Abstract
Astragalus L., is one of the largest genuses of flowering plants in the Leguminosae family. Roots of A. membranaceus Bge. var. mongholicus (Bge.) Hsiao, A. membranaceus (Fisch.) Bge. and its processed products are listed in the China Pharmacopeia for “qi deficiency” syndrome treatment. However, more and more researches on other species of Astragalus have been conducted recently. We summarize the recent researches of Astragalus species in phytochemistry and pharmacology. More than 200 constituents, including saponins and flavonoids, obtained from 46 species of Astragalus genus were collected for this article. In pharmacological studies, crude extracts of Astragalus, as well as isolated constituents showed anti-inflammatory, immunostimulant, antioxidative, anti-cancer, antidiabetic, cardioprotective, hepatoprotective, and antiviral activities. The goal of this article is to provide an overview of chemical and pharmacological studies on the Astragalus species over the last 10 years, which could be of value to new drug or food supplement research and development.
Keywords: traditional Chinese medicines, Astragalus genus, phytochemistry, biological activities, analyses
1. Introdution
Astragalus L., is one of the largest genuses of flowering plants in the Leguminosae family. As annual or perennial herbs, subshrubs, or shrubs, the plants of Astragalus L. are widely distributed throughout the temperate and arid regions. So far, the genus has been estimated to contain 2000–3000 species and more than 250 taxonomic sections in the world [1,2,3].
Some species of Astragalus in Asia are a source of the economically important natural product, gum tragacanth. In addition, the dried roots of some species grown in East Asia are well used in Traditional Chinese Medicines (TCM) as antiperspirants, diuretics, and tonics for a wide array of diseases such as empyrosis, nephritis, diabetes mellitus, hypertension, cirrhosis, leukaemia, and uterine cancer [4,5]. For example, the root of A. membranceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao (Radix Astragali) is a precious medicine in TCM, which has the properties of intensifying phagocytosis of reticuloendothelial systems, stimulating pituitary-adrenal cortical activity, and restoring depleted red blood cell formation in bone marrow. Also, it is famed for its antimicrobial, antiperspirant, anti-inflammatory, diuretic and tonic effects [6]. Some plants in the Astragalus genus are well known for their pharmacological properties, particularly hepatoprotective, immunostimulant, and antiviral activities [7]. While, the most common use of this genus is as forage for livestock and wild animals, some plants in this genus have been recognized as being used in foods, medicines, cosmetics, as substitutes for tea or coffee, or as sources of vegetable gums.
Saponins, flavonoids, and polysaccharides are believed to be the principle active constituents of Astragalus [8]. It also includes components such as anthraquinones, alkaloids, amino acids, β-sitosterol, and metallic elements.
Here, we have undertaken this review in an effort to summarize the available literatures on these promising bioactive natural products. The review focuses on the phytochemistry, biological activities, and analysis of the Astragalus genus.
2. Phytochemistry
As the summarized results shown in Table 1, Table 2 and Table 3 and Figure 1, Figure 2, Figure 3 and Figure 4, 46 kinds of Astragalus species have been studied for their chemical constituents in recent years [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79]. Also there have been more than 200 constituents obtained from them. Though the studies were for different species, the chemical compositions in Astragalus genus appeared highly uniform, which mainly include terpenoids, flavonoids, and polysaccharides. The interesting compounds, such as terpenoids and flavonoids are always in free or glycosidic forms. Meanwhile, we found that about 40 percent of the composition researches were focused on the aerial parts of Astragalus.
Table 1.
Cycloartane-type triterpenoids from the Astragalus genus (1–142).
| Compound’s Name | Species Resource | Parts Used | Reference | ||||
|---|---|---|---|---|---|---|---|
| 1 | 3-O-[β-d-Xylopyranosyl(1→2)-β-d-xylopyranosyl]-6-O-β-d-glucuronopyranosyl-3β,6α,16β,24(S),25-pentahydroxyxyxloartane | A. erinaceus | whole plant | [9] | |||
| 2 | Hareftoside A | A. erinaceus | whole plant | [9] | |||
| 3 | Hareftoside B | A. hareftae | whole plant | [10] | |||
| 4 | Cycloquivinoside A | A. chivensis | aerial parts | [11] | |||
| 5 | Astramembranosides B | A. membranaceus | roots | [12] | |||
| 6 | 3-O-[α-l-Rhamnopyranosyl(1→2)-β-d-xylopyranosyl]-6-O-β-d-glucopyranosyl-24-O-α-(4'-O-acetoxy)-l-arabinopyranosyl-16-O-acetoxy-3β,6α,16β,24S,25-pentahydroxycycloartane | A. wiedemannianus | whole plant | [13] | |||
| 7 | 3-O-[α-l-Rhamnopyranosyl(1→2)-β-d-xylopyranosyl]-6-O-β-d-glucopyranosyl-24-O-α-l-arabinopyranosyl-16-O-acetoxy-3β,6α,16β,24(S),25-pentahydroxycycloartane | A. wiedemannianus | whole plant | [13] | |||
| 8 | Cyclocanthogenin | A. unifoliolatus | epigeal parts | [14] | |||
| 9 | 3-O-β-d-Xylopyraosyl-24(S)-cycloart-3β,6α,16β,24,25-pentaol-25-O-β-d-glucopyranoside | A. ernestii | roots | [16] | |||
| 10 | Cyclocanthoside E | A. hareftae | whole plant | [10] | |||
| 11 | Cyclochivinoside B | A. chivensis | aerial parts | [15] | |||
| 12 | Cyclochivinoside C | A. chivensis | aerial parts | [20] | |||
| 13 | Caspicuside I | A. caspicus | roots | [6] | |||
| 14 | Oleifoliosides A | A. oleifolius | lower stem parts | [18] | |||
| 15 | Oleifoliosides B | A. oleifolius | lower stem parts | [18] | |||
| 16 | 3-O-[α-l-Rhamnopyranosyl(1→2)-α-l-arabinopyranosyl(1→2)-β-d-xylopyranosyl]-6-O-β-d-xylopyranosyl-3β,6α,16β,24(S),25-pentahydroxycycloartane | A. aureus | whole plant | [21] | |||
| 17 | 3,6-di-O-β-d-Xylopyranosyl-25-O-β-d-glucopyranosyl-3β,6α,16β,24(S),25-pentahydr-oxycycloartane | A. aureus | whole plant | [21] | |||
| 18 | 3-O-β-d-Xylopyranosyl-6,25-di-O-β-d-glucopyranosyl-3β,6α,16β,24(S),25-pentahydroxycycloartane | A. aureus | whole plant | [21] | |||
| 19 | 6-O-β-d-Glucopyranosyl-3β,6α,16β,24(S),25-pentahydroxycycloartane | A. aureus | whole plant | [21] | |||
| 20 | 3-O-[α-l-Arabinopyranosyl(1→2)-O-3-acetoxy-α-l-arabinopyranosyl]-6-O-β-d-glucopyranosyl-3β,6α,16β,24(S),25-pentahydroxycycloartane | A. icmadophilus | whole plant | [22] | |||
| 21 | 3-O-[α-l-Rhamnopyranosyl(1→2)-O-α-l-arabinopyranosyl(1→2)-O-β-d-xylopyranosyl]-6-O-β-d-glucopyranosyl-3β,6α,16β,24(S),25-pentahydroxycycloartane | A. icmadophilus | whole plant | [22] | |||
| 22 | 3-O-[α-l-Arabinopyranosyl(1→2)-O-3,4-diacetoxy-α-l-arabinopyranosyl]-6-O-β-d-glucopyranosyl-3β,6α,16β,24(S),25-pentahydroxycycloartane | A. icmadophilus | whole plant | [22] | |||
| 23 | 3-O-β-d-Xylopyranosyl-25-O-β-d-glucopyranosyl-3β,6α,16β,24(S),25-pentahydroxycycloartane | A. ernestii | roots | [16] | |||
| 24 | 3-O-β-d-Xylopyranosyl-16-O-β-d-glucopyranosyl-3β,6α,16β,24(S),25-pentahydroxycycloartane | A. amblolepis | roots | [17] | |||
| 25 | 3-O-[β-d-Glucuronopyranosyl(1→2)-β-d-xylopyranosyl]-25-O-β-d-glucopyranosyl-3β,6α,16β,24(S),25-pentahydroxy-cycloartane | A. amblolepis | roots | [17] | |||
| 26 | 3-O-β-d-Xylopyranosyl-24,25-di-O-β-d-glucopyranosyl-3β,6α,16β,24(S),25-pentahydr-oxy-cycloartane | A. amblolepis | roots | [17] | |||
| 27 | 6-O-α-l-Rhamnopyranosyl-16,24-di-O-β-d-glucopyranosyl-3β,6α,16β,24(S),25-pentahydroxy cycloartane | A. amblolepis | roots | [17] | |||
| 28 | 6-O-α-l-Rhamnopyranosyl-16,25-di-O-β-d-glucopyranosyl-3β,6α,16β,24(S),25-pentahydroxy cycloartane | A. amblolepis | roots | [17] | |||
| 29 | Cicerosides A | A. cicer | aerial parts | [7] | |||
| 30 | Cicerosides B | A. cicer | aerial parts | [7] | |||
| 31 | Cycloascidoside | A. ernestii | roots | [16] | |||
| 32 | Eremophiloside A | A. eremophilus | aerial parts | [24] | |||
| 33 | Eremophiloside B | A. eremophilus | aerial parts | [24] | |||
| 34 | Cycloascidoside A | A. mucidus | aerial parts | [25] | |||
| 35 | Cyclounifoliside C | A. unifoliolatus | epigeal parts | [14] | |||
| 36 | 3-O-[α-l-Arabinopyranosyl(1→2)-β-d-glucopyranosyl]-24-O-β-d-glucopyranosyl-3β,6α,16β,24(R),25-pentahydroxycycloartane | A. stereocalyx | roots | [26] | |||
| 37 | 3-O-[α-l-Arabinopyranosyl(1→2)-β-d-glucopyranosyl]-16-O-β-d-glucopyranosyl-3β,6α,16β,24(R),25-pentahydroxycycloartane | A. stereocalyx | roots | [26] | |||
| 38 | 3-O-{α-l-Rhamnopyranosyl(1→4)-[α-l-arabinopyranosyl(1→2)]-β-d-glucopyranosyl}-3β,6α,16β,24(R),25-pentahydroxycycloartane | A. stereocalyx | roots | [26] | |||
| 39 | 3-O-[α-l-Arabinopyranosyl(1→2)-β-d-xylopyranosyl]-16-O-β-d-glucopyranosyl-3β,6α,16β,20(S),24(R),25-hexahydroxycycloartane | A. stereocalyx | roots | [26] | |||
| 40 | 3-O-[α-l-Arabinopyranosyl(1→2)-β-d-xylopyranosyl]-3β,6α,16β,20(S),24(R),25-hexahydroxycycloartane | A. stereocalyx | roots | [26] | |||
