Abstract
Background
Klotho protein has been shown to act as a hormone on the cardiovascular system, and to have specific protective effects on vascular endothelial cells. The aim of this study was to investigate the mechanisms of the anti-oxidative and anti-apoptotic effects of klotho protein on hydrogen peroxide (H2O2)-induced apoptosis and endoplasmic reticulum oxidative stress in human umbilical vein endothelial cells (HUVECs).
Material/Methods
HUVECs were cultured in vitro and treated with H2O2. The MTT assay evaluated cell viability of H2O2-treated HUVECs, and flow cytometry measured cell apoptosis. An enzyme-linked immunosorbent assay (ELISA) measured the levels of nitric oxide (NO), tumor necrosis factor-alpha (TNF-α), and interleukin (IL)-6. Western blot was used to detect the expression of the proteins, 78 kD glucose-regulated protein (GRP78), CCAAT-enhancer-binding protein homologous protein (CHOP), caspase-3, caspase-9, caspase-12, and AKT. The effects of LY294002, a pharmacological inhibitor of PI3K, were evaluated.
Results
Klotho protein increased the viability of H2O2-treated HUVECs and reduced the expression of NO, TNF-α, and IL-6. Klotho protein reduced the rate of apoptosis of H2O2-treated HUVECs and downregulated the expression of proteins associated with endoplasmic reticulum oxidative stress, GRP78 and CHOP, and the expression of the apoptotic proteins, caspase-3, caspase-9, and caspase-12, and activated the phosphorylation of AKT. The addition of LY294002 inhibited klotho protein downregulation of GRP78, CHOP, caspase-3, caspase-9, and caspase-12 expression.
Conclusions
In HUVECs, klotho protein suppressed apoptosis mediated by endoplasmic reticulum oxidative stress by activation of the PI3K/AKT pathway.
MeSH Keywords: Apoptosis, Endoplasmic Reticulum Stress, Human Umbilical Vein Endothelial Cells
Background
Worldwide, ischemic heart disease due to atherosclerosis of the coronary arteries is the leading cause of morbidity and mortality [1,2]. Apoptosis of vascular endothelial cells, mediated by oxidative stress involving the endoplasmic reticulum has been reported to be associated with the development of atherosclerotic lesions [3]. Further studies on the molecular mechanisms of endothelial cell apoptosis may contribute to the further understanding of the pathogenesis of atherosclerosis. Apoptosis is recognized to be a therapeutic target in several disease processes. Therefore, the development of gene or drug treatments that target apoptotic signaling pathways is an important area for further research.
The endoplasmic reticulum is an important organelle in eukaryotic cells, which has roles in protein synthesis, folding, assembly, modification and protein transportation. The endoplasmic reticulum also participates in lipid synthesis and carbohydrate metabolism, regulates intracellular calcium concentrations, and is a cellular site for the storage of calcium ions. The endoplasmic reticulum may undergo stress that affects its function, most importantly, oxidative stress, but also stress due to ischemia, or imbalance in calcium homeostasis, which can lead to overexpression of proteins or result in the accumulation of unfolded and misfolded proteins in the endoplasmic reticulum, resulting in the condition termed ‘endoplasmic reticulum stress’ [4]. When endoplasmic reticulum stress is prolonged or severe, it will initiate cell apoptosis [5–7].
PI3K/AKT is a key signaling pathway that regulates cell proliferation, differentiation, apoptosis, and cell senescence [8]. Previously published studies have shown that the PI3K/AKT pathway plays an important role in protecting cells from endoplasmic reticulum stress, but the molecular mechanism remains unclear [9]. LY24402 is a specific PI3K/AKT inhibitor that inhibits the phosphorylation of AKT and down-regulates the expression of endoplasmic reticulum stress-related proteins, significantly reducing PI3K/AKT mediated cell defense. Therefore, the PI3K/AKT pathway plays an important role in protecting the cell from endoplasmic reticulum stress, making it a potential therapeutic target.
Klotho protein is secreted in the blood and can act as a hormone to regulate various target organs, with a protective effect on the cardiovascular system. A previously published study has shown that klotho protein has anti-oxidative, anti-inflammatory, anti-apoptotic effects on vascular endothelial cells [10].
Therefore, the aim of this study was to investigate the mechanisms of the anti-oxidative and anti-apoptotic effects of klotho protein on hydrogen peroxide (H2O2)-induced apoptosis and endoplasmic reticulum oxidative stress in human umbilical vein endothelial cells (HUVECs), including the role of the PI3K/AKT pathway.
