Abstract
This study investigated the antioxidative and obsteoblast differentiation promoting activity of the phenolics isolated from the 70% ethanol extract of the roots of Livistona chinensis. Two new phenolics, (2R,3R)-3,5,6,7,3',4'-hexahydroxyflavane (1), and phenanthrene-2,4,9-triol (2), together with six known phenolics 3–8, were isolated and identified on the basis of extensive spectroscopic analysis. The antioxidative and obsteoblast differentiation promoting abilities of the compounds 1–3, 7–8 were tested, the phenolics 1–3, 7 showed effects on proliferation of osteoblastic cells and antioxidative activity of 3.125–50 µg/mL. In addition, the phenolics 1–3 observably increased alkaline phosphatase activity, osteocalcin content and hydroxyproline content in osteoblastic cells. Phenolic 1 at 12.5 µg/mL concentration significantly increased the area of nodules by about 9.35-fold. The antioxidative activity results indicated that the anti-osteoporosis effects of these phenolics may be linked to a reduction of oxidative stress. The observed effects of these phenolics on bone formation by rat osteoblastic cells suggest that these phenolics may have beneficial effects on bone health.
Keywords: phenolic, flavane, Livistona chinensis, osteoporosis, oxidative stress
1. Introduction
Osteoporosis is a disease characterized by the loss of bone mass and degeneration of bone microstructure, resulting in an increased risk of fractures. Osteoporosis, called postmenopausal osteoporosis, is common in women after menopause [1]. It may also develop in men, especially in the aged man, which is called age-related bone loss [2]. Osteoporosis may significantly affect life expectancy and quality of life in humans.
Oxidative stress, resulting from excessive formation of reactive oxygen species (ROS) or dysfunction of antioxidant defense system, represents a major cause of age-associated pathological conditions including aging [3] and postmenopausal bone loss [4]. Oxidative stress is a pivotal pathogenic factor for age-related bone loss in mice, leading to an increase in osteoblast and osteocyte apoptosis and a decrease in osteoblast number and the rate of bone formation [5,6]. On the other hand, ROS is also involved in bone resorption with a direct contribution of osteoclast-generated superoxide to bone degradation [7,8]. Oxidative stress increases differentiation and function of osteoclasts [8].
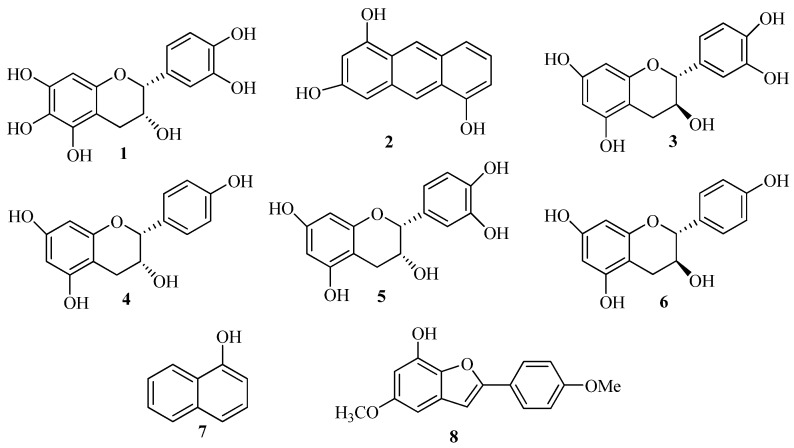
The genus Livistona is widely distributed in several ecosystems throughout the tropical zone, including upland hardwoods, flatwoods, and tropical hammocks. L. chinensis is commonly used for analgesic and hemostatic purposes [9,10]. Phytochemically, it has been reported to contain flavonoids, phenolics, ceramides and glycerides [11,12,13,14,15,16,17]. As part of our ongoing search for active anti-osteoporosis compounds from traditional medicinal plants, we investigated the constituents from the roots of L. chinensis. Eight phenolics, including two new phenolics, (2R,3R)-3,5,6,7,3',4'-hexahydroxyflavane (1), phenanthrene-2,4,9-triol (2), and six known compounds 3–8 was isolated and identified from the ethyl acetate-soluble fraction of a 70% ethanol extract. The structures of phenolics 1–8 are shown in Figure 1. Their potential osteogenic effects on the proliferation, differentiation and mineralization of osteoblastic cells were evaluated. To clarify the underlying mechanisms of action of the phenolics, we investigated whether protection against osteoporosis was linked to a reduction of oxidative stress.
Figure 1.
Chemical structures of the phenolics 1–8.
2. Results and Discussion
2.1. Structure Elucidation of the New Phenolics 1 and 2
Phenolic 1 was obtained as an amorphous red powder. The positive electrospray ionization mass spectrometry (ESI-MS) gave a [M+Na]+ ion at m/z 329 from which in combination with its 1H and 13C-NMR data the molecular formula of C15H14O7 was inferred. The 1H-NMR spectrum of 1 exhibited three meta-coupled doublets at δH 6.94 (1H, d, J =1.6 Hz, H-2'), 6.72 (1H, d, J = 8.4 Hz, H-5') and 6.76 (1H, dd, J = 8.4, 2.0 Hz, H-6'), consistent with a flavane 1',3',4'-trisubstituted B ring. One meta-coupled doublet at δH 5.91 (1H, s, H-8) was consistent with a flavane 5,6,7-trioxygenated A ring. These resonances, together with δH 4.77 (1H, brs, H-2), 4.14 (1H, m, H-3), 2.83 (1H, dd, J = 16.4, 4.8 Hz, H-4ax) and 2.70 (1H, dd, J = 16.8, 2.8 Hz, H-4eq) and the corresponding carbon signals in the HMQC spectrum revealed that 1 was 3,5,6,7,3',4'-hexahydroxyflavane. The following spectroscopic analysis made the absolute configurations at C-2 and C-3 assignable. The small coupling constants of H-2 and H-3 were indicative of the 2,3-cis relative configuration with the 2-phenyl group in a pseudo-equatorial orientation and the 3-hydroxy group in a pseudo-axial orientation, that’s to say, the configurations could be (2S,3S) or (2R,3R) [14]. By comparison with the literature values of 2R,3R-3,5,6,7,8,4'-hexahydroxyflavane, whose optical rotation value {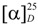 = −48.0 (c 0.30, MeOH)} indicated a 2R and 3R absolute configuration [14], the optical rotation value of 1 {
= −48.0 (c 0.30, MeOH)} indicated a 2R and 3R absolute configuration [14], the optical rotation value of 1 {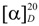 = −43.0 (c0.3, MeOH)} suggested the absolute configurations of C-2 and C-3 were (2R, 3R). Thus, the structure of 1 was elucidated to be 2R,3R-3,5,6,7,3',4'-hexahydroxyflavane.
= −43.0 (c0.3, MeOH)} suggested the absolute configurations of C-2 and C-3 were (2R, 3R). Thus, the structure of 1 was elucidated to be 2R,3R-3,5,6,7,3',4'-hexahydroxyflavane.
