H2O CH3OH |
[32,46] | Colourless powder | C5D5N | C5D5N | [32,56] |
| 6 | Leaves | H2O | [32] | / | C5D5N | C5D5N | [32] |
| 7 | Leaves Leaves |
H2O CH3OH |
[32,46] | / | C5D5N | C5D5N | [32] |
| 8 | Leaves | H2O | [56] | Colourless powder | C5D5N+D2O | C5D5N+D2O | [56] |
| 9 | Leaves | H2O | [56] | Colourless powder | C5D5N+D2O | C5D5N+D2O | [56] |
| 10 | Leaves | H2O:EtOH (2:3) | [35] | Amorphous white powder | C5D5N | C5D5N | [35] |
| 11 | Leaves | H2O:EtOH (2:3) | [35] | Amorphous white powder | C5D5N | C5D5N | [35] |
| 12 | Leaves | H2O:EtOH (2:3) | [35] | Amorphous white powder | C5D5N | C5D5N | [35] |
| 13 | Leaves | H2O:EtOH (2:3) | [35] | Amorphous powder | C5D5N | C5D5N | [35] |
| 14 | Leaves | H2O:EtOH (2:3) | [35] | Amorphous powder | C5D5N | C5D5N | [35] |
| 15 | Leaves | H2O:EtOH (2:3) | [36] | Amorphous white powder | C5D5N | C5D5N | [36] |
| 16 | Leaves | H2O:EtOH (2:3) | [36] | Amorphous white powder | C5D5N | C5D5N | [36] |
| 17 | Leaves | H2O:EtOH (2:3) | [36] | Amorphous white powder | C5D5N | C5D5N | [36] |
| 18 | Leaves | H2O:EtOH (2:3) | [36] | Amorphous white powder | C5D5N | C5D5N | [36] |
| 19 | Leaves | Microwave | [51] [32] |
/ | C5D5N | C5D5N CDCl3+CD3OD |
[31,56] [57] |
| By synthesis | |||||||
| 20 | By synthesis | [32] | / | C5D5N | C5D5N CD3OD |
[31,56] [57] |
|
| 21 | Aerial parts | CH2Cl2 | [58] | Amorphous powder | CD3OD | CD3OD | [58] |
| 22 | Aerial parts | CH2Cl2 | [58] | Amorphous powder | CD3OD | CD3OD | [58] |
| 23 | Aerial parts | CH2Cl2 | [58] | Amorphous powder | CD3OD | CD3OD | [58] |
| 24 | Aerial parts | CH2Cl2 | [58] | Amorphous powder | CD3OD | CD3OD | [58] |
| 25 | Aerial parts | CH2Cl2 | [58] | Amorphous powder | CD3OD | CD3OD | [58] |
| 26 | Aerial parts | CH2Cl2 | [58] | Amorphous powder | CD3OD | CD3OD | [58] |
| 27 | By synthesis | [59] | Micro-needles | C5D5N | C5D5N | [59] | |
| 28 | Leaves | H2O:EtOH (1:1) | [59] | Amorphous powder | C5D5N | C5D5N | [59] |
| 29 | Leaves | H2O:EtOH (1:1) | [59] | Amorphous powder | C5D5N | C5D5N | [59] |
| 30 | Leaves | H2O:EtOH (1:1) | [59] | Amorphous powder | C5D5N | C5D5N | [59] |
| 31 | Leaves | H2O:EtOH (1:1) | [59] | Amorphous powder | C5D5N | C5D5N | [59] |
| 32 | Leaves | CH3OH | [46] | Colourless fine crystals | C5D5N | C5D5N | [46] |
| 33 | Leaves | CH3OH | [46] | Colourless fine crystals | C5D5N | C5D5N | [46] |
| 34 | Leaves | CH3OH | [46] | Colourless fine crystals | C5D5N | C5D5N | [37] |
| 35 | Leaves | CH3OH | [46] | Colourless fine crystals | C5D5N | C5D5N | [37] |
| 36 | Leaves | CH3OH | [46] | Colourless fine crystals | C5D5N | C5D5N | [37] |
| 37 | Leaves | CH3OH | [46] | Colourless fine crystals | C5D5N | C5D5N | [37] |
| 38 | By synthesis | [34] | / | C5D5N | C5D5N | [34] | |
| 39 | Leaves | H2O:EtOH (1:1) | [34] | / | C5D5N | C5D5N | [34] |
| 40 | Leaves | CH3OH H2O:EtOH (1:1) |
[46] [34] |
/ | C5D5N | C5D5N | [34] |
| 41 | Leaves | H2O:EtOH (1:1) | [34] | / | C5D5N | C5D5N | [34] |
| 42 | Leaves | CH3OH H2O:EtOH (1:1) |
[46] [34] |
/ | C5D5N | C5D5N | [34] |
| 43 | Leaves | CH3OH H2O:EtOH (1:1) |
[46] [34] |
/ | C5D5N | C5D5N | [34] |
| 44 | Aerial parts | H2O | [32,60] | / | C5D5N | C5D5N | [32] |
| By synthesis | |||||||
| 45 | Aerial parts | H2O | [57] | Amorphous powder | CD3OD CDCl3 |
CD3OD CDCl3 |
[58] [61] |
| 46 | Aerial parts | CH2Cl2 | [60] | / | CDCl3 | CDCl3 | [62] |
| 47 | Aerial parts | CH2Cl2 | [60] | Amorphous powder | CD3OD | CD3OD | [60] |
| 48 | Aerial parts | CH2Cl2 | [60] | / | C5D5N | C5D5N | [32] |
| 49 | Aerial parts | CH2Cl2 | [60] | Amorphous powder | CD3OD | CD3OD | [60] |
| 50 | Aerial parts | CH2Cl2 | [60] | Amorphous powder | CD3OD | CD3OD | [60] |
| 51 | Aerial parts | CH2Cl2 | [60] | Amorphous powder | CD3OD | CD3OD | [60] |
| 52 | Aerial parts | CH2Cl2 | [60] | Amorphous powder | CD3OD | CD3OD | [60] |
| 53 | Aerial parts | CH2Cl2 | [60] | Amorphous powder | CD3OD | CD3OD | [60] |
Table 3.
Systematic names and physico-chemical characterization of triterpenes-1.