Material and Methods
Materials
Human umbilical vein endothelial cells (HUVECs) were obtained from the First Affiliated Hospital of Jinzhou Medical University. Fetal bovine serum (FBS), 0.25% trypsin containing ethylenediamine tetra-acetic acid (EDTA) and high glucose Dulbecco’s Modified Eagle Medium (DMEM) for cell culture were purchased from Hyclone Laboratories Inc. (Logan, UT, USA). Recombinant human klotho protein was purchased from PeproTech (Rocky Hill, NJ, USA). The MTT kit was purchased from Solaibio Science and Technology, Co., Ltd. (Beijing, China). Lactate dehydrogenase (LDH), malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione peroxidase (GSH-PX) detection kits were purchased from Nanjing Jiancheng Bio Company (Nanjing, China). The enzyme-linked immunosorbent assay (ELISA) kit for nitric oxide (NO) was purchased from Shanghai Yan Jin Biology Company (Shanghai, China). The ELISA kit for interleukin (IL)-6 and tumor necrosis factor-alpha (TNF-α) was purchased from Cheng Lin Biology Company (Beijing, China). The Annexin-V/propidium iodide (PI) cell apoptosis detection kit was purchased from Shanghai Yi Sheng Biotechnology Company (Shanghai, China). Primary antibodies to p-AKT, AKT, 78 kD glucose-regulated protein (GRP78), CCAAT-enhancer-binding protein homologous protein (CHOP), caspase-3, caspase-9, caspase-12 were purchased from Shenyang Wan Lei Biology Company. The horseradish peroxidase (HRP)-conjugated secondary antibody was purchased from EarthOx Life Sciences (Millbrae, CA, USA). The antibody to beta-actin was purchased from the SAB Signalway Antibody LLC (College Park, MD, USA). LY294002, a pharmacological inhibitor of PI3K, was purchased from the ApexBio (Walnut, CA, USA).
Cell culture of human umbilical vein endothelial cells (HUVECs)
HUVECs were cultured in high-glucose DMEM medium containing 10% FBS and 1% antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin) and at 37°C in 5% CO2 When the cells reached approximately 80% confluence, 0.25% trypsin was used to remove the cells. HUVECs in the logarithmic growth phase were used for the experiments. The vascular endothelial cell model of apoptosis mediated by endoplasmic reticulum stress was constructed by in vitro culture of HUVECs with 200 μmol/L hydrogen peroxide (H2O2) in cell culture medium. HUVECs were randomly divided into the following five groups: the H2O2-treated group (the model group); the klotho protein-treated group (treated with 50 μg/L or with 100 μg/L of klotho protein for 24 hours); the PI3K inhibitor group (treated with 100 μg/L klotho protein and the PI3K inhibitor, LY294002, for 24 hours); and the control group (with no H2O2 treatment).
The MTT assay to detect cell viability
The survival rate of cells in each group was determined by the MTT colorimetric assay, in which methyl tetrazolium blue was reduced to purple crystals by succinate dehydrogenase present in the mitochondria of living endothelial cells. The passaged cells were seeded in 96-well plates (1×105 cells/well) and treated according to their assigned experimental group. Only DMEM was added to the control group. MTT solution (20 μL) was added to each well, with a final concentration of 0.5 mg/ml with culture for a further 4 h. The supernatant was then discarded, 150 μL of dimethyl sulfoxide (DMSO) was added to each well, and the mixture was shaken for 15 min. The absorbance of the reaction in each well was read at a wavelength of 492 nm with a microplate reader. The percentage of HUVEC cell survival was expressed as a percentage of the control group.
Detection of apoptosis in HUVECs by flow cytometry
HUVECs in each study group were digested and centrifuged to collect the cells. HUVECs were resuspended in phosphate-buffered saline (PBS), washed twice, and adjusted to a cell concentration of 5×105 cells/L. To each study group of HUVECs, Annexin-V and PI were added and the reaction was performed for 15 minutes at room temperature in the dark. Then, 300 μL of binding buffer was added, and the apoptosis rate of each group of cells was measured by flow cytometry within 1 hour.
Detection of changes in reactive oxygen species (ROS) in HUVECs by dihydroethidium (DHE)-derived fluorescence probe detection
The logarithmic phase HUVECs were harvested and added to six-well plates with a concentration of 105 cells per well. The cells were cultured for 24 hours before the experimental treatments. Following treatment, the culture medium was removed and the cells were washed three times with PBS, and 10 μmol/L of dihydroethidium (DHE) was added. The HUVECs were incubated for 30 minutes at 37°C and viewed under a fluorescence microscope. The fluorescence imaging was analyzed using Image-Pro Plus 6 software. The results were expressed in units of relative fluorescence intensity.
Determination of the antioxidant index of HUVECs by measuring lactate dehydrogenase (LDH), malondialdehyde (MDA), superoxide dismutase (SOD), and reduced glutathione (GSH-PX)
According to the experimental design, the treated HUVECs in each group underwent analysis of the culture supernatant. A colorimetric method using a kit was used to detect the levels of lactate dehydrogenase (LDH), malondialdehyde (MDA), superoxide dismutase (SOD), and reduced glutathione (GSH-PX) in the cells, according to the manufacturer’s instructions. Superoxide anion (O2−), produced by the reaction between xanthine and xanthine oxidase, oxidizes hydroxylamine to form nitrite, which is purple-red under the action of a color-developing agent the absorbance was measured by a spectrophotometer. When sample contained SOD, it had a specific inhibitory effect on the superoxide anion radical, which reduced the formation of nitrite. When the absorbance value was lower than the absorbance value of the control, and a formula can be used to determine the SOD activity of the sample. GSH-PX can promote the reaction of H2O2 with GSH. The activity of GSH-PX was determined by the speed of enzymatic reaction. When the consumption of GSH in the enzymatic reaction was measured, the activity of the enzyme was determined. However, since the two reaction substrates can undergo a redox reaction without an enzyme, it was necessary to subtract the portion of the GSH reduction caused by the non-enzymatic reaction. The action of the anti-oxidant, reduced glutathione (GSH-PX), results in the production of 5-thio-2-nitrobenzoic acid, a stable yellow product, and the absorbance was measured at 412 nm to calculate the GSH-PX content.
