Abstract
The need to find new EGFR inhibitors for use in combination with radiotherapy in the treatment of solid tumors has drawn our attention to compounds derived from genistein, a natural isoflavonoid. The antiproliferative potential of synthetic genistein derivatives used alone or in combination with ionizing radiation was evaluated in cancer cell lines using clonogenic assay. EGFR phosphorylation was assessed with western blotting. Genistein derivatives inhibited clonogenic growth of HCT 116 cancer cells additively or synergistically when used in combination with ionizing radiation, and decreased EGFR activation. Our preclinical evaluation of genistein-derived EGFR inhibitors suggests that these compounds are much more potent sensitizers of cells to radiation than the parent isoflavonoid, genistein and indicate that these compounds may be useful in the treatment of colon cancer with radiation therapy.
Keywords: genistein glycoconjugates, epidermal growth factor receptor inhibition, radiotherapy, radiosensitization
1. Introduction
The epidermal growth factor receptor (EGFR) plays an important role in response of cancer cells to therapy. The binding of a ligand induces its homo- or heterodimerization with other members of the ErB family and leads to trans-autophosphorylation of tyrosines in the intracellular domain. This initiates phosphorylation of other intracellular proteins and adaptors, triggering activation of multiple signaling cascades governing cell proliferation, survival, invasion, adhesion, cell cycle progression, cell motility and DNA repair [1,2]. In cancer cells, EGFR signaling is often enhanced due to overexpression of ligands, the elevated level of the receptor, and the presence of activating mutations. The association between over-activation of EGFR and a worsened prognosis [3,4,5] has motivated many researchers to develop clinically useful inhibitors of its tyrosine kinase activity (TKI). Although the results of several clinical trials with use of available TKIs alone or in combination with chemo- and radiotherapy are encouraging [6,7,8], there are examples of disappointing outcomes [9]. Therefore, elaboration of new TKIs/new therapeutic modalities using known TKIs are strongly deserved.
The alternative to EGFR inhibitors currently used in cancer therapy, particularly with intended use in combination with other therapeutic modalities, may be non-toxic products of natural origin, such as general tyrosine kinase inhibitor, genistein or its derivatives. One of early observations relevant to possible anticancer applications of genistein was inhibitory activity of this compound on tyrosine kinases, including c-src i v-abl and EGFR [10]. Recent analysis suggested that genistein inhibited the activity of tyrosine kinase EGFR, PDGFR, insulin receptor, Abl, Fgr, Itk, Fyn and Src [11].
Genistein was shown to potentiate the effects of other tyrosine kinase inhibitors such as erlotinib, gefitinib and the monoclonal antibody cetuximab [12,13,14]. This isoflavonoid was reported to enhance the effects of radiotherapy in different cellular and animal models [15,16,17,18]. There were also indications that genistein played a protecting role against side effects evoked by radiotherapy, such as lung injury or cardiac dysfunction [19]. Genistein partially reduced the extent of fibrosis developing in mouse lungs after irradiation with no evidence of protection of small tumors in the lung [19]. Using genistein along with irradiation prevented the incidence of delayed lung injuries in mice [20] and aided early recovery of hematopoietic cells by stimulation of cytokins production [21].
Genistein inhibits the activity of tyrosine kinases at concentrations above 10 µM, which is difficult to achieve in vivo. Some efforts were made to obtain derivatives of genistein showing enhanced anti-kinase activity, but so far they have largely been unsuccessful [22,23]. The relatively simple structure of the genistein molecule and the many possibilities of its derivatization offer ample space for obtaining derivatives with improved activity or affecting new molecular targets [24]. In the previous work, we described glycoconjugates of genistein inhibiting cancer cell proliferation more efficiently than genistein [25,26,27,28,29]. The aim of this work was to evaluate EGFR tyrosine kinase inhibition by selected genistein derivatives and to explore the biological effects of these compounds used in combination with ionizing radiation.
2. Results and Discussion
2.1. Inhibition of Clonogenic Cell Survival
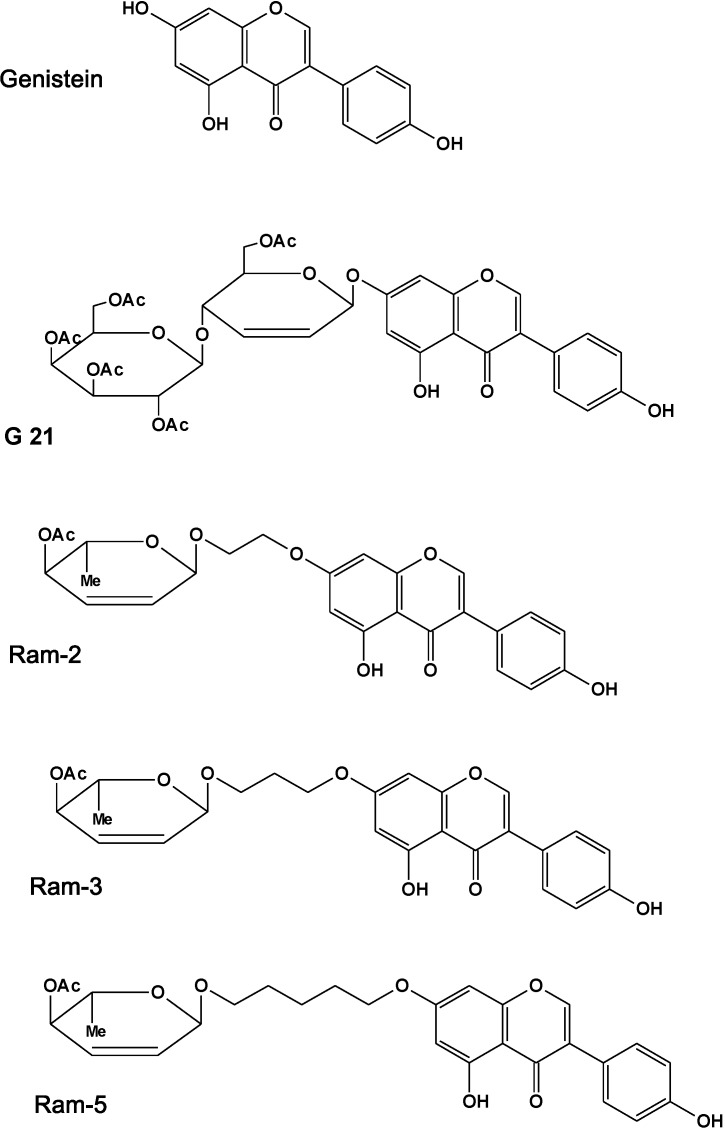
This work extends our previous findings of antiproliferative activity of sugar derivatives of genistein in cancer cell lines. Although the role a sugar moiety in many drugs seems to be important for their biological activity, only few selected categories of natural glycosides (e.g., different classes of antibiotics) have been examined in details for their pharmacological activity, and the studies comparing side by side activities of glycosides and their aglycons are scarce. In order to determine whether genistein and its four sugar derivatives (Figure 1) can potentiate the cytotoxic effect of radiation in cancer cells exhibiting high expression of EGFR protein and EGFR-dependent autocrine proliferation [30] we performed the clonogenic assay in the HCT 116 human colorectal line.