| 41 | 3-O-[α-l-Arabinopyranosyl(1→2)-β-d-glucopyranosyl]-3β,6α,16β,20(S),24(R),25-hexahdroxycycloartane | A. stereocalyx | roots | [26] | |||
| 42 | 3-O-β-d-Xylopyranosyl-3β,6α,16β,20(S),24(R),25-hexahydroxycycloartane | A. schottianus | roots | [29] | |||
| 43 | Cyclomacrogenin B | A. macropus | roots | [30,31] | |||
| 44 | Cyclomacroside E | A. macropus | roots | [32] | |||
| 45 | Cyclomacroside B | A. macropus | roots | [33] | |||
| 46 | Cyclomacroside D | A. macropus | roots | [31] | |||
| 47 | Mongholicoside A | A. membranace (Fisch.) Bge. var. mongholicus (Bge.) | aerial parts | [34] | |||
| 48 | Mongholicoside B | A. membranace (Fisch.) Bge. var. mongholicus (Bge.) | aerial parts | [34] | |||
| 49 | Askendoside K | A. taschkendicus | roots | [35] | |||
| 50 | Askendoside H | A. taschkendicus | roots | [36] | |||
| 51 | Cycloorbicoside D | A. orbiculatus | aerial parts | [37,38] | |||
| 52 | Cycloorbigenin C | A. taschkendicus | roots | [35,36] | |||
| A. orbiculatus | aerial parts | [37,38] | |||||
| 53 | Eremophiloside C | A. eremophilus | aerial parts | [24] | |||
| 54 | Eremophiloside D | A. eremophilus | aerial parts | [24] | |||
| 55 | Bicusposide F | A. bicuspis | whole plant | [39] | |||
| 56 | Bicusposide E | A. bicuspis | whole plant | [39] | |||
| 57 | Kahiricoside II | A. kahiricus | aerial parts | [40] | |||
| 58 | Kahiricoside III | A. kahiricus | aerial parts | [40] | |||
| 59 | Kahiricoside IV | A. kahiricus | aerial parts | [40] | |||
| 60 | Kahiricoside V | A. kahiricus | aerial parts | [40] | |||
| 61 | Secomacrogenin B | A. macropus | roots | [41] | |||
| 62 | Orbigenin | A. orbiculatus | aerial parts | [37,38] | |||
| 63 | Orbicoside | A. orbiculatus | aerial parts | [37,38] | |||
| 64 | 16-O-β-d-Glucopyranosyl-3β,6α,16β,25-tetrahydroxy-20(R),24(S)-epoxycycloartane | A. hareftae | whole plant | [10] | |||
| 65 | Astramembranosides A | A. membranaceus | roots | [12] | |||
| 66 | Cyclosiversioside F | A. oldenburgii | aerial parts | [42] | |||
| 67 | Astraverrucin IV | A. oldenburgii | aerial parts | [42] | |||
| 68 | Astragaloside VII | A. oldenburgii | aerial parts | [42] | |||
| 69 | 3-O-[α-l-Rhamnopyranosyl(1→2)-β-d-glucopyranosyl]-16-O-hydroxyacetoxy-3β,6α,16β,25-tetrahydroxy-20(R),24(S)-epoxycycloartane | A. angustifolius | whole plant | [45] | |||
| 70 | CyclolehmanosideC | A. lehmannianus | aerial parts | [46] | |||
| 71 | Armatoside II | A. armatus | roots | [47] | |||
| 72 | Acetylastragaloside I | A. baibutensis | roots | [48] | |||
| 73 | Astragaloside III | A. illyricus | roots | [49] | |||
| 74 | Cyclounifolioside B | A. illyricus | roots | [49] | |||
| 75 | Astraverrucin I | A. illyricus | roots | [49] | |||
| 76 | Trigonoside II | A. armatus | roots | [47] | |||
| 77 | Trojanoside H | A. stereocalyx | roots | [26] | |||
| 78 | Armatoside I | A. armatus | roots | [47] | |||
| 79 | Cyclosieversioside A | A. sieversianus | roots | [51] | |||
| 80 | Cyclosieversioside G | A. sieversianus | roots | [51] | |||
| 81 | Cyclosieversioside H | A. sieversianus | roots | [51] | |||
| 82 | 3-O-[α-l-Rhamnopyranosyl(1→2)-β-d-glucopyranosyl]-25-O-β-d-glucopyranosyl-20(R),24(S)-epoxy-3β,6α,16β,25-tetrahydroxycycloartane | A. wiedemannianus | whole plant | [13] | |||
| 83 | Cyclosiversigenin | A. orbiculatus | aerial parts | [52] | |||
| 84 | Brachyoside B | A. wiedemannianus | whole plant | [13] | |||
| 85 | Astragaloside II |
A. hareftae A. wiedemannianus |
whole plant whole plant |
[10] [13] |
|||
| 86 | Astrasieversianin X | A. wiedemannianus | whole plant | [13] | |||
| 87 | Astrasieversianin IX | A. sieversianus | roots | [51] | |||
| 88 | Caspicuside II | A. caspicus | roots | [6] | |||
| 89 | Baibutoside | A. baibutensis | roots | [48] | |||
| 90 | Astragalosides I | A. baibutensis | roots | [48] | |||
| 91 | Astraverrucin VII | A. verrucosus | aerial parts | [53] | |||
| 92 | Cycloaraloside D (Peregrinoside II) | A. verrucosus | aerial parts | [53] | |||
| 93 | Cycloaraloside C (Astrailienin A) | A. verrucosus | aerial parts | [53] | |||
| 94 | (20R,24S)-3-O-[α-l-Arabinopyranosyl(1→2)-β-d-xylopyranosyl]-20,24-epoxy-16-O-β-d-glucopyranosyl-3β,6α,16β,25-tetrahydroxycycloartane | A. halicacabus | whole plant | [27] | |||
| 95 | 3-O-[α-l-Arabinopyranosyl(1→2)-β-d-xylopyranosyl]-25-O-β-d-glucopyranosyl-3β,6α,16β,25-tetrahydroxy-20(R),24(S)-epoxycycloartane | A. campylosema Boiss. subsp. campylosema | roots | [28] | |||
| 96 | 3-O-[α-l-Arabinopyranosyl(1→2)-O-3-acetoxy-α-l-arabinopyranosyl]-6-O-β-d-glucopyranosyl-3β,6α,16β,25-tetrahydroxy-20(R),24(S)-epoxycycloartane | A. icmadophilus | whole plant | [22] | |||
| 97 | 20(R),24(S)-Epoxycycloartane-3β,6α,16β,25-tetraol-3-β-O-d-(2-O-acetyl)-xylopyranoside | A. bicuspis | whole plant | [39] | |||
| 98 | 3-O-[α-l-Rhamnopyranosyl(1→2)-β-d-glucopyranosyl]-16-O-hydroxyacetoxy-3β,6α,16β,23α,25-pentahydroxy-20(R),24(S)-epoxycycloartane | A. angustifolius | whole plant | [45] | |||
| 99 | 3-O-[α-l-Rhamnopyranosyl(1→2)-β-d-glucopyranosyl]-3β,6α,25-trihydroxy-20(R),24(S)-epoxycycloartane-16-one | A. angustifolius | whole plant | [45] | |||
| 100 | 3-O-[α-l-Arabinopyranosyl(1→2)-β-d-xylopyranosyl]-3β,6α,16β,23α,25-pentahydroxy-20(R),24(S)-epoxycycloartane | A. campylosema Boiss. subsp. campylosema | roots | [28] | |||
| 101 | 3-O-[α-l-Arabinopyranosyl(1→2)-β-d-xylopyranosyl]-16-O-hydroxyacetoxy-23-O-acetoxy-3β,6α,16β,23α,25-pentahydroxy-20(R),24(S)-epoxycycloartane | A. campylosema Boiss. subsp. campylosema | roots | [28] | |||
| 102 | Cyclogaleginoside E | A. galegiformis | stems | [54] | |||
| 103 | Cycloascualoside D | A. galegiformis | stems | [55] | |||
| 104 | Cyclogaleginoside C | A. galegiformis | stems | [55] | |||
| 105 | Cyclogalegigenin | A. galegiformis | stems | [54,55,57] | |||
| 106 | Cycloascauloside A | A. caucasicus | leaves | [56] | |||
| 107 | Cyclogaleginoside D | A. galegiformis | stems | [57] | |||
| 108 | 20(R),25-Epoxy-3-O-β-d-xylopyranosyl-24-O-β-d-glucopyranosyl-3β,6α,16β,24α-tetrahydroxycycloartane | A. schottianus | roots | [29] | |||
| 109 | 20(R),25-Epoxy-3-O-[-β-d-glucopyranosyl(1→2)]-β-d-xylopyranosyl-24-O-β-d-glucopyranosyl-3-β,6α,16β,24α-tetrahydroxycycloartane | A. schottianus | roots | [29] | |||
| 110 | Hareftoside C | A. hareftae | whole plant | [10] | |||
| 111 | Cylotrisectoside | A. dissectus | roots and stems | [43] | |||
| 112 | 3-O-[α-l-Arabinopyranosyl(1→2)-β-d-xylopyranosyl]-3β,6α,16β,24α-tetrahydroxy-20(R),25-epoxycycloartane | A. aureus | whole plant | [21] | |||
| 113 | 6-O-β-d-Glucopyranosyl-3β,6α,16β,24α-tetrahydroxy-20(R),25-epoxycycloartane | A. aureus | whole plant | [21] | |||
| 114 | 6-O-β-d-Xylopyranosyl-3β,6α,16β,24α-tetrahydroxy-20(R),25-epoxycycloartane | A. aureus | whole plant | [21] | |||
| 115 | 3-O-[α-l-Arabinopyranosyl(1→2)-O-β-d-xylopyranosyl]-6-O-β-d-glucopyranosyl-3β,6α,16β,24α-tetrahydroxy-20(R),25-epoxycycloartane | A. icmadophilus | whole plant | [22] | |||
| 116 | 3-O-[α-l-Rhamnopyranosyl(1→2)-O-α-l-arabinopyranosyl(1→2)-O-β-d-xylopyranosyl]-6-O-β-d-glucopyranosyl-3β,6α,16β,24α-tetrahydroxy-20(R),25-epoxycycloartane | A. icmadophilus | whole plant | [22] | |||
| 117 | Eremophiloside G | A. eremophilus | aerial parts | [24] | |||
| 118 | Eremophiloside E | A. eremophilus | aerial parts | [24] | |||
| 119 | Eremophiloside F | A. eremophilus | aerial parts | [24] | |||
| 120 | Eremophiloside H | A. eremophilus | aerial parts | [24] | |||
| 121 | Eremophiloside I | A. eremophilus | aerial parts | [24] | |||
| 122 | Eremophiloside J | A. eremophilus | aerial parts | [24] | |||
| 123 | Eremophiloside K | A. eremophilus | aerial parts | [24] | |||
| 124 | Cyclomacroside A | A. macropus | roots | [58] | |||
| 125 | Bicusposide D | A. bicuspis | whole plant | [39] | |||
| 126 | 3-O-[α-l-Arabinopyranosyl(1→2)-β-d-xylopyranosyl]-3β,6α,23α,25-tetrahydroxy-20(R),24(R)-16β,24;20,24-diepoxycycloartane | A. campylosema Boiss. subsp. campylosema | roots | [28] | |||
| 127 | Dihydrocycloorbigenin A | A. orbiculatus | aerial parts | [38] | |||
| 128 | Cycloorbigenin | A. orbiculatus | aerial parts | [38] | |||
| 129 | Cycloorbigenin B | A. orbiculatus | aerial parts | [38] | |||
| 130 | Cycloorbicoside A | A. orbiculatus | aerial parts | [38] | |||
| 131 | Cycloorbicoside B | A. orbiculatus | aerial parts | [38] | |||
| 132 | Cycloorbicoside C | A. orbiculatus | aerial parts | [38] | |||
| 133 | Cycloorbicoside G | A. orbiculatus | aerial parts | [38] | |||
| 134 | Tomentoside I | A. tomentosus | aerial parts | [59] | |||
| 135 | Deacetyltomentoside I | A. tomentosus | aerial parts | [59] | |||
| 136 | Tomentoside III | A. tomentosus | aerial parts | [59] | |||
| 137 | Tomentoside IV | A. tomentosus | aerial parts | [59] | |||
| 138 | Huangqiyenin E | A. membranaceus | leaves | [60] | |||
| 139 | Huangqiyenin F | A. membranaceus | leaves | [60] | |||
| 140 | Huangqiyegenin III | A. membranaceus | leaves | [60] | |||
| 141 | Huangqiyegenin IV | A. membranaceus | leaves | [60] | |||
| 142 | Trideacetylhuangqiyegenin III | A. membranaceus | leaves | [60] | |||
Table 2.