Detection of NO, TNF-α, and interleukin IL-6 by ELISA
The supernatant from each group of cultured HUVECs was collected. The levels of NO, TNF-α, and IL-6 in the supernatant were detected by an enzyme-linked immunosorbent assay (ELISA) kit. The kit was used at room temperature for 30 minutes, according to the manufacturer’s instructions. The absorbance measurements were plotted at 450 nm using a microplate reader and a standard curve was prepared to calculate the concentration of NO, TNF-α, and IL-6 according to the absorbance of each sample.
Western blot to detect protein expression
Following treatment of each group of HUVECs, according to the experimental design, the endothelial cells were washed twice with pre-cooled PBS, then added to the cell lysate and lysed for 15 minutes on the ice. The lysate was collected and centrifuged for 15 minutes, followed by removal of the supernatant. The BCA method was used to quantify the amount of protein. The total protein (20 μg) was added to each well and separated by electrophoresis on 4–12% gradient polyacrylamide gels and transferred to PVDF membranes. The membranes were blocked with 3% dried skimmed milk powder for 1 hour at room temperature. The membranes were incubated at 4°C overnight with the primary antibodies (1: 1000). After washing three times with TBST, the membranes were incubated with the secondary antibody (1: 5000) in TBST solution for 2 hours at room temperature. Thee membranes were washed, the chemiluminescence reagent was added and developed, and the result was detected using Image-J software. Beta-actin was used as an internal reference, and the relative expression of related proteins was reflected by the ratio of the target band to the internal reference gray value.
Statistical analysis
All data were analyzed by SPSS version17.0 software. Data were presented as the mean ±SD. The number of samples analyzed was five. For analysis of homogeneity of variance, one-way analysis of variance (ANOVA) was used for comparison between multiple groups. The differences were considered to be statistically significant with a P-value <0.05, and highly significant with a P-value <0.01.
Results
The effects of klotho protein on the viability of human umbilical vein endothelial cells (HUVECs) after hydrogen peroxide (H2O2) treatment
The results of the MTT assay showed that klotho protein significantly increased the viability of human umbilical vein endothelial cells (HUVECs) after hydrogen peroxide (H2O2) treatment (Figure 1). Compared with the HUVECS in the control group, the cell viability of HUVECS in the model group (H2O2-treated group) was significantly decreased (P<0.01). Compared with the model group (H2O2-treated group), the cell viability of the klotho protein-treated group increased gradually in a concentration-dependent manner (within a certain concentration range) (P<0.05). The cell viability of the LY294002-treated (10 μmol/L) group was significantly lower than that of the klotho protein-treated group (P<0.01). Compared with 100μg/L of klotho protein, 200 μg/L of klotho protein alone showed no significant changes in cell viability. These results indicated that klotho protein enhanced the viability of HUVECs after oxidative stress from the action of H2O2 (Figure 1).
Figure 1.
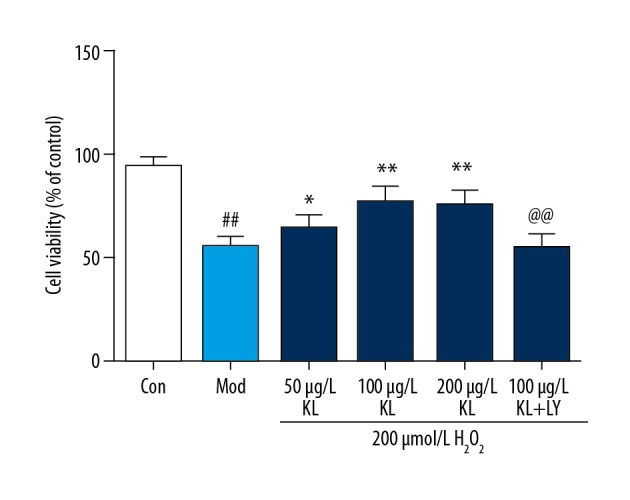
The effects of klotho protein treatment on the viability of human umbilical vein endothelial cells (HUVECs) treated with hydrogen peroxide (H2O2). Results are represented as the mean ±SD. Mod – the model group treated with 200 μmol/L of hydrogen peroxide (H2O2). The number of samples was five. # P<0.05. ## P<0.01 vs. the control. * P<0.05. ** P<0.01 vs. H2O2. @ P<0.05. @@ P<0.01 vs. 100 μg/L klotho protein. LY – LY294002, which is a PI3K inhibitor.