Phenolic 2 was obtained as an amorphous powder. Positive electrospray ionization mass spectrometry (ESI-MS) produced a [M+Na]+ ion at m/z 249, indicating a mass of 226, which is compatible with the molecular formula C14H10O3. Its molecular formula was confirmed by HR-ESIMS, which showed the ion [M+Na]+ at m/z 249.0637 (calcd. 249.0630). In the 1H-NMR spectrum, there are seven signals at δH 6.15 (1H, t, J = 5.6 Hz, H-11), 6.44 (1H, d, J = 2.0 Hz, H-14), 6.76 (1H, d, J = 8.8 Hz, H-10), 6.81 (1H, s, H-3), 6.92 (1H, s, H-5), 6.97 (1H, s, H-7) and 7.34 (1H, d, J = 8.4 Hz, H-12). The 13C-NMR spectrum included 14 nonequivalent carbon signals at δC 102.9–159.8 ppm, implying phenolic 2 is an anthracene compound. The counpling constant of every hydrogen mentioned above indicates the assignment of the hydroxyl groups at C-2, C-4 and C-9. From the data above, phenolic 2 was identified as a new compound, anthracene-2,4,9-triol. The known phenolics were identified, by comparing of their spectroscopic data with data previously reported in the literature, as (−)-catechin (3) [14], (−)-epiafzelechin (4) [14], (−)-epicatechin (5) [18], (−)-afzelechin (6) [18], naphthalen-2-ol (7) [19] and 7-hydroxy-5,4'-dimethoxy-2-arylbenzofuran (8) [15].
2.2. In Vitro Effect of the Phenolics 1–3, 7, 8 in Osteoblast Viability
When different concentration of the phenolics 1–3, 7–8 were cultured with rat osteoblast cells. Phenolic 8 inhibited the rat osteoblast cell growth and proliferation. However, phenolics 1 and 3 at concentration from 3.125 µg/mL to 50 µg/mL stimulated rat osteoblast cell growth and proliferation, and this effect appeared in a dose-dependent manner (Table 1). We observed a significant increase in promoting proliferative activity in cells treated with phenolic 1 after 2 days of treatment. Phenolic 1 at 25 µg/mL concentration showed the highest promoting proliferative activity (about 2.14-fold) at the second day after treatment, when compared with the negative control, which exhibited more potential promoting activity than positive control-resveratrol.
Table 1.
Effects of the phenolics 1–3, 7 on the proliferation of rat osteoblast cells after 48 h, as determined by MTT assay.
| Concentrations (μg/mL) | Cell Viability (%) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 7 | Positive Control | |
| 0 | 101.73 ± 6.54 | 104.55 ± 8.78 | 103.29 ± 10.85 | 101.29 ± 9.85 | 101.34 ± 13.23 |
| 3.125 | 158.43 ± 15.44 * | 111.75 ± 12.19 * | 163.99 ± 10.50 * | 107.99 ± 10.50 | 107.21 ± 9.56 |
| 6.25 | 181.54 ± 8.19 * | 125.07 ± 10.23 * | 171.45 ± 15.44 * | 111.45 ± 11.44 * | 115.25 ± 14.14 * |
| 12.5 | 208.88 ± 12.95 * | 129.13 ± 8.19 * | 186.90 ± 16.78 * | 116.90 ± 9.78 * | 169.32 ± 16.00 * |
| 25 | 314.67 ± 21.48 * | 134.23 ± 11.97 * | 214.22 ± 20.60 * | 121.22 ± 12.60 * | 197.66 ± 16.57 * |
| 50 | 228.94 ± 11.58 * | 125.76 ± 11.60 * | 175.73 ± 16.59 * | 115.73 ± 8.59 * | 153.23 ± 16.65 * |
Note: * p ˂ 0.05 vs. negative control.
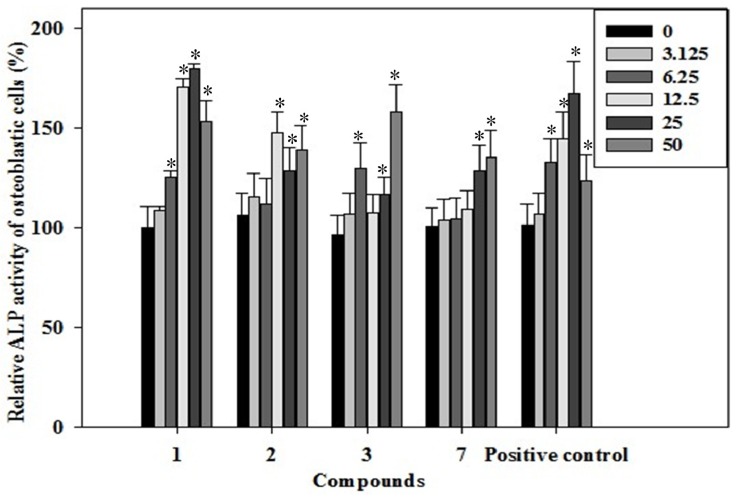
2.3. Effects of the Phenolics 1–3, 7 on ALP Activity of Rat Osteoblast
The phenotype of mature osteoblasts is characterized by their ability to synthesize and secrete molecules of the extracellular matrix. Osteoblasts regulate their mineralization of the formed matrix by producing alkaline phosphatase (ALP) [20]. This enzyme hydrolyzes phosphate esters to increase the local phosphate concentration and enhance mineralization of the extracellular matrix [21]. One of the characteristics of a mature osteoblast phenotype is the ability of the cells to synthesize ALP, which is considered an early marker of osteoblast differentiation. ALP is an early marker of osteogenic differentiation [22]. To determine whether the phenolics 1–3, 7 could stimulate osteogenic differentiation, the effects of the new flavane on bone formation early marker, ALP activity, was measured. Our data illustrated that treatment of rat osteoblast cells with the phenolics 1–3, 7 stimulated ALP activity in a dose-dependent manner. As shown in Figure 2, phenolic 1 at most of the treated concentrations increased the ALP activity compared with the negative control. At 7 day after treatment, phenolic 1 showed a dose-dependent increase in ALP activity by 8.8%, 25.8%, 70.4%, 80.1% and 53.3% at the concentrations of 3.125, 6.25, 12.5, 25 and 50 μg/mL, respectively, as compared to the control. Phenolic 2 demonstrated a dose-dependent increase in ALP activity by 11.5%, 11.7%, 48.0%, 28.8% and 39.3% at the concentrations of 3.125, 6.25, 12.5, 25 and 50 μg/mL, respectively, as compared to the control. Phenolic 3 significantly increased the ALP activity by 6.9%, 29.7%, 7.4%, 16.8% and 58.1% at concentrations of 3.125, 6.25, 12.5, 25 and 50 μg/mL, as compared to the control. Phenolic 7 significantly increased the ALP activity by 6.9%, 4.7%, 9.4%, 28.8% and 35.1% at concentrations of 3.125, 6.25, 12.5, 25 and 50 μg/mL, as compared to the control. At 25 μg/mL, the ALP activity of the new flavane was significantly increased by about 0.80-fold when compared with the negative control at 7 day after treatment. Its osteogenic effect was stronger than the positive control resveratrol at 25 μg/mL.
Figure 2.
The effects of the phenolics (1–3, 7) on ALP activity were assessed in rat osteoblast cells treated with the phenolics (1–3, 7) (3.125–50 µg/mL) for 7 days.
Note: * p ˂ 0.05 vs. negative control.