Oleanane triterpenoids from the Astragalus genus (143–161).
| Compound’s Name | Species Resource | Parts Used | Reference | |
|---|---|---|---|---|
| 143 | 3-O-[α-l-Rhamnopyranosyl(1→2)-β-d-xylopyranosyl(1→2)-β-d-glucuronopyranosyl]-21-O-α-l-rhamnopyranosyl-3β,21β,22α,24-tetrahydroxyolean-12-ene | A. tauricolus | whole plant | [61] |
| 144 | 3-O-[α-l-Rhamnopyranosyl(1→2)-β-d-glucopyranosyl(1→2)-β-d-glucuronopyranosyl]-21-O-α-l-rhamnopyranosyl-3β,21β,22α,24-tetrahydroxyolean-12-ene | A. tauricolus | whole plant | [61] |
| 145 | 3-O-[α-l-Rhamnopyranosyl(1→2)-β-d-glucopyranosyl(1→2)-β-d-glucuronopyranosyl]-3β,21β,22α,24,29-pentahydroxyolean-12-ene | A. tauricolus | whole plant | [61] |
| 146 | 3-O-[α-l-Rhamnopyranosyl(1→2)-β-d-xylopyranosyl(1→2)-β-d-glucuronopyranosyl]-22-O-α-l-rhamnopyranosyl-3β,22β,24-trihydroxyolean-12-ene | A. tauricolus | whole plant | [61] |
| 147 | 3-O-[α-l-Rhamnopyranosyl(1→2)-β-d-xylopyranosyl(1→2)-β-d-glucuronopyranosyl]-3β,21β,22α,24,29-pentahydroxyolean-12-ene | A. angustifolius | whole plant | [45] |
| 148 | 3-O-[α-l-Rhamnopyranosyl(1→2)-β-d-xylopyranosyl(1→2)-β-d-glucuronopyranosyl]-3β,22β,24-trihydroxyolean-12-en-29-oic acid | A. angustifolius | whole plant | [45] |
| 149 | 3-O-[α-l-Rhamnopyranosyl(1→2)-β-d-xylopyranosyl(1→2)-β-d-glucuronopyranosyl]-22-O-α-l-arabinopyranosyl-3β,22β,24-trihydroxyolean-12-ene | A. angustifolius | whole plant | [45] |
| 150 | 29- O-β-d-Glucopyranosyl-3β,22β,24,29-tetrahydroxy-olean-12-ene | A. angustifolius | whole plant | [45] |
| 151 | Soyasapogenol B | A. caprinus | roots | [62] |
| 152 | 3-O-[β-d-Xylopyranosyl(1→2)-O-β-d-glucopyranosyl(1→2)-O-β-d-glucuronopyranosyl] soyasapogenol B | A. hareftae | whole plant | [10] |
| 153 | 3-O-α-l-Rhamnopyranosyl(1→2)-β-d-glucuronopyranosyl]-22-O-β-d-apiofuranosyl soyasapogenol B | A. caprinus | roots | [62] |
| 154 | 3-O-[α-l-Rhamnopyranosyl(1→2)-β-d-xylopyranosyl(1→2)-β-d-glucuronopyranosyl]-29-O-β-d-glucopyranosyl-3β,22β,24-trihydroxyolean-12-en-29-oic acid | A. tauricolus | whole plant | [61] |
| 155 | 3-O-[α-l-Rhamnopyranosyl(1→2)-β-d-glucopyranosyl(1→2)-β-d-glucuronopyranosyl]-29-O-β-d-glucopyranosyl-3β,22β,24,-trihydroxyolean-12-en-29-oic acid | A. tauricolus | whole plant | [61] |
| 156 | 3-O-[β-d-Xylopyranosyl(1→2)-β-d-glucuronopyranosyl]-29-O-β-d-glucopyranosyl-3β,22β,24,-trihydroxyolean-12-en-29-oic acid | A. tauricolus | whole plant | [61] |
| 157 | 3-O-[α-l-Rhamnopyranosyl-(1→2)-β-d-glucopyranosyl-(1→2)-β-d-glucuronopyranosyl]-29-O-β-d-glucopyranosyl-3β,22β,24,29-tetrahydroxyolean-12-ene | A. tauricolus | whole plant | [61] |
| 158 | 3-O-[α-l-Rhamnopyranosyl-(1→2)-β-d-glucopyranosyl-(1→2)-β-d-glucuronopyranosyl]-3β,24-dihydroxyolean-12-ene-22-oxo-29-oic acid | A. tauricolus | whole plant | [61] |
| 159 | 3-O-[β-d-Glucopyranosyl-(1→2)-β-d-glucuronopyranosyl]-29-O-β-d-glucopyranosyl-3β,22β,24,-trihydroxyolean-12-en-29-oic acid | A. tauricolus | whole plant | [61] |
| 160 | Azukisaponin V | A. cruciatus | aerial parts and roots | [2] |
| 161 | Astragaloside VIII | A. flavescens | roots | [4] |
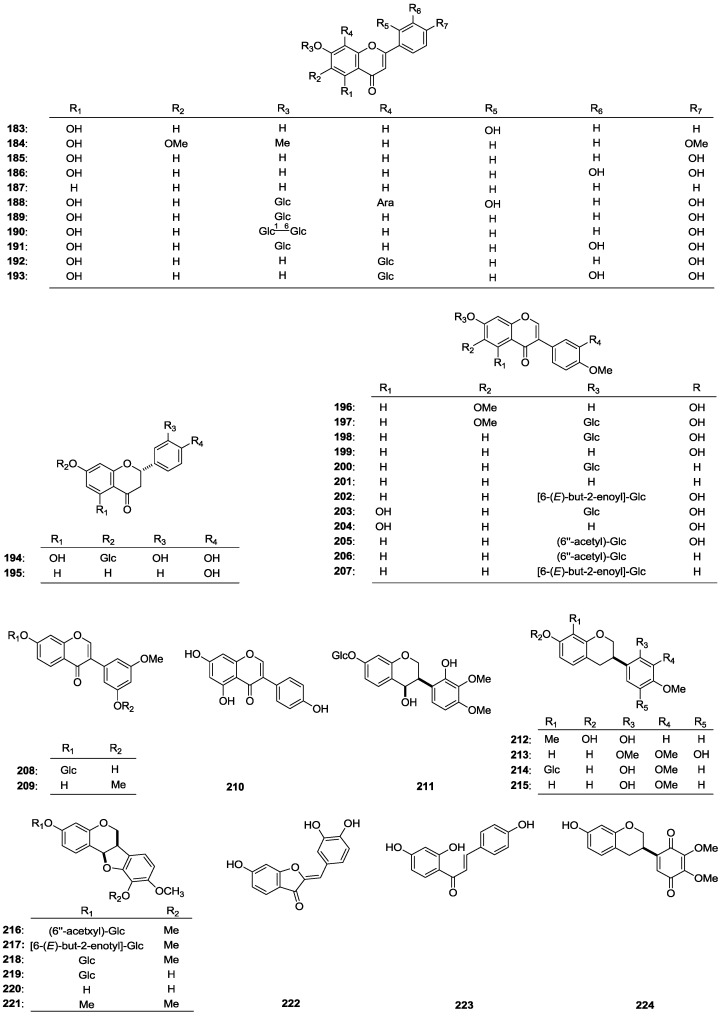
Table 3.
Flavonoids from the Astragalus genus (162–224).
| Compound’s Name | Species Resource | Parts Used | Reference | |
|---|---|---|---|---|
| 162 | Narcissin | A. cruciatus | aerial parts and roots | [2] |
| 163 | Nicotiflorin | A. cruciatus | aerial parts and roots | [2] |
| 164 | Kaempferol 3-O-α-l-rhamnopyranosyl(1→4)-α-l-rhamnopyranosyl(1→6)-β-d-glucopyranoside | A. cruciatus | aerial parts and roots | [2] |
| 165 | Microcephalin I | A. microcephalus | leaves | [65] |
| 166 | Microcephalin II | A. microcephalus | leaves | [65] |
| 167 | Kaempferol-3-O-α-l-rhamnoxyloside | A. microcephalus | leaves | [66] |
| 168 | Quercetin | A. asper | aerial parts | [64] |
| 169 | Quercimeritrin | A. asper | aerial parts | [64] |
| 170 | Rutin | A. cruciatus | aerial parts and roots | [2] |
| 171 | Quercetin-3-O-β-d-glucopyranoside | A. corniculatus | aerial parts | [63] |
| 172 | Kaempferol | A. corniculatus | aerial parts | [63] |
| 173 | Kaempferol-3-glucoside (Astragalin) | A. asper | aerial parts | [64] |
| 174 | Isorhamnetin | A. corniculatus | aerial parts | [63] |
| 175 | Quercetin-3-O-galactoside | A. corniculatus | aerial parts | [63] |
| 176 | Quercetin-3,7-di-β-d-glucopyranoside-4'-O-α-l-rhamnopyranoside | A. bombycinus | whole plant | [8] |
| 177 | Quercetin-3,7-di-O-β-d-glucopyranoside | A. bombycinus | whole plant | [8] |
| 178 | Quercetin 3-O-β-d-glucopyranoside-7-O-α-l-rhamnopyranoside | A. bombycinus | whole plant | [8] |
| 179 | Flagaloside C | A. galegiformis | leaves | [67] |
| 180 | Flagaloside D | A. galegiformis | leaves | [67] |
| 181 | Kaempferol 3-O-robinobioside | A. verrucosus | aerial parts | [53] |
| 182 | 7-O-Methyl-kaempferol-4'-β-d-galactopyranoside | A. hamosus | aerial parts | [68] |
| 183 | 5,7,2'-Trihydroxyflavone | A. cruciatus | aerial parts and roots | [2] |
| 184 | Salvigenin | A. propinquus | roots | [69] |
| 185 | Apigenin | A. bombycinus | whole plant | [8] |
| 186 | Luteolin | A. bombycinus | whole plant | [8] |
| 187 | 7-Hydroxyflavone | A. microcephalus | leaves | [66] |
| 188 | 5,2',4'-Trihydroxy-flavone-8-C-l-arabinopyranoside-7-O-β-d-glucopyranoside | A. bombycinus | whole plant | [8] |
| 189 | Apigenin 7-O-β-d-glucopyranoside | A. bombycinus | whole plant | [8] |
| 190 | Apigenin 7-O-gentobioside | A. bombycinus | whole plant | [8] |
| 191 | Luteolin 7-O-β-d-glucopyranoside | A. bombycinus | whole plant | [8] |