The effects of klotho protein on HUVEC functional factors and expression of inflammatory factors after H2O2 treatment
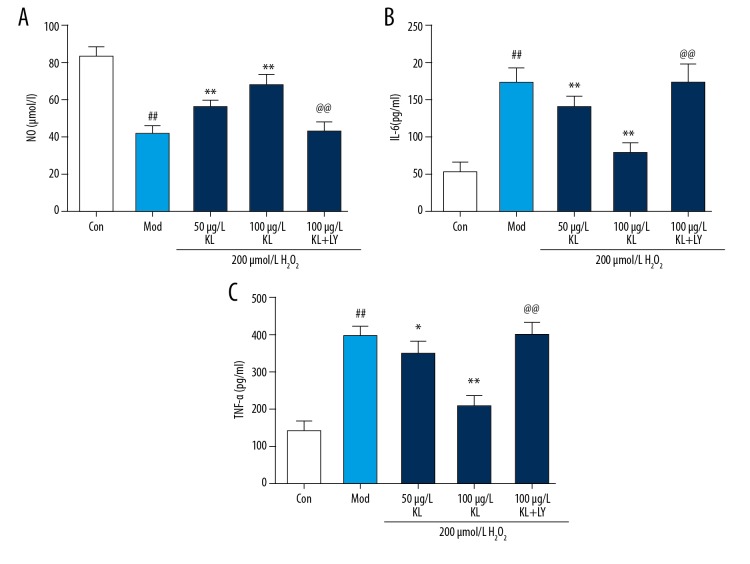
The ELISA method was used to detect the levels of NO (Figure 2A), IL-6 (Figure 2B) and TNF-α (Figure 2C) in the supernatant of cultured HUVECs in each group, Compared with the control group, the levels of NO (Figure 2A) in the HUVEC supernatant of the model group (H2O2-treated group) were significantly decreased, and the content of TNF-α (Figure 2C) and IL-6 (Figure 2B) were significantly increased (P<0.01). Compared with the model group (H2O2-treated group), the NO (Figure 2A) content in the cell supernatant of the klotho protein-treated group gradually increased with the concentration gradient, and the TNF-α (Figure 2C) and IL-6 (Figure 2B) levels gradually decreased, indication a concentration-dependent or dose-dependent protective effect of klotho protein (P<0.01). The content of NO in the supernatant of the LY294002-treated group (10 μmol/L) was lower than that of klotho protein-treated group, and the TNF-α and IL-6 levels were significantly higher compared with the klotho protein-treated group (P<0.01).
Figure 2.
The effects of klotho protein on human umbilical vein endothelial cell (HUVEC) functional factors, including nitric oxide (NO), and inflammatory factors, tumor necrosis factor-α (TNF-α) and interleukin (IL)-6 after hydrogen peroxide (H2O2) treatment. Enzyme-linked immunosorbent assay (ELISA) was used to detect the levels of nitric oxide (NO) (A), interleukin (IL)-6 (B), and tumor necrosis factor-α (TNF-α) (C) in the human umbilical vein endothelial cell (HUVEC) supernatant of each group. Results are represented as the mean ±SD. Mod – the model group, treated with 200 μmol/L of hydrogen peroxide (H2O2). The number of samples was five. # P<0.05. ## P<0.01 vs. control. * P<0.05. ** P<0.01 vs. H2O2. @ P<0.05. @@ P<0.01 vs. 100 μg/L klotho protein. LY – LY294002, which is a PI3K inhibitor.
The effects of treatment with klotho protein on reactive oxygen species (ROS) of HUVECs after hydrogen peroxide (H2O2) treatment
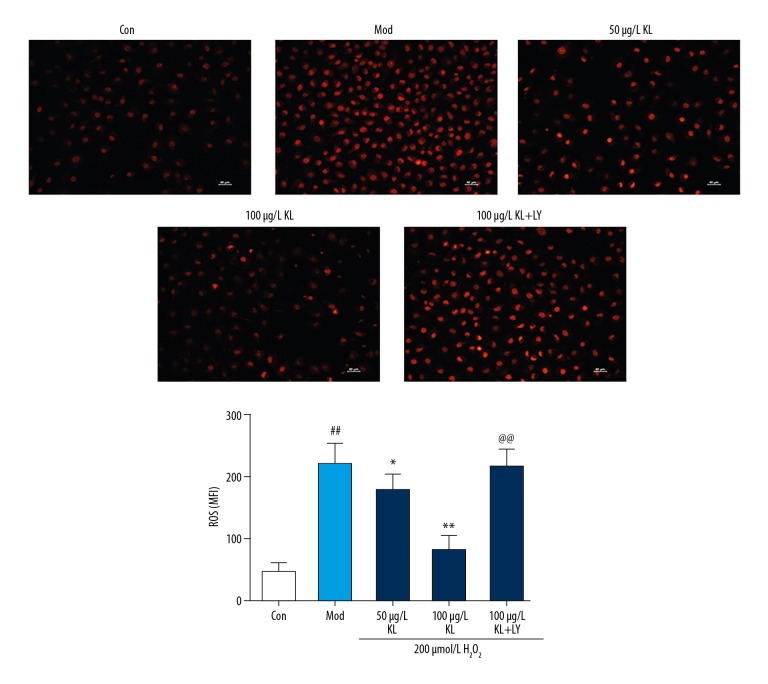
DHE fluorescence probe detection showed that HUVECs in the model group (H2O2-treated group) produced a large amount of ROS, which were significantly increased compared with the control group (P<0.05). However, the ROS content of the klotho protein group decreased gradually (P<0.05). Compared with the klotho protein-treated group, the ROS content in the LY294002-treated group (10 μmol/L) was significantly increased. Klotho protein reduced the production of ROS in HUVECs after H2O2 oxidative stress, and LY294002, a pharmacological inhibitor of PI3K, prevented this effect (Figure 3).
Figure 3.