Figure 1.
Chemical structure of genistein, G21, Ram-2, Ram-3, and Ram-5.
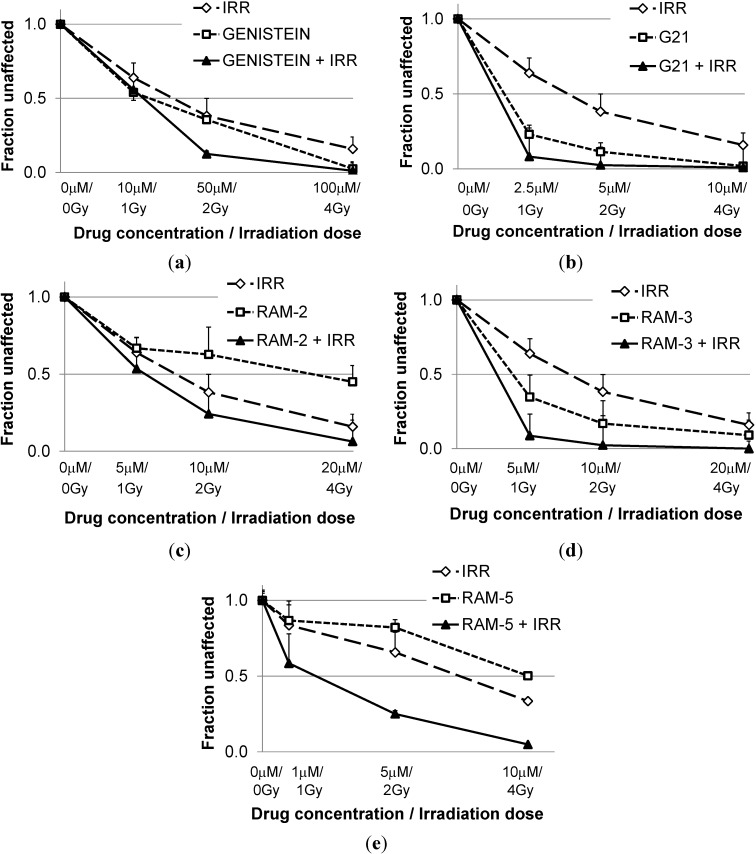
Concentration and dose-dependent inhibition of clonogenic survival of HCT 116 was assessed in a series of experiments, in which cells pretreated for 24 h with genistein or its derivatives were irradiated with a linear accelerator (Figure 2). To determine the type of additive effects from the tested derivatives and irradiation, the combination index (CI) was calculated (Table 1). This revealed that values of combination indices tended to decrease with growing effect. Genistein derivatives were much better sensitizers of cells to radiation then genistein. At the effect levels expressed as ED50 and ED75, interactions of genistein derivatives and radiation were mostly additive (while genistein was antagonistic), and at ED90 they turned out to be synergistic (CI < 0.7) (whereas genistein with radiation acted only additively). Here, we confirmed our previous results showing that modification of genistein with certain hexenoses may enhance antiproliferative activity of a derivative.
Figure 2.
Clonogenic survival of HCT 116 cell line after treatment with growing concentrations of genistein (a) or its derivatives, G21 (b), Ram-2 (c), Ram-3(d) and Ram-5 (e) and growing dose of radiation.
Table 1.
Values of combination index (CI) for drug-irradiation interactions at different effects: ED50, ED75 and ED90.
| CI * Values Calculated for Different Effects | |||
|---|---|---|---|
| ED50 | ED75 | ED90 | |
| Genist/Irr | 1.75 ± 0.25 | 1.36 ± 0.12 | 1.10 ± 0.19 |
| G21/Irr | 1.01 ± 0.54 | 0.86 ± 0.44 | 0.77 ± 0.35 |
| Ram-2/Irr | 1.14 ± 0.05 | 0.7±0.01 | 0.54±0.05 |
| Ram-3/Irr | 1.22 ± 0.16 | 0.72 ± 0.15 | 0.43 ± 0.12 |
| Ram-5/Irr | 0.63 ± 0.11 | 0.35 ± 0.08 | 0.21 ± 0.09 |
* CI is a quantitative measure of the degree of interaction between different treatments. When CI is equal to 0.9–1.1 it denotes additivity; If CI is greater than 1.1—antagonism; CI values between 0.9 and 0.7 indicate slight synergism; CI values less than 0.7—synergism. CI values were calculated according to the Chou and Talalay mathematical model for drug interactions [27] using the Calcusyn software on the base of the results of clonogenic assays. Data represent mean CI calculated from three independent experiments ± standard deviations.
Previous reports have indicated that genistein functions as a radiosensitizer in different cancer cells [15,16,17,18,31,32]. In this work we found that genistein used in combination with ionizing radiation produced weakly antagonistic or additive antiproliferative effects in HCT 116 cells, whereas its synthetic, glycosidic derivatives were much better radiosensitizers and acted synergistically when combined with radiation. The derivatives Ram-3 and Ram-5 were particularly efficient in cessation of clonogenic potential of irradiated cells. The most often cited biological pathways affected by genistein that lead to potentiation of radiotherapeutic effects are: inhibition of Nfkb, G2/M block of the cell cycle, inhibition of AKT and inhibition of tyrosine kinases [11,18]. However, the well documented activity of genistein on other molecular targets, such as estrogen receptors, DNA topoisomerases, protein kinase C or FGFR-1 may also influence radiotherapeutic response [33,34]. Although modulating effects of the tested derivatives on the pathways mentioned above are very likely, the presented work was focused on inhibition of EGFR by genistein derivatives, and evaluation of their preclinical activity in combination with ionizing radiation.
It is worth mentioning that the tested derivatives were apparently non-toxic for confluent and differentiated Caco-2 monolayers (in vitro model of human intestine) and they inhibited proliferation of normal cells (human keratinocyte cell line HaCaT) at higher concentration when compared with cancer cells.