| 192 | Apigenin-8-C-glucoside (Vitexin) | A. corniculatus | aerial parts | [63] |
| 193 | Luteolin-8-C-glucoside (Orientin) | A. corniculatus | aerial parts | [63] |
| 194 | Eriodyctiol-7-O-glucoside | A. corniculatus | aerial parts | [63] |
| 195 | Liquiritigenin | A. membranaceus | roots | [70] |
| 196 | Odoration | A. membranaceus var. mongholicus | roots | [71] |
| 197 | Odoration-7-O-β-d-glucopyranoside | A. mongholicus | aerial parts | [72] |
| 198 | Calycosin-7-O-β-d-glucopyranoside | A. ernestii | roots | [16] |
| 199 | Calycosin | A. membranaceus | roots | [70] |
| 200 | Ononin | A. membracaceus | roots | [50] |
| 201 | Formononetin | A. membranaceus | roots | [70] |
| 202 | Calycosin 7-O-β-d-{6''-[(E)-but-2-enoyl]}-glucoside | A. membracaceus | roots | [50] |
| 203 | Pratensein 7-O-β-d-glucopyranoside | A. membranaceus var. mongholicus | roots | [71] |
| 204 | Pratensein | A. verrucosus | aerial parts | [53] |
| 205 | Calycosin 7-O-β-d-(6''-acetyl)-glucoside | A. membracaceus | roots | [50] |
| 206 | 6ꞌꞌ-Acetylononin | A. membracaceus | roots | [50] |
| 207 | Ammopiptanoside A | A. membracaceus | roots | [50] |
| 208 | 7,5'-Dihydroxy-3'-methoxy-isoflavone-7-O-β-d-glucopyranoside | A. membranaceus var. mongholicus | roots | [71] |
| 209 | 7-Hydroxy-3',5'-dimethoxyisoflavone | A. peregrinus | aerial parts | [75] |
| 210 | Daidzein | A. bombycinus | whole plant | [8] |
| 211 | (3R,4R)-3-(2-Hydroxy-3,4-dimethoxy-phenyl)-chroman-4,7-diol-7-O-β-d-glucopyranoside | A. membranaceus | roots | [74] |
| 212 | (3R)-8,2'-Dihydroxy-7,4'-dimethoxyisoflavane | A. membranaceus | roots | [76] |
| 213 | (R)-3-(5-Hydroxy-2,3,4-trimethoxyphenyl)-chroman-7-ol | A. membracaceus | roots | [50] |
| 214 | Isomucronulatol 7-O-β-glucoside | A. membracaceus | roots | [50] |
| 215 | Isomucronulatol | A. membracaceus | roots | [50] |
| 216 | (–)-Methylinissolin 3-O-β-d-(6'-acetyl)-glucoside | A. membracaceus | roots | [50] |
| 217 | (–)-Methylinissolin 3-O-β-d-{6'-[(E)-but-2-enoyl]}-glucoside | A. membracaceus | roots | [50] |
| 218 | (–)-Methylinissolin 3-O-β-d-glucoside | A. membracaceus | roots | [50] |
| 219 | Licoagroside D | A. membracaceus | roots | [50] |
| 220 | Vesticarpan | A. membracaceus | roots | [50] |
| 221 | (–)-Methylinissolin | A. membracaceus | roots | [50] |
| 222 | Sulfuretin | A. microcephalus | leaves | [66] |
| 223 | Isoliquiritigenin | A. membranaceus | roots | [70] |
| 224 | Pendulone | A. membracaceus | roots | [50] |
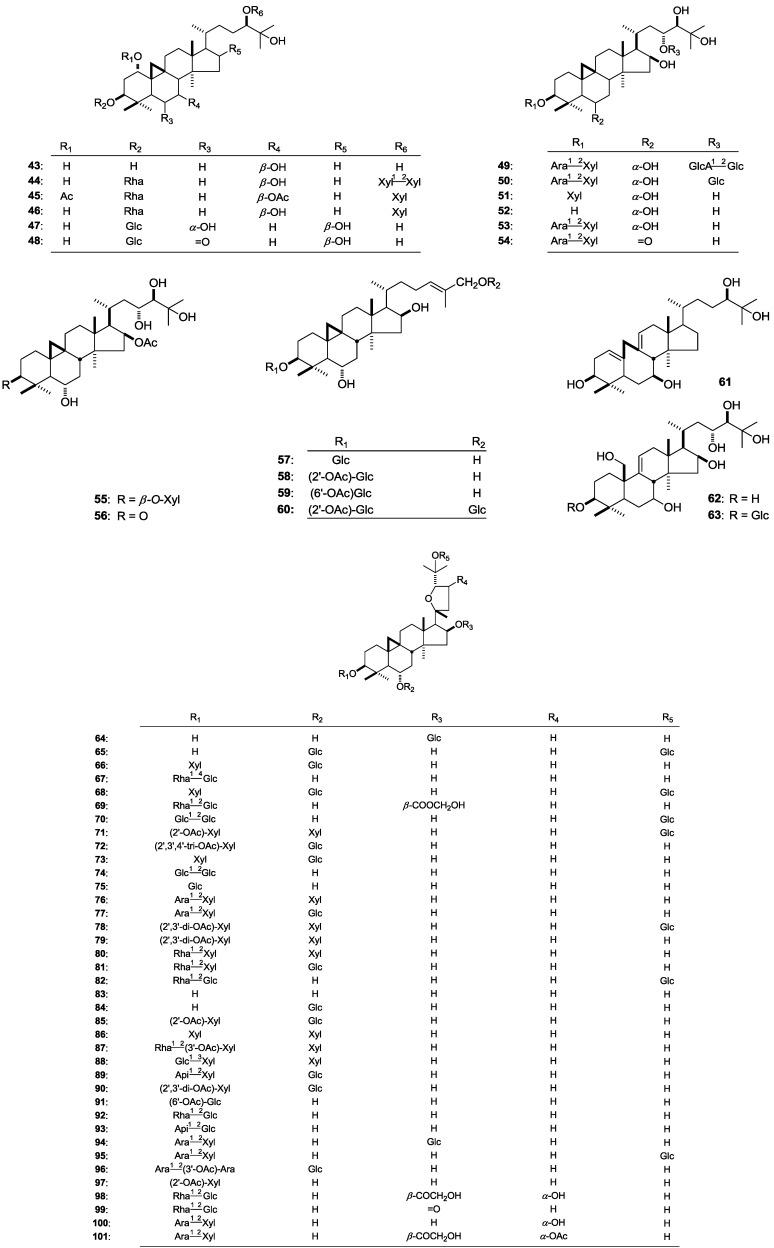
Figure 1.
The structures of compounds 1–142 obtained from the Astragalus genus.
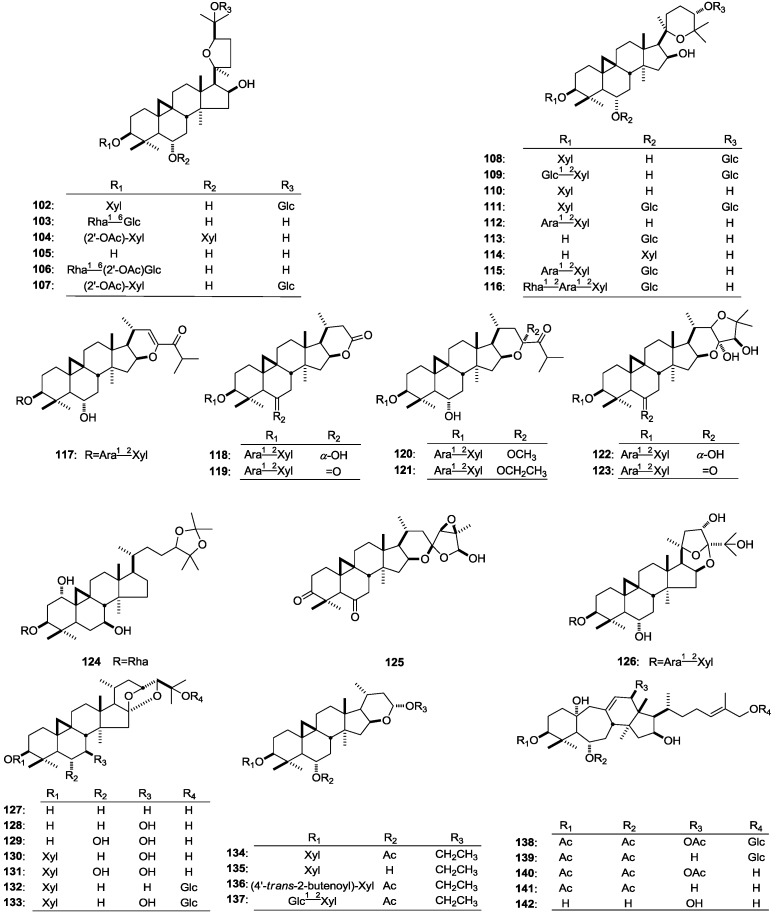
Figure 2.
The structures of compounds 143–161 obtained from the Astragalus genus.
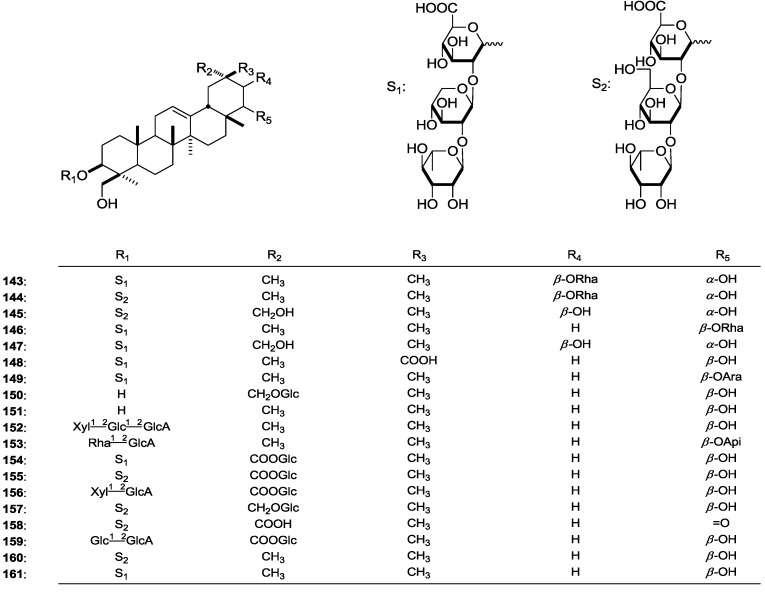
Figure 3.
The structures of compounds 162–224 obtained from the Astragalus genus.
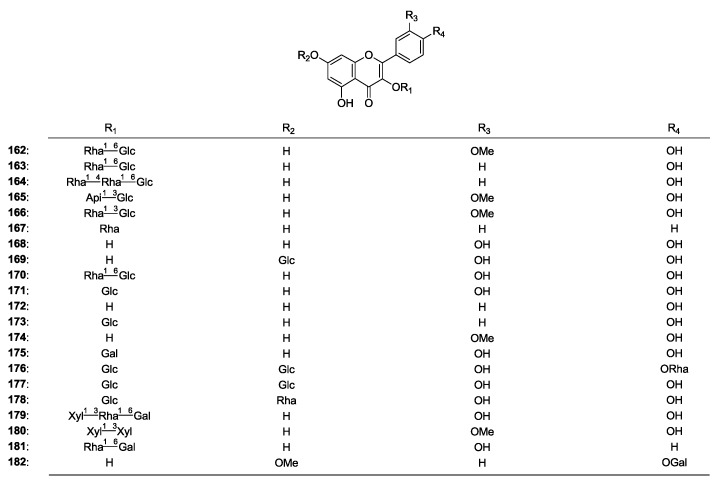
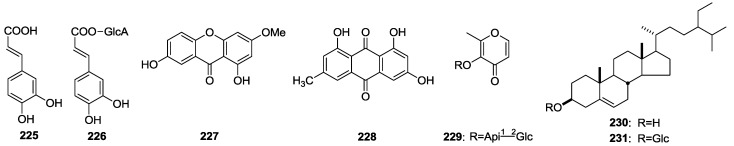
Figure 4.
The structures of compounds 225–231 obtained from the Astragalus genus.
2.1. Saponins
Saponin is the major chemical constituent type in the Astragalus genus. Cycloartane- and oleanane-type saponins from it showed interesting biological properties.
2.1.1. Cycloartane-Type Saponins
The plants of Astragalus genus have been proved to be the richest source of cycloartane-type saponins, possessing cardiotonic, hypocholesteremic, anti-depressive and antiblastic actions as well as immunomodulatory activity [7]. This promising spectrum of pharmacological effects led researchers to further search for structurally interesting cycloartane glycosides from the genus. Until now, more than 140 kinds of cycloartane-type saponins have been identified (Table 1, Figure 1). The main substituted sugar groups in them are β-d-glucopyranosyl (Glc), β-d-xylopyranosyl (Xyl), α-l-rhamnopyranosyl (Rha), or α-l-arabinopyranosyl (Ara). Additionally, β-d-glucuronopyranosyl (GlcA), β-d-fucopyranosyl (Fuc), β-d-apiofuranosyl (Api) and acetyl (Ac) groups were also found in cycloartane glycosides obtained from the Astragalus genus.
Commonly, the aglycon of cycloartane-type saponins such as 1–62 possess an acyclic side chain at the 17-position. For the 17-acyclic side chain substituted cycloartane-type saponins, obtained from the Astragalus genus, the 24-position is often replaced by oxygen containing groups, and there are two kinds of steric configuration constituents with 24S (1–28) [6,9,10,11,12,13,14,15,16,17,18,19,20,21,22] or 24R (29–48) [7,14,15,16,17,23,24,25,26,27,28,29,30,31,32,33,34].
If there are hydroxyls substituted in the 20- and 24-positions, it is easy to dehydrate and form a furan ring between them. So the 20,24-epoxycycloartane-type saponins like 64–107 are derived from secondary metabolic pathways. Also there are usually 20R, 24S (64–101) [6,10,12,13,22,26,27,28,39,42,43,44,45,46,47,48,49,50,51,52,53] or 20S,24R (102–107) [54,55,56,57] steric configurations.
Some uncommon cycloartane triterpene glycosides such as 108–142 were also isolated from the Astragalus genus. For example, the eremophilosides E–I (118–121) [24] are 16β,23-epoxycycloartanes. Among them, eremophilosides E (118) and F (119) are characterized as having an unusual loss of a four carbon side chain from C-24 to C-27 and a six-membered lactone ring between C-23 and C-16, while eremophilosides G–I (117, 120 and 121) show an unusual modification of the cyclic side chain. On the other hand, eremophilosides J (122) and K (123) are 16β,23; 22β,25-diepoxycycloartanes, which are highly oxygenated cycloartane triterpenes.