The effects of klotho protein treatment on reactive oxygen species (ROS) of human umbilical vein endothelial cells (HUVECs) after hydrogen peroxide (H2O2) treatment. Dihydroethidium (DHE)-derived fluorescence probe detection at 570 nm showed that human umbilical vein endothelial cells (HUVECs) in the model group, treated with hydrogen peroxide (H2O2) produced increased levels of reactive oxygen species (ROS), which were significantly increased compared with the untreated group. However, the ROS content of the klotho protein-treated group decreased gradually. Results are represented as the mean ±SD. Mod – the model group treated with 200 μmol/L of hydrogen peroxide (H2O2). The number of samples was five. # P<0.05. ## P<0.01 vs. control. * P<0.05. ** P<0.01 vs. H2O2. @ P<0.05. @@ P<0.01 vs. 100 μg/L klotho protein. LY – LY294002, which is a PI3K inhibitor.
The effects of klotho protein on the activity of lactate dehydrogenase (LDH), malondialdehyde (MDA), superoxide dismutase (SOD), and reduced glutathione (GSH-PX) in HUVECs after H2O2 treatment
Compared with the control group of HUVECs, the supernatant from the HUVECs in the model group (H2O2-treated group) showed a significant increase in the levels of lactate dehydrogenase (LDH) and malondialdehyde (MDA) (Figure 4A–4D) and a significant decrease in the activity of superoxide dismutase (SOD) and reduced glutathione (GSH-PX) (P<0.01). Different concentrations of klotho protein treatment of HUVECs in the model group (H2O2-treated group) resulted in a gradual decrease in levels of LDH and MDA (Figure 4A, 4B) and the activity of SOD and GSH-PX gradually increased (Figure 4C, 4D) (P<0.01). Compared with the klotho protein-treated group, the levels of LDH and MDA in the LY294002-treated group (10 μmol/L) significantly increased, while SOD and GSH-PX activities were significantly reduced (P<0.01).
Figure 4.
(A–D) The effects of klotho protein treatment on the activity of lactate dehydrogenase (LDH), malondialdehyde (MDA), superoxide dismutase (SOD), and reduced glutathione (GSH-PX) in human umbilical vein endothelial cells (HUVECs) after hydrogen peroxide (H2O2) treatment. Results are represented as the mean ±SD. Mod – the model group treated with 200 μmol/L of hydrogen peroxide (H2O2). The number of samples was five. # P<0.05. ## P<0.01 vs. control. * P<0.05. ** P<0.01 vs. H2O2. @ P<0.05. @@ P<0.01 vs. 100 μg/L klotho protein. LY – LY294002, which is a PI3K inhibitor.
The effects of klotho protein on apoptosis of HUVECs after H2O2 treatment
In this study, the total apoptosis rate of HUVECs was evaluated as the sum of the early apoptosis rate and the late apoptosis rate, represented by flow cytometry as the upper right quadrant (UR) + lower right quadrant (LR). Flow cytometry showed that the apoptosis rate of HUVECs in the model group (H2O2-treated group) was significantly higher compared with the control group (P<0.05), and the apoptosis rate of the klotho protein group treated with different concentrations gradually decreased (P<0.05). The apoptosis rate of HUVECs cells in the LY294002-treated group (10 μmol/L) was significantly lower than that of the klotho protein-treated group. These results indicated that klotho protein reduced the apoptosis rate of HUVECs cells after H2O2 treatment, but LY294002, a pharmacological inhibitor of PI3K, prevented this effect (Figure 5).
Figure 5.
The effects of klotho protein treatment on apoptosis of human umbilical vein endothelial cells (HUVECs) after H2O2 treatment measured by flow cytometry Results are represented as the mean ±SD. Mod – the model group treated with 200 μmol/L of hydrogen peroxide (H2O2). The number of samples was five. # P<0.05. ## P<0.01 vs. control. * P<0.05. ** P<0.01 vs. H2O2. @ P<0.05. @@ P<0.01 vs. 100 μg/L klotho protein. LY – LY294002, which is a PI3K inhibitor.
Klotho protein treatment reduced the expression of the proteins 78 kD glucose-regulated protein (GRP78), CCAAT-enhancer-binding protein homologous protein (CHOP), caspase-3, caspase-9, and caspase-12, and activated the phosphorylation of AKT
Western blot showed that the expression levels of the AKT protein in HUVECs in the model group (H2O2-treated group) was significantly lower compared with the control group (P<0.01) (Figure 6A). However, the expression levels of the AKT protein significantly increased with increasing concentrations of klotho protein treatment (P<0.01) (Figure 6A). In contrast, the expression levels of 78 kD glucose-regulated protein (GRP78), CCAAT-enhancer-binding protein homologous protein (CHOP), caspase-3, caspase-9, and caspase-12 protein in HUVECs in the model group (H2O2-treated group) were significantly greater compared with the control group (P<0.01). The expression of GRP78, CHOP, caspase-3, caspase-9, and caspase-12 protein in the group treated with klotho protein at different concentrations gradually decreased (P<0.01) (Figure 6B, 6C). Compared with the klotho protein-treated group, the expression level of AKT protein in the LY294002-treated group (10 μmol/L) was significantly reduced, and the expression levels of GRP78, CHOP, caspase-3, caspase-9, and caspase-12 proteins were significantly increased (P<0.01). Klotho protein treatment increased the expression of AKT and reduced the expression of GRP78, CHOP, caspase-3, caspase-9, and caspase-12, whereas treatment with LY294002, a pharmacological inhibitor of PI3K, prevented these effects (Figure 6A–6C). These results indicated that the mechanism of the inhibition of endoplasmic reticulum oxidative stress by klotho treatment may be related to the activity of the PI3K/AKT pathway.