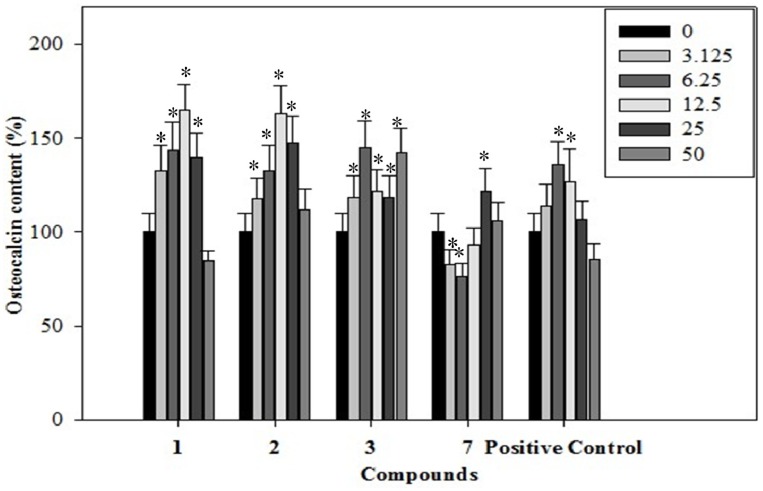
2.4. Effects of the Phenolics 1–3, 7 on Osteocalcin Content of the Rat Osteoblastic Cells
Osteocalcin is a noncollagenous protein found in bone and dentin. Osteocalcin is secreted solely by osteoblasts and thought to play a role in the body's metabolic regulation and is pro-osteoblastic, or bone-building, by nature. It is also implicated in bone mineralization and calcium ion homeostasis [23]. It is often used as a marker for the bone formation process. The effects of the phenolics 1–3, 7 on the bone formation marker osteocalcin were determined. Our data illustrated that treatment of rat osteoblast cells with the phenolics 1–3, 7 for 14 days stimulated osteocalcin secreting in a dose-dependent manner. As shown in Figure 3, phenolic 1 demonstrated a dose-dependent increase in osteocalcin content by 32.6%, 43.9%, 65.3% and 39.6% at the concentrations of 3.125, 6.25, 12.5 and 25 μg/mL, as compared to the control. Phenolic 2 demonstrated a dose-dependent increase in osteocalcin content by 18.0%, 32.8%, 63.1%, 47.6% and 11.7% at the concentrations of 3.125, 6.25, 12.5, 25 and 50 μg/mL, as compared to the control. Phenolic 3 demonstrated a dose-dependent increase in osteocalcin content by 18.3%, 45.0%, 21.5%, 18.4% and 42.1% at the concentrations of 3.125, 6.25, 12.5, 25 and 50 μg/mL, as compared to the control. Phenolic 7 demonstrated a dose-dependent increase in osteocalcin content by 21.6% and 5.8% at the concentrations of 25 and 50 μg/mL, as compared to the control. Treated with 12.5 μg/mL phenolic 1, the osteocalcin content was significantly increased by about 0.65-fold when compared with the negative control. Its osteogenic effect was stronger than the positive control resveratrol at 6.25 μg/mL.
Figure 3.
The effects of the phenolics (1–3, 7) on osteocalcin content were assessed in rat osteoblast cells treated with the phenolics (1–3, 7) (3.125–50 µg/mL) for 14 days.
Note: * p ˂ 0.05 vs. negative control.
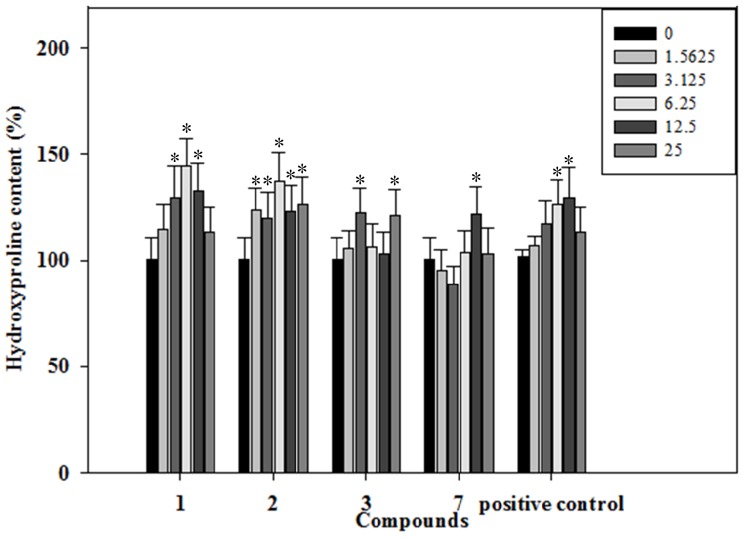
2.5. Effects of the Phenolics 1–3, 7 on Collagen Content of the Rat Osteoblastic Cells
Collagen type I is a marker of osteogenic maturity, and bone is a matrix with collagen type I [24]. Collagen content of the rat osteoblastic cells was determined by a hydroxyproline assay. The effects of the phenolics 1–3, 7 on bone formation of the intermediate-term marker, collagen, were determined. Our data illustrated that treatment of rat osteoblast cells with the phenolics 1–3, 7 for 22 days stimulated collagen secreting in a dose-dependent manner. As shown in Figure 4, phenolic 1 demonstrated a dose-dependent increase in collagen content by 14.3%, 29.5%, 44.4%, 32.7% and 13.2% at the concentrations of 1.5625, 3.125, 6.25, 12.5 and 25 μg/mL, as compared to the control. Phenolic 2 demonstrated a dose-dependent increase in collagen content by 23.3%, 19.5%, 37.5%, 22.7% and 26.3% at the concentrations of 1.5625, 3.125, 6.25, 12.5 and 25 μg/mL, as compared to the control. Phenolic 3 demonstrated a dose-dependent increase in collagen content by 5.2%, 22.4%, 6.4%, 2.7% and 21.2% at the concentrations of 1.5625, 3.125, 6.25, 12.5 and 25 μg/mL, as compared to the control. Phenolic 7 demonstrated a dose-dependent increase in collagen content by 3.4%, 21.6% and 3.2% at the concentrations of 6.25, 12.5 and 25 μg/mL, as compared to the control. Treated with 6.25 μg/mL phenolic 1, the collagen content was significantly increased by about 0.44-fold when compared with the negative control. Its osteogenic effect was stronger than the positive control resveratrol at 50 μg/mL.
Figure 4.
The effects of the phenolics (1–3, 7) on the hydroxyproline content were determined in rat osteoblast cells treated with the phenolics (1–3, 7) (1.5625–25 µg/mL) for 22 days.
Note: * p ˂ 0.05 vs. negative control.