| No. | Systematic Name | CAS | Molecular Formula | Molecular Weight | Melting Point °C | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | β-d-Glucopyranosiduronic acid, (3β,4α,16β,21β,22α)-28-(acetyloxy)-16,22,23-trihydroxy-21-[[(2E)-2-methyl-1-oxo-2-buten-1-yl]oxy]olean-12-en-3-yl | 122,168-40-5 | C43H66O14 | 806.97 | 211–212 | [31] | |||||||
| 2 | β-d-Glucopyranosiduronic acid, (3β,4α,16β,21β,22α)-28-(acetyloxy)-16,22,23-trihydroxy-21-[(2S)-2-methyl-1-oxobutoxy]olean-12-en-3-yl | 122,144-48-3 | C43H68O14 | 808.99 | 212–213 | [31] | |||||||
| 3 | β-d-Glucopyranosiduronic acid, (3β,4α,16β,21β,22α)-16,22,23,28-tetrahydroxy-21-[(2S)-2-methyl-1-oxobutoxy]olean-12-en-3-yl | 122,074-65-1 | C41H66O13 | 766.95 | 219–221 218–219 |
[56] [31] |
|||||||
| 4 | β-d-Glucopyranosiduronic acid, (3β,4α,16β,21β,22α)-16,22,23,28-tetrahydroxy-21-[[(2E)-2-methyl-1-oxo-2-buten-1-yl]oxy]olean-12-en-3-yl | 121,903-96-6 | C41H64O13 | 764.94 | 229–231 210–221 |
[56] [31] |
|||||||
| 5 | β-d-Glucopyranosiduronic acid, (3β,4α,16β,21β,22α)-16,23,28-trihydroxy-21,22-bis[[(2E)-2-methyl-1-oxo-2-butenyl]-oxy]olean-12-en-3-yl | 121,903-99-9 | C46H70O14 | 847.04 | 214–216 202–203 |
[56] [32] |
|||||||
| 6 | β-d-Glucopyranosiduronic acid, (3β,4α,16β,21β,22α)-16,22,23,28-tetrahydroxy-21-[[(2E)-2-methyl-1-oxo-2-butenyl]oxy]olean-12-en-3-yl 3-O-β-d-glucopyranosyl | 121,903-98-8 | C47H74O18 | 927.08 | 225–226 | [32] | |||||||
| 7 | β-d-Glucopyranosiduronic acid, (3β,4α,16β,21β)-16,21,23,28-tetrahydroxyolean-12-en-3-yl | 121,903-97-7 | C36H58O11 | 666.84 | 222–223 | [32] | |||||||
| 8 | β-d-Glucopyranosiduronic acid, [3β,4α,16β,21β(S),22α]-16,22,23,28-tetrahydroxy-21-(2-methyl-1-oxobutoxy)olean-12-en-3-yl 3-O-β-d-arabino-hexopyranos-2-ulos-1-yl | 131,653-19-5 | C47H74O18 | 927.08 | 218–220 | [56] | |||||||
| 9 | β-d-Glucopyranosiduronic acid, [3β,4α,16β,21β(E),22α]-16,22,23,28-tetrahydroxy-21-[(2-methyl-1-oxo-2-butenyl)oxy]-olean-12-en-3-yl 3-O-β-d-arabino-hexopyranos-2-ulos-1-yl | 131,653-20-8 | C47H72O18 | 925.06 | 222–224 | [56] | |||||||
| 10 | β-d-Glucopyranosiduronic acid, (3β,4α,16β,21β,22α)-28-(acetyloxy)-16,21,22,23-tetrahydroxyolean-12-en-3-yl | 147,934-05-2 | C38H60O13 | 724.86 | 210–212 | [35] | |||||||
| 11 | β-d-Glucopyranosiduronic acid, (3β,4α,16β,21β,22α)-16,22,23-trihydroxy-21,28-bis[[(2E)-2-methyl-1-oxo-2-butenyl]oxy]-olean-12-en-3-yl | 147,899-35-2 | C46H70O14 | 847.04 | 190–192 | [35] | |||||||
| 12 | β-d-Glucopyranosiduronic acid, (3β,4α,16β,21β,22α)-28-(acetyloxy)-16,22,23-trihydroxy-21-[[(2E)-2-methyl-1-oxo-2-butenyl]oxy]olean-12-en-3-yl 3-O-β-d-glucopyranosyl | 147,899-36-3 | C49H76O19 | 968.50 | 209–211 | [35] | |||||||
| 13 | β-d-Glucopyranosiduronic acid, (3β,4α,16β,21β,22α)-16,21,22,23-tetrahydroxy-28-[(2S)-2-methyl-1-oxobutoxy]olean-12-en-3-yl | 155,023-61-3 | C41H66O13 | 766.95 | 185–187 | [35] | |||||||
| 14 | β-d-Glucopyranosiduronic acid, (3β,4α,16β,21β,22α)-16,21,22,23-tetrahydroxy-28-[[(2E)-2-methyl-1-oxo-2-butenyl]oxy]olean-12-en-3-yl | 155,023-62-4 | C41H64O13 | 764.94 | 194–196 | [35] | |||||||
| 15 | β-d-Glucopyranosiduronic acid, (3β,4α,16β,21β,22α)-16,23,28-trihydroxy-22-[[(2E)-2-methyl-1-oxo-2-butenyl]oxy]-21-(2-methyl-1-oxobutoxy)olean-12-en-3-yl | 154,977-74-9 | C46H72O14 | 849.06 | / | / | |||||||
| 16 | β-d-Glucopyranosiduronic acid, (3β,4α,16β,21β,22α)-21,23,28-trihydroxy-16,22-bis[[(2E)-2-methyl-1-oxo-2-butenyl]oxy]olean-12-en-3-yl | 154,977-75-0 | C46H70O14 | 847.04 | 203–205 | [36] | |||||||
| 17 | Gymnemic acid XVII/β-d-Glucopyranosiduronic acid, (3β,4α,16β,21β,22α)-21-(benzoyloxy)-16,22,23,28-tetrahydroxyolean-12-en-3-yl | 154,977-76-1 | C43H62O13 | 786.94 | 211–213 | [36] | |||||||
| 18 | β-d-Glucopyranosiduronic acid, (3β,4α,16β,21β,22α)-28-(benzoyloxy)-16,21,22,23-tetrahydroxyolean-12-en-3-yl | 154,977-77-2 | C43H62O13 | 786.94 | 201–203 | [36] | |||||||
| 19 | Olean-12-ene-3,16,21,22,23,28-hexol, (3β,4α,16β,21β,22α) | 22,467-07-8 | C30H50O6 | 506.71 | 313–314 >300 328–335 |
[32,35] [56] [63] |
|||||||
| 20 | β-d-Glucopyranosiduronic acid, (3β,4α,16β,21β,22α)-3,16,21,22,23,28-hexahydroxyolean-12-en-3-yl | 50,647-08-0 | C36H58O11 | 666.84 | 230–231 | [32,35] | |||||||
| 21 | 3β,16β,23,28-Tetrahydroxyolean-12-ene | 42,483-24-9 | C30H50O4 | 474.72 | / | [58] | |||||||
| 22 | 3,16,23,28-O-Tetraacetyl 3β,16β,23,28-tetrahydroxyolean-12-ene | / | C38H59O7 | 627.87 | / | [58] | |||||||
| 23 | 21-O-(2S)-Methylbutanoyl 3β,16β,21β,22α,23,28-hexahydroxyolean-12-ene | / | C35H59O7 | 591.84 | / | [58] | |||||||
| 24 | 28-O-Acetyl 21-O-(2S)-methylbutanoyl 3β,16β,21β,22α,23,28-hexahydroxyolean-12-ene | / | C37H61O7 | 617.88 | / | [58] | |||||||
| 25 | 3,16,22,23,28-O-Pentaacetyl 21-O-(2S)-methylbutanoyl 3β,16β,21β,22α,23,28-hexahydroxyolean-12-ene | / | C45H68O11 | 785.01 | / | [58] | |||||||
| 26 | 21-O-Tigloyl 3β,16β,21β,22α,23,28-hexahydroxyolean-12-ene | / | C35H57O6 | 573.82 | / | [58] | |||||||