2.2. Inhibition of EGFR Phosphorylation in Cancer Cells Treated with Genistein Derivatives
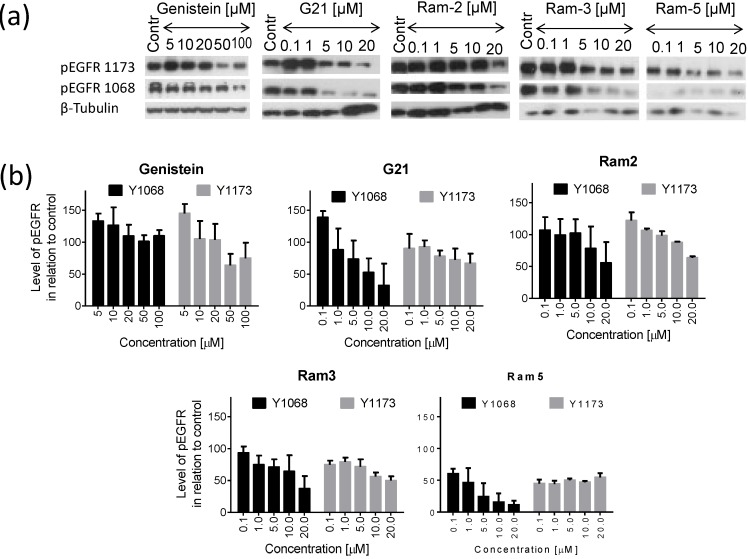
We analyzed the relative amount of pEGFR (Y1068 and Y1173) in cells treated for 24 h (Figure 3). Ram-2, Ram-3 and Ram-5 inhibited phosphorylation of EGFR both at the tyrosine 1068 and 1173 in a dose dependent manner, albeit G21 and genistein showed a biphasic response and at low concentrations the level of EGFR phosphorylation was higher than in the untreated control. The level of EGFR phosphorylation was reduced remarkably by Ram-5, which showed its inhibitory activity at very low concentration (0.1 µM). The general conclusion from the presented data is that all the tested sugar derivatives of genistein reduced the level of EGFR phosphorylation after 24 h treatment more efficiently than a parent drug, genistein. We also performed these experiments in DU 145 cell line and found a similar pattern of inhibition of EGFR phosphorylation, proving that the effect of the tested compounds is not limited to one cell line.
Figure 3.
Phosphorylation of EGFR in HCT 116 cells treated with genistein or its derivatives for 24 h. (a) western blots showing pEGFR 1068, pEGFR 1173 level and β-tubulin (used as a loading control); (b) charts presenting the results of densitometry. Bars show the mean values ± standard deviations of the band density normalized to the loading control in relation to untreated control (Contr) normalized to the loading control. Data from at least three independent experiments.
Inhibition of EGFR and several other tyrosine kinases by genistein was discovered a long time ago [28]. We currently know that genistein inhibits the activity of EGFR, PDGFR, insulin receptor, Abl, Fgr Itk, Fyn and Src [11]. Inhibition of EGFR by this isoflavonoid was confirmed in other studies [35]. Whereas a systematic search for optimum correlation between structure-activity relationship was performed for flavonoids as inhibitors of p56lck kinase [36,37], no such comprehensive study was done for tyrosine kinases and isoflavonoids. Experimental data obtained shortly after finding the inhibitory activity of genistein against EGFR indicate some structural features, relevant to activity of several isoflavones (genistein, daidzein, prunetin, genistin, biochanin A) [38]. The presence of a phenol group at C5 was suggested to be the structural feature critical for the inhibitory activity of genistein on tyrosine kinases. However, two other phenol groups at C7 and C4' were also cited as important for interactions with kinases [38]. Importantly, the large substituent, such as the glucose occurring at C7 position in genistin, was indicated as a factor in eliminating the inhibitory activity of the molecule. However, here we showed that substitution of genistein with certain sugar moieties did not eliminate inhibitory activity of genistein, and all the tested compounds were capable of EGFR phosphorylation inhibition in the concentration dependent manner.
2.3. Inhibition of EGFR Phosphorylation in Cancer Cells Treated with Genistein Derivatives and Ionizing Radiation
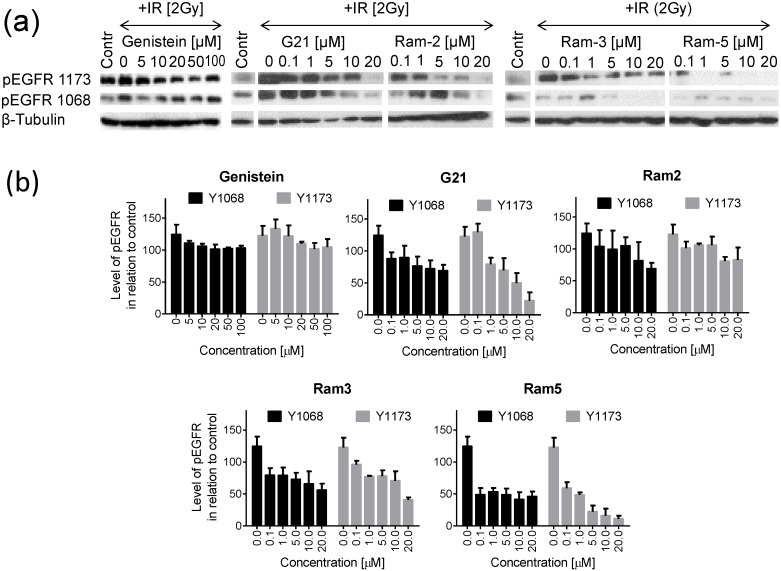
It is well established that EGFR activity is stimulated by ionizing radiation. In order to determine whether genistein and its sugar derivatives are capable of suppressing the radiation induced phosphorylation of EGFR, we incubated the HCT 116 cells with genistein, G21, Ram-2, Ram-3 and Ram-5 for 24 h and then irradiated cells with 2 Gy, as described in “Material and Methods” section. The level of pEGFR was analyzed using SDS PAGE and immunoblotting. It can be seen that in cells not exposed to genistein or its derivatives, irradiation caused a significant increase of pEGFR (Y1068) and pEGFR (Y1173) (Figure 4). In cells preincubated with genistein derivatives for 24 h before irradiation, the level of pEGFR was lowered remarkably. In contrast, in cells pretreated with genistein and then irradiated, the reduction of EGFR phosphorylation was very weak. The compound Ram-5 was most effective in prevention of irradiation-induced EGFR phosphorylation.