In the study of isoprenoid plants of the Astragalus genus like A. orbiculatus, researchers found several derivatives of 16β,23,16α,24-diepoxycycloartane (127–133) from its aerial parts [38], which have been found only in this species.
Additionally, some cycloartane-type saponin ethylacetals (134–142) were identified from the extracts of Astragalus genus [59,60].
2.1.2. Oleanane-Type Saponins
Apart from the cycloartane triterpene glycosides, many oleanane-type saponins shown in Table 2 were also isolated and identified from the Astragalus genus. Structure characterizations of this kind of saponin indicated they were substituted with a β-hydroxymethyl, instead of methyl in the 23-position.
2.2. Flavonoids
Just like many other herbs, Astragalus genus plants are also a rich source of flavonoids. The flavonoids in this genus include flavonols (162–182) [2,8,22,63,64,65,66,67,68], flavones (183–193) [2,8,53,63,66,69], flavonones (194–195) [65,70] and isoflavonoids (196–221) [16,50,53,65,66,70,71,72,73,74,75], which have many kinds of bioactivities.
In addition, some special flavonoids, such as sulfuretin (222) [65], isoliquiritigenin (223) [8], and pendulone (224) [50] have been obtained.
2.3. Polysaccharides
Yao et al., analyzed the monosaccharide compositions for the Radix Astragali polysaccharide by gas chromatography, and identified the monosaccharides in it as arabinose, fructose, glucose, and mannose. Their molar ratios calculated according to the equation was 1:10.309:24.667:0.462 [77]. Xu et al., isolated and purified two kinds of Astragalus polysaccharides (APS) (APS-I and APS-II) from the water extract of Radix Astragali. The research indicated that APS-I consisted of arabinose and glucose in the molar ratio of 1:3.45, with molecular weight 1,699,100 Da; meanwhile, APS-II consisted of rhamnose, arabinose and glucose in a molar ratio of 1:6.25:17.86 with molecular weight 1,197,600 Da [78].
2.4. Others
Undoubtedly, apart from the compounds mentioned above, others chemical constituents also exist in the Astragalus genus, including caffeic acid (225) [64], chlorogenic acid (226) [64], gentisin (227) [44], emodin (228) [44], 3-O-[β-d-apiofuranosyl(1→2)-O-β-d-glucopyranosyl] maltol (229) [27], β-sitosterol (230) [16,30,51,73] and β-sitosterol β-d-glycopyranoside (231) [51].
Certainly, some other constituents, such as amino acids and proteins have been obtained from Astragalus genus plants, which were also found to be rich in metal elements like Ca, Mg, Fe, Cu, Zn, and Mn [79].
3. Biological Activities of the Astragalus Genus
A. membranaceus, A. mongholicus and A. complanatus have been mainly used in folk medicine for their anti-inflammatory, immunostimulant, antioxidative, anti-cancer, antidiabetic, cardioprotective, hepatoprotective, and antiviral properties in recent years. The active constituents for the above-mentioned effects were proved to be polysaccharides, saponins, and flavonoids.
3.1. Anti-Inflammatory Activity
Astragalus extract, along with its polysaccharides, and saponins showed anti-inflammatory activity both in vitro and in vivo. Kim et al., found that the extract of A. membranaceus not only improved the atopic dermatitis skin lesions in 1-chloro-2,4-dinitrobenzene-induced mice as well as restoring nuclear factor-κB expression markedly, but also suppressed the expression of Th2 cytokines and significantly decreased the TNF-α level. They then figured out that A. membranaceus was effective for treating atopic dermatitis by regulating cytokines [80]. Ryu et al., verified that Astragali Radix had an anti-inflammatory effect mediated by the MKP-1-dependent inactivation of p38 and Erk1/2 and the inhibition of NFkappaB-mediated transcription [81]. As the main composition of Astragalus, Astragalus polysaccharides can effectively ameliorate the palmitate-induced pro-inflammatory responses in RAW264.7 cells through AMPK activity [82]. They also showed anti-inflammatory activity, along with structure protective properties for lipopolysaccharide-infected Caco2 cells [83]. On the other hand, the anti-inflammatory activity of saponins was also studied. The results, of agroastragalosides I, II, isoastragaloside II, and astragaloside IV showed the ability to inhibit lipopolysaccharide-induced nitric oxide production in RAW264.7 macrophages [84]. Meanwhile, astragaloside IV was shown to be a promising natural product with both healing and anti-scar effects for wound treatment [85], could be used as a novel anti-inflammatory agent, and attenuated diabetic nephropathy in rats by inhibiting NF-κB mediated inflammatory gene expression [86].
3.2. Immunoregulatory Activity
Qin et al., studied the effect of A. membranaceus extract on the advanced glycation end product-induced inflammatory response in α-1 macrophages. The results suggested that it could inhibit advanced glycation end product-induced inflammatory cytokine production to down-regulate macrophage-mediated inflammation via p38 mitogen-activated protein kinase and nuclear factor (NF)-κB signaling pathways [87]. Du et al., investigated the potential adjuvant effect of Astragalus polysaccharides on humoral and cellular immune responses to hepatitis B subunit vaccine. The result suggested that it was a potent adjuvant for the hepatitis B subunit vaccine and could enhance both humoral and cellular immune responses via activation of the Toll-like receptor 4 signaling pathway and inhibit the expression of transforming growth factor β [88]. Nalbantsoy et al., studied the in vivo effects of two Astragalus saponins on the immune response cytokines by using six to eight week old male Swiss albino mice. The results showed that astragaloside VII and macrophyllosaponin B showed powerful immunoregulatory effects without stimulation of inflammatory cytokines in mice, and had no significant effect on the inflammatory cellular targets in vitro [89]. Huang et al. found that astragaloside IV could rival the suppressing effect of high mobility group box 1 protein on immune function of regulatory T cells with dose-dependent in vitro [90].
3.3. Anti-Tumor Activity
Recently, many active screening results have indicated that Astragalus polysaccharides, saponins, and flavonoids have anti-tumor activities. Tian et al., investigated the adjunct anticancer effect of Astragalus polysaccharides on H22 tumor-bearing mice, and found that it exerted a synergistic anti-tumor effect with adriamycin and to alleviate the decrease in the sizes of the spleen and thymus induced by adriamycin in H22 tumor-bearing mice [91]. As a potential anti-tumor saponin, astragaloside IV could down-regulate Vav3.1 expression in a dose- and time-dependent manner [92]. Meanwhile, astragaloside II could down regulate the expression of the P-glycoprotein and mdr1 gene, which suggested it was a potent multidrug resistance reversal agent and could be a potential adjunctive agent for hepatic cancer chemotherapy [93]. On the other hand, the experimental data showed that the total flavonoids of Astragalus and calycosin could inhibit the proliferation of K562 cells [94].
3.4. Cardioprotection Activity
Ma et al., studied the cardio protective effect of the extract of Radix Astragali on myocardial ischemia and its underlying mechanisms in ROS-mediated signaling cascade in vivo by using different rat models, and drew the conclusion that the cardio protection was due to a protection of tissue structure and a decrease in serum markers of the ischemic injury [95]. The total flavonoids of A. mongholicus are the active components, which benefit cardiovascular disease attributed to the potent antioxidant activity in improving the atherosclerosis profile [96]. Isoflavones, calycosin and formononetin from the Astragalus root, could promote dimethylarginine dimethylaminohydrolase-2 protein and mRNA expressions in Madin Darby Canine Kidney (MDCK) II cells, and up regulate the neuronal nitric oxide synthase levels [97]. Astragaloside IV could prolong the action potential duration of guinea-pig ventricular myocytes, which might be explained by its inhibition of K+ currents [98].
3.5. Antidiabetic
The study of Liu et al., indicated that Astragalus polysaccharide could regulate part of the insulin signaling in insulin-resistant skeletal muscle, and could be a potential insulin sensitizer for the treatment of type 2 diabetes [99]. Zhou et al., found Astragalus polysaccharide could up regulate the expression of galectin-1 in muscle of type I diabetes mellitus mice [100]. Saponins and astragaloside IV could exert protective effects against the progression of peripheral neuropathy in streptozotocin-induced diabetes in rats [101]. In addition, astragaloside V was found to inhibit the formation of N-(carboxymethyl)lysine and pentosidine during the incubation of bovine serum albumin with ribose, which suggested that it might be a potentially useful strategy for the prevention of clinical diabetic complication by inhibiting advanced glycation end products [102].
3.6. Anti-Oxidative Activity
The anti-oxidative activities of some flavonoids and saponins from A. mongholicus have been studied. For example, formononetin, calycosin, calycosin-7-O-β-d-glucoside could scavenge 1,1-diphenyl-2-picrylhydrazyl free radicals in vitro. Formononetin and calycosin were found to inhibit xanthine/xanthine oxidase-induced cell injury significantly. Among them, calycosin exhibited the most potent antioxidant activity both in the cell-free system and in the cell system [73]. The compound 7,2-dihydroxy-3',4'-dimethoxyisoflavan-7-O-β-d-glucoside and calycosin-7-O-β-d-glucoside from A. membranaceus showed anti-lipid peroxidative activities [103]. The saponin, astragaloside IV can inhibit hepatic stellate cells activation by inhibiting generation of oxidative stress and associated p38 MAPK activation [104].
3.7. Anti-Aging
According to the study of the anti-aging effect of astragalosides, Lei et al., suggested that the mechanism might be related to the improvement of brain function and immunomodulatory effects [105]. Gao et al., concluded that Astragalus polysaccharides could lengthen the living time of mice, improve the activity of superoxide dismutase and decrease the malonaldehyde content in mice blood serum compared with the control group, which suggested that Astragalus polysaccharides have anti-aging effects [106].
3.8. Other Biological Activities
Additionally, according to previous research, the plants of Astragalus species also have pharmacological effects such as antiviral, hepatoprotective, neuron protective, and so on.
4. Analyses
Researchers have conducted numbers of qualitatively and quantitatively analytical experiments on the plants in the Astragalus genus by different methods. Among them, the analyses of flavonoids and saponins from the radix of A. membranaceus and A. mongholicus were well done.
Han et al., studied ultra-performance liquid chromatography for quantification of flavone in A. membranaceus, the outcome of which showed that the linear ranges of calycosin glycoside, formononetin glycoside, calycosin, and formononetin were 0.1313–1.313 g/L (r = 0.9997), 0.1186–1.186 g/L (r = 0.9994), 0.0206–0.206 g/L (r = 0.9995), and 0.0150–0.150 g/L (r = 0.9995), respectively. The average recoveries were 97.07%, 97.26%, 97.45% and 96.97% respectively [107]. The research results of Zhang et al., indicated that the content of flavonoids in Radix Astragali of different growth years increased along with the growth period, and the types of flavonoids remained the same [108]. By comparing the retention time and MS data with those obtained from the authentic compounds and the published data, Ye et al., identified a total of 19 compounds including 11 isoflavonoids and eight saponins [109].
In addition, analyses for other constituents in A. membranaceus and A. mongholicus and composition analysis for other species of Astragalus were also conducted. Huang et al., studied the water extract of Radix Astragali by infrared spectroscopy, and found it contained an abundance of polyose, and the residue from it included some substances such as starch, cellulose, and lignin. The study provided an effective reference for the further analyses of chemical components and the improvement of extraction-separation technologies of TCM [110]. Using solid phase microextraction followed by GC-MS analysis, Movafeghi et al., identified the volatile organic compounds in the leaves, roots and gum of A. compactus. As a result, a range of volatile compounds were recognized in different parts of it under optimized conditions, but tetradecane 1-chloro only existed in roots [111]. Sun et al., determined the chemical components of A. hamiensis with a systematic extract method and TLC, the results showed that it contained alkaloids, polysaccharides, glucosides, amino acids, steroids, terpenoids, oils, tannins, phenols, organic acids, flavonoids-chinones, cardiac glycosides, and coumarins. Futhermore, they found the alkaloids were mainly swainsonine and analogous indolizidine. Meanwhile, A. hamiensis was found to contain small amount of ermopsine and anagyrine, which belong to quinolizidine [112].