2.6. Effects of the Phenolics 1–3, 7 on the Formation of Mineralized Bone Nodules
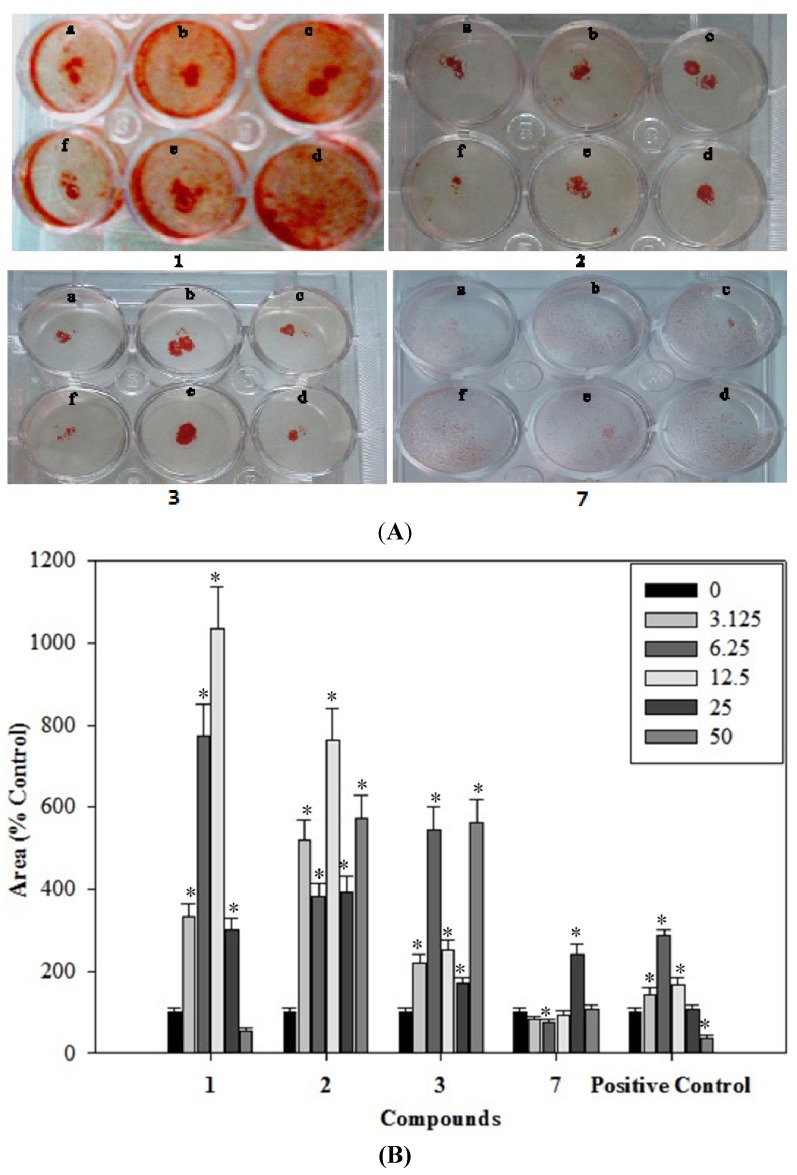
The mineralized bone nodules formed by rat osteoblast cells were observed after 20 days of treatment. The mineralized bone nodules could be visualized by the naked eye as red-purple spots after staining with alizarin red (Figure 5A). The addition of phenolic 1 at concentrations as low as 3.125 µg/mL to the culture media increased the formation of mineralized nodules compared with control. Phenolics 1–3, 7 caused a dose-dependent increase in the area of mineralized bone nodules as quantified using a Image-Pro Plus 6.0 software (Figure 5B). Phenolic 1 demonstrated a dose-dependent increase in the area of mineralized bone nodules by 232.6%, 673.9%, 935.3% and 199.6% at the concentrations of 3.125, 6.25, 12.5 and 25 μg/mL, as compared to the control. Phenolic 2 demonstrated a dose-dependent increase in the area of mineralized bone nodules by 518.0%, 282.8%, 663.1%, 290.6% and 571.7% at the concentrations of 3.125, 6.25, 12.5, 25 and 50 μg/mL, as compared to the control. Phenolic 3 demonstrated a dose-dependent increase in the area of mineralized bone nodules by 118.3%, 445.0%, 151.5%, 68.4% and 462.1% at the concentrations of 3.125, 6.25, 12.5, 25 and 50 μg/mL, as compared to the control. Phenolic 7 demonstrated a dose-dependent increase in the area of mineralized bone nodules by 141.6% and 5.8% at the concentrations of 25 and 50 μg/mL, as compared to the control. Analysis of the results from alizarin red staining showed that at 12.5 µg/mL, phenolic 1 increased the area of nodules by about 9.35-fold.
Figure 5.
Dose-dependent effects of the phenolics 1–3, 7 on the formation of mineralized nodules in a 12-well plate fixed on Day 20 and stained by alizarin red. (A) Cells were treated with the phenolics 1–3, 7 at concentrations of 3.125, 6.25, 12.5, 25, 50 and 0 µg/mL (from a to f) on day 20; and (B) Dose-dependent effects of the phenolics 1–3, 7 on the area of mineralized bone nodules stained by alizarin red.
Note: * p ˂ 0.05 vs. negative control.
2.7. Antioxidantive Activity of the Phenolics 1–3, 7
2.7.1. Protective Effects against H2O2-Induced Cell Injury in C2C12 Mouse Myoblast Cells
To investigate the protective effects of phenolics 1–3, 7 on H2O2-induced cytotoxicity, C2C12 mouse myoblast cells were cultured in the presence of 100 μM H2O2 with or without treatment with phenolics 1–3, 7. Treatment with phenolics 1–3, 7 increased the cell viability of C2C12 mouse myoblast cells compared to the H2O2-treated control (Table 2A). Treatment with phenolics 1–3, 7 decreased the number of apoptotic cells compared to the H2O2-treated control group (Table 2B). From the results, phenolics 1–3, 7 isolated from the roots of L. chinensis showed potential protective effects against H2O2-induced cell injury in C2C12 mouse myoblast cells. Flavane 1 at 50 µg/mL concentration showed the highest protective activity.
Table 2.
Protective effects of the phenolics 1–3, 7 on H2O2-induced cell injury in rat osteoblastic cells. Rat osteoblast cells were cultured in the presence of 100 μM H2O2 with or without treatment of the phenolics 1–3, 7 different concentrations. (A) The cell viability, and (B) the cell apoptosis, were assessed by MTT assay and flow cytometric analysis, respectively.