Figure 4.
Phosphorylation of EGFR in HCT 116 cells treated with genistein or its derivatives for 24 h, irradiated with 2 Gy and recovered for 24 h. (a) Western blots showing pEGFR 1068, pEGFR 1173 level and β-tubulin (used as a loading control); (b) Charts presenting the results of densitometry. Bars show the mean values ± standard deviations of the band density normalized to the loading control in relation to untreated control (neither drug-treated nor irradiated) (Contr) normalized to the loading control. Data from at least three independent experiments.
Although the degree of clonogenicity loss caused by the treatment with different derivatives and EGFR phosphorylation inhibition were not matching perfectly, we observed a clear correlating tendency between their intensity. Inhibition of EGFR phosphorylation by glycosidic derivatives of genistein seems to play a very important role in cancer cell elimination after radiotherapy and makes them promising agents for sensitization of a tumor to this therapeutic modality. Diminished phosphorylation of several tyrosines of EGFR abrogates the survival strategy leading to transmission of the mitogenic signals and evasion of apoptosis that tumor cells normally adopt after irradiation [2]. In our western blotting analyses all the tested genistein derivatives inhibited phosphorylation of tyrosines Y1068 and Y1173 in the EGFR, which may have important implications for cell fate after radiotherapy. These tyrosines are essential for binding the SH2 domain of Grb2 and provide docking sites for Shc [39,40]. Inhibiting interactions with those adaptor proteins hampers initiation of the signaling pathway of MAP kinase [41,42], thus stopping mitogenic stimulation. The capacity of EGFR inhibition seems to be of much relevance to clonogenic death after irradiation. The tested genistein derivatives decreased the level of phosphorylated EGFR in irradiated cells more profoundly than genistein. This correlated with higher antiproliferative effects in cells treated by the combination: genistein derivatives/irradiation when compared to genistein/irradiation.
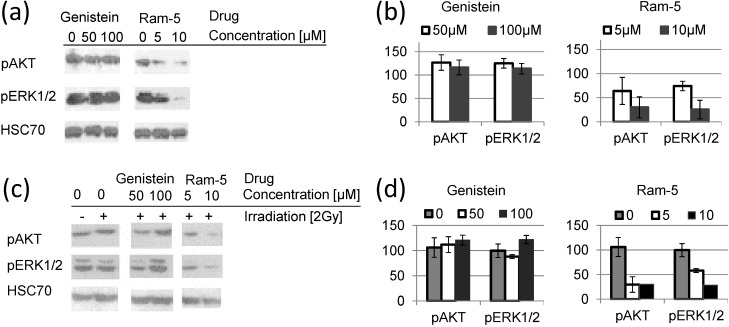
Next, we compared the activation of AKT and ERK in irradiated cells pretreated with genistein and the most potent derivative, Ram-5. The results presented in Figure 5. show that Ram-5 treatment, in contrast to genistein, leads to concurrent and prolonged inhibition of pAKT and pERK. The inhibitory effect observed after treatment of cells with Ram-5 also pertains after irradiation. Treatment of cells with genistein alone did not reduce the level of activated AKT and ERK1/2 (Figure 5). Moreover, we observed upregulation of activated AKT and ERK1/2 24 h after irradiation of the cells pretreated with genistein.
Figure 5.
Phosphorylation of AKT and ERK1/2 in HCT 116 cells treated with genistein or its derivative Ram-5 for 24 h without (a,b) or with subsequent irradiation (c,d). (a) Western blots showing pAKT, pERK1/2 and HSC70 (used as a loading control); (b) charts presenting the results of densitometry. Bars show the mean values ± standard deviations of the band density normalized to the loading control in relation to untreated control normalized to the loading control; (c) Western blots showing pAKT, pERK1/2 and HSC70 (used as a loading control); (d) Charts presenting the results of densitometry. Bars show the mean values ± standard deviations of the band density normalized to the loading control in relation to untreated control (neither drug-treated nor irradiated), normalized to the loading control. Data from at least three independent experiments.
Synergistic inhibition of HCT 166 cell proliferation by a combination of genistein derivatives and ionizing radiation has profound implications for further studies. This cell line expresses a mutated form of KRAS [43], playing a crucial role in cell proliferation, survival and metastasis in colon cancer [44]. KRAS is an intermediate signal transduction element in the EGFR pathway, activating MAPK and ERK, thus inhibition of EGFR only will not cause effective shutdown of the downstream signaling. For that reason, KRAS mutation is regarded as a contraindication for therapeutic use of EGFR inhibitors [45,46]. Our discovery that the Ram-5 compound, in contrast to genistein, inhibited constitutively active ERK1/2 and AKT makes this compound a much more promising agent for sensitization of cells to radiotherapy than was the parent isoflavone. Ionizing radiation causes compensatory activation of AKT and ERK1/2, fueling the survival machinery and protecting cells from toxic effects of the treatment. Moreover, ERK1/2 activation may promote the release of paracrine regulators, such as hereguline, causing secondary EGFR activation in irradiated cells [43]. The observed concurrent inhibition of EGFR, AKT and ERK1/2 by Ram-5 abrogates prosurvival signaling on many levels, thus potentiating the effects of radiation much more effectively than genistein. Although many reports show that genistein inhibits activity of Akt and ERK1/2 we did not observe this effect in the HCT 116 cell line. Our results showing the lack of genistein effects on pERK and pAkt in HCT 116 cells having KRAS mutation, and constitutively activated ERK1/2 and Akt, contribute to the concept that ERK1/2 and Akt inhibition by genistein is most likely a secondary effect of EGFR inhibition and occurs in cells with wild type KRAS. In this cellular context, the ability of Ram-5 to decrease the level of both pERK and pAKT suggests interaction of the compound with signaling elements downstream of KRAS. Apparently, Ram-5 exhibits an additional mode of activity to that described for genistein, but the mechanistic role of that compound in ERK1/2 and AKT signaling requires further studies.