Figure 6.
(A–C) The effects of klotho protein treatment on the expression of the proteins, 78 kD glucose-regulated protein (GRP78), CCAAT-enhancer-binding protein homologous protein (CHOP), and caspase-12. GRP78 and CHOP and caspase-12 protein were detected by Western blot. Results are represented as the mean ±SD. Mod – the model group treated with 200 μmol/L of hydrogen peroxide (H2O2). The number of samples was five. # P<0.05. ## P<0.01 vs. control. * P<0.05. ** P<0.01 vs. H2O2. @ P<0.05. @@ P<0.01 vs. 100 μg/L klotho protein. LY – LY294002, which is a PI3K inhibitor.
Discussion
Atherosclerosis of the coronary arteries leads to ischemic heart disease, and is a process that begins and progresses with changes in the endothelial cells that include apoptosis [11]. The endoplasmic reticulum stress-mediated apoptosis pathway is a recently described pathway, which has been shown in vascular endothelial cells, indicating that prevention or inhibition of this pathway in the vascular endothelium may prevent the development or progression of atherosclerosis [12]. Hydrogen peroxide (H2O2) is commonly used to induce experimental cell oxidative stress including oxidative damage and cell apoptosis [13]. The aim of this study was to investigate the mechanisms of the anti-oxidative and anti-apoptotic effects of klotho protein on H2O2-induced apoptosis and endoplasmic reticulum oxidative stress in human umbilical vein endothelial cells (HUVECs). The findings of this study showed that klotho protein suppressed apoptosis mediated by endoplasmic reticulum oxidative stress by activation of the PI3K/AKT pathway.
Klotho protein is a newly discovered anti-aging protein and is classified as membrane and secretory types. Klotho protein secreted in the blood can act as a hormone to regulate various target organs, and is mainly involved in the metabolism and homeostasis of calcium and phosphorus in the body and is also involved in insulin resistance and resistance to the effects of reactive oxygen species (ROS). Klotho protein can protect the vascular endothelium through its protective anti-inflammatory, anti-oxidation, and anti-apoptosis effects, and clinical studies on the potential effects of klotho protein on cardiovascular and cerebrovascular disease have been proposed [14].
In the present study, an in vitro model of apoptosis induced by endoplasmic reticulum oxidative stress in vascular endothelial cells was established with H2O2-treated HUVECs. The viability of HUVECs in the model group (H2O2-treated group) was significantly decreased and the apoptosis rate was significantly increased. Klotho protein treatment significantly increased the viability of HUVECs, decreased the apoptosis rate of HUVECs in a concentration-dependent manner, and treatment with LY294002, a pharmacological inhibitor of PI3K, prevented these effects. Klotho protein was shown to reduce endoplasmic reticulum stress-mediated apoptosis in HUVECs in vitro.
Previously published experimental and clinical studies have shown that oxidative stress is associated with endothelial cell abnormalities and with high-risk factors of atherosclerosis, including hypertension, hyperlipidemia, diabetes, and smoking [15]. Reactive oxygen species (ROS) are the products of oxidative stress and are a cause of endoplasmic reticulum stress. Also, endoplasmic reticulum stress can induce oxidative stress in a feedback manner. Expression levels of lactate dehydrogenase (LDH), malondialdehyde (MDA), superoxide dismutase (SOD), and reduced glutathione (GSH-PX) can reflect the antioxidant capacity of HUVECs. The integrity of the cell membrane after oxidative injury can be reflected by the amount of LDH leakage from the cell. MDA, as the final product of lipid peroxidation, can reflect the metabolism of the oxygen free radicals and the degree of free radical damage to cells.
In the present study, LDH and MDA increased in the model group (H2O2-treated group), while the content of LDH and MDA following treatment with different concentrations of klotho protein, decreased gradually. Klotho protein maintained the integrity of HUVEC cells and reduced the oxidative damage. Because SOD and GSH-PX, as antioxidant enzymes, would be expected to control cell damage caused by oxidative stress and repair damaged cells, their activity could reflect antioxidant capacity. In this study, the activity of SOD and GSH-PX in the model group (H2O2-treated group) decreased, while the SOD and GSH-PX activity of HUVECs in the klotho protein-treated group significantly increased significantly, which indicated that klotho protein might restore the antioxidant capacity of HUVECs cultured in vitro.
ROS, including O−2 and H2O2, can promote oxidative stress-induced endothelial cell injury and apoptosis [16]. In this study, H2O2 was shown to induce HUVECs to produce ROS, directly affected cell survival and function, and klotho protein treatment significantly reduced ROS production. Inflammatory factors, including interleukin (IL)-6 and tumor necrosis factor-alpha (TNF-α), have an important role in the initiation of atherosclerosis [17]. In this study, the levels of IL-6 and TNF-α in the model group (H2O2-treated group) were significantly increased, while the levels of IL-6 and TNF-α in the klotho protein-treated group were significantly decreased. These findings supported that klotho protein had anti-inflammatory effects in HUVECs in vitro.