| Concentrations (μg/mL) | (A) Cell Viability (%) | ||||
| 1 | 2 | 3 | 7 | Positive Control | |
| 0 | 211.23 ± 19.57 * | 205.92 ± 20.41 * | 211.45 ± 21.50 * | 197.99 ± 18.51 * | 210.12 ± 20.51 * |
| 100 μM H2O2 | 102.43 ± 8.34 | 103.66 ± 9.80 | 103.99 ± 8.08 | 102.50 ± 10.50 | 102.50 ± 9.65 |
| 100 μM H2O2 + 3.125 | 127.43 ± 6.34 | 113.66 ± 10.48 | 123.99 ± 10.09 | 106.81 ± 10.40 | 131.81 ± 12.40 |
| 100 μM H2O2 + 6.25 | 131.32 ± 11.57 * | 119.91 ± 10.50 | 126.02 ± 11.26 | 113.94 ± 11.33 | 163.94 ± 14.35 * |
| 100 μM H2O2 + 12.5 | 144.36 ± 18.83 * | 145.79 ± 12.61 * | 136.81 ± 13.33 * | 124.09 ± 12.64 * | 234.06 ± 21.63 * |
| 100 μM H2O2 + 25 | 198.44 ± 16.85 * | 154.01 ± 14.94 * | 188.02 ± 28.01 * | 134.24 ± 24.61 * | 499.24 ± 54.60 * |
| 100 μM H2O2 + 50 | 319.46 ± 23.20 * | 212.96 ± 19.07 * | 288.29 ± 22.14 * | 145.08 ± 16.06 * | 766.08 ± 79.15 * |
| Concentrations (μg/mL) | (B) Cell Apoptosis (%) | ||||
| 1 | 2 | 3 | 7 | Positive Control | |
| 0 | 2.50 ± 0.70 * | 2.55 ± 0.30 * | 2.40 ± 0.21 * | 2.04 ± 0.24 * | 2.35 ± 0.17 * |
| 100 μM H2O2 | 67.53 ± 4.56 | 66.87 ± 3.59 | 64.96 ± 6.73 | 68.67 ± 5.98 | 67.23 ± 4.39 |
| 100 μM H2O2 + 3.125 | 61.50 ± 5.73 | 62.50 ± 5.91 | 64.45 ± 3.34 | 66.50 ± 6.11 | 66.81 ± 6.41 |
| 100 μM H2O2 + 6.25 | 34.33 ± 3.44 * | 57.94 ± 5.34 * | 40.32 ± 3.57 * | 60.29 ± 6.07 | 61.94 ± 5.33 |
| 100 μM H2O2 + 12.5 | 9.56 ± 0.99 * | 46.09 ± 3.64 * | 13.36 ± 2.83 * | 55.13 ± 3.12 * | 13.09 ± 1.63 * |
| 100 μM H2O2 + 25 | 2.63 ± 0.19 * | 24.16 ± 2.15 * | 2.44 ± 0.19 * | 45.70 ± 4.13 * | 3.24 ± 0.20 * |
| 100 μM H2O2 + 50 | 1.92 ± 0.21 * | 10.68 ± 1.15 * | 1.46 ± 0.20 * | 23.89 ± 2.03 * | 0.68 ± 0.06 * |
Note: * p ˂ 0.05 vs. model group.
2.7.2. DPPH Free Radical-Scavenging Activity
From the results, it was found that phenolics 1–3, 7 isolated from L. chinensis showed obvious free radical scavenging activity on DPPH with IC50 values of 2.11 ± 0.18, 2.89 ± 0.28, 3.58 ± 0.18, 10.70 ± 1.18 μM, respectively. Meanwhile, phenolics 1–3 exhibited more potential antioxidant activity than positive control-quercetin (IC50: 5.40 ± 0.30 μM).
Osteoporosis is a degenerative bone disease characterized by low bone mass and structural deterioration of bone tissue, leading to bone fragility. Bone integrity requires a tight coupling between the activity of bone-forming osteoblasts and bone-resorbing osteoclasts [25]. Reactive oxygen species (ROS) are involved in osteogenesis including bone formation and resorption, which are associated with the aging process and may result in osteoporosis [26,27,28,29,30]. During bone formation, osteoblasts undergo a cascade of complex events that might include three phases: proliferation, osteogenic differentiation, and mineralization of extracellular matrix [31].
This study reported for the first time to investigate the osteogenic effects of the phenolics from the roots of L. chinensis. Two new phenolics were isolated from the the roots of L. chinensis. Anti-osteoporosis activity of the phenolics 4–6 was evaluated in our former research [32]. Phenolic 1 showed enhancing effects on proliferation of rat osteoblast cells at the tested concentrations (3.125–50 µg/mL). Phenolics 1–3 at the majority of the tested concentrations (3.125–50 µg/mL) increased the osteogenic differentiation. At 7 day after treatment, phenolic 1 at the concentration of 25 µg/mL increased the ALP activity to the highest level by 80.1%, compared to the control. Treated with 12.5 μg/mL phenolic 1, the osteocalcin content was significantly increased by about 0.65-fold when compared with the negative control. Collagen content of the rat osteoblastic cells was determined by hydroxyproline assay. Treated with the 6.25 μg/mL phenolic 1, the hydroxyproline content was significantly increased by about 0.44-fold when compared with the negative control. Phenolic 1 at 12.5 µg/mL increased the area of nodules by about 9.35-fold.
3. Experimental
3.1. General
Optical rotations were measured using a JASCO P-1030 (Tokyo, Japan) automatic digital polarimeter. NMR spectra (400 MHz for 1H NMR) were recorded on a Bruker DPX-400 spectrometer (Karlsruhe, Germany) using standard Bruker pulse programs. Chemical shifts were showed as the δ-value with reference to tetramethylsilane (TMS) as an internal standard. ESI-MS data were obtained on an Agilent 1200 HPLC/6410B TripleQuad mass spectrometer (Santa Clara, CA, USA), and HR-ESIMS were measured on a Bruker APEX II mass spectrometer (Bremen, Germany). Sephadex LH-20 (Pharmacia, Stockholm, Sweden), silica gel (Qingdao Ocean Chemical Co., Ltd, Qingdao, China), Octadecylsilanized (ODS) silica gel (Macherey-Nagel, Duren, Germany) were used for column chromatography. TLC was carried out on Silica gel 60 F254 (0.25 mm, Merck, Darmstadt, Germany), and RP-18 F254 (0.25 mm, Merck) plates, and spots were visualized by spraying with 15% H2SO4 followed by heating. HPLC was performed using an octadecylsilanized (ODS) silica gel column (XTerra 10 μm, 19 × 250 mm, Waters). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and trypsin-EDTA solution (1×) were obtained from Hyclone (Logan, UT, USA). Annexin-V/PI Apoptosis Detection Kit was purchased from Beyotime Institute of Biotechnology (Jiangsu, China). Hydroxyproline Assay Kit was purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). All other chemicals were analytical or HPLC grade and obtained from Shanghai Chemical Reagents Co., Ltd (Shanghai, China).
3.2. Plant Material
The fresh roots of Livistona chinensis (Jacq.) R. Br. were collected in Jiangmen, Guangdong Province, China, in September 2009, and were identified by Prof. Xiangjiu He, School of Pharmaceutical Sciences at Wuhan University. A voucher specimen (No. 20100115) is available at the School of Pharmaceutical Sciences, Wuhan University in Wuhan (430071), China.
3.3. Extraction and Isolation
The air-dried roots of L. chinensis (1.5 kg) were extracted with 70% EtOH (25 L × 3) at refluxed. Evaporation of the organic solvent under a vacuum at 55 °C yielded a crude extract (560 g). The concentrated brown syrup was resuspended in water and partitioned with chloroform (3 L × 3), ethyl acetate (3 L × 3) and water-saturated n-butanol (3 L × 3) gradually to afford 33.0 g, 35.3 g and 230.3 g of dried organic extracts, respectively. The ethyl acetate fraction with the most potential activity was fractionated over a silica gel (200 ~ 300 mesh) column eluting with a gradually amount of MeOH in CHCl3 to give 15 fractions. The CHCl3/MeOH (25:1) eluate was further purified on a silica gel column, eluted with CHCl3/MeOH (100:1→1:1), combined with preparative HPLC (28% methanol containing 0.1% CF3COOH, pH 3.0), yielding compounds 2 (56.79 mg), 5 (12.33 mg) and 7 (15.56 mg). The CHCl3/MeOH (10:1) eluate was subjected to an octadecylsilanized silica gel (ODS) column, followed by a preparative HPLC with 26% methanol (containing 0.1% CF3COOH, pH 3.0), yielding compound 6 (10.76 mg). The CHCl3/MeOH (15:1) eluate was subjected to silica gel and Sephadex LH-20 column chromatography, followed by preparative HPLC with 20% methanol (containing 0.1% CF3COOH, pH 3.0) to give phenolics 1 (15.56 mg), 3 (13.47 mg), 4 (21.34 mg) and 8 (132.46 mg).