| 27 | Olean-12-ene-3,16,22,23,28-pentol, (3β,4α,16β,22α) | 174,324-52-8 | C30H50O5 | 490.72 | 284–285 | [59] | |||||||
| 28 | β-d-Glucopyranosiduronic acid, (3β,4α,16β,22α)-16,23,28-trihydroxy-22-[[(2E)-2-methyl-1-oxo-2-butenyl]oxy]olean-12-en-3-yl 3-O-β-d-glucopyranosyl | 174,324-49-3 | C47H74O17 | 910.49 | 215–217 | [59] | |||||||
| 29 | β-d-Glucopyranosiduronic acid, (3β,4α,16β,22α)-16,22,23,28-tetrahydroxyolean-12-en-3-yl 3-O-β-d-glucopyranosyl | 174,324-48-2 | C42H68O16 | 828.45 | 221–222 | [59] | |||||||
| 30 | β-d-Glucopyranosiduronic acid, (3β,4α,16β,22α)-16,23,28-trihydroxy-22-[[(2E)-2-methyl-1-oxo-2-butenyl]oxy]olean-12-en-3-yl | 174,324-50-6 | C41H64O12 | 748.44 | 212–214 | [59] | |||||||
| 31 | β-d-Glucopyranosiduronic acid, (3β,4α,16β,22α)-16,22,23,28-tetrahydroxyolean-12-en-3-yl | 174,324-51-7 | C36H58O11 | 666.40 | 220–221 | [59] | |||||||
| 32 | β-d-Glucopyranosiduronic acid, (3β,4α,16β,21β,22α)-22-(acetyloxy)-16,23,28-trihydroxy-21-[[(2E)-2-methyl-1-oxo-2-buten-1-yl]oxy]olean-12-en-3-yl | 175,033-15-5 | C43H66O14 | 806.98 | 207.0–208.5 | [46] | |||||||
| 33 | β-d-Glucopyranosiduronic acid, (3β,4α,16β,21β,22α)-16-(acetyloxy)-22,23,28-trihydroxy-21-[[(2E)-2-methyl-1-oxo-2-buten-1-yl]oxy]olean-12-en-3-yl | 174,232-51-0 | C43H66O14 | 806.98 | 211.5–213.0 | [46] | |||||||
| 34 | β-d-Glucopyranosiduronic acid, (3β,4α,16β,21β,22α)-28-(acetyloxy)-21-(benzoyloxy)-16,22,23-trihydroxyolean-12-en-3-yl | 199,618-65-0 | C45H64O14 | 828.98 | 211.5–213.0 | [37] | |||||||
| 35 | β-d-Glucopyranoside, (3β,4α,16β,21β)-3,16,21,28-tetrahydroxyolean-12-en-23-yl O-β-d-xylopyranosyl-(1→6)-O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranosyl | 199,618-66-1 | C47H78O19 | 947.11 | 219.1–221.0 | [37] | |||||||
| 36 | β-d-Glucopyranoside, (3β,4α,16β)-3,16-dihydroxy-23-[(O-β-d-xylopyranosyl-(1→6)-O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranosyl)oxy]olean-12-en-28-yl 6-O-β-d-glucopyranosyl | 199,618-67-2 | C59H98O28 | 1255.39 | 202.8–204.1 | [37] | |||||||
| 37 | β-d-Glucopyranoside, (3β,4α,16β)-3,16-dihydroxy-23-[(O-β-d-xylopyranosyl-(1→6)-O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranosyl)oxy]olean-18-en-28-yl 6-O-β-d-glucopyranosyl | 199,618-68-3 | C59H98O28 | 1255.39 | 201.3–203.2 | [37] | |||||||
| 38 | β-d-Glucopyranoside, (3β,4α,16β)-3,16,28-trihydroxyolean-12-en-23-yl 6-O-β-d-glucopyranosyl | 133,629-85-3 | C42H70O14 | 799.00 | 173–175 | [34] | |||||||
| 39 | β-d-Glucopyranoside,(3β,4α,16β)-3,16,23-trihydroxyolean-12-en-28-yl | 133,629-80-8 | C36H60O9 | 636.86 | 184–185 | [34] | |||||||
| 40 | β-d-Glucopyranoside, (3β,4α,16β)-3,16-dihydroxyolean-12-ene-23,28-diyl bis | 133,629-81-9 | C42H70O14 | 799.00 | 190–192 | [34] | |||||||
| 41 | β-d-Glucopyranoside, (3β,4α,16β)-23-(β-d-glucopyranosyloxy)-3,16-dihydroxyolean-12-en-28-yl 6-O-β-d-glucopyranosyl | 133,629-82-0 | C48H80O19 | 961.14 | 203–205 | [34] | |||||||
| 42 | β-d-Glucopyranoside, (3β,4α,16β)-28-(β-d-glucopyranosyloxy)-3,16-dihydroxyolean-12-en-23-yl 6-O-β-d-glucopyranosyl | 133,629-83-1 | C48H80O19 | 961.14 | 201–203 | [34] | |||||||
| 43 | β-d-Glucopyranoside, (3β,4α,16β)-3,16-dihydroxyolean-12-ene-23,28-diyl bis[6-O-β-d-glucopyranosyl] | 133,629-84-2 | C54H90O24 | 1123.28 | 186–188 | [34,37] | |||||||
| 44 | Olean-12-ene-3,16,21,23,28-pentol, (3β,4α,16β,21β) | 19,942-02-0 | C30H50O5 | 490.72 | 290–291 | [32] | |||||||
| 45 | Olean-12-ene-3,16,23,28-tetrol, (3β,4α,16β) | 23,887-98-1 | C30H50O4 | 474.72 | [64] | ||||||||
| 46 | (3β-Olean-12-ene-3,23,28-triol | 35,043-82-4 | C30H50O3 | 458.72 | / | [62] | |||||||
| 47 | (3β,16β,21β-Olean-12-ene-3,16,21,23-tetrol | 1,447,214-81-4 | C30H50O4 | 474.72 | / | [62] | |||||||
| 48 | (3β,16β,21β-Olean-12-ene-3,16,21,23,28-pentol | 42,483-24-9 | C30H50O4 | 474.72 | / | [62] | |||||||
| 49 | (3β,16β,21α-Olean-12-ene-3,16,21,23,28-pentol | 1,447,214-84-7 | C30H50O5 | 490.72 | / | [62] | |||||||
| 50 | (3β,16β-Olean-13(18)-ene-3,16,23,28-tetrol | 26,540-63-6 | C30H50O4 | 474.72 | / | [62] | |||||||
| 51 | 16β,23,28-Tetrahydroxyolean-12-en-3-one | 1,447,214-87-0 | C30H48O4 | 472.70 | / | [62] | |||||||
| 52 | 16β,21β,23,28-Tetrahydroxyolean-12-en-3-one | 1,447,214-89-2 | C30H48O5 | 488.70 | / | [62] | |||||||
| 53 | 16 β,22α,23,28-Tetrahydroxyolean-12-en-3-one | 1,447,214-91-6 | C30H48O5 | 488.70 | / | [62] |
Table 4.
Physico-chemical characterization of triterpenes-2.
| No. | MS Analysis | IR υmax, cm−l | [α]D (c, MeOH) | Ref. |
|---|---|---|---|---|
| 1 | / | / | +36.7° (2.4) | [31] |
| 2 | / | / | +36.3° (1.5) | [31] |
| 3 | / | 3400 (OH), 1715 (C=O) | +9.6° (0.39) | [56] |
| 4 | / | 3400 (OH), 1700 (C=O) | +7.4° (0.21) | [56] |
| 5 | / FAB(+): 892 [M + 2Na]+ |
3400 (OH), 1700 (C=O) / |
+3.3° (0.30) +2.2° (3.6) |
[56] [32] |
| 6 | FAB(+): 972 [M + 2Na]+ | / | +11.7° (1.1) | [32] |
| 7 | FAB(+): 712 [M + 2Na]+ | / | +9.6° (5.7) | [32] |