5. Conclusions
As a TCM, the root of the Astragalus plant, Huang Qi, has been used in Chinese medicine for thousands of years. There are over two thousand species of Astragalus, among which only A. membranaceus and A. mongholicus are primarily used for medicinal purposes. In light of the considerable interest generated in the chemistry and pharmacological properties of the Astragalus plant, this review summarizes the retrieved literature published over the last 10 years dealing with several aspects including phytochemistry, bioactivity, and the research of the analysis.
In the field of phytochemistry and analysis, the chemical constituents from 46 kinds of Astragalus species have been studied. Although more than 200 compounds, including cycloartane-type saponins and flavonoids, were obtained from them, the systematic phytochemistry research needs to be improved. In addition, though the researchers conducted a number of qualitatively and quantitatively analytical experiments for flavonoids and saponins from the radix of A. membranaceus and A. mongholicus by different methods, the simultaneous analysis of two kinds of constituents was rarely found. In the field of pharmacology, the bioactivity evaluation of extracts and isolated compounds focused on anti-inflammatory, immunostimulant, antioxidative, anti-cancer, antidiabetic, cardioprotective, hepatoprotective, and antiviral, but anti-inflammation activity research of flavonoids was rare. Though a lot of results of pharmacological studies were carried out using crude extract of Astragalus species, the relationship between chemical constituents and activity is still unclear. Additionally, data on pharmacokinetics and bioavailability, especially related to the target tissue, need to be further supplemented.
Acknowledgments
This research was supported by the Program for New Century Excellent Talents in University (NCET-12-1069), Program for Tianjin Innovative Research Team in University (TD12-5033).
Author Contributions
Xiaoxia Li: Acquisition, interpretation of data and wrote the manuscript. References management; Lu Qu: Drafted the structural formulas. Classified the chemical components; Yongzhe Dong: Classified the Pharmacological literatures; Lifeng Han: Revising the review critically for important intellectual content; Erwei Liu: Drafted the structural formulas; Shiming Fang: References management; Yi Zhang: Obtained funding. Contributed to conception and design of the review; Tao Wang: Obtained funding. Overall responsibility.
Conflicts of Interest
The authors declare no conflict of interest.
Reference
- 1.Podlech D. The genus Astragalus L. (Fabaceae) in Europe with exclusion of the former Soviet Union. Feddes Repertor. 2008;119:310–387. doi: 10.1002/fedr.200811171. [DOI] [Google Scholar]
- 2.Benchadi W., Haba H., Lavaud C., Harakat D., Benkhaled M. Secondary metabolites of Astragalus cruciatus Link. and their chemotaxonomic significance. Rec. Nat. Prod. 2013;7:105–113. [Google Scholar]
- 3.Xu L., Podlech D.F. Astragalus Linnaeus, Sp. Pl. 2: 755. 1753. [(accessed on 15 March 2014)];Flora China. 2010 10:329–333. Available online: http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=102978. [Google Scholar]
- 4.Avunduk S., Mitaine-Offer A.-C., Alankus-Caliskan O., Miyamoto T., Senol S.G., Lacaille-Dubois M.A. Triterpene glycosides from the roots of Astragalus flavescens. J. Nat. Prod. 2008;71:141–145. doi: 10.1021/np0703500. [DOI] [PubMed] [Google Scholar]
- 5.Choudhary M.I., Jan S., Abbaskhan A., Musharraf S.G., Samreen, Sattar S.A., Atta-ur-Rahman Cycloartane triterpenoids from Astragalus bicuspic. J. Nat. Prod. 2008;71:1557–1560. doi: 10.1021/np800161j. [DOI] [PubMed] [Google Scholar]
- 6.Fathiazad F., Khosropanah M.K., Movafeghi A. Cycloartane-type glycosides from the roots of Astragalus. caspicus Bieb. Nat. Prod. Res. 2010;24:1069–1078. doi: 10.1080/14786410902975582. [DOI] [PubMed] [Google Scholar]
- 7.Linnek J., Mitaine-Offer A.-C., Miyamoto T., Tanaka C., Paululat T., Avunduk S., Alankus-Caliskan O., Lacaille-Dubois M.-A. Cycloartane glycosides from three species of Astragalus (Fabaceae) Helv. Chim. Acta. 2011;94:230–237. doi: 10.1002/hlca.201000157. [DOI] [Google Scholar]
- 8.Ibrahim L.F., Marzouk M.M., Hussein S.R., Kawashty S.A., Mahmoud K., Saleh N.A.M. Flavonoid constituents and biological screening of Astragalus bombycinus Boiss. Nat. Prod. Res. 2013;27:386–393. doi: 10.1080/14786419.2012.701213. [DOI] [PubMed] [Google Scholar]
- 9.Savran T., Gulcemal D., Masullo M., Karayildirim T., Polat E., Piacente S., Alankus-Caliskan O. Cycloartane glycosides from Astragalus erinaceus. Rec. Nat. Prod. 2012;6:230–236. [Google Scholar]
- 10.Horo I., Bedir E., Masullo M., Piacente S., Ozgokce F., Alankus-Caliskan O. Saponins from Astragalus hareftae (NAB.) SIRJ. Phytochemistry. 2012;84:147–153. doi: 10.1016/j.phytochem.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Naubeev T.K., Uteniyazov K.K., Isaev M.I., Kachala V.V., Shashkov A.S. Structure of cycloquivinoside A from the aerial part of Astragalus chiwensis. Chem. Nat. Compd. 2012;48:810–812. doi: 10.1007/s10600-012-0389-8. [DOI] [Google Scholar]
- 12.Kim J.S., Yean M.-H., Lee E.-J., Jung H.S., Lee J.Y., Kim Y.J., Kang S.S. Two new cycloartane saponins from the roots of Astragalus membranaceus. Chem. Pharm. Bull. 2008;56:105–108. doi: 10.1248/cpb.56.105. [DOI] [PubMed] [Google Scholar]
- 13.Polat E., Bedir E., Perrone A., Piacente S., Alankus-Caliskan O. Triterpenoid saponins from Astragalus wiedemannianus Fischer. Phytochemistry. 2010;71:658–662. doi: 10.1016/j.phytochem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 14.Kucherbaev K.D., Uteniyazov K.K., Kachala V.V., Saatov Z., Shashkov A.S. Triterpene Glycosides from plants of the Astragalus Genus. III. Structure of cyclounifolioside C from Astragalus unifoliolatus. Chem. Nat. Compd. 2003;38:447–449. doi: 10.1023/A:1022111726617. [DOI] [Google Scholar]
- 15.Naubeev T.K., Uteniyazov K.K., Kachala V.V., Shashkov A.S. Cyclochivinoside B from the aerial part of Astragalus chivensis. Chem. Nat. Compd. 2007;43:166–169. doi: 10.1007/s10600-007-0070-9. [DOI] [Google Scholar]
- 16.Sun L.-M., Wang X.-L., Deng W.-L., Ding L.-S., Peng S.-L. Chemical constituents from Astragalus ernestii. Zhongguo Tianran Yaowu. 2011;9:38–41. [Google Scholar]
- 17.Polat E., Caliskan-Alankus O., Perrone A., Piacente S., Bedir E. Cycloartane-type glycosides from Astragalus amblolepis. Phytochemistry. 2009;70:628–634. doi: 10.1016/j.phytochem.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Oezipek M., Doenmez A.A., Calis I., Brun R., Rueedi P., Tasdemir D. Leishmanicidal cycloartane-type triterpene glycosides from Astragalus oleifolius. Phytochemistry. 2005;66:1168–1173. doi: 10.1016/j.phytochem.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 19.Alaniya M.D., Kavtaradze N.S., Gigoshvili T.I., Lavoi S., Pichette A., Mshvildadze V.V. Cyclocanthoside E from Astragalus caucasicus. Chem. Nat. Compd. 2007;43:758–759. doi: 10.1007/s10600-007-0260-5. [DOI] [Google Scholar]
- 20.Naubeev T.K., Uteniyazov K.K. Structure of cyclochivinoside C from Astragalus chivensis. Chem. Nat. Compd. 2007;43:560–562. doi: 10.1007/s10600-007-0192-0. [DOI] [Google Scholar]
- 21.Gulcemal D., Alankus-Caliskan O., Perrone A., Ozgokce F., Piacente S., Bedir E. Cycloartane glycosides from Astragalus aureus. Phytochemistry. 2011;72:761–768. doi: 10.1016/j.phytochem.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Horo I., Bedir E., Perrone A., Oezgoekce F., Piacente S., Alankus-Caliskan O. Triterpene glycosides from Astragalus icmadophilus. Phytochemistry. 2010;71:956–963. doi: 10.1016/j.phytochem.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 23.Naubeev T.K., Zhanibekov A.A., Isaev M.I. Triterpene glycosides from Astragalus and their genins. XCIII. Cycloascidoside from Astragalus mucidus. Chem. Nat. Comp. 2012;48:813–815. doi: 10.1007/s10600-012-0390-2. [DOI] [Google Scholar]
- 24.Perrone A., Masullo M., Bassarello C., Bloise E., Hamed A., Nigro P., Pizza C., Piacente S. Unusual cycloartane glycosides from Astragalus eremophilus. Tetrahedron. 2008;64:5061–5071. doi: 10.1016/j.tet.2008.03.069. [DOI] [Google Scholar]
- 25.Naubeev T.K., Uteniyazov K.K., Isaev M.I. Triterpene glycosides from Astragalus and their genins. LXXXVIII. Cycloascidoside A, a new bisdesmoside of cycloasgenin C. Chem. Nat. Compd. 2011;47:250–253. doi: 10.1007/s10600-011-9894-4. [DOI] [Google Scholar]
- 26.Yalcin F.N., Piacente S., Perrone A., Capasso A., Duman H., Calis I. Cycloartane glycosides from Astragalus stereocalyx Bornm. Phytochemistry. 2012;73:119–126. doi: 10.1016/j.phytochem.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Djimtombaye B.J., Alankus-Caliskan O., Gulcemal D., Khan I.A, Anil H., Bedir E. Unusual secondary metabolites from Astragalus halicacabus Lam. Chem. Biodivers. 2013;10:1328–1334. doi: 10.1002/cbdv.201200436. [DOI] [PubMed] [Google Scholar]
- 28.Calis I., Doenmez A.A., Perrone A., Pizza C., Piacente S. Cycloartane glycosides from Astragalus campylosema Boiss. ssp. campylosema. Phytochemistry. 2008;69:2634–2638. doi: 10.1016/j.phytochem.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Karabey F., Khan I.A., Bedir E. Cycloartane-type glycosides from Astragalus schottianus. Phytochem. Lett. 2012;5:320–324. doi: 10.1016/j.phytol.2012.02.011. [DOI] [Google Scholar]
- 30.Iskenderov D.A., Isaev I.M., Isaev M.I. Triterpene glycosides from Astragalus and their genins. LXXVII. Cyclomacrogenin B, a new cycloartane triterpenoid. Chem. Nat. Compd. 2008;44:621–624. doi: 10.1007/s10600-008-9127-7. [DOI] [Google Scholar]
- 31.Iskenderov D.A., Isaev I.M., Isaev M.I. Triterpene glycosides from Astragalus and their genins. LXXX. Cyclomacroside D, a new bisdesmoside. Chem. Nat. Compd. 2009;45:55–58. doi: 10.1007/s10600-009-9256-7. [DOI] [Google Scholar]
- 32.Iskenderov D.A., Isaev I.M., Isaev M.I. Triterpene glycosides from Astragalus and their genins. LXXXV. Structure of cyclomacroside E. Chem. Nat. Compd. 2010;46:250–253. doi: 10.1007/s10600-010-9580-y. [DOI] [Google Scholar]