3.4. (2R,3R)-3,5,6,7,3',4'-Hexahydroxyflavane (1)
Amorphous colorless powder; 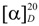 = −43.0 (c 0.3, MeOH). ESI-MS (positive-ion mode) [M+Na]+
m/z 329. 1H-NMR (CD3OD) δH 4.77 (1H, brs, H-2), 4.14 (1H, m, H-3), 2.83 (1H, dd, J = 16.4, 4.8 Hz, H-4ax), 2.70 (1H, dd, J = 16.8, 2.8 Hz, H-4eq), 5.91 (1H, s, H-8), 6.94 (1H, d, J =1.6 Hz, H-2'), 6.72 (1H, d, J = 8.4 Hz, H-5'), 6.76 (1H, dd, J = 8.4, 2.0 Hz, H-6'); 13C-NMR (CD3OD): δC 79.9 (C-2), 67.6 (C-3), 29.5 (C-4), 157.7(C-5), 145.8 (C-6), 146.1 (C-7), 96.6 (C-8), 158.0 (C-9), 100.3 (C-10), 132.4 (C-1'), 115.5 (C-2'), 157.4 (C-3'), 157.2 (C-4'), 116.1 (C-5'), 119.6 (C-6').
= −43.0 (c 0.3, MeOH). ESI-MS (positive-ion mode) [M+Na]+
m/z 329. 1H-NMR (CD3OD) δH 4.77 (1H, brs, H-2), 4.14 (1H, m, H-3), 2.83 (1H, dd, J = 16.4, 4.8 Hz, H-4ax), 2.70 (1H, dd, J = 16.8, 2.8 Hz, H-4eq), 5.91 (1H, s, H-8), 6.94 (1H, d, J =1.6 Hz, H-2'), 6.72 (1H, d, J = 8.4 Hz, H-5'), 6.76 (1H, dd, J = 8.4, 2.0 Hz, H-6'); 13C-NMR (CD3OD): δC 79.9 (C-2), 67.6 (C-3), 29.5 (C-4), 157.7(C-5), 145.8 (C-6), 146.1 (C-7), 96.6 (C-8), 158.0 (C-9), 100.3 (C-10), 132.4 (C-1'), 115.5 (C-2'), 157.4 (C-3'), 157.2 (C-4'), 116.1 (C-5'), 119.6 (C-6').
3.5. Anthracene-2,4,9-triol (2)
Amorphous colorless powder; ESI-MS (positive-ion mode) [M+Na]+ m/z 249. 1H-NMR (CD3OD) δH 6.81 (s, H-3), 6.92 (s, H-5), 6.97 (s, H-7), 6.76 (d, J = 8.8 Hz, H-10), 6.15 (t, J = 5.6 Hz, H-11), 7.34 (d, J = 8.4 Hz, H-12) and 6.44 (d, J = 2.0 Hz, H-14); 13C-NMR (CD3OD) δC 116.7 (C-1), 159.8 (C-2), 106.0 (C-3), 159.8(C-4), 130.8 (C-5), 141.6 (C-6), 102.9 (C-7), 128.9 (C-8), 158.6 (C-9), 106.0 (C-10), 128.9 (C-11), 116.7 (C-12), 127.2 (C-13), 129.4 (C-14).
3.6. Rat Osteoblast Cell Culture
Osteoblastic cells were enzymatically isolated from newborn rat calvaria by a previously described method with slight modifications [33]. The bone pieces were digested sequentially in a trypsin II-S (25 mg)-collagenase IA (70 mg) in 15 mL PBS solution at 37 °C for 30 min. Rat osteoblastic cells obtained from the last three digestion steps were pooled and plated together in a T25 tissue culture dish at a concentration of 50,000 cells/cm2 and cultured in DMEM supplemented with 10% FBS and 1% antibiotic (100 units/mL penicillin and 100 μg/mL streptomycin) at 37 °C in a 5% CO2 humid atmosphere. The medium was changed every 3 day to remove the non-adherent cells.
3.7. C2C12 Cell Culture
The C2C12 cell line was purchased from the American Type Culture Collection. Cells were cultured in basal medium, constituted with DMEM containing 10% fetal bovine serum (FBS, Gibco, Carlsbad, CA, USA) and 1% antibiotics (100 units/mL penicillin and 100 μg/mL streptomycin), for incubation at 37 °C in a 5% CO2 humidified atmosphere.
3.8. Cell Viability Assay
Cells were seeded in a 96-well plate at a density of 5 × 103 cells/well. The total volume was adjusted to 100 μL with growth medium. 24 h after the seeding, the cells were exposed to the phenolics or resveratrol (positive drug) of different concentrations. At two days after treatment, cell viability was examined using a standard MTT method [34]. Drug effect was expressed as percentage relative to the controls.
3.9. Alkaline Phosphatase (ALP) Activity
Cells were cultured in DMEM with 10% FBS and 1% antibiotic (100 units/mL penicillin and 100 μg/mL streptomycin) at 37 °C in a humid atmosphere containing 5% CO2. 1 mL of 5 × 104 cells/well of osteoblastic cells was seeded in 48-well plates. After 24 h, the culture medium with the phenolics or resveratrol (positive drug) at concentrations of 0, 3.125, 6.25, 12.5, 25 and 50 µg/mL was added.
The ALP activity of the samples was determined by a colorimetric assay [35]. At 7 days after treatment, cells seeded in 48-well plates were washed twice with 50 mM PBS (pH 7.4) and kept in 0.1% Triton X-100 lysis buffer overnight at –20 °C. The cells were later thawed. 300 µL of substrate buffer (6.7 mmol/L disodium p-nitrophenylphosphate hexahydrate, 25 mmol/L diethanolamine and 1 mmol/L MgCl2) was added in. After the mixtures were incubated at 37 °C for 30 min, we measured the absorbance at 405 nm.
3.10. Osteocalcin Assay
Osteoblastic cells (2 mL, 1 × 105 cells/well) were seeded in 6-well plates. After 24 h, the culture medium with the phenolics or resveratrol (positive drug) at 0, 3.125, 6.25, 12.5, 25, and 50 µg/mL concentrations was added in. Fourteen days after treatment, the conditioned media were collected for assessment. The concentration of the free osteocalcin was measured by radioimmunoassay (RIA) according to the manufacturer’s instructions (Tian Jin Nine Tripods Medical and Bioengineering Co, Ltd., Tianjin, China).