| 8 | HR-FAB(+): 949.4818 [M + Na]+ | 3450 (OH), 1730 (C=O) | +17.3° (0.74) | [56] |
| 9 | HR-FAB(+): 947.4681 [M + Na]+ | 3400 (OH), 1730 (C=O), 1700 (C=O) |
+11.4° (0.70) | [56] |
| 10 | FAB(−): 723[M − H]− | 3400 (OH), 1740 (C=O), 1610 (C=C), 1040 (OH) |
+14.9° (2.3) | [35] |
| 11 | FAB(−): 845 [M − H]− | 3400 (OH), 1740 (C=O), 1610 (C=C), 1040 (OH) |
+1.7° (5.3) | [35] |
| 12 | FAB(−): 967 [M − H]− | 3400 (OH), 1740 (C=O), 1720 (C=O), 1610 (C=C), 1040 (OH) |
+11.7° (3.6) | [35] |
| 13 | FAB(−): 765 [M − H]− | 3400 (OH), 1720 (C=O), 1600 (C=C), 1040 (OH) |
+21.5° (3.5) | [35,36] |
| 14 | FAB(−): 763 [M − H]− | 3380 (OH), 1705 (C=O), 1605 (C=C), 1060 (OH). |
+7.6° (1.8) | [35,36] |
| 15 | FAB(−): 847 [M − H]−, 747 [M − H − C5H8O2]−, 745 [M − H − C5H10O2]−, 645 [M − H − C5H8O2-C5H10O2]− |
3400 (OH), 1740 (C=O), 1720 (C=O), 1610 (C=C), 1040 (OH) |
+7.2° (1.52) | [36] |
| 16 | FAB(−): 845 [M − H]−, 745 [M − H − C5H8O2]−, 645 [M – H − 2C5H8O2]− |
3380 (OH), 1740 (C=O), 1650 (C=C), 1050 (OH) |
−6.8° (2.96) | [36] |
| 17 | FAB(−): 785 [M − H]−, 663 [M − H − C7H6O2]− | 3450 (OH), 1700 (C=O), 1720 (C=O), 1605(C=C), 1060 (OH) |
+7.1° (2.96) | [36] |
| 18 | FAB(−): 785 [M − H]−, 663 [M − H − C7H6O2]− | 3400 (OH), 1700 (C=O), 1650 (C=C), 1040 (OH) |
+6.4° (1.71) | [36] |
| 19 | FAB(+): 529 [M + Na]+
FAB(+): 506 [M]+, 488 [M − H2O]+ HR-ESI-MS: 507.3678 [M + H]+ |
/ / 3328, 1111, 1089, 1037 |
+53.5° (1.8) +53.9° (0.75) −1.2° (0.19) |
[32,35] [56] [58] |
| 20 | FAB(+): 705 [M + Na]+
728 [M + 2Na]+ |
/ / |
+8.4° (1.8) +8.4° (1.8) |
[35] [32] |
| 21 | ESI-MS: 475.2 [M + H]+. HR-ESI-MS: 475.3780 [M + H]+
FAB(+): 497 [M + Na]+ |
3345, 1132, 1077, 1038 | −0.67° (0.22) +32.0° (2.8) |
[58] [34,61] |
| 22 | HR-ESI-MS: 643.4204 [M + H]+ | 3333, 1758, 1754, 1117, 1091, 1033 | +50.0° (0.23) | [58] |
| 23 | HR-ESI-MS: 591.4254 [M + H]+ | 3370, 1747, 1118, 1096, 1046 | +3.5° (0.21) | [58] |
| 24 | HR-ESI-MS: 633.4360 [M + H]+ | 3352, 1746, 1113, 1091, 1041 | +16.5° (0.2 ) | [58] |
| 25 | HR-ESI-MS: 801.4782 [M + H]+ | 3355, 1764, 1750, 1113, 1090, 1042 |
+2.5° (0.21) | [58] |
| 26 | HR-ESI-MS: 589.4099 [M + H]+ | 3352, 17,252, 1113, 1093, 1041 |
+3.5° (0.22) | [58] |
| 27 | EI: 490 [M]+, 472 [M − H2O]+, 454 [M − 2H2O]+
441 [M − H2O − CH2OH]+, 436 [M − 3H2O]+ |
3350 (OH) | +51.5° (1.0) | [59] |
| 28 | FAB(+): 933 [M + Na]+. FAB(−): 909 [M − H]− | 3400 (OH), 1715 (C=O), 1600 (C=C) |
+15° (1.5) | [59] |
| 29 | FAB(−): 827 [M − H]− | 3420 (OH), 1710 (C=O) | +18.5° (1.0) | [59] |
| 30 | FAB(−): 747 [M − H]− | 3410 (OH), 1715 (C=O) | +12.5° (1.0) | [59] |
| 31 | FAB(−): 665 [M − H]− | 3425 (OH),1715 (C=O) | +8° (0.9) | [59] |
| 32 | HR-FAB(−): 805.4385 [M − H]−
HR-FAB(+): 829.4430 [M − Na]+ |
3453, 1721, 1649, 1040 | +4.7° (0.1) | [46] |
| 33 | HR-FAB(−): 805.4404 [M − H]−
HR-FAB(+): 829.4428 [M − Na]+ |
3445, 1718, 1649, 1044 | +6.6° (0.1) | [46] |
| 34 | HR-FAB(+): 829.4360 [M + Na]+
FAB(+): 851 [M + Na]+. FAB(−): 827 [M − H]− |
3445, 1718, 1649, 1044 | +6.6° (0.1) | [37] |
| 35 | HR-FAB(+): 969.5050 [M + Na]+
FAB(+): 991 [M + 2Na − H]+, 969 [M + Na]+. FAB(−): 945 [M − H]− |
3410, 1044 | +13.4° (0.1) | [37] |
| 36 | HR-FAB(−): 1253.6154 [M − H]−, FAB(+): 1277 [M + Na]+. FAB(−): 1253 [M − H]− |
3410, 1044 | +14.8° (0.1) | [37] |
| 37 | HR-FAB(−): 1253.6167 [M − H]−
FAB(+): 1277 [M + Na]+. FAB(−): 1253 [M − H]− |
3431, 1044 | −8.9° (0.1) | [37] |
| 38 | FAB(+): 821 [M + Na]+ | / | +12.1° (1.1) | [34,61] |
| 39 | FAB(+): 659 [M + Na]+ | / | +9.3° (3.5) | [34,61] |
| 40 | FAB(+): 821 [M + Na]+ | / | +1.9° (2.6) | [34,61] |
| 41 | FAB(+): 983 [M + Na]+ | / | −11.6° (1.1) | [34,61] |
| 42 | FAB(+): 98 3[M + Na]+ | / | −1.1° (1.9) | [34,61] |
| 43 | FAB(+): 1145 [M + Na]+ | / | −6.2°(1.9) | [34,61] |
| 44 | FAB(+): 712 [M+2Na]+ | / | +53.1° (2.4) | [32] |
| 45 | ESI-MS: 475.2 [M + H]+. HR-ESI-MS: 475.3780 [M + H]+ | 3333, 1758, 1754, 1117, 1091, 1033 |
−0.67° (0.22) | [36] |
| 46 | HR-FAB(+): 481.3720 [M + Na]+ | 3344, 2930, 2857, 1725, 1459, 756 |
/ | [62] |
| 47 | ESI-MS: 475.2 [M + H]+. HR-ESI-MS: 475.3769 [M + H]+ | 3347, 1130, 1080, 1035 | +20.5° (0.27) | [60] |
| 48 | / | / | +53.1° (2.4) | [32] |
| 49 | ESI-MS: 491.4 [M + H]+. HR-ESI-MS: 491.3728 [M + H]+ | 3330, 1115, 1095, 1035 | +32.3° (0.23) | [60] |
| 50 | ESI-MS: 475.5 [M + H]+. HR-ESI-MS: 475.3745 [M + H]+ | 3334, 1112, 1087, 1034 | −1.2° (0.19) | [60] |
| 51 | ESI-MS: 473.2 [M + H]+. HR-ESI-MS: 473.3620 [M + H]+ | 3376, 1722, 1118, 1090, 1045 | +25.5° (0.21) | [60] |
| 52 | ESI-MS: 489.2 [M + H]+. HR-ESI-MS: 489.3563 [M + H]+ | 3350, 1724, 1118, 1090, 1045 | +26.5° (0.22) | [60] |
| 53 | ESI-MS: 505.3 [M + H]+. HR-ESI-MS: 505.3500 [M + H]+ | 30, 1723, 1128, 1080, 1050 | +23.1° (0.27) | [60] |
Table 5.
Biological properties associated with the triterpenes with a focus on their potential chemotherapeutic applications.