NO is an important vasodilator factor released by vascular endothelial cells and can directly reflect the functional integrity of endothelial cells [18]. In the present study, the NO content in the model group (H2O2-treated group) was significantly reduced. The klotho protein-treated group had a significant increase in the NO content, suggesting that klotho protein restored normal NO production by HUVECs following H2O2 injury.
The GRP78 protein is a molecular chaperone of endoplasmic reticulum and is an important protein that is upregulated when endoplasmic reticulum stress is induced, having both a protective role and being a ‘signature’ protein for the detection of endoplasmic reticulum stress [19,20]. CHOP protein is an apoptosis signal molecule induced by endoplasmic reticulum stress, and when expressed in large quantities, causes arrest of cell growth and cell death [21]. CHOP protein expression is a direct consequence of endoplasmic reticulum stress-induced apoptosis, and mediates a several apoptotic signaling pathways, and acts as a common downstream apoptotic signaling molecule, and may reflect the endoplasmic reticulum stress-mediated apoptosis [22]. Caspase-12 belongs to the caspase family, and normally exists as an inactive zymogen, located on the outer membrane of the endoplasmic reticulum. When endoplasmic reticulum stress occurs, caspase-12 zymogen activates, and transports to the cytoplasm, activating caspase-9 and caspase-3, leading to cell apoptosis, which is why caspase-12 is an important marker of endoplasmic reticulum-induced apoptosis [23]. In this study, the expression of GRP78, CHOP, and caspase-12 protein in the model group (treated with 200 μmol/L of H2O2) were upregulated, while the in the klotho protein-treated group the expression of the proteins GRP78, CHOP and caspase-12 in HUVECs induced by H2O2 were down-regulated. Therefore, inhibition of apoptosis by klotho in endothelial cells occurs partly through inhibition of oxidative stress of the endoplasmic reticulum.
The PI3K/AKT signaling pathway is a key signaling cascade that promotes cell survival [24]. Following PI3K activation, phosphatidylinositol (3,4,5)-trisphosphate (PIP3) accumulates on the cell membrane and then increases proteins containing the pleckstrin homology domain (PH domain), including the inositol phosphate-dependent kinase-1 (PDK1) and AKT. PIP3-activated PDK1 also phosphorylates AKT at Thr308, together with Ser473 phosphorylation, to ensure complete AKT activation. This phenomenon eventually triggers many downstream signaling pathways involving cell proliferation, metabolism, and survival [25].
In the present study, klotho protein treatment prevented H2O2 induced AKT phosphorylation inhibition. LY-294002 is an inhibitor of PI3K, which inhibits the activation of downstream effectors (including AKT). In this study, LY294002 blocked the effects of klotho protein on AKT phosphorylation in HUVECs. Also, LY294002 partially inhibited klotho protein treatment associated down-regulation of the expression of the proteins GRP78, CHOP, and caspase-12. The MTT assay showed that LY294002 could reverse the protective effect of klotho protein on HUVECs. The findings of the present study are supported by those of a previous study, which showed that endoplasmic reticulum stress can be regulated through the PI3K/AKT pathway [26]. This study showed that klotho protein activated the PI3K/AKT pathway in HUVECs in vitro, which is a potential mechanism of inhibition of endoplasmic reticulum oxidative stress and associated apoptosis.
Conclusions
An in vitro study of the effects of endoplasmic reticulum oxidative stress caused by hydrogen peroxide (H2O2) treatment on human umbilical vein endothelial cells (HUVECs) and the effects of klotho protein showed that klotho protein had a direct protective effect on endothelial cells, from cell injury and apoptosis. Klotho protein reduced reactive oxygen species (ROS)-induced oxidative stress, reduced endoplasmic reticulum stress-mediated apoptosis, and reduced the release of inflammatory mediators through activation of the PI3K/AKT pathway.
Acknowledgments
The authors thank Bing Liu for his encouragement, assistance, and expert advice with this study.
Footnotes
Source of support: This study was supported by funding from the Science and Technology Foundation of Liaoning of China (No. 2012225019)
Conflicts of interest
None.