The use of small TKIs gains importance due to the fact, that clinically accepted antibodies targeting EGFR are not effective against tumors expressing the truncated form of EGFR, such as the mutated variant III of this receptor (EGFRvIII) [47,48]. Better control of cell proliferation may be achieved by treatment with combination of TKIs with different mode of action, i.e., the EGFR targeting antibodies together with small inhibitors affecting intracellular domain [49,50,51]. The use of genistein in combination with ionizing radiation seems to be a promising option, although, on the other hand, there are some controversies about therapeutic use of genistein. Recent discoveries indicate that EGFR-dependent signaling pathways may be involved in stimulation of cancer cell proliferation by low concentrations of genistein [52,53,54]. The enhancement of cancer cell proliferation at low concentrations of this isoflavonoid is sometimes used as an argument against genistein supplementation [52]. The compound Ram-5 attracts attention as a drug potentially useful for radio-sensitizing colon cancer cells due to the better characteristics of EGFR inhibition when compared with genistein. This compound does not stimulate EGFR phosphorylation at low concentration, in contrast to genistein, so it may have a better safety profile in vivo. In this context, the use of new derivatives of genistein lacking those low-concentration stimulatory effects on EGFR should not be controversial. In vitro results from the work here will create the basis for animal study to evaluate the efficacy of genistein derivatives in conjunction with radiation therapy in vivo.
3. Experimental Section
3.1. Chemicals
Genistein (5,7-dihydroxy-3-(4-hydroxyphenyl)chromen-4-one) was obtained from Pharmaceutical Research Institute (Warsaw, Poland). Genistein derivatives: G21 (7-O-(2,3,4,6-tetra-O-acetyl-β-d-galactopyranosyl)-(1→4)-(6-O-acetyl-hex-2-ene-α-d-erythropyranosyl)-3-(4-hydroksyphenyl)-chromen-4-one), Ram-2 (5-hydroxy-7-[(4-O-acetyl-2,3,6-trideoxy-α-l-erythrohex-2-enopyranosyl)-2-O-ethyl]-3-(4-hydroxyphenyl)chromen-4-one), Ram-3 (5-hydroxy-7-[(4-O-acetyl-2,3,6-trideoxy-α-l-erythrohex-2-enopyranosyl)-3-O-propyl]-3-(4-hydroxyphenyl)chromen-4-one) and Ram-5 (5-hydroxy-7-[(4-O-acetyl-2,3,6-trideoxy-α-l-erythrohex-2-enopyranosyl)-5-O-pentyl]-3-(4-hydroxyphenyl)chromen-4-one) were synthesized as described previously [25,26]. The structures of the tested compounds are shown in Figure 1. Genistein, G21, Ram-2, Ram-3 and Ram-5 stock solutions were prepared in dimethyl sulfoxide (DMSO), stored at −20 °C and diluted before use. Final concentration of DMSO in culture media did not exceed 0.5%.
3.2. Cell Lines and Culture Conditions
The HCT 116 colon cancer cell line and DU 145 prostate cancer cell line obtained from ATCC (American Type Culture Collection, Rockville, MD, USA) were routinely cultured in an RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS, ICN Pharmaceuticals, (Costa Mesa, CA, USA) and 1 μg/mL gentamicin (KRKA, Novo Mesto, Slovenia), at 37 °C in a humidified atmosphere containing 5% CO2 in the air. The cells were split every three days at 90% confluence. The cells were detached by rinsing with 0.02% ethylenediamine tetraacetic acid (EDTA) followed by incubation with 0.25% trypsin, and they were stored as frozen stocks in liquid nitrogen.
3.3. Clonogenic Assay
The cells were seeded at the density 1000 cells per a 6 cm plate (Nunc, Roskilde, Denmark). After 24 h, the medium was aspirated and replaced with a fresh one containing genistein or its derivatives. After 24 h, the treatment medium was replaced with a fresh one and cells were irradiated as described below.
3.4. Irradiation of Cells
The cells were irradiated with a 6 MV photon beam generated by a linear accelerator Clinac 2300 (Palo Alto, CA, USA) at a dose rate of 100 monitory units/min. The field was 30 cm × 30 cm, source-specimen distance (equivalent to source-skin distance, SSD) was 100.5 cm. The doses 1 Gy, 2 Gy or 4 Gy were equivalent to the doses measured under dosimetric conditions.
3.5. CI Calculation
Calculations of drug concentration/irradiation dose-effect and combination indices for different effects were calculated with Calcusyn software (Biosoft) using the median effect method described by Chou and Talalay [55]. The experiments enabling analysis of combination effects of drugs and irradiation had the checkerboard (latin square) design. In short, cells were treated with three serial growing concentrations of the drugs or the doses of radiation alone (the row and the column of the square formed an orthogonal series) or with the combination of the drug/radiation, used at the concentration/dose series forming the diagonal of the checkerboard.
3.6. Western Blotting
The cells were seeded in 10 cm culture plates and left for 24 h to allow the cells to attach to the bottom. They were then treated with a series of growing concentration of genistein, G21, Ram-2, Ram-3, Ram-5 for 24 h. In experiments testing the effect of combined treatment of genistein or its derivatives and ionizing radiation on EGFR phosphorylation medium containing isoflavones was aspirated and replaced with fresh medium without the tested drug, cells were irradiated with 2 Gy, as described above, and incubated for additional 24 h. Prior to the protein sample preparation cells were exposed to epithelial growth factor (EGF) at 50 ng mL in 0.1% bovine serum albumin (BSA) for 15 min and were lysed with the protein lysis buffer: Tris buffer pH 7.5 (50 mM), (1%) NP40 (Tergitol-type NP-40, nonyl phenoxylpolyethoxylethanol), NaCl (150 mM), ethylenediaminetetraacetic acid (EDTA, 1 mM), protease inhibitor solution (1:100, CompleteTM Roche, Basel, Switzerland) and phosphatase inhibitor solution (1:100; Sigma-Aldrich, Saint Louis, MO, USA). Subsequently, they were scraped, transferred to microtubes and centrifuged at 20,000 g for 10 min at 4 °C. The supernatant was collected, and the total protein content was quantified using a Bradford protein assay kit. The cell lysates were stored in −70 °C until use, and the proteins were separated by SDS/PAGE in 8% polyacrylamide gel at 100 V for 1.5 h, and transferred to nitrocellulose membrane (BA85; Schleicher and Schuell, Dassel, Germany). The blots were washed three times in TTBS (50 mM Tris/HCl, pH 7.5, 0.5% Tween-20, 0.15 M NaCl), blocked for 45 min at room temperature with 5% non-fat milk in TTBS and incubated overnight at 4 °C with the primary antibody. The antibodies used in the experiments were as follows: rabbit anti-EGFR (Cell Signaling, Danvers, MA, USA), rabbit and mouse anti-phospho-EGFR Y1068 (Cell Signaling), EGFR Y1173 (Millipore, Darmstadt, Germany), pAKT (Cell Signaling), pERK1/2 (Millipore), mouse-anti-β-tubulin (Sigma-Aldrich, Saint Louis, MO, USA), mouse-anti-actin (Cell Signaling), HSC70 (Santa Cruz, Dallas, TX, USA). After washing with TTBS, the blots were incubated with anti-rabbit or anti-mouse secondary antibody conjugated with horseradish peroxidase (HRP) for 1.5 h at room temperature. They were then washed in TTBS and the bands were visualized using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, Rockford, IL, USA).