| No. | Activity | Ref. |
|---|---|---|
| 1 | Antisweet activity Glucose uptake in rat small intestinal fragment Increase of serum glucose level in oral glucose-loaded rats Anti-hyperglycemic activity |
[31,35,38] [37,38] [46,47] [38] |
| 2 | Antisweet activity Glucose uptake in rat small intestinal fragment Increase of serum glucose level in oral glucose-loaded rats Anti-hyperglycemic activity |
[31,35,38] [37,38] [46,47] [38] |
| 3 | Antisweet activity Glucose uptake in rat small intestinal fragment Increase of serum glucose level in oral glucose-loaded rats Anti-hyperglycemic activity |
[30,31,35,38] [37,38] [46,47] [38] |
| 4 | Antisweet activity Glucose uptake in rat small intestinal fragment Increase of serum glucose level in oral glucose-loaded rats Anti-hyperglycemic activity Gut glycosidase inhibition |
[30,31,35,38] [37,38] [46,47] [38,65] [38] |
| 5 | Antisweet activity Increase of serum glucose level in oral glucose-loaded rats Anti-hyperglycemic activity Glucose uptake in rat small intestinal fragment |
[32,38] [46,47,65] [38] [38] |
| 6 | Antisweet activity | [32] |
| 7 | Antisweet activity Increase of serum glucose level in oral glucose-loaded rats |
[32] [46,47] |
| 10 | Antisweet activity | [35] |
| 11 | Antisweet activity | [35] |
| 12 | Antisweet activity | [35] |
| 13 | Antisweet activity | [35] |
| 14 | Antisweet activity | [35] |
| 15 | Antisweet activity | [36] |
| 16 | Antisweet activity | [36] |
| 17 | Antisweet activity | [36] |
| 18 | Antisweet activity | [36] |
| 19 | Antisweet activity | [31] |
| Pharmacokinetic study: determination of gymnemagenin in rat plasma using HPLC-MS/MS | [66] | |
| 20 | Antisweet activity | [31] |
| 27 | Inhibition of the 11β-hydroxysteroid dehydrogenase type 1 | [63,66] |
| 28 | Hypoglycemic and antihyperglycemic effect in rats | [61] |
| 29 | Hypoglycemic and antihyperglycemic effect in rats | [61] |
| 30 | Hypoglycemic and antihyperglycemic effect in rats | [61] |
| 31 | Hypoglycemic and antihyperglycemic effect in rats | [61] |
| 32 | Increase of serum glucose level in oral glucose-loaded rats | [46,47] |
| 33 | Increase of serum glucose level in oral glucose-loaded rats | [46,47] |
| 34 | Glucose uptake in rat small intestinal fragment | [37] |
| 35 | Glucose uptake in rat small intestinal fragment | [37] |
| 36 | Glucose uptake in rat small intestinal fragment | [37] |
| 37 | Glucose uptake in rat small intestinal fragment | [37] |
| 39 | Antisweet activity | [34] |
| 40 | Antisweet activity Glucose uptake in rat small intestinal fragment |
[34] [46,47] |
| 41 | Antisweet activity | [34] |
| 42 | Antisweet activity Glucose uptake in rat small intestinal fragment |
[34] [46,47] |
| 43 | Antisweet activity Glucose uptake in rat small intestinal fragment |
[34] [37,47,59] |
| 44 | Inhibitory effects on human tumor cell lines (A549, SK-OV-3, SK-MEL-2, and HCT15) in vitro using the sulforhodamine B (SRB) assay | [55] |
| 46 | In vivo antitumor-promoting activity in mouse skin tumor | [50] |
| Inhibition of the tumor-promoting action of 12-O-tetradecanoylphorbol 13-acetate | [48,49] | |
| Inhibition of phospholipid synthesis by 12-O-tetradecanoylphorbol-13-acetate | [67] | |
| In vitro in human uterus cancer cells | ||
| Anti-inflammatory activity and also to inhibit liver carcinogenesis and tumor growth | [51] |
Biological Effects of Triterpenoids
Diabetes mellitus (DM) is a disease caused by a deficiency or diminished effectiveness of endogenous insulin. It is characterised by hyperglycaemia, deranged metabolism and sequelae predominantly affecting the vasculature. There are two main types of diabetes mellitus: Type 1, in which the body does not produce sufficient insulin; Type 2, due to the resistance to the insulin, often initially with normal or increased levels of circulating insulin. In the UK, there were 2.9 million people with diabetes in 2011. It is estimated that 5 million people will have diabetes in the UK by 2025. It is also estimated that there are approximately 850,000 people who have undiagnosed diabetes. The average prevalence of diabetes in the UK is 4.45% of the population, but there are variations between countries and regions. The proportion of people with diabetes increases with age. However, the incidence of diabetes is increasing in all age groups. Type 1 diabetes is increasing in children (especially those < 5 years of age), and type 2 diabetes is increasing, particularly in black and minority ethnic groups. In general, the prevalence of diabetes mellitus and its percentages in different populations is almost the same throughout the world.
Compounds 1−12, 15−20, 39−43 were tested for their antisweet activity. The antisweet activity was tested on a group of volunteers who held a solution of the tested compounds (5 mL of the specific compounds solution in 0.01 M NaHCO3) in their mouth for 3 min, spat, and rinsed with distilled water. The subjects were directed to taste 10 sucrose solutions from 0.1 to 1.0 M. The activity of the compound tested was expressed at the maximum concentration of a sucrose solution whose sweetness was suppressed completely. 0.5 mM of a solution of gymnemic acids I (1) and II (2) led a complete suppression of sweetness induced by 0.4 mM sucrose. More precisely, application of a 1 mM solution of 1 and 2 orally led to a complete suppression of sweetness induced by 0.2 and 0.4 M sucrose, respectively [30]. A 1.0 mM solution of gymnemic acids III (3), IV (4), V (5) and VI (6) led to a complete suppression of sweetness induced by 0.4 mM sucrose [31,32]. The difference between the structures of gymnemic acid III and gymnemic acid IV is only the absence or presence of a double bond in the acyl group. Kurihara et al. [33] have suggested that the acyl groups might play an important role in the generation of the antisweet activity. However, it seems that acyl groups only increase the antisweet activity, but are not essential [34]. A 0.5 mM solution of gymnemic acids VIII (8), IX (9) and X (10) leads to a complete suppression of the sweet taste of 0.2 M sucrose. The results are similar to those for gymnemic acids III and IV, which are acylated at C21 [35].
In general, it has been found that the antisweet activity of these saponins decreases with the decreasing number of acyl groups. In fact, a 0.5 mM solution of each of the gymnemic acids XI (11) and XII (12), as with gymnemic acids I and II, are able to suppress the perception of sweetness due to 0.4 M sucrose. The results suggest the antisweet activity of a triterpenes is directly proportional to the number of acyl groups present in the molecule [35]. A 0.5 M solution of gymnemic acids XV (15), XVI (16), XVII (17), and XVIII (18) completely suppressed the sweet taste of 0.4 M sucrose, showing the same activity of gymnemic acids I and II [36]. Gymnemic acid VII (7), prosapogenin (20) and gymnemagenin (19) were not active at all [30,31,32]. In particular, gymnemic acid IV is a multidirectional antihyperglycaemic agent with antisweet activity [31], glucose uptake inhibitory activity, and gut glycosidase inhibitory action [37]. Moreover, the blood glucose lowering effect of gymnemic acid IV was higher than glibenclamide, which was used as a control in streptozotocin-diabetic mice [29]. Most of these pharmacological effects may synergistically contribute to alleviating type 2 diabetes-related symptoms. Thus, gymnemic acid IV may be used as a prophylactic against diabetes through its different mechanisms of action [38].
A 1.0 mM solution of compounds 41–43 completely reduced the perceived sweetness of 0.1 M sucrose; these results correspond to half of the activity of gymnemic acids I−VI. Compounds 39 and 40 were not active [34].
The literature suggests that the pharmacological effects of G. sylvestre extract or its mixture of triterpenoids occur through mechanisms such as modulation of incretin activity, stimulation of insulin secretion and release, regeneration of β-endocrinocyte Langerhans islets, activation of enzymes responsible for glucose utilisation, reduction of glucose and fatty acid assimilation in the small intestine, and interference with the sensation of sweetness. It is known that hormones that regulate the formation and secretion of hormones by pancreas islets are activated in response to the entry of food into the intestine. Release of specific and well-known gastrointestinal hormones (GIP) into the portal vein in response to the intraduodenal administration of d-glucose in the presence of G. sylvestre extract enriched in gymnemic acids/triterpenoids by inhibitors of certain proposed glucose sensors and transporters in the intestinal lumen has been studied experimentally [39]. Intraduodenal administration of d-glucose caused a dose-dependent increase in the concentration of portal immunoreactive GIP. This suggests that the extract of G. sylvestre leaves or its constituents increases GIP secretion by endocrine k-cells in the small intestine [40]. The literature suggests that the hypoglycaemic activity of G. sylvestre is due to stimulating the release of insulin (and possibly the regeneration of Langerhans islet β-cells) and enzymes responsible for glucose utilisation and inhibition of glucose absorption in the bowel [27,41,42,43]. This means that the hypertrophy of β-endocrinocytes most likely occurs due to the effect of G. sylvestre on the increased secretion of GIP [44]. An autogenic hormone in the blood that was correlated with the hypoglycaemic effect was observed in experimental animals with an experimental form of immunodependent DM during a study of G. sylvestre activity.
In Russia, tests of mixtures consisting of G. sylvestre extracts of various purities (certified preparations of G. sylvestre dry extracts) in various concentrations in combination with extracts that intensify the antioxidant effect (grape stem) and possess immunomodulating and regenerative properties are underway. More than 30 different combinations have been investigated in detail in vitro and in vivo. Considering these facts, it is obvious that G. sylvestre is a source of biologically active substances.