References
- 1.Herrington W, Lacey B, Sherliker P, et al. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res. 2016;118(4):535–46. doi: 10.1161/CIRCRESAHA.115.307611. [DOI] [PubMed] [Google Scholar]
- 2.Barquera S, Pedroza-Tobías A, Medina C, et al. Global overview of the epidemiology of atherosclerotic cardiovascular disease. Arch Med Res. 2015;46(5):328–38. doi: 10.1016/j.arcmed.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Ivanova EA, Orekhov AN. The role of endoplasmic reticulum stress and unfolded protein response in atherosclerosis. Int J Mol Sci. 2016;17(2) doi: 10.3390/ijms17020193. pii: E193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanemoto S, Nitani R, Murakami T, et al. Multivesicular body formation enhancement and exosome release during endoplasmic reticulum stress. Biochem Biophys Res Comm. 2016;480(2):166–72. doi: 10.1016/j.bbrc.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 5.Minamino T, Kitakaze M. ER stress in cardiovascular disease. J Mol Cell Cardiol. 2010;48(6):1105–10. doi: 10.1016/j.yjmcc.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 6.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7(9):880–85. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scull CM, Tabas I. Mechanisms of ER stress-induced apoptosis in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31(12):2792–97. doi: 10.1161/ATVBAHA.111.224881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCubrey JA, Lee JT, Steelman LS, et al. Interactions between the PI3K and Raf signaling pathways can result in the transformation of hematopoietic cells. Cancer Detect Prev. 2001;25(4):375–93. [PubMed] [Google Scholar]
- 9.Hu P, Han Z, Couvillon AD, et al. Critical role of endogenous AKT/IAPs and MEK1/ERK pathways in counteracting endoplasmic reticulum stress-induced cell death. J Biol Chem. 2004;279:49420. doi: 10.1074/jbc.M407700200. [DOI] [PubMed] [Google Scholar]
- 10.Ikushima M, Rakugi H, Ishikawa K, et al. Anti-apoptotic and anti-senescence effects of klotho on vascular endothelial cells. Biochem Biophys Res Commun. 2006;339(3):827–32. doi: 10.1016/j.bbrc.2005.11.094. [DOI] [PubMed] [Google Scholar]
- 11.Choy JC, Granville DJ, Hunt DW, McManus BM. Endothelial cell apoptosis: Biochemical characteristics and potential implications for atherosclerosis. J Molec Cell Cardiol. 2001;33(9):1673–90. doi: 10.1006/jmcc.2001.1419. [DOI] [PubMed] [Google Scholar]
- 12.Dickhout JG, Hossain GS, Pozza LM, et al. Peroxynitrite causes endoplasmic reticulum stress and apoptosis in human vascular endothelium: Implications in atherogenesis. Arterioscler Thromb Vasc Biol. 2005;25(12):2623–29. doi: 10.1161/01.ATV.0000189159.96900.d9. [DOI] [PubMed] [Google Scholar]
- 13.Yoshikawa A, Saito Y, Maruyama K. Attenuation of NF-κB and activation of Nrf2 signaling by 1,2,4-triazine derivatives, protects neuron-like PC12 cells against apoptosis. Apoptosis. 2010;15(6):738–51. doi: 10.1007/s10495-010-0496-6. [DOI] [PubMed] [Google Scholar]
- 14.Ikushima M, Rakugi H, Ishikawa K, et al. Anti-apoptotic and anti-senescence effects of klotho on vascular endothelial cells. Biochem Biophys Res Commun. 2006;339(3):827–32. doi: 10.1016/j.bbrc.2005.11.094. [DOI] [PubMed] [Google Scholar]
- 15.Förstermann U, Xia N, Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ Res. 2017;120(4):713–35. doi: 10.1161/CIRCRESAHA.116.309326. [DOI] [PubMed] [Google Scholar]
- 16.Piconi L, Quagliaro L, Assaloni R, et al. Constant and intermittent high glucose enhances endothelial cell apoptosis through mitochondrial superoxide overproduction. Diabetes Metab Res Rev. 2010;22(3):198–203. doi: 10.1002/dmrr.613. [DOI] [PubMed] [Google Scholar]
- 17.Deguchi J, Aikawa M, Tung CH, et al. Inflammation in atherosclerosis. Circulation. 2006;114(1):55–62. doi: 10.1161/CIRCULATIONAHA.106.619056. [DOI] [PubMed] [Google Scholar]
- 18.Triggle CR, Ding H, Anderson TJ, Pannirselvam M. The endothelium in health and disease: A discussion of the contribution of non-nitric oxide endothelium-derived vasoactive mediators to vascular homeostasis in normal vessels and in type II diabetes. Mol Cell Biochem. 2004;263(1):21–27. doi: 10.1023/B:MCBI.0000041845.62061.c9. [DOI] [PubMed] [Google Scholar]
- 19.Rao RV, Peel A, Logvinova A, et al. Coupling endoplasmic reticulum stress to the cell death program: The role of the ER chaperone GRP78. FEBS Lett. 2002;514(2):122–28. doi: 10.1016/s0014-5793(02)02289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minamino T, Komuro I, Kitakaze M. Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ Res. 2010;107(9):1071–82. doi: 10.1161/CIRCRESAHA.110.227819. [DOI] [PubMed] [Google Scholar]
- 21.Zinszner H, Kuroda M, Wang X, et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12(7):982–95. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bravo R, Gutierrez T, Paredes F, et al. Endoplasmic reticulum: ER stress regulates mitochondrial bioenergetics. Int J Biochem Cell Biol. 2012;44(1):16–20. doi: 10.1016/j.biocel.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakagawa T, Zhu H, Morishima N, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403(6765):98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 24.Shaw RJ, Cantley LC. Ras, PI(3)K, and mTOR signaling control tumor cell growth. Nature. 2006;441(7092):424–30. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 25.Alessandra G, Mingchuan L. Phosphoinositide 3-kinase: Friend and foe in cardiovascular disease. Front Pharmacol. 2015;6:169. doi: 10.3389/fphar.2015.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song Q, Han CC, Xiong XP, et al. PI3K-Akt-mTOR signal inhibition affects the expression of genes related to endoplasmic reticulum stress. Genet Mol Res. 2016;15(3) doi: 10.4238/gmr.15037868. [DOI] [PubMed] [Google Scholar]