3.11. Hydroxyproline Assay
Osteoblastic cells (2 mL, 1 × 105 cells/well) were seeded in 12-well plates. After 24 h, the culture medium with the phenolics or resveratrol (positive drug) at 0, 1.5625, 3.125, 6.25, 12.5, and 25 µg/mL concentrations was added. Twenty two days after treatment, cell samples were hydrolyzed in 6 N HCl (final concentration) for 12 h at 110 °C. The samples were filtered and vacuum dried. The residue was dissolved in water (200 µL). The hydroxyproline content was assayed according to the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
3.12. Determination and Quantification of Mineralized Bone Nodules
Osteoblastic cells (2 mL, 1 × 105 cells/well) were seeded in 12-well plates. After 24 h, osteogenic medium (10 mM L-glycerophosphate, 50 µg/mL ascorbic acid) with the phenolics or resveratrol (positive drug) was added. On Day 20, cells seeded in 24-well plates were fixed in 95% ethanol for 15 min and the mineralized bone nodules were visualized by alizarin red staining techniques [36]. The cells were stained with alizarin red (40 mM, pH 7.2) for 10 min and then rinsed them with PBS. Nodules were visualized using an inverted microscope. The area of mineralized nodules was quantified by the Image-Pro Plus 6.0 Software.
3.13. Flow Cytometric Analysis for Apoptosis
To investigate the protective effects of the new flavane on H2O2-induced cytotoxicity, C2C12 mouse myoblast cells were cultured in the presence of 100 μM H2O2 with or without treatment with the phenolics or quercetin (positive drug). Apoptosis was examined by Annexin V-fluorescein isothiocyanate staining (Beyotime Institute of Biotechnology, Jiangsu, China) according to the manufacturer’s instructions. Cells were seeded in 6-well plates. Two days after treatment, the cells were harvested by trypsinization, rinsed twice with PBS, and suspended in 500 µL of binding buffer. The suspended cells were incubated for 15 min at 4 °C with 5 µL Annexin V-FITC solution, and incubated for another 5 min at 4 °C after adding 10 µL of PI solution. The FITC fluorescence intensity of 10,000 cells was measured with a flow cytometer (Beckman-Coulter, Inc., Indianapolis, IN, USA). The apoptosis was expressed as the ratio of apoptotic cell count to total cell count.
3.14. DPPH Radical Scavenging Assay
The effect of the new flavane on DDPH radical was determined according to the method reported by Yen and Chen [37]. DPPH (50 mg/L) was dissolved in MeOH. The samples were dissolved in DMSO. The DPPH solution (995 μL) was mixed with 5 μL of the samples. The mixture was shaken and allowed to stand at room temperature in the dark for 20 min. The absorbance of the resulting solution was measured spectrophotometrically at 517 nm.
3.15. Statistical Analysis
All data were expressed as mean ± S.D. from at least three independent experiments, each performed in quintuplicate. The statistical significance among groups were performed by the SPSS 19.0 software and evaluated using variance (ANOVA) with Fisher’s PLSD test. The probabilities (P) less than 0.05 were considered statistically significant.
4. Conclusions
The findings of the present study showed that the phenolics in L. chinensis were able to activate simultaneously the rat osteoblastic cells at three phases. Phenolics 1–3 significantly promoted osteogenic proliferation, differentiation, and mineralization. ROS are also involved in bone resorption with a direct contribution of osteoclast-generated superoxide to bone degradation [8]. Oxidative stress is a pivotal pathogenic factor for age-related bone loss in mice, leading to an increase in osteoblast and osteocyte apoptosis and a decrease in osteoblast number and the rate of bone formation [38,39]. In addition, osteoblasts produce antioxidants such as glutathione peroxidase to protect against ROS [38,39]. The present study evaluated the antioxidative activity of phenolics through evaluating protecting activity against H2O2-induced apoptosis in C2C12 myogenic cells and DPPH free radical-scavenging activity. And it indicated that anti-osteoporosis effect of the phenolics isolated from the roots of L. chinensis might be linked to a reduction of oxidative stress. Further investigations are desirable to confirm this hypothesis based on findings of our current in vitro study.
Acknowledgments
This research was partly supported by the Start Fund of Guangdong Medical College (XB1302), National Natural Science Foundation of China (81102450 and 31301585), and Science andTechnology Innovation Fund of Guangdong Medical College (STIF 201104).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds (1–8) are available from the authors.
References
- 1.Ozgocmen S., Kaya H., Fadilliogl E., Aydogan R., Yilmaz Z. Role of antioxidant systems, lipid peroxidation, and nitric oxide in postmenopausal osteoporosis. Mol. Cell. Biochem. 2007;295:45–52. doi: 10.1007/s11010-006-9270-z. [DOI] [PubMed] [Google Scholar]
- 2.Overton T.R., Basu T.K. Longitudinal changes in radial bone density in older men. Eur. J. Clin. Nutr. 1999;53:211–215. doi: 10.1038/sj.ejcn.1600703. [DOI] [PubMed] [Google Scholar]
- 3.Linnane A.W., Eastwood H. Cellular redox regulation and prooxidant signaling systems, a new perspective on the free radical theory of aging. Ann. N. Y. Acad. Sci. 2006;1067:47–55. doi: 10.1196/annals.1354.008. [DOI] [PubMed] [Google Scholar]
- 4.Sendur O.F., Turan Y., Tastaban E., Serter M. Antioxidant status in patients with osteoporosis: A controlled study. Joint Bone Spine. 2009;76:514–518. doi: 10.1016/j.jbspin.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Mody N., Parhami F., Saraflan T.A., Demer L.L. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic. Biol. Med. 2001;31:509–519. doi: 10.1016/S0891-5849(01)00610-4. [DOI] [PubMed] [Google Scholar]
- 6.Fatokun A.A., Stone T.W., Smith R.A. Responses of differentiated MC3T3-E1 osteoblast-like cells to reactive oxygen species. Eur. J. Pharm. 2008;587:35–41. doi: 10.1016/j.ejphar.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Yang S., Madyastha P., Bingel S., Ries W., Key L. A new superoxide-generating oxidase in murine osteoclasts. J. Bio. Chem. 2001;276:5452–5458. doi: 10.1074/jbc.M001004200. [DOI] [PubMed] [Google Scholar]
- 8.Sontakke A.N., Tare R.S. A duality in the roles of reactive oxygen species with respect bone metabolism. Clin. Chim. Acta. 2002;318:145–148. doi: 10.1016/S0009-8981(01)00766-5. [DOI] [PubMed] [Google Scholar]
- 9.Healthy Ministry of Guangzhou Force Logistics . Common Chinese Herbal Medicine Handbook. People’s Health Publishing House; Beijing, China: 1969. [Google Scholar]