The very broad spectrum of pharmacological activity for G. sylvestre indicates that the use of its extract or its components at various doses and in various combinations improves the condition of latent forms of DM (prediabetes), treats insulin-independent DM, prolongs the action of hypoglycaemic preparations, and regenerates β-cells for insulin-dependent and insulin-independent DM [45]. Despite the results of these studies, only a few metabolites of G. sylvestre have been tested for their effects on glucose uptake. Among them, gymnemosaponin V (43) and gymnemic acids I-IV increase the amount of insulin in blood plasma in mice with streptozotocin-induced DM after their administration [35,38]. The presence of these compounds, but perhaps not only them, could explain that, when G. sylvestre extract was used for 21 days after streptozotocin intoxication, it reliably reduced the levels of glucose and HbAlc in blood plasma, increased the insulin content, and normalised the concentration of high-density lipoproteins (HDL) [28]. The inhibitory activity of some triterpene glycosides was examined to determine their impact on the increase in serum glucose level in oral glucose-loaded rats. Gymnemoside-b (33) and gymnemic acids III, V, and VII were found to exhibit slight inhibitory activity towards the increase of glucose absorption after a single administration of 100 mg/kg, but gymnemic acid I and gymnemasaponin, lacked this activity at the same dose. Although the above compounds are included in one of three categories (acylated polyhydroxyoleanane 3-O-glucuronide) of glucose absorption inhibitors, their activity is much less than that of their analogues [46]. Gymnemic acids II and III showed potent inhibitory activities on glucose uptake, which were almost equivalent to those of oleanolic acid-3-O-glucuronide and Escin Ia. Gymnemoside-f (37), gymnemic acid IV and gymnemasapoin V were also found to inhibit glucose uptake, while gymenomosides-c (34), -d (35), and -e (36) lacked this activity. Gymnemic acids II and III showed no effect on the serum glucose levels in oral glucose-loaded rats. They exhibited potent inhibitory activity towards glucose uptake in rat small intestine fragments [37]. Gymnemic acid V is normally considered to inhibit glucose absorption in the small intestine at a concentration of 100 mg/kg. The inhibitory effect is particularly marked after 2 h of administration, with values that are quite comparable in absolute terms to those of elatosides, escins, and senegasaponins, which were used as controls. Gymnemic acid I shows an effect almost 50% higher than that of the control after 2 h despite appearing to be less active after 30 min and 1 h. A very similar effect that is only slightly smaller than the control or gymnemic acid V was demonstrated for gymnemic acid VII and gymnemosides-a (32) and -b (33). In order of decreasing activity up to a value of approximately 30% of the control were gymnemic acid II, IV, gymnemasaponins IV and V, gymnesaponin II (40) and gymnemic acid III. However, no apparent structure-activity relationship was observed [47].
In recent years interest in cancer prevention has grown steadily and urgently, therefore it would be particularly important to identify molecules that are able to prevent or avoid the processes of carcinogenesis due to substances of which substantially we can’t do without or can’t avoid in our daily life. Several anti-inflammatory substances are well known to inhibit the action of tumour promoters in the mouse skin carcinogenesis. The compound 46 was found to inhibit the inflammatory reaction induced by tumour promoters. This anti-inflammatory activity may play an important role in the mechanism of antitumor promotion as it has already been demonstrated [48,49]. The effects of compound 46 on the growth of HepG2 cells were measured. Its inhibitory effect was remarkably effective (ID50 was 25 µM) as it induced apoptosis at high dose, and with a dose-dependent manner [50,51]. Moreover, the application of antitumor-promoting triterpenoids is highly promising for protection against tumour formation, and many triterpenoids were tested in vitro and in vivo against the action of tumour promoter, 12-O-tetradecanoylphorbol 13-acetate (TPA) induced Epstein-Barr virus (EBV) activation in Raji cells. Recently, ursolic and oleanolic acids have been reported to be inhibitors of TPA and the dose responses of the acids were very similar to those of the antitumor promoters, such as retinoic and glycyrrhetinic acids and their analogues [51,52]. Thus some glycyrrhetinic acid-related compounds were found to be inhibitors of tumour promoter-induced phenomenon in vitro. Among these compounds, the compound 46 also proved to have in vivo antitumor-promoting activity in mouse skin tumour formation induced by 7,12-dimethylbenz[a]anthracene plus tumor promoters TPA [50]. Although its mechanism is unknown, the modulation of phospholipid metabolism appeared to be a very interesting aspect was proved that the inhibitory potency of the triterpenoids for the TPA-enhanced phospholipid synthesis is closely associated with their antitumor-promoting activity. Finally, a sulforhodamine B bioassay was used to determine the cytotoxicity of compound 44. Its cytotoxic activity against four cultured human tumour cells was examined in vitro. The tumour cell lines were A549 (non small cell lung adenocarcinoma), SK-OV-3 (ovarian cancer cells), SK-MEL-2 (skin melanoma), and HCT15 (colon cancer cells) [53,54]. Doxorubicin was used as the positive control. The tested compound was essentially no cytotoxic [55]. A crude extract o mixture of compounds used in traditional medicine frequently contains components that have mutually opposing pharmacological activities beneficial for a specific disease. The higher potency of a crude drug is probably due to a synergistic effect among its component compounds, even though the activity of each compound is weak when used alone. This does not mean that it is not relevant to define a complete picture of the activities or biological properties of each compound. To identify a particular compound or a mixture of those with remarkable properties we should be able to deal with infusions or extracts that may have a different composition for an infinite number of reasons (location and/or harvesting season of the plant, particularly plant species, manner and time of extraction or partial purification etc.). Furthermore, the identification of one or of a few molecules suitable for the purpose would allow us to synthesize them, with a process as much as economically or timely convenient and to obtain pure products or well-defined composition.
Figure 2.
Chemical structures of compounds 37, 45, 47, 49–53
3. Conclusions
The market of natural substances is very attractive for its economic impact, which, on the other hand grows continuously. Research into new natural substances that can be used in the pharmaceutical, agrochemical and agro-industrial production of drugs, biopesticides and food additives has grown in recent years. Gymnema sylvestre is a relevant specific example of a plant very interesting for its numerous pharmacological properties, which include antidiabetic, anticarcinogenic, and neuroprotective effects, used as a medicinal plant in Asia for thousands of years. Its properties are attributed to triterpenoidic saponins. In light of the considerable interest generated in the chemistry and pharmacological properties of G. sylvestre triterpenes and their analogues, this review summarises the available literature on these promising bioactive natural products. The review shows the results about the isolation, chemistry and bioactivity of the triterpenoids oxidised at C-23, which are schematically presented in few tables, in particular, their isolation, distribution in different parts of the plant, and their NMR spectral data; their names and physico-chemical characterisation; and the biological properties associated with these compounds, with a focus on their potential chemotherapeutic applications.
Acknowledgments
This study was supported by AIPRAS Onlus (Associazione Italiana per la Promozione delle Ricerche sull’Ambiente e la Salute umana).