- 33.Iskenderov D.A., Isaev I.M., Isaev M.I. Triterpene glycosides from Astragalus and their genins. LXXXII. Cyclomacroside B, a new glycoside. Chem. Nat. Compd. 2009;45:511–513. doi: 10.1007/s10600-009-9397-8. [DOI] [Google Scholar]
- 34.Yu Q.T., Li P., Bi Z.M., Luo J., Gao X.D. Two new saponins from the aerial part of Astragalus membranaceus var. mongholicus. Chin. Chem. Lett. 2007;18:554–556. doi: 10.1016/j.cclet.2007.03.025. [DOI] [Google Scholar]
- 35.Isaev I.M., Iterpene gsaev M.I. Trilycosides from Astragalus and their genins. XC. Askendoside K from Astragalus taschkendicus. Chem. Nat. Compd. 2011;47:587–591. doi: 10.1007/s10600-011-0002-6. [DOI] [Google Scholar]
- 36.Isaev I.M., Isaev M.I. Triterpene glycosides from Astragalus and their genins. LXXXIX. Askendoside H from Astragalus taschkendicus. Chem. Nat. Compd. 2011;47:411–414. doi: 10.1007/s10600-011-9946-9. [DOI] [Google Scholar]
- 37.Isaev I.M., Mamedova R.P., Agzamova M.A., Isaev M.I. Triterpene glycosides and their genins from Astragalus. LXXIII. Stereochemistry of C-23 and C-24 in cycloartan and lanostan-16β,23,24,25-tetraols. Chem. Nat. Compd. 2007;43:115–116. doi: 10.1007/s10600-007-0048-7. [DOI] [Google Scholar]
- 38.Mamedova R.P., Agzamova M.A., Isaev M.I. Triterpene glycosides of Astragalus and their genins. LXV. Cycloartane and lanostane triterpenoids of Astragalus orbiculatus. Chem. Nat. Compd. 2003;38:354–355. doi: 10.1023/A:1021634210241. [DOI] [Google Scholar]
- 39.Jan S., Abbaskhan A., Musharraf S.G., Sattar S.A., Samreen, Resayes S.I., Al-Othman Z.A., Al-Majid A.M., Atta-ur-Rahman C.M.I. Three new cycloartane triterpenoids from Astragalus bicuspis. Planta Med. 2011;77:1829–1834. doi: 10.1055/s-0030-1271196. [DOI] [PubMed] [Google Scholar]
- 40.Radwan M.M., El-Sebakhy N.A., Asaad A.M., Toaima S.M., Kingston D.G.I. Kahiricosides II-V, cycloartane glycosides from an Egyptian collection of Astragalus kahiricus. Phytochemistry. 2004;65:2909–2913. doi: 10.1016/j.phytochem.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 41.Isaev I.M., Iskenderov D.A., Isaev M.I. Triterpene glycosides and their genins from Astragalus. LXXXIV. Secomacrogenin B, a new 9,10-seco-cycloartane. Chem. Nat. Compd. 2010;46:36–38. doi: 10.1007/s10600-010-9519-3. [DOI] [Google Scholar]
- 42.Naubeev T.K., Isaev M.I. Triterpene glycosides of Astragalus and their genins. XCII. Cycloartane glycosides from Astragalus oldenburgii. Chem. Nat. Compd. 2012;48:704–705. doi: 10.1007/s10600-012-0358-2. [DOI] [Google Scholar]
- 43.Sukhina I.A., Mamedova R.P., Agzamova M.A., Isaev M.I. Triterpene glucosides of Astragalus and their genins. LXXIV. Cyclotrisectoside, the first trisdesmoside of cyclocephalogenin. Chem. Nat. Compd. 2007;43:159–161. doi: 10.1007/s10600-007-0068-3. [DOI] [Google Scholar]
- 44.Zheng S., Wang Z. Chemical constituents of the roots of Astragalus membranace (Fisch.) Bge. var. mongholicus (Bge.) hisao. Shanghai Zhongyiyao Daxue Xuebao. 2011;25:89–94. [Google Scholar]
- 45.Gulcemal D., Masullo M., Bedir E., Festa M., Karayildirim T., Alankus-Caliskan O., Piacente S. Triterpene glycosides from Astragalus angustifolius. Planta Med. 2012;78:720–729. doi: 10.1055/s-0031-1298337. [DOI] [PubMed] [Google Scholar]
- 46.Zhanibekov A.A., Naubeev T.K., Uteniyazov K.K., Bobakulov Kh.M., Abdullaev N.D. Triterpene glycosides from Astragalus. Structure of cyclolehmanoside C from Astragalus lehmannianus. Chem. Nat. Compd. 2013;49:475–477. doi: 10.1007/s10600-013-0642-9. [DOI] [Google Scholar]
- 47.Semmar N., Tomofumi M., Mrabet Y., Lacaille-Dubois M.A. Two New acylated tridesmosidic saponins from Astragalus armatus. Helv. Chim. Acta. 2010;93:870–876. doi: 10.1002/hlca.200900303. [DOI] [Google Scholar]
- 48.Semmar N., Tomofumi M., Mrabet Y., Lacaille-Dubois M.A. Antitrypanosomal cycloartane glycosides from Astragalus baibutensis. Chem. Biodivers. 2006;3:923–929. doi: 10.1002/cbdv.200690094. [DOI] [PubMed] [Google Scholar]
- 49.Barbic M., Macabeo A.P.G., Kreft S., Heilmann J. Cycloastragenol glycosides from Astragalus illyricus. Biochem. Syst. Ecol. 2010;38:460–462. doi: 10.1016/j.bse.2010.03.016. [DOI] [Google Scholar]
- 50.Zhang L.J., Liu H.K., Hsiao P.C., Kuo L.M.Y., Lee I.-J., Wu T.S., Chiou W.F., Kuo Y.H. New isoflavonoid glycosides and related constituents from Astragali Radix (Astragalus membranaceus) and their inhibitory activity on nitric oxide production. J. Agric. Food Chem. 2011;59:1131–1137. doi: 10.1021/jf103610j. [DOI] [PubMed] [Google Scholar]
- 51.Iskenderov D.A., Keneshov B.M., Isaev M.I. Triterpene glycosides from Astragalus and their genins. LXXVI. Glycosides from Astragalus sieversianus. Chem. Nat. Compd. 2008;44:319–323. doi: 10.1007/s10600-008-9052-9. [DOI] [Google Scholar]
- 52.Isaev I.M., Iskenderov D.A., Isaev M.I. Triterpene glycosides from Astragalus and their genins. LXXXVII. Chemical transformation of cycloartanes. IX. Partial synthesis of cycloasalgenin. Chem. Nat. Compd. 2010;46:407–411. doi: 10.1007/s10600-010-9629-y. [DOI] [Google Scholar]
- 53.Pistelli L., Giachi I., Lepori E., Bertoli A. Further saponins and flavonoids from Astragalus verrucosus Moris. Pharm. Biol. 2003;41:568–572. doi: 10.1080/13880200390501370. [DOI] [Google Scholar]
- 54.Gigoshvili T.I., Alaniya M.D., Tsitsishvili V.G., Foure R., Debrauver L., Kemertelidze E.P. Structure of cyclogaleginoside E from Astragalus galegiformis. Chem. Nat. Compd. 2003;39:373–378. doi: 10.1023/B:CONC.0000003419.24465.3f. [DOI] [Google Scholar]
- 55.Alaniya M.D., Gigoshvili T.I. New cycloartane glycosides from Astragalus caucasicus and Astragalus galegiformis. Chem. Nat. Compd. 2012;48:914–916. doi: 10.1007/s10600-012-0424-9. [DOI] [Google Scholar]
- 56.Alaniya M.D., Chkadua N.F., Gigoshvili T.I., Kemertelidze E.P. Cycloascauloside A from Astragalus caucasicus leaves. Chem. Nat. Compd. 2006;42:445–448. doi: 10.1007/s10600-006-0177-4. [DOI] [Google Scholar]
- 57.Alaniya M.D., Gigoshvili T.I., Kavtaradze N.S. Cyclogaleginoside D from Astragalus galegiformis stems. Chem. Nat. Compd. 2006;42:310–312. doi: 10.1007/s10600-006-0107-5. [DOI] [Google Scholar]
- 58.Iskenderov D.A., Isaev I.M., Isaev M.I. Triterpene glycosides from Astragalus and their genins. LXXXIII. Structure of cyclomacroside A. Chem. Nat. Compd. 2009;45:656–659. doi: 10.1007/s10600-009-9437-4. [DOI] [Google Scholar]
- 59.Radwan M.M., Farooq A., El-Sebakhy N.A., Asaad A.M., Toaima S.M., Kingston D.G. Acetals of three new cycloartane-type saponins from Egyptian collections of Astragalus tomentosus. J. Nat. Prod. 2004;67:487–490. doi: 10.1021/np030395a. [DOI] [PubMed] [Google Scholar]
- 60.Kuang H., Okada Y., Yang B., Tian Z., Okuyama T. Secocycloartane triterpenoidal saponins from the leaves of Astragalus membranaceus Bunge. Helv. Chim. Acta. 2009;92:950–958. doi: 10.1002/hlca.200800341. [DOI] [Google Scholar]
- 61.Gulcemal D., Masullo M., Napolitano A., Karayildirim T., Bedir E., Alankus-Caliskan O., Piacente S. Oleanane glycosides from Astragalus tauricolus: Isolation and structural elucidation based on a preliminary liquid chromatography-electrospray ionization tandem mass spectrometry profiling. Phytochemistry. 2013;86:184–194. doi: 10.1016/j.phytochem.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 62.Mitaine-Offer A.C., Miyamoto T., Semmar N., Jay M., Lacaille-Dubois M.A. A new oleanane glycoside from the roots of Astragalus caprinus. Magn. Reson. Chem. 2006;44:713–716. doi: 10.1002/mrc.1809. [DOI] [PubMed] [Google Scholar]
- 63.Krasteva I., Nikolov S. Flavonoids in Astragalus corniculatus. Quim. Nova. 2008;31:59–60. doi: 10.1590/S0100-40422008000100012. [DOI] [Google Scholar]
- 64.Guzhva N.N. Flavonoids and hydroxycinnamic acids from Astragalus asper. Chem. Nat. Compd. 2010;46:303–304. doi: 10.1007/s10600-010-9597-2. [DOI] [Google Scholar]
- 65.Fathiazad F., Movafeghi A., Khosropanah M.K. Flavonol glycosides from the leaves of Astragalus microcephalus. Int. J. Biosci. 2012;2:23–28. [Google Scholar]
- 66.Kavtaradze N.S., Alaniya M.D., Mshvildadze V.D., Skhirtladze A.V., Lavoie S., Pichette A. Flavonoids from Astragalus microcephalus. Chem. Nat. Compd. 2011;46:971–973. doi: 10.1007/s10600-011-9800-0. [DOI] [Google Scholar]
- 67.Alaniya M.D., Kavtaradze N.S., Bassarello C., Skhirtladze A.V., Pizza C., Kutateladze I. Flavonoid glycosides from Astragalus galegiformis leaves. Chem. Nat. Compd. 2006;42:681–685. doi: 10.1007/s10600-006-0251-y. [DOI] [Google Scholar]
- 68.Alaniya M.D., Kavtaradze N.S., Bassarello C., Skhirtladze A.V., Pizza C., Kutateladze I. Flavonoids from Astragalus hamosus. Nat. Prod. Res. 2007;21:392–395. doi: 10.1080/14786410701236871. [DOI] [PubMed] [Google Scholar]
- 69.Chaturvedula V.S.P., Prakash I. Flavonoids from Astragalus propinquus. J. Chem. Pharm. Res. 2013;5:261–265. [Google Scholar]
- 70.Li W., Sun Y.N., Yan X.T., Yang S.Y., Kim S., Lee Y.M., Koh Y.S., Kim Y.H. Flavonoids from Astragalus membranaceus and their inhibitory effects on LPS-stimulated pro-inflammatory cytokine production in bone marrow-derived dendritic cells. Arch. Pharm. Res. 2014;37:186–192. doi: 10.1007/s12272-013-0174-7. [DOI] [PubMed] [Google Scholar]
- 71.Du X., Bai Y., Liang H., Wang Z., Zhao Y., Zhang Q., Huang L. Solvent effect in 1H-NMR spectra of 3'-hydroxy-4'-methoxy isoflavonoids from Astragalus membranaceus var. mongholicus. Magn. Reson. Chem. 2006;44:708–712. doi: 10.1002/mrc.1806. [DOI] [PubMed] [Google Scholar]
- 72.Bi Z.M., Yu Q.T., Li P., Lin Y., Gao X.D. Flavonoids from aerial parts of Astragalus membranaceus. Zhongguo Tianran Yaowu. 2007;5:263–265. [Google Scholar]
- 73.Yu D.H., Bao Y.M., Wei C., An L.J. Studies of chemical constituents and their antioxidant activities from Astragalus mongholicus Bunge. Biomed. Environ. Sci. 2005;18:297–301. [PubMed] [Google Scholar]
- 74.Liu W., Chen J., Zuo W.J., Li X., Wang J.H. A new isoflavane from processed Astragalus membranaceus. Chin. Chem. Lett. 2007;18:1092–1094. doi: 10.1016/j.cclet.2007.07.036. [DOI] [Google Scholar]