4. Conclusions
Genistein glycoconjugates potentiate effects of radiotherapy in synergistic or additive manner. These compounds prevent phosphorylation of EGFR induced by ionizing radiation and are potentially useful drugs for radio-sensitizing colon cancer cells.
Acknowledgments
AR acknowledges Professor Leszek Miszczyk, the Head of the Department of Radiotherapy, Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Gliwice Branch for his kind permission to use the linear accelerator for irradiation of cell cultures. The work was supported by POIG 01.01.02-14-102/09.
Author Contributions
The work presented here was carried out in collaboration between all authors. ZK, WS, GG, and AR defined the research subject. AR designed the experiments. AG and AR performed experiments, analyzed the data and interpreted the results. AR wrote the paper. All authors have contributed to the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds G21, Ram-2, Ram-3 and Ram-5 are available from the authors.
References
- 1.Ono M., Kuwano M. Molecular mechanisms of epidermal growth factor receptor (EGFR) activation and response to gefitinib and other EGFR-targeting drugs. Clin. Cancer Res. 2006;12:7242–7251. doi: 10.1158/1078-0432.CCR-06-0646. [DOI] [PubMed] [Google Scholar]
- 2.Thariat J., Milas L., Ang K.K. Integrating radiotherapy with epidermal growth factor receptor antagonists and other molecular therapeutics for the treatment of head and neck cancer. Int. J. Rad. Oncol. Biol. Phys. 2007;69:974–984. doi: 10.1016/j.ijrobp.2007.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicholson R.I., Gee J.M., Harper M.E. EGFR and cancer prognosis. Eur. J. Cancer. 2001;37(Suppl. 4):S9–S15. doi: 10.1016/S0959-8049(01)00231-3. [DOI] [PubMed] [Google Scholar]
- 4.Baiocchi G., Lopes A., Coudry R.A., Rossi B.M., Soares F.A., Aguiar S., Guimaraes G.C., Ferreira F.O., Nakagawa W.T. ErbB family immunohistochemical expression in colorectal cancer patients with higher risk of recurrence after radical surgery. Int. J. Colorectal Dis. 2009;24:1059–1068. doi: 10.1007/s00384-009-0702-6. [DOI] [PubMed] [Google Scholar]
- 5.Gravalos C., Jimeno A. HER2 in gastric cancer: A new prognostic factor and a novel therapeutic target. Ann. Oncol. 2008;19:1523–1529. doi: 10.1093/annonc/mdn169. [DOI] [PubMed] [Google Scholar]
- 6.Bonner J.A., Harari P.M., Giralt J., Azarnia N., Shin D.M., Cohen R.B., Jones C.U., Sur R., Raben D., Jassem J., et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 7.Cappuzzo F., Ciuleanu T., Stelmakh L., Cicenas S., Szczesna A., Juhasz E., Esteban E., Molinier O., Brugger W., Melezinek I., et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: A multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11:521–529. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 8.Herbst R.S., Sun Y., Eberhardt W.E., Germonpre P., Saijo N., Zhou C., Wang J., Li L., Kabbinavar F., Ichinose Y., et al. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): A double-blind, randomised, phase 3 trial. Lancet Oncol. 2010;11:619–626. doi: 10.1016/S1470-2045(10)70132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krause M., Gurtner K., Deuse Y., Baumann M. Heterogeneity of tumour response to combined radiotherapy and EGFR inhibitors: differences between antibodies and TK inhibitors. Int. J. Radiat. Biol. 2009;85:943–954. doi: 10.3109/09553000903232835. [DOI] [PubMed] [Google Scholar]
- 10.Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- 11.Yan G.R., Xiao C.L., He G.W., Yin X.F., Chen N.P., Cao Y., He Q.Y. Global phosphoproteomic effects of natural tyrosine kinase inhibitor, genistein, on signaling pathways. Proteomics. 2010;10:976–986. doi: 10.1002/pmic.200900662. [DOI] [PubMed] [Google Scholar]
- 12.Park S.J., Kim M.J., Kim Y.K., Kim S.M., Park J.Y., Myoung H. Combined cetuximab and genistein treatment shows additive anti-cancer effect on oral squamous cell carcinoma. Cancer Lett. 2010;292:54–63. doi: 10.1016/j.canlet.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Zhu H., Cheng H., Ren Y., Liu Z.G., Zhang Y.F., Luo B.D. Synergistic inhibitory effects by the combination of gefitinib and genistein on NSCLC with acquired drug-resistance in vitro and in vivo. Mol. Biol. Rep. 2012;39:4971–4979. doi: 10.1007/s11033-011-1293-1. [DOI] [PubMed] [Google Scholar]
- 14.Gadgeel S.M., Ali S., Philip P.A., Wozniak A., Sarkar F.H. Genistein enhances the effect of epidermal growth factor receptor tyrosine kinase inhibitors and inhibits nuclear factor kappa B in nonsmall cell lung cancer cell lines. Cancer. 2009;115:2165–2176. doi: 10.1002/cncr.24250. [DOI] [PubMed] [Google Scholar]
- 15.Hillman G.G., Forman J.D., Kucuk O., Yudelev M., Maughan R.L., Rubio J., Layer A., Tekyi-Mensah S., Abrams J., Sarkar F.H. Genistein potentiates the radiation effect on prostate carcinoma cells. Clin. Cancer Res. 2001;7:382–390. [PubMed] [Google Scholar]
- 16.Raffoul J.J., Wang Y., Kucuk O., Forman J.D., Sarkar F.H., Hillman G.G. Genistein inhibits radiation-induced activation of NF-kappaB in prostate cancer cells promoting apoptosis and G2/M cell cycle arrest. BMC Cancer. 2006;6 doi: 10.1186/1471-2407-6-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raffoul J.J., Banerjee S., Che M., Knoll Z.E., Doerge D.R., Abrams J., Kucuk O., Sarkar F.H., Hillman G.G. Soy isoflavones enhance radiotherapy in a metastatic prostate cancer model. Int. J. Cancer. 2007;120:2491–2498. doi: 10.1002/ijc.22548. [DOI] [PubMed] [Google Scholar]