Author Contributions
All authors contributed to the draft of the article and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Oksman-Caldentey K.M., Inze D. Plant cell factories in the post-genomic era: New ways to produce designer secondary metabolites. Trends Plant Sci. 2004;9:433–440. doi: 10.1016/j.tplants.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Duradeva N., Pichersky E. Metabolic engineering of plant volatiles. Curr. Opin. Biotech. 2008;19:1–9. doi: 10.1016/j.copbio.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Picman J., Picman A.K. Autotoxicity in Parthenium hysterophorus and its possible role in control of germination. Biochem. Sys. Ecol. 1984;12:287–292. doi: 10.1016/0305-1978(84)90051-6. [DOI] [Google Scholar]
- 4.Inderjit, Keating K.I. Allelopathy: Principles, procedures, processes, and promises for biological control. Adv. Agron. 1999;67:141–231. doi: 10.1016/S0065-2113(08)60515-5. [DOI] [Google Scholar]
- 5.Montesinos E. Development, registration and commercialization of microbial pesticides for plant protection. Int. Microbiol. 2003;6:245–252. doi: 10.1007/s10123-003-0144-x. [DOI] [PubMed] [Google Scholar]
- 6.Dayan F.E., Duke S.O. Biobased herbicides; Proceedings of the 233rd ACS National Meeting; Chicago, IL, USA. 25–29 March 2007; AGRO-200. [Google Scholar]
- 7.Macias F.A., Molinillo J.M.G., Varela R.M., Galindo J.C.G. Allelopathy—a natural alternative for weed control. Pest Manag. Sci. 2007;63:327–348. doi: 10.1002/ps.1342. [DOI] [PubMed] [Google Scholar]
- 8.Liu A.G., Cui Y., Zhu Y.D., Gao W., Kong C.H., Xu X.H. Allelochemicals and mechanism of weed-suppressing activity from allelopathic rice; Proceedings of the International Forum on Green Chemical Science & Engineering and Process Systems Engineering; Tianjin, China. 8–10 October 2006; pp. 546–549. [Google Scholar]
- 9.Kaufman P.B., Cseke L.J., Warber S., Duke J.A., Brielmann H.L. Natural Products from Plants. CRC Press; Boca Raton, FL, USA: 1999. [Google Scholar]
- 10.Fiehn O. Metabolomics-the link between genotypes and phenotypes. Plant Mol. Biol. 2002;48:155–171. doi: 10.1023/A:1013713905833. [DOI] [PubMed] [Google Scholar]
- 11.Dixon R.A. Natural products and plant disease resistance. Nature. 2001;411:843–847. doi: 10.1038/35081178. [DOI] [PubMed] [Google Scholar]
- 12.Canter P.H., Thomas H., Ernst E. Bringing medicinal plants into cultivation: Opportunities and challenges for biotechnology. Trends Biotechnol. 2005;23:180–185. doi: 10.1016/j.tibtech.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Rates S.M.K. Plants as sources of drugs. Toxicon. 2001;39:603–613. doi: 10.1016/S0041-0101(00)00154-9. [DOI] [PubMed] [Google Scholar]
- 14.Raskin I., Ribnicky D.M., Komarnytsky S., Ilic N., Poulev A., Borisjuk N., Brinker A., Moreno D.A., Ripoll C., Yakoby N., et al. Plants and human health in the twenty-first century. Trends Biotechnol. 2002;20:522–531. doi: 10.1016/S0167-7799(02)02080-2. [DOI] [PubMed] [Google Scholar]
- 15.Balandrin M.F., Klocke J.A. Biotechnology in Agriculture and Forestry. Volume 40. Springer Verlag; Berlin, Germany: 1981. Medicinal, Aromatic and Industrial Materials from Plants; pp. 1–35. [Google Scholar]
- 16.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2008;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 17.Dzubak P., Hajduch M., Vydra D., Hustova A., Kvasnica M., Biedermann D., Markova L., Urban M., Sarek A. Pharmacological activities of natural triterpenoids and their therapeutic implications. J. Nat. Prod. Rep. 2006;23:394–411. doi: 10.1039/b515312n. [DOI] [PubMed] [Google Scholar]
- 18.Dewick P.M. Medicinal Natural Products. 3rd Ed. John Wiley & Sons Ltd; Chichester, West Sussex, UK: 2009. p. 187. [Google Scholar]
- 19.Breitmaier E. Terpenes. Wiley-VCH; Weinheim, Germany: 2006. [Google Scholar]
- 20.Gills P.M., Jr. Revised section F: Natural products and related compounds. Pure Appl. Chem. 1999;71:587–593. [Google Scholar]
- 21.Fraga B.M. Natural sesquiterpenoids. Nat. Prod. Rep. 2008;25:1180–1209. doi: 10.1039/b806216c. [DOI] [PubMed] [Google Scholar]
- 22.Hanson J.R. Diterpenoids. Nat. Prod. Rep. 2007;24:1332–1341. doi: 10.1039/b705951p. [DOI] [PubMed] [Google Scholar]
- 23.Connolly J.D., Hill R.A. Triterpenoids. Nat. Prod. Rep. 2008;25:794–830. doi: 10.1039/b718038c. [DOI] [PubMed] [Google Scholar]
- 24.Lois L.M., Campos N., Putra S.R., Danielsen K., Rohmer M., Boronat A. Cloning and characterization of a gene from Escherichia coli encoding and transketolase-like enzyme that catalyze the synthesis of ᴅ-1-deoxyxylulose 5-phosphate, a common precursor for isoprenoid, thiamin and pyridoxol biosynthesis. Proc. Natl. Acad. Sci. USA. 1998;95:2015–2110. doi: 10.1073/pnas.95.5.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gildemeister E., Hoffmann F. Die ätherischen Öle. 4th ed. Akademie Verlag; Berlin, Germany: 1960. pp. 1–7. [Google Scholar]
- 26.Sandermann W. Naturharze, Terpentinöl, Tallöl. Springer Verlag; Berlin, Germany: 1960. [Google Scholar]
- 27.Osbourn A.E. Saponin and olant defence-a soap story. Trends Plant Sci. 1996;1:4–9. doi: 10.1016/S1360-1385(96)80016-1. [DOI] [Google Scholar]
- 28.Osbourn A.E. Preformed antimicrobial compound and plant defense against fungal attack. Plant Cell. 1996;8:1821–1831. doi: 10.1105/tpc.8.10.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harmatha J. Chemo-ecological role spirostanol saponins in the interaction between plants and insect. In: Olezsek W., Marston A., editors. Saponins in Food, Feedstuffs and Medicinal Plants. Kluwer Academic; Dordrecht, The Netherlands: 2000. [Google Scholar]
- 30.Yoshikawa K., Nakagawa M., Yamamoto R., Arihara S., Matsuura K. Studies on taste modifiers. II. Purification and structure determination of gymnemic acids, antisweet active principle from Gymnema sylvestre leaves. Tetrahedron Lett. 1989;30:1547–1550. doi: 10.1016/S0040-4039(00)99515-7. [DOI] [Google Scholar]
- 31.Yoshikawa K., Amimoto K., Arihara S., Matsuura K. Structure studies of new antisweet constituents from Gymnema sylvestre. Tetrahedron Lett. 1989;30:1103–1106. doi: 10.1016/S0040-4039(01)80371-3. [DOI] [Google Scholar]
- 32.Kazujo Y., Kayoko A., Shigenobu A., Kouji M. Gymenmic acids V, VI and VII from Gur-ma, the leaves of Gymnema sylvestre R. Br. Br. Chem. Pharm. Bull. 1989;37:852–854. doi: 10.1248/cpb.37.852. [DOI] [Google Scholar]
- 33.Kurihara K. Inhibition of cyclic 3',5'-nucleotide phosphodiesterase in bovine taste papillae by bitter taste stimuli. Febs Lett. 1972;27:279–281. doi: 10.1016/0014-5793(72)80640-9. [DOI] [PubMed] [Google Scholar]
- 34.Yoshikawa K., Arihara S., Matsuura K. A new type of antisweet principles occurring in Gymnema sylvestre. Tetrahedron Lett. 1991;32:789–792. doi: 10.1016/S0040-4039(00)74887-8. [DOI] [Google Scholar]
- 35.Yoshikawa K., Nakagawa M., Yamamoto R., Arihara S., Matsuura K. Antisweet natural products. V. Structures of gymnemic acids VIII-XII from Gymnema sylvestre R. Br. Chem. Pharm. Bull. 1992;40:1779–1782. doi: 10.1248/cpb.40.1779. [DOI] [Google Scholar]
- 36.Yoshikawa K., Kondo Y., Arihara S., Matsuura K. Antisweet natural products. IX. Structures of gymnemic acids XV-XVIII from Gymnema sylvestre R. Br. Chem. Pharm. Bull. 1993;41:1730–1732. doi: 10.1248/cpb.41.1730. [DOI] [Google Scholar]