- 75.Abd El-Latif R.R., Shabana M.H., El-Gandour A.H., Mansour R.M., Sharaf M. A new isoflavone from Astragalus peregrinus. Chem. Nat. Compd. 2003;39:536–537. doi: 10.1023/B:CONC.0000018105.23722.7d. [DOI] [Google Scholar]
- 76.Li R., Zhou Y, Qiao Li, Fu H., Pei Y. Separation and identification of chemical constituents from Astragalus membranaceus. Shenyang Yaoke Daxue Xuebao. 2007;24:20–22. [Google Scholar]
- 77.Yao D., Wang H. Monosaccharide composition in Radix Astragali polysaccharides by gas chromatography. Med. Plant. 2012;3:36–38. [Google Scholar]
- 78.Xu D.J., Xia Q., Wang J.J., Wang P.P. Molecular weight and monosaccharide composition of Astragalus polysaccharides. Molecules. 2008;13:2408–2415. doi: 10.3390/molecules13102408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li M., Liu X. Determination of eight metal elements in Astragalus by microwave digestion-flame atomic absorption spectrometry. Fenxi Kexue Xuebao. 2009;25:605–608. [Google Scholar]
- 80.Kim J.H., Kim M.H., Yang G., Huh Y., Kim S.H., Yang W.M. Effects of topical application of Astragalus membranaceus on allergic dermatitis. Immunopharmacol. Immunotoxicol. 2013;35:151–156. doi: 10.3109/08923973.2012.733708. [DOI] [PubMed] [Google Scholar]
- 81.Ryu M., Kim E.H., Chun M., Kang S., Shim B., Yu Y.B., Jeong G., Lee J.S. Astragali Radix elicits anti-inflammation via activation of MKP-1, concomitant with attenuation of p38 and Erk. J. Ethnopharmacol. 2008;115:184–193. doi: 10.1016/j.jep.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 82.Lu J., Chen X., Zhang Y., Xu J., Zhang L., Li Z., Liu W., Ouyang J., Han S., He X. Astragaluspolysaccharide induces anti-inflammatory effects dependent on AMPK activity in palmitate-treated RAW264.7 cells. Int. J. Mol. Med. 2013;31:1463–1470. doi: 10.3892/ijmm.2013.1335. [DOI] [PubMed] [Google Scholar]
- 83.Wang X., Li Y., Yang X., Yao J. Astragalus polysaccharide reduces inflammatory response by decreasing permeability of LPS-infected Caco2 cells. Int. J. Biol. Macromol. 2013;61:347–352. doi: 10.1016/j.ijbiomac.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 84.Lee D.Y., Noh H.J., Choi J., Lee K.H., Lee M.H., Lee J.H., Hong Y., Lee S.E., Kim S.Y., Kim G.S. Anti-inflammatory cycloartane-type saponins of Astragalus membranaceus. Molecules. 2013;18:3725–3732. doi: 10.3390/molecules18043725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen X., Peng L.H., Li N., Li Q.M., Li P., Fung K.P., Leung P.C., Gao J.Q. The healing and anti-scar effects of astragaloside IV on the wound repair in vitro and in vivo. J. Ethnopharmacol. 2012;139:721–727. doi: 10.1016/j.jep.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 86.Gui D., Huang J., Guo Y., Chen J., Chen Y., Xiao W., Liu X., Wang N. Astragaloside IV ameliorates renal injury in streptozotocin-induced diabetic rats through inhibiting NF-κB-mediated inflammatory genes expression. Cytokine. 2013;61:970–977. doi: 10.1016/j.cyto.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 87.Qin Q., Niu J., Wang Z., Xu W., Qiao Z., Gu Y. Astragalus membranaceus inhibits inflammation via phospho-p38 mitogen-activated protein kinase (MAPK) and nuclear factor (NF)-κB pathways in advanced glycation end product-stimulated macrophages. Int. J. Mol. Sci. 2012;13:8379–8387. doi: 10.3390/ijms13078379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Du X., Chen X., Zhao B., Lv Y., Zhang H., Liu H., Chen Z., Chen Y., Zeng X. Astragalus polysaccharides enhance the humoral and cellular immune responses of hepatitis B surface antigen vaccination through inhibiting the expression of transforming growth factor β and the frequency of regulatory T cells. FEMS Immunol. Med. Microbiol. 2011;63:228–235. doi: 10.1111/j.1574-695X.2011.00845.x. [DOI] [PubMed] [Google Scholar]
- 89.Nalbantsoy A., Nesil T., Yilmaz-Dilsiz O., Aksu G., Khan S., Bedir E. Evaluation of the immunomodulatory properties in mice and in vitro anti-inflammatory activity of cycloartane type saponins from Astragalus species. J. Ethnopharmcol. 2012;139:574–581. doi: 10.1016/j.jep.2011.11.053. [DOI] [PubMed] [Google Scholar]
- 90.Huang L.F., Yao Y.M., Li J.F., Zhang S.W., Li W.X., Dong N., Yu Y., Sheng Z.Y. The effect of Astragaloside IV on immune function of regulatory T cell mediated by high mobility group box 1 protein in vitro. Fitoterapia. 2012;83:1514–1522. doi: 10.1016/j.fitote.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 91.Tian Q.E., Li H.D., Yan M., Cai H.L., Tan Q.Y., Zhang W.Y. Astragaluspolysaccharides can regulate cytokine and P-glycoprotein expression in H22 tumor-bearing mice. World J. Gastroentero. 2012;18:7079–7086. doi: 10.3748/wjg.v18.i47.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Qi H., Wei L., Han Y., Zhang Q., Lau A.S., Rong J. Proteomic characterization of the cellular response to chemopreventive triterpenoid astragaloside IV in human hepatocellular carcinoma cell line HepG2. Int. J. Oncol. 2010;36:725–735. doi: 10.3892/ijo_00000548. [DOI] [PubMed] [Google Scholar]
- 93.Huang C., Xu D., Xia Q., Wang P., Rong C., Su Y. Reversal of P-glycoprotein-mediated multidrug resistance of human hepatic cancer cells by Astragaloside II. J. Pharm. Pharmacol. 2012;64:1741–1750. doi: 10.1111/j.2042-7158.2012.01549.x. [DOI] [PubMed] [Google Scholar]
- 94.Zhang D., Zhuang Y., Pan J., Wang H., Li H., Yu Y., Wang D. Investigation of effects and mechanisms of total flavonoids of Astragalus and calycosin on human erythroleukemia cells. Oxid. Med. Cell Longev. 2012;2012:209843. doi: 10.1155/2012/209843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ma X., Zhang K., Li H., Han S., Ma Z., Tu P. Extracts from Astragalus membranaceus limit myocardial cell death and improve cardiac function in a rat model of myocardial ischemia. J. Ethnopharmacol. 2013;149:720–728. doi: 10.1016/j.jep.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 96.Wang D., Zhuang Y., Tian Y., Thomas G.N., Ying M., Tomlinson B. Study of the effects of total flavonoids of Astragalus on atherosclerosis formation and potential mechanisms. Oxid. Med. Cell. Longev. 2012;2012 doi: 10.1155/2012/282383. doi:org/10.1155/2012/282383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bai F., Makino T., Kono K., Nagatsu A., Ono T., Mizukami H. Calycosin and formononetin from astragalus root enhance dimethylarginine dimethylaminohydrolase 2 and nitric oxide synthase expressions in Madin Darby Canine Kidney II cells. J. Nat. Med. 2013;67:782–789. doi: 10.1007/s11418-013-0749-0. [DOI] [PubMed] [Google Scholar]
- 98.Zhao M., Zhao J., He G., Sun X., Huang X., Hao L. Effects of astragaloside IV on action potentials and ionic currents in guinea-pig ventricular myocytes. Biol. Pharm. Bull. 2013;36:515–521. doi: 10.1248/bpb.b12-00655. [DOI] [PubMed] [Google Scholar]
- 99.Liu M, Wu K, Mao X, Wu Y, Ouyang J. Astragalus polysaccharide improves insulin sensitivity in KKAy mice: Regulation of PKB/GLUT4 signaling in skeletal muscle. J. Ethnopharmacol. 2009;127:32–37. doi: 10.1016/j.jep.2009.09.055. [DOI] [PubMed] [Google Scholar]
- 100.Zhou X., Xu Y., Yang G., Li F. Increased galectin-1 expression in muscle of Astragalus polysaccharide-treated Type 1 diabetic mice. J. Nat. Med. 2011;65:500–507. doi: 10.1007/s11418-011-0527-9. [DOI] [PubMed] [Google Scholar]
- 101.Yu J., Zhang Y., Sun S., Shen J., Qiu J., Yin X., Yin H., Jiang S. Inhibitory effects of astragaloside IV on diabetic peripheral neuropathy in rats. Can. J. Physiol. Pharmacol. 2006;84:579–587. doi: 10.1139/y06-015. [DOI] [PubMed] [Google Scholar]
- 102.Motomura K., Fujiwara Y., Kiyota N., Tsurushima K., Takeya M., Nohara T., Nagai R., Ikeda T. Astragalosides isolated from the root of astragalus radix inhibit the formation of advanced glycation end products. J. Agric. Food Chem. 2009;57:7666–7672. doi: 10.1021/jf9007168. [DOI] [PubMed] [Google Scholar]
- 103.Kim E.J., Yang K.S. Antilipidperoxidative activity of Astragalus membranaceus. Yakhak. Hoechi. 2005;49:11–19. [Google Scholar]
- 104.Li X., Wang X., Han C., Wang X., Xing G., Zhou L., Li G, Niu Y. Astragaloside IV suppresses collagen production of activated hepatic stellate cells via oxidative stress-mediated p38 MAPK pathway. Free Radic. Biol. Med. 2013;60:168–176. doi: 10.1016/j.freeradbiomed.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 105.Lei H., Wang B., Li W.P., Yang Y., Zhou A.W., Chen M.Z. Anti-aging effect of astragalosides and its mechanism of action. Acta Pharmacol. Sin. 2003;24:230–234. [PubMed] [Google Scholar]
- 106.Gao X., Li L., Liu B. Effect of Astragalus polysaccharides on stress response ability and regulation of free radicals in mice. Zhongguo Yufang Yixue Zazhi. 2010;11:120–121. [Google Scholar]
- 107.Han L., Yu C., Lin H. Ultra-performance liquid chromatography for quantification of flavone in Astragalus membranaceus and its preparation. Zhongguo Shiyan Fangjixue Zazhi. 2012;18:115–118. [Google Scholar]
- 108.Zhang Y., Liu N., Liu S., Yang S., Zhang S. Comparative study on flavonoids extracted from Astragalus membranaceus of different growth year. Yanbian Daxue Yixue Xuebao. 2011;34:34–37. [Google Scholar]
- 109.Ye G., Tang Y.H., Xia G.X., Sun Z.L., Li Z.X., Huang C.G. Characterization of anti-coxsackie virus B3 constituents of Radix Astragali by high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Biomed. Chromatogr. 2010;24:1147–1151. doi: 10.1002/bmc.1400. [DOI] [PubMed] [Google Scholar]
- 110.Huang D., Xu Y., Chen X. Analysis of Radix Astragali and its water extract by infrared spectroscopy. Guangpu Shiyanshi. 2012;29:2823–2826. [Google Scholar]
- 111.Movafeghi A., Djozan D., Razeghi J.A., Baheri T. Identification of volatile organic compounds in leaves, roots and gum of Astragalus compactus Lam. using solid phase microextraction followed by GC-MS analysis. Nat. Prod. Res. 2010;24:703–709. doi: 10.1080/14786410802361446. [DOI] [PubMed] [Google Scholar]
- 112.Sun L., Lu H., Zhao B., Huo X., Wan X., Liu Z., Wang Z. Preliminary chemical test of Astragalus hamiensis S.B.Ho and alkaloids analysis. Zhongguo Shouyi Xuebao. 2009;29:1217–1221. [Google Scholar]