- 18.Yashar C.M., Spanos W.J., Taylor D.D., Gercel-Taylor C. Potentiation of the radiation effect with genistein in cervical cancer cells. Gynecol. Oncol. 2005;99:199–205. doi: 10.1016/j.ygyno.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Para A.E., Bezjak A., Yeung I.W., van Dyk J., Hill R.P. Effects of genistein following fractionated lung irradiation in mice. Radiother. Oncol. 2009;92:500–510. doi: 10.1016/j.radonc.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Day R.M., Barshishat-Kupper M., Mog S.R., Mccart E.A., Prasanna P.G., Davis T.A., Landauer M.R. Genistein protects against biomarkers of delayed lung sequelae in mice surviving high-dose total body irradiation. J. Radiat. Res. 2008;49:361–372. doi: 10.1269/jrr.07121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh V.K., Grace M.B., Parekh V.I., Whitnall M.H., Landauer M.R. Effects of genistein administration on cytokine induction in whole-body gamma irradiated mice. Int. Immunopharmacol. 2009;9:1401–1410. doi: 10.1016/j.intimp.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Booth C., Hargreaves D.F., Hadfield J.A., Mcgown A.T., Potten C.S. Isoflavones inhibit intestinal epithelial cell proliferation and induce apoptosis in vitro. Br. J. Cancer. 1999;80:1550–1557. doi: 10.1038/sj.bjc.6690559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gargala G., Baishanbo A., Favennec L., Francois A., Ballet J.J., Rossignol J.F. Inhibitory activities of epidermal growth factor receptor tyrosine kinase-targeted dihydroxyisoflavone and trihydroxydeoxybenzoin derivatives on Sarcocystis neurona, Neospora caninum, and Cryptosporidium parvum development. Antimicrob. Agents Chemother. 2005;49:4628–4634. doi: 10.1128/AAC.49.11.4628-4634.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rusin A., Krawczyk Z., Grynkiewicz G., Gogler A., Zawisza-Puchalka J., Szeja W. Synthetic derivatives of genistein, their properties and possible applications. Acta Biochem. Pol. 2010;57:23–34. [PubMed] [Google Scholar]
- 25.Rusin A., Gogler A., Glowala-Kosinska M., Bochenek D., Gruca A., Grynkiewicz G., Zawisza J., Szeja W., Krawczyk Z. Unsaturated genistein disaccharide glycoside as a novel agent affecting microtubules. Bioorg. Med. Chem. Lett. 2009;19:4939–4943. doi: 10.1016/j.bmcl.2009.07.089. [DOI] [PubMed] [Google Scholar]
- 26.Rusin A., Zawisza-Puchalka J., Kujawa K., Gogler-Piglowska A., Wietrzyk J., Switalska M., Glowala-Kosinska M., Gruca A., Szeja W., Krawczyk Z., et al. Synthetic conjugates of genistein affecting proliferation and mitosis of cancer cells. Bioorg. Med. Chem. 2011;19:295–305. doi: 10.1016/j.bmc.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed A.A., Goldsmith J., Fokt I., Le X.F., Krzysko K.A., Lesyng B., Bast R.C., Priebe W. A genistein derivative, ITB-301, induces microtubule depolymerization and mitotic arrest in multidrug-resistant ovarian cancer. Cancer Chemother. Pharm. 2011;68:1033–1044. doi: 10.1007/s00280-011-1575-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gogler-Piglowska A., Rusin A., Bochenek D., Krawczyk Z. Aneugenic effects of the genistein glycosidic derivative substituted at C7 with the unsaturated disaccharide. Cell Biol. Toxicol. 2012;28:331–342. doi: 10.1007/s10565-012-9227-9. [DOI] [PubMed] [Google Scholar]
- 29.Rusin A., Chrubasik M., Papaj K., Grynkiewicz G., Szeja W. C-Glycosidic genistein conjugates and their antiproliferative activity. J. Chem. 2013;2013 doi: 10.1155/2013/951392. [DOI] [Google Scholar]
- 30.Sawhney R.S., Zhou G.H.K., Humphrey L.E., Ghosh P., Kreisberg J.I., Brattain M.G. Differences in sensitivity of biological functions mediated by epidermal growth factor receptor activation with respect to endogenous and exogenous ligands. J. Biol. Chem. 2002;277:75–86. doi: 10.1074/jbc.M103268200. [DOI] [PubMed] [Google Scholar]
- 31.Akimoto T., Nonaka T., Ishikawa H., Sakurai H., Saitoh J., Takahashi T., Mitsuhashi N. Genistein, a tyrosine kinase inhibitor, enhanced radiosensitivity in human esophageal cancer cell lines in vitro: Possible involvement of inhibition of survival signal transduction pathways. Int. J. Radiat. Oncol. 2001;50:195–201. doi: 10.1016/S0360-3016(00)01560-1. [DOI] [PubMed] [Google Scholar]
- 32.Toillon R.A., Magne N., Laios I., Lacroix M., Duvillier H., Lagneaux L., Devriendt D., van Houtte P., Leclercq G. Interaction between estrogen receptor alpha, ionizing radiation and (anti-) estrogens in breast cancer cells. Breast Cancer Res. Treat. 2005;93:207–215. doi: 10.1007/s10549-005-5148-0. [DOI] [PubMed] [Google Scholar]
- 33.Merajver Sd R.D., van Wassenhove L. Protein Kinase C in Cancer Signaling and Therapy. 1st ed. Springer; New York, NY, USA: 2010. PKC and breast cancer; p. 494. [Google Scholar]
- 34.Donnem T., Al-Shibli K., Al-Saad S., Busund L.T., Bremnes R.M. Prognostic impact of fibroblast growth factor 2 in non-small cell lung cancer coexpression with VEGFR-3 and PDGF-B predicts poor survival. J. Thorac. Oncol. 2009;4:578–585. doi: 10.1097/JTO.0b013e31819f2e38. [DOI] [PubMed] [Google Scholar]
- 35.Jakobkiewicz-Banecka J., Piotrowska E., Narajczyk M., Baranska S., Wegrzyn G. Genistein-mediated inhibition of glycosaminoglycan synthesis, which corrects storage in cells of patients suffering from mucopolysaccharidoses, acts by influencing an epidermal growth factor-dependent pathway. J. Biomed. Sci. 2009;2:16–26. doi: 10.1186/1423-0127-16-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nikolovska-Coleska Z., Suturkova L., Dorevski K., Krbavcic A., Solmajer T. Quantitative structure-activity relationship of flavonoid inhibitors of p56lck protein tyrosine kinase: A classical/quantum chemical approach. Quant. Struct.-Act. Relat. 1998;17:7–13. doi: 10.1002/(SICI)1521-3838(199801)17:01<07::AID-QSAR7>3.3.CO;2-E. [DOI] [Google Scholar]