- 37.Masuyuki Y., Toshiyuki M., Hisashi M. Medicinal foodstuffs. X. Structures of new triterpene glycosides, Gymnemosides-c, -d, -e, and -f, from the leaves of Gymnema sylvestre R. Br.: Influence of Gymnema glycosides on glucose uptake in rat small intestinal fragmentes. Chem. Pharm. Bull. 1997;45:2034–2038. doi: 10.1248/cpb.45.2034. [DOI] [PubMed] [Google Scholar]
- 38.Kimura I., Zasshi Y. Medical benefits of using natural compounds and their derivatives having multiple pharmacological actions. Actions. 2006;126:133–143. doi: 10.1248/yakushi.126.133. [DOI] [PubMed] [Google Scholar]
- 39.Henkel T., Brunne R.M., Muller H.M., Reichel F. Statistical investigation into the structural complementary of natural products and synthetic compound. Angew. Chem. Int. 1999;38:643–647. doi: 10.1002/(SICI)1521-3773(19990301)38:5<643::AID-ANIE643>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 40.Eggersdofer M. Ullmann’s Encyclopedia of Industrial Chemistry, Electronic Release. Wiley-VCH; Weinheim, Germany: 2005. Terpenes. [Google Scholar]
- 41.Duke S.O., Romagni J.G., Dayan F.E. Natural products as sources for new mechanisms of herbicidal action. Crop Prot. 2000;19:583–589. doi: 10.1016/S0261-2194(00)00076-4. [DOI] [Google Scholar]
- 42.Catchpole O.J., von Kamp J.C., Grey J.B. Extraction of squalene from shark liver oil in a packed column using supercritical carbon dioxide. Ind. Eng. Chem. Res. 1997;36:4318–4324. doi: 10.1021/ie9702237. [DOI] [Google Scholar]
- 43.Pietsch A., Jaeger P. Concentration of squalene from shark liver oil by short-path distillation. Eur. J. Lipid. Sci. Technol. 2007;109:1077–1082. doi: 10.1002/ejlt.200700039. [DOI] [Google Scholar]
- 44.Eisenreich W., Rohdich F., Bacher A. Deoxyxylulose phosphate pathway to terpenoids. Trends Plant Sci. 2001;6:78–84. doi: 10.1016/S1360-1385(00)01812-4. [DOI] [PubMed] [Google Scholar]
- 45.Stocklin W. Gymnemagenin, vermutliche struktur Glykoside und Aglykone, 289. Mitteilung. Helv. Chim. Acta. 1967;50:491–503. doi: 10.1002/hlca.19670500217. [DOI] [Google Scholar]
- 46.Yoshikawa M., Murakami T., Kadoya M., Li Y., Murakami N., Yamahara J., Matsuda H. Medicinal foodstuffs. IX. The inhibitors of glucose absorption from the leaves of Gymnema sylvestre R. Br. (Asclepiadaceae): Structures of gymnemosides a and b. Chem. Pharm. Bull. 1997;45:1671–1676. doi: 10.1248/cpb.45.1671. [DOI] [PubMed] [Google Scholar]
- 47.Murakami N., Murakami T., Kadoya M., Matsuda H., Yamahara J., Yoshikawa M. New hypoglycemic constituents in “gymnemic acid” from Gymnema sylvestre. Chem. Pharm. Bull. 1996;44:469–471. doi: 10.1248/cpb.44.469. [DOI] [PubMed] [Google Scholar]
- 48.Nishino H., Nishino A., Takayasu J., Hasegawa T., Iwashima A., Hirabayashi K., Iwata S., Shibata S. Inhibition of the tumor-promoting action of 12-O-tetradecanoylphorbol 13-acetate by some oleanane-type triterpenoid compounds. Cancer Res. 1988;48:5210–5521. [PubMed] [Google Scholar]
- 49.Shibata S. ACS Symposium Series Food Phytochemicals for Cancer Prevention II. Volume 547. American Chemical Society; Washington, DC, USA: 1994. Antitumor-promoting and anti-inflammatory activities of licorice principles and their modified compounds; pp. 308–321. [Google Scholar]
- 50.Nishino H., Shibata S., Hirabayashi K., Iwata S. Antitumor-promoting activity of glycyrrhetinic acid-related compounds. Kyoto-Furitsu Ika Daigaku Zasshi. 1986;95:1563–1566. [Google Scholar]
- 51.Satomi Y., Nishino H., Shibata S. Glycyrrhetinic and related compounds induce G1 arrest and apoptosis in human hepatocellular carcinoma HepG2. Anticancer Res. 2005;25:4043–4047. [PubMed] [Google Scholar]
- 52.Ohigashi H., Takamura H., Koshimizu H., Tokuda H., Ito Y. Search for possible antitumor promoters by inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced Epstein-Barr virus activation. Ursolic acid and oleanolic acid from an anti-inflammatory. Cancer Lett. 1986;30:143–151. doi: 10.1016/0304-3835(86)90082-0. [DOI] [PubMed] [Google Scholar]
- 53.Lee J.S., Yoo H.S., Suh Y.G., Jung J.K., Kim J.W. Structure-activity relationship of pentacylic triterpene esters from Uncaria rhynchophylla as inhibitors of phospholipase Cγ1. Planta Med. 2008;74:1481–1487. doi: 10.1055/s-2008-1081348. [DOI] [PubMed] [Google Scholar]
- 54.Skehan P., Stroreng R., Scudiero D., Monks A., Mcmahon J., Vistica D., Warren J.T., Bokesch H., Kenney S., Boyd M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Nat. Cancer. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 55.Lee I.K., Choi S.U., Lee K.R. Triterpene saponins from Pleurospermum kamtschaticum and their biological activity. Chem. Pharm. Bull. 2012;60:1011–1018. doi: 10.1248/cpb.c12-00274. [DOI] [PubMed] [Google Scholar]
- 56.Liu H.M., Kiuchi F., Tsuda Y. Isolation and structure elucidation of gymnemic acids, antisweet principles of Gymnema sylvestre. Chem. Pharm. Bull. 1992;40:1366–1375. doi: 10.1248/cpb.40.1366. [DOI] [PubMed] [Google Scholar]
- 57.Yoshisuke T., Fumiyuki K., Hong-Min L. Establishment of the structure of gymenmagenin by X-ray analysis and the structure of deacylgymnemic acid. Tetrahedron Lett. 1989;30:361–362. doi: 10.1016/S0040-4039(00)95202-X. [DOI] [Google Scholar]
- 58.Zarrelli A., Ladhari A., Haouala R., di Fabio G., Previtera L., DellaGreca M. New acylated oleane and lupane triterpenes from Gymnema sylvestre. Helv. Chim. Acta. 2013;96:2200–2206. doi: 10.1002/hlca.201300047. [DOI] [Google Scholar]
- 59.Sahu N.P., Mahato S.B., Sarkar S.K., Poddar G. Triterpenoid saponins from Gymnema sylvestre. Phytochemistry. 1996;41:1181–1185. doi: 10.1016/0031-9422(95)00782-2. [DOI] [PubMed] [Google Scholar]
- 60.Zarrelli A., Della Greca M., Ladhari A., Haouala R., Previtera L. New Triterpenes from Gymnema sylvestre. Helv. Chim. Acta. 2013;96:1036–1045. doi: 10.1002/hlca.201200331. [DOI] [Google Scholar]
- 61.Sarkar S.K. Potential Hypoglycemic and Antihyperglycemic Triterpenold Saponins from Gymnema sylvestre; Proceedings of 210th ACS National Meeting; Chicago IL, USA. August 1995; Book of Abstracts; AGFD-239. [Google Scholar]
- 62.García-Granados A., Lopez P.E., Melguizo E., Parra A., Simeo Y. Remote hydroxylation of methyl groups by regioselective cyclopalladation. Partial synthesis of hyptatic acid-A. J. Org. Chem. 2007;72:3500–3509. doi: 10.1021/jo070116e. [DOI] [PubMed] [Google Scholar]
- 63.Spasov A.A., Samokhina M.P., Bulanov A.E. Medicinal plants antidiabetic properties of Gymnema sylvestre (a review) Pharm. Chem. J. 2008;42:29–31. doi: 10.1007/s11094-008-0051-8. [DOI] [Google Scholar]
- 64.Ye W.C., Liu X., Zhao S.X., Che C.T. Triterpenes from Gymnema sylvestre growing in China. Biochem. Syst. Ecol. 2001;29:1193–1195. [Google Scholar]
- 65.Sugihara Y., Nojima H., Matsuda H., Murakami T., Yoshikawa M., Kimura I. Antihyperglycemic effects of gymnemic acid IV, a compound derived from Gymnema sylvestre leaves in streptozotocin-diabetic mice. J. Asian Nat. Prod. Res. 2000;2:321–327. doi: 10.1080/10286020008041372. [DOI] [PubMed] [Google Scholar]
- 66.Maser E., Blum A. Selektiver Inhibitor der 11β-Hydroxysteroid Dehydrogenase. DE 102004040690 A1. 2006 Mar 2;
- 67.Shibata S., Nishino H., Hirabayashi K., Iwata S. Epicarcinogen Inhibitors Containing Oleanane Triterpenes. JP 63057519 A. 1988 Mar 12;