- 10.Zhao G.P., Dai S., Chen E.S. Dictionary of Traditional Chinese Medicine. Shanghai Science and Technology Press; Shanghai, China: 2001. [Google Scholar]
- 11.Chen P., Yang J.S. Studies on chemical constituents of Livistona chinensis seeds. Chin. Tradit. Herb Drugs. 2007;8:665–667. [Google Scholar]
- 12.Chen P., Yang J.S. Studies on chemical constituents of Livistona chinensis seeds. Chin. Pharm. J. 2008;43:1669–1670. [Google Scholar]
- 13.Tao Y., Yang S.P., Zhang H.Y., Liao S.G., Wei W., Yan W., Wu Y., Tang X.C., Yue J.M. Phenolic compounds with cell protective activity from the fruits of Livistona chinensis. J. Asian Nat. Prod. Res. 2009;11:243–249. doi: 10.1080/10286020802684631. [DOI] [PubMed] [Google Scholar]
- 14.Zeng X.B., Qiu Q., Jiang C.G., Jing Y.T., Qiu G.F., He X.J. Antioxidant flavanes from Livistona chinensis. Fitoterpia. 2011;82:609–614. doi: 10.1016/j.fitote.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Zeng X.B., Wang Y.H., Qiu Q., Jiang C.G., Jing Y.T., Qiu G.F., He X.J. Bioactive phenolics from the fruits of Livistona chinensis. Fitoterapia. 2012;83:104–109. doi: 10.1016/j.fitote.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Zeng X.B., Xiang L.M., Li C.Y., Wang Y.H., Qiu G.F., Zhang Z.X., He X.J. Cytotoxic ceramides and glycerides from the roots of Livistona chinensis. Fitoterapia. 2012;83:609–616. doi: 10.1016/j.fitote.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Zeng X.B., Li C.Y., Wang H., Qiu Q., Qiu G.F., He X.J. Unusual lipids and acylglucosylsterols from the roots of Livistona chinensis. Phytochem. Lett. 2013;6:36–40. doi: 10.1016/j.phytol.2012.10.010. [DOI] [Google Scholar]
- 18.Waterman P.G., Faulkner D.F. (−)-Epiafzelechin from the Root Bark of Cassia sieberiana. Planta Med. 1979;37:178–179. doi: 10.1055/s-0028-1097322. [DOI] [Google Scholar]
- 19.Jin G.Z., Jin H.S., Jin L.L. Synthesis and antiproliferative activity of 1,4-bis(dimethylamino)-9,10-anthraquinone derivatives against P388 mouse leukemic tumor cells. Arch. Pharm. Res. 2011;34:1071–1076. doi: 10.1007/s12272-011-0704-0. [DOI] [PubMed] [Google Scholar]
- 20.Aubin J.E. Advances in osteoblast lineage. Biochem. Cell. Biol. 1998;76:899–910. doi: 10.1139/o99-005. [DOI] [PubMed] [Google Scholar]
- 21.Lian B.J., Stein G.S., Canalis E., Robey P.G., Boskey A.L. Bone Formation: Osteoblast Lineage Cells, Growth Factors, Matrix Proteins and the Mineralization Process. In: Favus M.J., editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Lippincott Williams & Williams; Philadelphia, PA, USA: 1999. p. 461. [Google Scholar]
- 22.Tong A.L., Chen L.L., Ding G.Z. Progress of mechanism of osteoblastic bone formation. Chin. J. Osteoporos. 1999;5:60–64. [Google Scholar]
- 23.Lee N.K., Sowa H., Hinoi E., Ferron M., Ahn J.D., Confavreux C., Dacquin R., Mee P.J., McKee M.D., Jung D.Y., et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakano S., Murata Y., Miura T. Collagen and alkaline phosphatase gene expression during bone morphogenetic protein (BMP)-induced cartilage and bone differentiation. Clin. Orthop. Relat. Res. 1993;292:337–344. [PubMed] [Google Scholar]
- 25.Riggs B.L., Melton L.J. The prevention and treatment of osteoporosis. N. Engl. J. Med. 1992;327:620–627. doi: 10.1056/NEJM199208273270908. [DOI] [PubMed] [Google Scholar]
- 26.Nohl H. Involvement of free radicals in ageing: A consequence or cause of senescence. Br. Med. Bull. 1993;49:653–657. doi: 10.1093/oxfordjournals.bmb.a072638. [DOI] [PubMed] [Google Scholar]
- 27.Basu K., Michaëlsson H., Olofsson H., Johansson S., Melhus H. Association between oxidative stress and bone mineral density. Biochem. Biophys. Res. Commun. 2001;288:275–279. doi: 10.1006/bbrc.2001.5747. [DOI] [PubMed] [Google Scholar]
- 28.Muthusami S., Ramachandran I., Muthusamy B., Vasudevan G., Prabhu V., Subramaniam V., Jagadeesan A., Narasimhan S. Ovariectomy induces oxidative stress and impairs bone antioxidant system in adult rats. Clin. Chim. Acta. 2005;360:81–86. doi: 10.1016/j.cccn.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Isomura H., Fujie K., Shibata K., Inoue N., Iizuka T., Takebe G., Takahashi K., Nishihira J., Izumi H., Sakamoto W. Bone metabolism and oxidative stress in postmenopausal rats with iron overload. Toxicology. 2004;197:93–100. doi: 10.1016/j.tox.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Yalin S., Bagis S., Aksit S.C., Arslan H., Erdogan C. Effect of free radicals and antioxidants on postmenopausal osteoporosis. Asian J. Chem. 2006;18:1091–1096. [Google Scholar]
- 31.Zhang D.W., Cheng Y., Wang N.L., Zhang J.C., Yang M.S., Yao X.S. Effects of total flavonoids and flavonol glycosides from Epimedium koreanum Nakai on the proliferation and differentiation of primary osteoblasts. Phytomedicine. 2008;15:55–61. doi: 10.1016/j.phymed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Zeng X.B., Su Y.J., Zheng Y.Y., Cui L. Osteogenic effects of the flavanes from green tea polyphenols. Acta Pharmacol. Sin. 2013;S2:34. [Google Scholar]
- 33.Declercq H., Vreken N.V., Maeyer E.D., Verbeeck R., Schacht E., Ridder L.D., Cornelissen M. Isolation, proliferation and differentiation of osteoblastic cells to study cell/biomaterial interactions: Comparison of different isolation techniques and source. Biomaterials. 2004;25:757–768. doi: 10.1016/S0142-9612(03)00580-5. [DOI] [PubMed] [Google Scholar]
- 34.He X.J., Liu R.H. Triterpenoids isolated from apple peels maybe responsible for their anticancer activity. J. Agric. Food Chem. 2007;55:4366–4370. doi: 10.1021/jf063563o. [DOI] [PubMed] [Google Scholar]
- 35.Rao L.G., Liu L.J., Murray T.M., McDermott E., Zhang X. Estrogen added intermittently but not continuously, stimulates differentiation and bone formation in SaOS-2 cells. Biol. Pharm. Bull. 2003;26:936–945. doi: 10.1248/bpb.26.936. [DOI] [PubMed] [Google Scholar]
- 36.Hale L.V., Ma Y.F., Santerre R.F. Semi-quantitative fluorescence analysis of calcein binding as a measurement of in vitro mineralization. Calcif. Tissue Int. 2000;67:80–84. doi: 10.1007/s00223001101. [DOI] [PubMed] [Google Scholar]
- 37.Yen G.C., Chen H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food. Chem. 1995;43:27–32. doi: 10.1021/jf00049a007. [DOI] [Google Scholar]
- 38.Manolagas S.C. De-fense! De-fense! De-fense: Scavenging H2O2 while making cholesterol. Endocrinology. 2008;149:3264–3266. doi: 10.1210/en.2008-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garrett J.R., Boyce B.F., Oreffo R.O., Bonewald L., Poser J., Mundy G.R. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J. Clin. Invest. 1990;85:632–639. doi: 10.1172/JCI114485. [DOI] [PMC free article] [PubMed] [Google Scholar]