- 37.Oblak M., Randic M., Solmajer T. Quantitative structure-activity relationship of flavonoid analogues. 3. Inhibition of p56lck protein tyrosine kinase. J. Chem. Inf. Comput. Sci. 2000;40:994–1001. doi: 10.1021/ci000001a. [DOI] [PubMed] [Google Scholar]
- 38.Ogawara H., Akiyama T., Watanabe S., Ito N., Kobori M., Seoda Y. Inhibition of tyrosine protein-kinase activity by synthetic isoflavones and flavones. J. Antibiot. 1989;42:340–343. doi: 10.7164/antibiotics.42.340. [DOI] [PubMed] [Google Scholar]
- 39.Batzer A.G., Rotin D., Urena J.M., Skolnik E.Y., Schlessinger J. Hierarchy of binding-sites for Grb2 and Shc on the epidermal growth-factor receptor. Mol. Cell. Biol. 1994;14:5192–5201. doi: 10.1128/mcb.14.8.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rojas M., Yao S.Y., Lin Y.Z. Controlling epidermal growth factor (EGF)-stimulated ras activation in intact cells by a cell-permeable peptide mimicking phosphorylated EGF receptor. J. Biol. Chem. 1996;271:27456–27461. doi: 10.1074/jbc.271.44.27456. [DOI] [PubMed] [Google Scholar]
- 41.Blenis J. Signal-transduction via the MAP kinases: Proceed at your own Rsk. Proc. Natl. Acad. Sci. USA. 1993;90:5889–5892. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ravichandran K.S. Signaling via Shc family adapter proteins. Oncogene. 2001;20:6322–6330. doi: 10.1038/sj.onc.1204776. [DOI] [PubMed] [Google Scholar]
- 43.Yacoub A., Miller A., Caron R.W., Qiao L., Curiel D.A., Fisher P.B., Hagan M.P., Grant S., Dent P. Radiotherapy-induced signal transduction. Endocr.-Related Cancer. 2006;13:S99–S114. doi: 10.1677/erc.1.01271. [DOI] [PubMed] [Google Scholar]
- 44.Tai C.J., Chang C.C., Jiang M.C., Yeh C.M., Su T.C., Wu P.R., Chen C.J., Yeh K.T., Lin S.H., Chen H.C. Clinical-pathological correlation of K-Ras mutation and ERK phosphorylation in colorectal cancer. Pol. J. Pathol. 2012;63:93–100. [PubMed] [Google Scholar]
- 45.Raponi M., Winkler H., Dracopoli N.C. KRAS mutations predict response to EGFR inhibitors. Curr. Opin. Pharmacol. 2008;8:413–418. doi: 10.1016/j.coph.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 46.Linardou H., Dahabreh I.J., Kanaloupiti D., Siannis F., Bafaloukos D., Kosmidis P., Papadimitriou C.A., Murray S. Assessment of somatic k-RAS mutations as a mechanism associated with resistance to EGFR-targeted agents: a systematic review and meta-analysis of studies in advanced non-small-cell lung cancer and metastatic colorectal cancer. Lancet Oncol. 2008;9:962–972. doi: 10.1016/S1470-2045(08)70206-7. [DOI] [PubMed] [Google Scholar]
- 47.Jutten B., Dubois L., Li Y.N., Aerts H., Wouters B.G., Lambin P., Theys J., Lammering G. Binding of cetuximab to the EGFRvIII deletion mutant and its biological consequences in malignant glioma cells. Radiother. Oncol. 2009;92:393–398. doi: 10.1016/j.radonc.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 48.Wheeler S.E., Suzuki S., Thomas S.M., Sen M., Leeman-Neill R.J., Chiosea S.I., Kuan C.T., Bigner D.D., Gooding W.E., Lai S.Y., et al. Epidermal growth factor receptor variant III mediates head and neck cancer cell invasion via STAT3 activation. Oncogene. 2010;29:5135–5145. doi: 10.1038/onc.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang S., Armstrong E.A., Benavente S., Chinnaiyan P., Harari P.M. Dual-agent molecular targeting of the epidermal growth factor receptor (EGFR): Combining anti-EGFR antibody with tyrosine kinase inhibitor. Cancer Res. 2004;64:5355–5362. doi: 10.1158/0008-5472.CAN-04-0562. [DOI] [PubMed] [Google Scholar]
- 50.Huether A., Hopfner M., Baradari V., Schuppan D., Scherubl H. EGFR blockade by cetuximab alone or as combination therapy for growth control of hepatocellular cancer. Biochem. Pharmacol. 2005;70:1568–1578. doi: 10.1016/j.bcp.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 51.Nakamura H., Takamori S., Fujii T., Ono M., Yamana H., Kuwano M., Shirouzu K. Cooperative cell-growth inhibition by combination treatment with ZD1839 (Iressa) and trastuzumab (Herceptin) in non-small-cell lung cancer. Cancer Lett. 2005;230:33–46. doi: 10.1016/j.canlet.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 52.Ju Y.H., Allred C.D., Allred K.F., Karko K.L., Doerge D.R., Helferich W.G. Physiological concentrations of dietary genistein dose-dependently stimulate growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in athymic nude mice. J. Nutr. 2001;131:2957–2962. doi: 10.1093/jn/131.11.2957. [DOI] [PubMed] [Google Scholar]
- 53.Nakamura H., Wang Y.W., Kurita T., Adomat H., Cunha G.R., Wang Y.Z. Genistein increases epidermal growth factor receptor signaling and promotes tumor progression in advanced human prostate cancer. PLoS One. 2011;6 doi: 10.1371/journal.pone.0020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qi W.T., Weber C.R., Wasland K., Savkovic S.D. Genistein inhibits proliferation of colon cancer cells by attenuating a negative effect of epidermal growth factor on tumor suppressor FOXO3 activity. BMC Cancer. 2011;11 doi: 10.1186/1471-2407-11-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chou T.C., Talalay P. Analysis of combined drug effects: A new look at a very old problem. Trends Pharmacol. Sci. 1983;4:450–454. doi: 10.1016/0165-6147(83)90490-X. [DOI] [Google Scholar]